Abstract
Background
Patients with unresectable melanoma or sarcoma hepatic metastasis have a poor prognosis with few therapeutic options. Percutaneous hepatic perfusion (PHP), isolating and perfusing the liver with chemotherapy, provides a promising minimally invasive management option. We reviewed our institutional experience with PHP.
Methods
We retrospectively reviewed patients with unresectable melanoma or sarcoma hepatic metastasis treated with PHP from 2008 to 2013 and evaluated therapeutic response, morbidity, hepatic progression free survival (hPFS), and overall survival (OS).
Results
Ten patients were treated with 27 PHPs (median 3). Diagnoses were ocular melanoma (n=5), cutaneous melanoma (n=3), unknown primary melanoma (n=1), and sarcoma (n=1). Median hPFS was 240 days, 9 of 10 patients (90%) demonstrated stable disease or partial response to treatment. At a median follow up of 11.5 months, 4 of 10 (40%) remain alive. There were no perioperative mortalities. Myelosuppresion was the most common morbidity, managed on an outpatient basis with growth factors. The median hospital stay was 3 days.
Conclusions
Patients with metastatic melanoma and sarcoma to the liver have limited treatment options. Our experience with PHP demonstrates promising results with minimal morbidity and should be considered (pending FDA approval) as a management option for unresectable melanoma or sarcoma hepatic metastasis.
Keywords: Percutaneous hepatic perfusion, Liver metastasis, Minimally invasive
Introduction
Hepatic metastases from most malignancies portend a very poor prognosis. Complete surgical resection offers the best improvement in overall survival; however, even with neoadjuvant down-staging and two-stage hepatectomies, only about 60% of patients will become resectable [1-3]. Ocular melanoma is an aggressive malignancy which metastasizes in approximately 50% of patients [16]. In those that develop metastatic disease, 95% will have hepatic metastases, and in 80% that is their only site of metastatic disease [17]. Median survival in these patients has been reported to be less than 9 months, and systemic treatment options are limited due to lack of efficacy with this histology [7]. Stage IV cutaneous melanoma has a long-term survival rate of approximately 10%, and 5 year survival with hepatic metastases has been reported to be 0%[18]. Soft tissue sarcoma metastatic to the liver has an equally dismal prognosis, with a median survival of 12 months [4].
For patients with unresectable liver-dominant disease, that either fails to respond or progresses through systemic treatment, multiple strategies for liver-directed therapy have been developed [4, 5]. Regional therapies for liver metastases such as hepatic arterial infusion of chemotherapy, transarterial chemoembolization, radiofrequency or microwave ablation, transarterial radioactive microspheres and selective external beam radiation, offer the ability to treat hepatic disease while minimizing systemic toxicity [6-8].
In patients with lesions too numerous to ablate or resect, chemotherapy infusion directly into the liver through the hepatic artery takes advantage of the fact that liver metastases derive their blood supply primarily from the hepatic artery, while normal hepatocytes are supplied by the portal vein [6, 8, 9]. Moreover, isolation of the liver from the systemic circulation allows for higher concentrations of chemotherapeutic agents to be reached within the tumors themselves, which has been shown in multiple studies to increase the effectiveness of the infused agent(s) [3, 8, 10, 11]. Isolated hepatic perfusion (IHP) of chemotherapy is an operative technique first described by Dr. Robert Ausman in 1961, and has evolved over the ensuing 50 years. IHP has been shown to be efficacious in the treatment of multiple tumor histologies, with partial response (PR) rates of 2-83% [8]. In patients with metastatic ocular melanoma treated with IHP, PR rates of 52-62% have been observed, with 10% complete responses described in one study [7, 12].
Though efficacious, IHP is a major operative procedure that can only be performed once, with average operative times of 8-9 hours, morbidities associated with laparotomy, and mortality rates as high as 33%[8]. The development of a minimally invasive, percutaneous approach to isolated hepatic perfusion began as an attempt to match the response rates seen with IHP, while minimizing the morbidity, mortality, hospital length of stay, and allowing for treatment repetition [3, 13, 14]. In a phase I dose-ranging study of percutaneous hepatic perfusion (PHP) with melphalan [15], 28 patients were treated with 74 PHP treatments with doses of melphalan ranging from 2.0 mg/kg to 3.5 mg/kg. Patients received treatment every 4-8 weeks for a total of 4 treatments, and responses to treatment were radiographically measured. The overall response rate for all those treated was 30%, and among the cohort of patients with metastatic ocular melanoma, the response rate was 50%. Evaluation of toxicities after PHP showed the treatment to be safe and well tolerated at a dose of 3 mg/kg with bone marrow toxicity (neutropenia, thrombocytopenia, and anemia) and mild hepatic toxicity being the most commonly identified morbidities. A phase III, multicenter randomized trial of percutaneous hepatic perfusion (PHP) using melphalan versus best alternative care (BAC) in patients with metastatic ocular or cutaneous melanoma isolated to the liver reported 8.0 months vs. 1.6 months (p<0.0001) hepatic progression-free survival (hPFS) and 6.7 months vs. 1.6 months (p <0.0001) overall progression-free survival (oPFS) in patients treated with PHP vs. BAC, respectively [19]. Here, we review our institutional experience with the management of patients with unresectable melanoma and sarcoma hepatic metastasis and report patient outcomes.
Methods
We retrospectively reviewed all patients treated with PHP between December 2008 and August 2013 at Moffitt Cancer Center. Approval for this retrospective study was obtained from the institutional review board.
Patient Selection
Patients in this series included unresectable, biopsy-proven hepatic metastases from ocular melanoma, cutaneous melanoma, unknown primary melanoma, or leiomyosarcoma. Disease was limited to the liver in all patients. Patients with previously treated extrahepatic metastases were not excluded. Patients received computed tomography (CT) scans of their chest, abdomen, and pelvis or full body PET/CT scans prior to treatment to assess tumor burden, and identify any other potential sites of metastatic disease. A dedicated 3 phase CT of the liver or MRI of the liver was used to gauge tumor burden and size of lesions in the liver for response to therapy based on Response Evaluation Criteria In Solid Tumors (RECIST).
Procedure in Detail
The PHP procedures were performed in the interventional radiology suite under general anesthesia which is recommended due to the transient hemodynamic changes seen during the procedure [20]. Briefly, 3 percutaneous catheters are placed by the interventional radiologist as follows: A 5 French femoral arterial sheath, an 18 French femoral venous sheath, and a 10 French internal jugular (IJ) central venous catheter. Systemic anticoagulation with heparin is performed with a goal of an activated clotting time of greater than 400 seconds. The chemotherapy infusion catheter is introduced through an arterial catheter placed through the sheath and advanced under fluoroscopic guidance into the proper hepatic artery distal to the takeoff of the GDA. Prior to chemotherapy infusion, visceral angiograms of the celiac axis and superior mesenteric arteries were performed to identify any arterial variations. Prophylactic coil embolization was performed if there was less than 1 cm of proper hepatic artery distal to the take-off of the gastroduodenal artery (GDA) to prevent retrograde flow of the chemotherapeutic drug into the GDA. Other aberrant branches (such as left gastric) that originated from the hepatic arteries were also embolized to prevent chemotherapy from reaching other abdominal viscera.
Next, the 16 French double-balloon hepatic isolation and aspiration catheter (Delcath Systems, Inc. NY, NY) is introduced through the venous sheath, and using fluoroscopic guidance is advanced into the retrohepatic inferior vena cava (IVC) (Fig. 1). The cephalad balloon of the IVC catheter is inflated in the right atrium and retracted until it forms a seal at the cavoatrial junction. The caudal balloon is inflated in the IVC below the level of the hepatic veins and above the level of the renal veins. Fenestrations in the catheter between the two occlusion balloons allow for venous outflow from the hepatic veins to be shunted extracorporeally, and using a perfusion bypass machine the venoveno bypass circuit passes blood through the chemotherapy filtration device, and then returns it via the IJ venous catheter thus completing the veno-veno bypass circuit.
Figure 1.
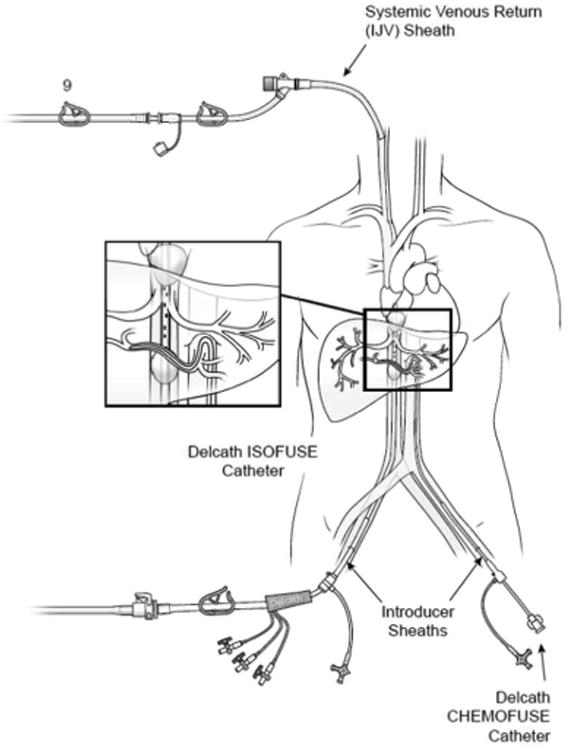
Diagram of the Delcath Hepatic Chemosat Delivery System. The enlarged box shows the double-balloon catheter in place in the inferior vena cava, which by occluding hepatic venous outflow allows for isolation of the liver from the systemic circulation. Also pictured in the enlarged box is the arterial cannula through which the chemotherapeutic agent (melphalan) is delivered into the hepatic artery. Hepatic venous outflow is collected via fenestrations between the two occlusion balloons, and pumped through an extracorporeal filtration system which removes melphalan from the effluent venous blood, then returns the filtered blood to the patient via a 10 French cannula placed into the internal jugular vein, thus completing the veno-veno bypass.
Prior to administering any chemotherapy, contrast media is infused through the fenestrations in the IVC catheter to ensure no leakage around either balloon occurs, thus verifying hepatic isolation (Fig. 2). Once the liver is isolated from the systemic circulation and veno-veno bypass is established, the chemotherapeutic agent can be administered through the arterial catheter. Melphalan, a nonspecific alkylating agent, is used due to its high first pass metabolism and high hepatic clearance rate. The total dose given was 3 mg/kg, and adjusted for the patient's ideal body weight. Generally, the dose was administered into the liver in a split fashion by advancing the arterial catheter into the right and left hepatic arteries to allow for 60% of the drug to be given into the right hepatic artery, and 40% to the left hepatic artery based on a generalized estimation of hepatic lobe volumes.
Figure 2.
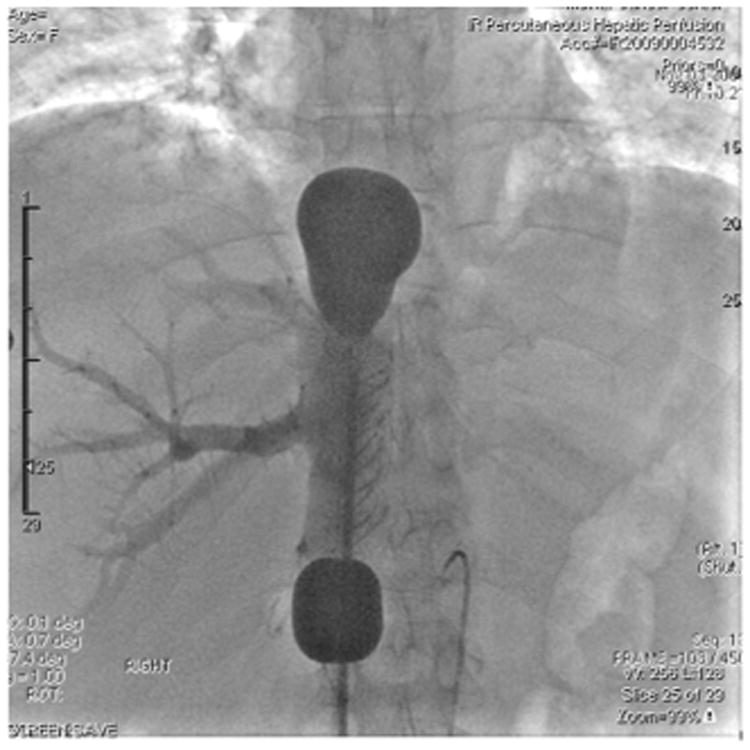
Venogram of the 16 French double-balloon hepatic isolation and aspiration catheter (Delcath Isofuse Isolation Aspiration Catheter) in place in the retrohepatic inferior vena cava with cephalad and caudal balloons inflated with contrast media to verify placement. The mushroom shape of the cephalad balloon verifies proper placement at the cavo-atrial junction. Contrast instilled through the catheter fenestrations between the occlusion balloons shows backfilling of the right hepatic venous system and verifies no leakage of blood around the balloons. Also seen in the image is the arterial infusion cannula in the hepatic artery.
Intra-arterial perfusion is performed for 30 minutes, followed by an additional 30 minutes on veno-veno bypass to allow for washout of any residual drug within the liver. Throughout the treatment, hemodynamic and arterial pressure monitoring is performed due to the drop in mean arterial pressure caused by the decreased venous return to the right atrium upon inflation of the double-balloon IVC catheter. Patients are supported with fluids, and typically the administration of vasopressive agents such as phenylephrine, vasopressin or norepinephrine are required at the initial opening of the filters, and are weaned off throughout the case.
Post-Operative Management
At the completion of the treatment, protamine sulfate is infused to reverse the heparinization, and transfusions of fresh frozen plasma as well as cryoprecipitate are provided as needed to replace clotting factors and fibrinogen removed by the filtration system. The patients are transferred to the post anesthesia care unit, and once their activated clotting time, prothrombin time, partial thromboplastin time and platelet levels return to baseline, the femoral venous and arterial sheaths are removed and pressure held at the insertion sites for 45 minutes. Patients were admitted to the hospital for postoperative monitoring, with the first 12-24 hours spent in the intensive care unit. Any adverse events or complications were evaluated and recorded.
Post-operative Patient Follow Up
After discharge from the hospital, patients were seen twice weekly with blood work to monitor their hepatic function tests as well as complete blood counts for the first 2-4 weeks. Neutropenia, thrombocytopenia or symptomatic anemia are treated with transfusions and stimulating factors (granulocyte stimulating factor, G-CSF) as needed. Repeat imaging of the liver was performed with a triple phase liver CT or dedicated MRI scan and whole body PET/CT scanning approximately 6 weeks after treatment to evaluate for response. Patients with stable disease or a partial response to treatment without development of new extra-hepatic metastatic disease were scheduled for repeat PHP treatments approximately every 6-8 weeks. Patients with progression of their hepatic disease, had their treatment discontinued and were referred for systemic therapy.
Data Collection
Demographics and clinicopathologic parameters were recorded. The percent of tumor response and hPFS were evaluated radiographically. Up to five target lesions within the liver were measured on CT or MRI imaging using RECIST criteria. Baseline imaging was obtained within 14 days prior to the first treatment and subsequent imaging within 3 days prior to the next treatment. Follow up imaging after completion of all treatments occurred every 12 weeks until progression. Follow up and overall survival data were also collected.
Results
Patient Characteristics
Between 2008 and 2013, 10 patients with unresectable melanoma (ocular or cutaneous) or sarcoma hepatic metastasis were treated with PHP. The median age of patients was 63 years, and there were 7 women and 3 men (Table 1). Three patients had primary cutaneous melanoma, 5 had ocular melanoma, 1 had melanoma of unknown primary, and 1 had metastatic leiomyosarcoma. The median time from primary tumor diagnosis to the diagnosis of hepatic metastases was 31 months (range 8-87 months). Some patients had been treated for their hepatic metastases with other modalities prior to PHP, including systemic chemotherapy, radiofrequency ablation, and transarterial chemoembolization (See Table 1). The 10 patients underwent 27 PHP treatments, a median of 3 per patient.
Table 1.
Clinicopathological factors and response to therapy for all patients treated with percutaneous hepatic perfusion (PHP).
| Patient | Sex | Age | Primary Tumor | Number of PHP Treatments | hPFS (days) | Tumor Response (% change) | Follow-Up (months) | Overall Survival (months) | Previous Treatment | Status |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 69 | Leiomyosarcoma | 4 | 420 | -10.1 | 40 | - | Doxorubicn/Ifosfamide | AWD |
| 2 | F | 55 | Ocular | 3 | 413 | -58.6 | 35 | 46.4 | none | DOD |
| 3 | F | 68 | Ocular | 1 | 0 | +84.9 | 6 | 10.0 | none | DOD |
| 4 | M | 77 | Ocular | 3 | 228 | -38.6 | 12 | 14.2 | Carboplatin/Taxol | DOD |
| 5 | F | 47 | Cutaneous | 4 | 252 | -11.1 | 11 | 11.0 | none | DOD |
| 6 | M | 73 | Ocular | 3 | 453 | -72.1 | 30 | 26.1 | none | DOD |
| 7 | M | 65 | Cutaneous | 2 | 1337** | -43.6 | 44 | - | none | AWD |
| 8 | F | 59 | Unknown Primary Melanoma | 3 | 181 | -4.0 | 9 | - | RFA, TACE | AWD |
| 9 | F | 51 | Cutaneous | 1 | 121 | -8.1 | 5 | 10.5 | Carboplatin/Taxol | DOD |
| 10 | F | 62 | Ocular | 3 | 166** | -33.3 | 5 | - | none | AWD |
No progression of hepatic disease to date,
hPFS = Hepatic progression free survival, RFA= Radiofrequency ablation, TACE = Transarterial chemoembolization, AWD = Alive with Disease, DOD = Dead of disease.
Best Response to Therapy PHP
Nine of 10 patients (90%) treated with PHP had stable disease or a partial response on follow up imaging (Fig. 3). The percent change in hepatic tumor volume was evaluated by triple-phase liver CT or MRI of the liver, with representative CT images provided for a patient with metastatic leiomyosarcoma response (Figure 4 panel a. showing hepatic metastasis prior to PHP and b. showing the same lesion after 3 PHP treatments.), and a patient with metastatic melanoma of unknown primary (Figure 4. Panel c. showing pretreatment metastasis and d. showing the same lesion after 3 PHP treatments). One patient had progression of liver metastases after the first PHP treatment and received no further PHPs. The median percent decrease in hepatic tumor volume in the 9 of 10 patients who had stable disease or a partial response was 33.3% for the entire cohort, 48.6% for those with ocular melanoma, 11.1% for those with cutaneous melanoma, 4.0% for the unknown primary melanoma, and 10.1% for the sarcoma (Fig. 3).
Figure 3.
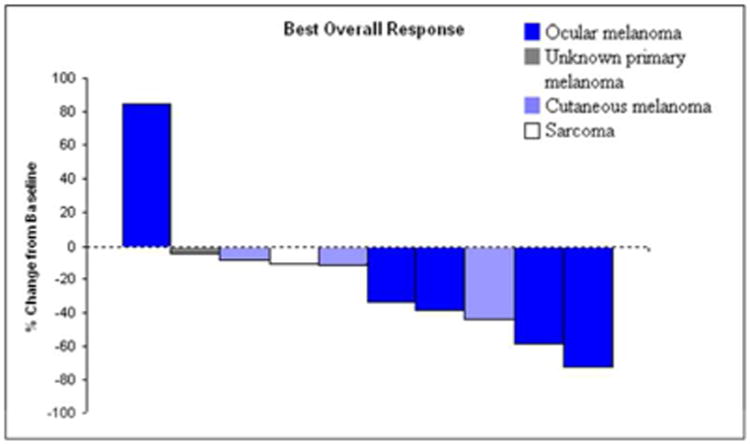
Waterfall plot of maximal tumor response to treatment in percent change from baseline in 10 patients treated with percutaneous hepatic perfusion (PHP). Response percentage was based on change in tumor size on serial imaging, as determined by a radiologist.
Figure 4.
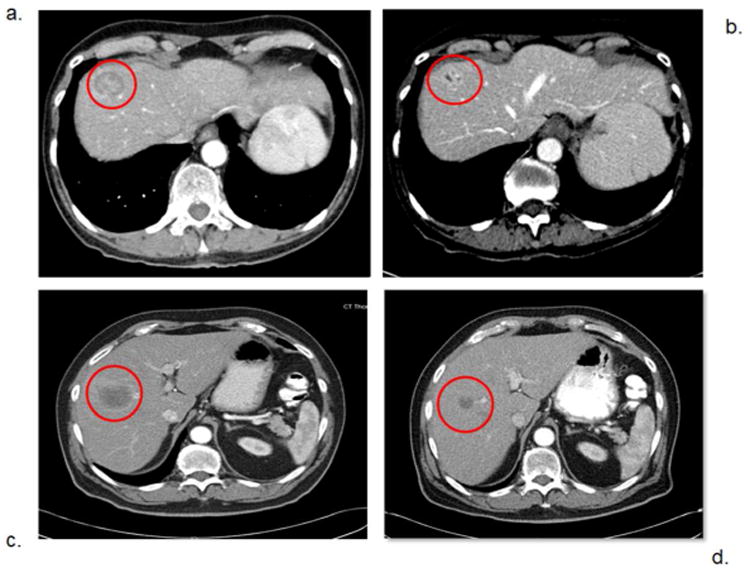
Computed Tomography (CT) images with arterial phase intravenous contrast of patients before and after percutaneous hepatic perfusion (PHP) treatment. Panel a. shows a liver metastasis in patient number 1 (from Table 1.) before treatment, and panel b. shows the same lesion after 3 PHP treatments. Panel c. shows a metastatic lesion in patient number 8 before treatment, and panel d. shows the same metastasis after 3 PHP treatments.
Hepatic Progression Free Survival
At a median follow up of 11.5 months (range 5-44 months); the median hPFS was 240 days. For the patients with cutaneous melanoma, the median hPFS was 252 days, while it was 228 days for patients with ocular melanoma, 181 days for the patient with unknown primary melanoma, and 420 days for the patient with sarcoma (Fig. 5). One ocular melanoma patient and one cutaneous melanoma patient have no progression of hepatic disease to date (hPFS = 166 and 1337, respectively), while one ocular melanoma patient progressed on initial post-procedure restaging imaging (Figure 3).
Figure 5.
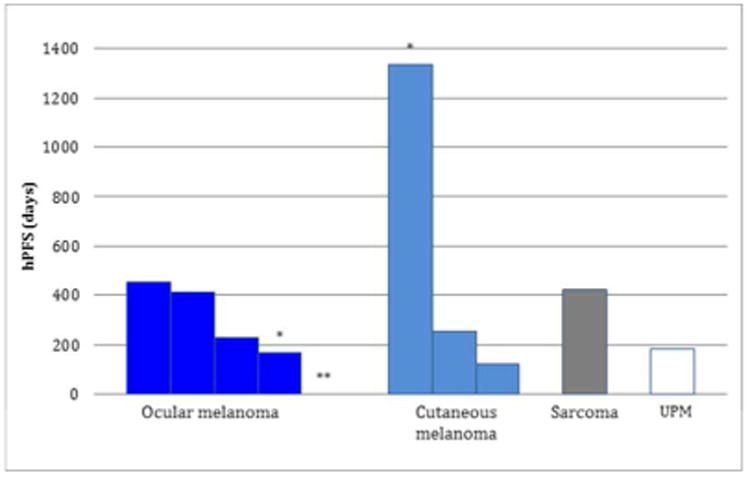
Days of hepatic progression free survival (hPFS) of 10 patients treated with percutaneous hepatic perfusion (PHP). Patients are grouped by primary tumor site. (UPM = unknown primary melanoma). * = patients who have yet to progress in their liver. ** = patient who did not respond to PHP treatment.
Complications
The 10 patients treated had a median post-operative hospital stay of 3 days. There were no treatment related mortalities. Myelosuppression was the most common adverse event and was treated on an outpatient basis in all cases. Myelosuppression was treated with GCSF growth factors and/or products (packed red blood cells (PRBCs) and platelets as needed. Growth factors were given to patients whose absolute neutrophil counts were below 1000 per uL, platelets were given to patients whose platelets counts were below 20,000 per uL and PRBCs were used selectively if any patient had symptomatic anemia at the discretion of the single surgical oncologist who treated each patient. Six of the 10 treated (60%) patients received filgrastim injections for neutropenia after at least one of their PHP treatments.
Seven patients (70%) were found to have mild elevations in their serum troponin levels after treatment. In all cases, these elevations were without EKG and echocardiographic evidence of myocardial ischemia, dyskinesia or dysfunction, and no patient required invasive cardiac intervention. In one patient, the 4th PHP treatment was aborted after the IVC balloons were inflated due to non-sustained ventricular tachycardia. No chemotherapy was given to this patient and no cardiac sequelae were seen. One patient developed a small apical pneumothorax, likely from the placement of the internal jugular venous catheter, which resolved without further intervention required. Two patients required readmission for an acute cough to rule out pneumonia and both were treated and discharged within 48 hours.
Survival Outcome
Six of the 10 patients (60%) have died from their disease (Table 1). Of those who have died of their disease, the median overall survival from the time of diagnosis of hepatic metastases was 12.6 months.
Of the 4 patients alive with disease, two have yet to show hepatic progression. The first patient has completed 3 PHPs and has achieved 166 days of hPFS, and has a 4th PHP planned based on restaging scans which will be performed shortly. The second patient, who has had a 1337 day hPFS response after 2 PHPs, has developed progressive disease in the lungs, abdomen and dermis for which he has received ipilimumab treatment and is currently without a recurrence in the liver.
Discussion
Regional therapies for metastatic cancer to the liver are attractive due to the potential to deliver high doses of chemotherapy or other agents directly at the tumors, minimizing systemic toxicities, all the while performing this with minimally invasive techniques. Regional hepatic perfusion with melphalan has been shown to delay the progression of hepatic metastatic melanoma in the open and minimally invasive percutaneous technique. In the current series, 9 of 10 patients had either stable disease or a partial response to therapy as evidenced by a reduction in aggregate tumor volume, with a median hPFS of 240 days or 7.9 months. Unlike open hepatic perfusion (IHP), the percutaneous procedure allows for repeated treatments, and can potentially be performed every 6-8 weeks, as long as the patient's disease is responding, new extra-hepatic metastases do not develop, and toxicities do not limit further treatments.
In these patients with stage IV disease, survival is often measured in months. As previously noted, ocular melanoma with hepatic metastasis has a reported median survival of 9 months [7]. Similarly, cutaneous melanoma with hepatic spread has a dismal prognosis with close to no survivors at 5 years reported in one study [18]. A treatment, like IHP or PHP, which may delay the progression of hepatic metastatic disease has tremendous potential.
The minimally invasive nature of the PHP procedure decreases some of the treatment-related morbidity, and eliminates the potential morbidity associated with an abdominal incision and exploration. In this series only two patients had treatment discontinued for reasons other than disease progression: one for myelosuppression (after a 3rd PHP), and one for ventricular arrhythmia during the balloon inflation on his 4th PHP. The treatment is associated with hemodynamic changes secondary to balloon occlusion of the IVC and chemofiltration, which are managed with fluid resuscitation and vasopressors as needed. We have found that a well prepared team including an anesthesiologist, nurse anesthetist, interventional radiologist and surgical oncologist who are all familiar with the procedure's hemodynamic shifts, is key to safely completing the treatment.
Immediately after treatment, clotting factors and platelets removed during the filtration process may have to be repleted to prevent bleeding complications before removal of the catheters. Our preparation for the procedure includes pre-ordering standard amounts of cryoprecipitate and platelets from the blood bank, which eliminates waiting time in the operating room, and contributes to added safety. There have been no major hemorrhagic complications in our treated patients.
Also noted were several patients who had a slight rise in their troponin levels in the days immediately following treatment. This occurred in 7 of 10 patients, but only 1 had levels greater than 1.0 ng/mL. None of the patients showed EKG or echocardiographic evidence of myocardial damage or dyskinesia. These small transient elevations are likely the result of cardiac stress due to the decreased venous return associated with the IVC balloon occlusion coupled with vasopressor use. We include cardiac risk stratification in our pre-treatment evaluation of all patients, and obtain cardiology clearance and consultation when indicated before or after treatment. Most importantly, no patients required invasive cardiac testing or treatment after undergoing PHP.
Adverse events in the days and weeks following treatment were minimal, and most were treated without readmission to the hospital. The most common morbidity associated with the procedure was myelosuppression, which was treated with transfusions and colony stimulating factors as needed. This bone marrow toxicity is the result of small amounts of melphalan in the blood after passing through the chemofiltration circuit. The efficiency of the filters used prior to January 2012 was close to 75%. A second-generation filter has been developed and is available for use in compassionate use and expanded access protocol patients since June of 2012, with the purported benefit of more efficient melphalan filtration. We were able to use the newer “Gen II” filter for the last 6 of the 27 PHP treatments performed at our institution. Three patients had at least one treatment using the Gen II filter, and only one required G-CSF after treatment. Interestingly, one of the patients received treatment one time with the original filter (Gen I) and then two additional treatments with the newer Gen II filter. This patient required transfusions and G-CSF after the initial treatment using the GEN I filter. (See Fig. 6.)
Figure 6.
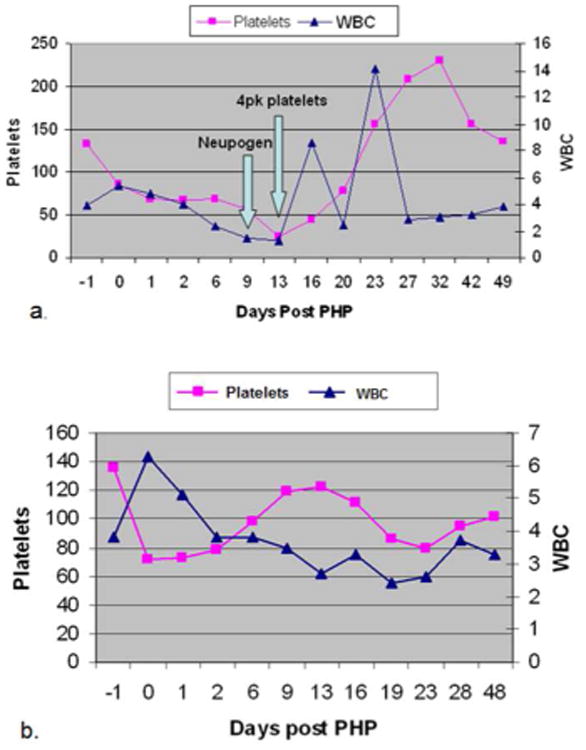
Line graphs showing serum platelet and white blood cell (WBC) levels in patient number 8 (from Table 1) before and after percutaneous hepatic perfusion (PHP) treatment. Panel a. shows levels after the first PHP treatment which utilized the first generation chemofiltration filter. Arrows indicate when platelets were transfused due to thrombocytopenia, and when Neupogen (WBC colony stimulating factor), was started due to leukopenia. Panel b. shows the same patient's serum platelet and WBC levels before and after her second PHP treatment which utilized the second generation chemofiltration filter. Note the levels did not fall below thresholds, and therefore no transfusions or Neupogen were given. (4pk = 4 pack unit of platelets).
In the future, improvements in chemofiltration could remove almost all risk of bone marrow suppression associated with the PHP treatment, thereby abrogating the most common post treatment morbidity. However, in this challenging field with limited treatment options, PHP may provide select patients with a benefit. It is therefore an important consideration in the management of stage IV disease and a promising avenue of research.
Conclusions
Percutaneous hepatic perfusion provides a safe and promising minimally invasive management option for unresectable melanoma or sarcoma hepatic metastasis in select patients. At our institution, PHP resulted in a median hPFS of 8 months and a prolonged hPFS of 44 months in one patient. Patients tolerate PHP well with minimal morbidity, most of which is myelosuppression that can be treated in the outpatient setting. Myelosuppression will likely become less of an issue in the future as the chemofiltration technology improves. In addition, PHP may be a consideration for other unresectable hepatic malignant lesions in the future, such as in metastatic colorectal cancer or hepatocellular carcinoma, especially as new filters for different chemotherapeutic agents are developed.
While the outcomes of our single institution experience with PHP are promising, a recently completed phase III clinical trial manuscript is being finalized which will detail the benefits, hPFS, overall survival and morbidities associated with PHP.
References
- 1.Adam R, Miller R, Pitombo M, et al. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232(6):777–85. doi: 10.1097/00000658-200012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruers T, Bleichrodt RP. Treatment of liver metastases, an update on the possibilities and results. Eur J Cancer. 2002;38(7):1023–33. doi: 10.1016/s0959-8049(02)00059-x. [DOI] [PubMed] [Google Scholar]
- 3.Savier E, Azoulay A, Huguet E, et al. Percutaneous isolated hepatic perfusion for chemotherapy: a phase 1 study. Arch Surg. 2003;138(3):325–32. doi: 10.1001/archsurg.138.3.325. [DOI] [PubMed] [Google Scholar]
- 4.Deneve JL, Choi J, Gonzalez RJ, et al. Chemosaturation with percutaneous hepatic perfusion for unresectable isolated hepatic metastases from sarcoma. Cardiovasc Intervent Radiol. 2012;35(6):1480–7. doi: 10.1007/s00270-012-0425-x. [DOI] [PubMed] [Google Scholar]
- 5.Han D, Beasley GM, Tyler DS, et al. Minimally invasive intra-arterial regional therapy for metastatic melanoma: isolated limb infusion and percutaneous hepatic perfusion. Expert Opin Drug Metab Toxicol. 2011;7(11):1383–94. doi: 10.1517/17425255.2011.609555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander HR, Jr, Butler CC. Development of isolated hepatic perfusion via the operative and percutaneous techniques for patients with isolated and unresectable liver metastases. Cancer journal (Sudbury, Mass) 2010;16(2):132–41. doi: 10.1097/PPO.0b013e3181db9c0a. [DOI] [PubMed] [Google Scholar]
- 7.Alexander HR, Jr, Libutti SK, Pingpank JF, et al. Hyperthermic isolated hepatic perfusion using melphalan for patients with ocular melanoma metastatic to liver. Clin Cancer Res. 2003;9(17):6343–9. [PubMed] [Google Scholar]
- 8.Grover A, Alexander HR., Jr The past decade of experience with isolated hepatic perfusion. Oncologist. 2004;9(6):653–64. doi: 10.1634/theoncologist.9-6-653. [DOI] [PubMed] [Google Scholar]
- 9.Lin G, Lunderquist A, Hagerstrand I, Boijsen E. Postmortem examination of the blood supply and vascular pattern of small liver metastases in man. Surgery. 1984;96(3):517–26. [PubMed] [Google Scholar]
- 10.Ku Y, Tominaga M, Iwasaki T, et al. Isolated hepatic perfusion chemotherapy for unresectable malignant hepatic tumors. Int J Clin Oncol. 2002;7(2):82–90. doi: 10.1007/s101470200011. [DOI] [PubMed] [Google Scholar]
- 11.Ravikumar TS, Dixon K. Isolated liver perfusion for liver metastases: pharmacokinetic advantage? Surg Oncol Clin N Am. 1996;5(2):443–9. [PubMed] [Google Scholar]
- 12.Alexander HR, Libutti SK, Bartlett DL, et al. A phase I-II study of isolated hepatic perfusion using melphalan with or without tumor necrosis factor for patients with ocular melanoma metastatic to liver. Clin Cancer Res. 2000;6(8):3062–70. [PubMed] [Google Scholar]
- 13.Beheshti MV, Denny DF, Jr, Glickman MG, et al. Percutaneous isolated liver perfusion for treatment of hepatic malignancy: preliminary report. J Vasc Interv Radiol. 1992;3(3):453–8. doi: 10.1016/s1051-0443(92)71988-5. [DOI] [PubMed] [Google Scholar]
- 14.Weinreich DM, Alexander HR., Jr Transarterial perfusion of liver metastases. Semin Oncol. 2002;29(2):136–44. doi: 10.1053/sonc.2002.31681. [DOI] [PubMed] [Google Scholar]
- 15.Pingpank JF, Libutti SK, Chang R, et al. Phase I study of hepatic arterial melphalan infusion and hepatic venous hemofiltration using percutaneously placed catheters in patients with unresectable hepatic malignancies. J Clin Oncol. 2005;23(15):3465–74. doi: 10.1200/JCO.2005.00.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kivela T, Eskelin S, Kujala E. Metastatic uveal melanoma. Int Ophthalmol Clin. 2006;46(1):133–49. doi: 10.1097/01.iio.0000195861.71558.13. [DOI] [PubMed] [Google Scholar]
- 17.Woodman SE. Metastatic uveal melanoma: biology and emerging treatments. Cancer journal (Sudbury, Mass) 2012;18(2):148–52. doi: 10.1097/PPO.0b013e31824bd256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pawlik TM, Zorzi D, Abdalla EK, et al. Hepatic resection for metastatic melanoma: distinct patterns of recurrence and prognosis for ocular versus cutaneous disease. Ann Surg Oncol. 2006;13(5):712–20. doi: 10.1245/ASO.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Pingpank JF, Hughes MS, Faries MB, et al. A phase III random assignment trial comparing percutaneous hepatic perfusion with melphalan (PHP-mel) to standard of care for patients with hepatic metastases from metastatic ocular or cutaneous melanoma. ASCO Meeting Abstracts. 2010;28(18_suppl):LBA8512. [Google Scholar]
- 20.Miao N, Pingpank JF, Alexander HR, Jr, et al. Percutaneous hepatic perfusion in patients with metastatic liver cancer: anesthetic, hemodynamic, and metabolic considerations. Ann Surg Oncol. 2008;15(3):815–23. doi: 10.1245/s10434-007-9781-1. [DOI] [PubMed] [Google Scholar]


