Abstract
Background
Gallbladder cancer (GBC) is a rare malignancy, yet certain groups are at higher risk. Knowledge of predisposing factors may facilitate earlier diagnosis by enabling targeted investigations into otherwise non-specific presenting signs and symptoms. Detecting GBC in its initial stages offers patients their best chance of cure.
Methods
PubMed was searched for recent articles (2008–2012) on the topic of risk factors for GBC. Of 1490 initial entries, 32 manuscripts reporting on risk factors for GBC were included in this review.
Results
New molecular perspectives on cholesterol cycling, hormonal factors and bacterial infection provide fresh insights into the established risk factors of gallstones, female gender and geographic locality. The significance of polyps in predisposing to GBC is probably overstated given the known dysplasia–carcinoma and adenoma–carcinoma sequences active in this disease. Bacteria such as Salmonella species may contribute to regional variations in disease prevalence and might represent powerful targets of therapy to reduce incidences in high-risk areas. Traditional risk factors such as porcelain gallbladder, Mirizzi's syndrome and bile reflux remain important as predisposing factors.
Conclusions
Subcentimetre gallbladder polyps rarely become cancerous. Because gallbladder wall thickening is often the first sign of malignancy, all gallbladder imaging should be scrutinized carefully for this feature.
Introduction
Gallbladder cancer (GBC) is a rare malignancy, although certain groups are more likely to develop this lethal disease.1–6 Knowledge of groups at high risk should facilitate earlier diagnosis by allowing targeted investigation into the otherwise non-specific symptoms and signs with which the disease most commonly presents. Detecting GBC in its initial stages offers patients their best chance for cure as spread to lymph nodes and dissemination occur quite early in the course of the disease (the frequency of nodal involvement is 12–20% in stage Ib tumours).7–9
The highest incidence of GBC is seen in females with gallstones in geographic locations such as northern India and Chile.4 Increasing age is a well-established risk factor across regions.4 Dietary and environmental factors also clearly play various roles (although directly causal agents are yet to be characterized) and obesity increases the risk by 15–66%.4 Controversy regarding the management of gallbladder polyps continues.5 In addition to these well-established risk factors, recent data referring to the significance of concomitant bacterial infection and hormonal factors provide new insights into causality.
Materials and methods
A literature search was performed in PubMed. A query using the search term ‘gallbladder cancer’ in literature published between January 2008 and July 2012 produced an initial result of 1490 entries. Only manuscripts describing carcinogenic factors and published in English were considered. Non-human and non-clinical studies were excluded, as were duplicate series and stand-alone abstracts. Only papers specifically addressing cases of GBC were included; papers identified with the initial search strategy that focused on only advanced biliary cancer or cholangiocarcinoma were excluded. The reference lists of each manuscript were also searched to identify additional articles that might have been missed with the initial search strategy.
Figure 1 illustrates the screening and inclusion procedures conducted according to the PRISMA (preferred reporting items for systematic reviews and meta-analyses) criteria. A total of 1302 articles were excluded based on their titles alone. The remaining 188 manuscripts were reviewed by two authors (CHCP and RTG) for relevance to the study. In addition to the 32 studies included from the literature search, nine references were included to add background information.1,7–14
Figure 1.
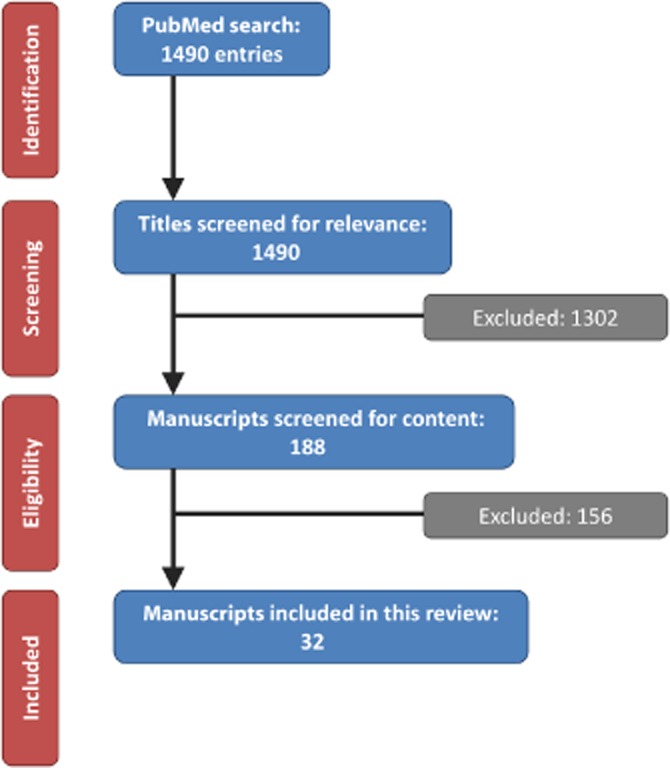
Flow chart showing the process by which manuscripts were selected for this review
Results
Gallstones
The association between the presence of gallstones and GBC is supported by Level II evidence (multiple-cohort studies). Stone characteristics may also play a role and larger, heavier stones are implicated in the occurrence of disease,15 as is the length of time for which stones are present.6 The presence of bacteria in the bile and gallstones in the gallbladder, and the synergistic consequence of GBC is a relatively new concept of considerable interest.14
Some authors have recently questioned whether the presence of gallstones is causative or merely correlative with regard to GBC.6 Certainly, the excretion by the liver of cholesterol into the bile (with the subsequent formation of gallstones) can parallel the hepatic excretion of other toxic compounds which may be carcinogenic.6 Patients genetically predisposed to gallstones as a result of an accelerated cholesterol metabolism may therefore also be at risk for excessive exposure of other toxic compounds to the gallbladder epithelium. Two candidates are the orphan nuclear receptor (ONR) family and the adenosine triphosphate (ATP)-binding cassette (ABC) transporter family.16 An interesting hypothesis introduced by Venniyoor16 connects the lower incidence of hepatocellular carcinoma and higher incidence of GBC seen in areas of northern India and South America, with epithelial exposure to aflatoxin. Overactivity of the ONR and ABC efflux pumps may reduce the exposure time of hepatocytes to aflatoxin, but increase the exposure of the gallbladder epithelium to the same agent, thus resulting in the observed shift in the incidences of these two anatomically related, but quite distinct, malignancies. If the function of these or similar molecular mechanisms actually increases risk for GBC, gallstone presence might simply reflect the overactivity of these pumps and, rather than the gallstones themselves being directly carcinogenic, the subsequently increased exposure to some other carcinogenic compound might represent the actual causative factor.16 Differences in environmental toxins would then explain geographic variations in the incidence of GBC, despite similar rates of cholelithiasis.16 This concept is still in its early development, but appears worthy of further investigation.
Polyps
The underlying issues driving the management of patients with gallbladder polyps concern the missing of a malignant diagnosis and the interval development of GBC.5 Level III evidence (heterogeneous case series) is available for this. Precursor lesions of tumours of the gallbladder and extrahepatic biliary ducts are, however, relatively uncommon and are certainly less studied than other gastrointestinal tract malignant precursors.
Although both an adenoma–carcinoma sequence and a dysplasia–carcinoma sequence are thought to operate in the context of GBC, the association between mass-forming pre-invasive neoplasia (adenoma) with adenocarcinoma is weaker for multiple reasons.1 Firstly, there is frequently no evidence of adenomatous tissue in GBC specimens. Secondly, the apparently indolent course of many incidentally detected adenomatous lesions, which never progress to carcinoma, argues against a clear malignant pathway of development.1 There is also no clear genetic sequence, as is described, for example, in colonic neoplasia. It is likely that the majority of occurrences of GBC arise from dysplastic flat lesions, not adenomatous polyps, as the majority of GBCs are morphologically flat and infiltrative, rather than polypoid in character.1
Interestingly, Asian series often report much higher rates of neoplastic polyps, with rates of GBC occurring in up to 15% of patients,17 by stark contrast with Western series, in which such reports are much rarer. For example, in a series of over 400 patients with polyps reported from the Memorial Sloan–Kettering Cancer Center, no cases of GBC and only a 2% rate of adenomata were found.5 This may simply represent differing incidences of the disease across continents.4,18 However, differences in definitions of what is included in any series of gallbladder lesions (particularly in terms of what is defined as a polyp and what is defined as a mass lesion) can also dramatically skew results and may explain the wide disparities in the rates observed. For example, the inclusion of sessile lesions of the gallbladder wall of >30 mm in size and pedunculated lesions of <10 mm will clearly result in a rate of malignancy that differs from that observed in a series that excludes the former lesion type.19 Large sessile masses are highly likely to represent GBC.20 The point at which a lesion should no longer be considered as a ‘polyp’ has yet to be clearly defined. A sessile mass replacing the gallbladder is unlikely to be mistaken for a benign polyp and its inclusion in studies that attempt to differentiate benign from malignant lesions will introduce a bias resulting in a higher rate of malignancy than is truly attributable to small pedunculated polyps. Studies that report malignancy rates in relation to polyps should define the size-based criteria used to establish the point at which a lesion ceases to be considered as a polyp and instead is regarded as a mass lesion or GBC. Studies assessing purely sessile lesions are lacking.
Predicting malignancy in polyps is difficult as the vast majority will be benign. The single factor that remains significant in all series is the increase in the rate of malignancy with increasing size of lesion.3,19,20 The corollary is naturally that small lesions are more likely to be benign. Even cohorts with particularly high percentages of malignancy in small polyps (Kwon et al. reported a rate of GBC of almost 10% for lesions of 10–20 mm in size20) describe a malignancy rate of only 1.3% for polyps of <10 mm. The growth rate of polyps has not been found to be helpful in differentiating neoplastic from non-neoplastic polyps21 and although other authors have suggested that newer imaging modalities can be used to detect subtle characteristics of polyps,22 there is no easily applied, unambiguous, definitive diagnostic characteristic that is sensitive and specific for neoplastic gallbladder polyps.
Spending inordinate amounts of time scrutinizing and agonizing over polyps, especially those of <10 mm in size, in order to pick out the 1% of malignant lesions is of questionable value, particularly in Western centres, in which the rate of malignancy is even lower.5 For many surgeons, the level of suspicion is far lower when preoperative gallbladder wall thickening is detected and, although this is a significantly more common radiological finding in GBC, it may be overlooked, with the result that patients are scheduled for laparoscopic cholecystectomy without further consideration. In general, incidental polyps of <10 mm are highly unlikely to harbour malignancy and can safely be managed expectantly.3
A more thoughtful approach would be to consider the pre-test probability of a particular polyp being malignant, and to tailor investigation and management accordingly. As Aldouri et al. have elegantly reported,2 the prevalence of malignancy in patients with gallbladder polyps was found to be significantly higher among patients in a high-risk group (subjects with an Indian ethnic background) compared with White subjects living in the UK (5.5% versus 0.08%). Risk stratification based on these types of consideration is much more likely to enable the appropriate selection of patients who require further investigation and intervention versus those who can be safely reassured.
Another subgroup of patients with polyps who are at higher risk for the development of GBC includes those with primary sclerosing cholangitis (PSC). This disease is known to increase the risk for GBC1 and, as Eaton et al.23 have suggested, it may be reasonable to lower the threshold for polyp size to 8 mm in patients with PSC. In that series of 57 patients with PSC the authors identified a high rate of post-laparoscopic cholecystectomy morbidity (33%) and no malignancy in polyps of <8 mm.23 They concluded that observation might be appropriate in patients with polyps of <8 mm in the absence of other concerning imaging or clinical features.23
Although there is no known association between GBC and acromegaly, an increased rate of polyps was recently reported in patients with this pituitary tumour (29% versus 4.6% in control subjects).24 This patient group is known to be at increased risk for colonic polyps and adenocarcinoma, but this is the first report to link gallbladder polyps with acromegaly. The significance of this finding remains unclear because all of the measured polyps were small (<6 mm). Further studies assessing risk for GBC in patients with acromegaly may well be justified, although it is extremely unlikely that these polyps are of any clinical significance based on size alone.
The role of bacteria
Knowledge of the carcinogenic influence of bacteria in GBC is limited to Level V evidence. In their excellent overview of the role of bacteria in carcinogenesis, Nath et al.25 outline two main pathways by which bacteria may play a role in the development of malignancy. Firstly, chronic inflammation caused by persistent bacterial infection may lead to carcinogenesis; alternatively, bacterial toxins and other metabolites may themselves be sufficiently carcinogenic to result in malignancy.
The biliary tract has been described as the ‘consummate example of inflammation-associated carcinoma’.1 However, the role in the process played by bacteria has been less thoroughly investigated. Both of the mechanisms described by Nath et al.25 may be significant for the causation of GBC. Stasis associated with cholelithiasis promotes bacterial infection. The combined effects of chronic mechanical irritation caused by stones and chronic inflammatory change associated with colonization (or subclinical secondary infection) may certainly be sufficiently carcinogenic to result in GBC.14 This might also explain why rates of GBC do not exactly correlate with rates of cholelithiasis; that is, an additional carcinogenic factor acting along with the stones may be necessary.16
There are also a number of potential routes by which various toxins and metabolites associated with bacterial infection may be implicated in the development of malignancy. Firstly, bacterial infection is known to promote the development of carcinogenic secondary bile acids via the action of the enzyme glucuronidase, which is known to be produced by bacteria such as Salmonella species.14 Secondly, glucuronidase can also deconjugate the glucuronide moiety from other xenobiotic and endogenous toxins metabolized by the liver and, in doing so, reactivate them.16 The natural concentrating function of the gallbladder might then amplify the carcinogenic effect exerted by these mediators.25
Salmonella typhi is a prime candidate for bacterial predisposition to GBC.4,25 In addition to the theoretical implication outlined above, empiric evidence is derived from the finding that Salmonella was the most common isolate in bile from a group of patients with GBC [in 16 of 40 patients (40%)] and was seen five times more commonly than in a comparison group of patients with simple cholelithiasis (8%).14 Culture-positive bile was more common in patients with GBC (65% of patients) than in either patients with simple cholelithiasis (42%) or control subjects without cholelithiasis (12%) in a high-risk area in northern India.14 Global variations in the epidemiology of GBC also correlate strongly with rates of cholelithiasis and Salmonella infection.26 Certain Helicobacter species have also attracted attention (Helicobacter bilius and Helicobacter hepaticus),27 although the supporting evidence is significantly weaker.4
Hormones
Female subjects are at increased risk for cholelithiasis and also have an increased risk for GBC [odds ratio (OR): 2–8].4 Whether this reflects only the incidence of gallstones (or whether there is a separate hormonal influence) remains to be proven, but multiple case–control and cohort studies have assessed female hormones and GBC and indicate that, overall, female hormones are likely to increase risk (based on Level III evidence).4 A recent case–control study of 78 women with and without GBC performed in India demonstrated a significantly increased risk for carcinogenesis in women with a younger age of menarche, greater number of pregnancies and older age at menopause28 and thus a longer duration of exposure to female sex hormones. Hormone replacement therapy (HRT) and use of oral contraceptives have both been linked to risk for GBC.29 An Italian case–control study of 31 GBC patients and 3702 control subjects suggested that women previously treated with HRT had an increased risk for GBC [OR = 3.2, 95% confidence interval (CI) 1.1–9.3], which appeared to increase with longer durations of therapy.30 During a prospective, population-based study of over 60 000 Chinese women, 54 developed GBC and oral contraceptive use was shown to increase risk (OR = 2.38, 95% CI 1.26–4.49).31
Park et al.32 identified that variants of the CYP1A1 gene, which plays a role in steroid hormone synthesis, were associated with both GBC and cholangiocarcinoma. The oestrogen and progesterone receptor status of resected GBC tissue has been examined on multiple occasions, frequently with conflicting results, as recently summarized by Barreto et al.33 These inconsistencies in receptor expression are more likely to represent diverse tumour biologies than the true absence of association and deserve further investigation.
There is also evidence that female gender and prolonged oestrogen exposure can result in the increased export of cholesterol and xenobiotics into bile.16 The toxic effect resulting from this might well underpin any potential link that may exist between gender and cancer. It is known that cholesterol per se can cause dysplasia of the gallbladder epithelium so that, independently of the formation of stones and hormones, increased cholesterol cycling in females may predispose to malignancy.16 Geographic variations in the incidence of GBC are more pronounced in women,4 and this too may be explained by a physiologic concentrating effect of enhanced hepatic excretion present in women that would magnify the potential carcinogenicity of any environmental toxins.
Historical and new risk factors
The radiographic finding of porcelain gallbladder has traditionally been considered a risk factor for GBC. A recent systematic review and case series including work published as far back as 1923 identified 140 cases of porcelain gallbladder in 60 665 cholecystectomies reported in seven studies (incidence: 0.2%).34 The rate of GBC among subjects with porcelain gallbladder was 15%, which, although the authors concluded it to be insufficiently high to warrant prophylactic cholecystectomy, is nonetheless nearly 100 times higher than the 0.11% rate of GBC reported in association with simple cholelithiasis.2 By contrast, none of the 13 patients with porcelain gallbladder in the case series included in this systematic review34 and, similarly, none of 12 patients with porcelain gallbladder reported in another contemporary series from South Korea35 had GBC. However, these two studies respectively included 35 and 117 patients with GBC, none of whom displayed any feature of porcelain gallbladder.34,35 With careful preoperative cross-sectional imaging using computed tomography or magnetic resonance imaging, in addition to ultrasound, and with the subsequent exclusion of T-stage II or III GBC, the laparoscopic route also need not be contraindicated in porcelain gallbladder, although it is potentially more difficult than cholecystectomy for non-porcelain gallbladder.
Mirizzi's syndrome represents another historical correlate of GBC. A recent case report described a patient in whom the malignancy was considered to be directly responsible for the syndrome by causing a previously mobile gallstone to become progressively impacted into the gallbladder neck and to compress the common hepatic duct as the cancer grew.36 The disproportionately high rate of Mirizzi's syndrome in other series of GBC patients (five incidences of GBC in 18 patients with Mirizzi's syndrome13) may reflect misdiagnosis wherein a GBC invading the porta hepatis is mistaken for gallstone-related pathology, but this case report36 clearly described the genuine coexistence of the two conditions.
Chronic reflux of enteric contents or pancreatic juice is thought to underlie the higher rate of cholangiocarcinoma following bilioenteric anastomoses, and the higher rates of both cholangiocarcinoma and GBC in association with an anomalous pancreaticobiliary duct junction (APBDJ) and/or choledochal cyst.11,37 In a recent case report, GBC was described as a longterm complication of cholecystoenteric anastomosis, probably caused by the same mechanism by which bilioenteric anastomosis predisposes to cholangiocarcinoma.37 Cholecystoenteric anastomosis is rare and is most commonly used in the setting of advanced malignancy for palliation. This bypass should not be performed in benign disease. Similarly, in a small study of children followed after correction of an APBDJ, all patients were found to have chronic inflammatory changes in the gallbladder, which may underlie a predisposition to subsequent GBC in such patients.38 In addition, in a Japanese study, seven of 26 patients with an APBDJ developed GBC, and seven of 48 patients with GBC were found to demonstrate this anatomical variant.39
Although adenomyomatosis is not thought to predispose to GBC, the presence of this pathological feature was found to be associated with more advanced T (tumour) and N (node) stages of malignancy in an analysis of 97 patients with GBC (in 25 of whom GBC was associated with adenomyomatosis).40 Adenomyomatosis may be identified using ultrasound, in which the gallbladder wall is shown to be thickened (segmental or diffuse) and may contain anechoic or echogenic foci, known as the ring-down reverberation artefact.12 Whether the progression of GBC leads to the development of adenomyomatosis in these cases or whether there is some association between Rokitansky–Aschoff sinus development and malignancy remains unclear. Until further data are available, the implication for clinical practice may be only that the preoperative detection of adenomyomatosis should perhaps stimulate further imaging to exclude GBC. Unfortunately, the differentiation of adenomyomatosis from GBC on imaging is extremely difficult.41
Conclusions
New insights into factors that predispose patients to GBC should help identify people at high risk for this disease and support appropriate investigations and treatment in these subjects at an earlier point in the natural history of the disease. Less concern about small polyps seems appropriate because the vast majority of these lesions do not become GBC. Gallbladder cancer more commonly arises from dysplastic, rather than adenomatous, lesions. Knowledge of the intriguing role that bacteria may play in the pathogenesis of this disease may prove to be a powerful weapon should more evidence accumulate implicating chronic infection by bacteria such as those of Salmonella species. Further understanding of the molecular events involved in cholesterol metabolism may also uncover more of the mechanical bases of the development of GBC. Finally, the traditional risk factors of porcelain gallbladder and Mirizzi's syndrome, as well as the significance of bile reflux following bilioenteric bypass or in association with an APBDJ, should also be kept in mind as these patients seem to have a much greater risk for GBC than the background population.
Conflicts of interest
None declared.
References
- 1.Adsay NV. Neoplastic precursors of the gallbladder and extrahepatic biliary system. Gastroenterol Clin North Am. 2007;36:889–900. doi: 10.1016/j.gtc.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Aldouri AQ, Malik HZ, Waytt J, Khan S, Ranganathan K, Kummaraganti S, et al. The risk of gallbladder cancer from polyps in a large multiethnic series. Eur J Surg Oncol. 2008;35:48–51. doi: 10.1016/j.ejso.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 3.Corwin MT, Siewert B, Sheiman RG, Kane RA. Incidentally detected gallbladder polyps: is follow-up necessary? Longterm clinical and US analysis of 346 patients. Radiology. 2011;258:277–282. doi: 10.1148/radiol.10100273. [DOI] [PubMed] [Google Scholar]
- 4.Eslick GD. Epidemiology of gallbladder cancer. Gastroenterol Clin North Am. 2010;39:307–330. doi: 10.1016/j.gtc.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Ito H, Hann LE, D'Angelica M, Allen P, Fong Y, Dematteo RP, et al. Polypoid lesions of the gallbladder: diagnosis and follow-up. J Am Coll Surg. 2009;208:570–575. doi: 10.1016/j.jamcollsurg.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Shrikhande SV, Barreto SG, Singh S, Udwadia TE, Agarwal AK. Cholelithiasis in gallbladder cancer: coincidence, cofactor, or cause! Eur J Surg Oncol. 2010;36:514–519. doi: 10.1016/j.ejso.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Lin HT, Liu GJ, Wu D, Lou JY. Metastasis of primary gallbladder carcinoma in lymph node and liver. World J Gastroenterol. 2005;11:748–751. doi: 10.3748/wjg.v11.i5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pawlik TM, Gleisner AL, Vigano L, Kooby DA, Bauer TW, Frilling A, et al. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg. 2007;11:1478–1486. doi: 10.1007/s11605-007-0309-6. ; discussion 1486–1487. [DOI] [PubMed] [Google Scholar]
- 9.Sikora SS, Singh RK. Surgical strategies in patients with gallbladder cancer: nihilism to optimism. J Surg Oncol. 2006;93:670–681. doi: 10.1002/jso.20535. [DOI] [PubMed] [Google Scholar]
- 10.Strong RW. Late bile duct cancer complicating biliary–enteric anastomosis for benign disease. Am J Surg. 1999;177:472–474. doi: 10.1016/s0002-9610(99)00087-2. [DOI] [PubMed] [Google Scholar]
- 11.Fieber SS, Nance FC. Choledochal cyst and neoplasm: a comprehensive review of 106 cases and presentation of two original cases. Am Surg. 1997;63:982–987. [PubMed] [Google Scholar]
- 12.Levy AD, Murakata LA, Rohrmann CA., Jr Gallbladder carcinoma: radiologic–pathologic correlation. Radiographics. 2001;21:295–314. doi: 10.1148/radiographics.21.2.g01mr16295. ; questionnaire 549–555. [DOI] [PubMed] [Google Scholar]
- 13.Redaelli CA, Buchler MW, Schilling MK, Krahenbuhl L, Ruchti C, Blumgart LH, et al. High coincidence of Mirizzi syndrome and gallbladder carcinoma. Surgery. 1997;121:58–63. doi: 10.1016/s0039-6060(97)90183-5. [DOI] [PubMed] [Google Scholar]
- 14.Sharma V, Chauhan VS, Nath G, Kumar A, Shukla VK. Role of bile bacteria in gallbladder carcinoma. Hepatogastroenterology. 2007;54:1622–1625. [PubMed] [Google Scholar]
- 15.Alvi AR, Siddiqui NA, Zafar H. Risk factors of gallbladder cancer in Karachi – a case–control study. World J Surg Oncol. 2011;9:164–168. doi: 10.1186/1477-7819-9-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venniyoor A. Cholesterol gallstones and cancer of gallbladder (CAGB): molecular links. Med Hypotheses. 2008;70:646–653. doi: 10.1016/j.mehy.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 17.Cha BH, Hwang JH, Lee SH, Kim JE, Cho JY, Kim H, et al. Preoperative factors that can predict neoplastic polypoid lesions of the gallbladder. World J Gastroenterol. 2011;17:2216–2222. doi: 10.3748/wjg.v17.i17.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butte JM, Matsuo K, Gonen M, D'Angelica MI, Waugh E, Allen PJ, et al. Gallbladder cancer: differences in presentation, surgical treatment, and survival in patients treated at centres in three countries. J Am Coll Surg. 2011;212:50–61. doi: 10.1016/j.jamcollsurg.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Park KW, Kim SH, Choi SH, Lee WJ. Differentiation of non-neoplastic and neoplastic gallbladder polyps 1 cm or bigger with multi-detector row computed tomography. J Comput Assist Tomogr. 2010;34:135–139. doi: 10.1097/RCT.0b013e3181b382d7. [DOI] [PubMed] [Google Scholar]
- 20.Kwon W, Jang JY, Lee SE, Hwang DW, Kim SW. Clinicopathologic features of polypoid lesions of the gallbladder and risk factors of gallbladder cancer. J Korean Med Sci. 2009;24:481–487. doi: 10.3346/jkms.2009.24.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin SR, Lee JK, Lee KH, Lee KT, Rhee JC, Jang KT, et al. Can the growth rate of a gallbladder polyp predict a neoplastic polyp? J Clin Gastroenterol. 2009;43:865–868. doi: 10.1097/MCG.0b013e31819359aa. [DOI] [PubMed] [Google Scholar]
- 22.Dutta U, Poornachandra KS. Gallbladder polyps: how to pick up the sinister ones. J Gastroenterol Hepatol. 2009;24:175–178. doi: 10.1111/j.1440-1746.2008.05769.x. [DOI] [PubMed] [Google Scholar]
- 23.Eaton JE, Thackeray EW, Lindor KD. Likelihood of malignancy in gallbladder polyps and outcomes following cholecystectomy in primary sclerosing cholangitis. Am J Gastroenterol. 2012;107:431–439. doi: 10.1038/ajg.2011.361. [DOI] [PubMed] [Google Scholar]
- 24.Annamalai AK, Gayton EL, Webb A, Halsall DJ, Rice C, Ibram F, et al. Increased prevalence of gallbladder polyps in acromegaly. J Clin Endocrinol Metab. 2011;96:1120–1125. doi: 10.1210/jc.2010-2669. [DOI] [PubMed] [Google Scholar]
- 25.Nath G, Gulati AK, Shukla VK. Role of bacteria in carcinogenesis, with special reference to carcinoma of the gallbladder. World J Gastroenterol. 2010;16:5395–5404. doi: 10.3748/wjg.v16.i43.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sacks A, Peller PJ, Surasi DS, Chatburn L, Mercier G, Subramaniam RM. Value of PET/CT in the management of primary hepatobiliary tumours, part 2. AJR Am J Roentgenol. 2011;197:260–265. doi: 10.2214/AJR.11.6995. [DOI] [PubMed] [Google Scholar]
- 27.Mishra RR, Tewari M, Shukla HS. Helicobacter species and pathogenesis of gallbladder cancer. Hepatobiliary Pancreat Dis Int. 2010;9:129–134. [PubMed] [Google Scholar]
- 28.Shukla VK, Chauhan VS, Mishra RN, Basu S. Lifestyle, reproductive factors and risk of gallbladder cancer. Singapore Med J. 2008;49:912–915. [PubMed] [Google Scholar]
- 29.Reshetnyak VI. Concept of the pathogenesis and treatment of cholelithiasis. World J Hepatol. 2012;4:18–34. doi: 10.4254/wjh.v4.i2.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marsden J, Sturdee D. Cancer issues. Best Pract Res Clin Obstet Gynaecol. 2009;23:87–107. doi: 10.1016/j.bpobgyn.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Dorjgochoo T, Shu XO, Li HL, Qian HZ, Yang G, Cai H, et al. Use of oral contraceptives, intrauterine devices and tubal sterilization and cancer risk in a large prospective study, from 1996 to 2006. Int J Cancer. 2009;124:2442–2449. doi: 10.1002/ijc.24232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park SK, Andreotti G, Sakoda LC, Gao YT, Rashid A, Chen J, et al. Variants in hormone-related genes and the risk of biliary tract cancers and stones: a population-based study in China. Carcinogenesis. 2009;30:606–614. doi: 10.1093/carcin/bgp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barreto SG, Haga H, Shukla PJ. Hormones and gallbladder cancer in women. Indian J Gastroenterol. 2009;28:126–130. doi: 10.1007/s12664-009-0046-8. [DOI] [PubMed] [Google Scholar]
- 34.Khan ZS, Livingston EH, Huerta S. Reassessing the need for prophylactic surgery in patients with porcelain gallbladder: case series and systematic review of the literature. Arch Surg. 2011;146:1143–1147. doi: 10.1001/archsurg.2011.257. [DOI] [PubMed] [Google Scholar]
- 35.Kim JH, Kim WH, Yoo BM, Kim MW. Should we perform surgical management in all patients with suspected porcelain gallbladder? Hepatogastroenterology. 2009;56:943–945. [PubMed] [Google Scholar]
- 36.Wijesuriya SR, Delriviere L, Mitchell A. Gall bladder cancer and Mirizzi syndrome: alternative explanation to the common belief. ANZ J Surg. 2010;80:116–117. doi: 10.1111/j.1445-2197.2009.05192.x. [DOI] [PubMed] [Google Scholar]
- 37.Pilgrim CH, Satgunaseelan L, Ward SM, Evans PM. Gallbladder carcinoma as a longterm complication of cholecystojejunostomy. J Gastrointest Surg. 2009;13:2330–2332. doi: 10.1007/s11605-009-0879-6. [DOI] [PubMed] [Google Scholar]
- 38.Ali AE, Blythe AI, Ford WD. Chronic inflammatory changes seen in gallbladders of patients with pancreatico-biliary malunion years after transduodenal sphincterotomy: is it a precursor for gallbladder carcinoma? Pediatr Surg Int. 2008;24:1005–1008. doi: 10.1007/s00383-008-2197-6. [DOI] [PubMed] [Google Scholar]
- 39.Inui K, Yoshino J, Miyoshi H. Diagnosis of gallbladder tumours. Intern Med. 2011;50:1133–1136. doi: 10.2169/internalmedicine.50.5255. [DOI] [PubMed] [Google Scholar]
- 40.Kai K, Ide T, Masuda M, Kitahara K, Miyoshi A, Miyazaki K, et al. Clinicopathologic features of advanced gallbladder cancer associated with adenomyomatosis. Virchows Arch. 2011;459:573–580. doi: 10.1007/s00428-011-1155-1. [DOI] [PubMed] [Google Scholar]
- 41.Sugita R, Yamazaki T, Furuta A, Itoh K, Fujita N, Takahashi S. High b-value diffusion-weighted MRI for detecting gallbladder carcinoma: preliminary study and results. Eur Radiol. 2009;19:1794–1798. doi: 10.1007/s00330-009-1322-9. [DOI] [PubMed] [Google Scholar]


