Abstract
BACKGROUND
Premenopausal women represent approximately 35% of new breast cancer diagnoses. Diagnosis and treatment may lead to substantial disruption in quality of life (QOL).
METHODS
Premenopausal patients (aged 18 to 50 years) treated for nonmetastatic breast cancer completed a mailed questionnaire. Multiple self-reported QOL measures and clinical data were collected. Cluster analysis and Cronbach’s α were used to validate the survey. Analysis of variance was performed for specific interventions. Lower interference scores conveyed higher QOL.
RESULTS
The response rate was 49.8%. Cronbach’s α was 0.96. Immediate contralateral prophylactic mastectomy (CPM) carried the highest interference (mean, 3.3148) with sexuality compared with no CPM (mean, 2.85) or delayed CPM (P = .03). Breast conservation had the least interference with appearance (P < .01) and work and finances (P = .02).
CONCLUSIONS
Therapeutic mastectomy and CPM with or without reconstruction may adversely affect QOL. These findings suggest that the choice and timing of interventions may significantly affect patient satisfaction.
Keywords: Breast cancer, Premenopausal, Quality of life, Prophylactic, mastectomy
One in 8 American women is projected to develop breast cancer over the course of her lifetime.1 Cancer clinical trials traditionally measure tumor response, freedom from disease, and survival, but evaluation of subjective morbidity and the impact of both intervention and disease on a patient’s lifestyle are increasingly recognized. Particularly among the breast cancer population, in which 5-year survival exceeds 88% for all comers,2 longevity after cancer diagnosis and treatment translates into a greater impact on the life of the survivor. The study of these events on a person’s general well-being has come to be known as measuring “quality of life” (QOL), which is multidimensional and can be difficult to define. Specific instruments for measuring QOL in patients with cancer have been developed.3–7 QOL issues for patients with breast cancer include such factors as pain, fear of recurrence, fatigue, and concerns common to a wide range of cancers, in addition to breast-specific concerns such as altered sense of femininity, feelings of decreased attractiveness, change in body image, and sexuality.4,8–10
Existing research suggests that in the long term, women with breast carcinoma report comparable levels of physical, social, and psychological well-being with those of unaffected women.3 However, in the short term (within 1 year of diagnosis), women with breast carcinoma, especially younger women, face substantial disruptions in their QOL. In general, younger women tend to suffer more psychological distress than older women3,11 and are more likely to be candidates for aggressive therapy affecting other aspects of QOL, such as physical and sexual functioning at a time in life when sexual issues are important to themselves and their partners.8,12 Multiple recent studies support the need for future research focusing on the psychosocial implications of breast carcinoma in younger women.3,13
Reproductive health concerns such as impaired fertility or premature ovarian failure are also common among young women shortly after breast cancer treatment.14 Young breast cancer survivors are less sexually active and have greater fertility concerns and more body image issues than healthy women in their same age range.4,8,12 The problems reported are related to both breast cancer treatment as well as emotional and social difficulties, which may be magnified by a breast cancer diagnosis.
The primary goal of this study was to examine short-term QOL impairment in premenopausal patients with breast cancer (diagnosed before the age of 50 years), including self-perception, views of their sexuality, impact of surgical and oncologic treatment, femininity, and changes in relationships with partners and other family members.
Methods
Patients treated at a single tertiary cancer center for breast cancer from 2005 to 2009 were identified through a query of the institutional breast cancer database after institutional review board approval. English-speaking, female patients diagnosed between the ages of 18 and 49 years with nonmetastatic breast cancer were identified for further study. All patients were ≥6 months from their last curative treatment (surgery, chemotherapy, or radiation), except for ongoing hormone therapy as indicated. An institutional review board–approved, mailed survey of 63 discrete items was sent up to 3 times to 300 women. Survey responses were scanned electronically and collated for further analysis. Demographic, treatment, and recurrence data were collected from a chart review of the respondents’ electronic medical records. No chart review was performed for nonresponders.
This study used 50 validated questions from other tools5–8 and 13 newly created, unvalidated items to examine QOL, particularly in the domain of relationships. The survey instrument was clustered into 8 domains (Table 1). Responses were recorded using a 5-item Likert-type scale ranging from “not at all” to “very much.” Fourteen additional questions encompassed demographics and treatment. Staging and specific treatment data were collected from the electronic medical record for correlation to patient-reported information.
Table 1.
Survey tool by QOL domains
| Domain | Question |
|---|---|
| Family/social network | Do you have trouble meeting the needs of your family because of your physical condition since your diagnosis of breast cancer? |
| How much emotional support do you get from your family since your diagnosis of breast cancer? | |
| How much emotional support do you get from your friends and neighbors since your diagnosis of breast cancer? | |
| How well did your family accept your breast cancer diagnosis? | |
| How much isolation do you feel is caused by your cancer? | |
| Appearance | Has your cancer or treatment caused changes in your appearance? |
| Do you see yourself less attractive since your diagnosis of breast cancer? Has your cancer or treatment caused changes in your appearance? | |
| Has your cancer or treatment caused changes in your self concept (the way you see yourself)? Do you see yourself less attractive since your diagnosis of breast cancer? | |
| Relationships | *Has your cancer or treatment caused changes in your self concept (the way you see yourself)? |
| *How stable was your relationship before your diagnosis? | |
| *How stable is your relationship now since your diagnosis? How stable was your relationship before your diagnosis? | |
| *How well did your partner accept your breast cancer diagnosis? How stable is your relationship now since your diagnosis? | |
| *Is your continuing health care interfering with your personal relationship? How well did your partner accept your breast cancer diagnosis? | |
| *Did your disease have any negative impact on your relationship? Is your continuing health care interfering with your personal relationship? | |
| *How much support do you get from your partner since your diagnosis of breast cancer? Did your disease have any negative impact on your relationship? | |
| *Is the amount of support you receive from your partner since your diagnosis of breast cancer sufficient to meet your needs? How much support do you get from your partner since your diagnosis of breast cancer? | |
| *Do you feel close to your partner since your diagnosis of breast cancer? Is the amount of support you receive from your partner since your diagnosis of breast cancer sufficient to meet your needs? | |
| *Since your diagnosis did you ever have the impression that your partner sees you less attractive? Do you feel close to your partner since your diagnosis of breast cancer? | |
| *Since your diagnosis has your partner ever mentioned that you are less attractive? Since your diagnosis did you ever have the impression that your partner sees you less attractive? | |
| Sexuality | Since your diagnosis has your partner ever mentioned that you are less attractive? |
| Have you been sexually active during the past year? | |
| If you answered yes to question #10 above, how satisfied are you with your sex life at this time? Have you been sexually active during the past year? | |
| Is your sexuality impacted by your cancer? If you answered yes to question #10 above, how satisfied are you with your sex life at this time? | |
| Do you worry about your sexual attractiveness since your diagnosis of breast cancer? Is your sexuality impacted by your cancer? | |
| Do you worry about your sexual attractiveness since your diagnosis of breast cancer? | |
| Work/finances | *Since your diagnosis have you been able to work either at home or outside of the home? |
| Is your work (including work in home) fulfilling since your diagnosis of breast cancer? Since your diagnosis have you been able to work either at home or outside of the home? | |
| To what degree has your breast cancer diagnosis and treatment interfered with your activities at home? Is your work (including work in home) fulfilling since your diagnosis of breast cancer? | |
| To what degree has your breast cancer diagnosis and treatment interfered with your employment? To what degree has your breast cancer diagnosis and treatment interfered with your activities at home? | |
| *How much financial burden have you incurred as a result of your breast cancer and treatment? To what degree has your breast cancer diagnosis and treatment interfered with your employment? | |
| *How much financial burden have you incurred as a result of your breast cancer and treatment? | |
| Physical symptoms | Are you fatigued since your diagnosis of breast cancer? |
| Has your appetite changed since your diagnosis? Are you fatigued since your diagnosis of breast cancer? | |
| Do you have aches or pain since your diagnosis of breast cancer? Has your appetite changed since your diagnosis? | |
| Are you experiencing sleep changes since your diagnosis of breast cancer? Do you have aches or pain since your diagnosis of breast cancer? | |
| Have you experienced weight gain since your diagnosis of breast cancer? Are you experiencing sleep changes since your diagnosis of breast cancer? | |
| Do you have vaginal dryness since your diagnosis of breast cancer? Have you experienced weight gain since your diagnosis of breast cancer? | |
| Do you have menopausal symptoms (e.g. hot flashes) since your diagnosis of breast cancer? Do you have vaginal dryness since your diagnosis of breast cancer? | |
| Have you experienced menstrual changes since your diagnosis of breast cancer? | |
| Do you have menopausal symptoms (e.g. hot flashes) since your diagnosis of breast cancer? | |
| Are you satisfied with your overall physical health since your diagnosis of breast cancer? Have you experienced menstrual changes since your diagnosis of breast cancer? | |
| Distress | Are you satisfied with your overall physical health since your diagnosis of breast cancer? |
| Did you feel distressed about the initial diagnosis? | |
| Did you feel distressed about the cancer chemotherapy? | |
| Did you feel distressed about the cancer radiation? | |
| Did you feel distressed about cancer surgery? | |
| Do you have anxiety since your diagnosis of breast cancer? | |
| Do you feel depressed since your diagnosis of breast cancer? | |
| How much uncertainty do you feel about your future since your diagnosis of breast cancer? | |
| Life with breast cancer | Have you accepted your breast cancer diagnosis? |
| How difficult is it for you to cope most of the days as a result of your breast cancer? | |
| How difficult is it for you to cope most of the days as a result of your treatment? | |
| Do you think your quality of life is good since your diagnosis of breast cancer? | |
| Do you feel happy since your diagnosis of breast cancer? | |
| Do you feel like you are in control of situations in your life since your diagnosis of breast cancer? | |
| How satisfying is your life since your diagnosis of breast cancer? | |
| How useful do you feel since your diagnosis of breast cancer? |
Previously unvalidated item.
Evaluation of the reliability and validity of the newly created questions was a secondary end point of this study. Statistical methods included Cronbach’s α to evaluate internal validity of the survey tool. Questions with poor item-to-total correlations were discarded. Cluster analysis was used to evaluate categorization of questions into specific QOL domains. The general linear model was used to compare QOL in women receiving different treatment modalities within the realms of surgery, systemic therapy, and radiation. On the basis of the wording of the QOL items, scores were expressed as interference scores, with higher scores corresponding to decreased and poorer QOL.
Results
Of 300 patients surveyed, 13 were undeliverable, and 143 responses (49.8%) were received. The overall Cronbach’s α for the questionnaire was 0.96, indicating a high degree of relationship among items. The mean age at diagnosis for the 143 respondents was 40.4 years (range, 26.6 to 48.1 years); the mean time from diagnosis to survey response was 35.8 months (range, 9.9 to 56.4 years). Half of the respondents (72 [50%]) were diagnosed between the ages of 41 and 50 years, 43% from 31 to 40 years, and the remaining 7% before 30 years of age. Age at diagnosis did not significantly affect any single domain or overall QOL. Eighty-six percent of respondents were Caucasian, 5.6% black, and 7.7% Asian, other, or unknown; 97% patients had at least a high school degree.
Eighty-three percent of respondents were married or partnered, 16% were single or divorced, and 2 patients (1.4%) were widowed. Partnered women had significantly lower overall QOL interference scores than unmarried women (P = .002), but marital status did not relate to interference on sexuality or sexual attractiveness. Eleven of 140 patients (7.7%) who responded had experienced changes in relationship status (either married or separated) since their treatment; there were too few events to evaluate the impact of this change on QOL. The vast majority of respondents were parents, with 81% having ≥1 child, although the number of children did not significantly affect QOL, including those with no children at all. Nearly 78% of respondents did express a desire for more children, although this did not appear to affect QOL across respondents (P = .45).
All of the patients surveyed presented with stage 0, I, II, or III breast cancer; 93.8% of respondents had invasive disease at diagnosis. Forty-six (32.2%) presented with stage I, 52 (36.4%) with stage II, and 36 (25.2%) with stage III disease. Eight of the 143 had developed either local recurrence or distant metastasis at the time of response. Patients with stage III and stage IV disease at the time of survey response trended toward a greater interference score compared with patients with early-stage disease, but this was not statistically significant overall (P = .33) and was significant only in the QOL domains of work and finances (P = .03). One hundred twenty-seven of the 143 respondents received chemotherapy in the neoadjuvant (30 patients [20.8%]) or adjuvant (97 patients [67.4%]) setting; 70% of patients received adjuvant hormonal therapy as well. Interestingly, the use of systemic therapy had no statistically significant impact on any single QOL domain or on overall QOL.
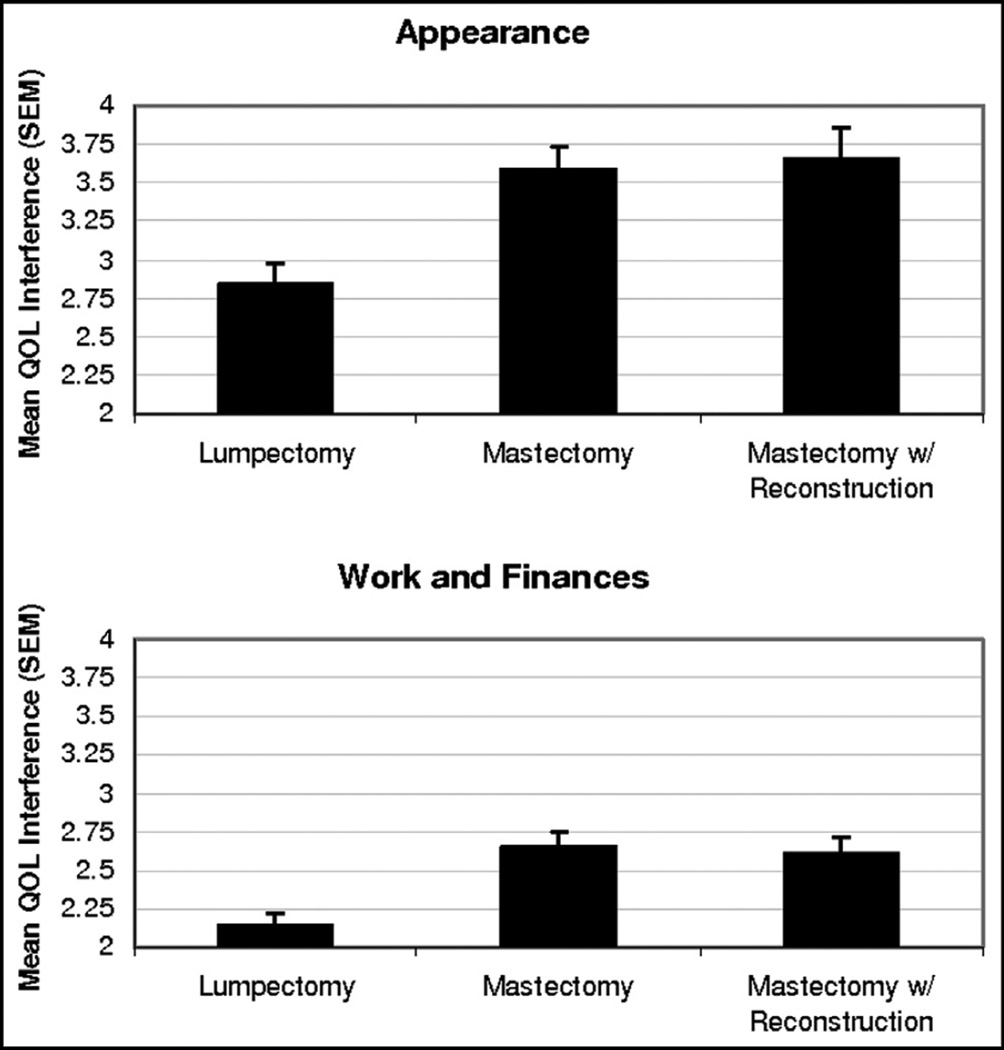
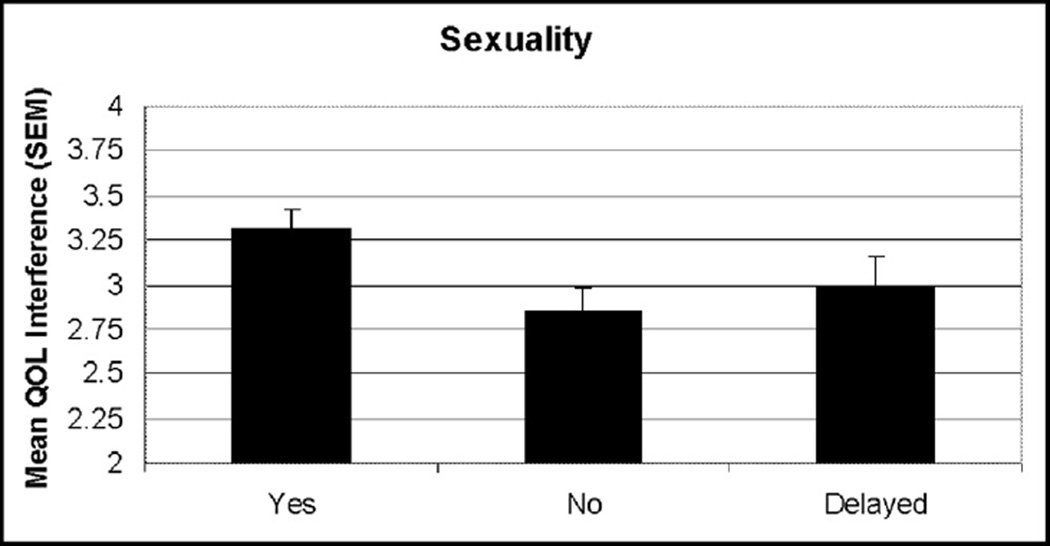
In contrast, local therapies did have a measurable and significant impact on specific QOL indicators. Surgical treatments clearly were related to QOL, particularly in the domains of appearance (P < .001) and work and finances (P = .02). Specifically, patients undergoing mastectomy (50 patients [34.7%]) and mastectomy with reconstruction (33 patients [23.2%]) reported significantly higher QOL interference (Fig. 1). The use of contralateral prophylactic mastectomy (CPM) also had a significant effect on sexuality (P =.03), with the 54 patients (37.5%) who underwent immediate CPM reporting higher interference scores than those undergoing delayed (13 patients [9.0%]) or no CPM (Fig. 2).
Figure 1.
Graph of QOL interference by surgery.
Figure 2.
Graph of QOL interference by use of CPM.
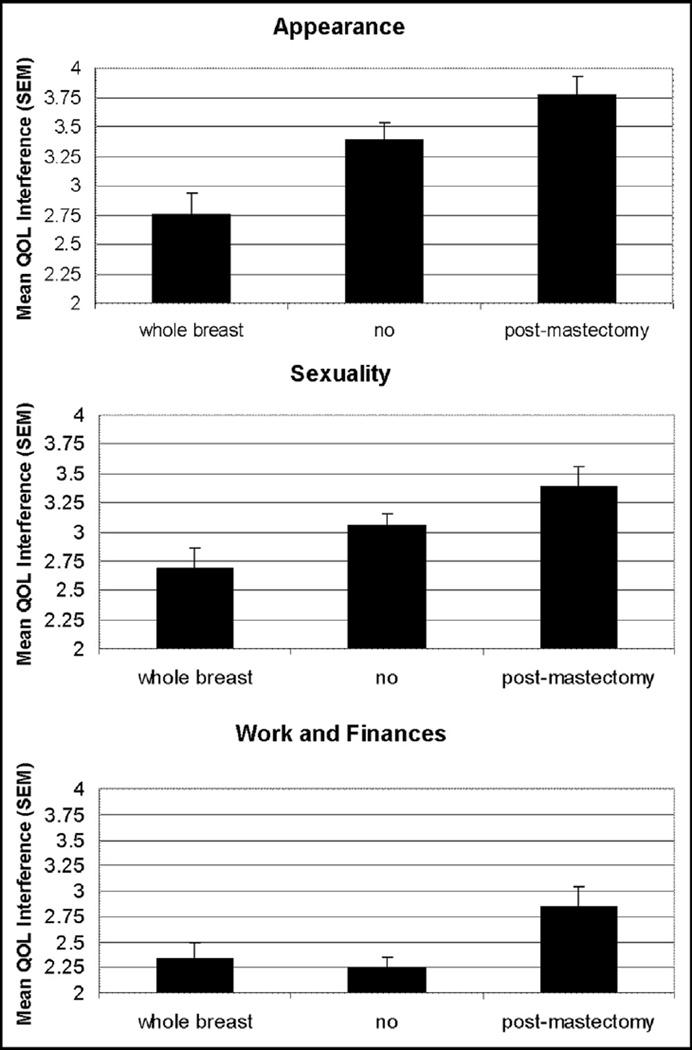
Radiation also affected QOL, particularly in the domains of appearance (P < .001), sexuality (P = .02), and work and finances (P = .03). Given that patients who received whole-breast irradiation were treated with lumpectomy and that many mastectomy patients did not receive adjuvant radiation at all, the effects of radiation on QOL are closely tied to surgical decision making. Not surprisingly, patients undergoing breast conservation followed by standard whole-breast irradiation or no radiation reported lower interference scores in all domains compared with women undergoing mastectomy. Among women treated with radiation, the 3 patients (2.1%) undergoing partial-breast irradiation (PBI) and 37 women (35.7%) treated with postmastectomy irradiation reported the highest interference scores (Fig. 3); women receiving postmastectomy irradiation reported interference scores comparable with those of PBI patients. On the basis of institutional radiation guidelines, PBI is generally limited to women aged ≥60 years, so the number of subjects and respondents who received PBI was very small.
Figure 3.
Graph of QOL interference by radiation type.
Comments
Younger patients with breast cancer face different stressors than many of their older counterparts. In this prospective, single-institution survey study, we developed and validated a QOL questionnaire tool and several novel questions to assess distinct QOL domains, in addition to evaluating the effects of specific demographic and treatment situations relative to QOL indicators.
Our 49.8% response rate may be related to the nature of the questionnaire and the timing of the study, as all of the patients selected were >6 months from treatment. Two patients declined to participate and were not counted among the respondents. We were unable to discern specific trends between responders and nonresponders.
The results of our survey suggest that the majority of our patients did not experience shifts in family or home as a consequence of their treatment, nor did the presence or absence of children affect QOL. This particular finding is different from those of other studies,9,15,16 which have noted that young cancer survivors express stress related to fertility loss after cancer treatment. For example, a systematic review of young breast cancer survivors’ QOL and health outcomes suggested women aged <50 years were more likely to have concerns about fertility that were not well addressed, thereby reducing perceived QOL.13,15,16 Our findings may be related to the fact that >80% of respondents already had ≥1 child at the time of treatment, as nulliparity affects the use of fertility preservation.17,18 Similar to other studies evaluating this special population, patients with long-term partners reported lower interference scores than unpartnered respondents. Patients did not report significant interference if they were unsure of further childbearing, although the need to address the effects of cancer treatment on fertility is unique to this age group and a growing area of investigation at our institution and others.
In this population, breast cancer treatment most frequently affected the domains of work and finance, appearance, and, in the case of CPM, sexuality. Surprisingly, neither use nor timing of systemic therapy had any effect on specific or overall QOL, even comparing those with and without treatment. These findings may be attributed to several factors. First, breast cancer treatment in the United States is expensive, with estimated lifetime costs ranging from $10,000 to $100,000 or more; treatment costs tend to be higher for those diagnosed at younger ages.19,20 These figures do not include the patient time cost of treatment. A recent Surveillance, Epidemiology and End Results/Medicare publication evaluating the patient-time cost of care for Medicare-age patients estimated the cost of treatment to be approximately $11,800 in the initial 12 months for patients with breast cancer aged >65 years.21 For women with working or childcare responsibilities, these costs are likely even higher. Because systemic therapy is generally well tolerated by younger women, these findings suggest that patient-time costs interfere more with QOL than treatment itself.
As previously noted, local-regional therapies appeared to have the greatest impact on QOL indicators. Recommendations for surgery and radiation are intertwined, especially in women who are candidates for breast conservation, and in this young population, the effects on QOL appear to be in line with other studies not targeted to patients by age. It is interesting to note the significantly higher interference scores reported by patients undergoing immediate CPM compared with those without prophylactic or with delayed CPM. The choice of CPM is driven by a multiplicity of factors, including fear of recurrence, overestimation of recurrence risk, cosmesis and symmetry, availability of reconstruction, and abnormal breast imaging results. Similar to other studies, women undergoing CPM reported negative effects on sexuality and appearance.22–24 These issues should be taken into consideration when counseling patients regarding the utility and the timing of procedures in an unaffected breast.
In this particular population, in which work and finances were frequently cited as areas of increased interference from treatment, it is not surprising to see that women undergoing breast-conserving surgery and whole-breast radiation reported lower interference scores than patients receiving postmastectomy irradiation or PBI. Because PBI is typically a twice-daily regimen and may involve the use of a temporary implantable device in the breast for the duration of treatment, it is not surprising that these patients reported higher interference scores than those receiving whole-breast irradiation, reinforcing the concept that patient-time costs may play a greater role in QOL for this group than treatment.
This study had several limitations. First, the patients surveyed were all treated at the same center, skewing the patient population with regard to age, level of education, stage at presentation, and study participation. The overall breast cancer population at our institution is younger and travels greater distances than state and nationwide. Given that younger patients generally present with higher stage disease, this likely also affects the applicability of the responses to the general (postmenopausal) breast cancer population. Furthermore, all patients were English speaking, as the survey was not administered in any other languages.
Another limitation is the response rate. We received 49.9% of the mailed surveys back, despite multiple attempts. This was likely multifactorial, including subjects with limited reading comprehension or insufficient mastery of English. At least 2 declined to participate because of the nature of the questionnaire. Finally, this was a 1-time, posttreatment survey, so pretreatment QOL data were not available for comparison. Future goals include a follow-up of the present study to assess QOL over time.
The results of this study should be considered when counseling young patients regarding local and regional therapies, particularly the timing and impact of CPM, a common procedure in this patient population. Further study is ongoing and warranted, particularly given the potential longevity and lasting effects on patients and their families.
Conclusions
This study suggests that young patients with breast cancer experience significant stress from changes in body habitus, altered sexuality, and disruptions different from older populations. In particular, the impact of local therapy and CPM on QOL and sexuality should be taken into consideration when discussing treatment options with this population. Further investigation of patient time costs in this diverse population are difficult but warrant further investigation and may strongly influence treatment recommendations and timing of interventions. Given these findings, patients should be counseled regarding the potential advantages of delaying prophylactic surgery decisions when discussing breast cancer treatment.
Acknowledgments
This study was supported by the Pain and Palliative Care Department at Moffitt Cancer Center (Tampa, FL).
Footnotes
The authors declare no conflicts of interest.
References
- 1.American Cancer Society. Breast cancer facts and figures 2011–12. Atlanta, GA: American Cancer Society; 2012. [Google Scholar]
- 2.Ries LAG, et al. SEER cancer statistics review 1975–2005. Available at: http://seer.cancer.gov/csr/1975_2005/.
- 3.Arora NK, Gustafson DH, Hawkins RP, et al. Impact of surgery and chemotherapy on the quality of life of younger women with breast carcinoma. Cancer. 2001;92:1288–1298. doi: 10.1002/1097-0142(20010901)92:5<1288::aid-cncr1450>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Avis NE, Crawford S, Manuel J. Quality of life among younger women with breast cancer. J Clin Oncol. 2005;23:3322–3330. doi: 10.1200/JCO.2005.05.130. [DOI] [PubMed] [Google Scholar]
- 5.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the functional assessment of cancer therapy breast quality-of-life instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 6.Levine MN, Guyatt GH, Gent M, et al. Quality of life in stage II breast cancer: an instrument for clinical trials. J Clin Oncol. 1988;6:1798–1810. doi: 10.1200/JCO.1988.6.12.1798. [DOI] [PubMed] [Google Scholar]
- 7.Sprangers MA, Groenvold M, Arraras JI, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14:2756–2768. doi: 10.1200/JCO.1996.14.10.2756. [DOI] [PubMed] [Google Scholar]
- 8.Fobair P, Stewart SL, Chang S, et al. Body image and sexual problems in young women with breast cancer. Psycho-Oncology. 2006;15:579–594. doi: 10.1002/pon.991. [DOI] [PubMed] [Google Scholar]
- 9.Schover LR. Premature ovarian failure and its consequences: vasomotor symptoms, sexuality, and fertility. J Clin Oncol. 2008;26:753–758. doi: 10.1200/JCO.2007.14.1655. [DOI] [PubMed] [Google Scholar]
- 10.Waljee JF, Hu ES, Ubel PA, et al. Effect of esthetic outcome after breast-conserving surgery on psychosocial functioning and quality of life. J Clin Oncol. 2008;26:3331–3337. doi: 10.1200/JCO.2007.13.1375. [DOI] [PubMed] [Google Scholar]
- 11.Nissen MJ, Swenson KK, Ritz LJ, et al. Quality of life after breast carcinoma surgery. Cancer. 2001;91:1238–1246. [PubMed] [Google Scholar]
- 12.Burwell SR, Case LD, Kaelin C, et al. Sexual problems in younger women after breast cancer surgery. J Clin Oncol. 2006;24:2815–2821. doi: 10.1200/JCO.2005.04.2499. [DOI] [PubMed] [Google Scholar]
- 13.Howard-Anderson J, Ganz PA, Bower JE, et al. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:386–405. doi: 10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- 14.Quinn G, Vadaparampil S, editors. Reproductive health and cancer. New York: Springer; 2012. [Google Scholar]
- 15.Schover LR, Rybicki LA, Martin BA, et al. Having children after cancer. Cancer. 1999;86:697–709. doi: 10.1002/(sici)1097-0142(19990815)86:4<697::aid-cncr20>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 16.Quinn GP, Vadaparampil ST, Gwede CK, et al. Discussion of fertility preservation with newly diagnosed patients: oncologists’ views. J Cancer Surviv. 2007;1:146–155. doi: 10.1007/s11764-007-0019-9. [DOI] [PubMed] [Google Scholar]
- 17.Goodman LR, Balthazar U, Kim J, et al. Trends of socioeconomic disparities in referral patterns for fertility preservation consultation. Hum Reprod. 2012;27:2076–2081. doi: 10.1093/humrep/des133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King JW, Davies MC, Roche N, et al. Fertility preservation in women undergoing treatment for breast cancer in the U.K.: a questionnaire study. Oncologist. 2012;17:910–916. doi: 10.1634/theoncologist.2012-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell J, Ramsey S. The costs of treating breast cancer in the US: a synthesis of published evidence. Pharmacoeconomics. 2009;27:199–209. doi: 10.2165/00019053-200927030-00003. [DOI] [PubMed] [Google Scholar]
- 20.Barron J, Quimbo R, Nikam PT, et al. Assessing the economic burden of breast cancer in a US managed care population. Breast Cancer Res Treat. 2008;109:367–377. doi: 10.1007/s10549-007-9650-4. [DOI] [PubMed] [Google Scholar]
- 21.Yabroff KR, Davis WW, Lamont EB, et al. Patient time costs associated with cancer care. J Natl Cancer Inst. 2007;99:14–23. doi: 10.1093/jnci/djk001. [DOI] [PubMed] [Google Scholar]
- 22.Brandberg Y, Sandelin K, Erikson S, et al. Psychological reactions, quality of life, and body image after bilateral prophylactic mastectomy in women at high risk for breast cancer: a prospective 1-year follow-up study. J Clin Oncol. 2008;26:3943–3949. doi: 10.1200/JCO.2007.13.9568. [DOI] [PubMed] [Google Scholar]
- 23.Frost MH, Slezak JM, Tran NV, et al. Satisfaction after contralateral prophylactic mastectomy: the significance of mastectomy type, reconstructive complications, and body appearance. J Clin Oncol. 2005;23:7849–7856. doi: 10.1200/JCO.2005.09.233. [DOI] [PubMed] [Google Scholar]
- 24.Unukovych D, Sandelin K, Liljegren A, et al. Contralateral prophylactic mastectomy in breast cancer patients with a family history: a prospective 2-years follow-up study of health related quality of life, sexuality and body image. Eur J Cancer. 2012;48:3150–3156. doi: 10.1016/j.ejca.2012.04.023. [DOI] [PubMed] [Google Scholar]