Abstract
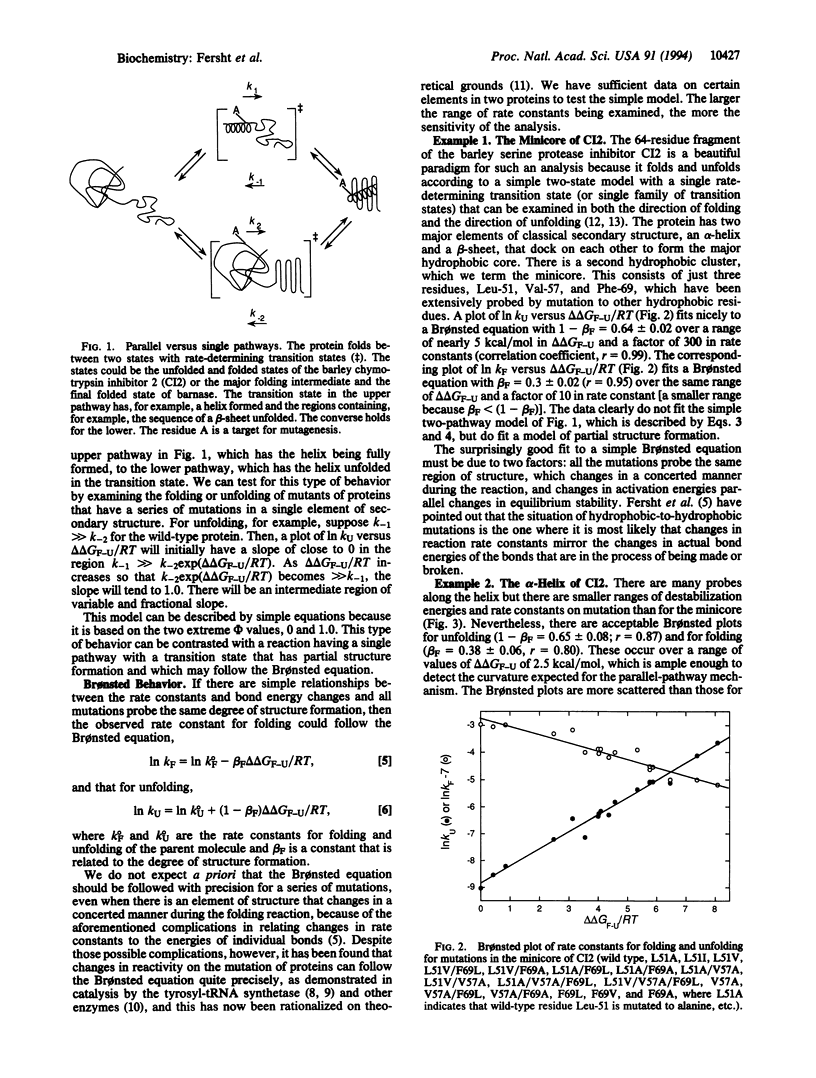
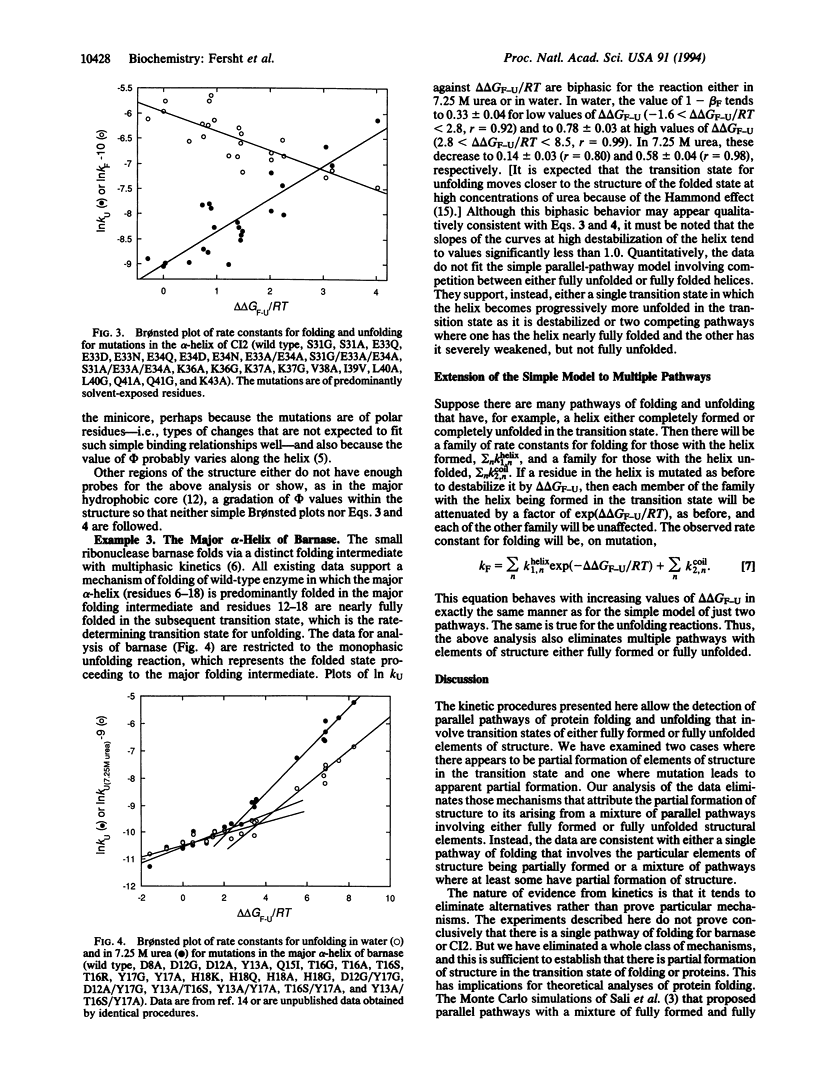
Protein engineering and kinetic experiments indicate that some regions of proteins have partially formed structure in the transition state for protein folding. A crucial question is whether there is a genuine single transition state that has interactions that are weakened in those regions or there are parallel pathways involving many transition states, some with the interactions fully formed and others with the structural elements fully unfolded. We describe a kinetic test to distinguish between these possibilities. The kinetics rule out those mechanisms that involve a mixture of fully formed or fully unfolded structures for regions of the barley chymotrypsin inhibitor 2 and barnase, and so those regions are genuinely only partially folded in the transition state. The implications for modeling of protein folding pathways are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avis J. M., Fersht A. R. Use of binding energy in catalysis: optimization of rate in a multistep reaction. Biochemistry. 1993 May 25;32(20):5321–5326. doi: 10.1021/bi00071a006. [DOI] [PubMed] [Google Scholar]
- Baldwin R. L. Protein folding. Matching speed and stability. Nature. 1994 May 19;369(6477):183–184. doi: 10.1038/369183a0. [DOI] [PubMed] [Google Scholar]
- Caflisch A., Karplus M. Molecular dynamics simulation of protein denaturation: solvation of the hydrophobic cores and secondary structure of barnase. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1746–1750. doi: 10.1073/pnas.91.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht A. R. Jubilee Lecture. Pathway and stability of protein folding. Biochem Soc Trans. 1994 May;22(2):267–273. doi: 10.1042/bst0220267. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Leatherbarrow R. J., Wells T. N. Structure-activity relationships in engineered proteins: analysis of use of binding energy by linear free energy relationships. Biochemistry. 1987 Sep 22;26(19):6030–6038. doi: 10.1021/bi00393a013. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Matouschek A., Serrano L. The folding of an enzyme. I. Theory of protein engineering analysis of stability and pathway of protein folding. J Mol Biol. 1992 Apr 5;224(3):771–782. doi: 10.1016/0022-2836(92)90561-w. [DOI] [PubMed] [Google Scholar]
- Fersht A. R. The sixth Datta Lecture. Protein folding and stability: the pathway of folding of barnase. FEBS Lett. 1993 Jun 28;325(1-2):5–16. doi: 10.1016/0014-5793(93)81405-o. [DOI] [PubMed] [Google Scholar]
- Harrison S. C., Durbin R. Is there a single pathway for the folding of a polypeptide chain? Proc Natl Acad Sci U S A. 1985 Jun;82(12):4028–4030. doi: 10.1073/pnas.82.12.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S. E., Fersht A. R. Folding of chymotrypsin inhibitor 2. 1. Evidence for a two-state transition. Biochemistry. 1991 Oct 29;30(43):10428–10435. doi: 10.1021/bi00107a010. [DOI] [PubMed] [Google Scholar]
- Jackson S. E., Fersht A. R. Folding of chymotrypsin inhibitor 2. 2. Influence of proline isomerization on the folding kinetics and thermodynamic characterization of the transition state of folding. Biochemistry. 1991 Oct 29;30(43):10436–10443. doi: 10.1021/bi00107a011. [DOI] [PubMed] [Google Scholar]
- Jackson S. E., elMasry N., Fersht A. R. Structure of the hydrophobic core in the transition state for folding of chymotrypsin inhibitor 2: a critical test of the protein engineering method of analysis. Biochemistry. 1993 Oct 26;32(42):11270–11278. doi: 10.1021/bi00093a002. [DOI] [PubMed] [Google Scholar]
- Li A., Daggett V. Characterization of the transition state of protein unfolding by use of molecular dynamics: chymotrypsin inhibitor 2. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10430–10434. doi: 10.1073/pnas.91.22.10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matouschek A., Fersht A. R. Application of physical organic chemistry to engineered mutants of proteins: Hammond postulate behavior in the transition state of protein folding. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7814–7818. doi: 10.1073/pnas.90.16.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matouschek A., Fersht A. R. Protein engineering in analysis of protein folding pathways and stability. Methods Enzymol. 1991;202:82–112. doi: 10.1016/0076-6879(91)02008-w. [DOI] [PubMed] [Google Scholar]
- Matouschek A., Kellis J. T., Jr, Serrano L., Bycroft M., Fersht A. R. Transient folding intermediates characterized by protein engineering. Nature. 1990 Aug 2;346(6283):440–445. doi: 10.1038/346440a0. [DOI] [PubMed] [Google Scholar]
- Matouschek A., Kellis J. T., Jr, Serrano L., Fersht A. R. Mapping the transition state and pathway of protein folding by protein engineering. Nature. 1989 Jul 13;340(6229):122–126. doi: 10.1038/340122a0. [DOI] [PubMed] [Google Scholar]
- Matouschek A., Serrano L., Fersht A. R. The folding of an enzyme. IV. Structure of an intermediate in the refolding of barnase analysed by a protein engineering procedure. J Mol Biol. 1992 Apr 5;224(3):819–835. doi: 10.1016/0022-2836(92)90564-z. [DOI] [PubMed] [Google Scholar]
- Otzen D. E., Itzhaki L. S., elMasry N. F., Jackson S. E., Fersht A. R. Structure of the transition state for the folding/unfolding of the barley chymotrypsin inhibitor 2 and its implications for mechanisms of protein folding. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10422–10425. doi: 10.1073/pnas.91.22.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford S. E., Dobson C. M., Evans P. A. The folding of hen lysozyme involves partially structured intermediates and multiple pathways. Nature. 1992 Jul 23;358(6384):302–307. doi: 10.1038/358302a0. [DOI] [PubMed] [Google Scholar]
- Sali A., Shakhnovich E., Karplus M. Kinetics of protein folding. A lattice model study of the requirements for folding to the native state. J Mol Biol. 1994 Feb 4;235(5):1614–1636. doi: 10.1006/jmbi.1994.1110. [DOI] [PubMed] [Google Scholar]
- Toney M. D., Kirsch J. F. Brønsted analysis of aspartate aminotransferase via exogenous catalysis of reactions of an inactive mutant. Protein Sci. 1992 Jan;1(1):107–119. doi: 10.1002/pro.5560010111. [DOI] [PMC free article] [PubMed] [Google Scholar]



