Abstract
Objectives
Recent improvements in surgical technique have extended the indications for liver resection. The aims of this study were to assess whether this extension is associated with a changing patient profile and to evaluate how this potential shift has influenced mortality after liver resection in order to define standard expectations for hepatectomy.
Methods
The characteristics and postoperative outcomes of all patients undergoing elective hepatectomy from 2000 to 2009 were reviewed retrospectively. Multivariate analysis was conducted to determine the factors associated with mortality in the subgroup of patients with malignant disease.
Results
Among the 2012 patients in whom hepatectomies were performed, the percentage of patients operated for malignancy increased from 66.4% in 2000 to 82.3% in 2009 (P < 0.001). These patients experienced higher mortality (4.5% versus 0.7%; P < 0.001), were significantly older, and displayed greater comorbidity and underlying parenchymal disease compared with those with benign lesions. Mortality over the entire study period was 3.5% and was fairly stable, dropping from 3.8% in 2000 to 3.1% in 2009 (P = 0.686). On multivariate analysis, age of >60 years, an American Society of Anesthesiologists score of ≥3, major resection, vascular procedure, severe fibrosis (F3–F4) and steatosis of >30% were associated with increased mortality in patients with malignant disease.
Conclusions
The profile of patients undergoing liver resection has changed and now includes more high-risk patients with diseased parenchyma undergoing major hepatectomy for malignancy. This change in patient profile is responsible for the stability in mortality rates over the years.
Introduction
During the last decades of the 20th century, several factors contributed to reduce mortality after hepatectomy from 5% to almost 0%.1–4 Among these factors, better knowledge of both liver anatomy and physiology, including of liver regeneration and preoperative volume modulation,5 better morphological assessment,6 advances in parenchymal transection with the selective use of vascular control7 and sophisticated perioperative management have all contributed to reduce the risks associated with liver resection. Although better characterization of liver lesions prevents the unnecessary resection of benign lesions, the number of hepatectomies carried out for malignancy is increasing. For example, both screening in high-risk individuals with viral hepatitis or metabolic syndrome, and more efficient chemotherapy regimens currently allow resection in increasing numbers of patients with hepatocellular carcinoma (HCC) and colorectal liver metastasis (CLM). However, such patients are more likely to demonstrate pathological changes in the underlying parenchyma, such as fibrosis, steatosis and chemotherapy-associated liver injury.8–10 It is therefore likely that such parenchymal changes, along with modifications in patient characteristics, may impede anticipated improvements in results after liver resection.11,12 Hence, the aims of the current study were to assess changes in both the profile of patients undergoing hepatectomy and factors predictive of mortality in these patients in a single tertiary care centre over a 10-year period in order to define standard expectations in hepatectomy.
Materials and methods
All patients who underwent elective liver resections between January 2000 and December 2009 at Beaujon Hospital, Clichy, France, were included. During the study period, 2012 elective hepatectomies were performed in 1958 patients. Data for this 10-year period were collected prospectively and analysed retrospectively. Patients who underwent resection after liver transplantation, biliary cyst fenestration or excisional biopsy were excluded. To assess a possible change in the patient profile and its influence on the evolution in mortality rates over time, the 2000–2009 study period was arbitrarily divided into two periods of equal duration (2000–2004 and 2005–2009) and data for the 1990s were extracted from analyses previously reported by the present group.13 Accordingly, several parameters, including preoperative characteristics (age >60 years, existence of an associated medical comorbidity), indication for liver resection (benign versus malignant disease), extent of resection and mortality rate were compared across these different periods.
Preoperative management
Standard preoperative workup included blood analysis with liver tests and imaging by computed tomography or magnetic resonance imaging in all patients. Tumour and parenchymal biopsies were performed selectively. Preoperative portal vein embolization (PVE) (n = 120) prior to right or extended right hepatectomy was undertaken when the anticipated future liver remnant was <25% of liver size in patients with strictly normal liver and <35–40% in patients with underlying liver disease.5 Preoperative PVE was preceded by transarterial chemoembolization in 36 HCC patients. No patients with cirrhosis of Child–Pugh classes B or C underwent surgery. Among 546 (27.1%) patients operated for CLM, 401 (73.4%) received neoadjuvant chemotherapy. Preoperative endoscopic or percutaneous biliary drainage was performed in 53 (2.6%) jaundiced patients with biliary tumours involving the confluence in order to achieve a preoperative bilirubin level of <50 μmol/l.
Operative procedure
Intraoperative assessment with conventional B-mode sonography, parenchymal transection and haemostasis was performed as previously described.13 However, since 2000, several techniques that were not commonly used during this group's previously reported experience, such as the laparoscopic approach and the hanging manoeuvre,14 were performed in 93 (4.6%) and 719 (66.5% of all major resections) patients, respectively. From 2000, only 44 patients (2.2%) were operated through thoracoabdominal incisions. The routine use of Doppler sonography greatly facilitated surgery in cases of extensive hepatectomy with involvement of the portal vein, major hepatic veins, vena cava or hepatic artery requiring vascular reconstruction. Major extrahepatic resections were undertaken in 257 patients (12.8%); these included colonic resections in 120 patients, pancreas or duodenum resections in 50 patients, stomach resections in 17 patients and other types of resections in the remaining 70 patients. Major resection was defined as the resection of three or more adjacent segments. Intraoperative variables analysed included operative time, estimated blood loss, transfusion requirements, duration of vascular clamping, extent of resection, and associated liver (vascular or biliary) and extrahepatic procedures.
Histological analysis
In addition to tumour characteristics, the underlying liver parenchyma was assessed for the existence of fibrosis graded from F0 to F4.15 Fibrosis of stages F3 and F4 was considered severe. Steatosis of >30% based on the percentage of hepatocytes with fat droplets was considered significant. In patients with CLM who received preoperative chemotherapy, chemotherapy-induced liver injury was considered significant if it resulted in chemotherapy-associated steatohepatitis (CASH)10 and/or sinusoidal obstruction syndrome (SOS) including lesions of grades 2 or 3 8,9 and nodular regenerative hyperplasia.16
Postoperative care
Postoperative complications were stratified according to the Clavien–Dindo classification.17 Major complications (Clavien–Dindo classes 3 and 4) and operative mortality (Clavien–Dindo class 5) were considered when they occurred within 90 days after surgery or at any time during postoperative hospitalization. Specific liver complications that frequently develop after major liver surgery were described as follows: (i) liver failure was defined according to the ‘50-50 criteria’ on postoperative day 5 as previously reported;18 (ii) ascites was defined as >10 ml/kg/day of drainage output from the abdomen after postoperative day 5,19 and (iii) bile leakage was defined as a bilirubin concentration in the drain fluid at least three times that of serum bilirubin on or after postoperative day 3 or as the need for radiological or operative intervention resulting from biliary collections or bile peritonitis.20 In the majority of patients, the diagnosis of biliary fistula was first suspected macroscopically and subsequently confirmed biologically.
Statistical analysis
Continuous variables are expressed as mean values with ranges and were compared using the Mann–Whitney U-test. Categorical variables were compared using the chi-squared test with Yates' correction or Fisher's exact test as appropriate. Univariate analysis was used to examine the relationships between mortality and several clinical, operative and pathological parameters. All variables achieving statistical significance at a level of 0.1 in the univariate analysis for predictive factors for mortality were considered for multivariate analysis. A backward variable procedure with a P-value cut-off of 0.05 was used to identify independent factors predictive of mortality in the whole group with malignant disease, as well as in the three subgroups of patients with HCC, CLM and biliary tumours, respectively. All statistical tests were two-sided. For all tests, statistical significance was defined by a P-value of <0.05. Data were analysed using spss Version 18.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Results
Indications for the 2012 elective hepatectomies performed during the study period are summarized in Table 1. The vast majority (72.2%) of liver resections were performed for malignant lesions, of which CLM represented the most common. Liver cell adenoma was the most frequently operated benign lesion. Whereas the overall number of hepatectomies remained stable during the study period, the rate of resection for benign disease significantly declined from 33.5% (61 of 182) in 2000 to 17.8% (40 of 225) in 2009 (P < 0.001).
Table 1.
Indications for liver resection in 2012 patients
| Indication | n (%) |
|---|---|
| Malignant (n = 1453) | |
| Primary tumours | 701 (48.2%) |
| Hepatocellular carcinoma | 450 (30.9%) |
| Biliary tumours | 194 (13.4%) |
| Intrahepatic carcinoma | 81 (5.6%) |
| Hilar carcinoma | 78 (5.4%) |
| Gallbladder carcinoma | 35 (2.4%) |
| Others | 57 (3.9%) |
| Secondary tumours | 752 (51.8%) |
| Colorectal metastases | 546 (37.6%) |
| Neuroendocrine metastases | 115 (7.9%) |
| Other metastases | 91 (6.3%) |
| Benign (n = 559) | |
| Adenoma | 129 (23.1%) |
| Focal nodular hyperplasia | 61 (10.9%) |
| Haemangioma | 32 (5.7%) |
| Hydatid and cystic disease | 77 (13.8%) |
| Polycystic liver disease | 40 (7.2%) |
| Living donor transplant | 93 (16.6%) |
| Hepatolithiasis | 32 (5.7%) |
| Others | 95 (16.9%) |
Patient characteristics
The mean age of patients was 54.4 years (range: 14–85 years) and 44.0% of patients displayed at least one associated medical comorbidity. As Table 2 shows, the comparison of preoperative variables between patients with malignant and benign disease, respectively, revealed that patients with malignancy were significantly older and displayed greater comorbidity, including metabolic factors. An abnormal underlying parenchyma was more frequently observed in patients with malignant lesions than in those with benign lesions. Specifically, severe fibrosis and significant chemotherapy-associated liver injury were present in 62.0% and 35.0% of HCC and CLM patients, respectively.
Table 2.
Preoperative characteristics
| Malignant disease group (n = 1453) | Benign disease group (n = 559) | P-value | |
|---|---|---|---|
| Age, years, mean (range) | 58.7 (14–85) | 42.8 (15–84) | <0.001 |
| Age >60 years, n (%) | 735 (50.6%) | 80 (14.3%) | <0.001 |
| Female gender, n (%) | 533 (36.7%) | 372 (66.5%) | <0.001 |
| BMI, kg/m2, mean (range) | 25.2 (14.6–45.4) | 24.2 (14.3–48.9) | <0.001 |
| BMI of >30 kg/m2, n (%) | 215 (14.8%) | 65 (11.6%) | 0.065 |
| Comorbidity, n (%) | 728 (50.1%) | 157 (28.1%) | <0.001 |
| Diabetes | 220 (15.1%) | 24 (4.3%) | <0.001 |
| Hypertension | 470 (32.3%) | 78 (13.9%) | <0.001 |
| Cardiac disease | 148 (10.2%) | 22 (3.9%) | <0.001 |
| Pulmonary disease | 156 (10.9%) | 36 (6.4%) | 0.003 |
| ASA class ≥3, n (%) | 103 (7.1%) | 10 (1.8%) | <0.001 |
| Underlying parenchyma, n (%) | |||
| Normal | 525 (36.1%) | 328 (58.7%) | <0.001 |
| Steatosis of >30% | 189 (13.1%) | 44 (7.9%) | 0.001 |
| Fibrosis of F1/F2 | 454 (31.3%) | 128 (22.9%) | <0.001 |
| Fibrosis of F3/F4 | 362 (24.9%) | 41 (7.3%) | <0.001 |
| Chemotherapy-induced injurya, n (%) | 249 (66.9%) | 0 | – |
Chemotherapy-induced liver injury was only observed in the group with colorectal liver metastases.
BMI, body mass index
ASA, American Society of Anesthesiologists.
Surgical procedures
Intraoperative data for the entire series are summarized in Table 3. Patients with malignant disease underwent more complex resections with higher rates of associated vascular, biliary and extrahepatic procedures, longer operative times and increased blood loss, and more frequently required transfusion as well as longer vascular occlusion. Major hepatectomy was performed in 1084 (53.9%) patients and included right or extended right hepatectomy in 696 (34.6%) cases. Although the rates of major hepatectomy were similar in the groups with benign and malignant disease, patients with malignant disease who underwent major resection had increased blood loss (883.6 ml versus 774.6 ml; P < 0.001) and more often required vascular occlusion (77.4% versus 63.2%; P < 0.001) compared with those undergoing major resection for benign disease. Of the 205 patients with malignant disease who required extrahepatic procedures, 80 patients with CLM underwent concomitant colonic resection. Of these, only 16 (20.0%) procedures were performed along with major hepatectomy. Among the 152 (10.5%) patients with malignant disease who required vascular reconstruction, 38 (25.0%) had biliary cancer, 56 (36.8%) had CLM, 34 (22.4%) had HCC and the remaining 24 (15.8%) had other types of hepatic malignancies. Almost 20% of the 194 patients with biliary cancer required vascular reconstruction involving the portal vein (n = 33), hepatic veins or inferior vena cava (n = 12) and/or the hepatic artery (n = 7).
Table 3.
Intraoperative characteristics
| Benign disease group (n = 559) | Malignant disease group | ||||
|---|---|---|---|---|---|
| Overall (n = 1453) | HCC (n = 450) | CLM (n = 546) | Biliary tumours (n = 194) | ||
| Major hepatectomy, n (%) | 299 (53.5%) | 783 (53.9%) | 206 (45.8%)a,b | 284 (52.1%)c | 151 (77.8%) |
| Associated procedures, n (%) | 60 (10.8%)d | 399 (27.5%) | 52 (11.6%)a,b | 141 (25.9%)c | 113 (58.2%) |
| Extrahepatic | 16 (2.7%)d | 205 (14.1%) | 19 (4.2%)a,b | 94 (17.2%)c | 20 (10.3%) |
| Vascular | 9 (1.6%)d | 153 (10.5%) | 34 (7.6%)b | 56 (10.3%)c | 38 (19.6%) |
| Biliary | 40 (7.2%) | 128 (8.8%) | 6 (1.3%)b | 12 (2.2%)c | 103 (53.1%) |
| Operative time, min, mean (range) | 306.55 (30–942)d | 325.18 (20–765) | 285.14 (60–765)a,b | 323.93 (20–690)c | 425 (160–755) |
| Estimated blood loss, ml, mean (range) | 525.21 (0–13000)d | 633 (14–11000) | 681.27 (0–11000) | 607.89 (0–6000) | 698.17 (0–3500) |
| Transfusion, n (%) | 86 (15.3%)d | 330 (22.7%) | 91 (20.2%)b | 114 (20.9%)c | 61 (31.4%) |
| Pedicle clamping, n (%) | 281 (50.3%)d | 930 (64.0%) | 290 (64.4%)b | 347 (63.5%)c | 141 (72.7%) |
| Duration, min, mean (range) | 37.4 (0–212)d | 42.2 (5–150) | 42.8 (1–150) | 40.8 (3–148) | 43.4 (7–140) |
Marks a significant difference between HCC and CLM.
Marks a significant difference between HCC and biliary tumours.
Marks a significant difference between CLM and biliary tumours.
Marks a significant difference between the benign and malignant disease groups.
HCC, hepatocellular carcinoma; CLM, colorectal liver metastasis.
Perioperative course
As Table 4 shows, 1134 (56.4%) patients experienced postoperative complications, including 420 (20.9%) of Clavien–Dindo class 3 or 4. Pulmonary complications were noted in 408 (20.3%) patients. Specific post-hepatectomy complications included ascites in 418 (20.8%), abdominal collection requiring drainage in 167 (8.3%), biliary fistula in 152 (7.6%) and liver failure in 41 (2.0%) patients. Among the 100 (5.0%) patients requiring reoperation, 26 underwent laparotomy for haemorrhage. The incidence of perioperative complications was significantly lower in the group with benign disease, in whom both intensive care unit and overall hospital stays were lower than in the malignant disease group. Specifically, in the malignant disease group, the incidence of major complications was 41.8% in the subgroup of patients with biliary malignancy, compared with 26.8% and 22.7% in patients who underwent liver resection for HCC or CLM, respectively (both P < 0.05). There were 70 postoperative deaths, giving overall perioperative mortality of 3.5%. Mortality was significantly lower in patients undergoing surgery for benign lesions compared with those with malignant disease (P < 0.001).
Table 4.
Postoperative outcomes
| Benign disease group (n = 559) | Malignant disease group (n = 1453) | CLM (n = 546) | HCC (n = 450) | Biliary tumours (n = 194) | |
|---|---|---|---|---|---|
| Mortality, n (%) | 39 (50.7%)a | 65 (4.5%) | 9 (1.7%)b | 29 (6.4%) | 16 (8.2%)d |
| Overall morbidity, n (%) | 267 (47.7%)a | 870 (59.9%) | 286 (52.3%)b | 927 (63.8%)c | 146 (75.3%)d |
| Clavien–Dindo class ≥3, n (%) | 97 (17.4%)a | 392 (27%) | 124 (22.7%) | 121 (26.8%)c | 81 (41.8%)d |
| Liver-specific complications, n (%) | |||||
| Pulmonary | 96 (17.1%)a | 120 (21.5%) | 100 (18.4%) | 98 (21.8%) | 54 (27.8%)d |
| Liver failure | 22 (0.4%)a | 39 (2.7%) | 13 (2.4%) | 9 (2.0%)c | 10 (5.2%)d |
| Ascites | 69 (12.4%)a | 135 (24.1%) | 98 (18%)b | 131 (29.1%) | 67 (34.5%)d |
| Biliary fistula | 39 (7.0%) | 113 (7.8%) | 112 (7.7%) | 28 (6.2%)c | 27 (13.9%)d |
| Reoperation, n (%) | 16 (2.9%) | 84 (5.8%) | 51 (3.5%)b | 31 (6.9%) | 16 (8.2%)d |
| Intensive care unit stay, days, mean (range) | 3.9 (1–52)a | 6.1 (1–67) | 5.5 (1–62) | 6.4 (1–57) | 7.4 (1–67)d |
| Hospital stay, days, mean (range) | 11.3 (1–90)a | 14.6 (1–96) | 13.4 (1–82) | 14.2 (3–84)c | 19.5 (2–81)d |
Marks a significant difference between the benign and malignant disease groups.
Marks a significant difference between CLM and HCC.
Marks a significant between HCC and biliary malignancy.
Marks a significant difference between biliary malignancy and CLM.
HCC, hepatocellular carcinoma; CLM, colorectal liver metastasis.
Patient-related factors associated with mortality
Four (0.7%) postoperative deaths were observed in the 559 patients operated for benign conditions. These deaths occurred in three patients who underwent major hepatectomy for hepatolithiasis and who succumbed to uncontrollable sepsis, and in one living donor after right hepatectomy, who was found to have previously undiagnosed myeloma.21 Overall mortality in patients with malignant disease was 4.5%. A subgroup analysis revealed a difference in mortality rates between distinct aetiologies: patients with CLM had a mortality rate of only 1.7%, which was significantly lower than the 6.4% rate in HCC patients (P < 0.001) and the 8.2% rate in patients with biliary tumours (P < 0.001). Multiple preoperative, operative and histological variables were associated with increased risk for mortality in univariate analysis (Table 5). The univariate analysis showed significance for male sex, age >60 years, existence of associated comorbidity, American Society of Anesthesiologists (ASA) score of ≥3, major resection, transfusion, blood loss of >1000 ml, vascular clamping, associated vascular or bile duct procedures, presence of severe fibrosis, steatosis of >30% and chemotherapy-induced liver injury in patients operated for CLM (P < 0.001). Within the study period, neither the 2000–2004 nor the 2005–2009 time interval proved to be a risk factor for mortality in univariate analysis (P = 0.261). In multivariate analysis (Table 5), common risk factors for mortality for all three types of malignancy were age >60 years, ASA score of ≥3, need for inflow clamping, increased blood loss, transfusion and steatosis of >30%. In patients with CLM, the presence of chemotherapy-induced liver injury [odds ratio (OR) 2.568, 95% confidence interval (CI) 1.312–9.467; P = 0.003] and need for associated vascular (OR = 10.048, 95% CI 5.26–18.371; P = 0.03) or biliary (OR = 13.125, 95% CI 2.488–69.795; P = 0.018) procedures were independent risk factors for mortality. In the subgroup of HCC patients, mortality was also increased in patients undergoing major resection and with underlying liver fibrosis of F3–F4 (OR = 4.101, 95% CI 1.402–11.997; P = 0.006), whereas in patients with biliary malignancy, mortality was also increased by an associated vascular procedure (OR = 11.116, 95% CI 2.083–19.332; P = 0.005).
Table 5.
Univariate and multivariable analysis for mortality in patients with malignant disease
| Malignant disease group | |||||
|---|---|---|---|---|---|
| n (%) | P-value (UV) | OR | 95% CI | P-value (MV) | |
| Male | 52 (5.7%) | 0.008 | – | – | NS |
| Age >60 years | 40 (5.4%) | 0.096 | 1.530 | 1.123–2.534 | 0.036 |
| ASA class ≥3 | 11 (9.7%) | <0.001 | 3.003 | 1.515–5.952 | 0.017 |
| BMI > 30 kg/m2 | 14 (6.5%) | 0.069 | – | – | NS |
| Blood loss >1000 ml | 26 (10.6%) | <0.001 | 3.963 | 2.315–6.783 | <0.001 |
| Transfusion | 44 (13.3%) | <0.001 | 7.692 | 4.538–13.043 | <0.001 |
| Major resection | 53 (6.8%) | <0.001 | 3.664 | 1.979–6.781 | 0.008 |
| Vascular procedure | 24 (15.6%) | <0.001 | 5.559 | 3.262–9.746 | <0.001 |
| Bile duct resection | 15 (11.7%) | <0.001 | – | – | NS |
| Fibrosis F3–F4 | 35 (9.6%) | <0.001 | 3.511 | 2.131–5.785 | <0.001 |
| Steatosis >30% | 16 (8.5%) | 0.005 | 2.273 | 1.257–4.111 | <0.001 |
| Vascular occlusion | 57 (6.1%) | <0.001 | 3.697 | 1.815–7.530 | <0.001 |
UV, univariate analysis
OR, odds ratio
95% CI, 95% confidence interval
MV, multivariate analysis
NS, not significant
ASA, American Society of Anesthesiologists
BMI, body mass index.
Relationship between evolution in the profile of liver resection patients and mortality
As Fig. 1 shows, rates of patients aged >60 years, patients with medical comorbidities, patients undergoing resection for malignant disease, patients undergoing major hepatectomy and patients displaying a diseased underlying parenchyma linearly increased over the study periods. Mortality rates did not differ significantly within the study period (3.8% in 2000–2004 versus 3.1% in 2005–2009; P = 0.334), nor between this centre's previously reported experience in the 1990s (4.4%)13 and the overall rate during 2000–2009 (3.5%) (P = 0.247).
Figure 1.
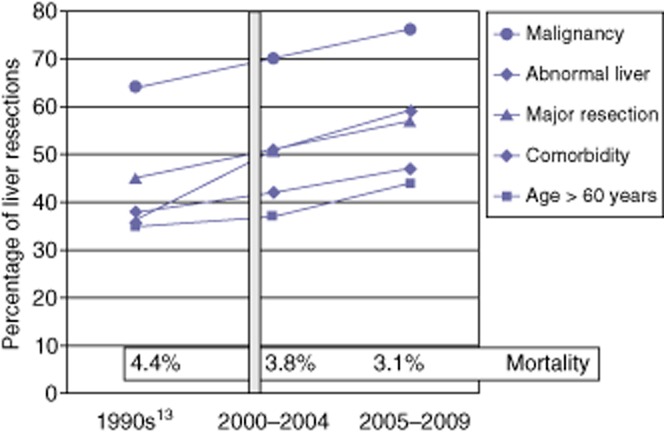
Data for patients undergoing liver resection during the 1990s, 2000–2004 and 2005–2009, showing rates of malignancy, abnormal liver, major resection, comorbidity and age >60 years
Discussion
The current study demonstrates that the extending of indications for liver resection has led to a linear increase in the rate of elderly patients displaying associated comorbidity and undergoing major resection for malignant lesions on diseased parenchyma. The present results furthermore emphasize that these changes in the patient profile are associated with the stabilizing of mortality within the study period and in comparison with data previously reported from this centre.13
In the present series, both the mortality rate of <1% and the severe morbidity rate of <20% observed in the 559 patients undergoing liver resection for benign conditions meet the expected targets of high-volume hepatopancreatobiliary units. Although most patients with benign lesions were younger, displayed less comorbidity and generally underwent liver resection with a normal hepatic parenchyma, more than half of them underwent major resections. This clearly illustrates that the improvements in postoperative outcomes following liver resection represent the consequence of refinements in both surgical technique and perioperative management.1–4 In this context, some authors have emphasized the benefits of laparoscopy and encourage its use.22 The present series included only a limited laparoscopic experience, which mainly concerned highly selected patients with benign lesions in normal parenchyma. This does not allow the drawing of any conclusions on the use of laparoscopic hepatectomy, not least because indications for the resection of benign liver lesions continue to decrease. Undoubtedly, both more precise preoperative diagnosis and better knowledge of the natural history of benign lesions have led to a decrease in the rate of liver resection for benign liver tumours, including adenoma, for which resection is now indicated only in patients with a specific immunohistochemical profile or with lesions of >5 cm in size.23 A similar trend was observed in living donor liver transplantation, in which both specific and persistent risks associated with major resection are likely to explain the decreasing use of this strategy as long as cadaveric grafts are available.24,25 In addition to one postoperative death observed in a living donor,21 three deaths occurred after major resection in patients with hepatolithiasis, a disease characterized by both chronic liver infection and abnormal underlying parenchyma.
In the present series, the risk for mortality was six-fold higher in patients operated for malignant disease than in those undergoing liver resection for benign lesions. This result was related to the combination of several known factors, including patients' comorbidity and underlying liver status, as well as some technical parameters. Although the influence of advanced age per se on the postoperative course after liver resection is still under debate,26–28 the current study confirms the impact of the increase in the incidence of associated medical comorbidity in parallel with increasing age on both mortality and morbidity rates in patients with malignant disease. One explanation for this is that these patients displayed significantly more metabolic risk factors, including diabetes, hypertension and dyslipidaemia. It is likely that the existence of such risk factors was responsible for a higher rate of underlying cardiorespiratory disease that led to the occurrence of more cardiorespiratory events.29–31 However, it is obvious that the metabolic syndrome itself accounted for increases in both underlying significant steatosis and steatohepatitis, which are now known to directly or indirectly adversely affect the postoperative course.32–34 Another explanation is that the very nature of the malignancy is associated with several pathological changes in the underlying liver, as was found in more than sixty percent of patients in the present series. It is indeed well known that most HCC lesions develop on diseased underlying parenchyma, including parenchyma with severe fibrosis and/or massive fatty infiltration,35–37 and that the widespread use of chemotherapy in patients with CLM is associated with an increasing rate of chemotherapy-associated liver injury, including SOS8,9 and CASH.10 In the current study, as in others,9,10,35–37 these underlying conditions were independent significant risk factors for postoperative mortality. The last plausible explanation is that the extending of indications for liver resection in patients with malignancy has resulted in the performance of more extensive and complex resections. In the present series, both major liver resection and associated vascular procedures were performed more frequently than in this centre's previously reported experience.13 This finding is likely to account for the fact that the oncological rules require an anatomic resection38–40 and it is therefore unlikely that rates of major liver resection in patients with HCC and biliary tumours will decrease. Nevertheless, despite a low rate of postoperative liver failure in the group of patients with malignant disease, major liver resection in patients with abnormal underlying parenchyma associated with vascular reconstruction yielded a dramatically high mortality rate.41 In such situations, a fine balance between caution and the necessary radical surgery should be sought, as in patients with initially unresectable CLM.42
This study has several limitations as a result of its retrospective nature. Although all resected patients were categorized as having Child–Pugh class A disease, no correlation between the accurate assessment of liver function and postoperative outcome could be demonstrated. Platelet counts,18,43,44 as well as indocyanine green (ICG) clearance3,4,45 are often used to estimate the risk for postoperative liver failure and early death. However, these tests may not adequately distinguish between cirrhosis and lesser forms of pathological changes45 and may therefore preclude resection in otherwise suitable candidates. Another drawback refers to the inability to determine the existence of features of non-alcoholic steatohepatitis (NASH) in the underlying liver in all of the patients in the present series. This series covers a 10-year period, at the beginning of which NASH was not routinely assessed. Several series have recently emphasized the negative impact of NASH on the postoperative course34,35 and it is likely that, given the influence of massive steatosis found in the present report, NASH would also have been an important risk factor for both morbidity and mortality.
In conclusion, as a result of the increased proportion of high-risk patients undergoing major resection for malignancy, mortality after liver resection is still far from zero. In order to improve results after liver resection, it is important that more conservative strategies that include parenchyma-sparing procedures as well as oncological laparoscopic approaches continue to be developed.
Conflicts of interest
None declared.
References
- 1.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection. Analysis of 1803 consecutive cases over the past decade. Ann Surg. 2002;236:397–407. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases. Analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–710. doi: 10.1097/01.sla.0000141195.66155.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 4.Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Yamashita K, et al. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg. 2010;211:443–449. doi: 10.1016/j.jamcollsurg.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mise Y, Hasegawa K, Satou S, Aoki T, Beck Y, Sugawara Y, et al. Venous reconstruction based on virtual liver resection to avoid congestion in the liver remnant. Br J Surg. 2011;98:1742–1751. doi: 10.1002/bjs.7670. [DOI] [PubMed] [Google Scholar]
- 7.Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, et al. Continuous versus intermittent portal trial clamping for liver resection. Ann Surg. 1999;229:369–375. doi: 10.1097/00000658-199903000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 9.Soubrane O, Brouquet A, Zalinski S, Terris B, Brézault C, Mallet V, et al. Predicting high grade lesions of sinusoidal obstruction syndrome related to oxaliplatin-based chemotherapy for colorectal liver metastases: correlation with post-hepatectomy outcome. Ann Surg. 2010;251:454–460. doi: 10.1097/SLA.0b013e3181c79403. [DOI] [PubMed] [Google Scholar]
- 10.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 11.Cescon M, Vetrone G, Grazi GL, Ramacciato G, Ercolani G, Ravaioli M, et al. Trends in perioperative outcome after hepatic resection. Analysis of 1500 consecutive unselected cases over 20 years. Ann Surg. 2009;249:995–1002. doi: 10.1097/SLA.0b013e3181a63c74. [DOI] [PubMed] [Google Scholar]
- 12.de Haas RJ, Wicherts DA, Andreani P, Pascal G, Saliba F, Ichai P, et al. Impact of expanding criteria for resectability of colorectal metastases on short- and longterm outcomes after hepatic resection. Ann Surg. 2011;253:1069–1079. doi: 10.1097/SLA.0b013e318217e898. [DOI] [PubMed] [Google Scholar]
- 13.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 14.Belghiti J, Guevara OA, Noun R, Saldinger PF, Kianmanesh R. Liver hanging manoeuvre: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg. 2001;193:109–111. doi: 10.1016/s1072-7515(01)00909-7. [DOI] [PubMed] [Google Scholar]
- 15.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 16.Wicherts DA, de Haas RJ, Sebagh M, Ciacio O, Lévi F, Paule B, et al. Regenerative nodular hyperplasia of the liver related to chemotherapy: impact on outcome of liver surgery for colorectal metastases. Ann Surg Oncol. 2011;18:659–669. doi: 10.1245/s10434-010-1385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The ‘50-50’ criteria on postoperative day 5. An accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–829. doi: 10.1097/01.sla.0000189131.90876.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishizawa T, Hasegawa K, Kokudo N, Sano K, Imamura H, Beck Y, et al. Risk factors and management of ascites after liver resection to treat hepatocellular carcinoma. Arch Surg. 2009;144:46–51. doi: 10.1001/archsurg.2008.511. [DOI] [PubMed] [Google Scholar]
- 20.Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Melloul E, Dondero F, Paugam-Burtz C, Bouadma L, Arnulf B, Belghiti J. Living liver donor death related to complications of myeloma. Liver Transpl. 2009;15:326–329. doi: 10.1002/lt.21685. [DOI] [PubMed] [Google Scholar]
- 22.Kneuertz PJ, Marsh JW. Jong MC, Covert K, Hyder O, Hirose K, et al. Improvements in quality of life after surgery for benign hepatic tumours: results from a dual centre analysis. Surgery. 2012;152:193–201. doi: 10.1016/j.surg.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Dokmak S, Paradis V, Vilgrain V, Sauvanet A, Farges O, Valla D, et al. A single-centre surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009;137:1698–1705. doi: 10.1053/j.gastro.2009.07.061. [DOI] [PubMed] [Google Scholar]
- 24.Belghiti J. Will improved donor safety increase liver donations? Transplantation. 2009;88:19–20. doi: 10.1097/TP.0b013e3181a9ea53. [DOI] [PubMed] [Google Scholar]
- 25.Organ Procurement and Transplantation Network. 2012. Living donor transplants in the US (1988–2012) . Available at http://optn.transplant.hrsa.gov/latestData/rptData.asp (last accessed 1 October 2012)
- 26.Menon KV, Al-Mukhtar A, Aldouri A, Prasad RK, Lodge PA, Toogood GJ. Outcomes after major hepatectomy in elderly patients. J Am Coll Surg. 2006;203:677–683. doi: 10.1016/j.jamcollsurg.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 27.Shirabe K, Kajiyama K, Harimoto N, Gion T, Tsujita E, Abe T, et al. Early outcome following hepatic resection in patients older than 80 years of age. World J Surg. 2009;33:1927–1932. doi: 10.1007/s00268-009-0122-3. [DOI] [PubMed] [Google Scholar]
- 28.Adam R, Frilling A, Elias D, Laurent C, Ramos E, Capussotti L, et al. Liver resection of colorectal metastases in elderly patients. Br J Surg. 2010;97:366–376. doi: 10.1002/bjs.6889. [DOI] [PubMed] [Google Scholar]
- 29.Bhayani NH, Hyder O, Frederick W, Schulick RD, Wolgang CL, Hirose K, et al. Effect of metabolic syndrome on perioperative outcomes after liver surgery: a National Surgical Quality Improvement Program (NSQIP) analysis. Surgery. 2012;152:218–226. doi: 10.1016/j.surg.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanwagner LB, Bhave M, Te HS, Feinglass J, Alvarez L, Rinella ME. Patients transplanted for non-alcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology. 2012;56:1741–1750. doi: 10.1002/hep.25855. [DOI] [PubMed] [Google Scholar]
- 31.Laish I, Braun M, Mor E, Sulkes J, Harif Y, Ben Ari Z. Metabolic syndrome in liver transplant recipients: prevalence, risk factors, and association with cardiovascular events. Liver Transpl. 2011;17:15–22. doi: 10.1002/lt.22198. [DOI] [PubMed] [Google Scholar]
- 32.de Meijer VE, Kalish BT, Puder M, Ijzermans JN. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br J Surg. 2010;97:1331–1339. doi: 10.1002/bjs.7194. [DOI] [PubMed] [Google Scholar]
- 33.McCormack L, Petrowsky H, Jochum W, Furrer K, Clavien PA. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case–control study. Ann Surg. 2007;245:923–930. doi: 10.1097/01.sla.0000251747.80025.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kooby DA, Fong Y, Suriawinata A, Gonen M, Allen PJ, Klimstra DS, et al. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–1044. doi: 10.1016/j.gassur.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Reddy SK, Marsh JW, Varley PR, Mock BK, Chopra KB, Geller DA, et al. Underlying steatohepatitis, but not simple hepatic steatosis, increases morbidity after liver resection: a case–control study. Hepatology. 2012;56:2221–2230. doi: 10.1002/hep.25935. [DOI] [PubMed] [Google Scholar]
- 36.Cauchy F, Zalinski S, Dokmak S, Fuks D, Farges O, Castera L, et al. Surgical treatment of HCC associated with the metabolic syndrome. Br J Surg. 2013;100:113–121. doi: 10.1002/bjs.8963. [DOI] [PubMed] [Google Scholar]
- 37.Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J. Risk of major liver resection in patients with underlying chronic liver disease. A reappraisal. Ann Surg. 1999;229:210–215. doi: 10.1097/00000658-199902000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imamura H, Matsuyama Y, Miyagawa Y, Ishida K, Shimada R, Miyagawa S, et al. Prognostic significance of anatomical resection and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma. Br J Surg. 1999;86:1032–1038. doi: 10.1046/j.1365-2168.1999.01185.x. [DOI] [PubMed] [Google Scholar]
- 39.Neuhaus P, Jonas S, Bechstein WO, Lohmann R, Radke C, Kling N, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–819. doi: 10.1097/00000658-199912000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agrawal S, Belghiti J. Oncologic resection for malignant tumours of the liver. Ann Surg. 2011;253:656–665. doi: 10.1097/SLA.0b013e3181fc08ca. [DOI] [PubMed] [Google Scholar]
- 41.Bachellier P, Rosso E, Pessaux P, Oussoultzoglou E, Nobili C, Panaro F, et al. Risk factors for liver failure and mortality after hepatectomy associated with portal vein resection. Ann Surg. 2011;253:173–179. doi: 10.1097/SLA.0b013e3181f193ba. [DOI] [PubMed] [Google Scholar]
- 42.Cauchy F, Aussilhou B, Dokmak S, Fuks D, Gaujoux S, Farges O, et al. Reappraisal of the risks and benefits of major liver resection in patients with initially unresectable colorectal liver metastases. Ann Surg. 2012;256:746–752. doi: 10.1097/SLA.0b013e3182738204. [DOI] [PubMed] [Google Scholar]
- 43.Yang T, Zhang J, Lu JH, Yang GS, Wu MC, Yu WF. Risk factors influencing postoperative outcomes of major hepatic resection of hepatocellular carcinoma for patients with underlying liver diseases. World J Surg. 2011;35:2073–2082. doi: 10.1007/s00268-011-1161-0. [DOI] [PubMed] [Google Scholar]
- 44.Sima CS, Jarnagin WR, Fong Y, Elkin E, Fischer M, Wuest D, et al. Predicting the risk of perioperative transfusion for patients undergoing elective hepatectomy. Ann Surg. 2009;250:914–921. doi: 10.1097/sla.0b013e3181b7fad3. [DOI] [PubMed] [Google Scholar]
- 45.Lam CM, Fan ST, Lo CM, Wong J. Major hepatectomy for hepatocellular carcinoma in patients with an unsatisfactory indocyanine green clearance test. Br J Surg. 1999;86:1012–1017. doi: 10.1046/j.1365-2168.1999.01204.x. [DOI] [PubMed] [Google Scholar]


