Abstract
BAG3, a member of the BAG co-chaperones family, is expressed in several cell types subjected to stressful conditions, such as exposure to high temperature, heavy metals, drugs. Furthermore, it is constitutively expressed in some tumors. Among the biological activities of the protein, there is apoptosis downmodulation; this appears to be exerted through BAG3 interaction with the heat shock protein (Hsp) 70, that influences cell apoptosis at several levels. We recently reported that BAG3 protein was detectable in the cytoplasm of reactive astrocytes in HIV-1-associated encephalopathy biopsies. Here we report that downmodulation of BAG3 protein levels allows caspase-3 activation by HIV-1 infection in human primary microglial cells. This is the first reported evidence of a role for BAG3 in the balance of death versus survival during viral infection.
INTRODUCTION
Cells infected by intracellular pathogens undergo apoptosis, as a consequence of either attack from immune system or activation of intrinsic apoptotic pathways (Xu et al., 2001; Waterhouse et al., 2004). The ability to trigger anti-apoptotic mechanisms constitutes an advantage for some intracellular pathogens, that by this means sustain the prolonged survival of their host cell (Hiscott et al., 2001; Xu et al., 2001; Hilleman, 2004). In HIV-1 infection, cells of the mononuclear phagocytic lineage, including blood monocytes, exert a major role as virus reservoir. Their long-term survival after HIV-1 infection and reduced sensitivity to apoptosis are of central importance in this respect (Herbein et al., 2002; Crowe et al., 2003; Aquaro et al., 2005).
Infection of the nervous system by HIV-1 is commonly associated with a syndrome of cognitive and motor abnormalities called HIV-1-associated dementia (HAD) (Gonzalez-Scarano and Martin-Garcia, 2005; Trujillo et al., 2005). HAD is caused by productive viral infection of brain mononuclear phagocytes (MPs) (perivascular and parenchymal brain macrophages and microglia) and sustained by paracrine neurotoxic responses. Indeed, HIV-1 enters the brain at the early stage of infection and resides primarily in a limited number of macrophages/microglia and astrocytes; their long-term survival after HIV-1 infection renders these cells an important reservoir of the virus (Mollace et al., 2001; Lum and Badley, 2003; Chipitsyna et al., 2004; Gonzalez-Scarano and Martin-Garcia, 2005; Speth et al., 2005). Neuronal cell death is induced by macrophage-produced cytokines (including tumor necrosis factor-related apoptosis-inducing ligand: TRAIL), arachidonic acid and its metabolites, nitric oxide, and viral proteins, including Tat, that can affect neighboring uninfected cells (Mollace et al., 2001; Peruzzi et al., 2002; Regulier et al., 2004; Aquaro et al., 2005; Huang et al., 2005; Jones and Power, 2006). The characterization of mechanisms that sustain cell survival in HIV-1-harboring cells (mononuclear leukocytes, microglial cells, astrocytes) and reduce their sensitivity to apoptosis induced by infection, ROS- and RNS-mediated oxidative stress, cytokines of the TNF family and other agents can contribute to our understanding of HIV-1-related pathogenesis and identification of novel target for therapies. Co-chaperone proteins that share the BAG domain are characterized by their interaction with heat shock proteins and other partners (steroid hormone receptors, Raf-1 and others), involved in regulating a number of cellular processes, including proliferation and apoptosis (Takayama et al., 1999; Doong et al., 2000; Takayama and Reed, 2001; Doong et al., 2002). Among BAG family members there is BAG3, also known as CAIR-1 or Bis (Lee et al., 1999; Antoku et al., 2001; Liao et al., 2001; Takayama and Reed, 2001; Briknarova et al., 2002; Doong et al., 2002; Lee et al., 2002a,b; Chroboczek et al., 2003; Doong et al., 2003; Pagliuca et al., 2003; Romano et al., 2003a,b; Bonelli et al., 2004; Chen et al., 2004; Homma et al., 2006; Chiappetta et al., 2007; Rosati et al., 2007a,b). BAG3 forms a complex with Hsp70 (Takayama et al., 1999), a protein that assists polypeptide folding, can mediate altered peptide delivery to proteasome (Young et al., 2003) and is able to modulate apoptosis by interfering with cytochrome c release, apoptosome assembly and other events in the death process (Beere, 2005). Notably, we observed that BAG3 expression can be induced by some stressful agents, such as high temperature or heavy metals (Pagliuca et al., 2003) mainly through the activation of heat shock factor (HSF)-1 (Franceschelli et al., 2008). In this report we investigated whether BAG3 protein levels influenced apoptotic events in microglial cells infected by HIV-1.
MATERIALS AND METHODS
Cells and HIV-1 infection
Primary human fetal microglial cells were prepared from 8- to 12-week-old human fetal brain tissue (purchased from Advanced Bioscience Resources Inc., Alameda, CA) by a modified procedure based on the methods by Cole and Vellis (1997). Human fetal microglial cells plated at 70% of confluency were infected with JR-FL of HIV (obtained from the AIDS Research and Reference Reagent Program, division of AIDS, NIAID, NIH). Briefly: 50 ng of p24 containing virus stock were added to every 1 × 106 cells. Cells were incubated with virus stock in a small volume of serum free media for 2 h at 37°C, the cells were then washed twice with PBS and a new fresh media containing 2% of FBS was added. Cells were harvested at indicated days and HIV-1 infection was assessed by Western blot for HIV-1 Tat.
Western blot and antibodies
Cells were harvested and whole cell lysates were obtained in TNN buffer (50 mM Tris PH 7,5, 150 mM NaCl, 0.5% NP40) supplemented with a protease inhibitor cocktail (Sigma–Aldrich Corp., St. Louis, MO), with five cycles of freeze and thawing. Protein amount was determined by Bradford assay and 30 μg of total protein were loaded in a SDS–PAGE and blotted on a nitrocellulose membrane. Total cell lysates were examined for the amount of BAG3 protein (monoclonal antibody AC-1: Alexis Biochemicals, San Diego, CA), anti-α-tubulin (Sigma), pAKT and cleaved caspase-3 (polyclonal antibody purchased from Cell Signaling Inc., Beverly, MA). Immunoreactivity was detected by sequential incubation with horseradish peroxidase-conjugated secondary antibody (GE HealthCare, Pittsburgh, PA) and enhanced chemiluminescence reagents (SuperSignal West Dura Extended Duration Substrate, Pierce, Rockford, IL) following standard protocols.
Adenoviral vectors
BAG3siRNA-Ad construct was made using the BD Adeno-X Expression Systems 2 PT3674-1 (Pr36024) and BD knockout RNAi Systems PT3739 (PR42756) (BD Biosciences-Clontech, Palo Alto, CA). We inserted a dsDNA oligonucleotide against a specific bag3 mRNA (5′-AAG GUU CAG ACC AUC UUG GAA-3) in a RNAi-ready pSIREN-DNR vector, designed to express a small hairpin RNA (shRNA) driven by the human Pol III-dependent U6 promoter. After ligation, this vector was used to transfer the shRNA expression cassette to the Adenoviral Acceptor Vector pLP-Adeno-X-PRLS viral DNA (BD Adeno-X Expression Systems 2), containing ΔE1/ΔE3 Ad5 genome, by Cre-loxP mediated recombination. An AdNull empty adenoviral vector was used as control. Infectious adenoviruses were propagated in the HEK-293 cell line and CsCl gradient purified; functional plaque-forming unit (pfu) titers were determined by limiting dilution plaque titration on HEK-293 cells according to standard techniques. Microglial cells were transduced by thawing the titrated virus stocks at 37°C, mixing the appropriate volume of virus (10 PFU for single cell) in serum free medium (Optimem: GIBCO SRL, Life Technologies, Milano, Italy) and adding the mixture to the target cells. After 1 h incubation, regular medium was added to the cells.
Analysis of hypodiploid (apoptotic) nuclei
Primary microglial cells were transduced with an adenoviral vector capable of expressing siRNA against bag3 gene (BAG3siRNA-Ad). As a control another set was transduced with empty adenoviral vector (AdNull). Forty-eight hours later cells were either mock infected or infected with the JRFL strain of HIV. Four days after HIV infection cells were collected, permeabilized and stained with propidium iodide and quantification of apoptotic cells was done by following standard guidelines recommended by Guava Technology and by using a Guava Citometer.
RESULTS AND DISCUSSION
To investigate the influence of BAG3 protein levels on apoptotic events in microglial cells harboring HIV-1, we infected human primary fetal microglial cells with the virus. BAG3 expression was increased in HIV-1-infected compared with uninfected cells (Fig. 1A). Those findings are in accord with reported results in reactive astrocytes from HIV-1-associated encephalopathy biopsies (Rosati et al., 2007a).
Figure 1.
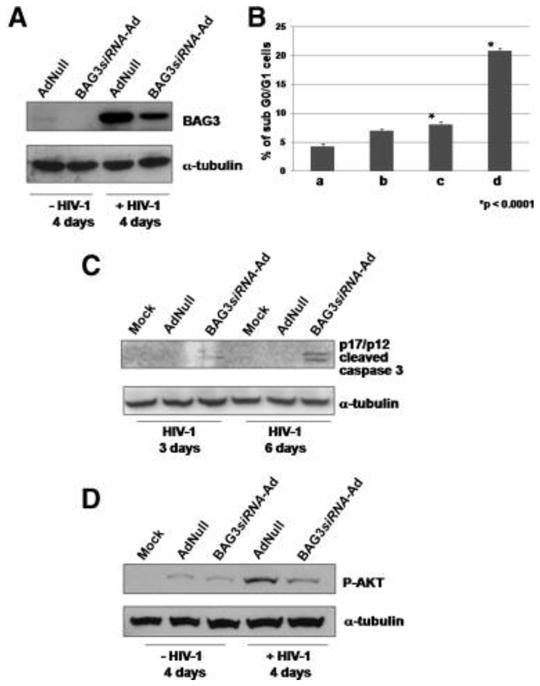
Primary human fetal microglial cultures were prepared from 8- to 12-week-old human fetal brain tissue. Adenoviral constructs carrying BAG3-specific siRNA (BAG3siRNA-Ad) or AdNull were made using the BDAdeno-X Expression Systems 2 and BDknockout RNAi Systems. Microglial cells were plated at 70% confluency and transductions were performed using 10 PFU for target cell. After 48 h, cells were infected with HIV-1 JRFL obtained from the AIDS Research and Reference Reagent Program, division of AIDS, NIAID, NIH. Whole cell lysates were obtained and analyzed by Western blot as described in Materials and Methods Section with the following antibodies: (A) anti-BAG3 monoclonal antibody (mAb) AC-1 (Alexis Corp., San Diego, CA); anti-alpha-tubulin mAb (Sigma); (C) anti-cleaved caspase 3 (Asp175) and (D) anti-P-AKT antibodies (Cell Signaling, Danvers, MA). B: Sub-Go/G1 cell population was measured after 4 days of HIV-1 infection in the following samples: (a) mock cells; (b) mock cells + HIV-1; (c) AdNull infected cells + HIV-1; (d) BAG3siRNA-Ad infected cells + HIV-1.
We reduced BAG3 expression by using an adenovirus carrying a BAG3-specific small interfering (si) RNA (BAG3siRNA-Ad); a void adenoviral vector (AdNull) was used as control. Forty-eight hours following adenoviral infections, microglial cells were infected with HIV (JR-FL) and harvested after 4 days. In BAG3siRNA-Ad-infected cultures, and not in AdNull-infected control cells, we observed a decrease in BAG3 protein levels (Fig. 1A). We then analyzed the percentage of hypodiploid (apoptotic) nuclei in those cells. We found that in the BAG3siRNA-Ad treated microglia infected by HIV-1, apoptosis was enhanced by more than 250% (P < 0.0001) in respect to AdNull treated microglia cells (Fig. 1B). Although the knockdown of BAG3 was only partial (Fig. 1A), the effect on apoptosis is clearly evident (Fig. 1B). This observation is in agreement with analogous findings in other cell systems (Rosati et al., 2007b) and strengthens the role of BAG3 in maintaining cell survival. To verify the activation of the apoptotic pathways, we then analyzed cell extracts for the presence of the activated form (p17/p12) of caspase-3, a pivotal actor of cell apoptosis. In uninfected cells or in cells infected with HIV-1 alone, we did not detect significant levels of activated caspase-3. On the other hand, when HIV-1-infected cells were treated with BAG3siRNA-Ad, activated caspase-3 was clearly evident, 3 and 6 days after HIV-1 infection (Fig. 1C); it was not detectable in control (Mock) or AdNull-treated cells. Therefore, it appeared that HIV-1 infection was able to activate caspase-3, but only in cells where BAG3 protein levels were downmodulated. Those results indicated that a pro-apoptotic impulse was produced by HIV-1 infection, but counteracted by BAG3 protein activity, in microglial cells. Among elements with a pro-survival role in HIV-1-infected cells, there is the protein kinase Akt (Yang et al., 2006; Zhao et al., 2007). BAG3 protein reportedly interferes with the Hsp70-mediated delivery of Akt kinase to proteasome sustaining its intracellular levels (Doong et al., 2003). We found that phospho (P)-Akt levels, raised in HIV-1-infected cells, were downmodulated by effect of BAG3siRNA-Ad (Fig. 1D). The influence of BAG3 on the level of Akt kinase activity is likely to be responsible, at least in part, for the apoptosis-modulatory effect of this co-chaperone.
The above reported observations indicate that BAG3 protein induction counteracts apoptotic events induced by HIV-1 in human primary microglial cells. It is well known that HIV-1 infection causes cell stress, and particularly oxidative stress, through more than one mechanism (Perl and Banki, 2000; Mollace et al., 2001; Gil et al., 2003; Pocernich et al., 2005). A few reports showed an increase in Hsp70 and/or Hsp27 levels in lymphocytes (Agnew et al., 2003) or blood (Espigares et al., 2006) from HIV-1-infected patients or in HIV-1 infected cells (Wainberg et al., 1997; Pocernich et al., 2005). bag3 gene is induced by stressful agents, mainly through the activation of heat shock factor (HSF)-1 (Franceschelli et al., 2008). Therefore, the increase in BAG3 expression is likely part of the cell’s response to virus infection and appears to represent a homeostatic mechanism for cell defence. The findings reported here provide a sound basis for investigating the role of BAG3 in maintaining an HIV-1 reservoir in infected cells which do not undergo apoptosis.
Acknowledgments
Work in part supported by funds from Italian Ministry for Research and Association for Cancer Research and a grant awarded by NIH to KK and MCT. This work is dedicated to the memory of Dr. Arturo Leone.
References
- Agnew LL, Kelly M, Howard J, Jeganathan S, Batterham M, Ffrench RA, Gold J, Watson K. Altered lymphocyte heat shock protein 70 expression in patients with HIV disease. AIDS. 2003;17:1985–1988. doi: 10.1097/00002030-200309050-00019. [DOI] [PubMed] [Google Scholar]
- Antoku K, Maser RS, Scully WJ, Jr, Delach SM, Johnson DE. Isolation of Bcl-2 binding proteins that exhibit homology with BAG-1 and suppressor of death domains protein. Biochem Biophys Res Commun. 2001;286:1003–1010. doi: 10.1006/bbrc.2001.5512. [DOI] [PubMed] [Google Scholar]
- Aquaro S, Ronga L, Pollicita M, Antinori A, Ranazzi A, Perno CF. Human immunodeficiency virus infection and acquired immunodeficiency syndrome dementia complex: Role of cells of monocyte-macrophage lineage. J Neurovirol. 2005;11:58–66. doi: 10.1080/13550280500513416. [DOI] [PubMed] [Google Scholar]
- Beere HM. Death versus survival: Functional interaction between the apoptotic and stress-inducible heat shock protein pathways. J Clin Invest. 2005;115:2633–2639. doi: 10.1172/JCI26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli P, Petrella A, Rosati A, Lerose R, Pagliuca G, Amelio T, Festa M, Martire G, Venuta S, Turco MC, Leone A. BAG3 protein regulates stress-induced apoptosis in normal and neoplastic leukocytes. Leukemia. 2004;18:358–360. doi: 10.1038/sj.leu.2403219. [DOI] [PubMed] [Google Scholar]
- Briknarova K, Takayama S, Homma S, Baker K, Cabezas E, Hoyt DW, Li Z, Satterthwait AC, Ely KR. The serine-rich domain from Crk-associated substrate (p130cas) is a four-helix bundle. J Biol Chem. 2002;277:31172–31178. [Google Scholar]
- Chen L, Wu W, Dentchev T, Zeng Y, Wang J, Tsui I, Tobias JW, Bennett J, Baldwin D, Dunaief JL. Light damage induced changes in mouse retinal gene expression. Exp Eye Res. 2004;79:239–247. doi: 10.1016/j.exer.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Chiappetta G, Ammirante M, Basile A, Rosati A, Festa M, Monaco M, Vuttariello E, Pasquinelli R, Arra C, Zerilli M, Todaro M, Stassi G, Pezzullo L, Gentilella A, Tosco A, Pascale M, Marzullo L, Belisario MA, Turco MC, Leone A. The antiapoptotic protein BAG3 is expressed in thyroid carcinomas and modulates apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand. J Clin Endocrinol Metab. 2007;92:1159–1163. doi: 10.1210/jc.2006-1712. [DOI] [PubMed] [Google Scholar]
- Chipitsyna G, Slonina D, Siddiqui K, Peruzzi F, Skorski T, Reiss K, Sawaya BE, Khalili K, Amini S. HIV-1 Tat increases cell survival in response to cisplatin by stimulating Rad51 gene expression. Oncogene. 2004;23:2664–2671. doi: 10.1038/sj.onc.1207417. [DOI] [PubMed] [Google Scholar]
- Chroboczek J, Gout E, Favier AL, Galinier R. Novel partner proteins of adenovirus penton. Curr Top Microbiol Immunol. 2003;272:37–55. doi: 10.1007/978-3-662-05597-7_2. [DOI] [PubMed] [Google Scholar]
- Cole R, Vellis J. Protocols for neural cell culture. Totowa, NJ: Humana Press; 1997. pp. 117–130. [Google Scholar]
- Crowe S, Zhu T, Muller WA. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14+ monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J Leukoc Biol. 2003;74:635–641. doi: 10.1128/JVI.76.2.707-716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doong H, Price J, Kim YS, Gasbarre C, Probst J, Liotta LA, Blanchette J, Rizzo K, Kohn E. CAIR-1/BAG-3 forms an EGF-regulated ternary complex with phospholipase C-gamma and Hsp70/Hsc70. Oncogene. 2000;19:4385–4395. doi: 10.1038/sj.onc.1203797. [DOI] [PubMed] [Google Scholar]
- Doong H, Vrailas A, Kohn EC. What’s in the ‘BAG’?—A functional domain analysis of the BAG-family proteins. Cancer Lett. 2002;188:25–32. doi: 10.1016/s0304-3835(02)00456-1. [DOI] [PubMed] [Google Scholar]
- Doong H, Rizzo K, Fang S, Kulpa V, Weissman AM, Kohn EC. CAIR-1/BAG-3 abrogates heat shock protein-70 chaperone complex-mediated protein degradation: Accumulation of poly-ubiquitinated Hsp90 client proteins. J Biol Chem. 2003;278:28490–28500. doi: 10.1074/jbc.M209682200. [DOI] [PubMed] [Google Scholar]
- Espigares E, Bueno A, Hernandez J, Garcia F, Luna JD, Espigares M, Galvez R. Levels of HSP70 in HIV(+) patients in different viroimmunological states. J Med Virol. 2006;78:318–323. doi: 10.1002/jmv.20542. [DOI] [PubMed] [Google Scholar]
- Franceschelli S, Rosati A, Le Rose R, Turco MC, Pascale M. bag3 gene expression is regulated by heat shock factor 1. J Cell Phisiol. 2008;215:575–577. doi: 10.1002/jcp.21397. [DOI] [PubMed] [Google Scholar]
- Gil L, Martinez G, Gonzalez I, Tarinas A, Alvarez A, Giuliani A, Molina R, Tapanes R, Perez J, Leon OS. Contribution to characterization of oxidative stress in HIV/AIDS patients. Pharmacol Res. 2003;47:217–224. doi: 10.1016/s1043-6618(02)00320-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Herbein G, Coaquette A, Perez-Becoff D, Pancino G. Macrophage activation and HIV infection: Can the Trojan horse turn into a fortress? Curr Mol Med. 2002;2:723–738. doi: 10.2174/1566524023361844. [DOI] [PubMed] [Google Scholar]
- Hilleman MR. Strategies and mechanisms for host and pathogen survival in acute and persistent viral infections. Proc Natl Acad Sci USA. 2004;101:14560–14566. doi: 10.1073/pnas.0404758101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J, Kwon H, Genin P. Hostile takeovers: Viral appropriation of the NF-kappaB pathway. J Clin Invest. 2001;107:143–151. doi: 10.1172/JCI11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma S, Iwasaki M, Shelton GD, Engvall E, Reed JC, Takayama S. BAG3 deficiency results in fulminant myopathy and early lethality. Am J Pathol. 2006;169:761–773. doi: 10.2353/ajpath.2006.060250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Erdmann N, Zhao J, Zheng J. The signaling and apoptotic effects of TNF-related apoptosis-inducing ligand in HIV-1 associated dementia. Neurotox Res. 2005;8:135–148. doi: 10.1007/BF03033825. [DOI] [PubMed] [Google Scholar]
- Jones G, Power C. Regulation of neural cell survival by HIV-1 infection. Neurobiol Dis. 2006;21:1–17. doi: 10.1016/j.nbd.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Lee JH, Takahashi T, Yasuhara N, Inazawa J, Kamada S, Tsujimoto Y. Bis, a Bcl-2-binding protein that synergizes with Bcl-2 in preventing cell death. Oncogene. 1999;18:6183–6190. doi: 10.1038/sj.onc.1203043. [DOI] [PubMed] [Google Scholar]
- Lee MY, Kim SY, Choi JS, Choi YS, Jeon MH, Lee JH, Kim IK, Lee JH. Induction of Bis, a Bcl-2-binding protein, in reactive astrocytes of the rat hippocampus following kainic acid-induced seizure. Exp Mol Med. 2002a;34:167–171. doi: 10.1038/emm.2002.24. [DOI] [PubMed] [Google Scholar]
- Lee MY, Kim SY, Shin SL, Choi YS, Lee JH, Tsujimoto Y, Lee JH. Reactive astrocytes express bis, a bcl-2-binding protein, after transient forebrain ischemia. Exp Neurol. 2002b;175:338–346. doi: 10.1006/exnr.2002.7903. [DOI] [PubMed] [Google Scholar]
- Liao Q, Ozawa F, Friess H, Zimmermann A, Takayama S, Reed JC, Kleeff J, Buchler MW. The anti-apoptotic protein BAG-3 is overexpressed in pancreatic cancer and induced by heat stress in pancreatic cancer cell lines. FEBS Lett. 2001;503:151–157. doi: 10.1016/s0014-5793(01)02728-4. [DOI] [PubMed] [Google Scholar]
- Lum JJ, Badley AD. Resistance to apoptosis: Mechanism for the development of HIV reservoirs. Curr HIV Res. 2003;1:261–274. doi: 10.2174/1570162033485203. [DOI] [PubMed] [Google Scholar]
- Mollace V, Nottet HSLM, Clayette P, Turco MC, Muscoli C, Salvemini D, Perno CF. Oxidative stress and neuroAIDS: Triggers, modulators and novel antioxidants. Trends Neurosci. 2001;24:411–416. doi: 10.1016/s0166-2236(00)01819-1. [DOI] [PubMed] [Google Scholar]
- Pagliuca MG, Lerose R, Cigliano S, Leone A. Regulation by heavy metals and temperature of the human BAG-3 gene, a modulator of Hsp70 activity. FEBS Lett. 2003;541:11–15. doi: 10.1016/s0014-5793(03)00274-6. [DOI] [PubMed] [Google Scholar]
- Perl A, Banki K. Genetic and metabolic control of the mitochondrial transmembrane potential and reactive oxygen intermediate production in HIV disease. Antioxid Redox Signal. 2000;2:551–573. doi: 10.1089/15230860050192323. [DOI] [PubMed] [Google Scholar]
- Peruzzi F, Gordon J, Darbinian N, Amini S. Tat-induced deregulation of neuronal differentiation and survival by nerve growth factor pathway. J Neurovirol. 2002;8:91–96. doi: 10.1080/13550280290167885. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, Sultana R, Mohmmad-Abdul H, Nath A, Butterfield DA. HIV-dementia, Tat-induced oxidative stress, and antioxidant therapeutic considerations. Brain Res Brain Res Rev. 2005;50:14–26. doi: 10.1016/j.brainresrev.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Regulier EG, Reiss K, Khalili K, Amini S, Zagury JF, Katsikis PD, Rappaport J. T-cell and neuronal apoptosis in HIV infection: Implications for therapeutic intervention. Int Rev Immunol. 2004;23:25–59. doi: 10.1080/08830180490265538. [DOI] [PubMed] [Google Scholar]
- Romano MF, Festa M, Pagliuca G, Lerose R, Bisogni R, Chiurazzi F, Storti G, Volpe S, Venuta S, Turco MC, Leone A. BAG3 protein controls B-chronic lymphocytic leukemia cell apoptosis. Cell Death Differ. 2003a;10:383–385. doi: 10.1038/sj.cdd.4401167. [DOI] [PubMed] [Google Scholar]
- Romano MF, Festa M, Petrella A, Rosati A, Pascale M, Bisogni R, Poggi V, Kohn EC, Venuta S, Turco MC, Leone A. BAG3 protein regulates cell survival in childhood acute lymphoblastic leukemia cells. Cancer Biol Ther. 2003b;2:508–510. doi: 10.4161/cbt.2.5.524. [DOI] [PubMed] [Google Scholar]
- Rosati A, Leone A, Del Valle L, Amini S, Khalili K, Turco MC. Evidence for BAG3 modulation of HIV-1 gene transcription. J Cell Physiol. 2007a;210:676–683. doi: 10.1002/jcp.20865. Direct Link. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati A, Ammirante M, Gentilella A, Basile A, Festa M, Pascale M, Marzullo L, Belisario MA, Tosco A, Franceschelli S, Moltedo O, Pagliuca G, Lerose R, Turco MC. Apoptosis inhibition in cancer cells: A novel molecular pathway that involves BAG3 protein. Int J Biochem Cell Biol. 2007b;39:1337–1342. doi: 10.1016/j.biocel.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Speth C, Dierich MP, Sopper S. HIV-infection of the central nervous system: The tightrope walk of innate immunity. Mol Immunol. 2005;42:213–228. doi: 10.1016/j.molimm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Takayama S, Reed JC. Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol. 2001;3:237–241. doi: 10.1038/ncb1001-e237. [DOI] [PubMed] [Google Scholar]
- Takayama S, Xie Z, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999;274:781–786. doi: 10.1074/jbc.274.2.781. [DOI] [PubMed] [Google Scholar]
- Trujillo JR, Jaramillo-Rangel G, Ortega-Martinez M, Penalva de Oliveira AC, Vidal JE, Bryant J, Gallo RC. International NeuroAIDS: Prospects of HIV-1 associated neurological complications. Cell Res. 2005;15:962–969. doi: 10.1038/sj.cr.7290374. [DOI] [PubMed] [Google Scholar]
- Wainberg Z, Oliveira M, Lerner S, Tao Y, Brenner BG. Modulation of stress protein (hsp27 and hsp70) expression in CD4+ lymphocytic cells following acute infection with human immunodeficiency virus type-1. Virology. 1997;233:364–373. doi: 10.1006/viro.1997.8618. [DOI] [PubMed] [Google Scholar]
- Waterhouse NJ, Clarke CJ, Sedelies KA, Teng MW, Trapani JA. Cytotoxic lymphocytes; instigators of dramatic target cell death. Biochem Pharmacol. 2004;68:1033–1040. doi: 10.1016/j.bcp.2004.05.043. [DOI] [PubMed] [Google Scholar]
- Xu XN, Screaton GR, McMichael AJ. Virus infections: Escape, resistance, and counterattack. Immunity. 2001;15:867–870. doi: 10.1016/s1074-7613(01)00255-2. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ikezoe T, Nishioka C, Bandobashi K, Takeuchi T, Adachi Y, Kobayashi M, Takeuchi S, Koeffler HP, Taguchi H. NFV, an HIV-1 protease inhibitor, induces growth arrest, reduced Akt signalling, apoptosis and docetaxel sensitisation in NSCLC cell lines. Br J Cancer. 2006;95:1653–1662. doi: 10.1038/sj.bjc.6603435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Barral JM, Ulrich Hartl F. More than folding: Localized functions of cytosolic chaperones. Trends Biochem Sci. 2003;28:541–547. doi: 10.1016/j.tibs.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Zhao T, Adams MH, Zou SP, El-Hage N, Hauser KF, Knapp PE. Silencing the PTEN gene is protective against neuronal death induced by human immunodeficiency virus type 1 Tat. J Neurovirol. 2007;13:97–106. doi: 10.1080/13550280701236841. [DOI] [PubMed] [Google Scholar]


