Abstract
Background
As the long-term survival of pancreatic head malignancies remains dismal, efforts have been made for a better patient selection and a tailored treatment. Tumour size could also be used for patient stratification.
Methods
One hundred and fourteen patients underwent a pancreaticoduodenectomy for pancreatic adenocarcinoma, peri-ampullary and biliary cancer stratified according to: ≤20 mm, 21–34 mm, 35–45 mm and >45 mm tumour size.
Results
Patients with tumour sizes of ≤20 mm had a N1 rate of 41% and a R1/2 rate of 7%. The median survival was 3.4 years. N1 and R1/2 rates increased to 84% and 31% for tumour sizes of 21–34 mm (P = 0.0002 for N, P = 0.02 for R). The median survival decreased to 1.6 years (P = 0.0003). A further increase in tumour size of 35–45 mm revealed a further increase of N1 and R1/2 rates of 93% (P < 0.0001) and 33%, respectively. The median survival was 1.2 years (P = 0.004). Tumour sizes >45 mm were related to a further decreased median survival of 1.1 years (P = 0.2), whereas N1 and R1/2 rates were 87% and 20%, respectively.
Discussion
Tumour size is an important feature of pancreatic head malignancies. A tumour diameter of 20 mm seems to be the cut-off above which an increased rate of incomplete resections and metastatic lymph nodes must be encountered and the median survival is reduced.
Introduction
Pancreatic head cancer, whether they are pancreatic ductal adenocarcinoma, distal cholangiocarcinoma or ampullary cancer, represents an ongoing diagnostic and therapeutic challenge. Its long-term outcome is still worse than most other gastrointestinal cancers, and has not much improved over the past 20 years.1–4
Modern imaging techniques are used to pre-operatively assess the local resectability of the primary tumour, i.e. invasion of vascular structures, and also to detect distant metastasis. Thereby, precise determination of the tumour size, duodenal infiltration and lymph node involvement often remains limited.5,6 In case of a given operative indication, surgeons largely base their decision of local resectability on radiological information. As segmental resection of the infiltrated portal/mesenteric vein is now routinely performed in many centres, resectability rates could have been greater. Of note, while mortality significantly decreased to < 5%, morbidity rates remain increased up to 50%.7–9 Many centres encounter an increasing number of pancreas resections caused by centralization of major surgery and by demographic changes as pancreatic head cancer has a close, age-related incidence.10–12 In spite of surgery remaining pivotal, adjuvant chemotherapy is an accepted adjunct as its beneficial effects have been proven by the European Study Group for Pancreatic Cancer studies.13,14 Until recently, chemotherapy was based on gemcitabine only but nowadays, more potent combination therapies such as folfirinox are increasingly being used.15,16
In a modern multidisciplinary approach, a precise tumour staging system considering all important risk factors is crucial as it represents the common basis for all specialists involved in the diagnosis and treatment to describe tumour-related issues. The findings of pre-operative imaging should be integrated into tumour classification systems in order to allow a careful patient selection, and an individual and risk-adjusted treatment planning, e.g. patients are excluded from a curative surgical approach if major visceral arteries are encased by the tumour, or neoadjuvant treatment will be indicated to downstage the primary tumour. Post-operatively, a precise tumour classification based on histopathological assessment allows reliable risk estimation for recurrence and survival, and therefore optimized adjuvant or palliative treatment options can be considered.
The TNM system considers infiltration in adjacent tissues and tumour size as criteria to describe primary tumours.17 Of note, the latter criterion is only used in pancreatic adenocarcinoma to define the T1 stage, whereas peri-ampullary and biliary tumours are solely characterized by infiltration of the duodenum and/or vascular structures. As a consequence, the majority of tumours are classified as T3.18–21 This patient group is probably very heterogeneous and, for practical reasons, it would be helpful to have other criteria, that are easy to assess, to further distinguish these patients.
Tumour size could have the potential to be used as such criteria as it can be considered as a surrogate parameter for tumour volume. Based on computational models, an increased tumour mass could impact on the occurrence of metastatic lymph nodes, resection margins and, finally, long-term survival.22 Therefore, the present study aimed to assess the impact of tumour size as an independent risk factor on the pre-operative predictability of lymph node metastasis and completeness of surgical resections (R0 versus R1/2) of pancreatic head cancer. In addition, long-term survival related to tumour size after a resection was also evaluated.
Methods
Patients and data collection
From our pancreas database containing 288 pancreatectomies between January 2000 and June 2011, 114 patients were identified who underwent a pancreaticoduodenectomy for pancreatic, ampullary and distal bile duct adenocarcinomas at the Department of Visceral Surgery, University Hospital of Lausanne (CHUV), Lausanne, Switzerland. Patients who were operated from 2000 to 2008 were retrospectively entered whereas since 2009 patients' data have been collected prospectively. There were 86 pancreatic ductal adenocarcinoma, 20 distal cholangiocarcinoma and 8 ampullary carcinoma. Not included were all cases of duodenal cancer, resections for benign diseases, patients with a total pancreatectomy and pancreatic left resection, as well as cases without clear determination of tumour size on pathological examination, mainly in the case of a tumour not expanding as a bulky mass but spreading tumours, e.g. malignant intraductal papillary mucinous neoplasia of the pancreas and distal cholangiocarcinoma. Pancreatic resections were always performed as a classic pancreaticoduodenectomy (no pylorus preservation) with standard lymph node dissection (anterior and posterior pancreaticoduodenal lymph nodes, nodes in the lower hepatoduodenal ligament and nodes along the right aspect of the superior mesenteric artery and vein).23 Survival data were obtained by review of hospital records, local tumour registry or by directly contacting the individual patient. Informed consent was obtained from all patients. The study was approved by the local Ethics Committee.
Definition of tumour size and groups
All tumour sizes were obtained from histopathological reports that always include measurement of the two greatest axis of the tumour on the fixated specimen. Formalin fixation is known to have a shrinkage effect on tissues. This shrinkage is lower for tumour tissue which contains less water and is estimated to be 10% less in vitro than in vivo.24 The larger diameter was considered as the reference value of the tumour size. Four groups of tumour size were defined. First, in a similar way to the American Joint Committee on Cancer (AJCC) classification, group 1 was defined as a tumour size of ≤20 mm. Many patients had tumours of about 30 mm, reflecting an arithmetic mean and a median tumour size of 32 and 30 mm, respectively of the entire cohort, thus all these patients were included in group 2 (21 mm to 34 mm). Group 3 comprised tumors measuring 35 mm to 45 mm. A fourth group of large tumors with a diameter > 45 mm was labelled as group 4. This way of grouping created two similar groups around the largest group of tumours of about 30 mm and the addition of a fourth group of large tumours (Fig. 1).
Figure 1.
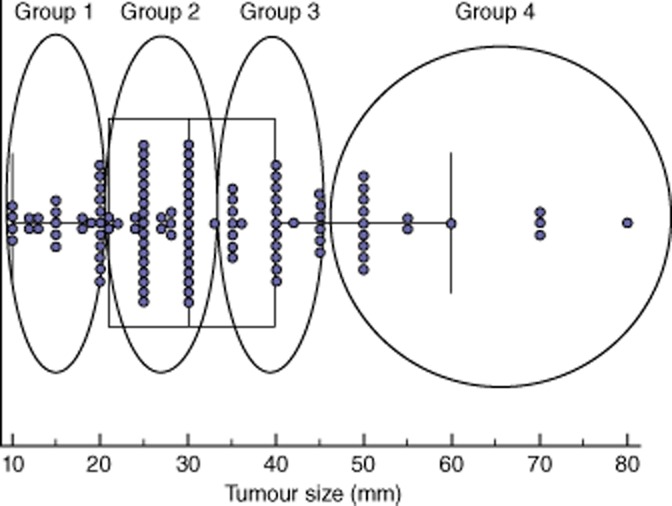
Distribution of tumour size in 114 resected specimens of pancreatic head tumours
AJCC staging and pathological parameters
Fifth, sixth and seventh editions of AJCC Cancer Staging Manuals were used as standards throughout the 12-year study period.17,25,26 Because of the modification of the T stage definition between the fifth and the sixth edition, pathology reports were revised and redefined according to the seventh edition. The seventh edition of the AJCC was also used for the prognostic group staging (stage IA, IB, IIA, IIB, III and IV). R status of the resection was recorded and defined as R0 if no tumour was present at the resection margin and R1 if margins contained microscopic tumour residue.
Data analysis
The correlation of tumour size with pathological results (N and R status) was assessed with stratification between predefined size groups. The chi-square test and Fisher's exact test were used on univariate analysis to detect the ability of tumour size to predict N positivity or R1 resection. Comparison of pathological characteristics between T3 tumours and the other T stage was also performed. The Mann–Whitney test and samples t-test were used to compare non-parametric independent samples. The impact of tumour size on survival was analysed and compared with AJCC prognostic groups. The median survival and overall survival rates were determined using Kaplan–Meier survival curves. Overall survival was defined as time span from the index operation to the date of death due to any reason. The log-rank test was used for comparison of different variables. Differences were considered significant at P < 0.05. The median follow-up time was 1.4 years. Statistics were performed using MedCalc software (MedCalc software, Mariakerke, Belgium).
Results
Patients' characteristics and peri-operative data of the 66 men and 48 women are shown in Table 1. The details of histopathological assessment with special emphasize on tumour size and staging are shown in Table 2. The majority of tumours were staged as T3 (73 of 114 specimen, 64%) and 78 patients (68%) were classified in the IIB AJJCC prognostic groups. The tumour size group classification showed a more equilibrated repartition of resected specimens between groups with the maximal number of cases in the group 2 (21–34 mm) containing 45 specimens (39.5%). The median and mean tumour sizes were 30 mm (range, 10–80 mm) and 32 ± 14 mm, respectively. Pancreatic adenocarcinoma were larger (median size of 30 mm) than ampullary adenocarcinoma and distal cholangiocarcinoma with median sizes of 15 mm (P = 0.0002) and 20 mm (P = 0.002), respectively. Overall, 87 specimens showed a metastatic lymph node (76.3%) and 86 resections were R0 (75.4%).
Table 1.
Patients characteristics and peri-operative results
| Gender | |
| Male | 66 |
| Female | 48 |
| Age (years), median (range) | 68 (39–85) |
| BMI (kg/m2), mean ± SD | 24 ± 3 |
| ASA score | |
| 1 | 8 (7.0%) |
| 2 | 76 (66.7%) |
| 3 | 27 (23.7%) |
| 4 | 3 (2.6%) |
| Mean operative time ± SD (min) | 352 ± 86 |
| Length of hospital stay (days) | |
| Median (range) | 18 (8–85) |
| Mortality (30 days and inhospital) | 6 (5.3%) |
| Morbidity (30 days and inhospital) | 67 (61.5%) |
Data are expressed as number of patients when not other indication.
BMI, Body Mass Index
ASA, American Society of Anesthesiologist
SD, standard deviation.
Table 2.
TNM classification, histopathological results and tumour size
| Overall (n = 114) | Pancreatic adenocarcinoma (n = 86 ) | Ampullary carcinoma (n = 20 ) | Distal bile duct adenocarcinoma (n = 8 ) | |
|---|---|---|---|---|
| TNM | ||||
| T1 | 4 | 3 | 1 | 0 |
| T2 | 29 | 21 | 6 | 2 |
| T3 | 73 | 58 | 9 | 6 |
| T4 | 8 | 4 | 4 | 0 |
| N0 | 27 (23.7%) | 14 (16.3%) | 10 | 3 |
| N1 | 87 (76.3%) | 72 (83.7%) | 10 | 5 |
| M1 | 2 | 2 | 0 | 0 |
| Stage | ||||
| IA | 3 | 2 | 1 | 0 |
| IB | 9 | 3 | 5 | 1 |
| IIA | 14 | 9 | 3 | 2 |
| IIB | 78 | 66 | 7 | 5 |
| III | 8 | 4 | 4 | 0 |
| IV | 2 | 2 | 0 | 0 |
| R status | ||||
| R0 | 86 (75.4%) | 27 (68.6%) | 20 | 7 |
| R1 | 28 (24.6%) | 59 (31.4%) | 0 | 1 |
| Tumour size (mm) | ||||
| Mean ± SD | 32 ± 14 | 34 ± 13 | 23 ± 16 | 23 ± 8 |
| Median (range) | 30 (10–80) | 30 (18–80) | 15 (10–70) | 20 (15–40) |
| Tumour size | ||||
| ≤20 mm | 27 (23.7%) | 10 | 12 | 5 |
| 21–34 mm | 45 (39.5%) | 40 | 3 | 2 |
| 35–45 mm | 27 (23.7%) | 23 | 3 | 1 |
| >45 mm | 15 (13.2%) | 13 | 2 | 0 |
SD, standard deviation.
Analysis of the large group of T3 tumours containing 73 specimens, showed that mean and median tumour size, N stage, R status and repartition of the specimen into the four size groups did not differ between T3 tumours and other stages of tumours (Table 3). This observation shows that T3 tumours have the same histopathological characteristics compared with other T stage specimens. Thus, T3 tumours are a heterogeneous group in which risk factors for outcome could be sort out.
Table 3.
Comparison between T3 tumours and other T stages
| T3 stage (n = 73) | T1, T2 and T4 stages (n = 41 ) | P-value | |
|---|---|---|---|
| Tumour size (mm) | |||
| Mean ± SD | 32 ± 14 | 30 ± 15 | 0.475a |
| Median (range) | 30 (10–80) | 28 (10–70) | 0.343b |
| N stage | |||
| N0 | 14 (19.2%) | 13 (31.7%) | 0.169c |
| N1 | 59 (80.8%) | 28 (68.3%) | |
| R status | |||
| R0 | 53 (72.6%) | 33 (80.5%) | 0.376c |
| R1 | 20 (27.4%) | 8 (19.5%) | |
| Tumour size | |||
| ≤20 mm | 15 (20.5%) | 12 (29.3%) | 0.715c |
| 21–34 mm | 29 (39.7%) | 16 (39.0%) | |
| 35–45 mm | 19 (26.0%) | 8 (19.5%) | |
| >45 mm | 10 (13.7%) | 5 (12.2%) |
Independent samples T-test.
Mann–Whitney test.
Fisher's exact test.
To analyse the value of tumour size compared with T stage as a marker of tumour aggressiveness or severity, correlations between these two parameters and N and R status were performed (Table 4). N positivity was significantly lower in tumours with a diameter ≤20 mm compared with larger tumours with 40.7%; N1 in group 1 versus 84.4%, 92.6% and 86.7% in groups 2, 3 and 4, respectively (P < 0.001, P < 0.001 and P = 0.008). Positive resections margins were also more frequent in larger tumours than in tumours of group size 1. T staging also correlated well with N and R positivity. T3 tumours of a diameter ≤20 mm had 53.3% of a risk to be N1 compared with 86.2%, 89.5% and 90.0% of T3 tumours of group size 2, 3 and 4, respectively (P = 0.028, P = 0.025 and P = 0.088). The probability of positive resection margins was also higher for large T3 tumours than small T3 tumours, but without reaching significance (Table 4).
Table 4.
Correlation between tumour characteristics and N and R status
| N0 | N1 | %N1 | P-value | R0 | R1 | %R1 | P-value | |
|---|---|---|---|---|---|---|---|---|
| Overall | 27 | 87 | 76.3 | 86 | 28 | 24.6 | ||
| Tumour size | <0.001b | 0.085b | ||||||
| ≤ 20 mm | 16 | 11 | 40.7 | 25 | 2 | 7.4 | ||
| 21–34 mm | 7 | 38 | 84.4 | <0.001a | 31 | 14 | 31.1 | 0.021a |
| 35–45 mm | 2 | 25 | 92.6 | <0.001a | 18 | 9 | 33.3 | 0.039a |
| >45 mm | 2 | 13 | 86.7 | 0.008a | 12 | 3 | 20.0 | 0.329b |
| TNM T stage | 0.045b | 0.101b | ||||||
| T1 | 3 | 1 | 25.0 | 4 | 0 | 0 | ||
| T2 | 9 | 20 | 69.0 | 0.125a | 25 | 4 | 13.8 | 1.000a |
| T3 | 14 | 59 | 80.8 | 0.032a | 53 | 20 | 27.4 | 0.568a |
| T4 | 1 | 7 | 87.5 | 0.067a | 4 | 4 | 50.0 | 0.208a |
| T3 stage | 0.025b | 0.519b | ||||||
| ≤20 mm | 7 | 8 | 53.3 | 13 | 2 | 13.3 | ||
| 21–34 mm | 4 | 25 | 86.2 | 0.028a | 19 | 10 | 34.5 | 0.171a |
| 35–45 mm | 2 | 17 | 89.5 | 0.025a | 14 | 5 | 26.3 | 0.426a |
| >45 mm | 1 | 9 | 90.0 | 0.088a | 7 | 3 | 30.0 | 0.358a |
Fisher's exact test.
Chi-square test.
The overall median survival was 1.8 years and 1-, 3- and 5-year survival were 71.3%, 30.2% and 17.5%, respectively. N stage, R status and tumour size were a significant prognostic factor of survival on univariate analysis, whereas AJCC prognostic groups and T stages showed a tendency to predict survival, without statistical significance (Table 5 and Fig. 2). Tumours of ≤20 mm had a better survival than larger tumours (median survival of 3.4 versus 1.3 years, P < 0.001). On multivariate analysis, a R0 resection was the only significant predictor of survival [hazard ratio (HR) 0.57, P = 0.037] whereas a tumour size ≤20 mm did not reach significance (HR 0.52, P = 0.071) (Table 6). The cut-off of ≤20 mm was also a significant factor of better survival for T3 tumours (median survival of 2.9 years for T3 tumours of ≤20 mm compared with 1.3 years for T3 tumours larger than 20 mm, P = 0.019), whereas N stage and R status were not significant predictors of long-term survival in the large group of T3 tumours (Table 7 and Fig. 3).
Table 5.
Survival after a pancreaticoduodenectomy
| N | Median survival (years) | 1-year survival (%) | 3-year survival (%) | 5-year survival (%) | P-valuea | |
|---|---|---|---|---|---|---|
| Overall | 103 | 1.8 | 71.3 | 30.2 | 17.5 | |
| AJCC stage | 0.062 | |||||
| IA | 3 | 5.4 | 100.0 | 100.0 | 66.8 | |
| IB | 9 | 3.4 | 100.0 | 53.4 | 35.7 | |
| IIA | 13 | 2.9 | 77.0 | 43.1 | 43.1 | |
| IIB | 68 | 1.7 | 68.9 | 26.5 | 7.8 | |
| III | 8 | 0.8 | 37.5 | 12.5 | 0 | |
| IV | 2 | 1.4 | 100.0 | 0 | 0 | |
| T stage | 0.076 | |||||
| T1 | 4 | 5.4 | 100.0 | 100.0 | 66.8 | |
| T2 | 26 | 2.2 | 84.7 | 35.2 | 17.5 | |
| T3 | 65 | 1.6 | 69.4 | 25.1 | 13.5 | |
| T4 | 8 | 0.8 | 37.5 | 12.5 | 0 | |
| Tumour size | 0.006 | |||||
| ≤20 mm | 26 | 3.4 | 96.2 | 52.3 | 40.6 | |
| 21–34 mm | 38 | 1.6 | 73.4 | 19.5 | 0 | |
| 35–45 mm | 26 | 1.2 | 49.9 | 18.4 | 6.2 | |
| >45 mm | 13 | 1.1 | 53.7 | 43.0 | 43.0 | |
| Tumour size | <0.001 | |||||
| ≤20 mm | 23 | 3.4 | 96.2 | 52.3 | 40.6 | |
| >20 mm | 77 | 1.3 | 62.4 | 21.7 | 8.2 | |
| N stage | 0.005 | |||||
| N0 | 26 | 3.4 | 88.3 | 53.5 | 41.2 | |
| N1 | 77 | 1.6 | 65.7 | 22.1 | 8.5 | |
| R status | 0.008 | |||||
| R0 | 26 | 2.2 | 76.6 | 36.9 | 23.4 | |
| R1 | 77 | 1.2 | 57.4 | 13.3 | 0 |
Log-rank test.
Figure 2.
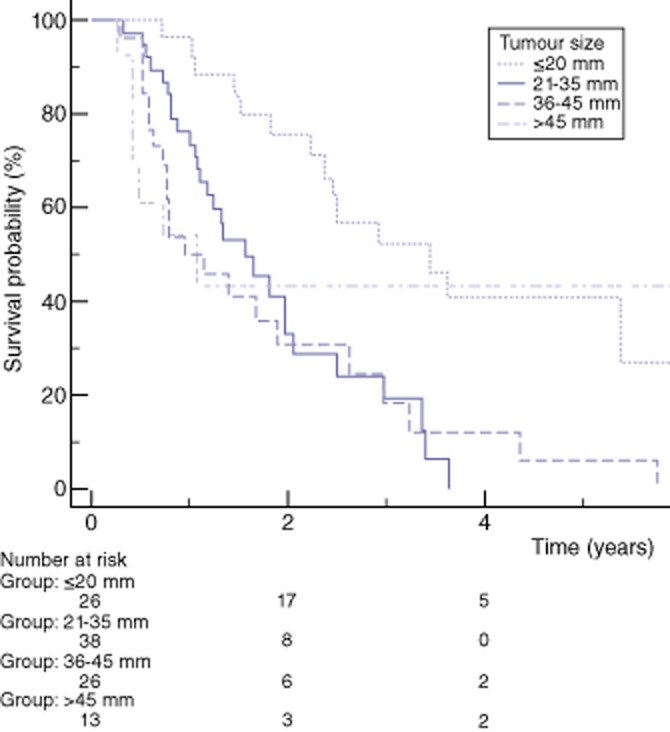
Kaplan–Meier estimated overall survival stratified by tumour size
Table 6.
Multivariate analysis of predictors for long-term survival
| Parameter | Subgroup | Hazard ratio (95% CI) | P-value |
|---|---|---|---|
| Tumour size (mm) | ≤20 mm, > 20 mm | 0.52 (0.25–1.05) | 0.071 |
| N stage | N0, N1 | 0.77 (0.37–0.96) | 0.485 |
| R status | R0, R1 | 0.57 (0.33–0.96) | 0.037 |
Table 7.
Survival after a pancreaticoduodenectomy for T3 tumours
| N | Median survival (years) | 1-year survival (%) | 3-year survival (%) | 5-year survival (%) | P-valuea | |
|---|---|---|---|---|---|---|
| Tumour size | 0.115 | |||||
| ≤20 mm | 14 | 2.9 | 92.7 | 44.8 | 44.8 | |
| 21–34 mm | 24 | 1.3 | 70.8 | 17.8 | 0 | |
| 35–45 mm | 18 | 1.7 | 61.1 | 27.7 | 9.2 | |
| >45 mm | 9 | 0.7 | 44.4 | 22.1 | 22.1 | |
| Tumour size | 0.019 | |||||
| ≤20 mm | 14 | 2.9 | 92.8 | 44.9 | 44.9 | |
| >20 mm | 51 | 1.3 | 62.5 | 18.8 | 6.2 | |
| N stage | 0.115 | |||||
| N0 | 13 | 2.9 | 76.9 | 43.3 | 43.3 | |
| N1 | 52 | 1.6 | 67.4 | 21.6 | 7.0 | |
| R status | 0.109 | |||||
| R0 | 47 | 1.6 | 72.2 | 31.3 | 19.6 | |
| R1 | 18 | 1.0 | 61.0 | 8.2 | 0 |
Log-rank test.
Figure 3.
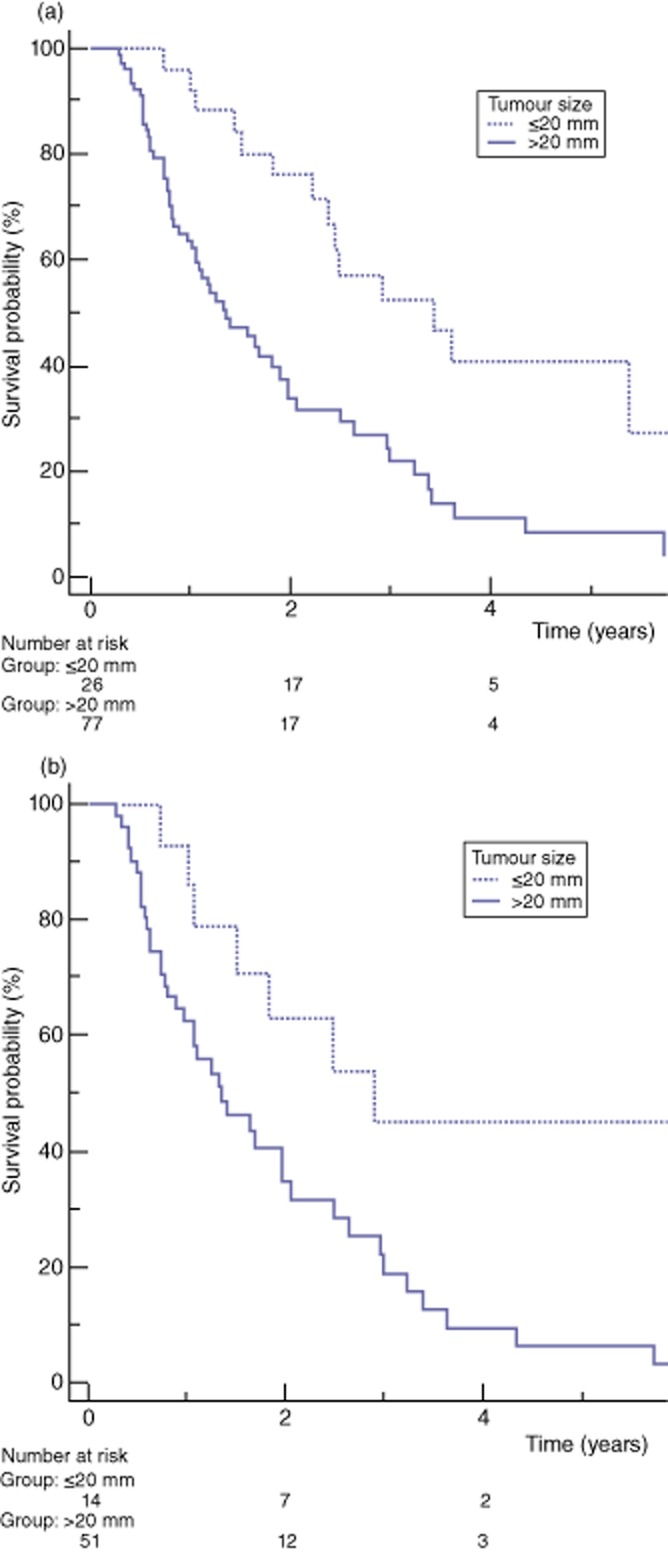
Kaplan–Meier estimated overall survival for tumour size ≤20 mm versus >20 mm. (a) All tumours. (b) T3 tumours only Definition of four size groups: Group 1: ≤20 mm; Group 2: 21–34 mm; Group 3: 35–45 mm; Group 4: > 45 mm
The same analysis was performed taking into account pancreatic ductal adenocarcinomas only (Table 8). The risk of a positive lymph node correlated well with a tumour size of pancreatic ductal adenocarcinoma as there were 50.0% N1 in group 1 versus 85.0%, 95.7% and 84.6% in group 2,3 and 4, respectively (P = 0.030, P = 0.005 and P = 0.169). The percentage of R1 resections was lower for pancreatic ductal adenocarcinomas of 20 mm or less than larger pancreatic ductal adenocarcinomas but this was not significant. The median survival after resection of pancreatic ductal adenocarcinomas of ≤20 mm was 6.9 years compared with 1.3 years for tumours larger than 20 mm (P = 0.021). The N stage was also a significant factor of survival (median survival of 3.4 years for N0 tumors compared with 1.3 years for N1, P = 0.047), whereas R0 resections showed a median survival of 1.6 years compared with 1.2 years after R1 resections (P = 0.065). Looking at T3 pancreatic ductal adenocarcinoma seemed to show the same tendency, but without reaching significance probably owing to the lower number of patients in each groups (Table 8).
Table 8.
Subanalysis of pancreatic ductal adenocarcinoma (n = 86)
| %N1 | P-value | %R1 | P-value | Median survival (years) | P-valuec | |
|---|---|---|---|---|---|---|
| Overall | 83.7 | 31.4 | 1.4 | |||
| Tumour size | 0.013b | 0.843b | 0.136 | |||
| ≤20 mm | 50.0 | 20.0 | 6.9 | |||
| 21–34 mm | 85.0 | 0.030a | 32.5 | 0.702a | 1.6 | 0.011 |
| 35–45 mm | 95.7 | 0.005a | 39.1 | 0.430a | 1.2 | 0.01 |
| >45 mm | 84.6 | 0.169a | 23.7 | 1.000a | 1.1 | 0.347 |
| Tumour size | 0.009b | 0.495b | 0.021 | |||
| ≤20 mm | 50.0 | 20.0 | 6.9 | |||
| >20 mm | 88.2 | 32.9 | 1.3 | |||
| N0 | 3.4 | |||||
| N1 | 1.3 | 0.047 | ||||
| R0 | 1.6 | |||||
| R1 | 1.2 | 0.065 | ||||
| T3 stage | 0.074b | 0.878b | 0.430 | |||
| ≤20 mm | 57.1 | 28.6 | 6.9 | |||
| 21–34 mm | 84.0 | 0.157a | 36.0 | 1.000a | 1.3 | 0.068 |
| 35–45 mm | 93.8 | 0.067a | 31.3 | 1.000a | 3.0 | 0.204 |
| >45 mm | 90.0 | 0.250a | 30.0 | 1.000a | 0.8 | 0.266 |
| ≤20 mm | 57.1 | 28.6 | 6.9 | |||
| >20 mm | 88.2 | 0.068a | 33.3 | 1.000a | 1.3 | 0.129 |
| N0 | 1.2 | |||||
| N1 | 1.3 | 0.277 | ||||
| R0 | 1.3 | |||||
| R1 | 2.0 | 0.234 |
Fisher's exact test.
Chi-square test.
Logrank test.
Discussion
Classification items of the AJCC staging system have been validated to predict outcome after resection of pancreas head malignancy,27 but many other factors also impact on long-term survival.28 Tumour size is of particular interest because it could be determined on pre-operative imaging modalities. The present study showed that tumour size of peri-ampullary tumours was a good indicator of tumour aggressiveness, i.e. predicting the probability of metastatic lymph nodes and resection margins, and correlated with long-term survival.
The role of tumour size as a possible prognostic factor for survival has already been assessed, and the most reported size cut-offs ranged from 20 to 30 mm.23,28–37 However, possible correlations between tumour size and other risk factors impacting on survival are largely unknown. Our finding that a larger tumour size was associated with a higher incidence of metastatic lymph nodes and positive resection margins at histopathological examination was at least partially confirmed by a recent study from De Jong et al. While they found that the cut-off value of 20 mm was not associated with a poor outcome, tumour size was strongly associated with the risk of other adverse prognostic factors.36
In our series, the risk for metastatic lymph nodes and positive resection margins was 40% and 7%, in the case of a tumour size ≤20 mm compared with > 80% and > 30% for larger tumours, respectively. The cut-off of 20 mm was highly significant predicting long-term survival with a median survival of 3.4 years for tumours ≤20 mm compared with 1.3 years for tumours >20 mm. As in many other series, the subgroup of T3 tumours comprised the majority of the resected tumours.18–21 Interestingly, the cut-off of 20 mm was a determinant risk factor for T3 tumours; and the long-term survival of T3 tumors ≤20 mm was 2.9 years compared with 1.3 years for T3 tumours > 20 mm.
In order to overcome the shortcomings of the TNM system, a specific nomogram including various tumour-related factors and clinical factors has been developed. As it is based on risk factors that are only known post-operatively, adapting the pre-operative treatment is not possible.18,20 However, based on computational models, the probability to harbour metastasis at an initial diagnosis was 28% for patients with a tumour of 10 mm, compared with 73% and 94% for a tumour of 20 and 30 mm, respectively.22 Therefore, the pre-operative estimated tumour size with its risk to be N positive and to obtain positive resection margins could be crucial information to develop an individualized pre-operative tumour treatment plan. This latter aspect will gain more importance as there is increasing evidence favouring neoadjuvant treatment using a modern chemotherapeutic regimen and radiotherapy.22,38–40
Pre-operative tumour size measurement of peri-ampullary tumours remains difficult.41,42 There are good indications that pancreatic adenocarcinoma are well defined with magnetic resonance imaging (MRI) and this modality has been shown to get a better tumour conspicuity than computed tomography (CT).5MRI should be accurate for precise pre-operative tumour measurement. MRI can be used to accurately detect an ampullary carcinoma43 and endosocpic ultrasound has also been shown to be accurate and to have a high agreement with pathology reports.44 The detection and measurement of an extrahepatic cholangiocarcinoma differs if it is periductal, intraductal or mass forming. In our series, mass-forming types were kept for analysis. This type of tumour can be well described and measured with MRI.45
There are some limitations to this present study. First, we included three different tumour entities that may have different characteristics, particularly regarding long-term survival. But in daily clinical practice, the exact tumour type often remains unknown pre-operatively; and the indication to resect a pancreatic head tumour is not dependent on the final histology but rather on local criteria of resectability. In addition, classification of the three tumour entities is very similar. Second, patients operated early in the series were assessed retrospectively. As the annual number of pancreatic head resections has markedly increased during recent years, most patients were prospectively documented. Third, we used the AJCC definition for R0, meaning that only completely tumor-free resection margins are considered as R0. This definition has recently been challenged and, nowadays, many centres re-define R0 as tumour cells detected up to 1 mm to the resection margins.46 As a consequence, the role of R0 status is currently re-evaluated, and its role as a prognostic factor may change.
In conclusion, tumour size is an important but underestimated risk factor to stratify patients at risk for positive lymph nodes and resection margins. It could be easily assessed pre-operatively in individual patients and may be helpful to tailor oncological treatment for patients with pancreatic head cancer.
Conflicts of interest
The authors declare no conflicts of interest or sources of funding for this research.
References
- 1.Winter JM, Brennan MF, Tang LH, D'Angelica MI, Dematteo RP, Fong Y, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19:169–175. doi: 10.1245/s10434-011-1900-3. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winter JM, Cameron JL, Olino K, Herman JM. Jong MC, Hruban RH, et al. Clinicopathologic analysis of ampullary neoplasms in 450 patients: implications for surgical strategy and long-term prognosis. J Gastrointest Surg. 2010;14:379–387. doi: 10.1007/s11605-009-1080-7. [DOI] [PubMed] [Google Scholar]
- 4.Choi SB, Park SW, Kim KS, Choi JS, Lee WJ. The survival outcome and prognostic factors for middle and distal bile duct cancer following surgical resection. J Surg Oncol. 2009;99:335–342. doi: 10.1002/jso.21238. [DOI] [PubMed] [Google Scholar]
- 5.Park HS, Lee JM, Choi HK, Hong SH, Han JK, Choi BI. Preoperative evaluation of pancreatic cancer: comparison of gadolinium-enhanced dynamic MRI with MR cholangiopancreatography versus MDCT. J Magn Reson Imaging. 2009;30:586–595. doi: 10.1002/jmri.21889. [DOI] [PubMed] [Google Scholar]
- 6.Tamm EP, Balachandran A, Bhosale PR, Katz MH, Fleming JB, Lee JH, et al. Imaging of pancreatic adenocarcinoma: update on staging/resectability. Radiol Clin North Am. 2012;50:407–428. doi: 10.1016/j.rcl.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Schafer M, Mullhaupt B, Clavien PA. Evidence-based pancreatic head resection for pancreatic cancer and chronic pancreatitis. Ann Surg. 2002;236:137–148. doi: 10.1097/00000658-200208000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teh SH, Diggs BS, Deveney CW, Sheppard BC. Patient and hospital characteristics on the variance of perioperative outcomes for pancreatic resection in the United States: a plea for outcome-based and not volume-based referral guidelines. Arch Surg. 2009;144:713–721. doi: 10.1001/archsurg.2009.67. [DOI] [PubMed] [Google Scholar]
- 9.McPhee JT, Hill JS, Whalen GF, Zayaruzny M, Litwin DE, Sullivan ME, et al. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007;246:246–253. doi: 10.1097/01.sla.0000259993.17350.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128–2137. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topal B, Van de Sande S, Fieuws S, Penninckx F. Effect of centralization of pancreaticoduodenectomy on nationwide hospital mortality and length of stay. Br J Surg. 2007;94:1377–1381. doi: 10.1002/bjs.5861. [DOI] [PubMed] [Google Scholar]
- 12.de Wilde RF, Besselink MG. Tweel I. Hingh IH. Eijck CH, Dejong CH, et al. Impact of nationwide centralization of pancreaticoduodenectomy on hospital mortality. Br J Surg. 2012;99:404–410. doi: 10.1002/bjs.8664. [DOI] [PubMed] [Google Scholar]
- 13.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 14.Neoptolemos JP, Stocken DD, Tudur Smith C, Bassi C, Ghaneh P, Owen E, et al. Adjuvant 5-fluorouracil and folinic acid vs observation for pancreatic cancer: composite data from the ESPAC-1 and -3(v1) trials. Br J Cancer. 2009;100:246–250. doi: 10.1038/sj.bjc.6604838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 16.Trouilloud I, Dubreuil O, Boussaha T, Lepere C, Landi B, Zaanan A, et al. Medical treatment of pancreatic cancer: new hopes after 10 years of gemcitabine. Clin Res Hepatol Gastroenterol. 2011;35:364–374. doi: 10.1016/j.clinre.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Byrd DR, Compton CC, Fritz AG, Green FL, Trotti A. AJCC Cancer Staging Manual. 7th edn. Chicago: Springer; 2010. [Google Scholar]
- 18.Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–298. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helm J, Centeno BA, Coppola D, Melis M, Lloyd M, Park JY, et al. Histologic characteristics enhance predictive value of American Joint Committee on Cancer staging in resectable pancreas cancer. Cancer. 2009;115:4080–4089. doi: 10.1002/cncr.24503. [DOI] [PubMed] [Google Scholar]
- 20.Ferrone CR, Kattan MW, Tomlinson JS, Thayer SP, Brennan MF, Warshaw AL. Validation of a postresection pancreatic adenocarcinoma nomogram for disease-specific survival. J Clin Oncol. 2005;23:7529–7535. doi: 10.1200/JCO.2005.01.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartwig W, Hackert T, Hinz U, Gluth A, Bergmann F, Strobel O, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2011;254:311–319. doi: 10.1097/SLA.0b013e31821fd334. [DOI] [PubMed] [Google Scholar]
- 22.Haeno H, Gonen M, Davis MB, Herman JM, Iacobuzio-Donahue CA, Michor F. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell. 2012;148:362–375. doi: 10.1016/j.cell.2011.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeo CJ. The Johns Hopkins experience with pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma. J Gastrointest Surg. 2000;4:231–232. doi: 10.1016/s1091-255x(00)80070-0. [DOI] [PubMed] [Google Scholar]
- 24.Hsu PK, Huang HC, Hsieh CC, Hsu HS, Wu YC, Huang MH, et al. Effect of formalin fixation on tumor size determination in stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:1825–1829. doi: 10.1016/j.athoracsur.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Fleming ID, Cooper JS, Henson DE, Hutter RV, Kennedy BJ, Murphy GP, et al. AJCC Cancer Staging Manual. 5th edn. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 26.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al. AJCC Cancer Staging Manual. 6th edn. Chicago: Springer; 2002. [Google Scholar]
- 27.Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110:738–744. doi: 10.1002/cncr.22852. [DOI] [PubMed] [Google Scholar]
- 28.Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP. 2008;9:99–132. [PubMed] [Google Scholar]
- 29.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. doi: 10.1097/00000658-199709000-00004. ; discussion 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benassai G, Mastrorilli M, Quarto G, Cappiello A, Giani U, Forestieri P, et al. Factors influencing survival after resection for ductal adenocarcinoma of the head of the pancreas. J Surg Oncol. 2000;73:212–218. doi: 10.1002/(sici)1096-9098(200004)73:4<212::aid-jso5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 31.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takai S, Satoi S, Toyokawa H, Yanagimoto H, Sugimoto N, Tsuji K, et al. Clinicopathologic evaluation after resection for ductal adenocarcinoma of the pancreas: a retrospective, single-institution experience. Pancreas. 2003;26:243–249. doi: 10.1097/00006676-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. ; discussion 1210–1191. [DOI] [PubMed] [Google Scholar]
- 34.Chen JW, Bhandari M, Astill DS, Wilson TG, Kow L, Brooke-Smith M, et al. Predicting patient survival after pancreaticoduodenectomy for malignancy: histopathological criteria based on perineural infiltration and lymphovascular invasion. HPB. 2010;12:101–108. doi: 10.1111/j.1477-2574.2009.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jamieson NB, Denley SM, Logue J, Mackenzie DJ, Foulis AK, Dickson EJ, et al. A prospective comparison of the prognostic value of tumor- and patient-related factors in patients undergoing potentially curative surgery for pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2011;18:2318–2328. doi: 10.1245/s10434-011-1560-3. [DOI] [PubMed] [Google Scholar]
- 36.de Jong MC, Li F, Cameron JL, Wolfgang CL, Edil BH, Herman JM, et al. Re-evaluating the impact of tumor size on survival following pancreaticoduodenectomy for pancreatic adenocarcinoma. J Surg Oncol. 2011;103:656–662. doi: 10.1002/jso.21883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayo SC, Nathan H, Cameron JL, Olino K, Edil BH, Herman JM, et al. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer. 2012;118:2674–2681. doi: 10.1002/cncr.26553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heinrich S, Schafer M, Weber A, Hany TF, Bhure U, Pestalozzi BC, et al. Neoadjuvant chemotherapy generates a significant tumor response in resectable pancreatic cancer without increasing morbidity: results of a prospective phase II trial. Ann Surg. 2008;248:1014–1022. doi: 10.1097/SLA.0b013e318190a6da. [DOI] [PubMed] [Google Scholar]
- 40.Papalezova KT, Tyler DS, Blazer DG, 3rd, Clary BM, Czito BG, Hurwitz HI, et al. Does preoperative therapy optimize outcomes in patients with resectable pancreatic cancer? J Surg Oncol. 2012;106:111–118. doi: 10.1002/jso.23044. [DOI] [PubMed] [Google Scholar]
- 41.Tummala P, Junaidi O, Agarwal B. Imaging of pancreatic cancer: an overview. J Gastrointest Oncol. 2011;2:168–174. doi: 10.3978/j.issn.2078-6891.2011.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shrikhande SV, Barreto SG, Goel M, Arya S. Multimodality imaging of pancreatic ductal adenocarcinoma: a review of the literature. HPB. 2012;14:658–668. doi: 10.1111/j.1477-2574.2012.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geier A, Nguyen HN, Gartung C, Matern S. MRCP and ERCP to detect small ampullary carcinoma. Lancet. 2000;356:1607–1608. doi: 10.1016/s0140-6736(05)74455-x. [DOI] [PubMed] [Google Scholar]
- 44.Artifon EL, Couto D, Jr, Sakai P, da Silveira EB. Prospective evaluation of EUS versus CT scan for staging of ampullary cancer. Gastrointest Endosc. 2009;70:290–296. doi: 10.1016/j.gie.2008.11.045. [DOI] [PubMed] [Google Scholar]
- 45.Li N, Liu C, Bi W, Lin X, Jiao H, Zhao P. MRCP and 3D LAVA imaging of extrahepatic cholangiocarcinoma at 3 T MRI. Clin Radiol. 2012;67:579–586. doi: 10.1016/j.crad.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 46.Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93:1232–1237. doi: 10.1002/bjs.5397. [DOI] [PubMed] [Google Scholar]


