Abstract
High resolution optical imaging by confocal reflectance microscopy (CRM) was investigated for revealing epithelial microstructure and alterations which may occur from vaginal microbicides. The vaginal tracts of Swiss Webster mice treated with medroxyprogesterone acetate were exposed in vivo to a 4% nonoxynyl-9 containing gel or saline for 4, 16, or 48 hours. The vaginal tract was removed and imaged by CRM without staining and imaged regions were then biopsied and processed for histology. In control mice, CRM revealed columnar epithelium and lamina propria with features resembling that of histology. CRM revealed exfoliated epithelium following 4 and 16 hour N-9 treatments and quantitative measurement of epithelial thickness revealed a decrease from approximately 41.7 ± 1.7 (SEM) in controls to 14.9 ± 4.5 and 24.4 ± 2.1 after 4 and 16 hours, respectively. Indication of inflammation at 4 hours was given through the presence of inflammatory infiltrates. After 48 hours the epithelium was regenerating. The timecourse of change in structure and epithelial thickness detected by CRM closely resembled that observed and measured by histology. This study demonstrates CRM can reveal epithelial structure and indicators of inflammation following treatment with N-9 and may be a useful imaging tool for evaluating effects of microbicides.
Keywords: confocal reflectance microscopy, nonoxynol-9, epithelial disruption, microbicides
Introduction
Topical microbicides offer the possibility of a female-controlled means for protection against sexually transmitted infections (STI). To date no microbicide has shown efficacy in Phase III trials. Of utmost importance in the development and evaluation of topical microbicides is safety, with recent efforts focused on the action of such agents on epithelial integrity. Such an emphasis became important after early microbicide candidates were shown to compromise the cervicovaginal epithelial barrier and to increase susceptibility to infection by STIs despite early promising in vitro evidence to the contrary. Among these agents is nonoxynol-9 (N-9), which initially was shown to be an effective antimicrobial candidate in vitro against a number of STIs including, HIV [1-3]. However N-9 failed to act as an effective microbicide in clinical trial [4] and in two efficacy trials frequent use was linked to an increase in HIV infection [5-6]. An additional microbicide candidate, cellulose sulfate, was also recently terminated in light of safety concerns [7]. These events have proven the impetus for an increased emphasis on new methods for preclinical microbicide safety assessment [8-10].
Factors such as epithelial disruption and recruitment of inflammatory infiltrates by topical agents may play a role in increased susceptibility to STIs [8]. The events are likely complex and enhanced understanding require methods with the ability to assess spatiotemporal responses of the vaginal epithelium to topical agents. Colposcopy is an accepted standard for assessing vaginal irritation by topical agents. This white light-assisted visualization method provides tissue level examination of the cervicovaginal surface and can identify gross findings such as petechaie, erythema, and ulcerations [11]. However, deep epithelial disruptions smaller than 1 cm in diameter cannot be distinguished from more superficial disruption unless there is bleeding [12] and microstructural indication of compromised barrier function cannot be assessed by this method. Few studies have correlated visual findings with biopsy, complicating the interpretation of colposcopic findings [12]. While colposcopy has been utilized in small animal models for preclinical microbicide safety studies, its ability to detect damage is limited as well. For example, in one study, colposcopy failed to detect N-9 toxicity despite evidence of epithelial exfoliation and inflammation indicated by cytokine levels and macrophage infiltration [9].
Additional methods for investigating the effects of microbicides include histopathology and cytokine mapping [9-8; 13-14]. Histopathology provides visualization of epithelial microarchitecture and indication of inflammation, however it requires ex vivo fixation and processing of tissue which can take several days to weeks before results are obtained. Cytokine sampling has the potential to provide molecular-specific indication of inflammation from vaginal lavage by revealing levels of proinflammatory markers such as IL-1, IL-6, and IL-8 believed to play a role in STI transmission [15]. The method does not give an indication of epithelial microstructure, and is not standardized, making interpretation of cytokine profiles difficult and limiting comparisons from studies performed by different groups [16]. Thus, while the discussed methods offer varied advantages, their limitations indicate the need for additional and improved monitoring methods to assess epithelial integrity and response to topical agents. Desired capabilities include the ability to assess microstructure in near-realtime or in real time and potentially give an indication of inflammatory response.
Confocal reflectance microscopy (CRM) allows the reconstruction of images based on reflected signal as a function of depth to provide high resolution images of tissue microstructure without the need for staining. To date the principle application of CRM has been to study the microstructural morphology of normal and diseased tissues, such as skin, towards the diagnosis of malignancy in vivo or as an ex vivo adjunct to surgery [17-22]. In mucosal tissue (oral and cervix), confocal reflectance combined with contrast agents has been investigated for noninvasive detection of neoplasia [23-24]. We believe that CRM could be used in preclinical microbicide safety testing to provide information on epithelial integrity and response to microbicide actions with 1) immediate feedback since CRM can be performed on fresh tissues, 2) high resolution to reveal focal and microstructural indicators of damage, and 3) depth-resolved capabilities to assess the full epithelium. Thus in these studies we investigate the use of CRM as a method to study both the cellular-level morphology of mouse vaginal mucosa and the mucosal architecture following treatment with N-9.
MATERIALS AND METHODS
Animal model and intravaginal inoculations
Female Swiss Webster mice [Harlan, Houston, TX] were pretreated with a single subcutaneous injection containing 2 mg medroxyprogesterone acetate one week prior to use. Medroxyprogesterone acetate treatment transforms the keratinizing stratified epithelium of the vaginal mucosa to a diestrous-like state in which the vaginal epithelium is thinned [25]. On the day of microbicide treatment the vaginal vault was gently swabbed with a premoistened swab and the animals administered 0.03 ml of Conceptrol™ containing 4% N-9 (hereafter referred to as N-9) or phosphate buffered saline (PBS). Studies were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and conformed to the Guide for the Care and Use of Laboratory Animals.
Confocal Reflectance Microscopy (CRM)
Animals were sacrificed and the vaginal tract removed for imaging 4 hours (n=4), 16 hours (n=5), or 48 hours (n=4) after N-9 treatment; 7 mice served as PBS treated controls. The excised cervicovaginal tract was opened longitudinally along the posterior side and the vaginal samples placed on the sample stage with the mucosal surface facing the microscope objective. CRM was performed using a Zeiss 410 Confocal Laser Scanning Microscope (Zeiss, Germany). A 40X, 0.8 N.A. water immersion objective lens was used for delivery and collection of light from the sample. The sample was illuminated with 568 nm light from a Krypton:Argon ion laser [Melles Griot, Inc. Carlsbad, CA.]. Remitted 568 nm light was detected using cooled photomultiplier tubes [R6060-11; Hamamatsu, Japan]. The imaging objective provided an x-y field-of-view of 320 × 320 μm. A pixel dwell time of 15.2 μs was used to obtain images. Z-stacks were collected using a 1 μm increment between z-planes and to a depth of 150 μm. Z-stacks were obtained in six areas of the vaginal tract. The microscopist was blinded to the treatment conditions. MetaMorph (version 7.1, Molecular Devices, Sunnyvale, CA) was used to process and reconstruct CRM z-stacks into two-dimensional (2D) cross-sectional views (x-z) and three-dimensional (3D) views. Prior to reconstruction, each image in the z-stack underwent background subtraction of a blank field. Cross-sectional (x-z) views allowed for depth assessment of fresh tissue CRM micrographs in the transverse direction, akin to histology sections.
Histological processing
Immediately after imaging, the entire vaginal tract was immersed in formalin for fixation (24 hours). Punch biopsies were obtained from imaged regions and embedded in paraffin, sectioned transversely, and serial sections stained with hemotoxylin and eosin (H&E) for histological examination.
Epithelial thickness determination
Quantitative determination of epithelial thickness on CRM micrographs were determined by measuring the full depth from the tissue surface to the z-plane where collagen fibers were first visible indicating the beginning of the lamina propria. For each field-of-view, eight-twelve randomly chosen regions were measured and averaged. H&E stained sections were examined in trans-illumination mode of a standard light microscope and the distance from the surface to the basement membrane was measured to determine epithelial thickness.
Statistical Analysis
Epithelial thickness was compared by two-tailed one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. All comparisons were two-tailed.
RESULTS
CRM and histology of vaginal tissue in PBS treated animals
Figure 1 shows representative CRM images from freshly excised vagina after treatment with PBS together with corresponding H&E stained sections. In PBS treated animals, x-y micrographs of the surface showed epithelial cells having a bright highly reflective cytoplasm with dark nuclei (Figure 1A) . These surface cells had a large cytoplasmic volume, consistent with the feature of mucosal surface columnar cells. The lamina propria was identified by fibrillar structure characteristic of extracellular matrix. The basement membrane separating epithelium from lamina propria was defined as being the transition between basal cells and extracellular matrix. The basal cells displayed little cytoplasm with the primary feature a dark nucleus. Cross-sectional (x-z), micrographs (Figure 1B) had a banded appearance comprised of a bright region at the surface arising from surface columnar cells, followed by a dark band resulting from the high nuclear density at the basal layer, and a second bright region from the lamina propria. Light attenuation with depth eventually resulted in the loss of total signal. The high-resolution 3-D reconstruction views (Figure 1C) showed characteristic round mucosal cells at the epithelial surface. Histology confirmed features identified by CRM. Columnar cells in H&E sections had a large cytoplasmic volume at the surface, and a small cytoplasm-nuclear ratio near the basal layer, in agreement with CRM findings. (Figure 1B) Both CRM and histology of vaginal tissue in PBS treated animals was comparable at all of the times examined.
Figure 1.
Vaginal tissue imaged by CRM and processed for histology post-imaging. (A) x-y micrographs taken at the surface and underlying lamina propria. Surface columnar cells appear morphologically round and are highly reflective (bright). Nuclei appear dark and basal cells have a large nucleus to cytoplasmic area. Primary feature of the lamina propria is fibrillar extracellular matrix. (B) CRM cross-sections reveal multilayered nature of epithelium with identifiable bright surface columnar cells, a dark basal layer, and bright lamina propria (total depth of cross-section image: 125 μm). Corresponding H&E section obtained from fresh tissue post-imaging confirms surface columnar cells with a large cytoplasmic volume and highly nuclear basal layer. (C) 3-D reconstruction viewed from an angle of 45° shows intact epithelium with the characteristic feature being rounded surface cells. Scale bars: 25 μm
Impact of N-9 treatment on CRM and histology
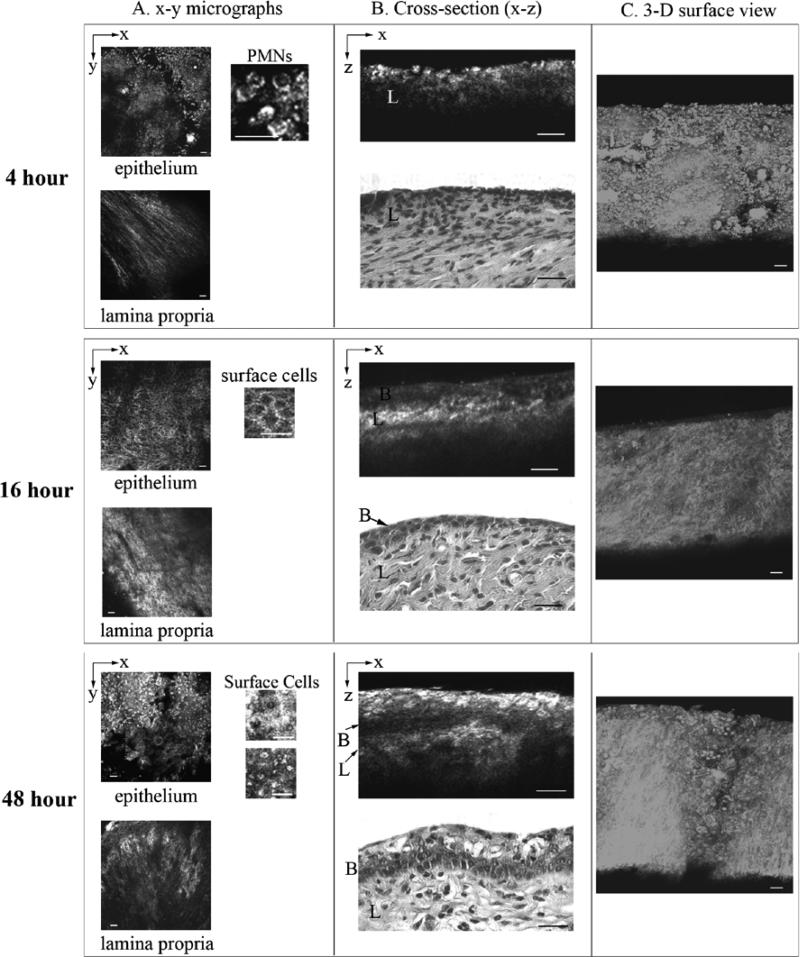
Figure 2 shows representative CRM images taken from freshly excised vagina at various times after treatment with N-9 and corresponding H&E stained sections. CRM micrographs taken 4 hours after N-9 treatment revealed substantial disruption of the epithelium (Figure 2a). The characteristic surface columnar cells seen in PBS controls were absent and the epithelium was thinned with only the basal layer remaining. This resulted in CRM cross-sections missing the first bright surface band seen in controls (Figure 2b). In two of the four animals, cellular infiltrates resembling polymorphonuclear neutrophils (PMNs) were observed by CRM – features of these cells matched those of PMNs imaged previously by CRM [19]. The surface view in the 3-D reconstruction (Figure 2B) showed a severely disrupted epithelium, void of the characteristic round mucosal cells found in controls and having surface debris and infiltrates. Corresponding histology, of the tissue showed that the majority of the epithelium was thinned and that there were regions where it was fully denuded (approximately 20% of the surface). H&E sections also showed evidence of inflammation with presence of PMNs confirming findings from CRM indicating an inflammatory response in addition to the thinned epithelium.
Figure 2.
CRM images taken from freshly excised vagina at (a) 4 hours, (b) 16 hours, and (c) 48 hours following intravaginal inoculation with N-9. In each case, the first column shows x-y micrographs taken of the surface and underlying lamina propria, the second column shows cross-sectional (x-z) views and H&E sections obtained from the imaged site (depth of image is 100 μm for 4 hours, and 125 μm for 16 and 48 hour cross-sections), and the third column is a 3-D reconstruction viewed from the surface at a tilt angle of 45°. Scale bars: 25 μm.
CRM of animals imaged 16 hours after N-9 treatment showed the epithelium denuded to the basal layer, however no inflammatory infiltrates were seen at this time. When viewed in 3D, the characteristic round mucosal cells of the controls were completely absent; instead the prominent feature was fibrous structure due to the lamina propria extracellular matrix. CRM findings were again confirmed by histology, which showed substantially thinned epithelium down to the basal layer in the majority of the surface in all samples. However, as with CRM imaging histology showed no evidence of infiltrates in any of the 16 hour samples.
Finally, in samples taken 48 hours after N-9 treatment, CRM revealed that the mucosal surface was undergoing re-epithelialization. Epithelial cells viewed by CRM were varied in morphology, but cells having bright cytoplasm were again present at the surface. In cross-section, the epithelium was thickened beyond the 4 and 16 hour treatments and surface cells appeared bright and round, although somewhat morphologically different from the surface cells in PBS treated animals. The 3D topographic view also differed markedly from the PBS controls during at this time. In particular cells at the surface did not have the round shape seen in controls. H&E sections at 48 hours revealed re-epithelialization of the vaginal surface and the epithelium no longer showed evidence of exfoliation in any of the samples. Surface epithelial cells were not columnar as in the PBS controls and cells differed in morphology throughout the depth of epithelium, again mirroring observations by CRM.
Measurement of changes in epithelial thickness
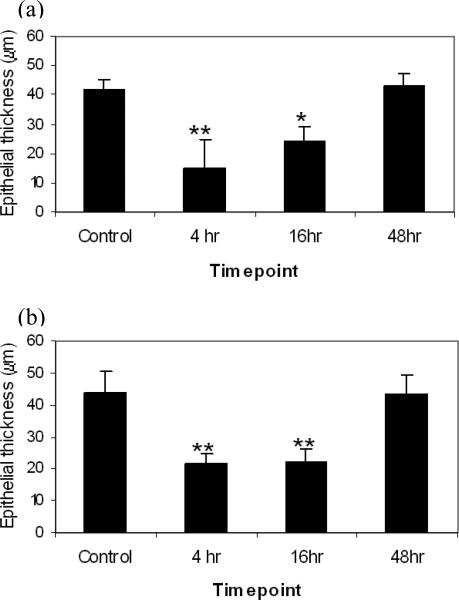
Cone et al., have previously shown a temporal correlation between changes in epithelial thickness and susceptibility to infection by herpes simplex virus type 2 in mice following N-9 treatment. Consequently, we examined whether CRM could be used to measure epithelial thickness of vaginal tissue and follow the thickness changes resulting from treatment with N9 over time. Figure 3A shows that using this method there was a significant decrease in epithelial thickness both 4 hours (p<0.001) and 16 hours (p<0.05) after N-9 treatment compared to controls but that the epithelium had returned to near baseline thickness 48 hours after N-9 treatment. Epithelial thickness measurements were also made from the corresponding histology samples (Figure 3b). Again the results were comparable with those seen by CRM with significant epithelial thinning at both 4 hours and 16 hours after N-9 treatment (p<0.001 each) and a return to near baseline levels at 48 hours.
Figure 3.
Timecourse of epithelial thickness measured by CRM on freshly excised vaginal tissue (a), and on H&E sections fixed and processed for histology following imaging (b). For each measurement technique epithelial thickness was compared by two-tailed one-way analysis of variance (ANOVA) followed by Tukey's post hoc test.* represents statistical significance with p<0.05 and ** with p<0.001.
DISCUSSION
This study evaluated the use of CRM as a means for revealing the subsurface microarchitecture of vaginal epithelium and for detecting epithelial changes resulting from topical treatment with microbicides. The goal of using such a technique would be as an adjunct to existing methods of assessment and that can be used on fresh tissue samples and provide near real-time monitoring of epithelial microstructural change in preclinical studies. Similarly, the method could be useful for noninvasive imaging on explant models used to evaluate tissue response to microbicides. In evaluating candidate microbicides, there is a real need to identify indicators of epithelial damage or toxicity which may be indicative of an increase of cervicovaginal susceptibility to infection with STI pathogens. Since there is evidence to suggest that disruption of the epithelial barrier is linked to increased susceptibility, direct imaging of epithelial microarchitecture could be an important tool in preclinical testing of candidate microbicides. A study by Cone et al., attempted to correlate the time course of toxic effects of N-9 on a mouse model for HSV-2 transmission with the time course of susceptibility to infection [9]. The factors examined were epithelial exfoliation, number of macrophages in the vaginal lumen, and levels of inflammatory cytokines. While there was an increase in levels of inflammatory cytokines and macrophages at times of increased susceptibility, the timecourse of epithelial thickness change was named as the factor which best correlated with the timecourse and duration of increased susceptibility. Another interesting result of that study was that colposcopy failed to detect visible signs of N-9 toxicity despite evidence of epithelial exfoliation and inflammation.
In preclinical safety evaluation of candidate microbicides, it is important to ensure testing is as rigorous as possible before candidate agents move into the clinical setting, particularly given the unexpected failure of previous candidate agents. It is an advantage to use models in which the epithelium is at its highest risk of damage to allow the most sensitive detection of microbicide-elicited effects. Medroxyprogesterone acetate pretreatment as used in these studies results in a diestrous-like state in which the vaginal epithelium is thinned [25]. It is expected that this pretreatment may result in more pronounced microbicide-elicited damage than may be seen in other phases of the estrous cycle, satisfying the desire of a sensitive preclinical model. It will be of interest in future studies to evaluate CRM in naturally cycling mice, despite the constantly changing epithelial structure.
In the present study CRM allowed specific epithelial structures to be identified in fresh unstained tissue, including surface mucous columnar cells, basal cells, and fibrillar extracellular matrix of the lamina propria to depths of 100-125 μm. Corresponding histology served as a means to corroborate these microstructural findings through visual comparison of cross-sectional x-z CRM micrographs with cross-sectional histology. For example the large mucous columnar cells present at the surface of the PBS control tissue in H&E sections had a similar morphology in CRM micrographs. CRM was also shown to follow changes in epithelium as a result of a single treatment with N-9 and these changes were mirrored by results from histology. Changes revealed by both CRM and histology included a loss in surface columnar cells and thinning in epithelium up to 16 hours after treatment and partial re-epithelialization after 48 hours as well as the presence of inflammatory infiltrates at 4 hours.
The timeline of change in vaginal epithelium revealed by CRM is consistent with published studies in which N-9 was shown to lead to epithelial disruption including sloughing and complete epithelial loss in uterine columnar epithelium [26] and cervicovaginal epithelium [14]. The study by Dayal et al. reported the disruption of uterine columnar epithelium following exposure to a gel containing 3.5% N-9 compared to a control gel or water after 24 hours, with epithelial regeneration in 48 hour samples and complete restoration by 72 hours. An interesting finding was that the regenerated epithelium consisted of cuboidal cells instead of the normal columnar cells. This emulates our results from CRM and histology, in the studies reported here in which the regenerating epithelium recovered the native thickness, but consisted of morphologically altered cells.
The ability to observe indicators of inflammation is an important characteristic of this imaging method, since an inflammatory response may be linked to increased susceptibility to infection by certain STIs, including HIV. Infiltrating inflammatory cells were observed by CRM at 4 hours. Infiltrates were also identified in H&E sections at this timepoint. Inflammatory infiltrates were not observed at 16 hours by CRM, and this was again confirmed by histology. Published studies have reported temporal modulations in inflammatory markers that coincide with the observed trend. Catalone et al., noted that a single dose of N-9 elicited an inflammatory response within 2-4 hours that accompanied columnar epithelial disruption and although the epithelium remained denuded at 8 hours, the inflammatory response was largely gone and remained absent at 24 hours [14]. Similarly, in the current study, inflammatory infiltrates were identified by CRM in 4 hour treatment samples but not at the longer timepoints of 16 hours and 48 hours.
Although the current studies were conducted on ex vivo tissue specimens, there is the potential for in vivo imaging in the future. With the advancement of emerging technologies such as confocal microendoscopy, in vivo preclinical and clinical assessment would be possible. CRM has been applied in vivo in the past in more accessible tissues such as skin and human oral mucosa to depths greater than 300 μm[18-24]. Recent developments in optical technology offer the promise of endoscopic in vivo imaging as well. Such advances include the development of fiber-optic confocal reflectance microscope systems with miniature imaging components [27-30]. Fluorescence-based confocal systems with endoscopic imaging capabilities and imaging probes small enough to fit within the lumen of small animal cervicovaginal tracts have also recently been developed [31]. These emerging technologies will also make possible repeated in vivo imaging for longitudinal studies.
In summary, CRM was successfully used to detect subsurface microarchitectural change in cervicovaginal mucosa treated with an N-9 containing contraceptive gel. CRM followed changes in epithelial structure (exfoliation, regeneration, cellular morphology changes) and detected the presence of an inflammatory response through the presence of inflammatory infiltrates. High resolution imaging by CRM or similar optical microscopy techniques may be a useful adjunct to traditional histology and colposcopy toward assessing microbicide effects on cervicovaginal tissue.
Acknowledgments
We thank Dr. Claudia Castro of the UTMB Department of Pathology for her assistance in reading histology for this study.
Sources of financial support (including grant numbers); This work was supported in part by the John Sealy Memorial Endowment Fund for faculty recruitment (# 6074-03) and NICHD (N01-HD-5-3407) and NIAID (R21AI07606202).
Footnotes
The authors do not have a commercial or other association that might pose a conflict of interest (e.g., pharmaceutical stock ownership, consultancy, advisory board membership, relevant patents, or research funding)
Presented in part: Microbicides 2006 International Meeting, 23-26 April 2006, Cape Town South Africa (poster presentation).
References
- 1.Singh B, Posti B, Cutler JC. Virucidal effects of certain chemical contraceptives on type 2 herpesvirus. Am J Obstet Gynecol. 1976;126:422–425. doi: 10.1016/0002-9378(76)90630-x. [DOI] [PubMed] [Google Scholar]
- 2.Polsksy B, Baron PA, Gold JWM, Smith JL, Jensen RH, Armstrong D. In vitro inactivation of HIV-1 by contraceptive sponge containing nonoxynol-9. Lancet. 1988;1:1456. doi: 10.1016/s0140-6736(88)92261-1. [DOI] [PubMed] [Google Scholar]
- 3.Malkovsky M, Newell A, Dalgleish AG. Inactivation of HIV by nonoxynol-9. Lancet. 1988;1:645. doi: 10.1016/s0140-6736(88)91440-7. [DOI] [PubMed] [Google Scholar]
- 4.Roddy RE, Zekeng L, Ryan KA, Tamoufe’ U, Weir SS, Wong EL. A controlled trial of nonoxynol 9 film to reduce male-to-femaile transmission of sexually transmitted diseases. NEJM. 1998;339(8):504–510. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- 5.Kreiss J, Ngugi E, Holmes K, Ndinya-Achola J, Waiyaki P, Roberts PL, Ruminjo I, Sajabi R, Kimata J, Fleming TR, et al. Efficacy of nonoxynol-9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA. 1992;268:477–482. [PubMed] [Google Scholar]
- 6.Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, Sirivongrangson P, Mukenge-Tshibaka L, Ettienge-Traore V, Uaheowitchai C, Karim SS, Masse B, Perriens J, Laga M, COL-1492 Study Group Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002:971–977. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 7.Ramjee G, Govinden R, Morar N, Mbewu A. South Africa's experience of the closure of the cellulose sulphate microbicide trial.(Policy Forum). Pl o S Medicine 47. 2007;7:1167. doi: 10.1371/journal.pmed.0040235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillier SL, Moench T, Shattock R, Black R, Reichelderfer P, Veronese F. In vitro and in vivo: the story of nonoxynol 9. J Acquir Immune Defic Syndr. 2005;39(1):1–8. doi: 10.1097/01.qai.0000159671.25950.74. [DOI] [PubMed] [Google Scholar]
- 9.Cone RA, Hoen T, Wong X, et al. Vaginal microbicides: detecting toxicities in vivo that paradoxically increase pathogen transmission. BMC Infect Dis. 2006;6:90. doi: 10.1186/1471-2334-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galen BT, Martin AP, Hazrati E, Garin A, Guzman E, Wilson SS, Porter DD, Lira SA, Keller MJ, Herold BC. A comprehensive murine model to evaluate topical vaginal microbicides: mucosal inflammation and susceptibility to genital herpes as surrogate markers of safety. JID. 2007;195:1332–9. doi: 10.1086/513279. [DOI] [PubMed] [Google Scholar]
- 11.Ballagh SA, Mauck CK, Henry D, Archer DF, Abercrombie T, Callahan MM, Gabelnick HL. A comparison of techniques to assess cervicovaginal irritation and evaluation of the variability between two observers. Contraception. 2004;70(3):241–9. doi: 10.1016/j.contraception.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Ballagh S. Factors affecting the reproducibility and validity of colposcopy for product development: review of current literature. J Acquir Immune Defic Syndr. 2004;37(S3):S152–5. [PubMed] [Google Scholar]
- 13.Doncel GF, Chandra N, Fichorova RN. Preclinical assessment of the proinflammatory potential of microbicide candidates. J Acquir Immune Defic Syndr. 2004 Oct;37(Suppl 3):S174–80. [PubMed] [Google Scholar]
- 14.Catalone BJ, Kish-Catalone TM, Budgeon LR, et al. Mouse model of cervicovaginal toxicity and inflammation for preclinical evaluation of topical vaginal microbicides. Antimicrob Agents Chemother. 2004;48:1837–1847. doi: 10.1128/AAC.48.5.1837-1847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fichorova RN. Guiding the vaginal microbicide trials with biomarkers of inflammation. J Acquir Immune Defic Syndr. 2004;37(Suppl 3):S184–93. [PMC free article] [PubMed] [Google Scholar]
- 16.Fichorova RN, Richardson-Harman N, Alfano M, Belec L, Carbonneil C, Chen S, Cosentino L, Curtis K, Dezzutti CS, Donoval B, Doncel GF, Donaghay M, Grivel JC, Guzman E, Hayes M, Herold B, Hillier S, Lackman-Smith C, Landay A, Margolis L, Mayer KH, Pasicznyk JM, Pallansch-Cokonis M, Poli G, Reichelderfer P, Roberts P, Rodriguez I, Saidi H, Sassi RR, Shattock R, Cummins JE., Jr Biological and Technical Variables Affecting Immunoassay Recovery of Cytokines from Human Serum and Simulated Vaginal Fluid: A Multicenter Study. Anal Chem. 2008 May 17; doi: 10.1021/ac702628q. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerger A, Horn M, Koller S, et al. Confocal examination of untreated fresh specimens from basal cell carcinoma: implications for microscopically guided surgery. Arch Dermatol. 2005;141:1269–1274. doi: 10.1001/archderm.141.10.1269. [DOI] [PubMed] [Google Scholar]
- 18.Gerger A, Koller S, Weger W, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006;107:193–200. doi: 10.1002/cncr.21910. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez S, Tannous Z. Real-time, in vivo confocal reflectance microscopy of basal cell carcinoma. J Am Acad Dermatol. 2002;47:869–874. doi: 10.1067/mjd.2002.124690. [DOI] [PubMed] [Google Scholar]
- 20.Horn M, Gerger A, Koller S, et al. The use of confocal laser-scanning microscopy in microsurgery for invasive squamous cell carcinoma. Br J Dermatol. 2007;156:81–84. doi: 10.1111/j.1365-2133.2006.07574.x. [DOI] [PubMed] [Google Scholar]
- 21.Rajadhyaksha M, Menaker G, Flotte T, et al. Confocal examination of nonmelanoma cancers in thick skin excisions to potentially guide mohs micrographic surgery without frozen histopathology. J Invest Dermatol. 2001;117:1137–1143. doi: 10.1046/j.0022-202x.2001.01524.x. [DOI] [PubMed] [Google Scholar]
- 22.Tannous Z, Torres A, Gonzalez S. In vivo real-time confocal reflectance microscopy: a noninvasive guide for Mohs micrographic surgery facilitated by aluminum chloride, an excellent contrast enhancer. Dermatol Surg. 2003;29:839–846. doi: 10.1046/j.1524-4725.2003.29219.x. [DOI] [PubMed] [Google Scholar]
- 23.Clark AL, Gillenwater AM, Collier TG, et al. Confocal microscopy for real-time detection of oral cavity neoplasia. Clin Cancer Res. 2003;9:4714–4721. [PubMed] [Google Scholar]
- 24.Drezek RA, Collier T, Brookner CK, et al. Laser scanning confocal microscopy of cervical tissue before and after application of acetic acid. Am J Obstet Gynecol. 2000;182:1135–1139. doi: 10.1067/mob.2000.104844. [DOI] [PubMed] [Google Scholar]
- 25.Whaley KJ, Barrat RA, Zeitlin L, Hoen TE, Cone RA. Nonoxynol-9 protects mice against vaginal transmission of genital herpes infections. J Infect Dis. 1993;168:1009–1011. doi: 10.1093/infdis/168.4.1009. [DOI] [PubMed] [Google Scholar]
- 26.Dayal MB, Wheeler J, Williams CJ, Barnhart KT. Disruption of the upper female reproductive tract epithelium by nonoxynol-9. Contraception. 2003;68:273–279. doi: 10.1016/s0010-7824(03)00178-1. [DOI] [PubMed] [Google Scholar]
- 27.Maitland KC, Gillenwater AM, Williams MD, El-Naggar AK, Descour MR, Richards-Kortum RR. In vivo imaging of oral neoplasia using a miniaturized fiber optic confocal reflectance microscope. Oral Oncol. 2008 Apr 5; doi: 10.1016/j.oraloncology.2008.02.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlson K, Chidley M, Sung KB, Descour M, Gillenwater A, Follen M, Richards-Kortum R. In vivo fiber-optic confocal reflectance microscope with an injection-molded plastic miniature objective lens. Appl Opt. 2005;44:1792–1797. doi: 10.1364/ao.44.001792. [DOI] [PubMed] [Google Scholar]
- 29.Chidley MD, Carlson KD, Richards-Kortum RR, Descour MR. Design, assembly, and optical bench testing of a high-numerical-aperture miniature injection-molded objective for fiber-optic confocal reflectance microscopy. Appl Opt. 2006;45(11):2545–54. doi: 10.1364/ao.45.002545. [DOI] [PubMed] [Google Scholar]
- 30.Sung KB, Liang C, Descour M, Collier T, Follen M, Malpica A, Richards-Kortum R. Near real time in vivo fibre optic confocal microscopy: sub-cellular structure resolved. J Microsc. 2002;207:137–145. doi: 10.1046/j.1365-2818.2002.01049.x. [DOI] [PubMed] [Google Scholar]
- 31.Laemmel E, Genet M, Le Goualher G, Perchant A, Le Gargasson JF, Vicaut E. Fibered Confocal FluorescenceMicroscopy (Cell-viZioTM) Facilitates Extended Imaging in the Field of Microcirculation: A Comparison with Intravital Microscopy. J. Vasc. Res. 2004;41:400–411. doi: 10.1159/000081209. [DOI] [PubMed] [Google Scholar]