Abstract
Lung neuroendocrine tumors are a heterogeneous subtype of pulmonary cancers representing approximately 20% of all lung cancers, including small-cell lung cancer (SCLC) and large-cell neuroendocrine carcinoma (LCNEC). The frequency appears to be approximately 3% for LCNEC. Diagnosis of LCNEC requires attention to neuroendocrine features by light microscopy and confirmation by immunohistochemical staining for neuroendocrine markers. Both SCLC and pulmonary LCNEC are high-grade and poor-prognosis tumors, with higher incidence in males and smokers and peripheral localization. LCNEC is very rare, and the precise diagnosis on small specimens is very difficult, so we have still too few data to define a standard of treatment for pulmonary LCNECs. Data of literature, most based on retrospective analysis, indicated a poor 5-year overall survival, with a high incidence of recurrence after surgery, even in stage I disease. Primary surgery should be the first option in all operable patients because there is no validate therapeutic approach for LCNEC due to lack of clinical trials in this setting. Neoadjuvant platinum-based regimens remain only an option for potentially resectable tumors. In advanced stages, SCLC-like chemotherapy seems the best option of treatment, with a good response rate but a poor overall survival (from 8 to 16 months in different case series). New agents are under clinical investigation to improve LCNEC patients’ outcome. We reviewed all data on treatment options feasible for pulmonary LCNEC, both for localized and extensive disease.
Keywords: Lung neuroendocrine tumors, Large-cell neuroendocrine carcinoma, Pathologic characterization, Cancer treatment
Lung neuroendocrine tumors are a heterogeneous group of cancers originating from neuroendocrine cells in the pulmonary and bronchial epithelium and represent 20% of all lung cancers.1
In the 1970s, pulmonary neuroendocrine tumors were classified into three histologically defined categories: typical carcinoids (TC), atypical carcinoids (AC), usually defined as carcinoids, and the more undifferentiated entity represented by small-cell lung cancer (SCLC).2 In 1991, Travis et al. introduced a new distinct category of lung cancer, defined as large-cell neuroendocrine carcinoma (LCNEC), which showed large cells with abundant cytoplasm, necrotic areas, a high mitotic rate, and neuroendocrine features. It shared some characteristics with SCLC, while differing because this latter presents smaller cells, with low nuclear/cytoplasm ratio and a different pattern of tissue invasiveness.3 Later in 1999 and 2004, the World Health Organization recognizes LCNEC as a variant of large cell carcinoma (LCC), a type of non–small-cell lung cancer (NSCLC) and one of the four major types of lung neuroendocrine tumors.4–6
Currently, LCNECs are considered as a separate entity for clinical characteristics, histology, prognosis, and survival.
INCIDENCE AND EPIDEMIOLOGY
Pulmonary LCNECs are rare tumors of the lung: in a series of surgically resected cases, the incidence of pulmonary LCNECs appeared to be between 2.1% and 3.5%. However, the frequency appears to be higher than estimated because of difficulties in diagnosis on cytological specimens.7
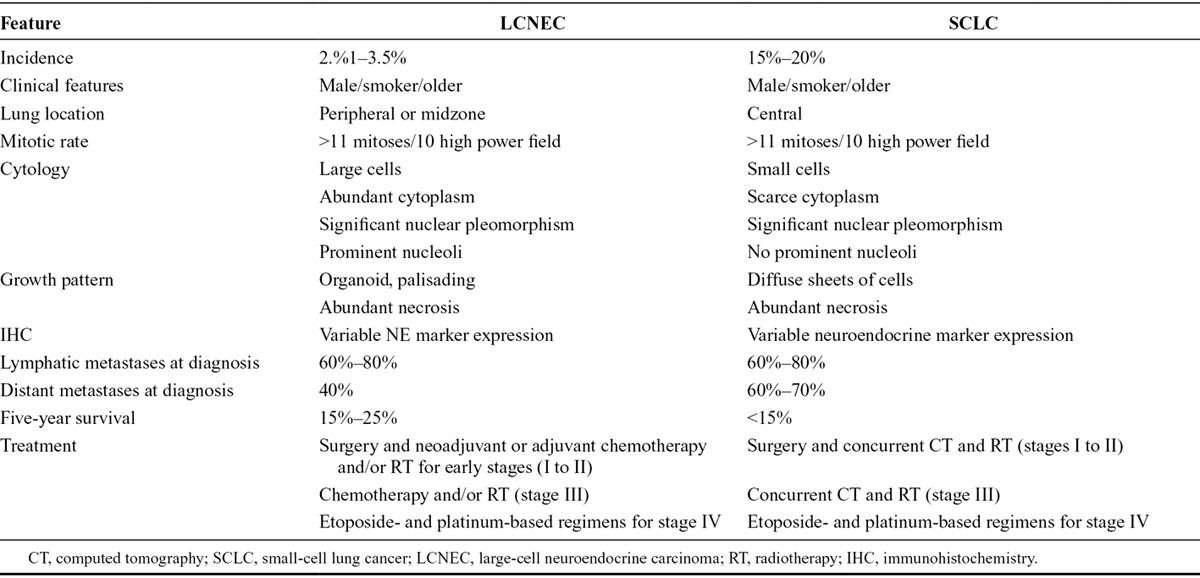
Unlike TCs and ACs, LCNECs are often associated with male sex, older age (median age is 65 years), and heavy smoking habit8–11 (Table 1).
Table 1.
Main Clinicopathologic Characteristics of Pulmonary LCNECsa

CLINICAL PRESENTATION
Several characteristics differentiate LCNECs from carcinoids (TCs and ATs), indicating a more aggressive behavior. Patients with LCNECs are poorly symptomatic; cough, hemoptysis, or postobstructive pneumonia are infrequent. Sometimes, patients present an asymptomatic nodule or chest pain, nonspecific flu-like symptoms, dyspnea, night sweats, and carcinoid syndrome. Paraneoplastic syndromes are quite uncommon. At the moment of diagnosis, among pulmonary neuroendocrine tumors, LCNEC present high rate of lymph node (60%–80%) and distant metastasis (40%), similar to SCLC8 (Table 1).
DIAGNOSIS AND STAGING
Diagnosis of LCNEC could be suggested by conventional radiograph of the chest and computed tomography scan. There are no specific findings in conventional radiographic examination; LCNECs often are peripherally located expansively growing lesions with irregular margins, with unspecific calcifications in 10%.12 Bronchoscopy and staging are recommended. International Association for the Study of Lung Cancer suggested application of tumor, node, metastasis (TNM) staging to predict prognosis for neuroendocrine tumors.13
Because neuroendocrine tumors frequently express somatostatin receptors (SSTR), mostly type 2 (68%),14 SSTR scintigraphy diagnostic techniques have been used for their imaging work-up. In particular, OctreoScan (indium 11-tagged diethylenetriaminepentaacetic acid pentreotide scintigraphy) targets with high-affinity SSTR2, SSTR3, and SSTR5, whereas 111In-DOTA-TOC (111In-DOTA-DPhe1-Tyr3-octreotide) and 111In-DOTA-LAN (111In-DOTA-lanreotide) targets, especially, with SSTR2 and SSTR5. These imaging procedures have been proposed to be used in preoperative staging and in postoperative follow-up of LCNEC, but there is still no evidence supporting their use in clinical practice, as it is for F-18 fluorodeoxyglucose positron emission tomography imaging, which is still controversial. Indeed, in neuroendocrine tumors, F-18 fluorodeoxyglucose positron emission tomography can have a minor sensitivity than 111In-DOTA-TOC and 111In-DOTA-LAN in detecting metastatic lesions, expecially for those located in mediastinum.15
Pulmonary LCNECs diagnosis often requires immunohistochemical staining and sometimes electronic microscopy to identify clear marks of neuroendocrine differentiation, which are difficult to perform on small biopsies or cytology specimens. Consequently, diagnosis is rarely enunciated without surgery.5
PATHOLOGIC CHARACTERIZATION
Histologic features of pulmonary LCNEC include large cell size (similar to three or more lymphocytes), areas of abundant necrosis, low nuclear/cytoplasm ratio, neuroendocrine differentiation growth pattern such organoid nests, trabecular, rosette and palisading features, a variably granular pattern of chromatin, clear or atypical nucleoli, and high mitotic rate (11 or more mitoses per 10 high-power fields)16–19 (Table 1).
Foci of squamous or adenomatous differentiation sometimes coexist in these tumors, creating mixed pathologic entities called “mixed LCNEC.” Although prospective data seem to be uncertain, mixed LCNEC exhibit an aggressive behavior, with a 5-year overall survival (OS) of 30%, quite similar to “pure LCNEC.”20
LCNEC and AC share some pathologic features, such as growth patterns and necrosis, so differential diagnosis may be challenging. For instance, AC presents fewer mitotic figures and LCNEC exhibit much more necrosis.18 Indeed, a mitotic rate of 11 mitoses or more per 10 high-power fields is a key factor to differentiate LCNEC and SCLC from AC.21 Moreover, with respect to basaloid carcinoma, it presents more often comedo-like necrosis compared with the abundant one of LCNEC, and in addition, it does not express generally neuroendocrine markers.18
To achieve a more precise diagnosis, a careful pathologic review is recommended because it is quite easy to mistake an LCNEC for a poorly differentiated NSCLC, an AC and even an SCLC. The diagnosis of LCNEC is difficult on small biopsy or cytological samples and often described as non–small-cell lung carcinomas-not otherwise specified: these two terms referred to two different entities, not interchangeable expecially for treatment.22
IMMUNOHISTOCHEMISTRY
Pathologic diagnosis of NSCLC routinely uses a panel of immunohistochemical markers: cytokeratin (CK) 5/6, protein (p) 63, and p40 are squamous markers and thyroid transcription factor-1, napsin A, and CK7 are adenocarcinoma markers, whereas chromogranin A, synaptophysin, and CD56 are usually considered neuroendocrine markers. All the previous often are common to several lines of differentiation and may significantly overlap. Nevertheless, by using this panel of markers, an unspecific diagnosis of LCC could be better specified. Thus, approximately 60% to 70% of LCC can be reclassified as poorly differentiated adenocarcinoma, 10% to 20% as squamous carcinoma, and 5% only as LCNEC.19
Regarding immunohistochemistry analysis, pulmonary LCNECs express typical neuroendocrine markers such as chromogranin, neuron-specific enolase, synaptophysin, and somatostatin, which are necessary to obtain the diagnosis, whereas they are negative for high-molecular-weight CKs, typically expressed by SCLC and other neuroendocrine tumors7 (Table 1).
Indeed, recent data show that LCNECs express higher levels of tropomyosin-related kinase B and brain-derived neurotrophic factor by immunohistochemistry than both non-neuroendocrine tumor and SCLC23 although none of them is clearly enough to help in the differential diagnosis between LCNEC and the other pulmonary neuroendocrine tumors.
MOLECULAR MARKERS
Pulmonary LCNEC proliferative rate is higher than classic LCC and other low-grade neuroendocrine tumors, such as carcinoids. As SCLC, they show higher expression of Ki-67, Bcl-2, and p21 and of telomerase activity, abnormal p53, and absent Rb.24 In particular, Onuki et al.25 analyzed loss of heterozygosity (LOH) at 10 chromosomal regions and p53 mutations in 59 neuroendocrine tumors, including 18 LCNEC. They found high frequencies of LOH and p53 alterations in LCNEC (83% and 72%, respectively): p53 alternations were in 23% LOH, 31% point mutations, and 46% both. Abnormalities of p53 seemed to be correlated to poor survival. These data derive from single, small-size retrospective studies, so it is impossible to obtain realistic recommendations for clinical practice.
The bigger genomic-based classification of lung cancer produced by the Clinical Lung Cancer Genome Project and Network Genomic Medicine identified important similarities between LCNEC and SCLC, regarding also the transcriptome, amplified and deleted regions and mutated genes. Therefore, the authors suggest that to combine immunohistochemical and genomic analysis to differentiate each other.26
DIFFERENTIAL DIAGNOSIS BETWEEN SCLC AND PULMONARY LCNEC
SCLC and pulmonary LCNEC share several characteristics such as high incidence in males and smokers, high mitotic rate, variable neuroendocrine marker expression, high grade, poor prognosis, and some genetic alterations (i.e., MEN1gene mutation). Indeed, these two histotypes are often combined in the single entity of high-grade neuroendocrine carcinoma (HGNEC).21
LCNEC can be distinguished from SCLC using some morphologic criteria: larger cell size, abundant cytoplasm, polygonal shape (versus fusiform), less evident nuclear molding, and less DNA deposits in blood vessels16 (Table 2).
Table 2.
Differential Diagnosis between SCLC and Pulmonary LCNEC
Nitadori et al.27 found a stronger CK7, CK18, E-cadherin, and beta-catenin expression in pulmonary LCNEC as compared with SCLC. However, despite the discrete body of evidence and studies, more prospective data are necessary.
PROGNOSIS AND SURVIVAL
Pulmonary LCNEC behaves biologically aggressive, similarly to SCLC. Stage by stage, survival curves of pulmonary LCNEC and SCLC overlap, and in addition, survival is lower than other NSCLCs. Prognosis is poor even in patients with potentially resectable stage I lung cancer with 5-year survival rates ranging from 27% to 67%.28
Regarding all stages, Iyoda et al.28,29 revealed a 5-year survival rate of 35.3% and a 5-year disease-free survival rate of 27.4% (Table 1); great part of relapses occurred within the first 2-year follow-up. One of the causes of this dramatic issue is the development of second primary cancers, synchronous or metachronous.
TREATMENT
There is no standard treatment of pulmonary LCNEC, and only few data are available primarily from case series. Because it is a very rare disease, randomized clinical trials are difficult to be conducted. Five-year OS remains poor, despite multimodal treatment in advanced stages and incidence of recurrence after surgery is high even in stage I disease.30
Surgical Management
Primary surgery should be the first option in operable patients (TNM stages I and II). This approach constitutes also the principal way to obtain an accurate diagnosis. Unfortunately, the majority of pulmonary LCNEC are not eligible for surgical resection because of local or systemic spread. Instead, in early stages, lobectomy or pneumonectomy are the preferred choices because they may improve survival in the absence of lymph node metastases at mediastinal sampling30 (Table 1).
Grand et al.31 conducted a retrospective analysis to compare pulmonary LCNEC with LCC outcomes. They did not evidence any significant difference in terms of type of surgical approach (resection, lobectomy, or pneumonectomy) and OS. By contrast, they identified the rate of visceral pleura invasion as a frequent finding in combined LCNEC compared with pure LCNEC.
Mazières et al.32 reported a case series of 18 patients with pulmonary LCNEC treated with radical surgery, followed by adjuvant radiotherapy in T3 and/or N2 cases. One-year survival rate was 27%, and there was no correlation with nodal status.
Zacharias et al.30 suggested that an extended complete anatomical resection with a systematic nodal dissection may influence survival.
Definitely, surgery may be curative in approximately 30% of cases; therefore, optimization of perioperative treatments could improve outcome.
Adjuvant Setting
All potentially resectable pulmonary LCNEC (stage I–III) should be operated.33 Perioperative, neoadjuvant,33 or adjuvant chemotherapeutic treatment could be valid options to prevent disease relapse.34
Indeed, a retrospective analysis of 144 surgically removed pulmonary LCNEC revealed a better outcome although not statistically significant, with preoperative or postoperative chemotherapy in stage I disease, suggesting that adjuvant therapy has a promising role in earliest diagnosed disease.35
The optimum regimen has not been established yet; moreover, it is not clear whether pulmonary LCNEC should be treated such as an SCLC.
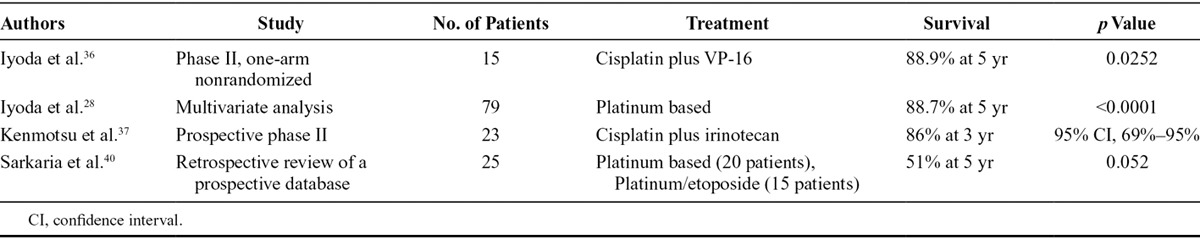
In 2006, Iyoda et al.36 conducted a prospective single-arm study of cisplatin–etoposide chemotherapy, the standard regimen for SCLC, as adjuvant treatment in completely resected pulmonary LCNEC comparing outcomes with historical data of patients treated without platinum in the same institution. Five-year OS favored the study arm (88.9% versus 47.4 %) and 2-year disease-free survival was 86.7% versus 47.8%. Three years later, the same group confirmed the superiority of platinum-based adjuvant chemotherapy over a nonplatinum adjuvant therapy or no adjuvant therapy. In addition, multivariate analysis showed that platinum-based adjuvant chemotherapy may have a significant impact on prognosis.28
More recently, a pilot adjuvant trial in HGNEC, including pulmonary LCNEC, enrolled patients to receive three to four cycles of cisplatin–irinotecan chemotherapy, after curative surgery. This study showed 3-year relapse-free survival of 74% and 3-year OS of 86%.37
On the basis of these studies, a phase III clinical trial of adjuvant cisplatin plus irinotecan versus etoposide has been designed and is still ongoing in Japan (Japan Clinical Oncology Group 1205/1206, UMIN000010298).38
Nevertheless, data presented are too much inadequate to provide a realistic recommendation because they all come from retrospective studies or studies having a too much little sample.
Currently, a defined biomarker of response to chemotherapy has been not yet identified. Skov et al.39 studied the expression of ERCC1, a member of the nucleotide excision repair system, involved in the repair of platinum-induced DNA damage and its correlation with chemosensitivity in a small cohort of patients with neuroendocrine lung tumors (SCLC, TC, AC, and LCNEC). Although the authors found a different expression of ERCC1 among neuroendocrine tumors with higher levels in low-grade neuroendocrine tumors (79% of TC and 67% of AC) and lower levels in SCLC and LCNEC (19% of LCNEC and 10% of SCLC), this difference did not affected the median survival. Probably, the study population was too much little to gain significance.
Sarkaria et al.40 examined the role of neoadjuvant platinum-based chemotherapy in LCNEC, without identifying a clear significance, but only a trend toward a better OS for patients treated with a multimodal treatment. Actually, it should not be used in such patients initially susceptible for surgery (Table 3).
Table 3.
Adjuvant and Neoadjuvant Setting
The role of radiotherapy32 in the treatment of local or advanced pulmonary LCNEC is still unclear, but some authors suggest its use in locally advanced disease setting. Prophylactic cranial irradiation, which is largely used in limited disease SCLC after partial or complete response (CR) to chemotherapy, is not currently recommended in pulmonary LCNEC patients.
Metastatic Setting
There is not a consensus on standard treatment for recurrent or advanced LCNEC. Positive results with SCLC-based regimes in neoadjuvant and perioperative setting encouraged to use this strategy also in unresectable disease.32,36,37
In 2005, Rossi et al.41 analyzed 83 cases of pulmonary LCNEC (65% metastatic patients), exploring clinical and therapeutic histories and performing immunohistochemical screening of several receptor tyrosine kinases to identify new potential therapeutic targets and better strategies of treatment. Review of clinical features confirmed prevalence of male sex, strong smoker habitus, median-age incidence, and peripheral location of lung lesion. Main sites of metastasization were brain, bone, and liver. Regarding chemotherapeutic strategies, their analysis confirmed in metastatic patients a greater efficacy of platinum–etoposide chemotherapy, with a response rate (RR) of 29%, including two cases of CRs and four partial responses (PRs); on the other hand, no CR or PR were observed in metastatic patients treated with different chemotherapeutic schemes. Immunohistochemical evaluation of receptor tyrosine kinases expression revealed positivity of platelet-derived growth factor receptor-β (81.9%), platelet-derived growth factor receptor-α (60.2%), met-protoncogene receptor tyrosyne kinase (47%), v-kit hardy-zuckerman 4 feline sarcoma viral oncogene homolog (62.7%), and stem cell factor (56.6%), which is c-KIT ligand always copresent with KIT. Interestingly, the only statistical significant correlation was found between MET positivity and OS, with a median OS of 18 and 24 months in MET positive and negative, respectively.41
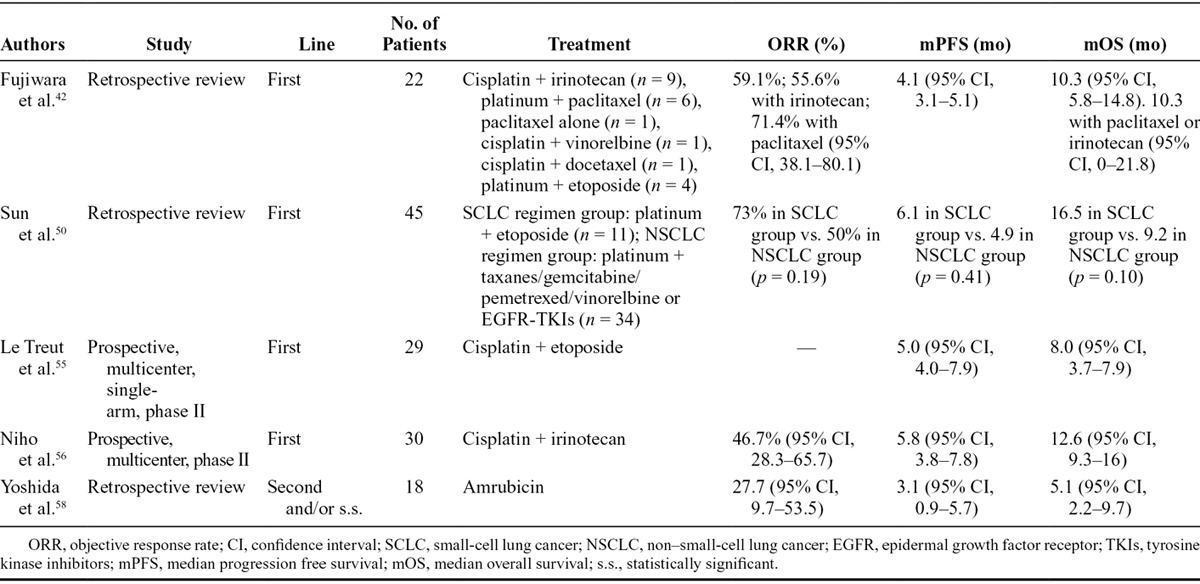
In 2007, Fujiwara et al.42 retrospectively analyzed outcomes from pulmonary LCNEC patients (22 patients, of which 20 were stage IIIB or IV or recurrent) receiving platinum plus irinotecan/taxanes/vinorelbine/etoposide or paclitaxel. PFS and objective response rate (ORR) on total patient population were 4.1 months and 59.1%, respectively. More efficacious regimes were platinum–paclitaxel (ORR, 71.4%) and platinum–irinotecan (ORR, 55.6%), with a median OS of 10.3 months and 1-year survival rate of 43%. All these results referred to a very small sample of patients: only four patients received platinum/etoposide, and survival of this subgroup was not reported.
Nevertheless, they are in agreement with those derived from the analysis of subgroups of LCNECs in previous studies.43–45 The efficacy of these drugs needs to be confirmed by prospective multi-institutional trials.
It is becoming increasingly evident that LCNEC tends to share several characteristics with SCLC also in terms of chemotherapy response,46,47 so efforts have been directed to clarify whether pulmonary LCNEC could be treated as SCLC, as NSCLC or as another variant of lung tumor.
Tokito et al.48 analyzed differences in treatment response to various schemes of chemotherapy of so called pulmonary “possible LCNEC.” The term “possible LCNEC” was introduced by Travis et al.47 referring to NSCLC positive to neuroendocrine markers and with neuroendocrine morphologic features on small samples derived from biopsies. He reviewed 24 “possible LCNEC” and 10 “defined LCNEC,” who underwent SCLC-like chemotherapy (platinum plus etoposide or irinotecan) in 67% and 60% of cases, respectively, finding no statistical differences in RR, PFS, or median survival time (median follow-up of 23.2 months). RR in “possible LCNEC” was 54%, similar to that obtained from previous analysis in the same setting: 50% by Igawa et al.46 and 61% by Shimada et al.49
Thus, considering that the clinical efficacy of chemotherapy for unresectable LCNEC has been shown to be comparable with that for extensive disease SCLC,46 new bigger and prospective trials for validation of SCLC-like approach both in defined and possible pulmonary LCNEC should be encouraged.
An interesting study on chemotherapy in pulmonary LCNEC patients has been published by Sun et al. 2 years ago.50 They conducted a retrospective analysis on 45 pulmonary LCNEC patients treated with chemotherapy, stratifying them by SCLC therapy or standard NSCLC therapy and by sex, age, smoking habit, and neuroendocrine immunohistochemical pattern. The choice of chemotherapeutic strategy depended on oncologist decisions, so patients received different treatments: SCLC strategy was platinum–etoposide/irinotecan in 24.4% of patients and NSCLC strategy was platinum-based doublet (with gemcitabine, vinorelbine, pemetrexed, or taxanes) in 68.9%; only one patient received vinorelbine–gemcitabine and two patients received a TKI. OS was 16.5 months versus 9.2 months for SCLC-like treated patients versus NSCLC-like group, respectively. Median PFS was 6.1 versus 4.9 months, respectively. RR was 73% versus 50% for the two populations of treatment; interestingly, the best RR was obtained with platinum-based regimens (60% overall, 41% when combined with gemcitabine, 7% to pemetrexed) compared with nonplatinum based (11%) and TKI (0%). Probably due to the small number of patients, these results were not statistically significant but underlined the importance to use platinum in first-line therapy for pulmonary LCNEC.
Several reports investigated the activity of other therapeutic agents in advanced setting. Among whom, pemetrexed efficacy in pulmonary LCNEC was found to be very poor, and this evidence should be ascribed to the major levels of thymidylate synthase expressed by this histotype compared with other NSCLC subtypes.51,52 Taxanes seemed to be more active, similarly to SCLC.53 Instead, poor efficacy of TKI could be linked to the low percentage of EGFR-activating mutations in pulmonary LCNEC.54
In 2013, two multicenter phase II trials evaluated cisplatin-based combination chemotherapy in unresectable pulmonary LCNEC.55,56
In the first one, conducted by Le Treut et al.,55 poor results on OS (8 months) were obtained with three to six cycles of cisplatin–etoposide chemotherapy in stage IIIB/IV LCNEC. It was a prospective, multicenter, single-arm, phase II study, with ORR as primary end point. Among 42 patients enrolled, only 29 diagnoses were centrally confirmed. In this subgroup stable disease occurred in 31% of patients, PR in 34% and progression disease in 35%; median PFS was 5 months and median OS was 8 months. All these results were not significantly different from total population and confirmed a worse prognosis.
In the second one, Niho et al.56 demonstrated that cisplatin–irinotecan could be an efficacious first-line chemotherapy option in stage IIIB or IV pulmonary LCNEC. Forty-four patients were enrolled in this single-arm study with RR as primary end point. Forty-one samples were centrally revised and reclassified as LCNEC (30 patients), SCLC (10 patients), and NSCLC with neuroendocrine structure (one patient). RR was 46.7% for LCNEC reclassified patients versus 80% in the 10 cases reclassified as SCLC. Median survival was 12.6 and 17.3 months, respectively, suggesting not only a worse prognosis of LCNEC compared with SCLC but also a minor chemoresponsiveness. Despite some limitations of this trial including statistical biases, small sample size (only 10 SCLC patients), and lack of information on second-line treatments, this is the first study that have evaluated prospectively this chemotherapeutic scheme in pulmonary LCNEC patients (Table 4) although it was as not designed to compare RR, PFS, and OS across different histology groups.
Table 4.
Metastatic Setting
TREATMENT AFTER FIRST LINE
Options for second-line treatment of SCLC are regimens including anthracyclines such as vinblastin, epirubicin/adriablastin, and cysplatinum.
The most investigated drug in this setting is amrubicin, a synthetic topoisomerase II inhibitor, extensively investigated in SCLC57 and currently approved for SCLC in Japan and not by U.S. Food and Drug Administration or European Medicines Agency.
In a retrospective revision, 18 LCNEC patients pretreated with platinum-based chemotherapy were treated from 2002 to 2008 with amrubicin single agent in second (72%) or subsequent lines of therapy (28%), with promising results. ORR resulted of 27.7% (5 PR, with disease control rate disease control rate of 61%), PFS was 3.1 months, and OS of 5.1 months58 (Table 3). Moreover, amrubicin treatment showed modest efficacy also in third/fourth lines of therapy.59
FUTURE DIRECTIONS
Few data are available on biological treatment in pulmonary LCNEC. Rossi et al.41 in 2005 analyzed molecular profile of 83 LCNEC patients and the correlation with clinical outcome, identifying a significant correlation between MET positivity and OS: median OS was 18 and 24 months in MET-positive and MET-negative samples, respectively.
Recent reports describe the presence of EGFR-activating mutations, involving exons 19 or 21 in mixed LCNECs with an adenocarcinoma component, indicating that EGFR mutations should be evaluated in this specific setting.60–63 Clinical responses to EGFR-targeted agent were, in fact, encouraging.
Angiogenesis is known to be one of the greater mechanism of tumor evolution; therefore, an important role could be played by inhibition of angiogenesis pathways, such as vascular endothelial growth factor, signal transducer and activator of transcription 1, and signal transducer and activator of transcription 3.64 Recently, Mairinger et al.65 explored how angiogenesis could be involved in LCNEC metastasization, hypothesizing the use of anti–angiogenetic-targeted drugs in association with chemotherapy.
Data of literature demonstrated a correlation between lymphangiogenesis and angiogenesis and hypoxia-inducible factor 1-α expression, a transcription factor that in response to hypoxia induces genes responsible of angiogenesis.66,67 In particular, overexpression of fibroblast growth factor and Fms-reLated Tyrosine kinase 4 and the reduced expression of Kinase insert Domain Receptor and hypoxia-inducible factor 1-α seemed to predict a trend toward malignant behavior and worse outcome.65
New agents under clinical development include nedaplatin, a platinum-based antineoplastic drug, in combination with irinotecan68 (Table 4).
Other innovative therapeutic targets could be represented by tropomyosin-related kinase B and brain-derived neurotrophic factor that are highly expressed in LCNEC as markers of invasiveness.22
To date, further studies are still needed to confirm positive data on all these drugs and especially better explore their use in pulmonary LCNEC to obtain the maximum effectiveness.
DISCUSSION
LCNEC of the lung is a rare tumor with a poor prognosis; because of biological and molecular features, they should be ascribed to the category of grade III neuroendocrine LCC, part of the neuroendocrine spectrum of lung cancer.
Previous studies have reported poor outcomes for patients with LCNEC, with 5-year survival rates ranging from 15% to 57%.7,8,20,23
Therefore, the prognosis of LCNEC patients has not changed. One of the main problems in the definition of the correct therapy is the lack of large phase II and III trials, which are very difficult to design and conduct because of the rarity of this tumor and the difficulties in the diagnosis. A way to overcome this issue may be the creation of large cooperative groups, which can accumulate enough patients to study LCNEC prospectively.
Current standard treatment for early-stage patients is radical surgery. Adjuvant chemotherapy, even in stage I patients, has shown benefit although the optimal schedule is yet to define.28,48 Neoadjuvant platinum-based regimens may be a feasible option for potentially resectable tumors.37 Rossi et al.41 recently demonstrated the efficacy of cisplatin plus etoposide in the adjuvant setting. Nevertheless, all these studies are retrospective.
Observation of clinical behavior and several genetic studies69,70 showed that LCNEC is very similar to SCLC. Furthermore, Filosso et al.71 reported preliminary data on the role of octreotide as from adjuvant therapy in LCNEC of the lung.
Irinotecan plus cisplatin combination was shown to be acceptable and feasible as adjuvant chemotherapy for completely resected HGNEC.37 Thus, a randomized phase III trial is ongoing in Japan to evaluate this combination in comparison with etoposide and cisplatin, for completely resected HGNEC (Japan Clinical Oncology Group 1205/1206).
In the advanced disease setting, the RR to cisplatin-based combinations chemotherapy was 50% in a series of 20 patients.72 Therefore, it is possible that a first-line NSCLC-like regimen should not be significantly inferior to an SCLC-like regimen.
Some reports showed the presence of EGFR-activating mutations in patients with pulmonary mixed LCNEC.60,63 It is common evidence that EGFR-TKIs are efficacious in tumors harboring an EGFR-activating mutation regardless of histology. However, these mutations seem to be extremely rare in LCNEC-pure type, whereas they could be identified in the variant with adenomatous component.
Prophylactic cranial irradiation is a useful treatment modality for SCLC,73 but its role in LCNEC should be an object of future research.
In conclusion, given the rarity of the neoplasm in object and the difficulty in obtaining a reliable diagnosis, especially on small biopsies, it is hopeful to create a cooperation between different hospitals to discuss diagnosis and treatment strategies and to conduct prospective randomized trials, with a number as larger as possible of patients.
ACKNOWLEDGMENT
This work has been supported by Associazione Italiana Per La Ricerca Sul Cancro (AIRC)—Project MFAG 2013-N.14392.
Footnotes
Disclosure: The authors declare no conflict of interest.
REFERENCES
- 1.Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2008;113:5–21. doi: 10.1002/cncr.23542. [DOI] [PubMed] [Google Scholar]
- 2.Arrigoni MG, Woolner LB, Bernatz PE. Atypical carcinoid tumors of the lung. J Thorac Cardiovasc Surg. 1972;64:413–421. [PubMed] [Google Scholar]
- 3.Travis WD, Linnoila RI, Tsokos MG, et al. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol. 1991;15:529–553. doi: 10.1097/00000478-199106000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628–1638. doi: 10.5858/2009-0583-RAR.1. [DOI] [PubMed] [Google Scholar]
- 5.Varlotto JM, Medford-Davis LN, Recht A, et al. Should large cell neuroendocrine lung carcinoma be classified and treated as a small cell lung cancer or with other large cell carcinomas? J Thorac Oncol. 2011;6:1050–1058. doi: 10.1097/JTO.0b013e318217b6f8. [DOI] [PubMed] [Google Scholar]
- 6.Younossian AB, Bründler MA, Tötsch M. Feasibility of the new WHO classification of pulmonary neuroendocrine tumours. Swiss Med Wkly. 2002;132:535–540. doi: 10.4414/smw.2002.09880. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez FG, Battafarano RJ. Large-cell neuroendocrine carcinoma of the lung: an aggressive neuroendocrine lung cancer. Semin Thorac Cardiovasc Surg. 2006;18:206–210. doi: 10.1053/j.semtcvs.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Sánchez de Cos Escuín J. Diagnosis and treatment of neuroendocrine lung tumors. Arch Bronconeumol. 2014;50:392–396. doi: 10.1016/j.arbres.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Travis WD, Rush W, Flieder DB, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 1998;22:934–944. doi: 10.1097/00000478-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Selvaggi G, Scagliotti GV. Histologic subtype in NSCLC: does it matter? Oncology (Williston Park) 2009;23:1133–1140. [PubMed] [Google Scholar]
- 11.Asamura H, Kameya T, Matsuno Y, et al. Neuroendocrine neoplasms of the lung: a prognostic spectrum. J Clin Oncol. 2006;24:70–76. doi: 10.1200/JCO.2005.04.1202. [DOI] [PubMed] [Google Scholar]
- 12.Oshiro Y, Kusumoto M, Matsuno Y, et al. CT findings of surgically resected large cell neuroendocrine carcinoma of the lung in 38 patients. AJR Am J Roentgenol. 2004;182:87–91. doi: 10.2214/ajr.182.1.1820087. [DOI] [PubMed] [Google Scholar]
- 13.Travis WD, Giroux DJ, Chansky K, et al. International Staging Committee and Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the inclusion of broncho-pulmonary carcinoid tumors in the forthcoming (seventh) edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2008;3:1213–1223. doi: 10.1097/JTO.0b013e31818b06e3. [DOI] [PubMed] [Google Scholar]
- 14.Papotti M, Croce S, Bello M, et al. Expression of somatostatin receptor types 2, 3, and 5 in biopsies and specimens of human lung tumours. Virchows Arch. 2001;439:787–797. doi: 10.1007/s004280100494. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues M, Traub-Weidinger T, Li S, et al. Comparison of 111In-DOTA-DPhe1-Tyr3-octreotideand 111In-DOTA-lanreotide scintigraphy and dosimetry in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2006;33:532–540. doi: 10.1007/s00259-005-0020-3. [DOI] [PubMed] [Google Scholar]
- 16.Travis WD, Brambilla E, Müller-Hermelink HK, et al. World Health Organization Classification of Tumours. Pathology & Genetics. Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2004. [Google Scholar]
- 17.Iyoda A, Hiroshima K, Nakatani Y, Fujisawa T. Pulmonary large cell neuroendocrine carcinoma: its place in the spectrum of pulmonary carcinoma. Ann Thorac Surg. 2007;84:702–707. doi: 10.1016/j.athoracsur.2007.03.093. [DOI] [PubMed] [Google Scholar]
- 18.Liang R, Chen TX, Wang ZQ, et al. A retrospective analysis of the clinicopathological characteristics of large cell carcinoma of the lung. Exp Ther Med. 2015;9:197–202. doi: 10.3892/etm.2014.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi G, Mengoli MC, Cavazza A, et al. Large cell carcinoma of the lung: clinically oriented classification integrating immunohistochemistry and molecular biology. Virchows Arch. 2014;464:61–68. doi: 10.1007/s00428-013-1501-6. [DOI] [PubMed] [Google Scholar]
- 20.Battafarano RJ, Fernandez FG, Ritter J, et al. Large cell neuroendocrine carcinoma: an aggressive form of non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;130:166–172. doi: 10.1016/j.jtcvs.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 21.Chen LC, Travis WD, Krug LM. Pulmonary neuroendocrine tumors: what (little) do we know? J Natl Compr Canc Netw. 2006;4:623–630. doi: 10.6004/jnccn.2006.0051. [DOI] [PubMed] [Google Scholar]
- 22.Pelosi G, Barbareschi M, Cavazza A, Graziano P, Rossi G, Papotti M. Large cell carcinoma of the lung: a tumor in search of an author. A clinically oriented critical reappraisal. Lung Cancer. 2015;87:226–231. doi: 10.1016/j.lungcan.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Odate S, Nakamura K, Onishi H, et al. TrkB/BDNF signaling pathway is a potential therapeutic target for pulmonary large cell neuroendocrine carcinoma. Lung Cancer. 2013;79:205–214. doi: 10.1016/j.lungcan.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Carvalho L. Reclassifying bronchial-pulmonary carcinoma: differentiating histological type in biopsies by immunohistochemistry. Rev Port Pneumol. 2009;15:1101–1119. [PubMed] [Google Scholar]
- 25.Onuki N, Wistuba II, Travis WD, et al. Genetic changes in the spectrum of neuroendocrine lung tumors. Cancer. 1999;85:600–607. doi: 10.1002/(sici)1097-0142(19990201)85:3<600::aid-cncr10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 26.The Clinical Lung Cancer Genome Project (CLCGP); Network Genomic Medicine (NGM) A genomics-based classification of human lung tumors. Sci Transl Med. 2013;5:209–213. doi: 10.1126/scitranslmed.3006802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nitadori J, Ishii G, Tsuta K, et al. Immunohistochemical differential diagnosis between large cell neuroendocrine carcinoma and small cell carcinoma by tissue microarray analysis with a large antibody panel. Am J Clin Pathol. 2006;125:682–692. doi: 10.1309/DT6B-J698-LDX2-NGGX. [DOI] [PubMed] [Google Scholar]
- 28.Iyoda A, Hiroshima K, Moriya Y, et al. Postoperative recurrence and the role of adjuvant chemotherapy in patients with pulmonary large-cell neuroendocrine carcinoma. J Thorac Cardiovasc Surg. 2009;138:446–453. doi: 10.1016/j.jtcvs.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 29.Iyoda A, Jiang SX, Travis WD, et al. Clinicopathological features and the impact of the new TNM classification of malignant tumors in patients with pulmonary large cell neuroendocrine carcinoma. Mol Clin Oncol. 2013;1:437–443. doi: 10.3892/mco.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zacharias J, Nicholson AG, Ladas GP, Goldstraw P. Large cell neuroendocrine carcinoma and large cell carcinomas with neuroendocrine morphology of the lung: prognosis after complete resection and systematic nodal dissection. Ann Thorac Surg. 2003;75:348–352. doi: 10.1016/s0003-4975(02)04118-8. [DOI] [PubMed] [Google Scholar]
- 31.Grand B, Cazes A, Mordant P, et al. High grade neuroendocrine lung tumors: pathological characteristics, surgical management and prognostic implications. Lung Cancer. 2013;81:404–409. doi: 10.1016/j.lungcan.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Mazières J, Daste G, Molinier L, et al. Large cell neuroendocrine carcinoma of the lung: pathological study and clinical outcome of 18 resected cases. Lung Cancer. 2002;37:287–292. doi: 10.1016/s0169-5002(02)00099-5. [DOI] [PubMed] [Google Scholar]
- 33.Fournel L, Falcoz PE, Alifano M, et al. Surgical management of pulmonary large cell neuroendocrine carcinomas: a 10-year experience. Eur J Cardiothorac Surg. 2013;43:111–114. doi: 10.1093/ejcts/ezs174. [DOI] [PubMed] [Google Scholar]
- 34.Saji H, Tsuboi M, Matsubayashi J, et al. Clinical response of large cell neuroendocrine carcinoma of the lung to perioperative adjuvant chemotherapy. Anticancer Drugs. 2010;21:89–93. doi: 10.1097/CAD.0b013e328330fd79. [DOI] [PubMed] [Google Scholar]
- 35.Veronesi G, Morandi U, Alloisio M, et al. Large cell neuroendocrine carcinoma of the lung: a retrospective analysis of 144 surgical cases. Lung Cancer. 2006;53:111–115. doi: 10.1016/j.lungcan.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Iyoda A, Hiroshima K, Moriya Y, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg. 2006;82:1802–1807. doi: 10.1016/j.athoracsur.2006.05.109. [DOI] [PubMed] [Google Scholar]
- 37.Kenmotsu H, Niho S, Ito T, et al. A pilot study of adjuvant chemotherapy with irinotecan and cisplatin for completely resected high-grade pulmonary neuroendocrine carcinoma (large cell neuroendocrine carcinoma and small cell lung cancer). Lung Cancer. 2014;84:254–258. doi: 10.1016/j.lungcan.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Eba J, Kenmotsu H, Tsuboi M, et al. Lung Cancer Surgical Study Group of the Japan Clinical Oncology Group; Lung Cancer Study Group of the Japan Clinical Oncology Group. A Phase III trial comparing irinotecan and cisplatin with etoposide and cisplatin in adjuvant chemotherapy for completely resected pulmonary high-grade neuroendocrine carcinoma (JCOG1205/1206). Jpn J Clin Oncol. 2014;44:379–382. doi: 10.1093/jjco/hyt233. [DOI] [PubMed] [Google Scholar]
- 39.Skov BG, Holm B, Erreboe A, Skov T, Mellemgaard A. ERCC1 and Ki67 in small cell lung carcinoma and other neuroendocrine tumors of the lung: distribution and impact on survival. J Thorac Oncol. 2010;5:453–459. doi: 10.1097/JTO.0b013e3181ca063b. [DOI] [PubMed] [Google Scholar]
- 40.Sarkaria IS, Iyoda A, Roh MS, et al. Neoadjuvant and adjuvant chemotherapy in resected pulmonary large cell neuroendocrine carcinomas: a single institution experience. Ann Thorac Surg. 2011;92:1180–1186. doi: 10.1016/j.athoracsur.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 41.Rossi G, Cavazza A, Marchioni A, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFR β, PDGFR α, and Met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol. 2006;34:8775–8785. doi: 10.1200/JCO.2005.02.8233. [DOI] [PubMed] [Google Scholar]
- 42.Fujiwara Y, Sekine I, Tsuta K, et al. Effect of platinum combined with irinotecan or paclitaxel against large cell neuroendocrine carcinoma of the lung. Jpn J Clin Oncol. 2007;37:482–486. doi: 10.1093/jjco/hym053. [DOI] [PubMed] [Google Scholar]
- 43.Bonomi P, Kim K, Fairclough D, et al. Comparison of survival and quality of life in advanced non-small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2000;18:623–631. doi: 10.1200/JCO.2000.18.3.623. [DOI] [PubMed] [Google Scholar]
- 44.Noda K, Nishiwaki Y, Kawahara M, et al. Japan Clinical Oncology Group. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 45.Kubota K, Watanabe K, Kunitoh H, et al. Japanese Taxotere Lung Cancer Study Group. Phase III randomized trial of docetaxel plus cisplatin versus vindesine plus cisplatin in patients with stage IV non-small-cell lung cancer: the Japanese Taxotere Lung Cancer Study Group. J Clin Oncol. 2004;22:254–261. doi: 10.1200/JCO.2004.06.114. [DOI] [PubMed] [Google Scholar]
- 46.Igawa S, Watanabe R, Ito I, et al. Comparison of chemotherapy for unresectable pulmonary high-grade non-small cell neuroendocrine carcinoma and small-cell lung cancer. Lung Cancer. 2010;68:438–445. doi: 10.1016/j.lungcan.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tokito T, Kenmotsu H, Watanabe R, et al. Comparison of chemotherapeutic efficacy between LCNEC diagnosed using large specimens and possible LCNEC diagnosed using small biopsy specimens. Int J Clin Oncol. 2014;19:63–67. doi: 10.1007/s10147-012-0509-2. [DOI] [PubMed] [Google Scholar]
- 49.Shimada Y, Niho S, Ishii G, et al. Clinical features of unresectable high-grade lung neuroendocrine carcinoma diagnosed using biopsy specimens. Lung Cancer. 2012;75:368–373. doi: 10.1016/j.lungcan.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Sun JM, Ahn MJ, Ahn JS, et al. Chemotherapy for pulmonary large cell neuroendocrine carcinoma: similar to that for small cell lung cancer or non-small cell lung cancer? Lung Cancer. 2012;77:365–370. doi: 10.1016/j.lungcan.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Monica V, Scagliotti GV, Ceppi P, et al. Differential thymidylate synthase expression in different variants of large-cell carcinoma of the lung. Clin Cancer Res. 2009;15:7547–7552. doi: 10.1158/1078-0432.CCR-09-1641. [DOI] [PubMed] [Google Scholar]
- 52.Jalal S, Ansari R, Govindan R, et al. Pemetrexed in second line and beyond small cell lung cancer: a Hoosier Oncology Group phase II study. J Thorac Oncol. 2009;4:93–96. doi: 10.1097/JTO.0b013e31818de1e6. [DOI] [PubMed] [Google Scholar]
- 53.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 54.Moore AM, Einhorn LH, Estes D, et al. Gefitinib in patients with chemo-sensitive and chemo-refractory relapsed small cell cancers: a Hoosier Oncology Group phase II trial. Lung Cancer. 2006;52:93–97. doi: 10.1016/j.lungcan.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Le Treut J, Sault MC, Lena H, et al. Multicentre phase II study of cisplatin-etoposide chemotherapy for advanced large-cell neuroendocrine lung carcinoma: the GFPC 0302 study. Ann Oncol. 2013;24:1548–1552. doi: 10.1093/annonc/mdt009. [DOI] [PubMed] [Google Scholar]
- 56.Niho S, Kenmotsu H, Sekine I, et al. Combination chemotherapy with irinotecan and cisplatin for large-cell neuroendocrine carcinoma of the lung: a multicenter phase II study. J Thorac Oncol. 2013;8:980–984. doi: 10.1097/JTO.0b013e31828f6989. [DOI] [PubMed] [Google Scholar]
- 57.Satouchi M, Kotani Y, Shibata T, et al. Phase III study comparing amrubicin plus cisplatin with irinotecan plus cisplatin in the treatment of extensive-disease small-cell lung cancer: JCOG 0509. J Clin Oncol. 2014;32:1262–1268. doi: 10.1200/JCO.2013.53.5153. [DOI] [PubMed] [Google Scholar]
- 58.Yoshida H, Sekine I, Tsuta K, et al. Amrubicin monotherapy for patients with previously treated advanced large-cell neuroendocrine carcinoma of the lung. Jpn J Clin Oncol. 2011;41:897–901. doi: 10.1093/jjco/hyr065. [DOI] [PubMed] [Google Scholar]
- 59.Harada T, Oizumi S, Ito K, et al. Hokkaido Lung Cancer Clinical Study Group. A phase II study of amrubicin as a third-line or fourth-line chemotherapy for patients with non-small cell lung cancer: Hokkaido Lung Cancer Clinical Study Group Trial (HOT) 0901. Oncologist. 2013;18:439–445. doi: 10.1634/theoncologist.2012-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakai Y, Yamasaki T, Kusakabe Y, et al. Large-cell neuroendocrine carcinoma of lung with epidermal growth factor receptor (EGFR) gene mutation and co-expression of adenocarcinoma markers: a case report and review of the literature. Multidiscip Respir Med. 2013;8:47–53. doi: 10.1186/2049-6958-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iyoda A, Travis WD, Sarkaria IS, et al. Expression profiling and identification of potential molecular targets for therapy in pulmonary large-cell neuroendocrine carcinoma. Exp Ther Med. 2011;2:1041–1045. doi: 10.3892/etm.2011.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Pas TM, Giovannini M, Manzotti M, et al. Large-cell neuroendocrine carcinoma of the lung harboring EGFR mutation and responding to gefitinib. J Clin Oncol. 2011;29:e819–e822. doi: 10.1200/JCO.2011.36.2251. [DOI] [PubMed] [Google Scholar]
- 63.Yanagisawa S, Morikawa N, Kimura Y, et al. Large-cell neuroendocrine carcinoma with EGFR mutation: possible transformation of lung adenocarcinoma. Respirology. 2012;17:1275–1277. doi: 10.1111/j.1440-1843.2012.02258.x. [DOI] [PubMed] [Google Scholar]
- 64.Dimova I, Popivanov G, Djonov V. Angiogenesis in cancer—general pathways and their therapeutic implications. J BUON. 2014;19:15–21. [PubMed] [Google Scholar]
- 65.Mairinger FD, Walter RF, Werner R, et al. Activation of angiogenesis differs strongly between pulmonary carcinoids and neuroendocrine carcinomas and is crucial for carcinoid tumorigenesis. J Cancer. 2014;5:465–471. doi: 10.7150/jca.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim JW, Koh Y, Kim DW, et al. Clinical implications of VEGF, TGF-β1, and IL-1β in patients with advanced non-small cell lung cancer. Cancer Res Treat. 2013;45:325–333. doi: 10.4143/crt.2013.45.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu B, Liu Q, Song Y, et al. Polymorphisms of HIF1A gene are associated with prognosis of early stage non-small-cell lung cancer patients after surgery. Med Oncol. 2014;31:877–886. doi: 10.1007/s12032-014-0877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kenmotsu Y, Oshita F, Sugiura M, et al. Nedaplatin and irinotecan in patients with large-cell neuroendocrine carcinoma of the lung. Anticancer Res. 2012;32:1453–1456. [PubMed] [Google Scholar]
- 69.Przygodzki RM, Finkelstein SD, Langer JC, et al. Analysis of p53, K-ras-2, and C-raf-1 in pulmonary neuroendocrine tumors. Correlation with histological subtype and clinical outcome. Am J Pathol. 1996;148:1531–1541. [PMC free article] [PubMed] [Google Scholar]
- 70.Hiroshima K, Iyoda A, Shibuya K, et al. Genetic alterations in early-stage pulmonary large cell neuroendocrine carcinoma. Cancer. 2004;100:1190–1198. doi: 10.1002/cncr.20108. [DOI] [PubMed] [Google Scholar]
- 71.Filosso PL, Ruffini E, Oliaro A, et al. Large-cell neuroendocrine carcinoma of the lung: a clinicopathologic study of eighteen cases and the efficacy of adjuvant treatment with octreotide. J Thorac Cardiovasc Surg. 2005;129:819–824. doi: 10.1016/j.jtcvs.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 72.Yamazaki S, Sekine I, Matsuno Y, et al. Clinical responses of large cell neuroendocrine carcinoma of the lung to cisplatin-based chemotherapy. Lung Cancer. 2005;49:217–223. doi: 10.1016/j.lungcan.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 73.Snider JW, III, Gondi V, Brown PD, Tome W, Mehta MP. Prophylactic cranial irradiation: recent outcomes and innovations. CNS Oncol. 2014;3:219–230. doi: 10.2217/cns.14.22. [DOI] [PMC free article] [PubMed] [Google Scholar]


