Abstract
Background
The aim was to assess the outcome of a total pancreatectomy (TP).
Methods
From 1993 to 2010, 56 patients underwent an elective TP for intraductal papillary mucinous neoplasia (n = 42), endocrine tumours (n = 6), adenocarcinoma (n = 5), metastases (n = 2) and chronic pancreatitis (n = 1). Morbidity and survival were analysed. Long-term survivors were assessed prospectively using quality-of-life (QoL) questionnaires.
Results
Five patients developed gastric venous congestion intra-operatively. Post-operative morbidity and mortality rates were 45% and 3.6%, respectively. An anastomotic ulcer occurred in seven patients, but none after proton pump inhibitor therapy. There were five inappropriate TPs according to definitive pathological examination. Overall 3- and 5-year survival rates were 62% and 55% respectively; five deaths were related to TP (two postoperative deaths, one hypoglycaemia, one ketoacidosis and one anastomotic ulcer). Prospective evaluation of 25 patients found that 14 had been readmitted for diabetes and that all had hypoglycaemia within the past month. The glycated haemoglobin (HbA1c) was 7.8% (6.3–10.3). Fifteen patients experienced weight loss. The QLQ-C30 questionnaire showed a decrease in QoL predominantly because of fatigue and diarrhoea, and the QLQ-PAN26 showed an impact on bowel habit, flatulence and eating-related items.
Discussion
Morbidity and mortality rates of TP are acceptable, although diabetes- and TP-related mortality still occurs. Endocrine and exocrine insufficiency impacts on the long-term quality of life.
Introduction
The first successful total pancreatectomies (TPs) were performed in the early 1940s in the USA.1 Enthusiasm for TP soon emerged, and some centres recommended TP in the treatment of pancreas carcinoma2 to improve long-term survival. Indeed, some surgeons considered that the high recurrence rate of a pancreatic carcinoma after a partial pancreatectomy could be explained in part by the high prevalence of a tumor3 multicentricity requiring total excision of the gland. Moreover, the absence of pancreatic anastomosis was considered as a means to reduce post-operative morbidity related to pancreatic fistulae. However, in studies published in the 1990s, TP has long been associated with unfavourable prejudice with a high peri-operative mortality rate4–6 and no clear benefit of survival.5,6
Nowadays, diffuse pancreatic disease represents the main indications for TP:7 intraductal papillary mucinous neoplasm (IPMN),8,9 end-stage chronic pancreatitis,10–12 neuroendocrine tumours, especially in the setting of multiple endocrine neoplasia or Von Hippel–Lindau disease8,13,14 and more rarely multiple metastatic lesions (mainly from renal cancer).13,14 The management of pancreatic insufficiency has improved over time and TP is now an option that should be considered to treat selected pancreatic diseases.
A few previous studies focused on long-term follow-up and quality of life (QoL) after TP13–15 and concluded that TP is now a viable option with an acceptable QoL. These results should be interpreted in the light of the type of QoL scale used and the indications for surgery. Recent data on technical complications and late pancreatic endocrine and exocrine insufficiency are also lacking therefore the current study was undertaken to evaluate these outcomes in a high-volume centre.
Patients and methods
Patient characteristics
Data of patients undergoing a TP between October 1993 and December 2010 in the department of HPB Surgery, Beaujon Hospital/University Paris VII, France, were collected retrospectively from clinical files. All patients with an elective TP (either one-step TP or completion pancreatectomy after a previous partial resection) were included. Patients who underwent salvage TP (because of severe pancreatic leakage after a first pancreatic resection) were excluded because of the high mortality and morbidity rates associated with this procedure and the potential to impact on the long-term QoL.12,13
Demographic data, indications for TP, peri-operative data (such as surgical technique, hospital mortality, morbidity and reoperation) and pathological examination results were recorded. A ‘pre-operative diagnosis’ was established according to imaging findings using thin slice contrast-enhanced spiral CT, completed by MRI or endoscopic ultrasound when appropriate. Fine-needle aspiration was not performed for cytological/histological diagnosis after 2003 as a result of disappointing results using this technique.16
TP technique
All TPs were performed as open procedures by two senior surgeons (A.S. and S.D.). The following parameters were collected: completion of a previous pancreatic resection, extension of a pancreatectomy for positive frozen section of the pancreatic margin, venous resection, extension of resection to adjacent organs, splenectomy and pylorus preservation. The spleen and pylorus were usually preserved when an invasive tumour was deemed localized far from these structures (i.e. in the pancreatic head and in the distal pancreas, for spleen and pylorus preservation, respectively). In patients primarily operated on for IPMN, a pancreatectomy was extended up to TP if a frozen section revealed at least: (i) IPMN adenoma on the main duct, or (ii) at least borderline IPMN (moderate dysplasia) on branch ducts, or (iii) invasive carcinoma.17 During pancreaticoduodenectomy, resection was extended to the left, preserving the splenic vessels, with additional pancreatic division and frozen section every 3 cm. During a distal pancreatectomy, an additional pancreatectomy was done by resection of the head/neck junction on the left side of the common bile duct and, if a frozen section revealed persistent IPMN lesions, by completion pancreatectomy.
Early post-operative medical management
After their discharge, patients were referred to an endocrinology department for the management of post-operative diabetes. Exocrine insufficiency was managed by oral pancreatic enzymes (25 000 units/tablet) three times a day during the main meals. Patients were aware of the need to adapt the number of pancreatic enzymes tablets/capsules to their fat intake, and to open the capsules or take granules if needed.
Follow-up
Most recent follow-up data were collected from clinical hospital files and/or telephone interviews with the patients' general practitioner, gastroenterologist or endocrinologist. All patients were periodically followed by an endocrinologist or gastroenterologist although this was normally undertaken locally. Living patients were asked to participate in long-term assessment, including blood tests and three questionnaires. Blood tests included glycated haemoglobin (HbA1c), liver function tests (LFTs), prothrombin time, creatinine, albumin, pre-albumin and orosomucoid. The last three tests were expressed as percentages of normal values according to gender and age as displayed by the assay office. Questionnaires included an institutional standardized questionnaire as well as two QoL questionnaires: the EORTC QLQ-C30 (version 3.0, French neutral) and the EORTC QLQ-PAN26 (French).
Questionnaires
The institutional standardized questionnaire collected information about sociodemographic data, exocrine function and endocrine function.
The European Organisation for Research and Treatment of Cancer ‘EORTC-QLQ-C30’18 is a questionnaire developed to assess the QoL of cancer patients. It includes multi- and single-item measures, including functional and symptom scales and global health status. A linear transformation was used to standardize the raw score, producing a score ranging from 0 to 100.
The module ‘EORTC-QLQ-PAN26’19 is specifically intended for pancreatic cancer patients at all disease stages, in order to measure health status and disease burden. The same linear transformation was used to standardize the raw score. Items regarding hepatic failure (nos 41 and 42) and ascites (no. 32) were deleted because of an absence of patients with ongoing liver metastases. Items regarding the side effects (nos 38, 43, 50) were deleted because of the absence of current treatment at the time of assessment. Items regarding sexuality (nos 55 and 56) were deleted because of the few percentage of answers.
Ethics
The design of the study was approved on 20 May 2011 by our institutional review board under the registration number IRB00006477 (Comité d'évaluation de l'éthique des projets de recherche biomédicale CEERB du GHU Nord). Patients who accepted the long-term follow-up (including institutional questionnaire, blood tests and QoL tests) all signed an informed consent.
Statistical analysis
Graph Pad Prism 5.0 software for Mac OS X (GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analysis. Qualitative data are expressed as absolute numbers and percentages, and quantitative data are expressed as median and range in square brackets. Comparisons of groups of patients were performed using the Mann–Whitney test and Fisher's exact test. Graphs of QoL parameters from QLQ-C30 and QLQ-PAN26 questionnaires are represented with bars for the mean and error bars for the standard error of the mean. Survival was estimated using the Kaplan–Meier method. Patients alive at the last follow-up or who were lost to follow-up were censored.
Results
Fifty-six patients undergoing an elective TP between 1993 and 2010 were identified. Forty-eight per cent of the patients (n = 27) were male, and the median age at the time of TP was 59 years [27–81].
Pre-operative data
A pre-operative diagnosis included non-invasive IPMN (n = 20, 36%), invasive IPMN (n = 22, 39%), neuroendocrine tumours (n = 6, 11%; including four associated with multiple endocrine neoplasia and one with nesidioblastosis), ductal adenocarcinoma without IPMN (n = 4, 7%), metastasis from renal carcinoma (n = 2), acinar cell carcinoma (n = 1) and familial chronic pancreatitis with intractable pain (n = 1).
In 39 of the 56 cases (70%), a TP was performed in one step, including 29 patients with a pre-operative indication of TP, and 10 patients with an intra-operative indication based on frozen section results. In 29% of the 56 cases (n = 16), a ‘completion pancreatectomy’ was performed after a previous pancreatic resection. The remaining patient had a three-step TP. For patients with a 2- (n = 16) or 3-step TP (n = 1), a previous resection was a pancreaticoduodenectomy (n = 7), distal pancreatectomy ± splenectomy (n = 7), central pancreatectomy (n = 2) and enucleation (n = 2). Eight of them had an invasive neoplasm (IPMN: n = 5, metastases from renal carcinoma: n = 2, endocrine carcinoma: n = 1) on the 1st-step specimen, and the median time elapsed between the two operations was 43 months [0.2–81].
Intra-operative data
Pylorus preservation was performed in 57% of patients (n = 32) and the spleen was preserved in 20% of patients (n = 11). A venous resection was performed in 11% (n = 6).
Intra-operative gastric venous congestion was observed in five patients (9%): (i) transient, after resection of splenic and left gastric veins (n = 1), (ii) permanent, requiring a subtotal gastrectomy (n = 1) or appropriate venous reconstructions (n = 3) of the splenic vein (n = 1), of the left gastric vein (n = 1), and of both the left and right gastric veins (n = 1).
Post-operative course
The overall morbidity rate was 45% (n = 25 patients) with an 8.9% relaparotomy rate (n = 5 patients). Details are given in Table 1. Two patients (3.6%) died post-operatively: one 72-year old female patient was re-operated on for a biliary fistula and died of septic shock 2 days after re-intervention, and one 81-year old woman died from acute respiratory distress syndrome and multiple organ failure at postoperative day 52.
Table 1.
Post-operative complications in 56 patients undergoing a total pancreatectomy (TP)
| Type of complication | Nb (%) | Treatment | |
|---|---|---|---|
| Medical/conservative | Re-intervention | ||
| Delayed gastric emptying | 9 (16%) | 9 | – |
| Sepsis/angiocholitis | 5 (9%) | 5 | – |
| Intra-abdominal abscess | 3 (5%) | 3 | – |
| Early anastomotic ulcer | 2 (4%) | – | 2a |
| Biliary fistula | 2 (4%) | – | 2 |
| Haemorrhage | 2 (4%) | 1 gastrointestinal haemorrhage | 1 intraabdominal bleeding |
| Pneumonia | 2 (4%) | 2 | – |
| Occlusion | 1 (2%) | – | 1 |
| Splenic infarction | 1 (2%) | 1 | – |
| Wound abscess | 1 (2%) | 1 (bed-side drainage) | – |
One patient was re-operated on for both an early anastomotic ulcer and a biliary fistula.
Comparison of the two patient cohorts was undertaken: the first 28 patients, operated on from 1993 to 2005, and the last 28 patients, from 2006 to 2010. The two post-operative deaths were observed during the second period of time. The overall morbidity rate was similar in both groups: 12/28 in the first period and 13/28 in the second period (P = 1.000), and did not differ according to the surgeon who performed the TP. Pylorus preservation was less frequently used in the first period (12/28 versus 20/28 in the second one, P = 0.057) and delayed gastric emptying was more frequently observed in the first period (8/28 versus 1/28 in the second, P = 0.025). However, a comparison of patients with pylorus preservation versus patients who had an antrectomy did not reveal any significant difference with regards to delayed gastric emptying (3/32 versus 6/24, respectively; P = 0.151).
Pathological examination
Of the 20 non-invasive diffuse IPMN diagnosed pre-operatively, 9 were confirmed by pathologic examination (Fig. 1), 6 were invasive at definitive pathological examination, 2 were non-invasive but localized at definitive pathological examination and 3 were ‘false’ IPMN (lupus pancreatitis n = 1, polycystic disease n = 1, obstructive pancreatitis owing to anastomotic stenosis after pancreatoduodenectomy for IPMN n = 1). Overall, according to the specimen definitive pathological examination, five inappropriate TPs (9%) have been performed (two localized IPMN and three ‘false’ IPMN).
Figure 1.
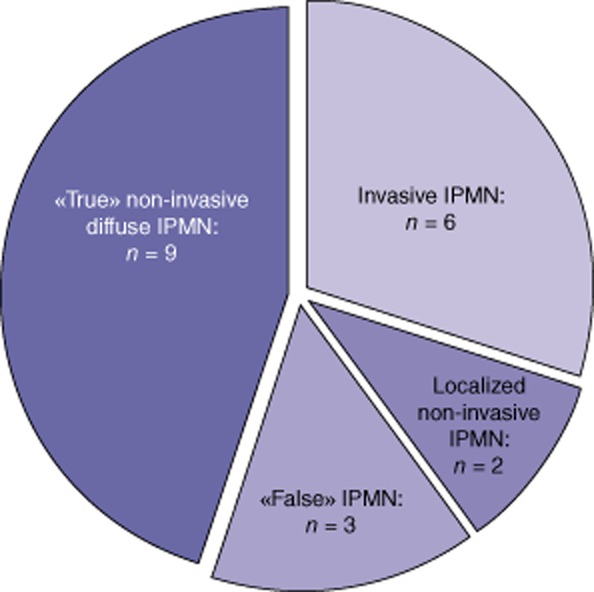
Definitive results of pathological examination of in the 20 patients undergoing a total pancreatectomy (TP) with the pre-operative diagnosis of non-invasive diffuse Intraductal papillary mucinous neoplasm (IPMN)
Long-term follow-up
The median follow-up of patients (except the two patients who died post-operatively) was 35 months [4–168], with one patient lost to follow-up after 18 months. Six patients (11%) were readmitted once for a hypoglycaemia coma and five patients (9%) presented with anastomotic ulcers, including two patients requiring re-operation.
Overall, seven patients (13%) experienced anastomotic ulcers: two in the early post-operative course and five during follow-up. All of these seven patients underwent a TP in the first period between 1993 and 2005 (P = 0.010 versus the second period) when permanent proton pump inhibitors (PPI) were not given routinely. No anastomotic ulcer was observed in the second period, after routine permanent PPI administration. Five out of 24 patients who had an antrectomy developed an anastomotic ulcer compared with two out of 32 patients with pylorus preservation (P = 0.114).
At the census date, the overall mortality rate was 47% (26/56 patients) with 20 deaths attributable to the underlying pancreatic disease, one to an endocarditis and five directly as a consequences of TP. The causes of death related to TP included: two early post-operative deaths (as described above), one perforated anastomotic ulcer, one hypoglycaemia and one ketoacidosis.
Overall, the median 3- and 5-year survivals for all patients (n = 56) were respectively 122 months, 62% and 55% (Fig. 2). For patients with IPMN (Fig. 3), the median, 3- and 5-year survivals were, respectively, 156 months, 90% and 90% for non-invasive IPMN, 20 months, 40% and 22% for invasive IPMN, and 46 months, 55% and 43% for all IPMN. Among the 15 patients with invasive IPMN who deceased from disease recurrence, 20% of them had an isolated regional recurrence and 80% presented metastatic lesions at time of recurrence. The three patients with ‘false’ IPMN are alive, including one who was re-operated for an anastomotic ulcer. Of the six patients with an endocrine tumour, two developed metastases: one patient died at 79 months and the other is currently awaiting a liver transplantation. Of the five patients with acinar cell carcinoma or adenocarcinoma without IPMN, four died: three from recurrence and one from ketoacidosis 5 months after surgery. Their median survival was 71 months [5–122]. The three remaining patients who underwent TP for renal cancer metastases and chronic pancreatitis are still living and are in a good general condition, with a follow-up of 32 and 7 months and 22 months, respectively.
Figure 2.
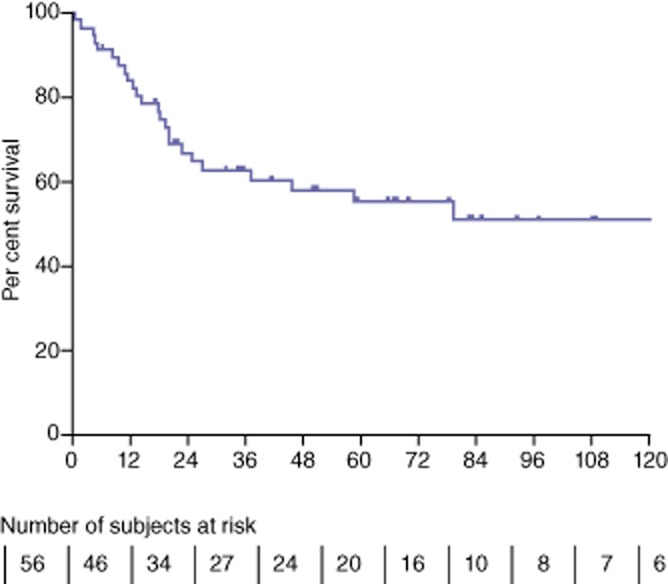
Overall survival
Figure 3.
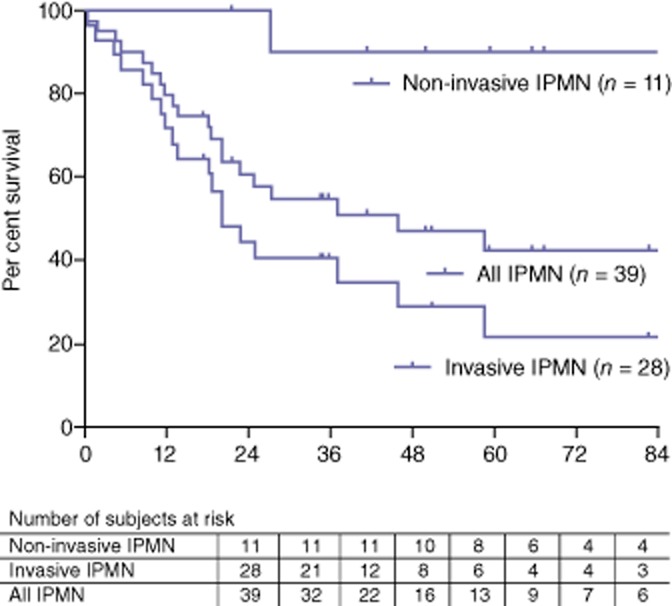
Survival of intraductal papillary mucinous neoplasm (IPMN) patients
Long-term prospective evaluation and QoL questionnaires
Among the 30 survivors, 25 agreed to participate prospectively in the study. Two declined to participate, one had severe comorbidity (renal cancer, renal failure and antiphospholipid antibody syndrome) affecting the QoL and was considered unsuitable for evaluation, one was lost to follow-up after a 18-month follow-up and one patient was living abroad and not contactable at the time of inclusion. The long-term assessment took place 66 months [7–168] after TP or completion of a pancreatectomy. Eleven patients (44%) were male and with a median age was 64 years [33–79]. Eighteen patients (72%) did not receive a higher education (college or university). Indications for TP were: non-invasive IPMN (n = 7), ‘false’ IPMN (n = 2), invasive IPMN (n = 8), endocrine tumour (n = 4), ductal adenocarcinoma (n = 1), metastases from renal cancer (n = 2) and chronic pancreatitis (n = 1). No patients are currently undergoing treatment for recurrence.
Exocrine function
Since a TP, 15 patients lost weight, with a median of 9 kg [2–14] and their weight at the time of analysis was 85% [78–97] of their pre-operative weight. Four patients gained weight (12.5 kg [4–22]). The 25 patients had 2 [1–5] stools per day, which were liquid in one-quarter of the patients. Seven patients had night stools, and for five patients diarrhoea stools were a limiting factor in everyday life. The median daily intake of pancreatic enzymes supplements was 6 [3–18] capsules (25 000 units per capsule) and 5 patients took other medications for intestinal transit, including loperamide (n = 1) and diosmectite (n = 1).
LFTs and prothrombin time were within normal ranges; particularly no cholestasis was noticed (data not shown). The renal function was usually preserved with a median serum creatinine of 82 μmol/l [50–163]. Nutrition markers included albumin of 100% [81–100], pre-albumin 100% [70–100] and orosomucoid 100% [63–100] of normal values.
Endocrine function
Patients assessed their glycaemia 5 times per day [3–10] and required 16 units [7–48] of long-acting insulin and 21 units [7–70] of rapid-acting insulin. One patient had an insulin pump. During the past month, they all had experienced hypoglycaemia (glycaemia<0.40 g/l) with a median of 10 times [1–36]. Fourteen patients have been readmitted since TP for diabetes equilibration, mostly (n = 8) for hypoglycaemic episodes. Ten patients had already had loss of consciousness due to hypoglycaemia. The median glycated haemoglobin was 7.8 [6.3–10.3] with no difference between non-malignant and malignant aetiologies (P = 0.550). Nine patients had a glycated haemoglobin level above 8%.
QoL questionnaires: EORTC-QLQ-C30 and QLQ-PAN 26
In the QLQ-C30 (Fig. 4), the global health status was scored 75% [17–100]. Functional scales scores had medians ranging from 83 to 100%, representing a high QoL in physical, role, emotional, cognitive and social functioning. Regarding symptom scales, scores were relatively low (corresponding to a high QoL) except for fatigue (median of 33%) and diarrhoea (33%).
Figure 4.
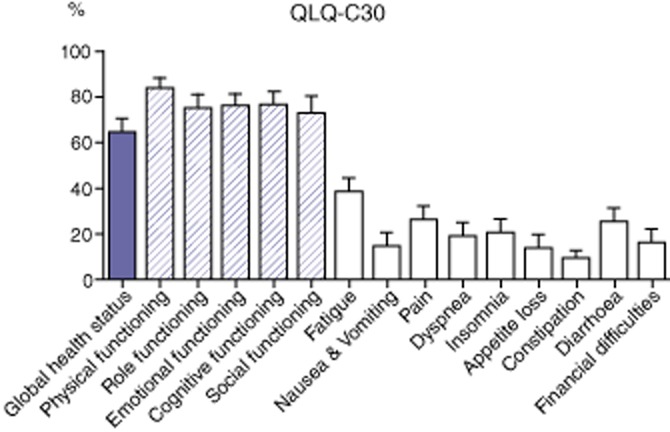
Results of QLQ-C30 quality-of-life (QoL) questionnaires QLQ-C30: a higher score represents better QoL for global health and functional scales (striped plots) and worse QoL for symptoms scales (white plots). Plots display the mean and error bars the standard deviation of the mean
In the QLQ-PAN26 score (Fig. 5), the most affected symptoms were altered bowel habits, flatulence and eating-related items. Patients were not highly satisfied with health care (information and support) with a median of 31%.
Figure 5.
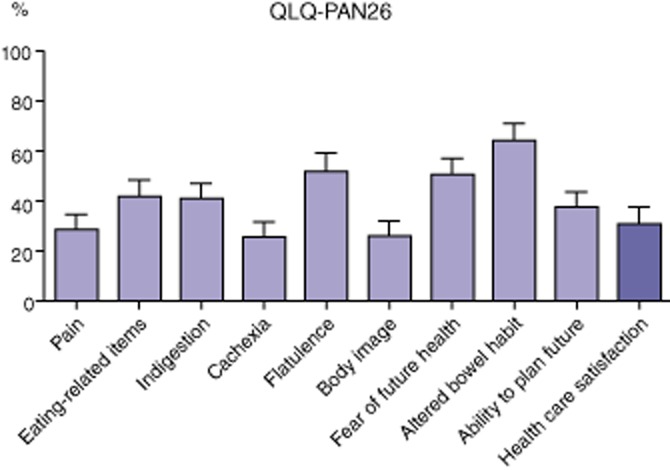
Results of QLQ-PAN26 quality-of-life (QoL) questionnairesQLQ-PAN26: a higher score represents better QoL for health care satisfaction and worse QoL for other items. Plots display the mean and error bars the standard deviation of the mean
Scores of global health status (QLQ-C30) and of health care satisfaction (QLQ-PAN26) were not affected by gender (P = 0.229 and P = 0.211, respectively), age below or above 60 years at the time of TP (P = 0.373 and P = 0.587, respectively) and a higher education level (P = 0.380 and P = 0.762, respectively).
Discussion
This study indicates that TP is a reasonable option in some diffuse pancreatic diseases, mainly IPMN, as operative mortality is low and long-term consequences of TP are now better managed, with an acceptable morbidity and QoL. Indications for TP should be carefully selected as the best long-term survival is observed for non-invasive IPMN whereas survival after TP for invasive IPMN or adenocarcinoma remains disappointing. TP can result in severe gastric congestion requiring some specific technical features, and the risk of an anastomotic ulcer can be prevented by routine permanent administration of PPI. However, TP should not be considered as a common procedure as diabetes management remains a challenge and exocrine insufficiency impacts on everyday life.
After the first enthusiasm for TP, results of large series of TP for pancreatic adenocarcinoma were published in the late 1980s and 1990s. They showed a high peri-operative mortality rate (13% in that of Launois et al.'s,4 17% in Baumel et al.'s5 and up to 27% in Ihse et al.'s series6) with no clear survival benefit on median survival (7 to 12 months).5,6 Furthermore, the frequency of tumour multicentricity of pancreatic carcinoma was then revised downward, as low as 6%.20 With the improving management of pancreatic fistulae, situations where a TP was performed in patients with a pancreatic remnant considered unsuitable for anastomosis had become exceptional. Nowadays, the role of TP in the management of pancreatic adenocarcinoma remains limited to an isolated positive neck margin,21 familial pancreatic cancer or underlying pancreatic disease such as IPMN.22 The long-term survival of patients with TP for adenocarcinoma seems to be similar to those of patients receiving pancreatoduodenectomy, as demonstrated in 2009 in the Surveillance, Epidemiology and End Results (SEER) database in the USA.23 As for an extended lymphadenectomy and increased soft tissue clearance, extension of a pancreatectomy does not improve survival24,25 comparatively to a pancreaticoduodenectomy and, therefore, should not ‘justify’ routine TP.
Regarding indications of TP, this series indicates that IPMN is currently the most frequent indication with good overall long-term results. In other series, 3-year survival rates range from 88%13 to 95%26 for non-invasive IPMN, and decreases to 45%8 to 49%26 in case of invasive component. This difference in the survival rate between invasive and non-invasive IPMN underlines the need to determine accurately the clinical and morphological features of invasive IPMN. When invasive IPMN is strongly suspected pre-operatively, the poor life expectancy and the long-term consequences of TP including decreased QoL should be taken into account. In this setting, a partial pancreatectomy, leaving a pancreatic remnant harbouring non-invasive lesions, could be an alternative to TP.27 Conversely, in patients with non-invasive IPMN, obtaining tumour-free margins probably allows a decrease in the rate of intra-pancreatic recurrence,28 as these patients have a long-life expectancy (Fig. 3). As a pre-operative diagnosis of invasive malignancy is still not reliable, as demonstrated in our series, an intra-operative frozen section could be performed not only on the pancreatic margin but also on the specimen to detect an invasive component. Thus, results of a frozen section could help the surgeon to more precisely decide if completion of a pancreatectomy is indicated.
Our study also suggests that, in patients with suspected diffuse IPMN, indications should be driven by histology as misdiagnoses can occur. In the present series, five TPs were inappropriately performed according to imaging findings mimicking diffuse IPMN. As a matter of fact, the diagnosis of IPMN is usually not supported pre-operatively by cytological or histological studies.16 As symptoms of IPMN are not specific, we now recommend performing a TP only after confirmation of both diagnosis of IPMN and extension into the whole gland, using intra-operative frozen sections with an accuracy of up to 94%.16 The pancreatic segments harbouring the most severe lesions should be identified by pre-operative workup and resected at first. The pancreatic remnant should then be resected only in case of worrisome findings on the pancreatic cut surface at frozen section examination.
Intra-operatively, we observed gastric venous congestion in five patients, requiring a subtotal gastrectomy or adequate venous reconstruction. Indeed, TP with an antrectomy and splenectomy leaves the stomach only vascularized by the left gastric vessels. The left gastric vein is sometimes difficult to preserve due to anatomical variations, inflammatory changes or tumour involvement.29 To our knowledge, the risk of gastric venous congestion during TP has not been precisely assessed, but only mentioned in one Japanese study.30 Indeed, gastric venous congestion may resolve spontaneously in some cases, as it occurred in one of our patient, so venous reconstruction might not be necessary in all patients. We believe that pylorus preservation combined with preservation of right gastric vessels can maintain venous drainage and should be considered in all patients undergoing TP or a partial pancreatectomy with a possible further pancreatic resection (mainly IPMN patients). Another important drawback of TP is the risk of an anastomotic ulcer, which can occur both in the early post-operative course and in the long term, as demonstrated in our series. This complication can be severe, resulting in reoperation or even death. The risk of an anastomotic ulcer after TP has been suggested since the 1980s31,32 and persisted in more recent series.33 We observed a anastomotic ulcer with or without pylorus preservation so this complication could not be prevented technically. However, we observed no anastomotic ulcer after routine administration of PPI hence we strongly recommend life-long treatment with PPI for all TP patients.
Pancreatic diabetes mellitus has for main characteristics a complete lack of endogenous insulin and glucagon, leading to frequent and deep states of hypoglycaemia with hyperglycaemic episodes that can be difficult to control (brittle diabetes34). In this study, 40% of our patients already had a loss of consciousness owing to hypoglycaemia, and a median of 10 hypoglycaemic episodes per month for all patients, which is consistent with previous studies.8 More than half of our patients were readmitted for diabetes equilibration, which is higher than previously described.13,35 We observed a glycated haemoglobin rate of 7.8%, also consistent with the literature, which reported rates ranging from 6.7% in benign disease13 to 9%.8 However, we did not find any difference between malignant and non-malignant disease, as observed by Muller et al.13 The severity of TP-induced diabetes justifies reinforcing patient education. We now do this routinely by referring the patient to a department of endocrinology directly from our surgical department once early post-operative surgical problems have been resolved. In this way, we aim to reinforce patients' information that has been scored unfavourably in the QLQ-PAN26. The patients' socio-economic level also should be taken into account to adapt their education for adequate diabetes management. However, we did not observe any difference with regards to long-term quality of life according to patients' age, gender or education level, suggesting that the determination of patients who will be able to manage pancreatic diabetes remains challenging.
In this series, the early post-operative mortality rate was 3.6%, which is in accordance with other published studies, from 2%26 to 8%36 (Table 2). Our data suggest that reported mortality of TP should also include long-term deaths from TP-related complications. This result is in accordance with some others series, which reported some deaths from hypoglycaemia during follow-up.15
Table 2.
Morbidity, mortality and survival after an elective a total pancreatectomy (TP) – main published series
| Author | Period of time | Aetiologies | Mortality rate (%) | Morbidity rate (%) | Diabetes | Survival rates | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Ductal carcinoma | Invasive IPMN | Non-invasive IPMN | Chronic Pancreatitis | Endocrine tumor | Renal cancer metastases | Other malignant | Other benign | ||||||
| Casadei et al.35 | 2006–2009 | 20 | 7 | 7 | 1 | 1 | 2 | 2 | 5% | 25%, of which: -hemorrhage=3 -biliary leakage=1 | - No death related to diabetes - 23% readmissions for glycemic control | - Ductal carcinoma: 3-y: 25% - Others: 3-y: 90%; 5-y: 70% | ||
| Muller et al.13 | 2001–2006 | 124 | 67 | 10 | 8 | 11 | 10 | 8 | 6 | 4 | 4.8% | 24% surgical 14.5% medical | - No death related to diabetes - 8.3% readmissions for diabetes control | - Malignant disease: 3-y: 37%a - Benign disease: 3-y: 88%a |
| Billings et al.15 | 1985–2002 | 99 | 33 | 17 | 9 | 20 | 20 | 5% | 32% | 3 deaths owing to hypoglycemia | - Malignant disease: 5-y: 34%b - Benign disease: 5y: 84%b | |||
| Schmidt et al.21 | 1992–2006 | 33 | 33 (all R0) | 6.1% | 48% | – | 3-y: 34% 5-y: 14% | |||||||
| Crippa et al.8 | 1996–2008 | 65 | 19 | 18 | 13 | 1 | 6 | 6 | 1 | 1 | 0 | 38.5%, of which: -hemorrhage =3 -biliary leakage=1 | No death owing to hypoglycemia | - All patients: 5-y: 71% - Ductal carcinoma: 3-y: 38%; 5-y: 0% - Invasive IPMN: 3-y: 56%; 5-y: 45% |
| Reddy et al.22 | 1970–2007 | 100 | 100 | 8% | ≈68% | – | 3-y: 27% 5-y: 19% | |||||||
| Stauffer et al.26 | 2002–2008 | 47 | 10 | 10 | 21 | 2 | 1 | 1 | 1 | 1 | 2% | 37% | 23/46 living patients were readmitted after discharge | 3-y survival: - All patients: 65% - Ductal carcinoma: 34%: - Invasive IPMN: 49% - Non-invasive IPMN: 95% |
| Present study | 1993–2010 | 56 | 4 | 28 | 11 | 1 | 6 | 2 | 1 | 3 | 3.6% | 45% | −1 death owing to hypoglycemia −1 death owing to ketoacidosis | - All patients: 3-y = 62%, 5-y = 55% - Invasive IPMN: 3-y = 40%, 5-y = 22% - Non-invasive IPMN: 3- and 5-y = 90% |
Including other patients with salvage TP.
Except patients who died in the postoperative period.
Although 68% of patients in the present study lost weight, which is within previously reported ranges13,35 (Table 3), evaluation of nutrition markers showed that no patient was suffering from malnutrition. In 1991, Dresler et al.34 first described the metabolic consequences of TP, in 45 patients who underwent TP from 1978 to 1988. They reported significant chronic diarrhoea in 10% of patients, with abnormal low levels of fat-soluble vitamins, magnesium and trace elements in individual patients in spite of a well-conducted multivitamin supplementation. In the same study, liver function tests (LFTs) showed elevated levels of serum transaminases (ASAT and ALAT), as well as alkaline phosphatase in almost all patients; three patients (6.6%) died from liver cirrhosis, not explained by alcoholism. This liver damage was not retrieved in other studies, but none of them, to our knowledge, reported LFTs or liver imaging results.8,13,15,22,35 In the present study, LFTs were within normal ranges, and no signs of hepatic dysfunction were observed. One hypothesis about liver dysfunction reported in Dresler's study is the presence of severe hepatic steatosis or steatohepatitis, which has already been reported after pancreatoduodenectomy in up to 33% of cases 6 months after surgery.36 The risk factors of hepatic steatosis are still unknown but a reduction in the post-operative body mass index greater than 3 kg/m237 or use of old pancreatic enzyme formulations can be hypothesized.
Table 3.
Endocrine and exocrine functions in the long-term after a a total pancreatectomy (TP) – Main published
| Authors | Period of time | N = | Median insulin units/d | Median hypo-glycaemia events | HbA1c | Median pancreatic lipase/d | Weight loss % of patients Nb of kg | Digestive symptoms |
|---|---|---|---|---|---|---|---|---|
| Crippa S et al.8 | 1996–2008 | 45 | 32 [18–52] | 2 [0–5] per week | 56% between 7 and 9 11% above 9% | 80 000 units [30 000–160 000] | 45% 5 kg [1–18] | Abdominal pain 22% Diarrhoea 13% |
| Muller et al.13 | 2001–2006 | 67 | 6.7% in benign disease 7.5% in malignant disease | 41% 13.5 kg [6–30] | Diarrhoea 41% Moderate to severe abdominal pain 15% | |||
| Billings et al.15 | 1985–2002 | 27 | 32 [2–66] | 7.4% (5.0–11.3] | 14 capsulesa [0–36] | 70% 12 kg [2–31] | ||
| Casadei et al.35 | 2006–2009 | 13 | 25 [20–52] | 4 [1–10] hypo + hyperglycemias per week | 8% [5.2–10.3] | 8 capsulesa [6–11] | 85% 15 kg [1–32] | |
| Present study | 1993–2010 | 25 | 16 [7–48] of long-acting insulin 21 [7–70] of rapid-acting insulin | 10 times [1–36] per month | 7.8 [6.3–10.3] 9 patients >8% | 150 000 units [75 000–450 000] | 60% 9 kg [2–14] | 2 [1–5] stools/day 28% with night stools |
Number of pancreatic enzyme units not specified.
We also observed long-term altered bowel habits in 20% to 25% of our patients. Indeed, of the 25 patients we evaluated, 7 had night stools and 5 described diarrhoea stools as a limiting factor in everyday life. Diarrhoea after TP results mainly from exocrine insufficiency. After TP, a specific attention should be given to the patients’ education with regards to exocrine insufficiency and pancreatic enzymes (to adapt the number of pancreatic enzymes tablets/capsules, to open the capsules or take granules if needed). Another explanation for the diarrhoea is the risk of nervous damage around the superior mesenteric artery (SMA).38 Indeed, dissection of the SMA during TP is more extensive than during a pancreaticoduodenectomy, resulting in impaired bowel control, as strongly suggested in previous studies that compared a regional versus extended lymphadenectomy during a pancreaticoduodenectomy.36,38
Only three previous studies have evaluated the long-term QoL after TP.13,15,35 Tables 4 and 5 summarize the results of these studies and of the present one. Although not fully comparable, these data showed similar scores for global health status (61 to 64%). Most impaired items after TP were symptoms scales, with a decrease of quality of life focusing mainly on fatigue, pain and diarrhoea as in Casadei's study.35 Main differences between our patients and reference patients concern eating and transit-related items like diarrhoea, vomiting and loss of appetite. We herein reported better QoL scores with the QLQ-PAN26 questionnaire than Billings et al.15 regarding body image, eating restrictions, pain and health care satisfaction. This decrease in QoL seems an unavoidable consequence of TP and should be explained pre-operatively to the patient for more objective information. The relatively low score of health care satisfaction should encourage us to reinforce the delivered information before and after surgery.
Table 4.
Long-term assessment of quality-of-life with standardized questionnaires QLQ-C30 – Review of the Literature
| Scales | Casadei35 13 patients Median [range] | Muller13 46 patients Mean ± standard deviation | Present study 25 patients Mean ± standard deviation Median [range] | |
|---|---|---|---|---|
| Global health | 75 [0–83] | 61 ± 20 | 64 ± 32 75 [17–100] | |
| Function scales | Physical functioning | 80 [0–100] | 78 ± 23 | 84 ± 21 93 [33–100] |
| Role functioning | 83 [0–100] | 67 ± 29 | 75 ± 33 83 [0–100] | |
| Emotional functioning | 75 [17–100] | 70 ± 22 | 76 ± 28 92 [8–100] | |
| Cognitive functioning | 100 [0–100] | 80 ± 26 | 76 ± 32 83 [0–100] | |
| Social functioning | 100 [0–100] | 67 ± 32 | 73 ± 37 100 [0–100] | |
| Symptom scales | Fatigue | 33 [0–89] | 38 ± 26 | 38 ± 32 33 [0–100] |
| Nausea/vomiting | 0 [0–67] | 6 ± 17 | 15 ± 27 0 [0–100] | |
| Pain | 0 [0–83] | 23 ± 27 | 26 ± 30 17 [0–100] | |
| Dyspnoea | 33 [0–100] | 14 ± 30 | 19 ± 33 0 [0–100] | |
| Insomnia | 33 [0–100] | 29 ± 30 | 20 ± 32 0 [0–100] | |
| Loss of appetite | 0 [0–100] | 16 ± 27 | 13 ± 30 0 [0–100] | |
| Constipation | 0 [0–33] | 8 ± 21 | 9 ± 18 0 [0–100] | |
| Diarrhoea | 33 [0–67] | 28 ± 32 | 25 ± 32 33 [1–100] | |
| Financial difficulties | 0 | 20 ± 27 | 16 ± 29 0 [0–100] | |
A higher score represents better QoL for global health and functional scales and worse QoL for symptoms scales.
Table 5.
Long-term assessment of quality-of-life with standardized questionnaires QLQ-PAN26
| Billings et al.15 27 patients Mean ± standard deviation | Present study 25 patients Mean ± standard deviation | |
|---|---|---|
| Body image | 75 ± 6 | 27 ± 39 |
| Pain | 72 ± 5 | 29 ± 32 |
| Side-effect of treatment | 71 ± 5 | NA |
| Altered bowel habit | 62 ± 3 | 64 ± 33 |
| Eating restrictions | 57 ± 5 | 42 ± 35 |
| Sexuality | 54 ± 9 | NA |
| Health care satisfaction | 18 ± 5 | 31 ± 38 |
A higher score represents better QoL for health care satisfaction and worse QoL for other items.
In conclusion, morbidity and mortality after TP are acceptable, although diabetes- and TP-related mortality still occurs. Indications for TP should be driven by histology, as misdiagnoses can occur. Specific attention should be paid to gastric venous drainage, highlighting the importance of pylorus preservation and adequate venous reconstruction if necessary. Definitive PPI administration is strongly recommended in order to prevent anastomotic ulcer. Long-term assessment shows that exocrine insufficiency impacts on everyday life in 20% of patients, but nutrition issues after TP are now better managed. TP-induced diabetes remains difficult to balance with frequent hypoglycaemias and hospital readmissions, underlying the importance of diabetic education after TP.
Conflicts of interest
No funding – no conflict of interest.
References
- 1.Priestley JT, Comfort MW, Radcliff J. Total pancreatectomy for hyperinsulinism due to an islet-cell adenoma. Ann Surg. 1944;119:211–221. doi: 10.1097/00000658-194402000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins JJ, Jr, Craighead JE, Brooks JR. Rationale for total pancreatectomy for carcinoma of the pancreatic head. N Engl J Med. 1966;274:599–602. doi: 10.1056/NEJM196603172741103. [DOI] [PubMed] [Google Scholar]
- 3.Tryka AF, Brooks JR. Histopathology in the evaluation of total pancreatectomy for ductal carcinoma. Ann Surg. 1979;190:373–379. doi: 10.1097/00000658-197909000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Launois B, Franci J, Bardaxoglou E, Ramee MP, Paul JL, Malledant Y, et al. Total pancreatectomy for ductal adenocarcinoma of the pancreas with special reference to resection of the portal vein and multicentric cancer. World J Surg. 1993;17:122–126. doi: 10.1007/BF01655724. [DOI] [PubMed] [Google Scholar]
- 5.Baumel H, Huguier M, Manderscheid JC, Fabre JM, Houry S, Fagot H. Results of resection for cancer of the exocrine pancreas: a study from the French Association of Surgery. Br J Surg. 1994;81:102–107. doi: 10.1002/bjs.1800810138. [DOI] [PubMed] [Google Scholar]
- 6.Ihse I, Anderson H, Andren-Sandberg A. Total pancreatectomy for cancer of the pancreas: is it appropriate? World J Surg. 1996;20:288–294. doi: 10.1007/s002689900046. [DOI] [PubMed] [Google Scholar]
- 7.Heidt DG, Burant C, Simeone DM. Total pancreatectomy: indications, operative technique, and postoperative sequelae. J Gastrointest Surg. 2007;11:209–216. doi: 10.1007/s11605-006-0025-7. [DOI] [PubMed] [Google Scholar]
- 8.Crippa S, Tamburrino D, Partelli S, Salvia R, Germenia S, Bassi C, et al. Total pancreatectomy: indications, different timing, and perioperative and long-term outcomes. Surgery. 2011;149:79–86. doi: 10.1016/j.surg.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi K, Konomi H, Kobayashi K, Ogura Y, Sonoda Y, Kawamoto N, et al. Total pancreatectomy for intraductal papillary-mucinous tumor of the pancreas: reappraisal of total pancreatectomy. Hepatogatsroenterology. 2005;52:1585–1590. [PubMed] [Google Scholar]
- 10.Braasch JW, Vito L, Nugent W. Total pancreatectomy for end-stage chronic pancreatitis. Ann Surg. 1978;188:317–321. doi: 10.1097/00000658-197809000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexakis N, Ghaneh P, Connor S, Raraty M, Sutton R, Neoptolemos JP. Duodenum- and spleen-preserving total pancreatectomy for end-stage chronic pancreatitis. Br J Surg. 2003;90:1401–1408. doi: 10.1002/bjs.4324. [DOI] [PubMed] [Google Scholar]
- 12.Janot MS, Belyaev O, Kersting S, Chromik AM, Seelig MH, Sülberg D, et al. Indications and early outcomes for total pancreatectomy at a high-volume pancreas center. HPB Surg. 2010;135:345–349. doi: 10.1155/2010/686702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller MW, Friess H, Kleeff J, Dahmen R, Wagner M, Hinz U, et al. Is there still a role for total pancreatectomy? Ann Surg. 2007;246:966–975. doi: 10.1097/SLA.0b013e31815c2ca3. [DOI] [PubMed] [Google Scholar]
- 14.Casadei R, Monari F, Buscemi S, Laterza M, Ricci C, Rega D, et al. Total pancreatectomy: indications, operative technique, and results. a single centre experience and review of literature. Updat Surg. 2010;62:41–46. doi: 10.1007/s13304-010-0005-z. [DOI] [PubMed] [Google Scholar]
- 15.Billings BJ, Christein JD, Harmsen WS, Harrington JR, Chari ST, Que FG, et al. Quality-of-life after total pancreatectomy: is it really that bad on long-term follow-up? J Gastrointest Surg. 2005;9:1059–1067. doi: 10.1016/j.gassur.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Maire F, Couvelard A, Hammel P, Ponsot P, Palazzo L, Aubert A, et al. Intraductal papillary mucinous tumors of the pancreas: the preoperative value of cytologic and histopathologic diagnosis. Gastrointest Endosc. 2003;58:701–706. doi: 10.1016/s0016-5107(03)02032-7. [DOI] [PubMed] [Google Scholar]
- 17.Couvelard A, Sauvanet A, Kianmanesh R, Hammel P, Colnot N, Lévy P, et al. Frozen sectioning of the pancreatic cut surface during resection of intraductal papillary mucinous neoplasms of the pancreas is useful and reliable: a prospective evaluation. Ann Surg. 2005;242:774–778. doi: 10.1097/01.sla.0000188459.99624.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 19.Fitzsimmons D, Johnson CD, George S, Payme S, Sandberg AA, Bassi C, et al. Development of a disease specific quality of life (QoL) questionnaire module to supplement the EORTC core cancer QoL questionnaire, the QLQ-C30 in patients with pancreatic cancer. EORTC Study Group on Quality of Life. Eur J Cancer. 1999;35:939–941. doi: 10.1016/s0959-8049(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 20.Motojima K, Urano T, Nagata Y, Shiku H, Tsurifune T, Kanematsu T. Detection of point mutations in the Kirtsen-ras oncogene provides evidence for multicentricity of pancreatic carcinoma. Ann Surg. 1993;17:138–143. doi: 10.1097/00000658-199302000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt CM, Glant J, Winter JM, Kennard J, Dixon J, Zhao Q, et al. Total pancreatectomy (R0 resection) improves survival over subtotal pancreatectomy in isolated neck margin positive pancreatic adenocarcinoma. Surgery. 2007;142:572–580. doi: 10.1016/j.surg.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Reddy S, Wolfgang CL, Cameron JL, Eckhauser F, Choti MA, Schulick RD, et al. Total pancreatectomy for pancreatic adenocarcinoma: evaluation of morbidity and long-term survival. Ann Surg. 2009;250:282–287. doi: 10.1097/SLA.0b013e3181ae9f93. [DOI] [PubMed] [Google Scholar]
- 23.Nathan H, Wolfgang CL, Edil BH, Choti MA, Herman JM, Schulick RD, et al. Peri-operative mortality and long-term survival after total pancreatectomy for pancreatic adenocarcinoma: a population-based perspective. J Surg Oncol. 2009;99:87–92. doi: 10.1002/jso.21189. [DOI] [PubMed] [Google Scholar]
- 24.Pedrazzoli S, DiCarlo V, Dionigi R, Mosca F, Pederzoli P, Pasquali C, et al. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Ann Surg. 1998;228:508–517. doi: 10.1097/00000658-199810000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riall TS, Cameron JL, Lillemoe KD, Campbell KA, Sauter PK, Coleman J, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma-part 3: update on 5-year survival. J Gastrointest Surg. 2005;9:1191–1204. doi: 10.1016/j.gassur.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 26.Stauffer JA, Nguyen JH, Heckman MG, Grewal MS, Dougherty M, Gill KR, et al. Patient outcomes after total pancreatectomy: a single centre contemporary experience. HPB. 2009;11:483–492. doi: 10.1111/j.1477-2574.2009.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raut CP, Cleary KR, Staerkel GA, Abbruzzese JL, Wolff RA, Lee JH, et al. Intraductal papillary mucinous neoplasms of the pancreas: effect of invasion and pancreatic margin status on recurrence and survival. Ann Surg Oncol. 2006;13:582–594. doi: 10.1245/ASO.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 28.White R, D'Angelica M, Katabi N, Tang L, Klimstra D, Fong Y, et al. Fate of the remnant pancreas after resection of noninvasive intraductal papillary mucinous neoplasm. J Am Coll Surg. 2007;204:987–993. doi: 10.1016/j.jamcollsurg.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 29.Rebibo L, Chivot C, Fuks D, Sabbagh C, Yzet T, Regimbeau JM. Three-dimensional computed tomography analysis of the left gastric vein in a pancreatectomy. HPB. 2012;14:414–421. doi: 10.1111/j.1477-2574.2012.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugiyama M, Atomi Y. Pylorus-preserving total pancreatectomy for pancreatic cancer. World J Surg. 2000;24:66–71. doi: 10.1007/s002689910013. [DOI] [PubMed] [Google Scholar]
- 31.Grant CS, Van Heerden JA. Anastomotic ulceration following subtotal and total pancreatectomy. Ann Surg. 1979;190:1–5. doi: 10.1097/00000658-197907000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott HW, Jr, Dean RH, Parker T, Avant G. The role of vagotomy in pancreaticoduodenectomy. Ann Surg. 1980;191:688–696. doi: 10.1097/00000658-198006000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Safioleas MC, Moulakakis KG, Andromanakos NP, Lygidakis NJ. How necessary is vagotomy after pancreaticoduodenectomy and total pancreatectomy. Hepatogastroenterology. 2005;52:251–252. [PubMed] [Google Scholar]
- 34.Dresler CM, Fortner JG, McDermott K, Bajorunas DR. Metabolic consequences of (regional) total pancreatectomy. Ann Surg. 1991;214:131–140. doi: 10.1097/00000658-199108000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casadei R, Ricci C, Monari F, Laterza M, Rega D, D'Ambra D, et al. Clinical outcome of patients who underwent total pancreatectomy. Pancreas. 2010;39:546–547. doi: 10.1097/MPA.0b013e3181c2dcd3. [DOI] [PubMed] [Google Scholar]
- 36.Nimura Y, Nagino M, Takao S, Takada T, Miyazaki K, Kawarada Y, et al. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: long-term results of a Japanese multicenter randomized controlled trial. J Hepatobiliary Pancreat Sci. 2012;19:230–241. doi: 10.1007/s00534-011-0466-6. [DOI] [PubMed] [Google Scholar]
- 37.Okamura Y, Sugimoto H, Yamada S, Fujii T, Nomoto S, Takeda S, et al. Risk factors for hepatic steatosis after pancreatectomy: a retrospective observational cohort study of the importance of nutritional management. Pancreas. 2012;41:1067–1072. doi: 10.1097/MPA.0b013e31824c10ab. [DOI] [PubMed] [Google Scholar]
- 38.Farnell MB, Pearson RK, Sarr MG, DiMagno EP, Burgart LJ, Dahl TR, et al. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005;138:628–630. doi: 10.1016/j.surg.2005.06.044. [DOI] [PubMed] [Google Scholar]


