Abstract
Background
The activity of neurogenic differentiation 1 (Neurod1) decreases after morphine administration, which leads to impairments of the stability of dendritic spines in primary hippocampal neurons, adult neurogenesis in mouse hippocampi, and drug-associated contextual memory. The current study examined whether Neurod1 could affect the development of opioid tolerance.
Methods
Lentivirus encoding Neurod1, microRNA-190 (miR-190), or short hairpin RNA against Neurod1 was injected into mouse hippocampi separately or combined (more than eight mice for each treatment) to modulate Neurod1 activity. The antinociceptive median effective dose values of morphine and fentanyl were determined with tail-flick assay and used to calculate development of tolerance. Contextual learning and memory were assayed using the Morris water maze.
Results
Decrease in NeuroD1 activity increased the initial antinociceptive median effective dose values of both morphine and fentanyl, which was reversed by restoring NeuroD1 activity. In contrast, decrease in NeuroD1 activity inhibited development of tolerance in a time-dependent manner, paralleling its effects on the acquisition and extinction of contextual memory. In addition, only development of tolerance, but not antinociceptive median effective dose values, was modulated by the expression of miR-190 and Neurod1 driven by Nestin promoter.
Conclusions
Neurod1 regulates the developments of opioid tolerance via a time-dependent pathway through contextual learning and a short-response pathway through antinociception.
Keywords: Analgesia, learning, Neurod1, opioid, tolerance, water maze
Neurogenic differentiation 1 (Neurod1), a basic helix-loop-helix transcriptional factor, was first identified to convert ectoderm into neurons in Xenopus embryos and plays critical roles in the development of the pancreas, cerebellum, and hippocampus (1–3). NeuroD1 also functions in maintaining the dendritic morphology of granule neurons (4). Reducing the activity of NeuroD1 by microRNA-190 (miR-190) or by inhibiting calcium-calmodulin kinase II α reduces dendritic spine stability in primary hippocampal neurons (5). NeuroD1 has also been identified to regulate adult neurogenesis in the subgranular zone of hippocampal dentate gyrus (DG) (6). Reducing NeuroD1 activity in DG with lentivirus encoding miR-190, a microRNA targeting Neurod1, impairs adult neurogenesis and the abilities of mice to retain drug-associated contextual memory (7). Because adult neurogenesis is associated with contextual learning and memory (8,9), which is implicated in development of opioid tolerance (10,11), we hypothesized that NeuroD1 could be one of the many transcription factors involved in development of opioid tolerance.
In regulating NeuroD1 activity, opioid agonists exhibit biased agonism. Both morphine and fentanyl reduce NeuroD1 phosphorylation by inhibiting calcium-calmodulin kinase II α activity. However, fentanyl, but not morphine, increases NeuroD1 protein level by suppressing the expression of miR-190. Morphine decreases the overall activity of NeuroD1, whereas fentanyl maintains it at the basal level (5,12). The inhibitory effects of morphine on NeuroD1 activity were applied to explain its ability to decrease adult neurogenesis in the hippocampus (7,13,14).
We examined opioid antinociception tolerance after manipulating NeuroD1 activity in the hippocampus with a previous reported lentivirus system (5,7). Although the hippocampus is not considered as the major brain structure in mediating opioid antinociception, it has been implicated in the affective-motivation component of pain sensation. Noxious stimulation increased the level of early inducible genes and changed synaptic plasticity in the hippocampus (15,16). Injection of L-arginine, a precursor of nitric oxide, reversed morphine-induced antinociception (17). Microinjection of thioperamide, a histamine H3 receptor antagonist, into the DG increased histamine-induced antinociception of the formalin-induced pain, whereas both H1 and H2 receptor antagonists, chlorpheniramine and ranitidine, blocked the histamine effect (18). Direct injection of carbachol, morphine, or bicuculline into the dorsal hippocampus promoted antinociception (19). These studies and others provide supportive evidence for the role of the hippocampus in pain sensation. Because both mu-opioid receptor (Oprm1) and Neurod1 are expressed at high levels within the hippocampus (1,20), and the hippocampus may contribute to the development of antinociception tolerance by supporting adult neurogenesis and associative learning, which also involves NeuroD1 (8–11), we targeted the hippocampus in studies to explore the connection between NeuroD1 and opioid antinociception tolerance.
Methods and Materials
Animal Studies
Our studies followed the guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols were approved by the Committee on the Ethics of Animal Experiments of the University of Minnesota or equivalent committee of Guangzhou Institutes of Biomedicine and Health. All efforts were made to minimize animal discomfort.
Tail-flick tests were done between 1:00 P.M. and 4:00 P.M. Mice were placed in the experimental room 2 hours beforehand for acclimatization. Tails were placed over an analgesia meter (Columbus Instruments, Columbus, Ohio), and radiant heat intensity was adjusted for 3 to 5 sec baseline latency. Cutoff time was 12 sec to minimize tail damage. Tail-withdrawal responses were recorded 30 min after morphine or 15 min after fentanyl injection subcutaneously. Percent of maximum possible effect was calculated as (measured latency−baseline latency) * 100/(12−baseline latency).
The up-and-down method was used to determine median effective dose (ED50) values (21,22). The first animal was given a dose of drug close to the anticipated ED50 value and evaluated in the tail-flick test. If the percent of maximum possible effect value was >50% or <50%, the dose for the next animal was incrementally decreased or increased (by log dose of .05). In general, the test was concluded after six animals (counted from the last of the first several mice that had responses all >50% or <50%). The ED50 values and standard errors (n = 6) were calculated from tables provided by Dixon (21,22). Degree of opioid tolerance (n = 2 for statistic analysis) was determined by comparing the ED50 values before and after certain opioid usages.
Lentiviruses were generated as described previously (5), and short hairpin RNA was designed to complement the 203–223 nucleotide sequence of the mouse Neurod1 messenger (m)RNA (NM_010894.2). Concentrated lentiviruses with titers at about 6 × 108 transducing units/mL were administered into DG of mice. Mice were anesthetized with 90 mg/kg ketamine and 10 mg/kg xylazine. Concentrated virus (1 μL) was injected −2.1 mm posterior to the bregma, ±1.1 mm lateral to the midline, and 1.7 mm below the meniscus in a 1-min period.
The Morris water maze (MWM) test was carried out as reported (23). In the learning section, the mice were trained for 5–7 days (until the escape latency reached the level on the fifth day of control group). In the extinction section, probe tests were performed every other 4 days afterward until the mice spent almost equal time in all four quadrants.
Neuron Stem Cells and Primary Neurons
After meninges were stripped off, the remaining tissues of the brain of E13.5 embryos were cut into pieces, rinsed with phosphate-buffered saline, and dissociated with .05% trypsin ethylenediaminetetraacetic acid (Gibco, Grand Island, New York) for 10–15 min. The dissociated cells were resuspended (Dulbecco’s modified Eagle’s medium F12 [Gibco] supplemented with N2 [Gibco], B27 [Gibco], 20 ng/mL basic fibroblast growth factor [R&D, Minneapolis, Minnesota], and 20 ng/mL epidermal growth factor [Invitrogen, Grand Island, New York]). After the adherence of fibroblasts, the supernatant was cultured to form neurospheres. Cultured neurospheres were trypsinized into single cells and seeded into plates coated with Matrigel (BD Biosciences, San Jose, California). Primary hippocampal neuron cultures were prepared as described previously (24).
Others
Immunoblotting was performed as described previously (25). Anti-β-actin and anti-NeuroD1 (1:1000; Cell Signaling Technology, Beverly, Massachusetts) were used to determine NeuroD1 protein levels. Anti-NeuroD1 (1:250) and protein G agarose (Invitrogen) were used to precipitate Neurod1 before using Anti-phos-Ser (1:500; Cell Signaling Technology) to determine the amounts of phosphorylated NeuroD1. Binding assay was applied to determine the amounts of OPRM1 on membrane isolated from mice hippocampi (26).
Total RNAs including microRNAs were extracted with miR-Neasy Mini Kit (Qiagen, Alameda, California) and reverse transcribed with miScript II RT reverse transcription kit (Qiagen). Quantitative polymerase chain reaction was done with miScript SYBR Green PCR Kit (Qiagen) in a CFX96 Real-Time system (Bio-Rad Laboratories, Hercules, California). β-actin was the internal control and had cycle number around 16. The cycle numbers of Neurod1, miR-190, Doublecortin, and Neurod4 were between 24 and 27. The cycle numbers of Oprm1 were around 28. The nucleotide sequences of the primers used are summarized in Table S1 in Supplement 1.
Statistics
Experiments were repeated at least three times except for behavior studies. Because of the methods used, n for ED50 values and ratios were 6 and 2, respectively. Error bars represent standard errors except in Figure 3E and Figures S1 and S2 in Supplement 1, where the error bars represent standard deviations.
Figure 3.
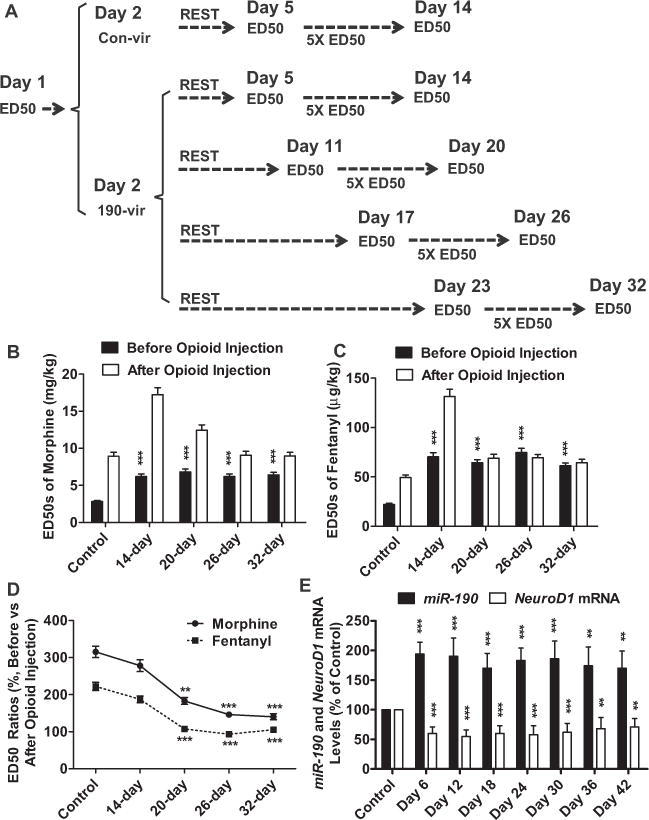
Decrease in NeuroD1 activity affected development of tolerance in a time-dependent manner. (A) Mice were injected with 190-vir on day 2 and divided into four groups to begin daily opioid injection at different time points. (B–D) The ED50 values were determined before and after opioid injection and sum-marized for morphine (B) and fentanyl (C). Different experimental groups were indicated by “14-day,” “20-day,” “26-day,” and “32-day.” After comparing the ED50 values (day 14 vs. day 5, day 20 vs. day 11, day 26 vs. day 17, day 32 vs. day 23), the abilities of decrease in NeuroD1 activity to affect opioid tolerance development were summarized (D). (E) The levels of Neurod1 mRNA and miR-190 were determined at indicated time points after 190-vir injection by quantitative polymerase chain reaction. The cycle numbers for β-actin were around 16. The cycle numbers of Neurod1 and miR-190 were between 24 and 27. One-way analysis of variance with Dunnett test as post hoc was used for comparisons between the basal level (control) and all other data in the same data set. Only significant differences were listed. *p < .05, **p < .01, ***p < .001. mRNA, messenger RNA other abbreviations as in Figure 1.
Results
Decrease in NeuroD1 Activity Affects Opioid Antinociception and Tolerance
Lentivirus encoding control vector (con-vir), miR-190 (190-vir), Neurod1 (nd-vir), or short hairpin RNA against Neurod1 (nd-sh-vir) was stereotactically injected into mice hippocampi as described in Methods and Materials. Similar to previous reports (5,7), 190-vir and nd-sh-vir decreased the protein and mRNA levels of Neurod1, the amount of phosphorylated Neurod1, and the expression of two Neurod1 downstream targets, Doublecortin and Neurod4 (27), whereas nd-vir had the opposite effects and could rescue the effects of 190-vir almost completely (Figure S1 in Supplement 1). The lentiviruses 190-vir and nd-sh-vir decreased Neurod1 activity, and nd-vir could block such decrease.
A 20-day experiment was performed (Figure 1A). On day 1, the antinociceptive ED50 values of morphine and fentanyl were determined. On day 2, the mice were divided into six groups and stereotactically injected with saline, con-vir, 190-vir, 190-vir plus nd-vir, nd-vir, or nd-sh-vir. After an 8-day rest, the ED50 values of opioids were determined again in each group on day 11. As summarized in Figure 1B and C, 190-vir increased the ED50 values of morphine and fentanyl to about 2.4-fold and 2.9-fold of basal levels, respectively. Injecting nd-vir with 190-vir could block these effects significantly, although nd-vir alone did not affect ED50 values. In addition, nd-sh-vir also increased ED50 values significantly (Figure 1B, C). The observed effects of 190-vir on opioid antinociception were mainly due to the 190-vir-mediated decrease in NeuroD1 activity in the hippocampus. For better understanding of the results, all the ED50 values are listed in Table S2 in Supplement 1.
Figure 1.
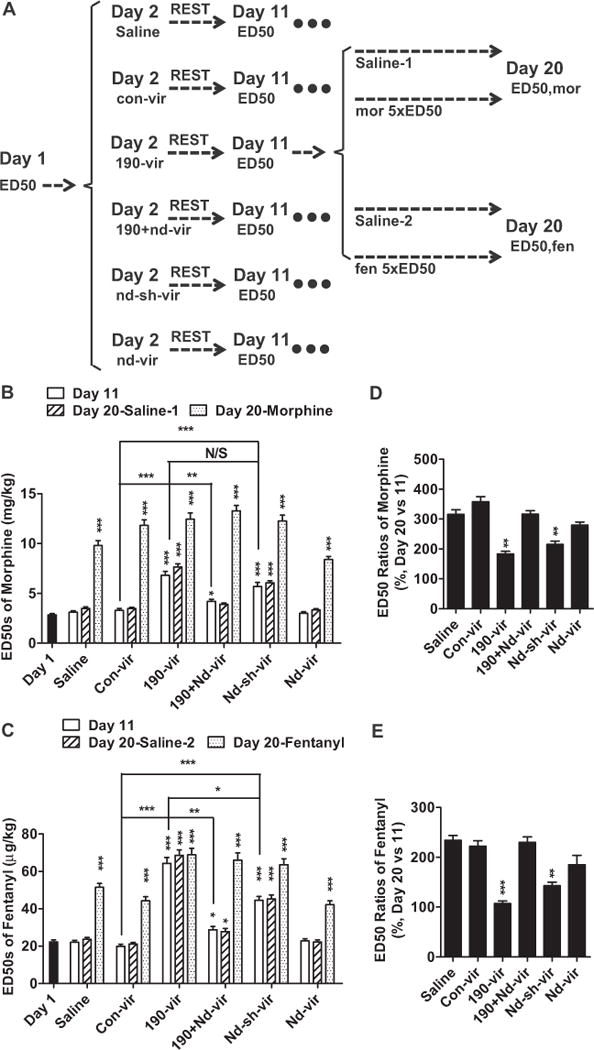
Decrease in NeuroD1 activity affected opioid antinociception and tolerance development. (A) Mice were injected with saline or different lentivirus on day 2 and received daily injection of saline or opioid (morphine or fentanyl) from day 12 to day 19. Six mice groups were divided into four subgroups, with more than eight mice in each subgroup. (B, C) The ED50 values of opioids were determined on day 1, day 11, and day 20 and summarized for morphine (B) and fentanyl (C). (D, E) Tolerance or ED50 ratios were calculated by normalizing ED50 values on day 20 to ED50 values on day 11. The results were also summarized for morphine (D) and fentanyl (E). One-way analysis of variance with Dunnett test as post hoc was used for comparisons between the basal level—day 1 (B, C) and saline (D, E)—and all other data in the same data set. Only significant differences were listed. *p < .05, **p < .01, ***p < .001. Additional comparisons were performed between indicated lanes. 190-vir, miR-190; con-vir, control vector; ED50, median effective dose; fen, fentanyl; mor, morphine; nd-sh-vir, short hairpin RNA against Neurod; nd-vir, Neurod1; N/S, not significant.
Each group of mice was divided into four subgroups, saline-1, saline-2, morphine, and fentanyl. The mice in the saline subgroups were injected with .3 mL saline subcutaneously three times a day from day 12 to day 19. The mice in the morphine and fentanyl subgroups were injected subcutaneously with 5 × ED50 doses of morphine and fentanyl under the same paradigm (Figure 1A). The ED50 values on day 11 were used for dose calculation to ensure that mice in different groups were treated with equivalent antinociceptive doses of opioids. On day 20, ED50 values of morphine were determined in saline-1 and morphine subgroups, and ED50 values of fentanyl were determined in saline-2 and fentanyl subgroups. Morphine ED50 values were increased by 8-day morphine injection more significantly in mice injected with saline (315 ± 15%), con-vir (358 ± 17%), 190-vir plus nd-vir (316 ± 12%) or nd-vir (280 ± 11%) than in mice injected with 190-vir (183 ± 9%) or nd-sh-vir (231 ± 11%) (Figure 1D). Similar attenuations in the degrees of tolerance to fentanyl were also observed in mice injected with 190-vir or nd-sh-vir compared with mice injected with saline, con-vir, or 190-vir plus nd-vir (Figure 1E), indicating decrease in NeuroD1 activity in the hippocampus inhibits development of tolerance after long-term morphine or fentanyl treatment.
Mice injected with either con-vir or 190-vir were used for additional analysis following modified procedures (Figure 2A). Mice were injected subcutaneously three times a day from day 12 to day 19 with 2 × ED50, 5 × ED50 determined, or 10 × ED50 doses that were determined on day 11. The antinociceptive ED50 values were determined on day 20 (Figure 2B–E). As expected, the higher the doses of opioids used during day 12 to day 19, the larger the increases in ED50 values or the higher degrees of tolerance observed in mice injected with con-vir. However, such dose-dependent development of opioid antinociceptive tolerance was significantly impaired in mice injected with 190-vir (Figure 2B–E). The lack of dose-dependent development of tolerance in mice injected with 190-vir was more pronounced with the fentanyl injections, where minimal increase in the ED50 value was observed even in mice treated with 10 × ED50 dose (Figure 2D, E). Because 5 × ED50 doses were not in plateau area of the dose-response curves, they were used in the following experiments to induce significant but modest tolerance.
Figure 2.
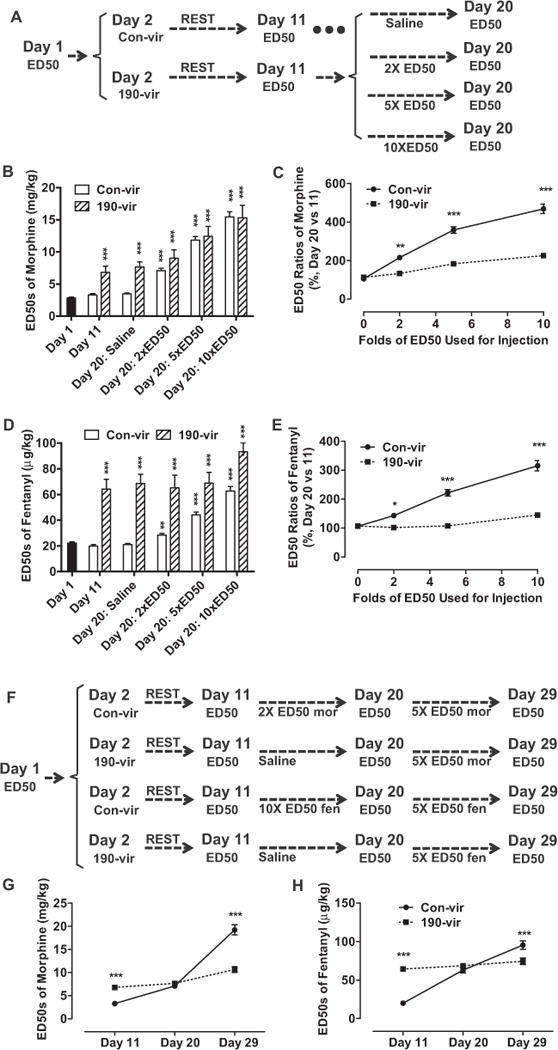
Dose-dependent development of tolerance was affected by 190-vir. (A–E) Mice with con-vir or 190-vir were injected with different doses (2 × ED50, 5 × ED50, or 10 × ED50) of opioids from day 12 to day 19. The ED50 values were determined on day 11 and day 20 (A) and summarized for morphine (B) and fentanyl (D). The dose-dependent curves for tolerance development were also summarized for morphine (C) and fentanyl (E). (F–H) Mice were injected with con-vir or 190-vir on day 2, with opioids (2 × ED50 [day 11] of morphine or 10 × ED50 [day 11] of fentanyl) or saline from day 12 to day 19, and with 5 × ED50 doses (day 20) from day 21 to day 28 (F). The ED50 values were determined on day 11, day 20, and day 29 and summarized for morphine (G) and for fentanyl (H). One-way analysis of variance with Dunnett test as post hoc was used for comparisons between the basal level—day 1 (B, D) and 0 × ED50 (C, E)—and all other data in the same data set (B–D). Two-way analysis of variance with Bonferroni test as post hoc was used for comparisons between the two data sets at every time point (G, H). Only significant differences were listed. *p < .05, **p < .01, ***p < .001. Abbreviations as in Figure 1.
The observed ability of 190-vir to inhibit development of tolerance might be due to the ceiling effect during tolerance development or the difficulty to increase ED50 values further from already elevated ED50 values as a result of virus injections. To eliminate this possibility, mice with con-vir were injected with 2 × ED50 doses of morphine (determined on day 11) from day 12 to day 19, whereas mice with 190-vir were injected with saline (Figure 2F). These different treatments resulted in similar ED50 values in both groups on day 20 (Figure 2G). The two groups of mice then were injected with 5 × ED50 doses of morphine (determined on day 20) from day 21 to day 28. When the ED50 values of morphine were measured on day 39, the ED50 increase in mice injected with con-vir was significantly larger than in mice injected with 190-vir. Because the ED50 values in both groups are close to each other on day 20, the ability of 190-vir to impair further tolerance development was not due to the ceiling effect (Figure 2G). When 10 × ED50 doses of fentanyl were used from day 12 to day 19, similar results were observed (Figure 2F, H).
Time-Dependent Regulation of Opioid Antinociception and Tolerance
The daily opioid injection was changed from day 12–19 to day 6–13, day 18–25, or day 24–31 after 190-vir injection on day 2 (Figure 3A). As indicated in Figure 3B and C, the antinociceptive ED50 values of opioids on day 5, day 11, day 17, or day 23 were similar but significantly higher than the basal level, suggesting the antinociceptive ED50 values were affected by the decrease in NeuroD1 activity immediately and constantly.
The later the daily opioid injections were initiated after 190-vir injection, smaller differences in the ED50 values before and after opioid injection were observed (Figure 3B–D), suggesting the development of tolerance is affected by NeuroD1 activity in a time-dependent manner. This temporal effect was not due to the differences in Neurod1 expression because both Neurod1 expression and miR-190 expression were constant from day 5 to day 42 after 190-vir injection (Figure 3E). Decrease in NeuroD1 activity not only increases antinociceptive ED50 values immediately but also impairs development of tolerance in a time-dependent manner.
Decrease in NeuroD1 Activity Affects Tolerance Development but Not Antinociception via Adult Neurogenesis
There were 2 weeks required for the decrease in NeuroD1 activity to affect tolerance development (Figure 3), which appeared to correlate with the peak of Neurod1 expression during adult neurogenesis (6,28,29). Nestin promoter was used to control the expression of miR-190 (Nes-190-vir) and Neurod1 (Nes-nd-vir). Nes-190-vir and Nes-nd-vir expressed miR-190 and Neurod1 only in neural stem cells (NSCs) and not in primary hippocampal neurons (Figure 4A, B). In addition, Nestin expression overlaps with the temporary upregulation of Neurod1 during neurogenesis (6). These two new viruses enabled us to restrict the modulations on NeuroD1 activity in NSCs or during adult neurogenesis without affecting mature neurons.
Figure 4.
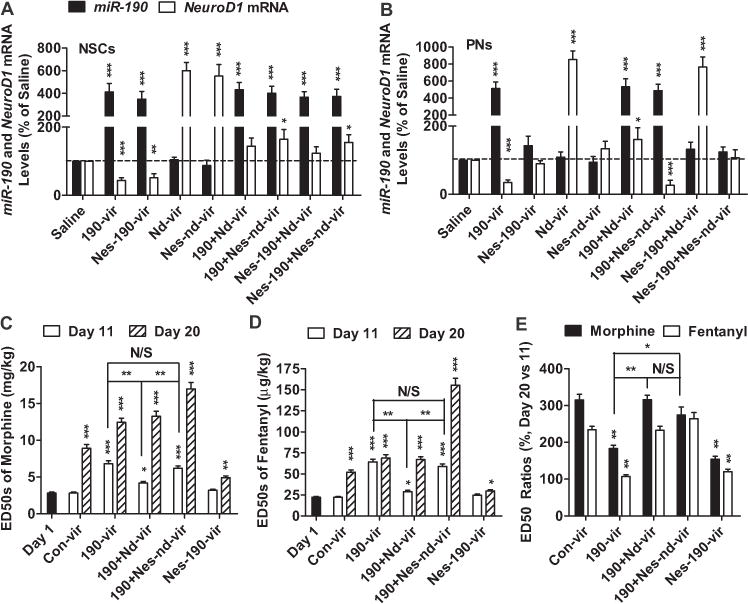
Decrease in NeuroD1 activity affected opioid tolerance via NSCs. (A, B) NSCs (A) and PNs (B) were infected with different combinations of saline, 190-vir, Nes-190-vir, nd-vir, and Nes-nd-vir. The levels of Neurod1 mRNA and miR-190 were determined 3 days after infection by quantitative polymerase chain reaction. The cycle numbers for β-actin were around 16. The cycle numbers of Neurod1 and miR-190 were between 24 and 27. (C–E) Five groups of mice injected with con-vir, 190-vir, 190-vir+nd-vir, 190-vir+Nes-nd-vir, and Nes-190-vir were tested following similar procedures in Figure 1A. The ED50 values on day 1, day 11, and day 20 were summarized for morphine (C) and fentanyl (D). The ratios of ED50 values on day 20 to values on day11 were also listed (E). One-way analysis of variance with Dunnett test as post hoc was used for comparisons between the basal level—saline (A, B), day 1 (C, D), and con-vir (E)—and all other data in the same data set. Only significant differences were listed. *p < .05, **p < .01, ***p < .001. Additional comparisons were performed between indicated lanes. mRNA, messenger RNA; NSCs, neural stem cells; PNs, primary hippocampal neurons; other abbreviations as in Figure 1.
By using a similar protocol to that shown in Figure 1A, 190-vir injection increased the antinociceptive ED50 values of opioids, which could be reversed by coinjection with nd-vir but not by coinjection with Nes-nd-vir. In contrast to 190-vir, Nes-190-vir did not alter the antinociceptive ED50 values of opioids (Figure 4C, D). As for antinociception tolerance development, injection of both 190-vir and Nes-190-vir impaired tolerance development. Both nd-vir and Nes-nd-vir could rescue the impairments in tolerance induced by 190-vir (Figure 4C–E). The development of tolerance, but not opioid antinociception, was regulated by NeuroD1-mediated adult neurogenesis.
Contextual Learning Connects Adult Neurogenesis with Tolerance Development
Because opioid tolerance has long been considered to involve associative learning (10,11) and NeuroD1 activity is critical for maintaining contextual memory (7), we hypothesized that decrease in NeuroD1 activity affects development of tolerance by impairing adult neurogenesis and subsequent contextual learning. As indicated in Figure 5A and C, the rates to learn the position of the submerged platform in all six groups of mice injected with various viruses were similar to each other when the MWM tests were initiated on day 7 or day 14 after virus injection. However, when the extinction of learned memory was determined after successful acquisition of contextual memory, 190-vir or nd-sh-vir accelerated the extinction, which could be partially blocked by the coinjection with nd-vir (Figure 5B, D).
Figure 5.
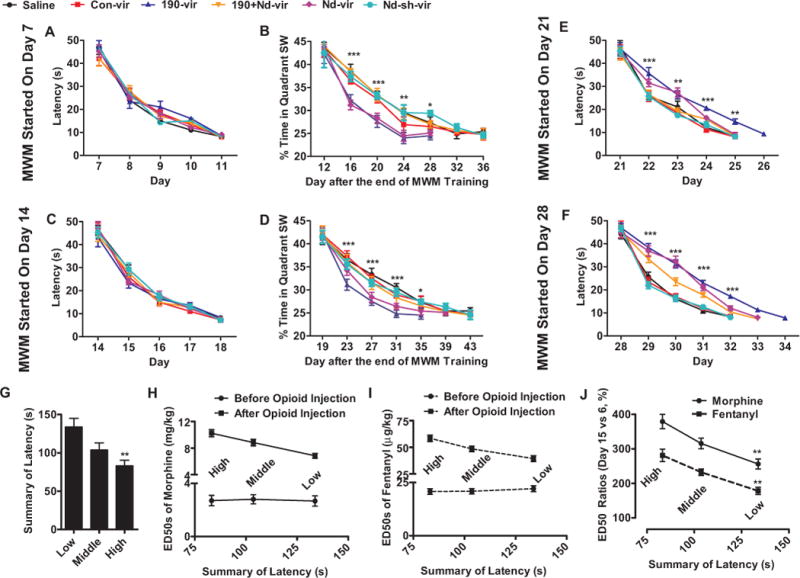
Decrease in NeuroD1 activity affected contextual learning. (A–F) Lentivirus (con-vir, 190-vir, 190-vir+nd-vir, nd-vir, and nd-sh-vir) and saline were injected into dentate gyrus of mice on day 2. The MWM tests began at different time points—day 7 (A), day 14 (C), day 21 (E), or day 28 (F). The learning section lasted for 5 days for mice injected with saline but for 5–7 days for other groups of mice depending on when the average latencies to find the platform were not significantly different from that of mice injected with saline on the fifth day. One day after the learning section, the extinctions of the learned contextual memory were monitored until the total extinction (percentage times in quadrant SW were not significantly different from 25% in two continuous probe tests) (B, D). (G–J) Mice were divided into three groups depending on their performance in MWM tests. One third of mice with smallest summaries of latencies in 5-day training were grouped as “High,” whereas the other two thirds of mice were grouped as “Middle” and “Low” (G). The ED50 values were calculated on day 6 and day 15 to determine the tolerance induced by the daily opioid injection (5 × ED50 doses determined on day 6, three times a day) from day 7 to day 14. The ED50 values (H, I) and tolerance development (J) were summarized. One-way analysis of variance with Dunnett test as post hoc was used for comparisons between the low group and another two groups (I). Two-way analysis of variance with Bonferroni test as post hoc was used to do comparisons between con-vir and 190-vir (A–F) and between morphine and fentanyl (J). Only significant differences were listed. *p < .05, **p < .01, ***p < .001. MWM, Morris water maze; SW, Southwest, in which quadrant the escaping platform was placed; other abbreviations as in Figure 1
Contextual memory acquisition in mice injected with 190-vir and nd-sh-vir was impaired when the MWM tests were initiated on day 21, and such impairments increased when MWM tests began on day 28 (Figure 5E, F). Although the mice in different groups finally learned the platform position after different numbers of training days, these mice should not be considered to have similar levels of contextual memory. Extinction studies were not performed with these mice. In addition, current observations were consistent with our previous report that contextual memory extinction, but not acquisition, is affected 2 weeks after decreasing NeuroD1 activity (7).
We further examined the relationship between contextual memory acquisition and development of tolerance. We divided the mice into three separate groups based on their performance in the MWM test (i.e., the total amount of time [latency] needed for the mice to learn platform position) (Figure 5G). All three groups of mice exhibited similar antinociceptive ED50 values (Figure 5H, I). However, mice that performed better in the MWM test (“High” performers) developed a significantly higher degree of tolerance to both opioids (Figure 5H–J), which further confirmed the correlation between contextual learning and development of tolerance.
Overexpression of Neurod1 Impairs Ability of Morphine to Induce Tolerance
Because morphine, but not fentanyl, decreases NeuroD1 activity and adult neurogenesis (7,12), it is reasonable to suggest that these morphine-induced decreases contribute to the development of tolerance to morphine. Because decrease in NeuroD1 activity increases antinociceptive ED50 values immediately, this pathway should contribute at least partially to the development of morphine tolerance. However, because the effects of 190-vir or nd-sh-vir required a minimal of 21 days for detection (Figures 3 and 5), decrease in NeuroD1 activity requires weeks to affect development of tolerance by modulating adult neurogenesis and contextual memory. This pathway might contribute to the development of morphine tolerance only when morphine treatment is long enough.
To test this hypothesis, mice were injected with 5 × ED50 doses of morphine from day 2 to day 9. The MWM tests were performed on day 10, day 16, day 23, or day 29 (Figure 6A). When the MWM tests were started on day 10 or day 16, morphine treatment did not affect contextual memory acquisition, similar to our previous observations (7). However, significant impairments in contextual memory acquisition were observed when the MWM tests were started on day 23 or day 29 (Figure 6B), suggesting that at least 2 weeks are required for morphine-induced decrease in NeuroD1 activity to affect adult neurogenesis and subsequent contextual memory acquisition.
Figure 6.
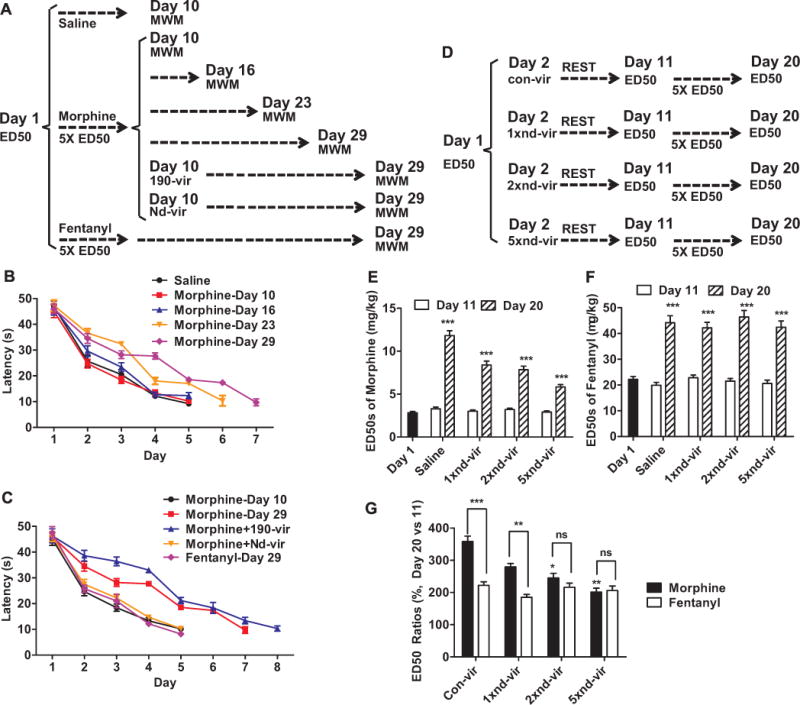
Decrease in NeuroD1 activity accounted for the different abilities of opioids to induce tolerance. (A–C) Mice were injected daily with 5 × ED50 doses on day 1 (three times a day) from day 2 to day 9 and subjected to MWM tests beginning on day 29. The mice injected with morphine were divided into six groups; four groups of mice underwent MWM tests beginning on day 10, day 16, day 23, or day 29, whereas the other two groups of mice had 190-vir or nd-vir injection on day 10 and underwent MWMs test beginning on day 29 (A). The acquisition of contextual memory was summarized (B, C). (D–F) Mice injected with con-vir, nd-vir, 2× nd-vir, or 5× nd-vir on day 2 were used for experiments following the similar procedures in Figure 1A (D). The ED50 values on day 11 and day 20 were summarized for morphine (E) and for fentanyl (F). Tolerance development was also summarized (G). One-way analysis of variance with Dunnett test as post hoc was used for comparisons between the basal level—day 1 (E, F) and con-vir (G)—and all other data in the same data set. Only significant differences were listed. *p < .05, **p < .01, ***p < .001. Additional comparisons were performed between indicated lanes. MWM, Morris water maze; ns, not significant; other abbreviations as in Figure 1.
Two groups of mice injected with 5 × ED50 doses of morphine from day 2 to day 9 received 190-vir or nd-vir injection on day 10, and an additional group of mice were injected with 5 × ED50 antinociceptive doses of fentanyl from day 2 to day 9 (Figure 6A). The MWM tests performed on day 29 confirmed that the impairments in contextual memory were due to the morphine-induced decrease in NeuroD1 activity (Figure 6C). This impairment in performance of the MWM test was not observed in mice injected with fentanyl.
Morphine, but not fentanyl, decreases NeuroD1 activity. In addition, when morphine treatment is not long enough, morphine-induced decrease in NeuroD1 activity is able to affect antinociception only, but not tolerance development. It is reasonable to suggest that the overexpression of Neurod1 via nd-vir may prevent the development of morphine, but not fentanyl, tolerance. This hypothesis was tested by injecting different amounts of nd-vir before opioid administration (Figure 6D). Independent of the nd-vir doses injected, the antinociceptive ED50 values of both morphine and fentanyl were not significantly altered on day 11 (Figure 6E, F). However, morphine tolerance exhibited a negative correlation with the virus dose, whereas fentanyl tolerance remained the same in all virus doses tested (Figure 6F, G). This result not only provided a new method to prevent the development of morphine tolerance but also indicated a possible explanation for the higher degree of tolerance observed with morphine compared with an equivalent dose of fentanyl (30).
Discussion
In our studies, decrease in NeuroD1 activity impaired opioid antinociception immediately, whereas development of tolerance was decreased in a time-dependent manner (Figure 3). In addition, 190-vir and nd-vir driven by Nestin promoter affected development of tolerance but not antinociception (Figure 4). We concluded that decrease in NeuroD1 activity not only increases antinociceptive ED50 values immediately by affecting mature neurons but also impairs antinociceptive tolerance development in a time-dependent manner by influencing hippocampal contextual memory. These results are consistent with the critical contributions of NeuroD1 to both the morphologic and the functional maintenance of existing mature neurons and the generation of new neurons in the hippocampus (4,5,7).
Although neither the ascending nor the descending nociceptive pathway includes hippocampus, the involvement of limbic structures in the affective-motivation component of pain sensation has been suggested. Alterations in nociceptive responses have been shown to reflect changes in the hippocampal cholinergic or GABAergic transmission (19). The colocalization of opioid receptors with gamma-aminobutyric acid receptors within the hippocampus structures (31) and the observed opioid inhibition of the GABAergic interneurons resulting in the excitation of the pyramidal cells (32,33) suggest the opioidergic transmission within the hippocampus would also influence the nociceptive response, which is supported by the observed antinociceptive responses on direct injection of morphine into the hippocampus structures (34).
One possible scenario to account for the observed increase in antinociceptive ED50 values after decreasing NeuroD1 activity in hippocampus is the reduced amount of synaptic content of OPRM1 or the postsynaptic spine densities. This hypothesis is supported by the high expression level of OPRM1 in the hippocampus (20), the location of OPRM1 in dendritic spines of primary hippocampal neurons (24), and the contributions of NeuroD1 to dendritic spine stability (5). Whether the decrease in OPRM1 synaptic content would result in increase in the agonist ED50 values is debatable. Although the decrease in NeuroD1 activity with 190-vir reduces dendritic spine stability and leads to a 20% decrease in dendritic spine density (5), even with OPRM1 clustering in the dendritic spines, not all dendritic spines express both OPRM1 and NeuroD1. In our studies, neither OPRM1 mRNA nor the amount of OPRM1 on membrane was affected significantly by 190-vir or nd-sh-vir that decreased NeuroD1 activity (Figure S2). The probable scenario could be that the decrease in NeuroD1 activity leads to the subsequent decrease in spine density within the hippocampus structures, which is the reason for the alteration in cholinergic, GABAergic, histaminergic, or other neurotransmissions that have been shown to influence pain sensation.
On one hand, decrease in NeuroD1 activity increases opioid ED50 values immediately, and the development of morphine tolerance could be partially attributed to the morphine-induced decrease in NeuroD1 activity. On the other hand, decrease in NeuroD1 activity also impairs the development of tolerance in a time-dependent manner as summarized in Figure 3. Because it requires 2–3 weeks for decrease in NeuroD1 activity to affect contextual memory and associative tolerance (Figure 6), influences on contextual memory should not contribute to morphine tolerance during short-term morphine treatment. In a scenario in which morphine is administered for <1 week, morphine-induced decrease in NeuroD1 activity would contribute to morphine tolerance only by impairing morphine antinociception and not by affecting contextual learning.
Because fentanyl does not decrease NeuroD1 activity or increase antinociceptive ED50 values subsequently, its ability to induce tolerance should be lower than that of morphine (30). The correlation between high receptor internalization and low tolerance development was explained at least partially (Figure S3) (35–37). In contrast to morphine, opioids (fentanyl and etorphine) that induce high levels of receptor phosphorylation and receptor internalization use a β-arrestin-dependent pathway to induce extracellular signal-regulated kinase phosphorylation and its nuclear translocation (25,38). Phosphorylated extracellular signal-regulated kinase in the nucleus reduces the expression of miR-190 via Yin Yang1 and counteracts with the inhibition of calcium-calmodulin kinase II α to maintain NeuroD1 activity around basal level (12,39).
By using Nestin promoter to limit the expression of miR-190 and Neurod1 in NSCs, we demonstrated that the increase in antinociception ED50 values can be rescued by nd-vir but not Nes-nd-vir (Figure 4) and should not involve the NeuroD1 control of NSC or progenitor cell differentiation and maturation into mature neurons. In contrast, the decrease in development of antinociception tolerance with the reduction of NeuroD1 activity can be rescued by either nd-vir or Nes-nd-vir (Figure 4) and involves the NeuroD1 actions in NSCs, progenitor cells, or mature neurons. These observations clearly support our hypothesis that decrease in acute opioid agonist antinociceptive potency is a reflection of the immediate effect of decreased NeuroD1 activity. The failure of Nes-nd-vir to rescue the 190-vir effect is due to the restrictive action of Nes-nd-vir within the hippocampal NSCs or progenitor cells. Because both nd-vir and Nes-nd-vir can rescue the 190-vir inhibitory effects on tolerance development, and such rescue is time dependent, we conclude that the increase in NeuroD1 activity must due to the action of the transcription factor in the adult neurogenesis process. The involvement of adult neurogenesis in the formation and extinction of contextual memory (40–42) also points to the critical aspect of associative tolerance in the overall development of opioid antinociception tolerance.
In conclusion, our studies established two distinct mechanisms used by Neurod1 to regulate opioid tolerance: increase in ED50 values and alteration of associative tolerance secondary to changes in contextual memory. Our studies also provided a new hypothesis to explain the prevailing observations that morphine has a higher ability to induce tolerance than fentanyl: their differential regulation of miR-190 level leads to differences in alterations in ED50 values.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China Grant No. 31100773, National Basic Research Program of China Grant No. 2010CB945402, Guangzhou International Science and Technology Cooperation Projects from Bureau of Science and Information Technology of Guangzhou Municipal Government Grant No. 2012J5100007, Guangdong Natural Science Foundation Grant No. S2012010010087, and National Institutes of Health Grant No. DA031442-A1.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2014.05.013.
References
- 1.Lee JK, Cho JH, Hwang WS, Lee YD, Reu DS, Suh-Kim H. Expression of neuroD/BETA2 in mitotic and postmitotic neuronal cells during the development of nervous system. Dev Dyn. 2000;217:361–367. doi: 10.1002/(SICI)1097-0177(200004)217:4<361::AID-DVDY3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Chae JH, Stein GH, Lee JE. NeuroD: The predicted and the surprising. Mol Cells. 2004;18:271–288. [PubMed] [Google Scholar]
- 3.Cho JH, Tsai MJ. The role of BETA2/NeuroD1 in the development of the nervous system. Mol Neurobiol. 2004;30:35–47. doi: 10.1385/MN:30:1:035. [DOI] [PubMed] [Google Scholar]
- 4.Gaudilliere B, Konishi Y, de la Iglesia N, Yao G, Bonni A. A CaMKII-NeuroD signaling pathway specifies dendritic morphogenesis. Neuron. 2004;41:229–241. doi: 10.1016/s0896-6273(03)00841-9. [DOI] [PubMed] [Google Scholar]
- 5.Zheng H, Zeng Y, Chu J, Kam AY, Loh HH, Law PY. Modulations of NeuroD activity contribute to the differential effects of morphine and fentanyl on dendritic spine stability. J Neurosci. 2010;30:8102–8110. doi: 10.1523/JNEUROSCI.6069-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Bohlen Und Halbach O. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 2007;329:409–420. doi: 10.1007/s00441-007-0432-4. [DOI] [PubMed] [Google Scholar]
- 7.Zheng H, Zhang Y, Li W, Loh HH, Law PY. NeuroD modulates opioid agonist-selective regulation of adult neurogenesis and contextual memory extinction. Neuropsychopharmacology. 2013;38:770–777. doi: 10.1038/npp.2012.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 10.Siegel S. Morphine tolerance acquisition as an associative process. J Exp Psychol Anim Behav Process. 1977;3:1–13. doi: 10.1037//0097-7403.3.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Siegel S, Hinson RE, Krank MD. The role of predrug signals in morphine analgesic tolerance: Support for a Pavlovian conditioning model of tolerance. J Exp Psychol Anim Behav Process. 1978;4:188–196. doi: 10.1037//0097-7403.4.2.188. [DOI] [PubMed] [Google Scholar]
- 12.Zheng H, Zeng Y, Zhang X, Chu J, Loh HH, Law PY. Mu-opioid receptor agonists differentially regulate the expression of miR-190 and NeuroD. Mol Pharmacol. 2010;77:102–109. doi: 10.1124/mol.109.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisch AJ, Harburg GC. Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology. Hippocampus. 2006;16:271–286. doi: 10.1002/hipo.20161. [DOI] [PubMed] [Google Scholar]
- 15.Khanna S, Sinclair JG. Noxious stimuli produce prolonged changes in the CA1 region of the rat hippocampus. Pain. 1989;39:337–343. doi: 10.1016/0304-3959(89)90047-X. [DOI] [PubMed] [Google Scholar]
- 16.Pearse D, Mirza A, Leah J. Jun, Fos and Krox in the hippocampus after noxious stimulation: Simultaneous-input-dependent expression and nuclear speckling. Brain Res. 2001;894:193–208. doi: 10.1016/s0006-8993(01)01993-x. [DOI] [PubMed] [Google Scholar]
- 17.Hashemi M, Karami M, Zarrindast MR, Sahebgharani M. Role of nitric oxide in the rat hippocampal CA1 in morphine antinociception. Brain Res. 2010;1313:79–88. doi: 10.1016/j.brainres.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Khalilzadeh E, Tamaddonfard E, Farshid AA, Erfanparast A. Microinjection of histamine into the dentate gyrus produces antinociception in the formalin test in rats. Pharmacol Biochem Behav. 2010;97:325–332. doi: 10.1016/j.pbb.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Favaroni Mendes LA, Menescal-de-Oliveira L. Role of cholinergic, opioidergic and GABAergic neurotransmission of the dorsal hippocampus in the modulation of nociception in guinea pigs. Life Sci. 2008;83:644–650. doi: 10.1016/j.lfs.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, et al. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon WJ. The up-and-down method for small samples. J Am Stat Assoc. 1965;60:967–978. [Google Scholar]
- 22.Dixon WJ. Staircase bioassay: The up-and-down method. Neurosci Biobehav Rev. 1991;15:47–50. doi: 10.1016/s0149-7634(05)80090-9. [DOI] [PubMed] [Google Scholar]
- 23.Vorhees CV, Williams MT. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao D, Lin H, Law PY, Loh HH. Mu-opioid receptors modulate the stability of dendritic spines. Proc Natl Acad Sci U S A. 2005;102:1725–1730. doi: 10.1073/pnas.0406797102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng H, Loh HH, Law PY. Beta-arrestin-dependent mu-opioid receptor-activated extracellular signal-regulated kinases (ERKs) translocate to nucleus in contrast to G protein-dependent ERK activation. Mol Pharmacol. 2008;73:178–190. doi: 10.1124/mol.107.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng H, Pearsall EA, Hurst DP, Zhang Y, Chu J, Zhou Y, et al. Palmitoylation and membrane cholesterol stabilize mu-opioid receptor homodimerization and G protein coupling. BMC Cell Biol. 2012;13:6. doi: 10.1186/1471-2121-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo S, Lim JW, Yellajoshyula D, Chang LW, Kroll KL. Neurogenin and NeuroD direct transcriptional targets and their regulatory enhancers. EMBO J. 2007;26:5093–5108. doi: 10.1038/sj.emboj.7601923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, et al. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Duttaroy A, Yoburn BC. The effect of intrinsic efficacy on opioid tolerance. Anesthesiology. 1995;82:1226–1236. doi: 10.1097/00000542-199505000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Kalyuzhny AE, Wessendorf MW. Relationship of mu- and delta-opioid receptors to GABAergic neurons in the central nervous system, including antinociceptive brainstem circuits. J Comp Neurol. 1998;392:528–547. [PubMed] [Google Scholar]
- 32.Svoboda KR, Lupica CR. Opioid inhibition of hippocampal interneurons via modulation of potassium and hyperpolarization-activated cation (Ih) currents. J Neurosci. 1998;18:7084–7098. doi: 10.1523/JNEUROSCI.18-18-07084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swearengen E, Chavkin C. Comparison of opioid and GABA receptor control of excitability and membrane conductance in hippocampal CA1 pyramidal cells in rat. Neuropharmacology. 1989;28:689–697. doi: 10.1016/0028-3908(89)90152-4. [DOI] [PubMed] [Google Scholar]
- 34.Tamaddonfard E, Erfanparast A, Farshid AA, Khalilzadeh E. Interaction between histamine and morphine at the level of the hippocampus in the formalin-induced orofacial pain in rats. Pharmacol Rep. 2011;63:423–432. doi: 10.1016/s1734-1140(11)70508-4. [DOI] [PubMed] [Google Scholar]
- 35.Koch T, Widera A, Bartzsch K, Schulz S, Brandenburg LO, Wundrack N, et al. Receptor endocytosis counteracts the development of opioid tolerance. Mol Pharmacol. 2005;67:280–287. doi: 10.1124/mol.104.004994. [DOI] [PubMed] [Google Scholar]
- 36.Narita M, Suzuki M, Narita M, Niikura K, Nakamura A, Miyatake M, et al. Mu-opioid receptor internalization-dependent and -independent mechanisms of the development of tolerance to mu-opioid receptor agonists: Comparison between etorphine and morphine. Neuroscience. 2006;138:609–619. doi: 10.1016/j.neuroscience.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 37.Zuo Z. The role of opioid receptor internalization and beta-arrestins in the development of opioid tolerance. Anesth Analg. 2005;101:728–734. doi: 10.1213/01.ANE.0000160588.32007.AD. [DOI] [PubMed] [Google Scholar]
- 38.Zheng H, Chu J, Zhang Y, Loh HH, Law PY. Modulating micro-opioid receptor phosphorylation switches agonist-dependent signaling as reflected in PKCepsilon activation and dendritic spine stability. J Biol Chem. 2011;286:12724–12733. doi: 10.1074/jbc.M110.177089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng H, Chu J, Zeng Y, Loh HH, Law PY. Yin Yang 1 phosphorylation contributes to the differential effects of mu-opioid receptor agonists on microRNA-190 expression. J Biol Chem. 2010;285:21994–22002. doi: 10.1074/jbc.M110.112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aimone JB, Deng W, Gage FH. Resolving new memories: A critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernandez-Rabaza V, Llorens-Martin M, Velazquez-Sanchez C, Ferragud A, Arcusa A, Gumus HG, et al. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience. 2009;159:59–68. doi: 10.1016/j.neuroscience.2008.11.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


