Abstract
Background
Prothrombin time-international normalized ratio (PT-INR) is widely utilized to guide plasma therapy and initiation of thromboprophylaxis after a hepatectomy. Thrombelastography (TEG) monitors shear elasticity, which is sensitive to cellular and plasma components in blood, allowing for functional assessment of the life of the clot. The objective of this study was to prospectively compare PT-INR and TEG in liver resection patients.
Methods
Forty patients were enrolled before undergoing an elective hepatectomy. Patients underwent a liver resection utilizing a low central venous pressure (CVP) anaesthetic technique and intermittent Pringle manoeuver. PT-INR and TEG were drawn prior to incision, post-operatively, and post-operative days 1, 3 and 5.
Results
All post-operative PT-INR values increased significantly when compared with pre-operative PT-INR (P < 0.01). The time of onset to clot (R-value) decreased significantly at the post-operative time point (P = 0.04), consistent with a relative hypercoagulability. Subsequent R-values were not different compared with the pre-operative R-value. The strength of the clot (maximum amplitude, MA) was unchanged when comparing pre- and post-operative time points.
Discussion
In spite of an elevation in PT-INR, patients undergoing a liver resection demonstrated a brief hypercoagulable state, followed by normal coagulation function based on TEG. These data call into question the practice of utilizing PT-INR to guide plasma transfusion and timing of prophylactic anticoagulation after a liver resection.
Introduction
A partial hepatectomy is a common surgical therapy for both benign and malignant hepatic disease. Advances in surgical technique over the past 30 years have led to decreased peri-operative blood product transfusion, morbidity and mortality. While a post-operative elevation in prothrombin time-international normalized ratio (PT-INR) has been well characterized, the clinical significance of a prolonged PT-INR in the post-operative period remains poorly understood.1–3
Previous studies have suggested that changes in liver function tests may vary and clinically correlate with the extent of a hepatic resection. Liver parenchymal enzymes such as serum glutamic oxaloacetic transaminase (SGOT), alkaline phosphatase, and total bilirubin all increase after both anatomic resections and smaller wedge resections.1 Liver resection leads to a transient decrease in synthetic function of the liver, resulting in impaired synthesis of clotting factors and regulatory proteins in the coagulation cascade.4 An associated elevation of the PT-INR after a liver resection is a common finding, and has been reported in multiple previous studies.1–7 Prospective studies have shown that the PT-INR remains elevated until post-operative day 5 compared with the pre-operative value.3
The clinical implications of an elevated PT-INR are numerous. Elevation of PT-INR frequently leads to post-operative transfusion of fresh frozen plasma (FFP) in liver resection patients. A transfusion is associated with the risks of infection, fluid overload, anaphylaxis and transfusion-related acute lung injury (TRALI).8,9 Thromboprophylaxis is considered the standard of care after major abdominal operations owing to the associated risk of a deep vein thrombosis (DVT) and pulmonary embolism (PE) and it is frequently delayed because of an elevated PT-INR.10–12 While reported rates of venous thromboembolism (VTE) vary in the literature, data exist suggesting that withholding thromboprophylaxis leads to a higher rate of clinically significant VTE.
Thrombelastography (TEG) is a point-of-care test that measures shear elasticity of a blood clot using whole blood, making it sensitive to changes in both plasma and cellular components in blood. TEG has been studied in trauma,13 liver transplantation,14,15 orthopaedic,16 urological17 and cardiac patients.18 Previous studies have demonstrated that TEG is superior to PT-INR for evaluating the coagulation status in critically ill patients.19–21 When comparing TEG to INR in living donor liver transplantation, Cerutti et al. demonstrated a hypercoagulable state in some patients in spite of an elevated PT-INR.14
Alterations in coagulation after a partial hepatectomy remain poorly understood and difficult to predict. The impaired synthesis of pro- and anticoagulant factors after liver resection results in a functional coagulation status that is largely unknown. The aim of this study was to characterize the peri-operative coagulation profile of patients undergoing a liver resection using both routine coagulation tests and TEG.
Methods
Patients
This study was designed as a prospective, non-interventional trial. The protocol was approved by the Institutional Review Board at Oregon Health & Science University. Patients scheduled to undergo an elective anatomic hepatic resection were screened. Informed consent was obtained from all patients prior to participation in the study. Demographics were collected from the patients including: age, gender, diagnosis, presence of cirrhosis, American Society of Anesthesiologists Physical Status Classification (ASA Class) and body mass index (BMI).
Standard laboratory tests
PT-INR, activated partial thromboplastin time (aPTT), platelet and fibrinogen assays were performed at the Oregon Health & Science University Core Laboratory. These studies were obtained at the pre-operative clinic appointment or on the day of surgery prior to the operation, 5 h after the completion of the operation (±3 h), and on post-operative days 1, 3 and 5.
Thrombelastography
Blood samples were obtained from participants on the day of surgery prior to the operation, 5 h after the completion of the operation (±3 h), and on the mornings of post-operative days 1, 3 and 5. Studies were performed on fresh non-citrated specimens utilizing kaolin to accelerate clotting. Heparinase-containing cups were also used if the patient was receiving prophylactic, low-molecular-weight heparin (LMWH). The Thrombelastograph Haemostasis Analyzer 5000 (Haemoscope, Skokie, IL, USA) was utilized for the analyses. TEG values were recorded and analyzed. Caregivers were blinded to the results of the TEGs and they were not used to make clinical decisions in this study.
Operative procedure
Administration of anaesthesia and peri-operative care was routinely performed by the attending anaesthesiologist. Epidural analgesia was offered to patients as deemed appropriate by the attending anaesthesiologist. Low central venous pressure (CVP) techniques were used during a partial hepatectomy, and an intermittent Pringle manoeuver was performed at the preference of the attending surgeon. The target CVP was less than 5 mmHg. Operation, operative time, estimated blood loss and Pringle time were recorded.
Post-operative course
All patients were taken to the intensive care unit post-operatively for routine care. Patients were monitored prospectively for blood product transfusion and venous thromboembolism (VTE). Patients in the intensive care unit underwent weekly screening duplex ultrasound exams per institutional protocol. Patients on the acute care units underwent duplex ultrasound examinations if symptoms of VTE developed.
Statistical analysis
Parametric, normally distributed data were compared using Student's t-test, and values are presented as mean ± standard error of the mean (SEM). Non-normally distributed data were compared using the Mann–Whitney U-test, and values are presented as median with interquartile range (IQR). Normally distributed data comparison within groups utilized a paired t-test, whereas non-parametric data comparisons within groups were assessed using a Kruskal–Wallis test. Statistical package software SPSS, version 19.0 (SPSS Inc., Chicago, IL, USA) was utilized for all statistical comparisons. Statistical significance was defined as a P-value <0.05.
Results
Forty patients were enrolled in the study after informed consent was obtained. Five patients were excluded after undergoing a minor hepatectomy (four patients) or a palliative hepaticojejunostomy for metastatic disease (one). Of the remaining 35 patients, the mean age was 59.9 years (Table 1). Eighteen patients were male. Thirty-one of the 35 patients underwent a partial hepatectomy for malignancy, most commonly metastatic colorectal cancer (16 of 35, Table 1). Five patients had evidence of cirrhosis on final pathology, and the most common ASA classification was 3 (24 of 35, Table 1).
Table 1.
Patient demographics including mean age, gender, diagnosis, pathological presence of cirrhosis, body mass index (BMI) and American Society of Anesthesiologists (ASA) physical status class
| Demographics | |
|---|---|
| Age – year, mean (range, SD) | 59.9 (32–88, 12.7) |
| Male – no. of patients | 18 |
| Diagnosis – no. of patients | |
| Metastatic colorectal cancer | 16 |
| Hepatocellular carcinoma | 8 |
| Cholangiocarcinoma | 3 |
| Gallbladder cancer | 2 |
| Other | 6 |
| Cirrhosis – no. of patients | 5 |
| BMI – mean (range, SD) | 26.5 (19.7–34.4, 4.5) |
| ASA Class – no. of patients | |
| 2 | 6 |
| 3 | 24 |
| 4 | 3 |
Includes focal nodular hyperplasia, renal cell carcinoma, neuroendocrine tumour, hepatoma and hepatic hemangioma.
The distribution of operative procedures is listed in Table 2. The most common operation performed was a right hepatectomy (16 of 35). The median estimated blood loss was 400 ml (range 50–18 000), and the mean operative time was 303.6 ± 114 min. Twelve patients underwent an intermittent Pringle manoeuver, with a mean total time of 16.8 min.
Table 2.
Operative details including operation performed, estimated blood loss, operating room time and use of a Pringle manoeuver
| Operative details | |
|---|---|
| Operation – no. of patients | |
| Right hepatectomy | 17 |
| Left hepatectomy | 7 |
| Extended right hepatectomy | 2 |
| Left lateral segmentectomy | 2 |
| Minor resection (2 or fewer sections) | 7 |
| Estimated blood loss (ml) – median (range) | 400 (50–18,000) |
| Operating room time (min) – mean (range, SD) | 303.6 (177–648, 114) |
| Pringle Manoeuver | |
| No. of patients | 12 |
| Length of time (min) – mean (range, SD) | 16.75 (8–32, 8.8) |
Pre-operative PT-INR was found to be within normal limits for all patients (Table 3). After the operation, PT-INR values were noted to be significantly elevated at all time points, with a peak median value of 1.4 on post-operative day 3 (Fig. 1). Values remained elevated through post-operative day 5, at the completion of the study.
Table 3.
Standard coagulation assays
| Time point | INR (IQR) | aPTT (IQR) | Fibrinogen (IQR) | Platelets (IQR) |
|---|---|---|---|---|
| (Ref range 0.8–1.2) | (Ref range 26–36 s) | (Ref range 200–450 mg/dl) | (Ref range 150–400 k/cu mm) | |
| Pre-operative | 1.0 (1.0, 1.1) | 30.1 (27.8, 32.3) | 412 (310, 468) | 184 (161, 256) |
| Post-operative | 1.2 (1.2, 1.3)a | 29.1 (26.7, 31.1)b | 294 (230, 347)a | 183 (139, 262) |
| POD #1 | 1.4 (1.2, 1.4)a | 30.0 (27.6, 31.7) | 294 (255, 367)a | 165 (132, 214)a |
| POD #3 | 1.4 (1.3, 1.5)a | 34.1 (32.5, 36.3)a | 451 (314, 540)b | 134 (93, 192)a |
| POD #5 | 1.3 (1.2, 1.4)a | 32.9 (30.9, 37.1)b | 448 (403, 552)b | 176 (115, 214)a |
International normalized ratio (INR) rose significantly after a hepatectomy. Activated partial thromboplastin time (aPTT) remained within normal limits. Fibrinogen decreased dramatically, and then rose beyond pre-operative values. Platelets decreased post-operatively, but trended towards recovery at the end of the study.
IQR, interquartile range; POD, post-operative day.
P < 0.01.
P < 0.05.
Figure 1.
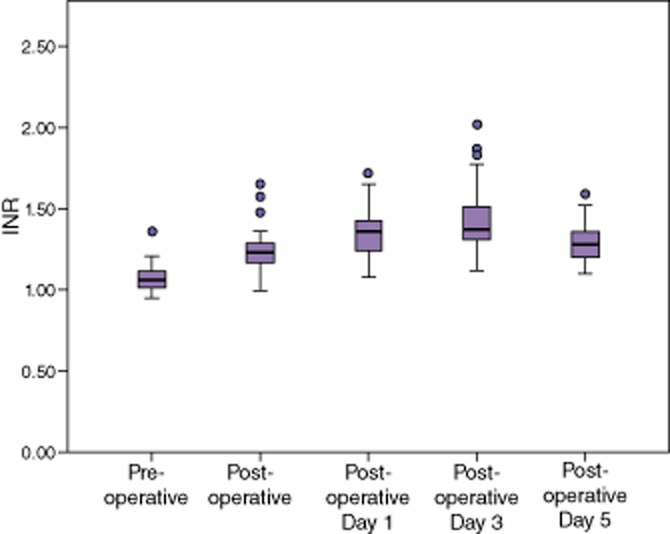
International normalized ratio (INR) rose significantly after a hepatectomy, suggesting coagulopathy
The median aPTT was found to decrease significantly after the operation (Table 3). It subsequently rose in the post-operative setting, peaking at post-operative day 3 at 34.1. In spite of a significant elevation on post-operative days 3 and 5, the aPTT was found to be within the normal range for the duration of the study (Fig. 2).
Figure 2.
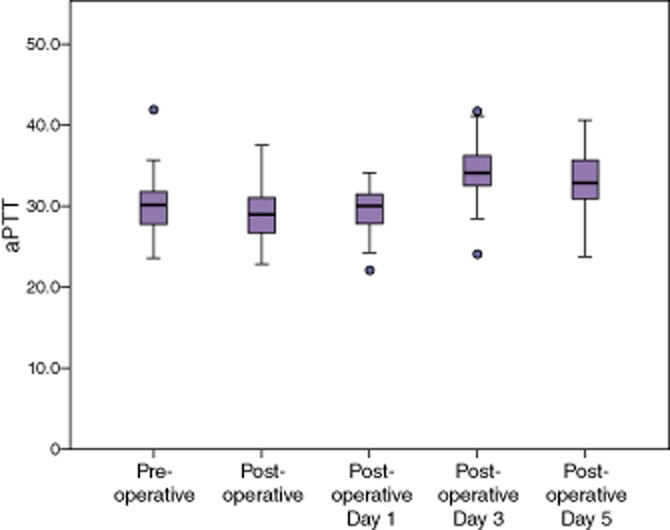
Median activated partial thromboplastin time (aPTT) remained within normal limits in spite of statistically significant changes
Fibrinogen decreased significantly after a partial hepatectomy (Table 3), but remained within normal limits. By post-operative day 3, the fibrinogen had increased above pre-operative levels and remained significantly elevated on post-operative day 5 (Fig. 3). Platelet counts remained unchanged immediately after a partial hepatectomy, but decreased significantly on post-operative day 1 (Table 3, Fig. 4). Counts continued to decrease on post-operative day 3, but recovered to normal levels on post-operative day 5.
Figure 3.
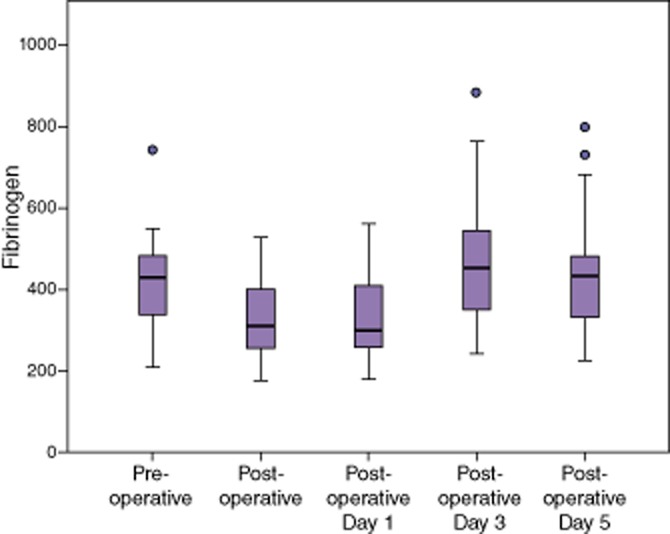
Fibrinogen declined after a hepatectomy, then rose above pre-operative values
Figure 4.
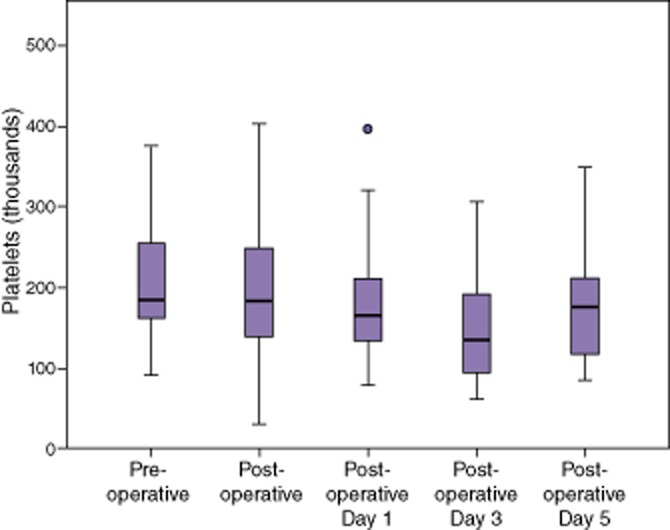
Platelets declined on post-operative days 1 and 3, but began to trend up by post-operative day 5
The time to initiation of a clot (R-value) was noted to be within the normal range pre-operatively for all patients (Table 4). At the post-operative time point, the R-value was significantly lower, but still within the normal limits. No statistically significant differences in R-value were noted at the remaining time points. The strength of clot (maximum amplitude, MA) did not change significantly at any time point (Table 4). The rate at which the clot strengthened (α-Angle) and the complete coagulation profile as assessed by TEG (coagulation index, CI) were unchanged at all time points.
Table 4.
Thrombelastography assays: R-time = time to initiation of clot
| Time point | R (Mean, minutes) | MA (Mean, mm) | Angle (Mean, deg) | CI (Mean) |
|---|---|---|---|---|
| (Ref range 4–9 min) | (Ref range 55–74 mm) | (Ref range 59–74) | (Ref range −3–+3) | |
| Pre-op | 6.9 ± 2.5 | 68.5 ± 6.2 | 61.5 ± 9.2 | 0.0 ± 2.1 |
| Post-op | 6.0 ± 1.5a | 67.4 ± 9.8 | 61.0 ± 8.2 | 0.3 ± 2.4 |
| POD #1 | 6.1 ± 2.4 | 68.9 ± 8.7 | 62.1 ± 8.7 | 0.6 ± 2.8 |
| POD #3 | 7.2 ± 2.4 | 68.9 ± 7.5 | 61.6 ± 10 | −0.1 ± 2.8 |
| POD #5 | 6.4 ± 1.3 | 69.4 ± 6.7 | 65.4 ± 5.7 | 0.8 ± 1.4 |
P = 0.04.
MA, maximum amplitude, maximum strength of the clot; Angle, α-Angle, rate of clot strengthening; CI, coagulation index.
Four patients in the study underwent a fresh frozen plasma transfusion post-operatively. Of those, one received FFP immediately after the operation for an elevated intra-operative PT-INR. Two patients had a TEG and standard coagulation assays performed on the morning of the transfusion (Table 5). TEG on both of those patients demonstrated s normal R-value and MA, suggesting normal coagulation. A third patient was transfused on post-operative day 2, a day in which TEG was not performed, and developed a febrile non-haemolytic reaction.
Table 5.
Coagulation assays prior to transfusion of fresh frozen plasma (FFP)
| FFP transfusion | Pt 1a | Pt 2 |
|---|---|---|
| PT-INR at transfusion | 2.05 | 2.07 |
| Morning PT-INR | 1.5 | 1.4 |
| aPTT | 24.7b | 30.9 |
| Fibinogen | 252 | 294 |
| Platelets | 130 | 214 |
| R-value | 5.7 | 8.2 |
| MA | 65.4 | 59.4 |
Patient 1 developed a pulmonary embolism 3 months after the operation.
aPTT below the normal range suggesting a hypercoagulable state.
PT-INR, Prothrombin time-international normalized ratio; aPTT, activated partial thromboplastin time.
Two patients had venous thromboembolic events after a partial hepatectomy. Of these, one was a pulmonary embolism approximately 3 months after the operation, in a patient who received FFP for an elevated INR. At the time of FFP transfusion, that patient had a normal TEG and a shortened aPTT. LMWH prophylaxis was started on this patient on the evening of post-operative day 2. The second patient developed a deep vein thrombosis at the site of a central venous catheter on post-operative day 9, after LMWH prophylaxis was initiated on post-operative day 4. While this patient's post-operative day 1 INR was 1.65, the R-time by TEG was 3.7 min, suggesting a transient hypercoagulable state by TEG. On post-operative day 5, after initiation of LMWH, the R-time was found to be 18.8 min by standard kaolin TEG and 7.3 min by heparinase TEG.
Discussion
PT-INR has been used to guide FFP transfusion and the initiation of thromboembolic prophylaxis after a partial hepatectomy, in spite of an incomplete understanding of the effect of a partial hepatectomy on the coagulation cascade. Multiple previous studies have shown that TEG is a better predictor of hypercoagulable and coagulopathic states when compared with PT-INR in cardiac and trauma patients.9,13,19–21 In the largest currently published series on TEG in partial hepatectomy, Cerruti et al. published an observational study of 10 patients undergoing a right hepatectomy for living donor liver transplantation.14 In their study, 4 out of 10 patients demonstrated hypercoagulability on TEG by post-operative day 5 and 6 out of 10 patients were hypercoagulable on post-operative day 10. One patient who demonstrated hypercoagulability on day 5 by TEG developed a DVT on day 8.
In the present study, the PT-INR demonstrated a coagulopathic state with median values persisting above the normal limits of the test at the completion of the study. In contrast, TEG demonstrated normal coagulation function in these patients, with a transient relative hypercoagulability immediately after a partial hepatectomy. aPTT measurements were similar to the TEG findings, demonstrating a transient decrease but remaining within the normal limits for the duration of the study.
The difference observed may be attributable to the nature of the tests. Unlike traditional coagulation assays, which measure components of blood and portions of the coagulation pathway separately, TEG uses whole blood to measure functional coagulation. This allows for active involvement of the red blood cell, white blood cell, platelet and plasma components of coagulation. Additionally, TEG measures the rate of formation, stabilization and lysis of the clot, to give a more complete picture of the coagulation status of the patient. PT-INR specifically measures factors II, V, VII, X and fibrinogen activity using platelet-poor plasma, which have been previously shown to decrease in the setting of a partial hepatectomy.22
The transient decreases in fibrinogen and platelet counts suggest the formation of fibrin–platelet complexes, and the successful formation of a blood clot. Both fibrinogen and platelets are inflammatory markers, and both demonstrate a subsequent rise consistent with a post-operative pro-inflammatory state.
Normal coagulation is likely maintained by a combination of factors. While poorly understood, it is commonly accepted that a pro-coagulant state occurs after major abdominal surgery. In addition, the decreased synthetic function of the liver after a partial hepatectomy leads to a concomitant decrease in both pro- and anti-coagulant factors. Additionally, previous studies have demonstrated a transient increase in factor VIII and von Willenbrand's factor, which are not synthesized in the liver.22 In decreasing all hepatically synthesized factors concurrently, and up-regulating non-hepatically synthesized factors, the coagulation system appears to maintain a near-normal coagulation.
With regard to the use of FFP in the treatment of presumed coagulopathy associated with elevation of the PT-INR, four patients were transfused with FFP post-operatively in the present study. Two of the patients had both TEG and complete coagulation assays performed on the morning prior to the transfusion of FFP (Table 4), and received plasma solely for elevated INR and concern for bleeding risk. In spite of an elevated PT-INR in both patients at the time of the morning lab draws and in the afternoon prompting a transfusion, neither demonstrated evidence of coagulopathy by TEG. Of these two patients, one actually had an aPTT value that was below normal limits, suggesting hypercoagulability, and was found to have an incidentally noted sub-segmental PE on chest CT approximately 3 months after a partial hepatectomy. No patients in the study had clinical evidence of persistent bleeding owing to coagulopathy.
After a partial hepatectomy, thromboembolic prophylaxis is frequently delayed based on the belief that an elevated PT-INR is associated with ‘auto-prophylaxis’ against thromboembolism. The results presented suggest that in spite of an elevated PT-INR, these patients maintained a normal coagulation function, and did not have an inherent coagulopathy that would preclude the formation of deep vein thrombosis. In fact, of the two patients with VTE, both demonstrated evidence of hypercoagulability on assays drawn concurrently with elevated PT-INR. While these patients did receive thromboembolic prophylaxis prior to the diagnosis of VTE, in both cases there was evidence of hypercoagulability prior to the initiation of LMWH. The median PT-INR peaked at 1.4, which some hepato-biliary surgeons would consider clinically insignificant. It should be noted that this is a median among 35 patients, meaning half of these patients demonstrated a PT-INR above this value, and would represent a group where concern for bleeding may lead to a delay in thromboembolic prophylaxis or treatment with FFP.
Several limitations exist in this study. While it is the largest series directly comparing TEG to PT-INR in patients after a partial hepatectomy, at 35 patients it remains inadequately powered to compare the effects of potential confounders in this patient population. These include usage or duration of the Pringle manoeuver, the presence of pre-existing liver disease, duration of surgery, extent of the resection and blood loss. Enrolment of additional patients would permit a multivariable analysis to analyse the potential effects of these factors. Finally, this study contains a heterogeneous series of operations and indications, both malignant and benign.
In conclusion, patients undergoing a partial hepatectomy for benign and malignant disease demonstrated normal functional coagulation after surgery, in spite of an an elevation in the PT-INR. While PT-INR is a strong indicator of synthetic function of the liver, TEG better characterizes functional coagulation in these patients. As it is an inadequate predictor of functional coagulation, PT-INR should not be used to guide the decision to transfuse FFP or to initiate or delay thromboembolic prophylaxis after a partial hepatectomy.
Acknowledgments
This study was funded by the Medical Research Foundation of Oregon MRF 212.
Conflicts of interest
None declared.
References
- 1.Pelton JJ, Hoffman JP, Eisenberg BL. Comparison of liver function tests after hepatic lobectomy and hepatic wedge resection. Am Surg. 1998;64:408–414. [PubMed] [Google Scholar]
- 2.Weinberg L, Scurrah N, Gunning K, McNicol L. Postoperative changes in prothrombin time following hepatic resection: implications for perioperative analgesia. Anaesth Intensive Care. 2006;34:438–443. doi: 10.1177/0310057X0603400405. [DOI] [PubMed] [Google Scholar]
- 3.Siniscalchi A, Begliomini B, De Pietri L, Braglia V, Gazzi M, Masetti M, et al. Increased prothrombin time and platelet counts in living donor right hepatectomy: implications for epidural anesthesia. Liver Transpl. 2004;10:1144–1149. doi: 10.1002/lt.20235. [DOI] [PubMed] [Google Scholar]
- 4.Mammen EF. Coagulopathies of liver disease. Clin Lab Med. 1994;14:769–780. [PubMed] [Google Scholar]
- 5.Borromeo CJ, Stix MS, Lally A, Pomfret EA. Epidural catheter and increased prothrombin time after right lobe hepatectomy for living donor liver transplantation. Anesth Analg. 2000;91:1139–1141. doi: 10.1097/00000539-200011000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Matot I, Scheinin O, Eid A, Jurim O. Epidural anesthesia and analgesia in liver resection. Anesth Analg. 2002;95:1179–1181. doi: 10.1097/00000539-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Wheatley RG, Schug SA, Watson D. Safety and efficacy of postoperative epidural analgesia. Br J Anaesth. 2001;87:47–61. doi: 10.1093/bja/87.1.47. [DOI] [PubMed] [Google Scholar]
- 8.MacLennan S, Williamson LM. Risks of fresh frozen plasma and platelets. J Trauma. 2006;60(6 Suppl):S46–S50. doi: 10.1097/01.ta.0000199546.22925.31. [DOI] [PubMed] [Google Scholar]
- 9.Park MS, Martini WZ, Dubick MA, Salinas J, Butenas S, Kheirabadi BS, et al. Thromboelastography is a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67:266–275. doi: 10.1097/TA.0b013e3181ae6f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris-Stiff AG, White A, Gomez GD, Toogood G, Lodge JP, Prasad KR. Thrombotic complications following liver resection for colorectal metastases are preventable. HPB. 2008;10:311–314. doi: 10.1080/13651820802074431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy SK, Turley RS, Barbas AS, Steel JL, Tsung A, Marsh JW, et al. Post-operative pharmacologic thromboprophylaxis after major hepatectomy. J Gastrointest Surg. 2011;15:1602–1610. doi: 10.1007/s11605-011-1591-x. [DOI] [PubMed] [Google Scholar]
- 12.Aloia TA, Fahy BN, Fischer CP, Jones SL, Duchini A, Galati J, et al. Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB. 2009;11:510–515. doi: 10.1111/j.1477-2574.2009.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martini WZ, Cortez DS, Dubick MA, Park MS, Holcomb JB. Thromboelastography is better than PT, aPTT, and activated clotting time in detecting clinically relevant clotting abnormalities after hypothermia, hemorrhagic shock, and resuscitation in pigs. J Trauma. 2008;65:535–543. doi: 10.1097/TA.0b013e31818379a6. [DOI] [PubMed] [Google Scholar]
- 14.Cerutti E, Stratta C, Romagnoli R, Schellino MM, Skurzak S, Rizzetto M, et al. Thromboelastogram monitoring in the perioperative period of hepatectomy for adult living liver donors. Liver Transpl. 2004;10:289–294. doi: 10.1002/lt.20078. [DOI] [PubMed] [Google Scholar]
- 15.Kang YG, Martin DJ, Marquez J, Lewis JH, Bontempo FA, Shaw BW, et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888–896. [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson D, Cooke EA, McNally MA, Wilson HK, Yeates A, Mollan RAB. Changes in coagulability as measured by thrombelastography following surgery for proximal femoral fracture. Injury. 2001;32:765–770. doi: 10.1016/s0020-1383(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 17.Bell CR, Cox DJ, Murdock PJ, Sullivan ME, Pasi KJ, Morgan RJ. Thrombelastographic evaluation of coagulation in transurethral prostatectomy. Br J Urol. 1996;78:737–741. doi: 10.1046/j.1464-410x.1996.19313.x. [DOI] [PubMed] [Google Scholar]
- 18.Spiess BD, Tuman KJ, McCarthy RJ, DeLaria GA, Schillo R, Ivankovich AD. Thromboelastography as an indicator of post-cardiopulmonary bypass coagulopathies. J Clin Monit. 1987;3:25–30. doi: 10.1007/BF00770880. [DOI] [PubMed] [Google Scholar]
- 19.Van PY, Cho SD, Underwood SJ, Morris MS, Watters JM, Schreiber MA. Thrombelastography versus antifactor Xa levels in the assessment of prophylactic-dose enoxaparin in critically ill patients. J Trauma. 2009;66:1509–1515. doi: 10.1097/TA.0b013e3181a51e33. [DOI] [PubMed] [Google Scholar]
- 20.Watters JM, Tieu BH, Differding JA, Muller PJ, Schreiber MA. A single bolus of 3% hypertonic saline with 6% dextran provides optimal initial resuscitation after uncontrolled hemorrhagic shock. J Trauma. 2006;61:75–81. doi: 10.1097/01.ta.0000222723.54559.47. [DOI] [PubMed] [Google Scholar]
- 21.Schreiber MA, Differding J, Thorborg P, Mayberry JC, Mullis RJ. Hypercoagulability is most prevalent early after injury and in female patients. J Trauma. 2005;58:475–480. doi: 10.1097/01.ta.0000153938.77777.26. [DOI] [PubMed] [Google Scholar]
- 22.Bezeaud A, Denninger MH, Dondero F, Saada V, Venisse L, Huisse MG, et al. Hypercoagulability after partial liver resection. Thromb Haemost. 2007;98:1252–1256. [PubMed] [Google Scholar]


