Abstract
BACKGROUND
Calmodulin activation by Ca2+, its translocation to the nucleus, and stimulation of phosphorylation of CREB (P-CREB) is necessary for new gene expression and has been linked to long term potentiation, a process important in memory formation. Since isoflurane is known to affect memory we tested whether isoflurane interfered with the translocation of calmodulin to the neuronal cell nucleus and attenuated the formation P-CREB.
METHODS
SH-SY5Y cells, a human neuroblastoma cell line, were cultured. Cells were depolarized with KCl and the phosphorylation of CREB examined by Western Blotting, ELISA, and immunocytochemistry. The translocation of calmodulin from the cytosol to the nucleus was also examined following depolarization. Cells were depolarized and lysed and fractionated by centrifugation to determine the amount of CaM translocated to the nucleus. CaM was localized by immunocytochemistry and quantitated by Western blotting and imaging. Prior to and during KCl depolarization cells were exposed to isoflurane, isoflurane plus Bay K8644, nitrendipine, and ω-conotoxin GVIa, respectively.
RESULTS
P-CREB increased following KCl depolarization. The increase of P-CREB peaked at depolarization duration of 30 seconds. The increase in P-CREB formation was inhibited by nitrendipine but not ω-conotoxin, and by isoflurane in a concentration dependent fashion. Pretreatment with the L-type Ca2+ channel agonist, Bay K8644, attenuated the inhibition of P-CREB formation by isoflurane. CaM presence in the nucleus occurred following KCl depolarization. CaM translocation was inhibited by nitrendipine, and attenuated by isoflurane. Bay K8644 pretreatment decreased the isoflurane inhibition of CaM translocation to the nucleus.
CONCLUSIONS
Our data demonstrate that isoflurane inhibits CaM translocation and P-CREB formation. This most likely occurs through isoflurane inhibition of Ca2+entry through L-type Ca2+ channels.
INTRODUCTION
Neurons translate electrical activity into chemical information via Ca2+ signaling. Upon depolarization of a neuronal cell, Ca2+ enters through voltage-gated Ca2+ channels. There are several sub-types of Ca2+ channels that open at different voltages and have different characteristics and functions. The L-type channel (Cav) is a high voltage activated channel that can be blocked with high specificity by dihydropyridines such as nitrendipine (NTP) and is found predominantly in the cell bodies and proximal dendrites of neurons.(1) The L-type channel has been shown to be important in the transfer of extracellular signals to the cell nucleus. The movement of Ca2+ through L-type Ca2+ channels in certain neuronal cells is specifically linked to the binding of Ca2+ to calmodulin and the rapid translocation of calmodulin to the cell nucleus.(2) This process leads to the activation of the transcription factor, cyclic AMP response element binding protein (CREB), through phosphorylation by CaM sensitive kinases and results in the stimulation of new gene expression.(3) This series of events links Ca2+ entry to nuclear signaling.
We have previously shown in SH-SY5Y cells, a human neuroblastoma cell line, that about one third of the depolarization induced Ca2+ transient is initiated by Ca2+ entry through the L-type channel.(4) The entry of Ca2+ through the L-type channel is reversibly inhibited by halothane (5) and isoflurane (this study) at clinically relevant concentrations. Furthermore, it has been shown that the administration of 1.2% isoflurane and N2O to rats results in a significant decrease compared to control in CREB phosphorylation (P-CREB) in the rat hippocampus. (6) Others have shown that [Ca2+]cyt elevation in SH-SY5Y cells results in the formation of P-CREB. (7) These considerations lead us to question whether isoflurane exposure of SH-SY5Y cells would result in inhibition of P-CREB formation and whether that inhibition is linked to Ca2+/CaM interaction and translocation.
METHODS
Cell culture and treatment
Undifferentiated human SH-SY5Y neuroblastoma cells were maintained in 75-cm2 flasks for 14–20 days in RPMI 1640 medium which contained 12% fetal bovine serum, 100 u/ml of penicillin and 100 mg/ml of streptomycin in a 37°C humidified incubator consisting of 5% CO2. All cell culture related reagents were purchased from Invitrogen, Carlsbad, CA.. The cells were centrifuged at 1900g for 5 min after washing with Dulbecco’s phosphate buffered saline (DPBS). The cells were rinsed once with incubation buffer (IBG) containing (in mM) 140 NaCl, 5 KCl, 5 NaHCO3, 1 MgCl2, 10 Hepes, 10 glucose, pH 7.4. The cell pellet was resuspended in IBG buffer and approximately 1.25x 106 cells added to each microcentrifuge tube. The cell suspension was incubated in IBG at room temperature for 10 min. After incubation without or with 10 mM nitrendipine (NTP) (5 min) or 100 nM ω-conotoxin GVIa (CTX) (5 min) or 0.2–0.8 mM isoflurane (ISO) (10min) at 37°C, 1.5 mM CaCl2 was added to the cell suspension. 3 minutes later the reaction was initiated with the addition of KCL (100 mM, 30sec). The reaction was stopped by placing the cell suspension on ice.
For Western Blot and enzyme linked immunosorbant assay (ELISA), following KCl stimulation, the reaction was stopped by dropping the reaction tubes containing the cell suspension into liquid nitrogen.
Measurement of L-type Ca2+ Currents
Ca2+ currents were measured in SH-SY5Y cells as previously described (5). A cell-attached single channel patch was used to measure the isoflurane effect. The bath solution contained (in mM): 120 KAspartate, 25 KCl, 2 MgCl2, 0.5 CaCl2, 2 EGTA, 2 Hepes, and 1μM tetrodotoxin (TTX) at pH 7.4. The pipette solution contained (in mM): 2 CaCl2, 110 Sucrose, 23 TEACl, 2 EGTA, 2 Hepes, and 100μM picrotoxinin, 0.1μM CTX, 1μM TTX at pH 7.4. Calcium currents were recorded in response to 150 ms test pulse to −10mV applied at every 4 seconds from a −60 mV holding potential. Wash (bath solution) and isoflurane solution (in bath solution) were applied directly on top of the cell through a millimanifold (MLF-4 from ALA Scientific, NY; dead space < 50 μl) at a 2.8 μl/sec flow rate. Data was filtered at 2 kHz and acquired at 20 kHz. Current traces in the figure were filtered at 500 Hz. Data analysis was done using Clampfit 9. 2 software (Molecular Devices, Sunnyvale, CA). Anesthetic application and measurement were as previously described. (5)
Fluorescence Measurement of Ca2+ Transients
SH-SY5Y cells in confluent culture were loaded with 5 μM fura-2 for 30 min at 37°C as previously described.(4) Following washing and suspension cells were equilibrated for 5 min before the addition of 1.5 mM CaCl2, following an additional 5 min 100 mM KCl was added to the reaction mixture. Fluorescence ratios were monitored at 510 nm after excitation at 340 and 380 nm. Cytosolic Ca2+ concentration was determined from the fluorescence ratios based as previously described. (4)
Isoflurane Addition and Measurement
An aliquot of pure isoflurane or an aliquot of a saturated solution of isoflurane (13.4 mM) was added to a suspension of cells with a Hamilton syringe as previously described to achieve the desired concentration. For cells attached on coverslips, each coverslip was mounted in a sealed chamber with 1 ml of liquid. An aliquot of saturated solution of isoflurane was injected into the sealed chamber and incubated with shaking for 10 min. The concentration of the isoflurane in the buffer solution was measured by gas chromatography as previously described. (4)
Immuno-Assay
CREB phosphorylation was measured by both Western Blot and ELISA method.
The ELISA immunoassay kit was purchased from Biosource International, Inc (Camarillo, CA). The anti-CREB antibody was from Cell Signaling Technology (Danvers, MA). The anti-P-CREB (Ser 133) antibody was from Cell Signaling Technology and Chemicon(Temecula, Ca.). The anti-Calmodulin antibody was from ABR (Golden, CO).CREB
Immunoprecipitation and Western Blotting
The frozen SH-SY5Y cell suspension was thawed at room temperature for 20 min and sonicated 3 times for 10 seconds on ice. Cell lysates were then incubated on ice with 2 μl/sample of undiluted monoclonal anti-P-CREB antibody for 2 hours. Immune complexes were collected on protein G-Sepharose (Sigma, St. Louis, Mo.) minicolumns and washed four times with cold PBS by centrifugation and then eluted with denature solution containing 0.6M Tris/HCl, 1% SDS, 10% sucrose, 0.5% β-Mercaptoethanol and 0.5% Bromophenol blue. The mixture was loaded on to the 10% SDS-PAGE gel for electrophoresis and then transferred to a PVDF membrane (Amersham Biosciences, Bucks, UK). Non-specific binding sites on the membrane were blocked with 5% skimmed milk. The membrane was then probed first with anti-P-CREB (Ser133) antibody on the immunoblots and then with secondary antibody, which conjugated with horseradish peroxidase and developed with Opti-4CN Kit (BioRad, Hercules, Ca.) for colorimetric detection.
P-CREB Quantification by ELISA
Quantification of CREB phosphorylation was performed by a non-competitive enzyme linked immunosorbant assay (ELISA) on soluble fractions obtained from cell lysates. The lysates were loaded onto the wells of the microtiter strips which were coated with monoclonal antibody specific for CREB. During the 2 hrs incubation at room temperature, the P-CREB antigen from the lysate was bound to the immobilized antibody. After washing for 4 times with buffer, the detection antibody specific for P-CREB at serine 133 was added. After 1 hr incubation followed by 4 washings, the horseradish peroxidase-labeled anti-rabbit IgG (anti-rabbit-IgG-HRP) was added. 30min later the complex was washed with substrate solution, and then the stabilized chromogen added. After a 30min incubation the stop solution (2M sulfuric acid) was added and the absorbance at 450nm was read by a BioRad micro-plate reader.
Fluorescent Immunocytochemistry
The SH-SY5Y cells were grown on coverslips placed in 12-well plates. After treatment as described above, they were fixed with 4% paraformaldehyde in PBS and permeabilized with 0.1% Triton X-100 for 45min. The cells were exposed to 3% horse serum in PBS/TBS followed by incubation with anti-P-CREB (Cell Signaling Technology) or anti-CaM (ABR) primary antibody (1:100), for 1 hr at 37°C, respectively. Cells were then incubated with an Alexa Fluor 568 goat anti-mouse IgG as the secondary antibody (Invitrogen) (1:500) for 1hour at room temperature. After washing with PBS/TBS, the stained cells were mounted on glass slides with mounting medium (Polysciences). CREB phosphorylation and CaM localization were examined by fluorescence imaging. All images were taken with a high resolution digital B/W CCD camera (ORCA-ER, Hamamatsu Photonics K.K., Hamamatsu City, Japan) connected to a confocal module CARV (Atto Instruments, Rockville, Md.). A Zeiss Axiovert S100 (inverted type) microscope with a Plan-Apo 63X objective (Carl Zeiss Jena GmbH, Jena, Germany) was used.
Nuclear protein preparation
After treatment as described above, the cells, at 0°C to 4°C in the presence of fresh 1x protease inhibitor cocktail (PIERCE #78410, Rockford, Il.) and 1mM dithiothreitol (Sigma), were centrifuged at 1900g for 10 min and washed once with 5x pellet volume of DPBS. The cell pellets were rapidly resuspended in 5x pellet volume of hypotonic buffer containing 10mM Hepes, pH7.9 at 4°C, 1.5mM MgCl2, 10mM KCl and then centrifuged at 1900g for 5 min. The pelleted cells were resuspended in 3x pellet volume of hypotonic buffer on ice for 10 min. The suspension was homogenized with 10 up-down strokes using a plastic pestle. The nuclei were collected by centrifugation at 3300g for 15 min. The nuclei were then resuspended in one half pellet volume of low-salt buffer containing 20mM Hepes, 25% glycerol, 1.5mM MgCl2, 0.2mM EDTA, pH 7.9 at 4°C. An additional one half pellet volume of low-salt buffer but containing 1.6mM KCL was added in a dropwise fashion. The nuclei were extracted for 30 min with continuous gentle stirring. The extract was centrifuged at 17500g for 40 min and the supernatant saved. Protein concentration of the supernatant was determined by the Lowry method.
CaM Detection by Western Blotting
The nuclear extract was mixed with denaturing solution containing 0.6M Tris/HCl, 1% SDS, 10% sucrose, 0.5% β-Mercaptoethanol and 0.5% Bromophenol blue. After boiling for 5 min and a brief centrifugation, the supernatant was loaded onto a 4–20% SDS-PAGE pre-made gel (Invitrogen) for electrophoresis and then transferred to a PVDF membrane pre-treated with 100% methanol. Non-specific binding sites on the membrane were blocked with 5% skim milk. The membrane was probed with anti-calmodulin (Invitrogen) antibody that recognized both Ca2+ free and Ca2+-bound CaM in the One-step Western Blot Kit (Columbia Bio LLC, Elmhurst, NY) and developed with SuperSignal West Pico Chemiluminescent Substrate kit (PIERCE, Rockford, IL) for detecting horseradish peroxides on immunoblots. The exposed and developed film was scanned and bands quantified by using NIH ImageJ.
RESULTS
Figure 1A. demonstrates the isoflurane-mediated inhibition of Ca2+ currents through single L-type Ca2+channels in SH-SY5Y cells. The averaged currents and individual traces clearly demonstrate the inhibition with isoflurane and the remarkable burst of channel activity when isoflurane is washed away.
Figure 1. Isoflurane inhibits L-type Ca2+ currents and nitrendipine inhibits CREB phosphorylation.
A. Isoflurane inhibits channel activity of Bay-K modified L-type calcium channels in SH-SY5Y cells. Current traces for a cell attached patch containing a single L-type Ca2+ channel before, during, and following the removal of 0.6mM isoflurane (sequence shown on top of figure). Top: for clarity we only show the 9 traces (out of the 32 collected in each run) that displayed the most channel activity during each run. Middle: the mean current traces for the 32 current traces in each condition. Below: the open channel probability (Po) during the 32 traces. B. Nitrendipine (NTP) inhibition of CREB phosphorylation. A representative Western blot of P-CREB from SH-SY5Y cell homogenate following a 3 min incubation with 1.5 mM CaCl2 (Ctrl-Ca), CaCl2 plus 100 mM KCl to depolarize the cells (KCl) or CaCl2 and 10 μM NTP 5 min prior to the addition of 100 mM KCl (NTP+KCl). C. SH-SY5Y cells exposed to the same treatment as in B. but fixed with 4% paraformaldehyde and incubated with anti-P-CREB antibody as the primary antibody and Alexa Fluor 568 goat anti-mouse IgG as the secondary antibody.
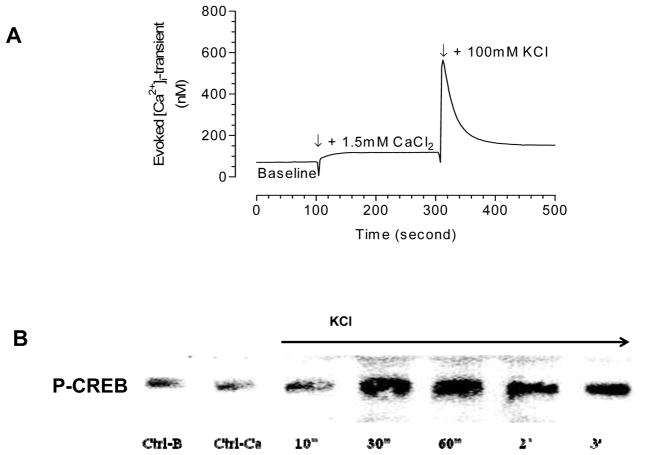
The phosphorylation of CREB (P-CREB) has been shown to be related to Ca2+ entry through L-type channels during depolarization. We have demonstrated that depolarization with 100 mM KCl induced an increase in P-CREB (Figure 1B & C) and that exposure of cells to nitrendipine (NTP) inhibited the phosphorylation of CREB (Figure 1B & C).. Figure 2A. demonstrates the increase in cell Ca2+ upon depolarization with 100 mM KCl. Upon addition of 100 mM KCl the cytosolic Ca2+ transient increases rapidly. We have shown previously that the transient is almost totally dependent on Ca2+ entry through L- and N-type Ca2+ channels.(4) The phosphorylation of CREB has been shown to be quite rapid in other systems and it occurs rapidly in SH-SY5Y cells, with peak phosphorylation achieved at 30 seconds (Figure 2B & 3A). Despite the increase in P-CREB, no evident change occurred in the amount of CREB as measured by Western blot (Figure 3A & B).
Figure 2. The time-course of KCl-stimulated P-CREB formation.
A. The cytosolic Ca2+ transient in SH-SY5Y following a 100 mM KCl stimulus. B. A representative western blot demonstrating the time-dependence of P-CREB formation following a 100 mM KCl stimulus.
Figure 3. Quantification of the KCl-evoked time-dependent changes in P-CREB and CREB.
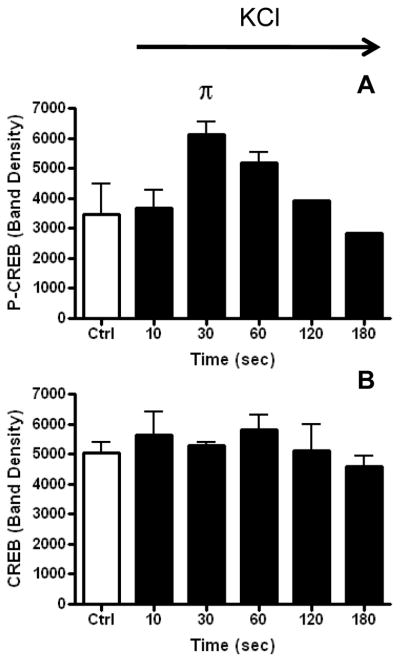
Graph of band density analysis of Western blots quantifying over time the amount of P-CREB (3A) and CREB (3B) following KCl stimulation. The bars represent the mean and SEM for control, and 10, 30, and 60 seconds (n=3), and the mean and range for 120 and 180 seconds (n=2). The effect of time on P-CREB formation was evaluated by one-way ANOVA and shown to be statistically significant with P<0.05. Dunnet’s post test demonstrated that only the P-CREB value at 30 sec was statistically significantly different from the control
The exposure of cells to isoflurane prior to and during depolarization resulted in a concentration dependent inhibition of KCl-evoked CREB phosphorylation (Figure 4). Figure 5 demonstrates a similar finding using immunocytochemistry and an antibody to P-CREB. KCl-induced depolarization causes a marked increase in P-CREB fluorescence and exposure to isoflurane inhibits that increase. Exposure of cells to Bay K 8644, an L-type Ca2+ channel agonist, even in the presence of isoflurane, leads to an increase in P-CREB fluorescence relative to isoflurane alone. This result suggests that the isoflurane inhibition of P-CREB fluorescence is related to the inhibition of the entry of Ca2+ through the L-type channel since Bay K 8644 can partially overcome isoflurane inhibition as has been shown in other systems (8).
Figure 4. Isoflurane inhibition of KCl-stimulated P-CREB formation.
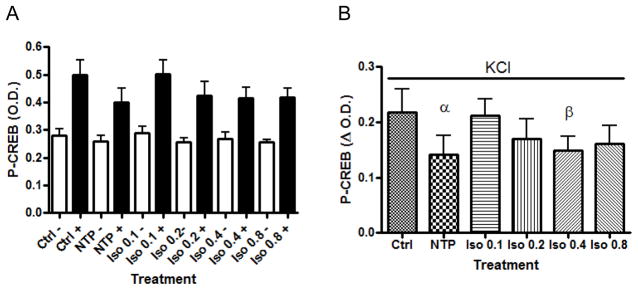
P-CREB formation was measured by ELISA. A. P-CREB formation in the absence and presence of KCl and the effect of treatment with NTP and increasing concentrations of isoflurane. The error bars represent the SEM and n=6 for each sample. The inhibitory effect of isoflurane on P-CREB formation in the presence of KCl was found to be significant by one-way ANOVA, P < 0.0001. B. Values of P-CREB for each treatment. The P-CREB value obtained after subtracting the minus KCl value from the plus KCl value for each treatment (data figure 4A.) Each bar represents the mean plus the SEM, n=6 (P<0.05 Ctrl vs NTP; P< 0.5 Ctrl vs 0.4 mM Iso, paired t-test).
Figure 5. Isoflurane inhibits P-CREB formation and this effect is partially reversed by Bay K 8644.
Fluorescent images of 100 mM KCl stimulated P-CREB formation and its inhibition by increasing concentrations of isoflurane. The Ca2+agonist, Bay K 8644 (5 μM), reversed the inhibition by 0.8mM isoflurane. Each bar in the graph represents the average fluorescence in all of the cells in each panel plus or minus the SEM measured by Image J software. The green color is a pseudocolor arbitrarily chosen.
Others have shown that CREB phosphorylation is linked to the entry of Ca2+, its binding to CaM, and the translocation of the complex to the cell nucleus. In SH-SY5Y cells a similar process apparently occurs. Following exposure to KCl, cells were rapidly fixed and permeabilized and exposed to CaM antibody followed by exposure to an Alexa-Fluor 568 goat anti-mouse IgG secondary antibody. Figure 6A demonstrates the marked increase in CaM fluorescence following KCl depolarization. The CaM fluorescence is essentially absent when KCl depolarization occurs in the presence of the L-type Ca2+ channel antagonist nitrendipine (NTP). Exposure to CTX, the N-type Ca2+ channel blocker, during KCl depolarization does not prevent the CaM fluorescence from appearing. We have previously shown in SH-SY5Y cells that 70% of Ca2+ entry upon depolarization occurs through N-type channels. These results suggest that Ca2+ entering through the L-type Ca2+ channel and not the N-type Ca2+ channel activates CaM and allows its translocation to the nucleus as others have found (9).
Figure 6. Calmodulin translocation to the cell nucleus.
A. Fluorescent images of CaM translocation to the nucleus of SH-SY5Y cells following a 100 mM KCl stimulus. Translocation was inhibited by NTP but not CTX. Bay K 8644 partially reversed the NTP-meditated inhibition of KCl stimulated CaM translocation. B. Inhibition of KCl stimulated CaM translocation by increasing concentrations of isoflurane. Bay K 8644 attenuated the inhibition of CaM translocation by 0.8 mM isoflurane. Each bar in the graphs represents the average fluorescence in all of the cells in each panel plus or minus the SEM measured by Image J software. The orange color is a pseudocolor arbitrarily chosen.
We then examined the effect of increasing concentrations of isoflurane on CaM fluorescence. As can be seen in Figure 6B following KCl depolarization there was a decrease in CaM fluorescence with increasing concentrations of isoflurane. We questioned whether the effect of isoflurane related to inhibition of Ca2+ entry through the L-type channel or some other downstream process such as Ca2+ binding to CaM or translocation of Ca2+/CaM to the nucleus. In order to test these possibilities we exposed cells to Bay K 8644 and isoflurane prior to KCl stimulation. As can be seen in figure 6B, Bay K 8644 exposure attenuated the inhibition of Ca2+/CaM fluorescence by 0.8 mM isoflurane.
A further demonstration of the activation and translocation of Ca2+/CaM was done through cell fractionation and isolation of the nuclear fraction. Cells were exposed briefly to KCl in the presence and absence of either NTP or increasing concentrations of isoflurane. The nuclear fraction was isolated and immunoblots for CaM obtained. Figure 7 demonstrates the increased amount of CaM found in the nucleus upon KCl exposure and the NTP- and isoflurane-evoked inhibition of the KCl-evoked CaM increase, supporting the view that NTP and isoflurane have an inhbitiory effect on Ca2+/CaM translocation.
Figure 7. Isoflurane-meditated inhibition of CaM translocation to the nucleus.

A. Quantification of Western blots from 5 independent experiments comparing the nuclear transfer of CaM when exposed to 1.5 mM CaCl2 (±) NTP or increasing concentrations of isoflurane prior to a 30 second stimulation with 100 mM KCl. The mean plus SEM of CaM translocated to the nuclear fraction of SH-SY5Y cells is plotted by treatment. (P<0.001 one-way ANOVA; P<0.001 KCl vs. 0.8 mM isoflurane (#). P< 0.05 KCl vs. NTP (^); P<0.05, Iso 0.8 vs Iso 0.2 and Iso 0.8 vs. Iso 0.4 and Iso 0.8 vs. NTP (*) by Newman-Keuls Multiple Comparison Test) B. A representative Western blot of nuclear fractions of SH-SY5Y cells that were treated as indicated in A.
DISCUSSION
In this study we have shown that exposure of SH-SY5Y cells to isoflurane inhibits the L-type Ca2+ channel current, the appearance of CaM fluorescence, and the phosphorylation of CREB in the cell nucleus. Other investigators have demonstrated that CaM translocation is initiated by the entry of Ca2+ through the L-type voltage gated Ca2+ channel and the subsequent binding of Ca2+ to CaM. (10) We have shown that isoflurane inhibits the entry of Ca2+ through the L-type channel and that the inhibition of Ca2+ entry is linked to the decreased translocation of CaM and the lower degree of phosphorylation of CREB. Two bits of evidence support this contention. First, NTP, the dihydropyridine blocker of the L-type channel, prevents the appearance of CaM fluorescence in the nucleus. CTX, an N-type Ca2+ channel blocker, does not prevent the appearance of CaM fluorescence in the nucleus, suggesting that Ca2+ entering through the L- but not the N-type channel has privileged access to CaM and activates its movement to the nucleus presumably in its Ca2+-bound state. Secondly, the L-type Ca2+ channel agonist, Bay K 8644, is able to mostly overcome the inhibition of both NTP and isoflurane. When Bay K 8644 is added to the solution containing isoflurane, we once again see CaM fluorescence in the nucleus which is absent when cells are exposed to isoflurane alone.
In SH-SY5Y cells we have shown that one-third of the Ca2+ transient induced by KCl depolarization results from the entry of Ca2+ through the L-type channel.(4) The Ca2+ which enters through the L-type channel then stimulates the release of Ca2+ from the caffeine-sensitive Ca2+ store. The current evidence does not allow one to distinguish between the activation of CaM by Ca2+ that enters directly through the L-type channel or the Ca2+ that is released from the caffeine-sensitive Ca2+ store. CaM has been shown to be bound to the L-type channel (11) but it also has been shown to be a modulator of the ryanodine receptor which is the conduit that releases Ca2+ from the caffeine-sensitive Ca2+ store (12) Since the L-type channel and the ryanodine receptor are in close proximity and recent evidence estimates the CaM concentration in the near vicinity of the L-type channel to be 2.5 mM, determining which of these two sources of Ca2+ binds to and activates CaM will require further investigation. (11) However, Ca2+ entry through the N-type Ca2+ channel also evokes the release of Ca2+ from the caffeine sensitive Ca2+ store but has no effect on P-CREB or CaM translocation suggesting a unique role of the L-type channel.
It is also possible that other sites along the path from extracellular Ca2+ to phosphorylation of CREB might be altered by isoflurane. The binding of Ca2+ to CaM might be inhibited by isoflurane. However, that seems unlikely in light of in vitro evidence that at the concentrations of isoflurane used in this study the affinity of CaM for Ca2+ is actually increased (13). However, the mechanism of rapid transport of Ca2+/CaM to the nucleus from the plasma membrane is believed to be a facilitated transport and might be a site at which isoflurane is exerting its inhibitory effect.(2) Finally, Ca2+/CaM activation of a kinase in the nucleus might be another isoflurane-sensitive site. The kinase involved in the phosphorylation of CREB is CaM kinase IV (14) and at present there is no evidence regarding the effect of isoflurane on this kinase.
CREB activation of new transcription requires phosphorylation of serine-133.(15) The phosphorylation of CREB has been directly linked to entry of Ca2+ through the L-type channel and association of that Ca2+ with CaM. CREB phosphorylation is stimulated by synaptic activity including those that support long-term potentiation and long-term depression (16). It has further been shown that P-CREB is reduced in the hippocampus of mice that have been anesthetized with isoflurane and N2O. (17) Our demonstration of isoflurane suppression of Ca2+/CaM translocation and CREB phosphorylation in a neuronal model system suggests a link between the effects of isoflurane on memory and the molecular processes involved. It also suggests that anesthesia with volatile anesthetics might have a considerable effect on gene transcription.
Implications Statement.
The demonstration of isoflurane suppression of Ca2+/CaM translocation and cyclic AMP response element binding protein (CREB) phosphorylation in a neuronal model system suggests a link between the effects of isoflurane on memory and the molecular processes involved.
Acknowledgments
Financial Support:
Supported by the NYU Anesthesiology Research Fund and in part by NIGMS and NINDS grant P30 NS050276 and NCRR Shared Instrumentation Grant S10 RR017990 to T.A.N.
Footnotes
Disclaimers:
None
Conflict of Interest:
None
References
- 1.Tsien RW, Lipscombe D, Madison D, Bley K, Fox A. Reflections on Ca2+-channel diversity, 1988–1994. Trends Neurosci. 1995;18:52–4. [PubMed] [Google Scholar]
- 2.Deisseroth K, Heist EK, Tsien RW. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- 3.Mermelstein PG, Deisseroth K, Dasgupta N, Isaksen AL, Tsien RW. Calmodulin priming: nuclear translocation of a calmodulin complex and the memory of prior neuronal activity. Proc Natl Acad Sci U S A. 2001;98:15342–7. doi: 10.1073/pnas.211563998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu F, Zhang J, Recio-Pinto E, Blanck TJ. Halothane and isoflurane augment depolarization-induced cytosolic Ca2+ transients and attenuate carbachol-stimulated Ca2+ transients. Anesthesiology. 2000;92:1746–56. doi: 10.1097/00000542-200006000-00035. [DOI] [PubMed] [Google Scholar]
- 5.Nikonorov IM, Blanck TJ, Recio-Pinto E. The effects of halothane on single human neuronal L-type calcium channels. Anesth Analg. 1998;86:885–95. doi: 10.1097/00000539-199804000-00038. [DOI] [PubMed] [Google Scholar]
- 6.Culley DJ, Yukhananov RY, Xie Z, Reddy G, Rudolph ET, Crosby G. Altered hippocampal gene expression 2 days after general anesthesia in rats. Eur J Pharmacol. 2006;549:71–8. doi: 10.1016/j.ejphar.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Tolbert LM, Carlson KW, Sadee W. Nuclear Ca2+/calmodulin translocation activated by mu-opioid (OP3) receptor. J Neurochem. 2000;74:1418–25. doi: 10.1046/j.1471-4159.2000.0741418.x. [DOI] [PubMed] [Google Scholar]
- 8.Kanaya N, Kawana S, Tsuchida H, Miyamoto A, Ohshika H, Namiki A. Comparative myocardial depression of sevoflurane, isoflurane, and halothane in cultured neonatal rat ventricular myocytes. Anesth Analg. 1998;87:1041–7. doi: 10.1097/00000539-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Liang H, DeMaria CD, Erickson MG, Mori MX, Alseikhan BA, Yue DT. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron. 2003;39:951–60. doi: 10.1016/s0896-6273(03)00560-9. [DOI] [PubMed] [Google Scholar]
- 10.Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- 11.Mori MX, Erickson MG, Yue DT. Functional stoichiometry and local enrichment of calmodulin interacting with Ca2+ channels. Science. 2004;304:432–5. doi: 10.1126/science.1093490. [DOI] [PubMed] [Google Scholar]
- 12.McPherson PS, Campbell KP. Characterization of the major brain form of the ryanodine receptor/Ca2+ release channel. J Biol Chem. 1993;268:19785–90. [PubMed] [Google Scholar]
- 13.Levin A, Blanck TJ. Halothane and isoflurane alter the Ca2+ binding properties of calmodulin. Anesthesiology. 1995;83:120–6. doi: 10.1097/00000542-199507000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–14. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 15.West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 2002;3:921–31. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- 16.Bito H, Deisseroth K, Tsien RW. Ca2+-dependent regulation in neuronal gene expression. Curr Opin Neurobiol. 1997;7:419–29. doi: 10.1016/s0959-4388(97)80072-4. [DOI] [PubMed] [Google Scholar]
- 17.Simon W, Hapfelmeier G, Kochs E, Zieglgansberger W, Rammes G. Isoflurane blocks synaptic plasticity in the mouse hippocampus. Anesthesiology. 2001;94:1058–65. doi: 10.1097/00000542-200106000-00021. [DOI] [PubMed] [Google Scholar]