Abstract
Objectives
The most frequent presentation of chemotherapy-related toxicity in colorectal liver metastases (CRLM) is sinusoidal obstruction syndrome (SOS). The purpose of the present study was to identify preoperative factors predictive of SOS and to establish associations between type of chemotherapy and severity of SOS.
Methods
A retrospective study was carried out in a tertiary academic referral hospital. Patients suffering from CRLM who had undergone resection of at least one liver segment were included. Grading of SOS on the non-tumoral liver parenchyma was accomplished according to the Rubbia-Brandt criteria. A total of 151 patients were enrolled and divided into four groups according to the severity of SOS (grades 0–3).
Results
Multivariate analysis identified oxaliplatin and 5-fluorouracil as chemotherapeutic agents responsible for severe SOS lesions (P < 0.001 and P = 0.005, respectively). Bevacizumab was identified as having a protective effect against the occurrence of SOS lesions (P = 0.005). Univariate analysis identified the score on the aspartate aminotransferase : platelets ratio index (APRI) as the most significant biological factor predictive of severe SOS lesions. Splenomegaly is also significantly associated with the occurrence of severe SOS lesions.
Conclusions
The APRI score and splenomegaly are effective as factors predictive of SOS. Bevacizumab has a protective effect against SOS.
Introduction
Chemotherapy is increasingly used as part of an integrated multimodal approach to treating colorectal liver metastases (CRLM). Tumour response rates have improved tremendously over recent decades and now reach 80% as a result of the combination of current chemotherapeutic agents including oxaliplatin and irinotecan and, more recently, biological agents such as cetuximab and bevacizumab.1–4 These effective chemotherapies are increasingly used prior to liver surgery for CRLM. Initially, preoperative chemotherapy was administered in unresectable patients and succeeded in converting 9–40% of initially unresectable metastases to resectable status.2,5 In current practice, preoperative chemotherapy is often administered to patients with resectable CRLM to reduce the risk for tumour relapse3,6 and to identify patients in whom disease will progress rapidly during chemotherapy. The latter factor helps to avoid futile hepatic surgery being offered to patients with poor prognoses.7 Some chemotherapeutic agents, especially oxaliplatin-based agents, may have significant toxicity on normal hepatic parenchyma. Indeed, sinusoidal obstruction syndrome (SOS) was first reported in 2004 by Rubbia-Brandt et al.8 Since then, several clinical series have confirmed the presence of SOS lesions in patients treated with oxaliplatin and have reported their potential clinical impact in the context of liver surgery, including an increased risk for intraoperative bleeding and postoperative liver insufficiency.9–13 In view of these potential adverse effects, a more cautious approach to the preoperative administration of chemotherapy has been employed. Increased use of preoperative liver function tests, such as the indocyanine green (ICG) test, preoperative liver biopsy14,15 and portal vein embolization (PVE)15,16 has been proposed. Preoperative factors predictive of SOS may be very helpful in the identification and better selection of patients who are at risk for severe SOS. The primary objective of the present study was to evaluate whether preoperative biological factors, liver function tests and scoring systems, respectively, were able to predict the occurrence of SOS lesions. The secondary objective was to evaluate the potential protective effect of bevacizumab against the occurrence of SOS lesions.
Materials and methods
Patients
Between January 2000 and January 2011, 302 patients underwent liver resection for CRLM in the Department of Abdominal Surgery and Transplantation, Saint-Luc University Hospital, Brussels, Belgium.
A retrospective review of data for those patients was conducted. A total of 151 (50%) patients were included for further analysis because they were identified as having undergone resection of at least one liver segment and thus to have provided enough non-tumoral liver tissue to facilitate a representative pathological analysis. Grading of SOS on the non-tumoral liver parenchyma was accomplished according to the criteria defined by Rubbia-Brandt et al.8 Percutaneous PVE was carried out when the future liver remnant was estimated to represent <30% of the total liver volume or when it represented <1% of the patient's body weight. The size of the spleen was measured on preoperative magnetic resonance imaging or computed tomography and was recorded. Splenomegaly was defined as a spleen major axis (craniocaudal) of >13 cm.17
Chemotherapy
Only chemotherapy administered within 6 months prior to liver surgery was taken into consideration. Type, duration and the interval between the administration of chemotherapy and liver resection were recorded. Liver resection was performed at least 4 weeks after the last course of chemotherapy and at least 6 weeks after the last administration of bevacizumab.
Preoperative assessment of liver function
Preoperative serum biologic data including platelet count, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (AP), γ-glutamyl-transferase (GGT) and direct bilirubin were collected. An ICG test, currently used to assess liver function,18,19 was used in the majority of patients and especially in those who underwent a major hepatectomy (resection of at least three liver segments) or those who received more than three courses of chemotherapy. Data on ICG test values were missing in 27 patients who underwent minor hepatectomy. The AST : platelet ratio index (APRI) score and a score based on the relationships among four regression coefficients (Fib-4 score), initially used to predict fibrosis in patients suffering from hepatitis C, were calculated according to the appropriate formulae:20–22
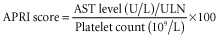 and
and 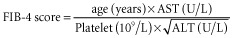 .
.
Pathological examination
All formalin-fixed, paraffin-embedded archival samples of the non-tumoral liver parenchyma were reviewed by one pathologist who was unaware of the clinical and biological patient data. Morphological analyses were based on haematoxylin and eosin (H&E) and reticulin-stained slides. Sinusoidal congestion was graded from 0 to 3 according to the severity of findings, as proposed in the original publication by Rubbia-Brandt et al.,8 in which grade 0 = absent, grade 1 = mild (one third of the lobule is affected), grade 2 = moderate (two thirds of the lobule are affected), and grade 3 = severe (the whole lobule is affected). Nodular regenerative hyperplasia (NRH) was defined as distinct nodular hyperplasia on H&E staining, clarified by reticulin staining, and was considered in the analysis of the present results as disease coexistent with SOS.9,16
Statistics
Statistical analyses for comparisons were performed using the Yates trend test.
Univariate or multivariate regressions for ordered categorical data were used to determine the relationships between the severity of SOS lesions and preoperative dichotomic variables, including gender, PVE, chemotherapy agents and splenomegaly.23 Ordinary univariate and multivariate regressions were used to determine the relationships between continuous variables (including age, serum platelet count, AST, ALT, GGT, AP, direct bilirubin, and liver function evaluation tests such as the ICG test, APRI and Fib-4 scores) and severity of SOS lesions. A P-value of <0.05 was considered to indicate statistical significance.
Results
Patients
A total of 151 patients met the criteria for inclusion and thus their data were included for analysis. Thirty-five patients (Group 1) did not exhibit any SOS lesions, 49 patients (Group 2) presented mild (grade 1) SOS lesions, 51 patients (Group 3) presented moderate (grade 2) SOS lesions and 16 patients (Group 4) demonstrated severe (grade 3) SOS lesions. Four patients with SOS grade 2 and one patient with SOS grade 3 presented coexistent NRH (7%). Univariate analysis of demographic and clinical features as potentially predictive factors of SOS injury is detailed in Table 1. Two patients presenting with splenomegaly had NRH.
Table 1.
Univariate analysis of demographic and clinical features and chemotherapeutic agents that represent factors predictive of the occurrence of sinusoidal obstruction syndrome (SOS)
| Group 1 | Group 2 | Group 3 | Group 4 | P-value | |
|---|---|---|---|---|---|
| (SOS grade 0, n = 35) | (SOS grade 1, n = 49) | (SOS grade 2, n = 51) | (SOS grade 3, n = 16) | ||
| Age, years, mean (range) | 64 (27–75) | 65 (14–82) | 65 (47–86) | 64 (40–73) | 0.130 |
| Gender, male/female, n | 21/14 | 19/30 | 26/25 | 9/7 | 0.890 |
| Portal vein embolization, n (%) | 9 (26%) | 20 (41%) | 20 (39%) | 7 (44%) | 0.200 |
| Splenomegaly (>13 cm), n (%) | 0 | 0 | 2 (4%) | 2 (13%) | 0.009 |
| Chemotherapy, n (%) | 24 (69%) | 45 (92%) | 46 (90%) | 15 (94%) | 0.007 |
| FOLFOX, patients, n (%) | 11 (31%) | 28 (57%) | 29 (57%) | 9 (56%) | 0.005 |
| Number of cycles, mean (range) | 6 (3–12) | 8 (3–21) | 9 (2–18) | 7 (4–24) | 0.210 |
| Interval between chemotherapy and surgery, months, mean (range) | 1 (1–6) | 2 (1–6) | 1 (1–6) | 1 (1–5) | 0.180 |
| FOLFIRI, patients, n (%) | 13 (37%) | 20 (41%) | 15 (29%) | 5 (31%) | 0.410 |
| Number of cycles, mean (range) | 7 (4–35) | 6 (2–26) | 8 (2–36) | 8 (3–20) | 0.520 |
| Interval between chemotherapy and surgery, months, mean (range) | 1 (1–5) | 1 (1–5) | 2 (1–6) | 1 (1–6) | 0.13 |
| Bevacizumab, patients, n (%) | 9 (26%) | 8 (16%) | 3 (6%) | 0 | 0.005 (protective effect) |
| Number of cycles, mean (range) | 10 (4–16) | 8 (6–12) | 7 (6–10) | – | 0.220 |
| Interval between chemotherapy and surgery, months, mean (range) | 2 (1–6) | 2 (1–3) | 3 (2–4) | – | 0.800 |
| Cetuximab, patients, n (%) | 4 (11%) | 8 (16%) | 5 (10%) | 1 (6%) | 0.470 |
| Number of cycles, mean (range) | 12 (6–18) | 6 (2–26) | 8 (4–36) | 20 | 0.340 |
| Interval between chemotherapy and surgery, months, mean (range) | 2 (1–2) | 1 (1–6) | 1 (1–2) | 1 | 0.240 |
| 5-Fluorouracil, patients, n (%) | 0 | 0 | 3 (6%) | 4 (25%) | <0.001 |
| Number of cycles, mean (range) | NA | NA | 8 (6–12) | 7 (2–12) | 0.480 |
| Interval between chemotherapy and surgery, months, mean (range) | NA | NA | 1 (1–2) | 1 (1–2) | 0.180 |
PVE, portal vein embolization
5-FU, 5-fluorouracil
FOLFOX, folinic acid, 5-FU, oxaliplatin
FOLFIRI, folinic acid, 5-FU, irinotecan
NA, not available.
Chemotherapy
Data on chemotherapeutic agents are detailed in Table 1. Multivariate analysis performed on chemotherapy data showed that the administration of either 5-fluorouracil (5-FU) (P < 0.001) or oxaliplatin-based chemotherapy (P = 0.005) was a significant independent factor predicting the presence of SOS lesions.
Biological variables
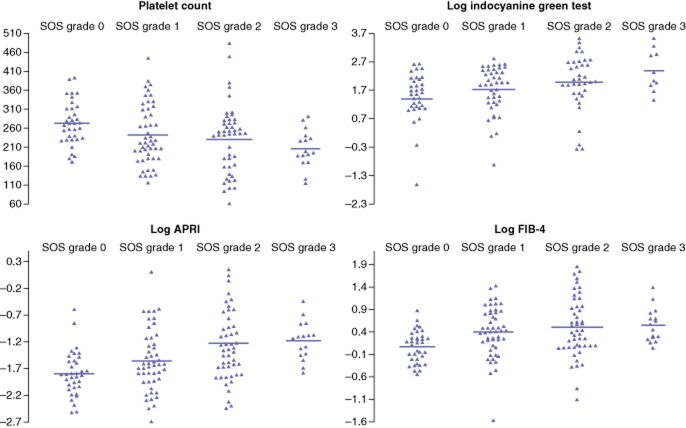
Results of univariate analysis for biological predictive factors are shown in Table 2. Although median preoperative platelet counts were significantly lower in Groups 3 and 4 than in Groups 1 and 2, values remained within the normal range. Four of the 35 patients in Group 1 had a slightly elevated ICG test (10.8–13.8%) and two of 35 patients had an APRI score of >0.36, but none of the 35 patients in Group 1 had an abnormal preoperative platelet count (<150 000/mm3). By contrast, abnormal platelet counts were observed in seven of 49 (14%) patients in Group 2, 11 of 51 (22%) patients in Group 3 and two of 16 (13%) patients in Group 4 (P = 0.029). Among patients for whom data on preoperative ICG tests were available, abnormal ICG test values (>10%) were found in four of 35 patients in Group 1, 11 of 49 patients in Group 2, 15 of 51 patients in Group 3 and five of 16 patients in Group 4 (P = 0.044). Of the five patients presenting with NRH (3%), three had an abnormal ICG test (values of 13%, 13% and 29%, respectively) and two presented both abnormal platelet values (94 × 109/l and 126 × 109/l, respectively) and high APRI scores (0.56 and 1.77, respectively) and Fib-4 scores (4.32 and 4.50, respectively). Abnormal direct bilirubin and GGT values were found in only two patients with NRH.
Table 2.
Univariate analysis of biological predictive factors and sinusoidal obstruction syndrome (SOS) by grade
| Preoperative biological variables | Group 1 | Group 2 | Group 3 | Group 4 | P-value |
|---|---|---|---|---|---|
| (SOS grade 0, n = 35) | (SOS grade 1, n = 49) | (SOS grade 2, n = 51) | (SOS grade 3, n = 16) | ||
| Mean (range) | Mean (range) | Mean (range) | Mean (range) | ||
| AST (normal: <50 U/l) | 25 (15–60) | 27 (15–92) | 30 (15–274) | 32 (21–100) | 0.130 |
| ALT (normal: <50 U/l) | 26 (14–78) | 25 (10–72) | 31 (11–277) | 30 (19–123) | 0.300 |
| AP (normal: 30–120 U/l) | 80 (29–218) | 99 (36–336) | 82 (49–672) | 93 (72–220) | 0.400 |
| GGT (normal: <50 U/l) | 45 (19–195) | 51 (10–327) | 59 (15–377) | 80 (19–377) | 0.033 |
| Direct bilirubin (normal: <0.3 mg/dl) | 0.1 (0–0.2) | 0.1 (0–0.4) | 0.1 (0–0.5) | 0.1 (0–0.3) | 0.018 |
| Platelet count (103) (normal: 150–300 × 103/ml) | 272 (171–393) | 222 (116–445) | 246 (62–484) | 197 (94–290) | 0.003 |
| Indocyanine green test (normal: <10%) | 4.8 (0.1–13.8) | 6.9 (0.4–16.7) | 7.7 (0.7–34) | 10.2 (3.9–34) | <0.001 |
| APRI score | 0.163 (0.080–0.556) | 0.198 (0.068–1.119) | 0.265 (0.087–4.529) | 0.325 (0.169–0.643) | <0.001 |
| Fib-4 score | 1.173 (0.580–2.394) | 1.504 (0.209–4.157) | 1.544 (0.330–6.395) | 1.658 (1.045–4.025) | <0.001 |
AST, aspartate aminotransferase
ALT, alanine aminotransferase
AP, alkaline phosphatase
GGT, γ-glutamyl-transferase
APRI, AST : platelet ratio index.
Data regarding biological factors that were significantly predictive of SOS and scores in univariate analysis are illustrated in Fig. 1.
Figure 1.
Significant biological factors predictive of sinusoidal obstruction syndrome (SOS) lesions in univariate analysis (Table 2). APRI, aspartate aminotransferase : platelets ratio index
Bevacizumab and sinusoidal lesions
Data on bevacizumab are detailed in Tables 1 and 3.
Table 3.
Occurrence of sinusoidal obstruction syndrome (SOS) by grade in patients treated with oxaliplatin with and without bevacizumab
| Oxaliplatin alone (n = 67) | Oxaliplatin + bevacizumab (n = 10) | P-value | |
|---|---|---|---|
| Group 1 (SOS grade 0, n = 35), n | 7 | 4 | 0.003 |
| Group 2 (SOS grade 1, n = 49), n | 23 | 5 | |
| Group 3 (SOS grade 2, n = 51), n | 28 | 1 | |
| Group 4 (SOS grade 3, n = 16), n | 9 | 0 |
Prevalences of grade 2 and 3 SOS lesions (Groups 3 and 4) were significantly lower when bevacizumab was administered (P = 0.005). Moreover, the prevalence of severe SOS lesions was significantly higher in the group of patients who received preoperative oxaliplatin-based chemotherapy alone compared with patients who received oxaliplatin-based chemotherapy with bevacizumab (P = 0.003). Finally, multivariate analysis identified bevacizumab as a chemotherapeutic agent that is protective against SOS lesions (P = 0.004).
Discussion
Chemotherapy-induced liver injury is a problem in patients undergoing surgery for CRLM, in whom an increase in postoperative morbidity has been observed.10–12 Patients who are treated with oxaliplatin-based chemotherapy have been found to display an increased need for perioperative transfusions,24,25 higher perioperative morbidity,11,24,26 especially in major hepatectomies,12,26 and a longer postoperative hospital stay.12,24 These consequences increase with the number of cycles of chemotherapy administered.11,24 Although oxaliplatin-based chemotherapy is the chemotherapeutic agent most often incriminated in SOS, the present authors have previously reported that severe SOS lesions were also encountered in patients treated with 5-FU or irinotecan-based chemotherapy.10 In the present study, both oxaliplatin-based chemotherapy and 5-FU were significantly associated with severe (grades 2 and 3) SOS lesions. However, the administration of irinotecan-based chemotherapy was not significantly correlated with grade 2 and 3 SOS lesions.
Perioperative morbidity and mortality after major hepatectomy are often related to postoperative liver failure. Outcomes following major liver resection have improved in recent decades, partially as a result of the better selection and preparation of patients through the use of preoperative liver volume assessment and selected use of PVE.12,26–28 However, liver volume is only one of the criteria to be taken into consideration in the preoperative strategy to avoid postoperative liver failure. Indeed, the quality of the liver parenchyma and liver function must also be considered. Until now, factors predictive of SOS lesions had not been widely identified. Aloia et al.24 reported an increased rate of GGT in both the pre- and postoperative periods in patients with SOS. Nakano et al.12 reported that female gender, the administration of six or more cycles of oxaliplatin-based chemotherapy, abnormal preoperative AST values (>36 IU/l) and abnormal preoperative ICG test values (>10%) were preoperative factors significantly associated with SOS lesions. In addition, the benefit of a preoperative liver needle biopsy to evaluate the severity of SOS in patients who receive high doses of chemotherapy is uncertain as a result of the heterogeneous distribution of lesions within the liver. Indeed, Rubbia-Brandt et al. reported that SOS lesions were predominantly visible in the subcapsular region, but were haphazardly distributed and affected nodules were intermingled with intact parenchyma.29 Recently, Soubrane et al.26 reported that a low preoperative platelet count and a high APRI score were the most reliable indicators of the severity of SOS, and provided area under the curve (AUC) values of 0.84 and 0.85, respectively, in receiver operating characteristic (ROC) analyses. The cut-off APRI score has been evaluated as 0.36 according to these data. This score is very interesting because it is readily available (it is calculated in preoperative laboratory tests including AST serum level and platelet count) at low cost.
In the present study, univariate analysis showed that direct bilirubin (P = 0.018), GGT (P = 0.033), platelet count (P = 0.003), ICG test (P < 0.001), Fib-4 score (P < 0.001) and APRI score (P < 0.001) represent factors that are significantly predictive of severe SOS. Nodular regenerative hyperplasia is the most severe form of SOS and describes a proliferative process in which regenerative nodules replace the normal liver architecture, inducing compression on the surrounding parenchyma, which exhibits congestion and sinusoidal dilatation. Portal hypertension (PHT) is commonly observed in NRH.9,16 Diffuse NRH is associated with increased postoperative hepatic morbidity.16 Preoperative factors predictive of NRH would be very helpful in the detection of these very high-risk patients. In the present study, ICG test values, APRI scores and Fib-4 scores were identified as the factors that best predicted NRH. This contradicts the results of Wicherts et al.,16 who reported non-altered ICG test values in patients with NRH. The latter authors identified preoperative elevated levels of GGT and total bilirubin as factors predictive of NRH.16 In the present study, a minority of patients with NRH (two of five) had abnormal GGT and direct bilirubin values. A low platelet count was found in 48% of patients with NRH in the series reported by Wicherts et al.16 and in 40% of patients with NRH in the present study. This may be explained by platelet trapping related to splenomegaly caused by PHT.
Overman et al.30 reported an increase in spleen size of ≥50% in 24% of patients treated with FOLFOX [folinic acid (leucovorin), 5-FU, oxaliplatin] correlated with high-grade hepatic sinusoidal injury at the time of liver resection and with a higher rate of thrombocytopoenia. In the present series, splenomegaly was also significantly associated with high-grade (2 and 3) sinusoidal lesions and with a low platelet count.
Secondly, data from the present study, like those reported previously,4,29 corroborate the protective effect of bevacizumab in reducing the incidence of SOS lesions significantly. The mechanism by which this protective effect is engaged remains unclear. Many authors have reported that the preoperative administration of bevacizumab does not increase postoperative morbidity in patients undergoing liver surgery for CRLM,31–33 provided that 6–8 weeks are allowed to elapse between the end of bevacizumab administration and liver surgery. Additionally, bevacizumab has also been reported to have a protective clinical impact on postoperative liver failure after liver resection and on the incidence of SOS lesions.4,34
In conclusion, as the present authors have previously reported,10 severe SOS lesions occurred in patients who received oxaliplatin-based chemotherapy and 5-FU chemotherapy. Univariate analysis identified APRI score as the most significant biological factor predictive of severe SOS lesions. Splenomegaly is also significantly associated with the occurrence of severe SOS lesions and/or NRH. Finally, bevacizumab has a protective effect against SOS lesions.
Special attention should be given to patients who have been treated with oxaliplatin-based chemotherapy without bevacizumab who present preoperatively with a low platelet count, an APRI score of >0.36 and/or splenomegaly because these individuals are at risk for severe SOS lesions. In such patients in whom major hepatectomy is planned, particular attention should be given to preoperative preparation according to liver sector volumetry in order that more liberal right PVE can be planned.
Acknowledgments
The authors are very grateful to Dr Carola Dahrenmoller, Division of Hepatobiliary and Pancreatic Surgery, Department of Abdominal Surgery and Transplantation, Saint-Luc University Hospital, Brussels, Belgium, for the linguistic revision of the current manuscript.
Conflicts of interest
None declared.
References
- 1.Adam R. Colorectal cancer with synchronous liver metastases. Br J Surg. 2007;94:129–131. doi: 10.1002/bjs.5764. [DOI] [PubMed] [Google Scholar]
- 2.Nordlinger B, Van CE, Rougier P, Kohne CH, Ychou M, Sobrero A, et al. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer. 2007;43:2037–2045. doi: 10.1016/j.ejca.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomized controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribero D, Wang H, Donadon M, Zorzi D, Thomas MB, Eng C, et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer. 2007;110:2761–2767. doi: 10.1002/cncr.23099. [DOI] [PubMed] [Google Scholar]
- 5.Van CE, Nordlinger B, Adam R, Kohne CH, Pozzo C, Poston G, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42:2212–2221. doi: 10.1016/j.ejca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Gruenberger B, Scheithauer W, Punzengruber R, Zielinski C, Tamandl D, Gruenberger T. Importance of response to neoadjuvant chemotherapy in potentially curable colorectal cancer liver metastases. BMC Cancer. 2008;8:120. doi: 10.1186/1471-2407-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, et al. Tumour progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1061. doi: 10.1097/01.sla.0000145964.08365.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le CM, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 9.Hubert C, Sempoux C, Horsmans Y, Rahier J, Humblet Y, Machiels JP, et al. Nodular regenerative hyperplasia: a deleterious consequence of chemotherapy for colorectal liver metastases? Liver Int. 2007;27:938–943. doi: 10.1111/j.1478-3231.2007.01511.x. [DOI] [PubMed] [Google Scholar]
- 10.Hubert C, Fervaille C, Sempoux C, Horsmans Y, Humblet Y, Machiels JP, et al. Prevalence and clinical relevance of pathological hepatic changes occurring after neoadjuvant chemotherapy for colorectal liver metastases. Surgery. 2010;147:185–194. doi: 10.1016/j.surg.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Karoui M, Penna C, Min-Hashem M, Mitry E, Benoist S, Franc B, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1–7. doi: 10.1097/01.sla.0000193603.26265.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakano H, Oussoultzoglou E, Rosso E, Casnedi S, Chenard-Neu MP, Dufour P, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247:118–124. doi: 10.1097/SLA.0b013e31815774de. [DOI] [PubMed] [Google Scholar]
- 13.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 14.Chun YS, Laurent A, Maru D, Vauthey JN. Management of chemotherapy-associated hepatotoxicity in colorectal liver metastases. Lancet Oncol. 2009;10:278–286. doi: 10.1016/S1470-2045(09)70064-6. [DOI] [PubMed] [Google Scholar]
- 15.Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–286. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 16.Wicherts DA, de Haas RJ, Sebagh M, Ciacio O, Levi F, Paule B, et al. Regenerative nodular hyperplasia of the liver related to chemotherapy: impact on outcome of liver surgery for colorectal metastases. Ann Surg Oncol. 2011;18:659–669. doi: 10.1245/s10434-010-1385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor AJ, Dodds WJ, Erickson SJ, Stewart ET. CT of acquired abnormalities of the spleen. AJR Am J Roentgenol. 1991;157:1213–1219. doi: 10.2214/ajr.157.6.1950868. [DOI] [PubMed] [Google Scholar]
- 18.Hemming AW, Scudamore CH, Shackleton CR, Pudek M, Erb SR. Indocyanine green clearance as a predictor of successful hepatic resection in cirrhotic patients. Am J Surg. 1992;163:515–518. doi: 10.1016/0002-9610(92)90400-l. [DOI] [PubMed] [Google Scholar]
- 19.Leevy CM, Smith F, Longueville J, Paumgartner G, Howard MM. Indocyanine green clearance as a test for hepatic function. Evaluation by dichromatic ear densitometry. JAMA. 1967;200:236–240. [PubMed] [Google Scholar]
- 20.Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology. 2007;45:297–306. doi: 10.1002/hep.21520. [DOI] [PubMed] [Google Scholar]
- 21.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 22.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple non-invasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 23.Snell EJ. A scaling procedure for ordered categorical data. Biometrics. 1964;20:592–607. [Google Scholar]
- 24.Aloia T, Sebagh M, Plasse M, Karam V, Levi F, Giacchetti S, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24:4983–4990. doi: 10.1200/JCO.2006.05.8156. [DOI] [PubMed] [Google Scholar]
- 25.Mehta NN, Ravikumar R, Coldham CA, Buckels JA, Hubscher SG, Bramhall SR, et al. Effect of preoperative chemotherapy on liver resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:782–786. doi: 10.1016/j.ejso.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Soubrane O, Brouquet A, Zalinski S, Terris B, Brezault C, Mallet V, et al. Predicting high grade lesions of sinusoidal obstruction syndrome related to oxaliplatin-based chemotherapy for colorectal liver metastases: correlation with post-hepatectomy outcome. Ann Surg. 2010;251:454–460. doi: 10.1097/SLA.0b013e3181c79403. [DOI] [PubMed] [Google Scholar]
- 27.Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49–57. doi: 10.1097/SLA.0b013e31815f6e5b. [DOI] [PubMed] [Google Scholar]
- 28.Hemming AW, Reed AI, Howard RJ, Fujita S, Hochwald SN, Caridi JG, et al. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003;237:686–691. doi: 10.1097/01.SLA.0000065265.16728.C0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubbia-Brandt L, Lauwers GY, Wang H, Majno PE, Tanabe K, Zhu AX, et al. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology. 2010;56:430–439. doi: 10.1111/j.1365-2559.2010.03511.x. [DOI] [PubMed] [Google Scholar]
- 30.Overman MJ, Maru DM, Charnsangavej C, Loyer EM, Wang H, Pathak P, et al. Oxaliplatin-mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J Clin Oncol. 2010;28:2549–2555. doi: 10.1200/JCO.2009.27.5701. [DOI] [PubMed] [Google Scholar]
- 31.Kesmodel SB, Ellis LM, Lin E, Chang GJ, Abdalla EK, Kopetz S, et al. Preoperative bevacizumab does not significantly increase postoperative complication rates in patients undergoing hepatic surgery for colorectal cancer liver metastases. J Clin Oncol. 2008;26:5254–5260. doi: 10.1200/JCO.2008.17.7857. [DOI] [PubMed] [Google Scholar]
- 32.D'Angelica M, Kornprat P, Gonen M, Chung KY, Jarnagin WR, DeMatteo RP, et al. Lack of evidence for increased operative morbidity after hepatectomy with perioperative use of bevacizumab: a matched case–control study. Ann Surg Oncol. 2007;14:759–765. doi: 10.1245/s10434-006-9074-0. [DOI] [PubMed] [Google Scholar]
- 33.Reddy SK, Morse MA, Hurwitz HI, Bendell JC, Gan TJ, Hill SE, et al. Addition of bevacizumab to irinotecan- and oxaliplatin-based preoperative chemotherapy regimens does not increase morbidity after resection of colorectal liver metastases. J Am Coll Surg. 2008;206:96–106. doi: 10.1016/j.jamcollsurg.2007.06.290. [DOI] [PubMed] [Google Scholar]
- 34.Klinger M, Eipeldauer S, Hacker S, Herberger B, Tamandl D, Dorfmeister M, et al. Bevacizumab protects against sinusoidal obstruction syndrome and does not increase response rate in neoadjuvant XELOX/FOLFOX therapy of colorectal cancer liver metastases. Eur J Surg Oncol. 2009;35:515–520. doi: 10.1016/j.ejso.2008.12.013. [DOI] [PubMed] [Google Scholar]