Abstract
HIV-1 gene transcription is controlled by the cooperation of viral and host factors which bind to specific DNA sequences within the viral promoter spanning the long terminal repeat, LTR. Previously we showed that the St. John's Wort DING phosphatase, p27SJ, suppresses HIV-1 gene transcription by binding to the viral protein Tat and preventing its nuclear import. Here, we describe the inhibitory effect of p27SJ on the phosphorylation of the C-terminal domain (CTD) of RNA polymerase II (RNAPII). This inhibition leads to the suppression of the association of RNAPII with the LTR. Inhibition of binding of RNAPII to LTR by p27SJ resulted in the suppression of LTR transcription elongation and a decrease in LTR transcriptional activity. Another form of the St. John's Wort DING phosphatase, p38SJ, also suppressed binding of RNAPII to the LTR, reduced transcription elongation and was even more powerful than p27SJ in inhibiting the transcriptional activity of the LTR. Our data suggest a possible mechanism by which the p27SJ/p38SJ DING phosphatase can regulate HIV-1 LTR expression by inhibiting phosphorylation of the CTD of RNAPII and suppressing LTR transcription elongation.
Keywords: p27SJ, DING proteins, RNA Polymerase II CTD, HIV-I
The transcription of the integrated HIV-1 proviral genome is controlled by the cooperation of viral and host regulatory trans-acting protein factors that bind to specific cis-acting DNA sequences within the viral promoter spanning the long terminal repeat, LTR (Nabel and Baltimore, 1987). A major player in this regulation is the HIV-1 transactivator protein Tat, which is a powerful stimulator of viral gene expression. HIV-1 mRNAs contain a sequence in their 5′ untranslated region known as TAR that is responsible for trans-activation by Tat and cooperates with promoter-binding proteins that interact with RNA polymerase II (RNAPII) to activate its processivity and thus enhance transcription elongation. This mechanism for this involves the binding of Tat to the positive transcription elongation factor b (pTEFb) and activation of its kinase activity resulting in the phosphorylation of the C-terminal domain (CTD) of RNAPII and an induction of HIV-1 transcription (Barboric and Peterlin, 2005; Barboric et al, 2001, 2007; Peterlin and Price, 2006; Zhou et al, 2003; Zhou and Yik, 2006).
Human RNAPII contains 52 repeats in the CTD (Peterlin and Price, 2006) and is phosphorylated from initiation to termination during transcription at serine 2, serine 5 and serine 7 of the repeat sequence (Tyr-Ser-Pro-Thr-Ser-Pro-Ser). Proteins that bind to the CTD can stimulate the polymerase activity of RNAPII and the phosphorylation of the CTD is necessary for the productive elongation and 3′ end processing of transcripts (McCracken et al, 1997; Phatnani and Greenleaf, 2006). It has also been shown that dephosphorylation of RNA polymerase II CTD leads to the inhibition of its association with the LTR (Marshall et al, 2000). Thus the inhibition or reversal of RNAPII phosphorylation would be expected to lead to its inhibition resulting in a suppression of HIV-1 transcription. Since we had previously found that the protein p27SJ inhibits HIV-1 transcription (Darbinian-Sarkissian et al, 2006) and possesses phosphatase activity (Darbinian et al, 2009), we were interested in examining a role for this mechanism.
p27SJ was cloned from a cDNA library prepared from the plant Hypericum perforatum, commonly known as St. John's Wort (Darbinian-Sarkissian et al, 2006). This protein suppresses transcription of the HIV-1 genome in several human cell types including primary culture of microglia and astrocytes. p2SJ associates with the C/EBPβ transcription factor and HIV-1 Tat suggesting a potential for the development of a therapeutic advance based on p27SJ protein to control HIV-1 transcription and replication in cells associated with HIV-1 infection in the brain. To this end, p27SJ protein fused to secretion/uptake signals has allowed us to create a bi-directional protein transduction system that produces an “armed” p27SJ derivative that inhibits Tat-induced activation of the LTR and decreases the level of viral replication in promonocytic cells including U-937 and T-lymphocytic cells (Darbinian et al, 2008). p27SJ belongs to a group of DING proteins, which are characterized by their conserved N-terminus that starts with the amino acid sequence DINGG, are widely distributed in prokaryotes, animals and plants and include phosphate-binding proteins and phosphatases (Berna et al, 2008; Perera et al, 2008). Interestingly, an anti-viral factor named HRF (HIV-1 Resistance Factor) secreted by HIV-1 resistant cells (Lesner et al, 2005, Simm et al, 2002, Simm et al, 2007) was recently identified as a DINGG protein and designated X-DING-C (Lesner et al, 2009). Additionally, p27SJ is homologous to the recently discovered human phosphate-binding apolipoprotein, HPBP (Diemer et al, 2008; Alam et al, 2009), human synovial fluid protein p205 (Hain et al 1996; Adams et al, 2002; et al, 1999), and prokaryotic phosphate-binding proteins including alkaline phosphatases from Pseudomonas species (Berna et al., 2008). Recently, we found that recombinant p27SJ has phosphatase activity in vitro (Darbinian et al, 2009).
Since p27SJ is associated with the suppression of HIV-1 gene transcription by binding to HIV-1 Tat (Darbinian-Sarkissian et al, 2006, Darbinian et al, 2008) and exhibits phosphatase activity (Darbinian et al, 2009), we examined the possibility that p27SJ may function through the dephosphorylation of the CTD of RNAPII.
Materials and Methods
Cell culture
U-87 MG, a cell line derived from a human glioblastoma, was obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (Life Technologies, Inc.) and antibiotics (100 U/ml penicillin and 10 mg/ml streptomycin) at 37°c in a humidified atmosphere containing 7% CO2. A p27SJ inducible cell line was established from U-87 MG cells based on the pTetOn Gene Expression System (BD Biosciences Clontech, Palo Alto, CA) as we have previously described (Darbinian-Sarkissian, 2006). Expression of p27SJ was induced by treatment of cells with 1 μg/ml doxycycline (Dox) for 48 hours.
Plasmids
CFP-Tat was created by cloning a BamHI-EcoRI Tat fragment from CMV-Tat into BglII-EcoRI-digested pECFP-C1 expression vector (Darbinian-Sarkissian et al, 2006). HIV-LTR (-476/+66) was generated by PCR amplification of the LTR and cloned into HindIII–KpnI site of pGL3-basic vector (Promega Corp., Madison, WI, USA) as previously described (Darbinian-Sarkissian et al, 2006). The sequence of all plasmids was verified by DNA sequencing. Oligonucleotides were prepared commercially by Oligos Etc, Inc. (Wilsonville OR).
Oligonucleotides
Luciferase primers for RT-PCR were: 1932(Reverse) - tta cac ggc gat ctt tcc gcc ctt; 1380(Reverse) - tgg aac aac ttt acc gac cgc gcc; 430(Reverse) - gga gct ttt ttt gca cgt tca aaa. The HIV-LTR primer for RT-PCR was: +18/+43(Forward) aga tct gag cct ggg agc tct cgg. HIV-LTR primers for ChIP were: -453(Forward) - tgg aag ggc taa ttc act ccc aac; +43(Reverse) ccg aga gct ccc agg ctc aga tct
Transfections
U-87 MG cells were transfected using FuGENE 6 transfection reagent (Roche, Inc., Indianapolis, IN). In brief, 2 × 105 cells were plated on a 60 mm plate and grown overnight. Transfection was carried out with 0.5 μg of HIV-LTR-luciferase reporter construct, in the presence and absence of 1 μg of YFP-p65 or CFP-Tat. Vector plasmid was added to equalize the total amount of DNA in each transfection mixture. At 36 h post-transfection and Dox treatment, cell extracts were prepared and equal amounts of proteins were assayed for luciferase activity. Each transfection was repeated a minimum of three times with different plasmid preparations.
RNA preparation
Total RNA was extracted from callus tissue or human cells using the RNAqueous® total RNA Isolation Kit (Ambion, Austin, TX, USA) according to the manufacturer's directions. Approximately 1 μg of RNA was used for cDNA synthesis using reverse transcriptase (Roche Molecular Biochemicals, Indianapolis, IN, USA). The cDNA was amplified by 28 cycles of PCR with Taq DNA polymerase. The amplified DNA was analyzed by DNA gel electrophoresis using 1.5 % agarose gel.
RT-PCR
The SuperScript III One-Step RT-PCR System with Platinum Taq (Invitrogen, Carlsbad, CA, USA) was used. One microgram of plant or human total RNA and primers specific to p27SJ or prokaryotic DING genes were used to amplify eukaryotic full-length DING cDNAs. Cycling conditions were optimized for DING primers. To achieve efficient cDNA synthesis, three-step cycling with separate annealing and extension steps were performed: 1) cDNA synthesis and pre-denaturation at 55°C for 30 minutes; 2) 40 cycles of PCR amplification with 15-second denaturing at 94°C, 30-second annealing at 60°C and 1 minute extension at 68°C; and 3) final extension at 68°C for 5 minutes. The amplified DNA was gel purified and cloned into the TA cloning vector (Invitrogen, Carlsbad, CA, USA). Recombinant clones were identified by blue/white screening in the presence of isopropyl-1-thio-β-D-galactopyrasine (IPTG) and 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (XGal) and were screened for the presence of inserts by using appropriate restriction enzymes.
ChIP Assay
For ChIP assays, cells were transfected with 1 μg of pGL3-Luc LTR plasmid, CFP-Tat and YFP-p65 expression plasmids using FuGENE 6 transfection reagent (Roche, Inc., Indianapolis, IN). After 48 h of Dox treatment, cells were subjected to crosslinking with 1% formaldehyde and immunoprecipitated using anti-pCTD antibody, followed by PCR amplification with primers flanking the TAR region (Upstate Cell Signaling Solutions, Charlottesville, VA, USA).
Preparation of protein extracts and immunoblot analysis
For preparation of whole-cell extract, cells were washed with ice-cold phosphate-buffered saline (PBS) and solubilized in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1 % Nonidet P-40 and 1% protease inhibitors cocktail (Sigma). Cell debris was removed by centrifugation for 5 min at 4°C. Fifty micrograms of proteins were eluted with Laemmli sample buffer, heated at 95°C for 10 min, and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For Western blot analysis, protein samples were resolved by SDS-PAGE and transferred to supported nitrocellulose membranes as described previously (Darbinian-Sarkissian et al, 2006). Proteins were visualized with the enhanced chemiluminescence detection system, ECL+ according to the manufacturer's instructions (Amersham Pharmacia), and exposed to X-ray film.
Antibodies
Antibody specific for p27SJ (anti-p27SJ rabbit polyclonal antibody) was obtained from Lampire Biological Laboratories, Inc., Pipersville, PA. Anti-p-CTD, RNAPII, Grb2, and Lamin A antibodies were from Santa Cruz Biotechnologies, Inc. (Santa Cruz, CA). Antibody specific for CFP-Tat (Living Colors) was purchased from BD Biosciences Clontech. Anti-p65 (F-6) antibody was purchased from Santa Cruz. Anti-Myc-tag antibody was purchased from Invitrogen. Expression of the YFP-fusion proteins was examined by Western blot analysis, using Living Colors full-length monoclonal antibody (BD Biosciences Clontech).
Immunoprecipitation/Western blot analysis
Approximately 300 μg of extracts (from cells producing p27SJ induced by Dox treatment) were incubated with 2 μl of anti-p-CTD RNAPII, anti-Myc antibody (Invitrogen) or polyclonal anti-p27SJ antibody overnight at 4°c. In all, 30 μl of Protein A-Sepharose were added, the samples were incubated at 4°c for 1 h, and resins were pelleted, washed, and immunoprecipitated bound complexes were resolved by SDS-PAGE and analyzed by Western blot utilizing anti-p27SJ antibody.
Cloning of cDNA encoding full-length p38SJ
Since p27SJ is an incomplete form of p38SJ lacking the C-terminus, we adopted an RT-PCR approach using forward primers designed from the known p27SJ sequence and reverse primers designed from the sequence of a Pseudomonas DING protein. After generating partial clones by RT-PCR from total RNA isolated from H. perforatum, two fragments (1-788 and 788-1179) were ligated to generate cDNA clone predicted to encode full-length 364 amino-acid, 38 kDa p38SJ (GenBank: AAW57408.2). For expression in human cells, the cDNA was cloned into pcDNA6 (Invitrogen).
Results
This study was aimed to investigate several aspects of HIV-1 regulation and the mechanism of action of p27SJ: phosphorylation and activation of RNAPII, binding of RNAPII to the HIV-1 LTR and regulation of transcription elongation. First, we investigated if phosphorylation of the CTD of RNAPII is affected by p27SJ.
p27SJ inhibits RNAPII CTD phosphorylation
To investigate whether the phosphatase activity of p27SJ affects the phosphorylation of the CTD of RNAPII, we used a p27SJ-inducible cell line derived from human U-87 MG glioblastoma cells. Western blot analysis was performed with extracts from cells treated with and without doxycycline, which induces expression of p27SJ (Fig. 1A). Doxycycline decreased the phosphorylation of the CTD of RNAPII as detected by Western blot with a phospho-specific antibody but did not alter the overall level of RNAPII expression. The expression of p27SJ also inhibited the phosphorylation of the CTD of RNAPII that is induced by HIV-1 Tat (Fig. 1B). The NF-κB subunit p65 induced CTD phosphorylation and this was especially pronounced in the presence of Tat (Fig. 1C, lanes 1-4). However, the induction of CTD phosphorylation was reversed by the expression of p27SJ (Fig. 1C, lanes 5-8). Thus, phosphorylation of the RNAPII CTD is inhibited by p27SJ and this is not affected by the presence of Tat and/or p65.
Figure 1. Effect of p27SJ on CTD RPII phosphorylation.
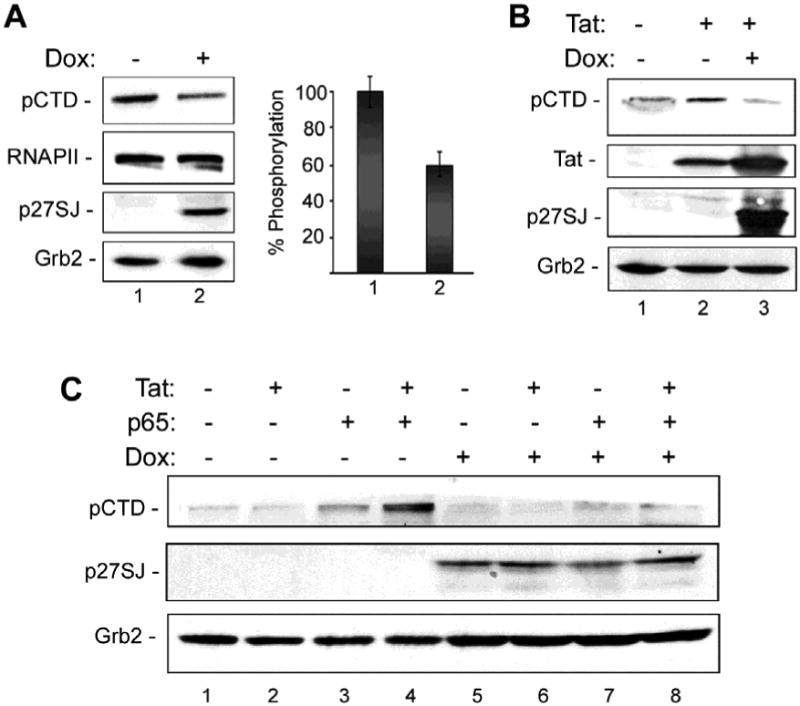
A. Western-blot analysis of extracts from the cells expressing p27SJ (Dox+, lane 2) or without p27SJ (Dox-, lane 1). Levels of phosphorylated CTD RPII were analyzed by densitometry and shown in % (bottom panel). Grb2 in served as a loading control. B. Western blot analysis of the phosphorylation of the CTD RNAPII in cells expressing Tat and/or p27SJ. C. Western blot analysis of extracts from cells expressing Tat and/or the p65 subunit of NF-κB p65 and treated or untreated with Dox to induce p27SJ.
Physical interaction of p27SJ and RNAPII
Since the level of CTD RPII phosphorylation is reduced by p27SJ, this suggest that the two proteins may physically interact. To determine if the dephosphorylation of the CTD of RNAPII occurs via direct interaction of p27SJ and RNAPII CTD, we carried out reciprocal immunoprecipitation/Western blot experiments (Fig. 2A and B). As shown in Fig. 2A, immunoprecipitation with antibody to phospho-CTD co-precipitated a band of p27SJ (molecular weight 27,000 in lane 4) that is slightly heavier than the IgG light chain and which was not seen with non-immune rabbit serum or in the absence of doxycycline. Similarly, immunoprecipitation with antibody to p27SJ co-precipitated a band of phospho-CTD, which has a molecular weight of about 200,000 (Fig. 2B, lane 6) and is heavier than the IgG heavy chain. Again, this was not seen with non-immune rabbit serum or in the absence of doxycycline. These data provide evidence that RNAPII physically associates with p27SJ suggesting that the dephosphorylation of RNAPII occurs through direct binding of RNAPII and p27SJ.
Figure 2. Reciprocal IP/Western analysis for the association of phosphorylated RNAPII in p27SJ-expressing cells.
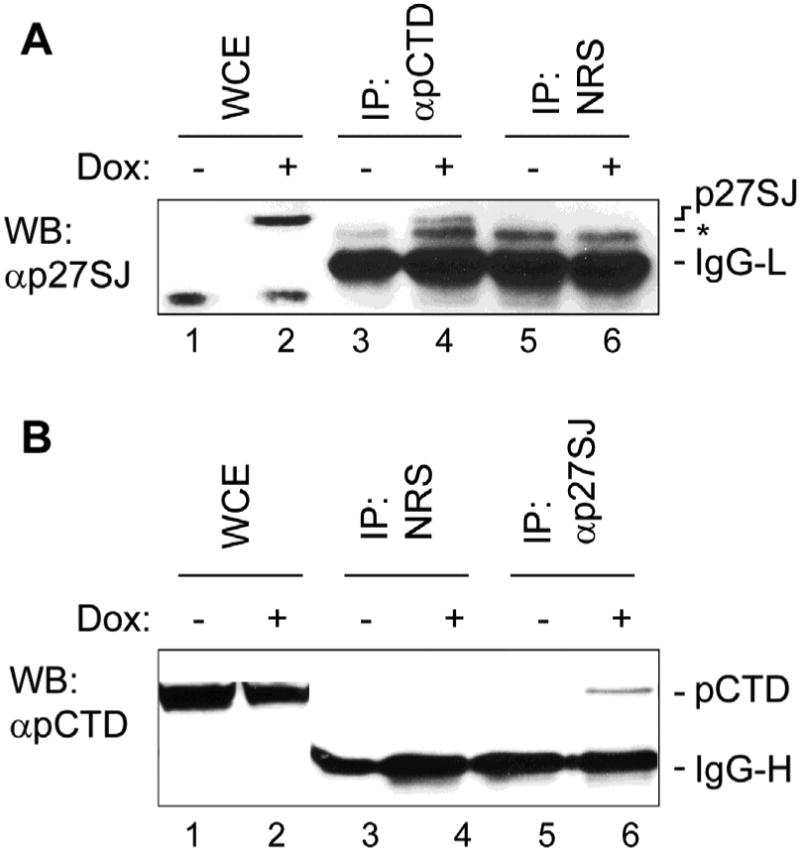
A. Results from IP/western-blot analysis of extracts from the cells expressing p27SJ. Lysates were immunoprecipitated with anti-p-CTD RNAPII and analyzed by Western blot assay using anti-p27SJ antibody. The negative control was nonimmune rabbit serum. B. Results from IP/western-blot analysis of extracts from the cells expressing p27SJ. Lysates were immunoprecipitated with anti-p27SJ antibody and analyzed by Western blot assay using anti-p-CTD RNAPII. The negative control was nonimmune rabbit serum.
p27SJ inhibits binding of RNAPII to the HIV-1 LTR
To investigate if p27SJ-mediated dephosphorylation of CTD can affect its biological activity, we employed a chromatin immunoprecipitation (ChIP) assay. Proteins are cross-linked to DNA followed by immunoprecipitation and the DNA associated with the immunoprecipitated protein analyzed by PCR. Normally, active RNAPII will bind to the HIV-1 LTR. However, if the CTD is dephosphorylated, this would be expected to result in the suppression of the binding of RNAPII to the LTR. Control cells and cells that were transfected with p65 and/or Tat and treated with and without doxycycline were treated with formaldehyde as cross-linking agent and immunoprecipitated using anti-phospho-CTD antibody. After decross-linking, the DNA in the immune complex was analyzed by PCR using primers specific for the HIV-1 LTR to determine whether the CTD was physically associated LTR in complex. In doxycycline-treated cells, the induction of p27SJ results in a dramatic reduction in phosphorylated RNAPII bound to the LTR in vivo (Fig. 3, compare lanes 1-4 to lanes 5-8).
Figure 3. Effect of p27SJ expression on the association of phospho-CTD RPII with the HIV-1 LTR.
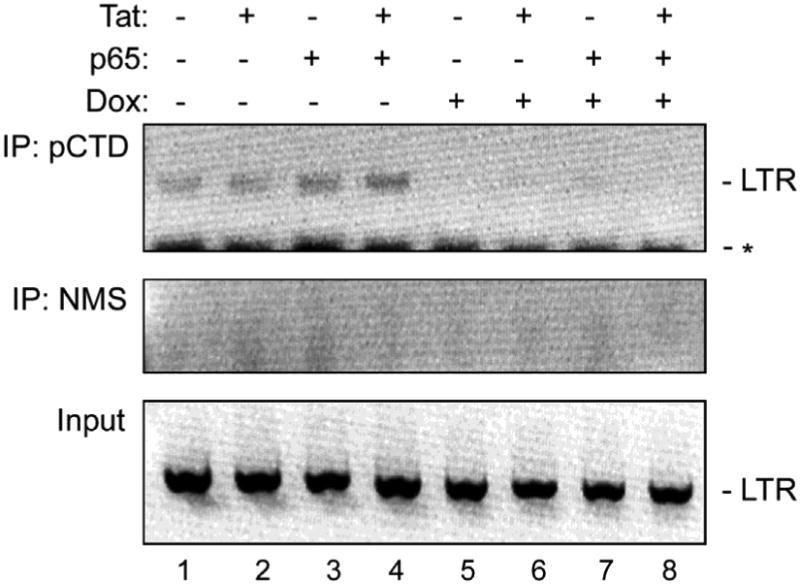
A ChIP assay was performed to measure the level of binding of immunoprecipitated phosphorylated RNAPII CTD to the LTR in cells expressing p27SJ in the presence and absence of Tat and/or p65. Immunoprecipitation using nonimmune mouse serum was the negative control (middle panel). Input DNA was the positive control (lower panel). The images of each of the ethidium bromide-stained gels were inverted using Adobe Photoshop for clarity of presentation.
p27SJ inhibits transcription elongation from the HIV-1 LTR
The results from the previous experiments indicate that the phosphorylation of the CTD of RNAPII is suppressed in the presence of p27SJ causing an inhibition of binding to the HIV-1 LTR. Thus, we expected that this would result in the suppression of HIV-1 LTR transcription. In order to directly examine this, an elongation study was performed by purifying RNA and employing an reverse-transcriptase/PCR (RT/PCR). Different primers were designed with the forward primer located at the TAR region of the HIV-1 LTR and reverse primers situated at different distances along the luciferase gene (Fig. 4A). The three amplicons that were examined were designated LUC1, LUC2 and LUC3 and the respective cDNAs were analyzed by agarose gel electrophoresis, ethidium bromide staining followed by image inversion in Adobe Photoshop to allow the bands to be seen clearly (Fig. 4B). The total RNA samples from the control and doxycycline-treated cells were also analyzed by agarose gel to verify RNA integrity and equivalent RNA inputs (Fig. 4C). When the gel shown in Fig. 4B was quantitated, it was found that the inhibitory effect of p27SJ was dependent on the position of the reverse primer with closest (LUC1) only showing only slight inhibition (0.5%) and the farther primers showing greater inhibition (35-40%) as indicated in the right hand side of Fig. 4A. No product was detected in the experiments without reverse transcriptase enzyme (Fig. 4B, lanes 7 and 8). We conclude that p27SJ inhibits transcription elongation from the HIV-1 LTR.
Figure 4. Analysis of the effect of p27SJ expression on LTR transcription elongation.
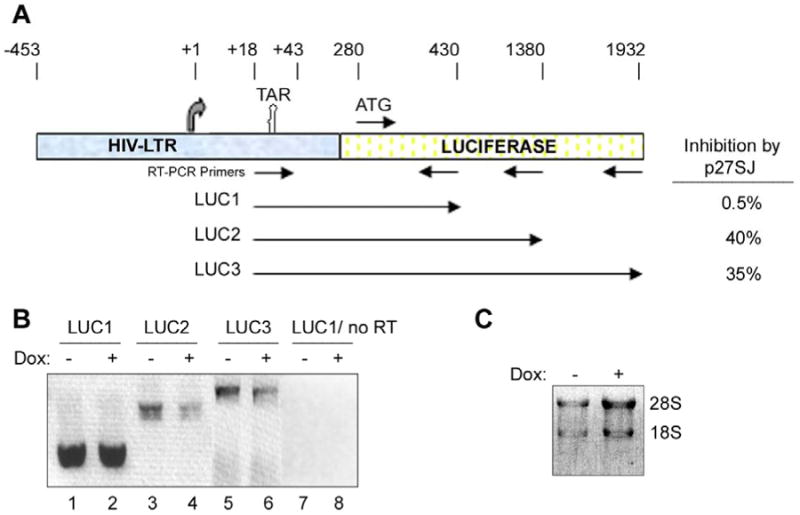
A. Schematic representation of the HIV-LTR-Luciferase reporter plasmid containing the luciferase gene under the control of LTR that was used in the transcription elongation studies. The sites of the primers used in the RT-PCR elongation assays are specified as are the positions of the three amplicons. B. Effect of p27SJ on LTR transcription elongation was analyzed in RT-PCR assays for the three amplicons. The negative control was performed without reverse transcriptase. The image of the ethidium bromide-stained gel was inverted using Adobe Photoshop for clarity of presentation. C. The integrity and loading of each of the RNA samples from cells with or without p27SJ was examined by agarose gel electrophoresis and ethidium bromide staining. The image of the gel was inverted using Adobe Photoshop for clarity of presentation.
p38SJ also inhibits HIV-1 transcription and binding of RNAPII to the HIV-1 LTR
The original clone of p27SJ derived from cDNA from Hypericum perforatum, also known as St. John's Wort, (Darbinian-Sarkissian et al, 2006) consisted of the N-terminal 263 amino acids of the full-length protein p38SJ (GenBank: AAW57408.1). Recently, we have cloned a cDNA encoding all 364 amino acids of the full-length protein p38SJ (GenBank: AAW57408.2) as described in Materials and Methods. To examine the effect of p38SJ, we performed another RT-PCR analysis of the LUC2 amplicon (Fig. 4A) with the p27SJ-inducible cell line and transfection of a p38SJ expression plasmid (Fig. 5A). As expected, the strongest signal was observed for cells expressing p65 and Tat (lane 4) and low levels were observed in cells transfected with p38SJ expression plasmid (lanes 5-8) even when p65 and Tat were present (lane 8). In cells expressing both p38SJ plus p27SJ, no reverse transcript was detectable in all cases (lanes 9-12). Ethidium staining of the ribosomal RNAs was used to verify RNA integrity and equivalent loading (Fig. 5A, lower panel).
Figure 5. Analysis of the effect of p38SJ expression on LTR transcription elongation and the association of phospho-CTD RPII with the LTR.
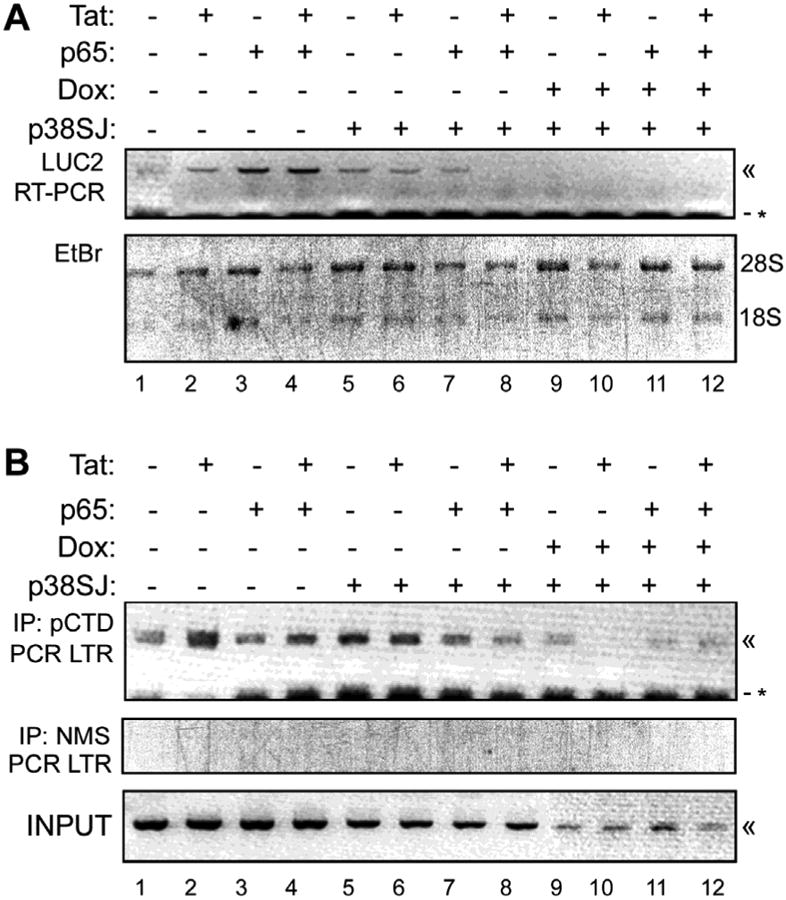
A. The effect of p38SJ on LTR transcription elongation in the presence and absence of Tat and/or p65 and/or p27SJ (Dox-treated) was analyzed in RT-PCR assays for the LUC2 amplicon. « - indicate the PCR product. The negative control was performed without reverse transcriptase. * - indicates a non-specific band. The image of the ethidium bromide-stained gel was inverted using Adobe Photoshop for clarity of presentation. The integrity and loading of each of the RNA samples from cells with or without p27SJ was examined by agarose gel electrophoresis and ethidium bromide staining (lower panel). The image of the gel was inverted using Adobe Photoshop for clarity of presentation. B. A ChIP assay was performed to measure the level of binding of immunoprecipitated phosphorylated RNAPII CTD to the LTR in cells expressing p38SJ in the presence and absence of Tat and/or p65 and/or p27SJ (Dox-treated). Immunoprecipitation using nonimmune mouse serum was the negative control (middle panel). Input DNA was the positive control (lower panel). The images of each of the ethidium bromide-stained gels were inverted using Adobe Photoshop for clarity of presentation.
Next, we examined the effect of p38SJ on the binding of RNAPII to the LTR using a ChIP assay as described for Figure 3. In general the effect of p38SJ was negative, especially in the presence of p65 and Tat (Fig. 5B, compare lanes 4 and 8) and when p38SJ and p27SJ were expressed together (lanes 9-12). The middle panel of Fig. 5B shows the nonimmune rabbit serum control and bottom panel shows the input. Taken together these data indicate a negative effect of p38SJ on RNAPII binding to the HIV-1 LTR.
p27SJ and p38SJ inhibit the promoter activity of the HIV-1 LTR
The effects of p27SJ and p38SJ on the promoter activity of the HIV-1 LTR were examined using the HIV-1 LTR-luciferase reporter construct in the p27SJ-inducible cell line (Fig. 6). In this experiment, Tat, p65 and both together stimulated promoter activity 6-, 1.6- and 8-fold respectively (columns 1-4). In the presence of p27SJ, i.e., the addition of Dox, there was about a 40-50% inhibition in each respective case (columns 5-8). For p38SJ, the inhibition was about 98% inhibition in each respective case (columns 9-12) and when both p27SJ and p38SJ were present, there was about 99.8% inhibition in each respective case (columns 12-16). We conclude that both p27SJ and p38SJ strongly inhibit the promoter activity of the HIV-1 LTR, even in the presence of the HIV-1 transcriptional activators NF-κB p65 and/or HIV-1 Tat. Of note, the full-length p38SJ has a significantly stronger suppressive effect than its foreshortened form, p27SJ.
Figure 6. Effect of p27SJ and p38SJ on the promoter activity of the HIV-1 LTR.
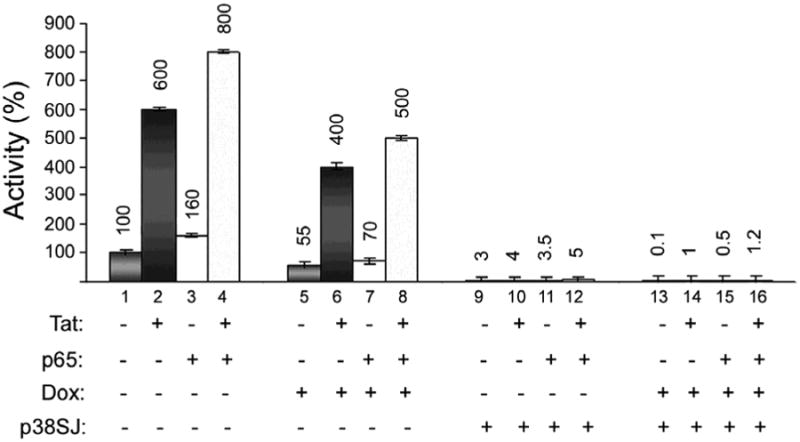
The effects of p27SJ and p38SJ on the promoter activity of the HIV-1 LTR were examined using the HIV-1 LTR-luciferase reporter construct in the p27SJ-inducible cell line (Fig. 6). In this experiment, the effect of Tat, p65 and both together were measured with and without doxycycline to induce p27SJ. Each column was normalized to the control in column 1 (100%), i.e., in the absence of Tat, p65, p27SJ (-Dox) and p38SJ.
Discussion
The cDNA for p27SJ was first isolated and cloned in 2006 and encodes a novel protein from St. John's Wort, Hypericum perforatum (Genbank: AAW57408.1; Darbinian-Sarkissian et al, 2006). p27SJ protein belongs to the DINGG family of proteins, a recently discovered protein family named for their four conserved N-terminal amino-acid sequences, DINGG. Some of the DING proteins with phosphate-binding domain, are related to alkaline phosphatases, and play key roles in various human diseases, including viral infections (Berna et al, 2009a,b). Although the DING protein family is ubiquitous in eukaryotes, their genes are lacking from genomic databases, which has considerably limited functional studies.
Here, we demonstrated the inhibitory effect of p27SJ on HIV-1 LTR transcriptional elongation. The mechanism for this appears to be dephosphorylation of the CTD of RNAPII and to involve the direct interaction of p27SJ with RNAPII. Since p27SJ can also bind to Tat, this suggests that p27SJ interferes with the formation of transcriptionally active complexes of RNAPII mediated by Tat activation of pTEFb. Likely as a consequence of this, occupancy of the HIV-1 LTR and transcriptional elongation are inhibited in cells that express p27SJ. Importantly, the inhibitory effect of p27SJ was dominant even in the presence of powerful transactivators of HIV-1 LTR transcription, HIV-1 Tat and the p65 subunit of NF-κB. While the action of p27SJ occurs at least in part through its action on the CTD of RNAPII, it is possible that other mechanisms are also involved since it has been found that p27SJ can inhibit the phosphorylation of other important cellular regulatory proteins suck as Erk1/2 (Darbinian et al, 2009).
In the present study, we also report the cloning and sequencing of p38SJ, a full length version of the St. John's Wort DINGG protein, of which p27SJ represents a C-truncated version. This is also active in the suppression of HIV-1 transcription. Indeed, in the HIV-1 LTR transcription reporter assay presented in Figure 6, p38SJ gave 97% inhibition while p27SJ gave 45%. Hence p38SJ is a more potent suppressor of HIV-1 gene expression than p27SJ. Before we had cloned the cDNA for p38SJ, we already had evidence for its biological activity in human cells. We purified p38SJ directly from a callus culture of H. perforatum and showed that treatment of neuronal cells with p38SJ protects cells against injury induced by exposure to ethanol (Amini et al, 2009). Further, pre-treatment of neuronal cells with p38SJ diminished the level of Bax and caspase 3 cleavage and increased the activity of superoxide dismutase.
In the case of p27SJ, we have investigated the potential for the development of a therapeutic approach to control HIV-1 transcription and replication in cells by creating a derivative of the p27SJ protein fused to secretion/uptake signals. This represents a bi-directional protein transduction system producing an “armed” p27SJ derivative that inhibits Tat-induced activation of the LTR and decreases the level of viral replication in promonocytic cells including U-937 and T-lymphocytic cells (Darbinian et al, 2008). Since p38SJ is more effective than p27SJ in suppressing HIV-1 gene expression, the cloning of p38SJ may facilitate its development as a potent anti-HIV therapeutic.
Acknowledgments
We wish to thank past and present members of the Department of Neuroscience and Center for Neurovirology for their continued support, insightful discussion, and sharing of reagents and ideas. We are particularly grateful for the collaboration with Dr. Yuri Popov from Yerevan State University, Armenia who provided the starting materials that led to the isolation of the p38SJ protein in our laboratory. We also wish to thank C. Schriver for editorial assistance. This work was supported by grants awarded by NIH to KK and SA.
References
- Adams L, Davey S, Scott K. The DING protein: an autocrine growth-stimulatory protein related to the human synovial stimulatory protein. Biochim Biophys Acta. 2002;1586:254–64. doi: 10.1016/s0925-4439(01)00104-1. [DOI] [PubMed] [Google Scholar]
- Alam M, Mahajan M, Raziuddin M, Singh TP, Yadav S. Proteomics-based approach for identification and purification of human phosphate binding apolipoprotein from amniotic fluid. Genet Mol Res. 2009;8:929–937. doi: 10.4238/vol8-3gmr620. [DOI] [PubMed] [Google Scholar]
- Amini S, Merabova N, Khalili K, Darbinian N. p38SJ, a novel DINGG protein protects neuronal cells from alcohol induced injury and death. J Cell Physiol. 2009;221:499–504. doi: 10.1002/jcp.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboric M, Peterlin BM. A New Paradigm in Eukaryotic Biology: HIV Tat and the Control of Transcriptional Elongation. PLoS Biol. 2005;3:e76. doi: 10.1371/journal.pbio.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboric M, Nissen RM, Kanazawa S, Jabrane-Ferrat N, Peterlin BM. NF-kappaB binds P-TEFb to stimulate transcriptional elongation by RNA polymerase II. Mol Cell. 2001;8:327–337. doi: 10.1016/s1097-2765(01)00314-8. [DOI] [PubMed] [Google Scholar]
- Barboric M, Yik JH, Czudnochowski N, Yang Z, Chen R, Contreras X, Geyer M, Matija Peterlin B, Zhou Q. Tat competes with HEXIM1 to increase the active pool of P-TEFb for HIV-1 transcription. Nucleic Acids Res. 2007;35:2003–2012. doi: 10.1093/nar/gkm063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna A, Bernier F, Chabrière E, Perera T, Scott K. DING proteins; novel members of a prokaryotic phosphate-binding protein superfamily which extends into the eukaryotic kingdom. Int J Biochem Cell Biol. 2008;40:170–175. doi: 10.1016/j.biocel.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Berna A, Bernier F, Chabrière E, Elias M, Scott K, Suh A. For whom the bell tolls? DING proteins in health and disease. Cell Mol Life Sci. 2009a;66:2205–2218. doi: 10.1007/s00018-009-0006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna A, Scott K, Chabrière E, Bernier F. The DING family of proteins: ubiquitous in eukaryotes, but where are the genes? Bioessays. 2009b;31:570–580. doi: 10.1002/bies.200800174. [DOI] [PubMed] [Google Scholar]
- Darbinian N, Czernik M, Darbinyan A, Elias M, Chabrière E, Bonasu S, Khalili K, Amini S. Evidence for phosphatase activity of p27SJ and its impact on the cell cycle. J Cell Biochem. 2009;107:400–407. doi: 10.1002/jcb.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbinian N, Popov Y, Khalili K, Amini S. Creation of a bi-directional protein transduction system for suppression of HIV-1 expression by p27SJ. Antiviral Res. 2008;79:136–141. doi: 10.1016/j.antiviral.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbinian-Sarkissian N, Darbinyan A, Otte J, Radhakrishnan S, Sawaya BE, Arzumanyan A, Chipitsyna G, Popov Y, Rappaport J, Amini S, Khalili K. p27(SJ), a novel protein in St John's Wort, that suppresses expression of HIV-1 genome. Gene Ther. 2006;13:288–295. doi: 10.1038/sj.gt.3302649. [DOI] [PubMed] [Google Scholar]
- Diemer H, Elias M, Renault F, Rochu D, Contreras-Martel C, Schaeffer C, Van Dorsselaer A, Chabrière E. Tandem use of X-ray crystallography and mass spectrometry to obtain ab initio the complete and exact amino acids sequence of HPBP, a human 38-kDa apolipoprotein. Proteins. 2008;71:1708–1720. doi: 10.1002/prot.21866. [DOI] [PubMed] [Google Scholar]
- Hain NA, Stuhlmuller B, Hahn GR, Kalden JR, Deutzmann R, Burmester GR. Biochemical characterization and microsequencing of a 205-kDa synovial protein stimulatory for T cells and reactive with rheumatoid factor containing sera. J Immunol. 1996;157:1773–80. [PubMed] [Google Scholar]
- Lesner A, Shilpi R, Ivanova A, Gawinowicz MA, Lesniak J, Nikolov D, Simm M. Identification of X-DING-CD4, a new member of human DING protein family that is secreted by HIV-1 resistant CD4(+) T cells and has anti-viral activity. Biochem Biophys Res Commun. 2009;389:284–289. doi: 10.1016/j.bbrc.2009.08.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesner A, Li Y, Nitkiewicz J, Li G, Kartvelishvili A, Kartvelishvili M, Simm M. A soluble factor secreted by an HIV-1-resistant cell line blocks transcription through inactivating the DNA-binding capacity of the NF-kappa B p65/p50 dimer. J Immunol. 2005;175:2548–2554. doi: 10.4049/jimmunol.175.4.2548. [DOI] [PubMed] [Google Scholar]
- Marshall NF, Dahmus ME. C-terminal domain phosphatase sensitivity of RNA polymerase II in early elongation complexes on the HIV-1 and adenovirus 2 major late templates. J Biol Chem. 2000;275:32430–32437. doi: 10.1074/jbc.M005898200. [DOI] [PubMed] [Google Scholar]
- McCracken S, Fong N, Yankulov K, Ballantyne S, Pan G, Greenblatt J, Patterson SD, Wickens M, Bentley DL. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature. 1997;385:357–361. doi: 10.1038/385357a0. [DOI] [PubMed] [Google Scholar]
- Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Perera T, Berna A, Scott K, Lemaitre-Guillier C, Bernier F. Proteins related to St. John's Wort p27SJ, a suppressor of HIV-1 expression, are ubiquitous in plants. Phytochemistry. 2008;69:865–872. doi: 10.1016/j.phytochem.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Peterlin BM, Price DH. Controlling the elongation phase of transcription with P-TEFb. Mol Cell. 2006;23:297–305. doi: 10.1016/j.molcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Phatnani HP, Greenleaf AL. Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 2006;20:2922–2936. doi: 10.1101/gad.1477006. [DOI] [PubMed] [Google Scholar]
- Simm M. The innate cellular responses to HIV-1 invasion: emerging molecules of ancient defense mechanisms. Arch Imunol Ther Exp (Warsz) 2007;55:131–138. doi: 10.1007/s00005-007-0023-9. [DOI] [PubMed] [Google Scholar]
- Simm M, Miller LS, Durkin HG, Allen M, Chao W, Lesner A, Potash MJ, Volsky DJ. Induction of secreted human immunodeficiency virus type 1 (HIV-1) resistance factors in CD4-positive T lymphocytes by attenuated HIV-1 infection. Virology. 2002;294:1–12. doi: 10.1006/viro.2001.1300. [DOI] [PubMed] [Google Scholar]
- Zhou M, Deng L, Kashanchi F, Brady J, Shatkin A, Kumar A. The Tat/Tar-dependent phosphorylation of RNA polymerase II C-terminal domain stimulates cotranscriptional capping of HIV-1 mRNA. Proc Natl Acad Sci USA. 2003;100:12666–12671. doi: 10.1073/pnas.1835726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Yik JH. The Yin and Yang of P-TEFb regulation: implications for human immunodeficiency virus gene expression and global control of cell growth and differentiation. Microbiol Mol Biol Rev. 2006;70:646–659. doi: 10.1128/MMBR.00011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


