Abstract
The multispecific efflux transporter, P-glycoprotein, plays an important role in drug disposition. Substrate translocation occurs along the interface of its transmembrane domains. The rotational C2 symmetry of ATP-binding cassette transporters implies the existence of two symmetry-related sets of substrate-interacting amino acids. These sets are identical in homodimeric transporters, and remain evolutionary related in full transporters, such as P-glycoprotein, in which substrates bind preferentially, but nonexclusively, to one of two binding sites. We explored the role of pore-exposed tyrosines for hydrogen-bonding interactions with propafenone type ligands in their preferred binding site 2. Tyrosine 953 is shown to form hydrogen bonds not only with propafenone analogs, but also with the preferred site 1 substrate rhodamine123. Furthermore, an accessory role of tyrosine 950 for binding of selected propafenone analogs is demonstrated. The present study demonstrates the importance of domain interface tyrosine residues for interaction of small molecules with P-glycoprotein.
Introduction
ATP-binding cassette (ABC) proteins form one of the largest families of transmembrane proteins. The human genome contains 48 genes encoding for ABC proteins, the majority of which are transporters. Mutations in at least 17 ABC transporters have been linked to disease etiologies (Linton et al., 2011). The minimal functional unit of a transporter consists of four domains: two transmembrane domains, which form the solute conduits, and two nucleotide binding domains (NBDs), which provide the energy for solute translocation by ATP binding and hydrolysis. Human P-glycoprotein (P-gp, ABCB1) is a multidrug resistance transporter, which plays a central role in drug disposition. Therefore, early profiling of developmental compounds includes routine screening for P-gp substrate properties (Giacomini et al., 2010).
A mechanistic model for cargo transport of ABC efflux transporters remains elusive, despite a large body of biochemical evidence. The present study characterizes the contribution of hydrogen-bonding interactions between propafenone type ligands and selected pore-exposed tyrosine OH groups. Propafenones have been characterized extensively in previous quantitative structure–activity relationship studies and demonstrated to be both substrates and inhibitors of P-gp (Schmid et al., 1999). Tyrosine residues are known to play a pivotal role for molecular recognition in biological systems, including domain interfaces and active site interactions. Tyrosines are amphipathic residues, capable of forming hydrophobic, hydrogen-bonding, π-π, and π-cation interactions. They were previously shown to make a large contribution to protein stability (Pace et al., 2001), but nevertheless contribute to structural plasticity of binding regions (Mian et al., 1991). The rigidity of the aromatic ring is associated with a reduced loss of conformational entropy upon immobilization in binding interfaces (Koide and Sidhu, 2009). The amphipathic nature of tyrosines allows them to readily tolerate changes in the polarity of their environment (MacCallum et al., 2007). Molecular dynamics simulations suggest that rotamers adopt different positions in the drug-binding pocket that allow them to contribute to polyspecificity (Liu et al., 2013). The known requirement for hydrogen-bonding interaction of P-gp substrates (Schmid et al., 1999; Gatlik-Landwojtowicz et al., 2006; Cramer et al., 2007) prompted us to investigate the importance of tyrosine hydroxyl groups for interaction with propafenone analogs.
A combination of photolabeling and mass spectrometry enabled us to demonstrate a dual interaction mode of P-gp with substrates in two rotationally symmetric positions. These involve tyrosine residues, which in the present study were mutated to phenylalanine to assess the contribution of tyrosine hydroxyl groups to hydrogen bond formation with propafenone type ligands. Data indicate an important role of these residues for interaction with propafenones as well as with the paradigmatic P-gp substrate rhodamine123 (rh123), thus providing additional experimental evidence for the dual interaction mode of P-gp with solutes and drugs.
Materials and Methods
Sequence Alignments and Homology Modeling
Generation of the ABCB1 model was previously described (Stockner et al., 2009). Briefly, ClustalW was used to obtain multiple sequence alignments of ABCB proteins. The models of human P-gp were based on the crystal structures of the outward-facing structure of Sav1866 from Staphylococcus aureus and the inward-facing structure of ABCB1 from Caenorhabditis elegans [PDB ID 2HYD, 3.0 Å resolution (Dawson and Locher, 2006); and PDB ID 4F4C, 3.4 Å resolution (Jin et al., 2012)] using the MODELLER software (version 9v12) (Sali and Blundell, 1993; Martí-Renom et al., 2000). The N terminus before the elbow helix as observed in the C. elegans ABCB1 structure was not included in the model and the interrupted helix 10 was replaced by a de novo model of an ideal helix. This replacement is supported by the observation of a contiguous helix 10 in all other structures from the ABCB transporter family. Initial models were further optimized by relaxation simulations of a membrane inserted transporter.
Knockdown of Endogenous P-gp in Human Embryonic Kidney 293 Cells
Construction and Prevalidation of Small Hairpin RNA Vectors
Human embryonic kidney 293 (HEK293) cells endogenously express P-gp at a level corresponding to approximately 5% of transiently expressed protein. To avoid interference from endogenous P-gp in functional assays, the transporter was knocked down by transduction with pLKO.1 lentiviral vectors (Moffat et al., 2006) containing P-gp small hairpin (shRNA) constructs targeted toward the 3′ untranslated region of the endogenous sequence as described by Addgene (http://www.addgene.org/plko; Addgene, Cambridge MA). Briefly, five specific oligonucleotides (Sigma-Aldrich, St. Louis, MO) targeting the 3′-untranslated region of the ABCB1 gene were introduced into the AgeI–EcoRI sites of pLKO.1 (plasmid 10878; Addgene). The following primers were used: ABCB1_1_forward, ccggAAGAGGTATCTGTTTAACATTctcgagAATGTTAAACAGATACCTCTTtttttg; ABCB1_1_reverse, aattcaaaaaAAGAGGTATCTGTTTAACATTctcgagAATGTTAAACAGATACCTCTT; ABCB1_2_forward, ccggGAATTATGAAGAGGTATCTGTctcgagACAGATACCTCTTCATAATTCtttttg; ABCB1_2_reverse, aattcaaaaaGAATTATGAAGAGGTATCTGTctcgagACAGATACCTCTTCATAATTC; ABCB1_3_forward, ccggGAACAGAGTGAGAGACATCATctcgagATGATGTCTCTCACTCTGTTCtttttg; ABCB1_3_reverse, aattcaaaaaGAACAGAGTGAGAGACATCATctcgagATGATGTCTCTCACTCTGTTC; ABCB1_4_forward, ccggGTGGAGAGAAATCATAGTTTActcgagTAAACTATGATTTCTCTCCACtttttg; ABCB1_4_reverse, aattcaaaaaGTGGAGAGAAATCATAGTTTActcgagTAAACTATGATTTCTCTCCAC; ABCB1_5_forward, ccggGACTGTATGAGATGTTAAATActcgagTATTTAACATCTC ATACAGTCtttttg; and ABCB1_5_reverse, aattcaaaaaGACTGTATGAG ATGTTAAATActcgagTATTTAACATCTCATACAGTC.
A nontargeting shRNA vector (plasmid 1864; Addgene) was used as a negative control. All shRNA expression cassettes were verified by sequencing. To test for efficiency and specificity, the five candidate shRNA constructs (numbers 1–5) were analyzed for target mRNA degradation using the Dual-Luciferase Reporter Assay System (Promega, Mannheim, Germany) according to the manufacturer’s recommendations. The inhibitory effects generated by shRNA constructs were expressed as normalized ratios between the activities of the reporter luciferase gene (firefly) and the luciferase reporter target gene fusion (Renilla) relative to the negative control vector containing scrambled shRNA (plasmid 1864; Addgene). The two most effective constructs (numbers 1 and 4) targeting endogenous P-gp were used to stably transduce HEK293 cells (see Supplemental Fig. 1 for further details).
Viral Particle Production and Target Cell Infection
Described shRNA-pLKO.1 constructs were cotransfected with the packaging plasmid pPax2 (plasmid 12260; Addgene) and the envelop plasmid pMD2.G (plasmid 12259; Addgene) into HEK293 cells using Lipofectamine 2000 (Invitrogen/LifeTech Austria, Vienna, Austria). Virus was harvested 72 hours after transfection and concentrated using a PEG virus precipitation kit (BioVision, Milpitas, CA). Infections of HEK293 cells were carried out in the presence of 10 μg/ml hexadimethrine bromide (polybrene; Sigma-Aldrich). After transduction, cells were selected with 2 μg/ml puromycin.
Construction of P-gp Mutants
The following primers were used for generation of the Y307F, Y310F, Y307F/Y310F, Y950F, Y953F, and Y950F/Y953F mutations of hexa–His-tagged human P-gp in the entry vector pENTR4: Y307F-forward, 5′-CTTTCCTGCTGATCTTTGCATCTTATGCTCTGGCC-3′; Y307F-reverse, 5′-GGCCAGAGCATAAGATGCAAAGATCAGCAGGAAAG-3′; Y310F-forward, 5′-CTTTCCTGCTGATCTATGCATCTTTTGCTCTGGCC-3′; Y310F-reverse, 5′-GGCCAGAGCAAAAGATGCATAGATCAGCAGGAAAG-3′; Y307F/Y310F-forward, 5′-CTTTCCTGCTGATCTTTGCATCTTTTGCTCTGGCC-3′; Y307F/Y310F-reverse, 5′-GGCCAGAGCAAAAGATGCAAAGATCAGCAGGAAAG-3′; Y950F-forward, 5′-TCACCCAGGCAATGATGTTTTTTTCCTATGCTGGATG-3′; Y950F-reverse, 5′-CATCCAGCATAGGAAAAAAACATCATTGCCTGGGTGA-3′; Y953F-forward, 5′-ACCCAGGCAATGATGTATTTTTCCTTTGCTGGATGTTTC-3′; Y953F-reverse, 5′-GAAACATCCAGCAAAGGAAAAATACATCATTGCCTGGGT-3′; Y950F/Y953F-forward, 5′-CCTTCACCCAGGCAATGATGTTTTTTTCCTTTGCTGGATGTTTCC-3′; and Y950F/Y953F-reverse, 5′-GGAAACATCCAGCAAAGGAAAAAAACATCATTGCCTGGGTGAAGG-3′. Furthermore, Q132R and Q773R mutations were introduced to the constructs above by using the following: Q132R-forward, 5′-GCTGCTTACATTCGTGTTTCATTTTG-3′; Q132R-reverse, 5′-CAAAATGAAACACGAATGTAAGCAGC-3′; Q773R-forward, 5′-CAT TTTTCCTTCGAGGTTTCACATTTG-3′; and Q773R-reverse, 5′-CA TTTTTCCTTCGAGGTTTCACATTTG-3′.
Gateway cloning technology (Hartley et al., 2000) was applied to shift wild-type and mutant P-gp constructs to pCEP4 destination vector.
Surface Expression of Wild-Type P-gp and Mutants
HEK293 cells were transiently transfected with wild-type or mutant P-gp constructs using TurboFect transfection reagent (Fisher Scientific, Vienna, Austria) according to the manufacturer’s instructions. Expression was determined by using the mouse monoclonal P-gp–specific MRK16 antibody (5μg/ml) (Kamiya Biomedical Company, Seattle, WA), IgG2A (2.5 μg/ml) as the control antibody, and a Becton Dickinson FACSCalibur flow cytometer (BD Biosciences, Vienna, Austria) as described previously (Parveen et al., 2011).
Continuous Monitoring of rh123 Zero trans Efflux
Cells were trypsinized, centrifuged at 500g, and washed with phosphate-buffered saline. Cells at 106 per data point (0.5 × 106/ml) were resuspended in Dulbecco’s modified Eagle’s medium (DMEM), pH 7.8, containing rh123 (Sigma-Aldrich) at a final concentration of 0.2 μg/ml (0.53 μM) and incubated at 37°C under gentle continuous agitation for 30 minutes. Loading was terminated by chilling tubes on ice and cells were washed twice with ice-cold DMEM, pH 7.4. Efflux was initiated by resuspending the cell pellet in DMEM, pH 7.4, prewarmed to 37°C. Efflux was monitored continuously over 5 minutes in a Becton Dickinson FACSCalibur flow cytometer at 37°C using a temperature-controlled water jacket. Viable cells were selected by setting appropriate gates for forward and side scatter. The excitation and emission wavelengths were 488 and 534 nm, respectively. Data points were exported to a graphic user interface programmed in LabView 2013 (National Instruments, Salzburg, Austria), which allows the import of flow cytometry standard files. Average mean fluorescence units values were computed for selected time intervals (default 1-second intervals) and these mean fluorescence units values were displayed as a function of time. When selecting 1-second intervals, the time course consists of 300 individual data points, each averaging about 3000 gated events. First-order rate constants (k values) were calculated from an exponential fit according to the following equation:
where a is the difference between the zero and infinite time point of the curve, e is the Euler number, k is the first-order rate constant, t is the time in seconds, and c is the background fluorescence of cells (refer to Supplemental Fig. 2 for further details). Transport rates were calculated from k values normalized to surface expression, which was determined by MRK16 staining. Fractional transport rates were calculated for each individual experiment.
Inhibition Assays
Cells were loaded with rh123 as described above and the cell pellet was resuspended in medium prewarmed to 37°C that contained either no inhibitor or compounds GPV005 [1-2-(2-hydroxy-3-(piperidin-1-yl)propoxy)phenyl)-3-phenylpropan-1-one], GPV031 [1-(2-(3-(4-(4-fluoro phenyl)piperazin-1-yl)-2-hydroxypropoxy)phenyl)-3-phenylpropan-1-one], or GPV366 [N-(2-hydroxy-3-(2-(3-phenylpropanoyl)phenoxy)propyl)-N-propylbenzamide] (refer to Supplemental Fig. 3 for structures) at various concentrations ranging from 4.6 nM to 90 μM, depending on solubility and expected potency. Eight concentrations (serial 1:3 dilution) were tested for each propafenone analog. Inhibition of rh123 efflux by these compounds was monitored continuously over 5 minutes at 37°C. First-order rate constants (k) were plotted as a function of inhibitor concentration and IC50 values were calculated for each compound by nonlinear regression analysis.
Statistical Analysis
All data are expressed as the mean ± S.D. Averages of IC50 values were compared using one-way analysis of variance (GraphPad Prism, version 5; GraphPad Software, Inc., La Jolla, CA). Post hoc Tukey analyses were carried out to find groups whose mean differences were significant.
Results
Selection of Residues and Design of Mutants
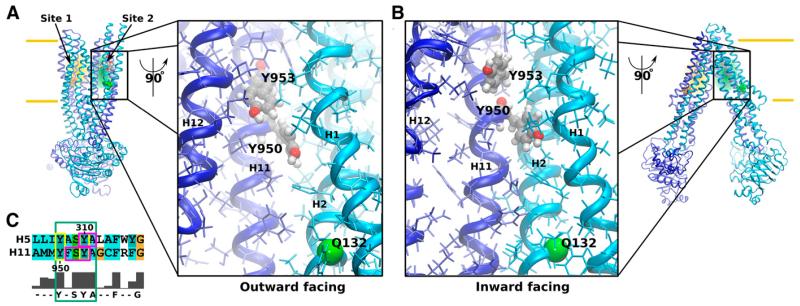
Our group previously demonstrated that photoactivated propafenone derivatives label residues in pseudosymmetric positions including helices 5 and 11 (Parveen et al., 2011) (Fig. 1). These results imply the existence of two substrate-transporter interaction modes. Full transporters have arisen from half transporters by gene duplication. In homodimeric half transporters, each conformation of any set of amino acid residues is represented twice. On theoretical grounds, binding of ligands would therefore be possible in either of two modes. In full transporters, these sets of amino acids were reshaped by evolution making them similar, but nonidentical (Parveen et al., 2011). To designate sets of interacting amino acid residues, the term site is subsequently used. Please note that this term is only meant to refer to sets of ligand-interacting amino acid residues in pseudosymmetric positions of the transporter that might either be separated in space or partially or fully overlapping.
Fig. 1.
Locations of site 1 (orange; preferred rh123 site) and site 2 (green; preferred propafenone, verapamil, and vinblastine site) are indicated in the homology models of P-gp. (A) Location in the outward-facing P-gp structure and in a close up of site 2 in a 90° rotated transporter. Site 2 is viewed from the pore. Residues Q773 (orange) and Q132 (green) lie proximal in the path taken by transported compounds. When mutated to arginine, these residues decrease the binding probability of protonatable compounds in either of the two sites. (B) The same representation of overall structure and a close up of site 2 for the inward-facing conformation. (C) The sequence alignment of helices 5 and 11 of P-gp. The cyan box shows the conserved Y(F)xSYA motif, and yellow boxes highlight mutated residues tyrosine Y950 and Y953. Residues which were previously shown to be strongly labeled by photoactivated propafenones are indicated in magenta.
Accordingly, rh123 was shown to prefer the first interaction site (site 1), whereas propafenones, verapamil, and vinblastine have a preference for the second site (site 2). Introduction of arginine residues in positions 773 and 132 changes the binding probability for protonatable compounds in site 1 or 2 by charge repulsion. Previous experiments identified site 2 tyrosine residue Y953 in helix 11 as being photolabeled by propafenones (Pleban et al., 2005; Parveen et al., 2011). The conserved residue Y953 is located in a consensus 950Y(F) xSYA954 motif that is also found in site 1. This amino acid residue lies at the apex of site 2 (Fig. 1) and represents a potential interaction partner for propafenone type ligands.
Pore-exposed tyrosine residue Y950, which lies one helical turn away from Y953 toward the cell interior, was also mutated to phenylalanine because it was considered a potential additional interaction partner for propafenones. This residue is a phenylalanine in some species, but a tyrosine in humans. Interaction of propafenones with the transporter was quantified by exploring the potential of the compounds to inhibit rh123 efflux. This proved necessary because propafenone analogs used in this study rapidly diffuse through biomembranes and thus a net transport is not measureable (Schmid et al., 1999).
Position and local geometry of site 2 are illustrated in Fig. 1, A and B, and compared between the outward-(model based on Sav1866 from S. aureus) (Dawson and Locher, 2006) and the inward-facing structures (model based on ABCB1 from C. elegans) (Jin et al., 2012). The local geometry at site 2 remained remarkably similar between the two structures. We observed a reduction in the distance between transmembrane helices 1 and 12 by approximately 2 Å during the transition from the inward to the outward-facing structure, whereas accessibility to residues Y950 and Y953 was comparable. The hydroxyl functional groups of both tyrosine residues remained water exposed in both conformations and would therefore be able to form hydrogen bonds to a ligand binding to site 2.
Effect of the Removal of Y950 and Y953 OH Groups on rh123 Efflux
P-gp mutants were first characterized for their ability to transport rh123 to assess any potential effect of changes in its transport rate on evaluation of propafenones. Rh123 is a preferred site 1 substrate, but is also transported via site 2. First-order rate constants were normalized by expression to account for differences in the amount of protein present at the plasma membrane, which differs as a result of using a transient expression system (refer to Supplemental Fig. 4 for the linear relationship between first-order rate constants and expression). The (normalized) transport rates are therefore independent of the amount of protein expressed. All mutants were detected at the plasma membrane, although the expression levels of Q132R/Y950F, Q773R/Y950F, and Y307F/Y310F were reduced (Supplemental Fig. 5). Correct formation of the contact interfaces between helices 2/11 and 5/8 is an important requirement for proper engagement of coupling helices 2 and 4 into the sockets formed between the core and the α-helical domain of NBD1 and NBD2, respectively. Less efficient folding of these mutants, which reside in the 2/11 and 5/8 interfaces, is likely the cause of lower plasma membrane expression.
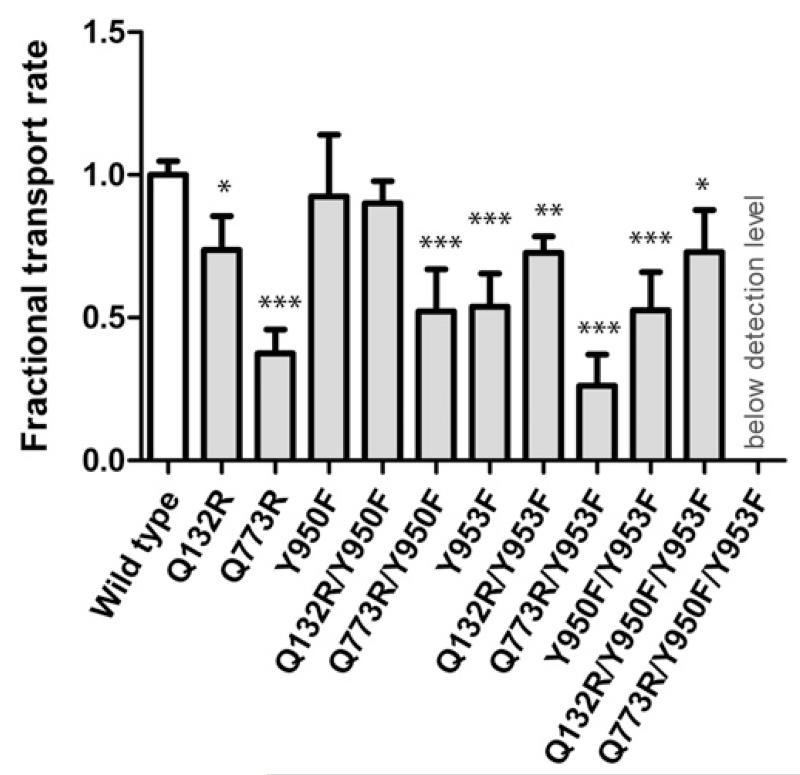
Mutant Y950F showed a similar transport rate as wild-type protein, whereas mutant Y953F showed a significantly decreased rate (54% ± 12% of wild-type) (Fig. 2). The double mutant Y950F/Y953F showed a decrease that was comparable with that observed for the Y953F single mutant alone. Thus, only the OH group of residue Y953, but not that of Y950, contributes to rh123 transport.
Fig. 2.
Bar graph showing changes in the fractional transport rate of mutants in comparison with wild-type. Rh123 efflux was monitored continuously for 5 minutes. First-order rate constants (k) were calculated from an exponential fit and normalized to surface expression that was determined by MRK16 staining. Q773R/Y950F/Y953F was expressed at the surface, but no rh123 efflux was detectable. Each value represents the mean ± S.D. of at least three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001, compared with wild-type P-gp).
Access of rh123 to binding sites 1 and 2 can be controlled by the introduction of positive charges (arginines) in the access path to the ligand binding sites. We previously showed that the Q132R mutation blocks access to site 2 for rh123, whereas the Q773R mutation prevents rh123 access to site 1 (Parveen et al., 2011). The effect of the Y953F mutation on rh123 efflux should be abolished by introducing selector residue R132 and should be more pronounced when deselecting site 1 by introducing selector residue R773. Introduction of arginine residues in positions 132 or 773 led to a decrease in rh123 efflux to 75% ± 11% and 37% ± 8% of wild-type, respectively (Fig. 2). These data are in agreement with our previous results, in which the same rank order of transport activity was found. As expected, the presence of the selector residue R132 abrogated the effect of single and double tyrosine mutation on transport activity. This is illustrated by comparable transport activity of the Q132R/Y950F, Q132R/Y953F, and Q132R/Y950F/Y953F mutants. These results also demonstrate that Y to F mutations in positions 950 and 953 do not perturb protein mechanics. This is an important finding because impairment of function is a frequently encountered limitation of site directed mutagenesis (Ito et al., 2001; Koike et al., 2002; Swartz et al., 2013).
By contrast, transport rates were found to be lower in the Q773R/Y953F mutant compared with the Q773R mutant alone, although this decrease did not reach statistical significance. Indeed, no decrease in transport activity was observed for the Y950F mutant introduced in the R773 background. In summary, these results demonstrate that the hydroxyl group of residue 953, but not that of 950, plays a role for rh123 transport.
Interestingly, neither single nor double mutation of corresponding residues Y307 and Y310 in binding site 1 changed rh123 efflux (Supplemental Fig. 6). These findings indicate that hydrogen-bonding interactions are of minor importance for interaction of rh123 with tyrosines located on helix 5. This clearly reflects evolutionary divergence of the two sites. Although aromatic interactions likely are important, these were not subject of the present study.
Role of Y950 and Y953 for the Formation of Hydrogen Bonds with Propafenone Analogs
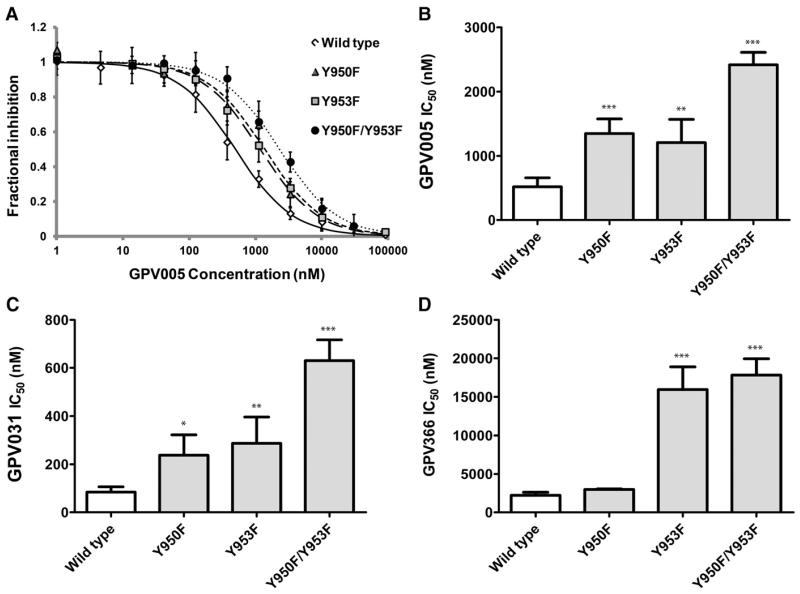
Protonatable propafenones GPV005 and GPV031 were chosen to be studied with wild-type protein and in the R132 background. The nonprotonatable acid amide GPV366 served as a control that is not influenced by the selector residue R132. Representative examples of concentration response curves for compound GPV005 are shown for wild-type and single and double tyrosine mutants (Fig. 3A). The wild-type showed an IC50 value of 518 ± 141 nM, whereas the values for the single mutants Y950F and Y953F were 1348 ± 229 and 1207 ± 362 nM, respectively. The double mutant had an IC50 value of 2418 ± 194 nM (Fig. 3B; refer to Supplemental Tables 1 and 2 for numerical values and statistical significance of differences in IC50 values, respectively). An analogous pattern was seen for GPV031 (wild-type, 85 ± 22 nM; Y950F, 238 ± 84 nM; Y953F, 287 ± 109 nM; and Y950F/Y953F, 630 ± 87 nM) (Fig. 3C). The fold change in IC50 values was thus similar for both compounds. Data suggest hydrogen-bonding interactions of compounds with both tyrosine OH groups, because a comparable increase in IC50 values was observed for each of them and an additive effect was seen when both of them were mutated. Figure 3D and Supplemental Table 1 summarize data for the nonprotonatable acid amide GPV366. In contrast with protonatable propafenones, a higher IC50 value was observed for the Y953F, but not for the Y950F, single mutant. The fold change for the double mutant was comparable with that observed for the Y953F single mutant (wild-type, 2239 ± 391 nM; Y950F, 2984 ± 79 nM, n.s.; Y953F, 15,946 ± 2941 nM, 7.1-fold change relative to wild-type; and Y950F/Y953F, 17,819 ± 2106 nM, 8-fold change).
Fig. 3.
Inhibition of rh123 efflux was measured in the absence and presence of eight serial dilutions of GPV005. (A) Open diamonds, wild-type; filled circles, Y950F/Y953F; triangles, Y950F; squares, Y953F. IC50 values for GPV005 (B), GPV031 (C), and GPV366 (D) were determined by fitting hyperbolic concentration response curves to data points by nonlinear regression analysis and calculation of 50% occupancy values. Mean ± S.D. values were calculated from at least three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001, compared with wild-type P-gp.
Effect of Site 2 Tyrosine Mutants in the R132 Background
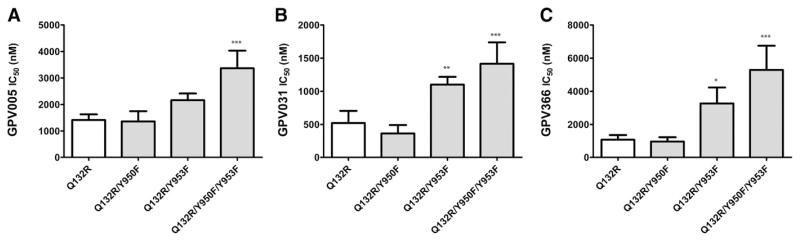
The at least 3-fold difference in IC50 values for wild-type protein (GPV005, 518 ± 141 nM; and GPV031, 85 ± 22 nM) (Fig. 3, B and C) and the Q132R mutant (GPV005, 1414 ± 215 nM; and GPV031, 521 ± 183 nM) (Fig. 4, A and B) demonstrates that an arginine residue in position 132 prevents access of protonatable compounds to site 2. Introducing tyrosine mutations in the R132 background should in principle make IC50 values of protonatable compounds independent of the presence of site 2 mutations. Indeed, data in Fig. 4, A and B, and Supplemental Table 1 show that for the Q132R/Y950F mutant, IC50 values were similar to those observed in the Q132R background alone. However, IC50 values were still higher in the Q132R/Y953F mutation. This increase was not significant for GPV005, but reached significance for GPV031. Thus, removal of the hydroxyl group of tyrosine 953 still affected IC50 values of protonatable propafenones, indicating that protonatable compounds were still able to access site 2, although to a lesser extent. Incomplete prevention of access is a result of ligand properties and not the transporter itself, because both GPV005 and GPV031 have a pKa (Ecker et al., 1999), which results in charged and uncharged species to coexist under physiologic conditions (further details are given in Discussion).
Fig. 4.
IC50 values for inhibition of rh123 efflux was measured for GPV005 (A), GPV031 (B), and GPV366 (C) by fitting hyperbolic concentration response curves to data points by nonlinear regression analysis and calculation of 50% occupancy values. The tyrosine mutants were generated in the Q132R mutation background. Mean ± S.D. values were calculated from at least three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001, compared with Q132R mutation.
For the nonprotonatable acid amide GPV366, a higher IC50 value was observed in the Q132R/Y953F mutant (Q132R, 1074 ± 282 nM; and Q132R/Y953F, 3261 ± 965 nM), whereas the Y950F mutation in the same background did not affect potency (966 ± 259 nM) (Fig. 4C). Although the pattern was similar to that observed in the wild-type background, the fold change was lower in the Q132R background and the IC50 value was somewhat lower in the protein containing the Q132R mutation than for wild-type. A possible explanation for this effect is the lack of competition of GPV366 in the preferred binding site 2 with rh123, because rh123 binding to site 2 is abolished by the Q132R mutation.
The higher IC50 values in the Q132R/Y950F/Y953F mutant were not due to an additive effect of the two tyrosine mutations on binding of propafenones. Therefore, the observed effect cannot be explained by a contribution from tyrosine mutations on small molecule binding and must have other reasons. Certainly, the effect is not brought about by a global perturbance of protein structure and function, because the Q132R mutant and the Q132R/Y950F/Y953F triple mutant showed comparable rh123 transport rates (Fig. 2). All three mutations are located at the contact interface of helices 2 and 11 and might thus locally influence the geometry of binding site 2 in the triple mutant. Importantly, this effect does not have a bearing on the interpretation of results in this study.
Discussion
The presence of tyrosines in binding cavities is important both for specificity and affinity of solutes toward proteins (Koide and Sidhu, 2009). This has been illustrated for a number of bacterial, rodent, and human transporters. Wu et al. (2008) studied the importance of tyrosine residues for the interaction of the bacterial exporter QacA from S. aureus with solutes. Mutation of residue Y410 in the drug-binding pocket to phenylalanine led to a decrease in the transport rate of QacA for several substrates, including monovalent dyes. A study on the human ABCC1 transporter (multidrug resistance protein 1) found that mutation of two cytoplasmic loop tyrosines (Y1189 and Y1190) to alanine or serine, respectively, resulted in a 50% reduced transport rate for different organic anion substrates, particularly glutathione (Conseil et al., 2005). Mammalian ABC transporters are rich in aromatic residues in the apical region of the central cavity, as illustrated by the crystal structure of mouse ABCB1, protein homology models of human ABCB1, and the related human hepatic transporter ABCB4 (Gutmann et al., 2010), as well as the crystal structure of ABCB10 (Shintre et al., 2013). Interactions formed at the apex of the central cavity may be a prerequisite for substrate-induced nucleotide occlusion in one of the composite nucleotide binding sites, which, in turn, affords the outward-facing conformation and substrate release (Tombline et al., 2005, 2006; Sauna et al., 2007).
Our study of the importance of the hydrogen-bonding capability of Y950 and Y953 was motivated by three lines of evidence: 1) the critical role that tyrosines play in molecular recognition; 2) the projected location of these residues in binding site 2; and 3) the presence of a conserved Y(F)xSYA motif, in which the second tyrosine is photolabeled by propafenones (Parveen et al., 2011). Residue Y953 seems to be highly conserved in multiple sequence alignments of annotated P-gp orthologs, whereas substitutions of Y950 by phenylalanine are more frequent.
To address the role of hydrogen-bonding interactions of these two tyrosines for rh123 efflux, the transport rate of this preferred site 1 substrate was determined either in the wildtype or the respective selector mutation background. Residues Q132 and Q773 are located below and outside the binding sites, and replacement by arginine was previously shown to direct protonatable compounds away from one and toward the respective alternate site by charge repulsion. Experimental evidence indicates Y953 to represent a hydrogen-bonding partner for rh123, because a significant decrease in transport rates in the wild-type and the Q773R backgrounds was observed. Identical transport rates were found for the Q132R and the Y953F mutants in the Q132R background. This finding was expected because rh123 always carries one positive charge at physiologic pH (Shinomiya et al., 1992) and introducing selector residue R132 would abolish any influence that mutations in the site, which is now inaccessible for ligands, would have.
For reasons detailed above in Results, hydrogen-bonding interactions of tyrosines with propafenone analogs GPV005, GPV031, and GPV366 were assessed by determining IC50 values for rh123 efflux inhibition. Both residues Y950 and Y953 were demonstrated to play a role as hydrogen-bonding partners for the protonatable propafenones GPV005 and GPV031, whereby the effect was additive in the double mutant. GPV366 showed a hydrogen-bonding interaction with residue Y953, whereas residue Y950 did not contribute. As expected, the double mutant showed an IC50 value that was comparable with that of the Y953F mutant.
In principle, access of charged propafenones to preferred site 2 should be prevented by introduction of the selector mutation R132. IC50 values were therefore expected to be the same in single and double tyrosine mutants generated in the Q132R background. Indeed, no difference was observed between the Q132R single mutant and the Q132R/Y950F double mutant. However, higher IC50 values were found for the Y953F mutant, although less pronounced than in the wild-type background. These results indicate that propafenones can still reach site 2. Propafenones coexist in protonated and nonprotonated forms at physiologic pH. The uncharged ligand species can still have access to site 2, which has been deselected for the protonated ligand species by introducing the Q132R mutation. The permanently charged rh123 is entirely prevented from reaching site 2 by introducing selector residue R132. The pKa value of GPV005 is 8.4 and that of GPV031 is 7.3 (Ecker et al., 1999). This allows estimating the percentage of protonated species of GPV005 and GPV031 at intracellular pH. Accordingly, the charged species amounts to >90% and 60–70%, respectively. GPV005 would therefore be prevented from accessing site 2 to a larger extent than GPV031. Indeed, results in Fig. 4, A and B, show that this is the case. For GPV005, mutant Q132R/Y953F showed a 1.5-fold higher IC50 value than the Q132R mutation, whereas the fold changes were 2.1-fold for GPV031 and 3.0-fold for uncharged GPV366. From experiments in the wild-type background (Fig. 3), an equal contribution of residues Y950 and Y953 was expected. However, such an effect was not observed in the Q132R background (Fig. 4, A and B). Residue Y950, therefore, lost its importance as an interaction partner, possibly due to the close proximity of residues Q132 and Y950, which are predicted in models to have a C-a distance of approximately 7 Å. Although residue Q132 has been shown not to be a direct interaction partner for propafenone type ligands (Parveen et al., 2011), a steric effect of the arginine side chain is likely, but is only seen for those propafenone analogs that interact with Y950 (GPV005 and GPV031, but not GPV366).
The triple Q132R/Y950F/Y953F mutant showed a further increase in IC50 values for all compounds, which is obviously not due to interaction with tyrosines. As discussed in Results, this finding is hypothesized to be due to local changes in site 2. The fact that the extent of deselecting inversely correlates with the fold change (2.3-fold for GPV005, 2.7-fold for GPV031, and 4.9-fold for GPV366) supports this hypothesis.
Interaction of propafenones with the transporter was assessed by determining IC50 values for rh123 efflux inhibition. In these experiments, both the interaction of P-gp with rh123 and with propafenones contributes to the outcome of the experiments. We therefore addressed the question of whether a change in rh123 efflux would affect propafenone IC50 values in the way we observed. When the transporter is partially impaired, an inhibition should be easier to accomplish. In contrast, our experiments showed the inverse (i.e., an increase in IC50 values, rather than a decrease). Therefore, the effects seen for the mutants are certainly not due to the observed decrease in rh123 flux, because in that case they should show the opposite to what we observed.
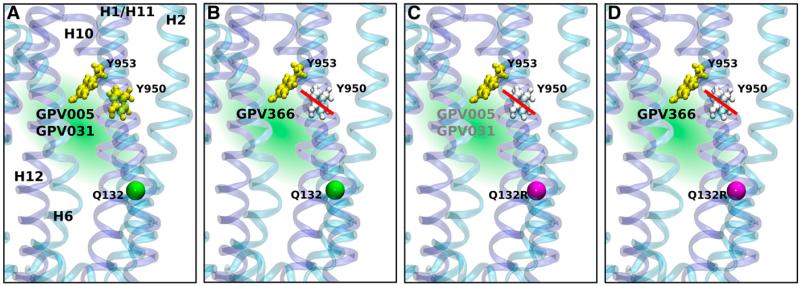
Figure 5 summarizes results in a schematic way. Tyrosines are shown in yellow when they are found important for hydrogen bond formation and in gray when they are not. Tyrosines Y950 and Y953 are hydrogen bonding with protonatable compounds GPV005 and GPV031 (Fig. 5A), whereas only residue Y953 is important for interaction with the nonprotonatable acid amide GPV366 (Fig. 5B). When the wild-type glutamine in position 132 is replaced by arginine, the probability of protonatable compounds to reach site 2 is decreased. This is indicated by gray font color of compounds GPV005 and GPV031. In addition, residue Y950 loses its importance as a residue that forms hydrogen bonds with propafenones (Fig. 5C). Compound GPV366 only interacts with residue Y953 (Fig. 5D).
Fig. 5.
Schematic presentation of the importance of tyrosine hydroxyl groups (tyrosines, yellow) for interaction with propafenone analogs in the outward-facing P-gp structure (template Sav1866) (A and B); Cα atom of residue Q132 (shown as a green sphere) and after introduction of the R132 selector mutation (Cα atom of residue Q132 shown in magenta) (C and D). The hydroxyl groups of both Y950 and Y953 are able to form hydrogen bonds with protonatable propafenones GPV005 and GPV031 (A), whereas compound GPV366 only forms hydrogen bonds with residue Y953 (B). The gray color of residue Y950 and red bar indicate that hydrogen bonds of residue Y950 are not important for interaction. The presence of the selector residue R132 (C and D) eliminates the importance of the hydroxyl group of Y950 for propafenone analog binding, whereas the interaction of residue Y953 continues to contribute to the interaction. Use of gray type indicates incomplete prevention of access of compounds GPV005 and GPV031 in C.
Our results are in agreement with earlier work on tyrosine residues in mouse and human P-gp. These previous studies evaluated toxicity (a composite of the influence of mutation on trafficking and function) (Hanna et al., 1996) and ATPase activity of reconstituted P-gp (Loo and Clarke, 2000), respectively. A direct evaluation of transport activity has not been performed in earlier work. Hanna et al. (1996) studied alanine mutants of tyrosine residues Y946 and Y949 in mouse mdr3. These residues are found in analogous position to tyrosines Y950 and Y953 in the human sequence. The ability of mutants to confer resistance to actinomycin D, adriamycin, colchicine, and vinblastine was determined. In these experiments, mutations to alanine probed for both hydrogen-bonding and aromatic interactions. The Y949A mutation increased toxicity by colchicine and adriamycin, the Y946A made cells more sensitive to vinblastine, and both mutants increased sensitivity toward actinomycin D. These data are in agreement with the finding that drugs, which we previously found to preferentially bind to site 1 or site 2, respectively, are affected by mutation of tyrosine residues. Loo and Clarke (2000) demonstrated altered ATPase stimulation of the human protein for the Y953A mutant by verapamil, colchicine, and vinblastine, but did not present evidence for a direct involvement in drug binding.
A dual substrate binding mode is rooted in the rotational C2 symmetry of human ABC transporters. In this article, the contribution of active site tyrosine residues for substrate interaction with propafenones has been studied with respect to their preferred interaction site 2. Propafenones were used as model compounds because of the availability of photolabeling data, which previously showed a dual pseudosymmetric mode of interaction with P-gp. The photolabeled tyrosine residue Y953 is shown to form hydrogen bonds with protonatable and nonprotonatable propafenones, as well as with preferred site 1 substrate rh123. We provide proof of principle that the preferred propafenone binding site can be studied individually and the influence of mutation can be resolved in a site-specific manner. We believe that dual symmetry-related sites present in other human ABC exporters may potentially be linked to disease etiologies. One candidate is the human bile salt export pump, which specifically transports conjugated bile acids, but, at the same time, is inhibited by numerous systemically administered drugs.
Supplementary Material
Acknowledgments
This work was supported by the Austrian Science Fund [Special Research Fund Grant 35-3509 and Stand-Alone Project 23319]; and the European Cooperation in Science and Technology [Action Grant CM0902]. N.K. acknowledges receipt of a scholarship from the Austrian Federal Ministry of Science and Research.
ABBREVIATIONS
- ABC
ATP-binding cassette
- DMEM
Dulbecco’s modified Eagle’s medium
- GPV005
1-2-(2-hydroxy-3-(piperidin-1-yl)propoxy) phenyl)-3-phenylpropan-1-one
- GPV031
1-(2-(3-(4-(4-fluorophenyl)piperazin-1-yl)-2-hydroxypropoxy)phenyl)-3-phenylpropan-1-one
- GPV366
N-(2-hydroxy-3-(2-(3-phenylpropanoyl)phenoxy)propyl)-N-propylbenzamide
- HEK293
human embryonic kidney 293
- NBD
nucleotide binding domain
- P-gp
P-glycoprotein
- rh123
rhodamine123
- shRNA
small hairpin RNA
Footnotes
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Conseil G, Deeley RG, Cole SP. Role of two adjacent cytoplasmic tyrosine residues in MRP1 (ABCC1) transport activity and sensitivity to sulfonylureas. Biochem Pharmacol. 2005;69:451–461. doi: 10.1016/j.bcp.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Cramer J, Kopp S, Bates SE, Chiba P, Ecker GF. Multispecificity of drug transporters: probing inhibitor selectivity for the human drug efflux transporters ABCB1 and ABCG2. ChemMedChem. 2007;2:1783–1788. doi: 10.1002/cmdc.200700160. [DOI] [PubMed] [Google Scholar]
- Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- Ecker G, Huber M, Schmid D, Chiba P. The importance of a nitrogen atom in modulators of multidrug resistance. Mol Pharmacol. 1999;56:791–796. [PubMed] [Google Scholar]
- Gatlik-Landwojtowicz E, Aänismaa P, Seelig A. Quantification and characterization of P-glycoprotein-substrate interactions. Biochemistry. 2006;45:3020–3032. doi: 10.1021/bi051380+. [DOI] [PubMed] [Google Scholar]
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, et al. International Transporter Consortium Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann DA, Ward A, Urbatsch IL, Chang G, van Veen HW. Understanding polyspecificity of multidrug ABC transporters: closing in on the gaps in ABCB1. Trends Biochem Sci. 2010;35:36–42. doi: 10.1016/j.tibs.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna M, Brault M, Kwan T, Kast C, Gros P. Mutagenesis of transmembrane domain 11 of P-glycoprotein by alanine scanning. Biochemistry. 1996;35:3625–3635. doi: 10.1021/bi951333p. [DOI] [PubMed] [Google Scholar]
- Hartley JL, Temple GF, Brasch MA. DNA cloning using in vitro sitespecific recombination. Genome Res. 2000;10:1788–1795. doi: 10.1101/gr.143000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Oleschuk CJ, Westlake C, Vasa MZ, Deeley RG, Cole SP. Mutation of Trp1254 in the multispecific organic anion transporter, multidrug resistance protein 2 (MRP2) (ABCC2), alters substrate specificity and results in loss of methotrexate transport activity. J Biol Chem. 2001;276:38108–38114. doi: 10.1074/jbc.M105160200. [DOI] [PubMed] [Google Scholar]
- Jin MS, Oldham ML, Zhang Q, Chen J. Crystal structure of the multidrug transporter P-glycoprotein from Caenorhabditis elegans. Nature. 2012;490:566–569. doi: 10.1038/nature11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide S, Sidhu SS. The importance of being tyrosine: lessons in molecular recognition from minimalist synthetic binding proteins. ACS Chem Biol. 2009;4:325–334. doi: 10.1021/cb800314v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K, Oleschuk CJ, Haimeur A, Olsen SL, Deeley RG, Cole SP. Multiple membrane-associated tryptophan residues contribute to the transport activity and substrate specificity of the human multidrug resistance protein, MRP1. J Biol Chem. 2002;277:49495–49503. doi: 10.1074/jbc.M206896200. [DOI] [PubMed] [Google Scholar]
- Linton KJ, Zolnerciks JK, Schmitt L. The ABC Transporters of Human Physiology and Disease: Genetics and Biochemistry of ATP Binding Cassette Transporters. World Scientific Publishing; London: 2011. [Google Scholar]
- Liu M, Hou T, Feng Z, Li Y. The flexibility of P-glycoprotein for its polyspecific drug binding from molecular dynamics simulations. J Biomol Struct Dyn. 2013;31:612–629. doi: 10.1080/07391102.2012.706079. [DOI] [PubMed] [Google Scholar]
- Loo TW, Clarke DM. Identification of residues within the drug-binding domain of the human multidrug resistance P-glycoprotein by cysteine-scanning mutagenesis and reaction with dibromobimane. J Biol Chem. 2000;275:39272–39278. doi: 10.1074/jbc.M007741200. [DOI] [PubMed] [Google Scholar]
- MacCallum JL, Bennett WF, Tieleman DP. Partitioning of amino acid side chains into lipid bilayers: results from computer simulations and comparison to experiment. J Gen Physiol. 2007;129:371–377. doi: 10.1085/jgp.200709745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí-Renom MA, Stuart AC, Fiser A, Sánchez R, Melo F, Sali A. Comparative protein structure modeling of genes and genomes. Annu Rev Biophys Biomol Struct. 2000;29:291–325. doi: 10.1146/annurev.biophys.29.1.291. [DOI] [PubMed] [Google Scholar]
- Mian IS, Bradwell AR, Olson AJ. Structure, function and properties of antibody binding sites. J Mol Biol. 1991;217:133–151. doi: 10.1016/0022-2836(91)90617-f. [DOI] [PubMed] [Google Scholar]
- Moffat J, Grueneberg DA, Yang X, Kim SY, Kloepfer AM, Hinkle G, Piqani B, Eisenhaure TM, Luo B, Grenier JK, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- Pace CN, Horn G, Hebert EJ, Bechert J, Shaw K, Urbanikova L, Scholtz JM, Sevcik J. Tyrosine hydrogen bonds make a large contribution to protein stability. J Mol Biol. 2001;312:393–404. doi: 10.1006/jmbi.2001.4956. [DOI] [PubMed] [Google Scholar]
- Parveen Z, Stockner T, Bentele C, Pferschy S, Kraupp M, Freissmuth M, Ecker GF, Chiba P. Molecular dissection of dual pseudosymmetric solute translocation pathways in human P-glycoprotein. Mol Pharmacol. 2011;79:443–452. doi: 10.1124/mol.110.067611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleban K, Kopp S, Csaszar E, Peer M, Hrebicek T, Rizzi A, Ecker GF, Chiba P. P-glycoprotein substrate binding domains are located at the transmembrane domain/transmembrane domain interfaces: a combined photoaffinity labeling-protein homology modeling approach. Mol Pharmacol. 2005;67:365–374. doi: 10.1124/mol.104.006973. [DOI] [PubMed] [Google Scholar]
- Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Sauna ZE, Kim IW, Nandigama K, Kopp S, Chiba P, Ambudkar SV. Catalytic cycle of ATP hydrolysis by P-glycoprotein: evidence for formation of the E. S reaction intermediate with ATP-gamma-S, a nonhydrolyzable analogue of ATP. Biochemistry. 2007;46:13787–13799. doi: 10.1021/bi701385t. [DOI] [PubMed] [Google Scholar]
- Schmid D, Ecker G, Kopp S, Hitzler M, Chiba P. Structure-activity relationship studies of propafenone analogs based on P-glycoprotein ATPase activity measurements. Biochem Pharmacol. 1999;58:1447–1456. doi: 10.1016/s0006-2952(99)00229-4. [DOI] [PubMed] [Google Scholar]
- Shinomiya N, Tsuru S, Katsura Y, Sekiguchi I, Suzuki M, Nomoto K. Increased mitochondrial uptake of rhodamine 123 by CDDP treatment. Exp Cell Res. 1992;198:159–163. doi: 10.1016/0014-4827(92)90162-2. [DOI] [PubMed] [Google Scholar]
- Shintre CA, Pike AC, Li Q, Kim JI, Barr AJ, Goubin S, Shrestha L, Yang J, Berridge G, Ross J, et al. Structures of ABCB10, a human ATP-binding cassettetransporter in apo- and nucleotide-bound states. Proc Natl Acad Sci USA. 2013;110:9710–9715. doi: 10.1073/pnas.1217042110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockner T, de Vries SJ, Bonvin AM, Ecker GF, Chiba P. Data-driven homology modelling of P-glycoprotein in the ATP-bound state indicates flexibility of the transmembrane domains. FEBS J. 2009;276:964–972. doi: 10.1111/j.1742-4658.2008.06832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz DJ, Weber J, Urbatsch IL. P-glycoprotein is fully active after multiple tryptophan substitutions. Biochim Biophys Acta. 2013;1828:1159–1168. doi: 10.1016/j.bbamem.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombline G, Donnelly DJ, Holt JJ, You Y, Ye M, Gannon MK, Nygren CL, Detty MR. Stimulation of P-glycoprotein ATPase by analogues of tetramethylrosamine: coupling of drug binding at the “R” site to the ATP hydrolysis transition state. Biochemistry. 2006;45:8034–8047. doi: 10.1021/bi0603470. [DOI] [PubMed] [Google Scholar]
- Tombline G, Muharemagić A, White LB, Senior AE. Involvement of the “occluded nucleotide conformation” of P-glycoprotein in the catalytic pathway. Biochemistry. 2005;44:12879–12886. doi: 10.1021/bi0509797. [DOI] [PubMed] [Google Scholar]
- Wu J, Hassan KA, Skurray RA, Brown MH. Functional analyses reveal an important role for tyrosine residues in the staphylococcal multidrug efflux protein QacA. BMC Microbiol. 2008;8:147. doi: 10.1186/1471-2180-8-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.