Abstract
Objective
To determine the cost-effectiveness of split IVF-intracytoplasmic sperm injection (ICSI) for the treatment of couples with unexplained infertility.
Design
Adaptive decision model.
Setting
Academic infertility clinic.
Patient(s)
A total of 154 couples undergoing a split IVF-ICSI cycle and a computer-simulated cohort of women <35 years old with unexplained infertility undergoing IVF.
Intervention(s)
Modeling insemination method in the first IVF cycle as all IVF, split IVF-ICSI, or all ICSI, and adapting treatment based on fertilization outcomes.
Main Outcome Measure(s)
Live birth rate, incremental cost-effectiveness ratio (ICER).
Result(s)
In a single cycle, all IVF is preferred as the ICER of split IVF-ICSI or all ICSI ($58,766) does not justify the increased live birth rate (3%). If two cycles are needed, split IVF/ICSI is preferred as the increased cumulative live birth rate (3.3%) is gained at an ICER of $29,666.
Conclusion(s)
In a single cycle, all IVF was preferred as the increased live birth rate with split IVF-ICSI and all ICSI was not justified by the increased cost per live birth. If two IVF cycles are needed, however, split IVF/ICSI becomes the preferred approach, as a result of the higher cumulative live birth rate compared with all IVF and the lesser cost per live birth compared with all ICSI.
Keywords: Cost-effectiveness, split IVF-ICSI, unexplained infertility
Up to 30% of infertile couples are diagnosed with unexplained infertility given the lack of pregnancy despite evidence of ovulation, patent fallopian tubes, adequate sperm production, and appropriate pattern of coitus (1). Couples with unexplained infertility may harbor reproductive disorders related to oocyte quality, tubal function, or sperm function that cannot be diagnosed by the standard infertility evaluation. In vitro fertilization is an effective therapeutic option when other treatments have failed and may provide additional diagnostic information regarding gamete function (2).
Total failed fertilization is observed in 8.4%–22.7% of couples with unexplained infertility that underwent conventional IVF (3–6). Fertilization failure is observed more often in couples with unexplained infertility than other infertile subgroups, suggesting that defects in fertilization may be the cause of infertility in some couples. Intracytoplasmic sperm injection (ICSI) is usually recommended for subsequent IVF cycles when poor or failed fertilization has occurred with conventional IVF, although fertilization failure may not always be repetitive (7, 8).
Strategies to minimize or eliminate fertilization failure in the first IVF cycle in couples with unexplained infertility include all ICSI or split IVF-ICSI, as practiced in our own center in recent years (9). Split IVF-ICSI involves randomly allocating sibling oocytes to conventional IVF or ICSI. This approach is more advantageous than IVF alone or ICSI alone given the ability to diagnose fertilization defects and to identify couples who will benefit from ICSI in subsequent IVF cycles and perhaps avoiding unnecessary ICSI in many couples. It has been suggested that ICSI should not be routinely performed in non-male factor infertility given that clinical outcomes are not improved (10, 11). In addition, ICSI in male factor infertility has been associated with an increased risk of sex and autosomal chromosomal aberrations, congenital anomalies, and imprinting disorders and it is unknown whether these risks are associated with ICSI in non-male factor infertility (12). Intracytoplasmic sperm injection also adds expense and laboratory time compared with conventional IVF (10). Despite these concerns, the use of ICSI in the United States for non-male factor infertility has dramatically increased (13).
In the absence of a clinical guideline regarding insemination method in couples with unexplained infertility undergoing IVF, we developed an adaptive decision analysis comparing all IVF, split IVF-ICSI, and all ICSI in the first cycle and adapted subsequent treatment based on fertilization outcomes. We then sought to determine whether split IVF-ICSI is a cost-effective approach.
MATERIALS AND METHODS
Split IVF-ICSI
To inform fertilization probabilities for the split IVF-ICSI arm of the decision analysis, we analyzed fertilization outcomes for all couples with unexplained infertility who underwent split IVF-ICSI in their first cycle at the Center for Reproduction & Infertility from 2006–2010. Unexplained infertility was diagnosed when there was no conception despite evidence of ovulation, patent fallopian tubes, and adequate sperm production. Institutional review board approval was obtained from Women & Infants’ Hospital of Rhode Island.
Patients underwent down-regulation with luteal leuprolide acetate (LA) or received a midfollicular GnRH antagonist to inhibit ovulation. The FSH doses were titrated based on response, which was monitored by serial ultrasounds and serum E2 measurements. Human chorionic gonadotropin was administered when at least two follicles reached mean diameters of 18 mm. Transvaginal ultrasound-guided retrieval was performed approximately 36 hours after hCG administration. If nine or more oocytes were retrieved, cumulus-oocyte complexes were randomly allocated to fertilization by conventional IVF or by ICSI. If less than nine oocytes are retrieved, all oocytes were fertilized by ICSI and these couples were not included in the analysis. Four hours after retrieval, the ICSI cumulus-oocyte complexes were treated with 80 IU/mL hyaluronidase to remove the granulosa cells (GCs) and assess for nuclear maturation. Mature oocytes (metaphase II) were injected using a standard ICSI procedure. The IVF oocytes were inseminated with 50,000 motile sperm per oocyte. Sixteen to 19 hours later, the oocytes were inspected for two pronuclear (2PN) fertilization.
Outcomes analyzed included fertilization rate (number of 2PN zygotes per the number of oocytes allocated to conventional IVF or ICSI), transfer rate (number of transferred embryos per total number of embryos available by insemination method), and cryopreservation rate (number of cryopreserved embryos per the total number of embryos available by insemination method). Outcomes were compared using Student’s t test.
Adaptive Decision Model
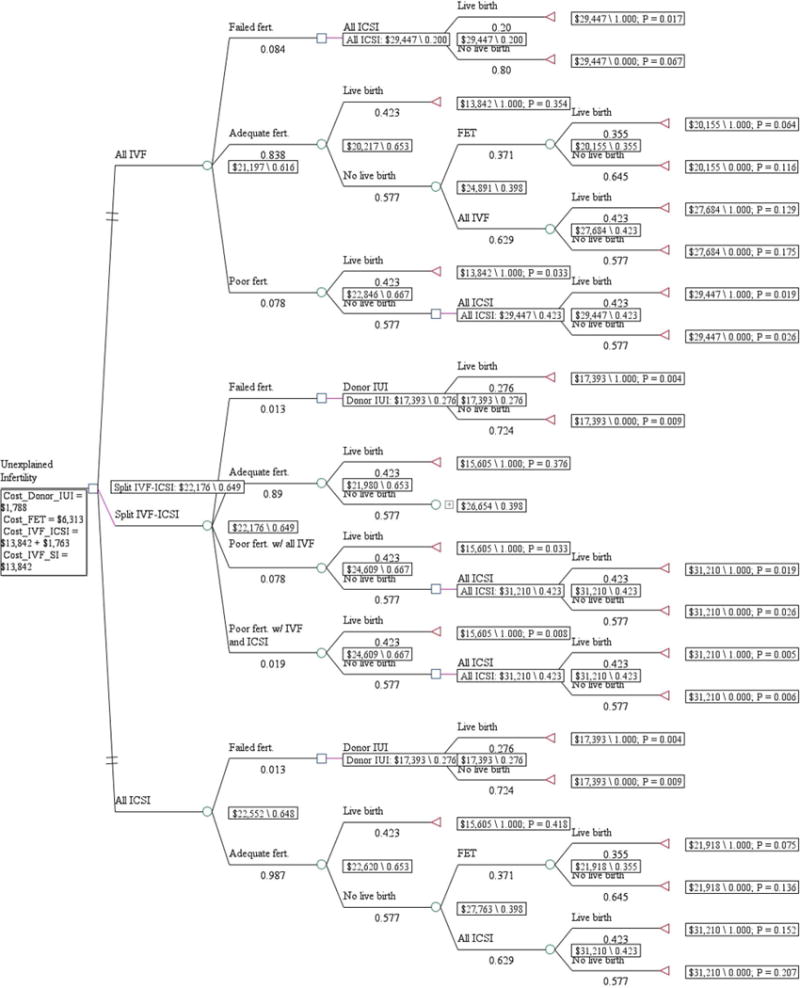
A decision analysis model (TreeAge Pro 2011) was constructed that compared all conventional IVF, split IVF-ICSI, and all ICSI for couples with unexplained infertility (Fig. 1). Fertilization outcomes included adequate fertilization (fertilization rate, R20%), poor fertilization (fertilization rate, >0 and <20%), or failed fertilization. In the event of poor or failed fertilization with conventional IVF, we assume that ICSI would be performed on all oocytes in the next cycle (Fig. 2). If adequate fertilization with conventional IVF was observed, we postulate that conventional IVF alone would be performed in the next cycle. If failed fertilization occurred with conventional IVF and ICSI, we propose that donor insemination would be recommended. The model is adaptive because it considers the outcomes of two fresh cycles—a fresh cycle followed by a frozen ET or a fresh cycle followed by a donor insemination cycle—updating the decision rules based on the extant evidence.
Figure 1.
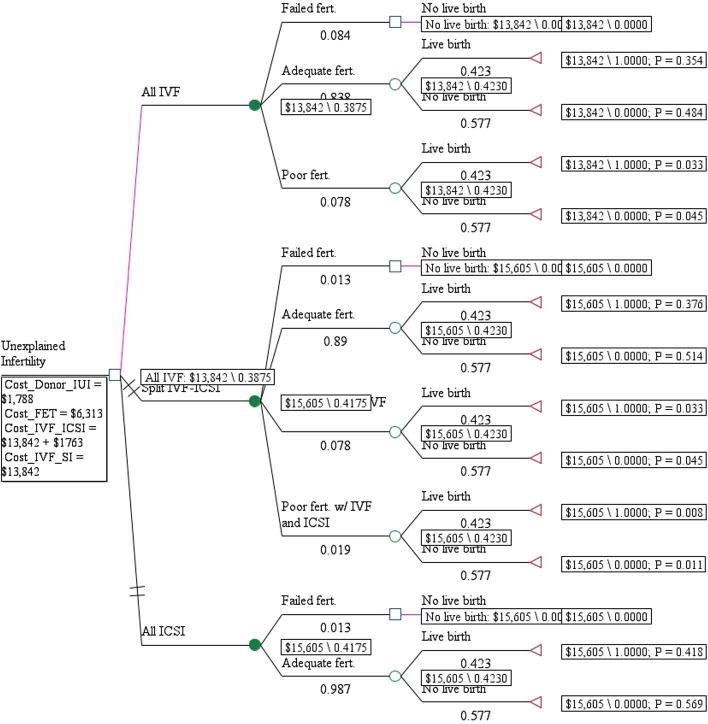
Figure 2.
The probability inputs for fertilization rates and live birth rates for women were based on data from our center, published data, and the 2009 Society for Assisted Reproductive Technology (SART) national data summary (Table 1). Basecase data for failed fertilization and fertilization rates reflect our center’s experience, but to our knowledge, this data set is the largest series available for couples with unexplained infertility undergoing split IVF-ICSI. Published data from populations with similar characteristics were used to inform the ranges. Live birth rates and ranges for fresh and thawed non-donor cycles in women <35 years old with unexplained infertility were selected from the 2009 SART national data summary (14). Given that a randomized controlled trial of couples with unexplained infertility undergoing IVF found that success rates were not significantly different during the first three IVF cycles, the same live birth rate and ranges were selected for the first and second IVF cycles in the model if fertilization occurred in the first cycle (15). In the event of failed fertilization with conventional IVF, lower pregnancy rates (PRs) in subsequent IVF/ICSI cycles have been reported and these data were applied in the model (16, 17). In the event of failed fertilization with conventional IVF and ICSI, donor insemination is recommended and PRs in this subgroup were based on data from donor sperm IUI cycles after ICSI failure (18). We determined the probability that non-pregnant couples with cryopreserved embryos would pursue a fresh IVF cycle or a frozen ET for subsequent treatment based on observations from our own cohort.
Table 1.
Probability and cost variables.
| Base case | Range | Reference | |
|---|---|---|---|
| Probability variables | |||
| Fertilization rates | |||
| Failed fertilization with conventional IVF | 0.084 | 0.084–0.227 | text, 3–6 |
| Failed fertilization with ICSI | 0.013 | 0–0.036 | text, 3–6 |
| Poor fertilization with conventional IVF | 0.078 | NA | text |
| Poor fertilization with conventional IVF and ICSI | 0.019 | NA | text |
| Live birth rates | |||
| IVF for unexplained infertility, aged <35 y | 0.423 | 0.41–0.436 | 14 |
| Frozen ET for unexplained infertility, aged <35 y | 0.365 | NA | 14 |
| First donor sperm IUI cycle after ICSI failure | 0.276 | NA | 14 |
| Cost variables | |||
| IVF | 13,842 | 6,920–27,685 | 20, 21 |
| ICSI | 1,763 | 1,141–4,280 | 22 |
| Embryo cryopreservation | 788 | 393–1,578 | 20 |
| Frozen ET cycle | 6,313 | 3,155–12,626 | 20 |
| Donor insemination | 1,788 | 1,114–3,607 | 22 |
Note: Cost variables are presented in 2012 US dollars. ICSI = intracytoplasmic sperm injection; NA = not available.
Vitek. Cost-effectiveness analysis of split IVF-ICSI.
The cost of IVF and ICSI are adjusted to constant 2012 US dollars using the Consumer Price Index (19) (Table 1). The direct cost of IVF with respect to the procedure, medication, and cryopreservation costs were verified by Little et al. (20) by surveying five geographically diverse IVF centers in 2005. In addition, the American Society for Reproductive Medicine (ASRM) reports a similar estimate for direct IVF costs (21). The direct cost and range for ICSI was determined by a survey of 30 IVF centers by Resolve: The National Infertility Association in 2006 (22). The cost of split IVF-ICSI was considered to be equivalent to the cost of performing all ICSI. The direct cost and range of frozen ET cycle was reported by Little et al. (20). The direct cost and range of donor IUI was based on the cost at our center and data from a survey by Resolve (22).
The cumulative probabilities for live birth and the estimated costs for one or two treatment cycles were determined for each scenario (Table 2). The incremental cost-effectiveness ratio represents the additional cost per live birth, and was calculated by dividing the differences in cost between the two scenarios by the difference in live birth rates between scenarios. A treatment was considered “dominated” if it was equally or less effective than the alternative but was more costly. One-way sensitivity analysis was performed by varying the cost of ICSI during two cycles within the reported range (Supplemental Fig. 1, available online).
Table 2.
Cost-effectiveness results after one or two cycles.
| Cost | Incremental cost | Effectiveness | Incremental effectiveness | Incremental cost-effectiveness ratio | |
|---|---|---|---|---|---|
| One cycle | |||||
| All IVF | 13,842 | 0.388 | |||
| Split IVF-ICSI | 15,605 | 1,763 | 0.418 | 0.03 | 58,766 |
| All ICSI | 15,605 | 1,763 | 0.418 | 0.03 | 58,766 |
| Two cycles | |||||
| All IVF | 21,197 | 0.616 | |||
| Split IVF-ICSI | 22,176 | 979 | 0.649 | 0.033 | 29,666 |
| All ICSI | 22,552 | 1,355 | 0.648 | 0.032 | 42,343 |
Note: ICSI = intracytoplasmic sperm injection.
Vitek. Cost-effectiveness analysis of split IVF-ICSI. Fertil Steril 2013.
RESULTS
Split IVF-ICSI Results
One hundred fifty-four couples with unexplained infertility underwent split IVF-ICSI cycles at the Center for Reproduction & Infertility during 2006–2010. The average age of the female patient was 34 (±4.3) years. The average number of oocytes retrieved was 17.3 (range, 9–40). The number of oocytes randomly allocated to conventional IVF or ICSI was 1,283 and 1,377, respectively. There was no significant difference in the fertilization rate between conventional IVF and ICSI (52.5% vs. 55.5%; P=.118). Two couples (1.3%) experienced failed fertilization with both conventional IVF and ICSI. Eleven couples (7.1%) had failed fertilization of oocytes allocated to conventional IVF and adequate fertilization of oocytes allocated to ICSI. An additional 15 couples experienced poor fertilization (fertilization rate, >0 and <20%) with conventional IVF. Of these 15 couples, 3 couples also experienced poor fertilization with ICSI. There was no significant difference in the transfer rate (12.5% vs. 14.5%; P=.14) or cryopreservation rate (10.0% vs. 11.1%; P=.39) of embryos fertilized by conventional IVF or ICSI. The average number of embryos transferred was 2.12 (±0.7). Embryo transfers consisted of mixed IVF and ICSI fertilized embryos in 44.8% of cycles, ICSI-only embryos in 33.1% of cycles and IVF-only embryos in 22.1% of cycles. Six live births resulted from cycles with failed (n=4) or poor fertilization (n=2) with conventional IVF, in which ET would likely not have occurred had ICSI not been performed.
Cost-Effectiveness Results
Single cycle analysis
The cost-effectiveness results are presented in Figure 1 and Table 2. For a single cycle, the cost per live birth in the all conventional IVF arm is $13,842 and was associated with a 38.8% likelihood of live birth. Both split IVF-ICSI and all ICSI was associated with an incremental cost of $1,763 and a 3.0 percentage point increase in cumulative live birth (from 38.8%–41.8%) when compared with all conventional IVF. Despite the increase in cumulative live birth with split IVF-ICSI and all ICSI, all conventional IVF was the preferred fertilization method given the substantial increase in cost per live birth (incremental cost-to-effectiveness ratio of $58,766) associated with split IVF-ICSI and all ICSI.
Two cycle analysis
If a second cycle becomes necessary (Fig. 2 and Table 2), the cost per live birth in the all conventional IVF arm was $21,197 and was associated with a 61.6% cumulative likelihood of live birth during two treatment cycles. By selectively applying ICSI to couples who are likely to benefit in the second cycle, split IVF-ICSI was associated with an incremental cost of $979 and a 3.3 percentage point increase in cumulative live birth (from 61.6%–64.9%). When all ICSI is universally applied to all couples in the second cycle, the associated incremental cost was $1,355 with a 3.2 percentage point increase in cumulative live birth (from 61.6%–64.8%). With an incremental cost-effectiveness ratio of $29,666 for split IVF-ICSI compared with an incremental cost-effectiveness ratio of $42,343 with all ICSI, split IVF-ICSI was the preferred method of insemination if two cycles become necessary.
Sensitivity Analysis
A one-way sensitivity analysis was performed varying the cost of ICSI for two cycles (Supplemental Fig. 1). When varying the cost of ICSI by $500 increments across the range, split IVF-ICSI dominated ICSI given that split IVF-ICSI is less expensive with a similar efficacy, except when the cost of all ICSI is at the minimum of the range ($1,141). In Supplemental Figure 1, we plot the net monetary benefits over a range of IVF-ICSI costs ($13,842–$15,605) showing that there is a threshold point (split IVF-ICSI = $14,125.67) when split IVF-ICSI becomes the dominant strategy.
DISCUSSION
We report that 7.1% of couples with unexplained infertility undergoing split IVF-ICSI experienced failed fertilization with conventional IVF and that four live births resulted among patients in this cohort in which ET would not have occurred had ICSI not been performed. By analyzing incremental cost-effectiveness ratios using randomized clinical data and plausible cost ranges in a decision model, we have demonstrated that all IVF for couples with unexplained infertility was the dominant strategy if a single cycle was performed. However, split IVF-ICSI was the dominant strategy if two cycles become necessary under most plausible cost ranges and using the results from the first cycle to determine the appropriate method of fertilization for the second cycle. By minimizing fertilization failure, split IVF-ICSI increases cumulative pregnancy rates by 3.3 percentage points over two treatment cycles when compared with all conventional IVF. Split IVF-ICSI was more cost effective than performing all ICSI in two cycles by identifying couples who would benefit from ICSI and limiting subsequent treatment with ICSI to only these couples. We also found that performance of all ICSI was a dominated strategy as it was more costly with similar efficacy to split IVF-ICSI. In sensitivity analyses, we found that split IVF-ICSI dominated all ICSI for the majority of plausible cost ranges.
A higher fertilization rate with ICSI compared with all conventional IVF has been reported in couples with unexplained infertility, although a prospective randomized trial of IVF versus ICSI for couples with unexplained infertility found no difference in fertilization rates (4, 6, 10). We found no significant difference in the fertilization rate with ICSI compared to IVF. We included all oocytes that were randomly allocated to ICSI to determine the fertilization rate, although immature oocytes were not injected, which may have decreased our fertilization rate with ICSI as compared to other reports. We observed a lower rate of fertilization failure with conventional IVF than reported in split IVF-ICSI studies, which may also account for the finding that our fertilization rates were not different. Similar to other reports, we found no difference in the transfer rate or cryopreservation rate of embryos fertilized by ICSI or conventional IVF suggesting that insemination method does not alter embryo development (7, 10,23). Data regarding the effect of insemination method on implantation rate, clinical PR, or live birth rate are not reported in the literature given that studies of split IVF-ICSI have included mixed transfers of embryos fertilized by IVF or ICSI. A randomized trial of IVF or ICSI for nonmale factor infertility found no difference in clinical PRs or live birth rates (10). Based on these findings, we assumed that live birth rate was not altered by insemination method in the adaptive decision model presented.
Although ICSI may not improve clinical outcomes for couples with unexplained fertility that do not exhibit fertilization defects, split IVF-ICSI can help identify couples who benefit from ICSI. Fertilization failure was averted by ICSI in 7.1% of cycles in our split IVF-ICSI cohort and four live births resulted. The adaptive decision model demonstrated that split IVF-ICSI would increase the cumulative live birth rate by 5.3% (0.033/0.616; 0.033 is the difference in percentage points of the estimated effectiveness, and 0.616 is the cumulative live birth rate for the all conventional IVF arm) during two cycles when compared with all conventional IVF by minimizing fertilization failure in the first cycle and using diagnostic information gained from the split to determine subsequent treatment (Table 2). Split IVF-ICSI saved $12,677 per live birth (in terms of incremental cost-effectiveness ratios) during two treatment cycles when compared with all ICSI by limiting subsequent treatment with ICSI to couples with fertilization defects. Additional cost savings would be gained if more than two cycles were needed.
Although clinical studies of split IVF-ICSI have shown that this strategy minimizes fertilization failure, the adaptive decision model demonstrates the impact of using the diagnostic information gained from the split to alter subsequent treatment to improve cumulative PRs and reduce costs. A strength of the decision model was the use of our data set on split IVF-ICSI outcomes to inform the base-case probabilities as it represents the largest reported experience with split IVF-ICSI for unexplained infertility. The sensitivity analysis covers the range of plausible assumptions based on our clinical practice and the published evidence.
Our split IVF-ICSI data was limited to couples with nine or more oocytes retrieved. It was our belief that the value of the diagnostic information obtained from split IVF-ICSI would be diminished for couples with less than nine oocytes retrieved given the limited numbers of observations. The decision model may not apply to couples with low oocyte yield as they may benefit from ICSI to maximize fertilization. All couples in our data set were diagnosed with unexplained infertility. Although it is possible that couples with mild male factor infertility were diagnosed with unexplained infertility, significant male factor cases would not have been eligible for split IVF-ICSI in our practice and would have been treated with all ICSI. A potential weakness of our data set is that oocytes were randomly allocated to IVF or ICSI by the embryologists and bias toward allocating mature-appearing oocytes to ICSI may have been introduced. Nonetheless, we did not see a difference in the rate of fertilization between IVF and ICSI, suggesting that this possible bias had no discernible impact on our data set.
A limitation of the decision model includes a lack of reported live birth rates for couples with poor or failed fertil ization with IVF who subsequently conceive with ICSI. Therefore reported clinical PRs were used. In addition, we assumed that couples with failed fertilization with both IVF and ICSI would proceed with donor IUI given our clinical experience, although one report (8) suggests that fertilization failure may not be universal with subsequent IVF or ICSI attempts.
The billing for split IVF-ICSI is not standardized. We assumed that the cost of split IVF-ICSI is the same as all ICSI for the decision model based on the practice at our own institution. If the cost of split IVF-ICSI is lower than all ICSI, our results underestimate the savings that split IVF-ICSI could offer during two cycles. The sensitivity analysis identified that the split IVF-ICSI is preferred except when ICSI is at the minimum cost in the range tested.
Selective application of ICSI in couples with fertilization defects is ideal given the lack of improved clinical outcomes for all couples with unexplained infertility, the unknown risks associated with ICSI in non-male factor infertility, and the cost of the technology. Our research suggests that split IVF-ICSI was the preferred insemination method in the initial treatment of couples with unexplained infertility if two or more cycles are necessary as it improves cumulative live birth rates compared with all conventional IVF and was more cost effective than all ICSI during multiple treatments.
Supplementary Material
References
- 1.Practice Committee of the American Society of Reprodutive Medicine. Effectiveness and treatment for unexplained infertility. Fertil Steril. 2006;86:S111–4. doi: 10.1016/j.fertnstert.2006.07.1475. [DOI] [PubMed] [Google Scholar]
- 2.Isaksson R, Tiitinen A. Present concept of unexplained infertility. Gynecol Endocrinol. 2004;18:278–90. doi: 10.1080/0951359042000199878. [DOI] [PubMed] [Google Scholar]
- 3.Aboulghar MA, Mansour RT, Serour GI, Sattar MA, Amin YM. Intracytoplasmic sperm injection and conventional in vitro fertilization for sibling oocytes in cases of unexplained infertility and borderline semen. J Assist Reprod Genet. 1996;13:38–42. doi: 10.1007/BF02068867. [DOI] [PubMed] [Google Scholar]
- 4.Hershlag A, Paine T, Kvapil G, Feng H, Napolitano B. In vitro fertilizationintracytoplasmic sperm injection split: an insemination method to prevent fertilization failure. Fertil Steril. 2002;77:229–32. doi: 10.1016/s0015-0282(01)02978-8. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz A, Remohi J, Minguez Y, Guanes PP, Simon C, Pellicer A. The role of in vitro fertilization and intracytoplasmic sperm injection in couples with unexplained infertility after failed intrauterine insemination. Fertil Steril. 1997;68:171–3. doi: 10.1016/s0015-0282(97)81497-5. [DOI] [PubMed] [Google Scholar]
- 6.Jaroudi K, Al-Hassan S, Al-Sufayan H, Al-Mayman H, Qeba M, Coskun S. Intracytoplasmic sperm injection and conventional in vitro fertilization are complementary techniques in management of unexplained infertility. J Assist Reprod Genet. 2003;20:377–81. doi: 10.1023/A:1025433128518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Westerlaken L, Helmerhorst F, Dieben S, Naaktgeboren N. Intracytoplasmic sperm injection as a treatment for unexplained total fertilization failure or low fertilization after conventional in vitro fertilization. Fertil Steril. 2005;83:612–7. doi: 10.1016/j.fertnstert.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 8.Kinzer DR, Barrett CB, Powers RD. Prognosis for clinical pregnancy and delivery after total fertilization failure during conventional in vitro fertilization or intracytoplasmic sperm injection. Fertil Steril. 2008;90:284–8. doi: 10.1016/j.fertnstert.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Quaas A, Dokras A. Diagnosis and treatment of unexplained infertility. Rev Obstet Gynecol. 2008;1:69–76. [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharya S, Hamilton MP, Shaaban M, Khalaf Y, Seddler M, Ghobara T, et al. Conventional in-vitro fertilisation versus intracytoplasmic sperm injection for the treatment of non-male-factor infertility: a randomised controlled trial. Lancet. 2001;357:2075–9. doi: 10.1016/s0140-6736(00)05179-5. [DOI] [PubMed] [Google Scholar]
- 11.Check JH, Yuan W, Garberi-Levito MC, Swenson K, McMonagle K. Effect of method of oocyte fertilization on fertilization, pregnancy and implantation rates in women with unexplained infertility. Clin Exp Obstet Gynecol. 2011;38:203–5. [PubMed] [Google Scholar]
- 12.Practice Committee of the American Society of Reprodutive Medicine. Intracytoplasmic sperm injection (ICSI) Fertil Steril. 2008;90:S187. doi: 10.1016/j.fertnstert.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 13.Jain T, Gupta RS. Trends in the use of intracytoplasmic sperm injection in the United States. N Engl J Med. 2007;357:251–7. doi: 10.1056/NEJMsa070707. [DOI] [PubMed] [Google Scholar]
- 14.Society for Assisted Reproductive Technology. National data summary. Available at: https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?Clin_icPKID=0. Accessed August 1, 2011.
- 15.Goldman MBR, Alper MM, Thornton KL, Reindollar RH. Pregnancy rates across multiple treatment cycles: data from the fast track and standard treatment (FASTT) trial. Fertil Steril. 2010;94:S1. doi: 10.1016/j.fertnstert.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Tomas C, Orava M, Tuomivaara L, Martikainen H. Low pregnancy rate is achieved in patients treated with intracytoplasmic sperm injection due to previous low or failed fertilization in in-vitro fertilization. Hum Reprod. 1998;13:65–70. doi: 10.1093/humrep/13.1.65. [DOI] [PubMed] [Google Scholar]
- 17.Miller KF, Falcone T, Goldberg JM, Attaran M. Previous fertilization failure with conventional in vitro fertilization is associated with poor outcome of intracytoplasmic sperm injection. Fertil Steril. 1998;69:242–5. doi: 10.1016/s0015-0282(97)00465-2. [DOI] [PubMed] [Google Scholar]
- 18.Viloria T, Garrido N, Minaya F, Remohi J, Munoz M, Meseguer M. Report of results obtained in 2,934 women using donor sperm: donor inseminationversus in vitro fertilization according to indication. Fertil Steril. 2011;96:1134–7. doi: 10.1016/j.fertnstert.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 19.United Stated Department of Labor, Bureau of Labor Statistics. CPI inflaation calculator. Available at: http://www.bls.gov/data/inflation_calculator.htm. Accessed August 1, 2011.
- 20.Little SE, Ratcliffe J, Caughey AB. Cost of transferring one through five embryos per in vitro fertilization cycle from various payor perspectives. Obstet Gynecol. 2006;108:593–601. doi: 10.1097/01.AOG.0000230534.54078.b3. [DOI] [PubMed] [Google Scholar]
- 21.American Society for Reproductive Medicine. Patient resources. Available at: http://www.asrm.org/awards/index.aspx?id=3012. Accessed August 1, 2011.
- 22.Resolve: The National Infertility Association. The cost of infertility treatment. Available at: http://www.resolve.org/family-building-options/insurance_coverage/the-costs-of-infertility-treatment.html. Accessed August 1, 2011.
- 23.Foong SC, Fleetham JA, O’Keane JA, Scott SG, Tough SC, Greene CA. A prospective randomized trial of conventional in vitro fertilization versus intracytoplasmic sperm injection in unexplained infertility. Fertil Steril. 2006;23:137–40. doi: 10.1007/s10815-005-9008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


