Abstract
Objectives
Twin-reversed arterial perfusion (TRAP) sequence affects 1% of monochorionic twin pregnancies and is caused by abnormal vascular connections between a pump twin and an acardiac mass. The effects of abnormal vascular connections on cerebral vasculature in the pump twin are unknown. We hypothesize that abnormal cerebral vascular impedance, as assessed by the pulsatility index (PI), is present in pump twins and that fetal intervention alters cerebral impedance.
Methods
Fetal echocardiograms performed between 2010 and 2013 in pregnancies diagnosed with TRAP (n = 19), recorded at presentation, and uncomplicated monochorionic twin pregnancies (controls, n = 18; 36 fetuses) were analyzed. In all subjects, the middle cerebral artery (MCA)-PI, combined cardiac output (CCO) and cardiothoracic ratio were calculated, and the values for cases and controls were compared.
Results
The mean gestational age at the time of echocardiography was 20 weeks in both groups. MCA-PI was lower in TRAP cases than in controls (1.55 (95%CI, 1.47–1.64) vs 1.74 (95% CI, 1.65–1.82), respectively; P = 0.004). CCO in TRAP cases was mildly elevated for gestational age (199.7 (95% CI, 138.4–261.1) mL/min) compared with that of controls (131.4 (95% CI, 102.2–160.7) mL/min). In six TRAP cases with a second echocardiogram available, the mean MCA-PI increased after intervention, from 1.5 (95%CI, 1.3–1.7) to 1.8 (95% CI, 1.4–2.2).
Conclusions
TRAP pump twins have lower cerebral vascular impedance than do controls, suggestive of a brain-sparing effect. MCA-PI appeared to increase in a small group of pump twins after intervention. These findings suggest a fetal cerebral autoregulatory response to a high cardiac output state that begins to change after fetal intervention. The long-termimplications for neurodevelopmental outcome warrant further study.
Keywords: cerebral vascular impedance, fetal cardiology, neurodevelopment, TRAP
INTRODUCTION
Twin-reversed arterial perfusion (TRAP) sequence, previously termed acardiac twinning, is a relatively uncommon complication of monochorionic twin pregnancies, affecting 1% of all monochorionic twin gestations1. TRAP sequence occurs when a normal or ‘pump’ twin perfuses an acardiac mass via placental vascular connections that are found only in monochorionic placentae. The pump twin perfuses the acardiac mass with deoxygenated blood in a retrograde manner via a placental arterioarterial anastomosis and the acardiac mass returns further deoxygenated blood back to the pump twin through a direct venovenous anastomosis2,3. Historically, there has been a 50% mortality rate for pump twins if the condition is left untreated, due to high-output cardiac failure and subsequent development of hydrops1,4,5. Fetal intervention by way of radiofrequency ablation (RFA) of flow within the acardiac mass (cord occlusion) has improved the survival rate of the pump twin to as high as 90% in experienced centers6.
Despite this improvement in survival rate, the effects of abnormal placental connections and a high cardiac output state on a pump twin’s fetal cerebral blood flow and vasculature, prior to, and following, occlusive intervention are unknown. We hypothesize that the pump twin exhibits abnormal cerebral hemodynamics in response to a high cardiac output state and that these changes are reversible with fetal intervention. Our aims were: (1) to compare the cerebral vascular impedance of pump twins to that of healthy monochorionic twins; (2) to determine if there is a correlation between cerebral vascular impedance and markers of a high cardiac output state; and (3) to assess the change in cerebral vascular impedance before and after fetal intervention.
METHODS
The clinical and sonographic data of fetuses with TRAP that were evaluated by the Fetal Treatment Center at the University of California San Francisco between 2010 and 2013 were reviewed. Inclusion criteria for the study consisted of fetuses with a diagnosis of TRAP that underwent both an ultrasound examination and fetal echocardiography prior to any intervention. Gestational age-matched monochorionic–diamniotic twin fetuses with normal cardiovascular anatomy, normal uteroplacental function and no extracardiac anatomic abnormalities were included as normal controls for comparison. Normal monochorionic twin pregnancies were chosen to comprise the control group, as there are probably inherent differences in cerebral Doppler patterns in twin pregnancies as compared to singletons7. Twin fetuses with possible twin–twin transfusion syndrome or significant growth discordance (> 20% difference in estimated fetal weight) were excluded. For the TRAP group of fetuses, information obtained from the obstetric ultrasound included the weight ratio between the acardiac and pump twin (AC:PT ratio). The longitudinal, transverse and anteroposterior dimensions (cm) of the acardiac mass were measured by ultrasound, and the volume of the mass was estimated from the formula for the volume of a spheroid – V = π/6 × (d1d2d3) (cm3) – which was converted to an estimated weight (g).
Obstetric history charts were reviewed to assess whether a fetal intervention had been performed and, if performed, the timing of the procedure was noted. Our center offers in-utero therapy for TRAP pregnancies in the form of intrafetal RFA. Indications for intervention in our center during the time period of this study included a large AC:PT ratio without compromise, clear evidence of fetal compromise, or echocardiographic findings of compromise6,8,9. RFA is used to obliterate flow at the insertion site of the umbilical vessels of the acardiac mass to prevent ongoing hemodynamic burden for the pump twin. The percutaneous intervention is performed under real-time ultrasound guidance using either a 14-or 17-gauge device and the appropriate radiofrequency generator (RITA Medical Systems, Fremont, CA, USA). Successful procedures demonstrate an absence of flow on Doppler interrogation of the vessels to and from the acardiac mass. Commencing in 2012, postintervention fetal echocardiography for TRAP pregnancies became a standard protocol in our center to monitor the improvement in fetal hemodynamics with intervention; therefore a subset of subjects that underwent a fetal intervention had a follow-up fetal echocardiogram available for review.
All subjects (TRAP and control) underwent a complete standard-of-care fetal echocardiogram. This included multiple tomographic views of the fetal heart according to American Society of Echocardiography guidelines10 as well as color Doppler and pulsed-wave Doppler examination of the umbilical cord, venous structures and the middle cerebral artery (MCA). Studies were performed on Sequoia C512 or S2000 ultrasound systems (Siemens, Mountain View, CA, USA). The images were stored digitally in standard DICOM format. In all fetuses, Doppler interrogation of the MCA was performed as part of the clinical protocol. Vascular impedance was expressed as the pulsatility index (PI), calculated using the following equation: PI=(peak systolic velocity – end-diastolic velocity)/time-averaged mean velocity; three measurements were obtained and the results were averaged for analysis. Cardiothoracic ratio (CTR) was determined by dividing the area occupied by the heart in diastole by the thoracic area in a standard axial image of the fetal thorax. Combined cardiac output (CCO) was calculated by summing the individual outputs of the right and left ventricles, derived from the equation: ventricular output=velocity time integral × heart rate × semilunar valve area. The cardiovascular profile score was calculated for every pump twin as described previously11. All measurements and calculations were repeated for those subjects that had data available from a fetal echocardiogram recorded within 48 h after fetal intervention.
Statistical analysis
All continuous variables were normally distributed, therefore, for each parameter, the mean and 95%CIs were calculated. For each parameter comparison was made between the TRAP group and control group using two-sample Student’s t-test. The relationship between MCA-PI and CCO and CTR was assessed by Pearson’s correlation. Finally, descriptive analysis was used to compare parameters for the TRAP subjects with pre-and postintervention echocardiography data.
RESULTS
Included in the study were 19 twin pregnancies with TRAP and 18 (36 fetuses) uncomplicated monochorionic–diamniotic twin pregnancies. Both TRAP and control subjects had one fetal echocardiogram available, for a total of 37 echocardiographic evaluations. Characteristics of the TRAP groups are shown in Table 1. There was no difference in gestational age between the TRAP and control subjects at the time of fetal echocardiography. The mean estimated weight ratio between the acardiac mass and pump twin in the TRAP group was 0.87 (95% CI, 0.65–1.10) and there was no evidence of hydrops or significant cardiovascular compromise (all had a cardiovascular profile score > 8). Fifteen TRAP pregnancies underwent RFA of the acardiac twin at a mean gestational age of 20.2 ± 2.8weeks. Overall survival up to delivery for pump twins was 95% (n = 18), with a mean birth weight of 2722±723 g (Table 1).
Table 1.
Obstetric characteristics of 19 subjects with twin-reversed arterial perfusion (TRAP) sequence included in the study
| Characteristic | TRAP subjects (n = 19) |
|---|---|
| AC:PT ratio* | 0.87 ± 0.45 |
| Fetal intervention | 15 (79) |
| GA at intervention (weeks) | 20.2 ± 2.8 |
| Pump-twin survival | 18 (95) |
| Pump-twin birth weight (g) | 2722 ± 723 |
Data are given as mean ± SD or n (%).
Sonographic estimated weight ratio between acardiac (AC) and pump twin (PT). GA, gestational age.
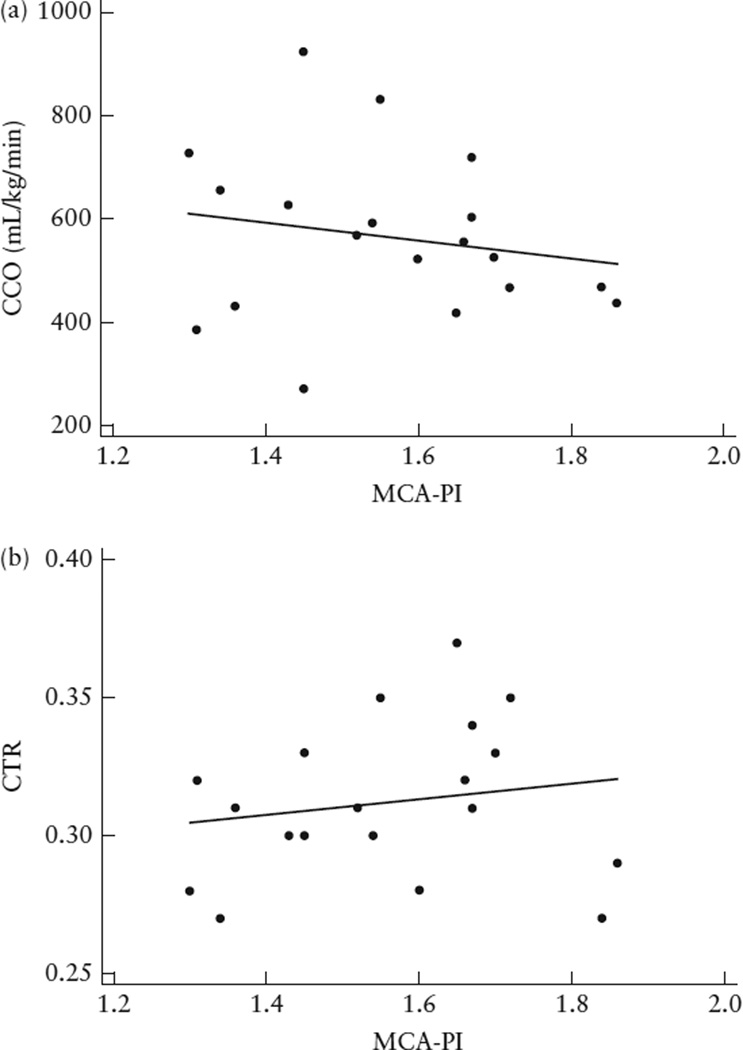
The cerebral vascular impedance in TRAP pregnancies as measured by PI was lower than in controls (P < 0.01), indicating increased cerebral vasodilation (Table 2). Furthermore, MCA Doppler patterns in TRAP pump twins exhibited a lower peak systolic velocity and more diastolic flow when compared with those of controls. The mean CCO among TRAP subjects was elevated, at 199.7 mL/min (95% CI, 138.4–261.1 mL/min), which is at the 95th percentile for gestational age, and was higher than that of controls (P = 0.022). Similarly, the mean CTR among TRAP subjects was larger than that of controls (P < 0.01), although still within the normal range (0.31 (95% CI, 0.30–0.33)). Correlation statistics did not demonstrate a relationship between MCA-PI and CCO, indexed to fetal weight, or between MCA-PI and CTR (Figure 1).
Table 2.
Comparison between measured parameters in monochorionic (MC) control twins and twin-reversed arterial perfusion (TRAP) pump twins
| Parameter | MC control twins (n = 36) |
TRAP pump twins (n = 19) |
P* |
|---|---|---|---|
| GA (weeks) | 20.3 (19.4 – 21.2) | 19.9 (18.7 – 21.2) | 0.63 |
| MCA-PI | 1.74 (1.65 – 1.82) | 1.55 (1.47 – 1.64) | 0.004 |
| CCO (mL/min) | 131.4 (102.2 – 160.7) | 199.7 (138.4 – 261.1) | 0.022 |
| CTR | 0.28 (0.28 – 0.29) | 0.31 (0.30 – 0.33) | <0.001 |
Data are given as mean (95% CI).
Significance level P < 0.05 (two-sample Student’s t-test). CCO, combined cardiac output; CTR, cardiothoracic ratio; GA, gestational age at first echocardiogram; MCA-PI, middle cerebral artery pulsatility index.
Figure 1.
Scatterplots showing relationship between middle cerebral artery pulsatility index (MCA-PI) and combined cardiac output (CCO) indexed to estimated fetal weight (a) and cardiothoracic ratio (CTR) (b) in 19 subjects with twin-reversed arterial perfusion sequence. There was no significant correlation (Pearson’s correlation) between fetal MCA-PI and hemodynamic markers of a high cardiac output state (CCO: r = −0.18, P = 0.45; CTR: r = 0.17, P = 0.49).
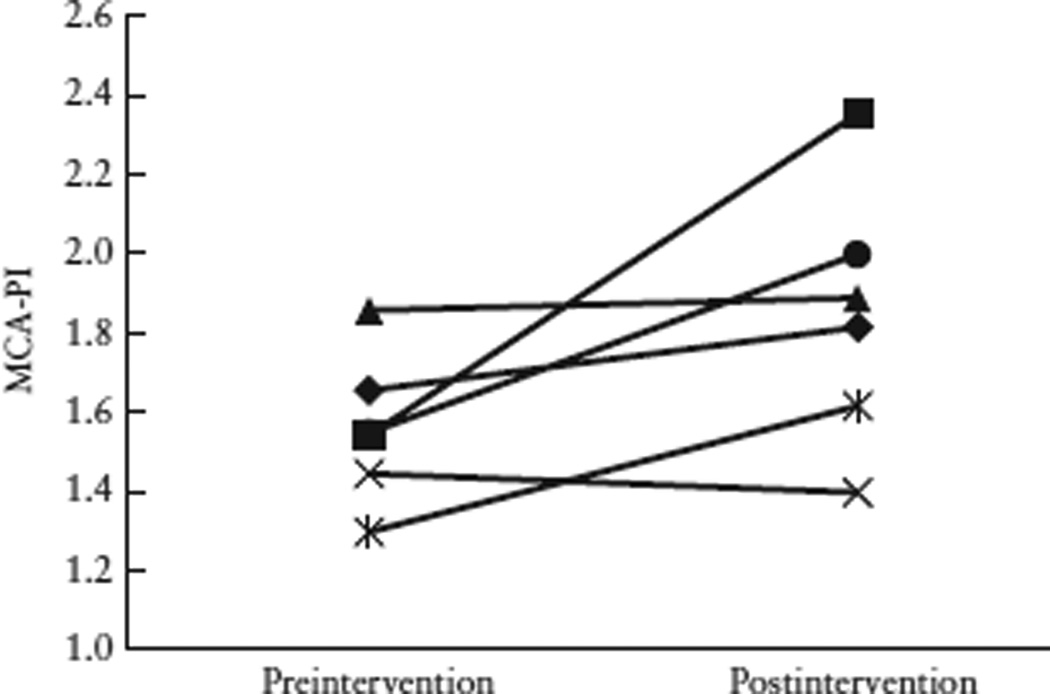
A subset of TRAP subjects (n=6) underwent a postintervention fetal echocardiogram within 48 h of RFA. Among these six subjects, there was no change in CTR from the pre- to postintervention echocardiogram (mean CTR preintervention 0.31 (95% CI 0.29–0.32); mean CTR postintervention 0.31 (95% CI, 0.29–0.35)). However, the mean MCA-PI increased after the intervention, from 1.5 (95% CI, 1.3–1.7) to 1.8 (95% CI, 1.4–2.2) (Figure 2).
Figure 2.
Pre- and postintervention middle cerebral artery pulsatility index (MCA-PI) in six twin-reversed arterial perfusion pump-twin subjects. Mean MCA-PI was higher after intervention: preintervention mean, 1.5 (95% CI, 1.3–1.7) vs postintervention mean, 1.8 (95% CI, 1.4–2.2).
DISCUSSION
Our study demonstrates that TRAP pump twins in a high cardiac output state have lower cerebral vascular impedance than do control twins of uncomplicated monochorionic pregnancies. However, there appears to be no significant correlation between cerebral vascular impedance and fetal hemodynamic markers of a high cardiac output state. Finally, in a small subset of pump twins that underwent postintervention fetal echocardiography, there appears to be a trend towards an increased MCA-PI after fetal intervention.
Our findings highlight a number of physiological considerations. Owing to abnormal placental vascular connections and the presence of the acardiac mass, TRAP pump twins exist in a high cardiac output state. The mean CCO in the TRAP pump fetuses was significantly higher than in unaffected monochorionic twin controls. In many untreated cases, the pump twin can progress to developing high-output cardiac failure and subsequent hydrops, which has been associated with poor outcomes1,3,12. In fact, studies have demonstrated that an elevated CCO and CTR predict those at high risk of cardiovascular compromise and/or poor outcome even prior to the onset of hydrops9,13. The TRAP subjects in our series did not demonstrate apparent cardiovascular compromise despite having an elevated CCO (all had a cardiovascular profile score > 8, with no evidence of hydrops), suggesting that the cerebral vascular changes can develop before significant heart failure and overt cardiac compromise. Interestingly, our findings demonstrate that there is no significant correlation between cerebral vascular impedance and fetal hemodynamic markers of a high cardiac output state (CCO and CTR). This may be secondary to our small sample size, or could be suggestive of an alternative etiology for the lower cerebral vascular impedance seen in pump twins.
The cerebral vasodilation seen in the pump twins may be secondary to unique and abnormal placental vascular connections, particularly arterioarterial and venovenous connections. Specifically, relatively deoxygenated blood is transported from the pump twin via its umbilical artery toward the shared placenta. Some blood does not perfuse a placental cotyledon but rather is diverted via an arterioarterial anastomosis and supplies, via retrograde flow in the umbilical artery, the acardiac mass. This blood is then further deoxygenated as it perfuses the acardiac mass. It then returns via retrograde flow in the acardiac mass’s umbilical vein, passes through a venovenous anastomosis and enters the umbilical vein, returning blood to the pump twin’s circulation. This circuit results in a decrease in the total oxygen delivery to the one potentially viable fetus. This may trigger the autoregulatory capacities of the cerebral vasculature in the pump twin, leading to cerebral vasodilation in an attempt to increase total oxygen delivery to the brain.
Vasodilation of the cerebral vasculature has been identified in other fetal disease states, including growth restriction and congenital heart disease14–16. Fetuses with placental insufficiency and growth restriction demonstrate decreased cerebral vascular impedance as assessed by the MCA-PI, which may represent an effective protective mechanism against significant neurological injury17,18. However this compensation may be inadequate, as some studies suggest that growth-restricted fetuses with lower MCA Doppler-derived cerebral vascular impedance born prematurely have more neurobehavioral abnormalities later in life19. Similarly, fetuses with hypoplastic left heart syndrome (HLHS) have been shown to have lower MCA Doppler-derived cerebral vascular impedance than do normal fetuses16,20–22, with evidence of abnormal neurodevelopmental outcomes23. In HLHS, these findings are thought to be due to decreased total blood flow to the brain and therefore decreased total oxygen delivery24,25. Although these are very different disease states in the fetus, all present with brain-sparing that is suggestive of some degree of overlap in etiology, as well as in ultimate neurodevelopmental outcomes.
Fetal intervention for TRAP sequence at our center has increased the survival rate of the pump twin to as high as 90%6. This success has been replicated in larger multicenter studies, establishing the benefit of fetal intervention, particularly for cases with a large acardiac mass (AC:PT ratio > 50%)8. Studies have also demonstrated no difference in the survival rate of the pump twin with expectant management for cases with an AC:PT ratio < 50%26; therefore, most centers, including ours, currently offer intervention only for cases with an AC:PT ratio > 50%. Additionally, the optimal timing for intervention has not been established with certainty. A large multicenter study showed less intrauterine fetal demise when intervention was performed after 19 weeks’ gestation than when it was performed before 18 weeks8. However, a recent study demonstrated that survival is no different when prophylactic intervention is performed earlier in gestation and that rates of premature delivery are lower27. Our study demonstrates that the mean MCA-PI increased in a small subset of pump twins after intervention, suggesting an improvement in cerebral hemodynamics. However, this finding deserves further study in a larger group of subjects. It remains to be seen whether the timing of intervention can impact on other parameters, such as neurodevelopmental outcome, given the possible changes in cerebral hemodynamics with intervention. Similarly, although survival is not affected by expectant management for those cases with an AC:PT ratio < 50%, both the short- and long-term neurodevelopmental outcomes in this cohort of largely untreated patients are unknown.
Our study is limited by its retrospective nature and small sample size. In addition, the TRAP cases included were likely to be at the severe end of the spectrum, as our institution is a referral center for fetal intervention. Furthermore, postintervention fetal echocardiography was performed only in a subset of subjects after it became part of the standard protocol, thus our conclusions regarding postintervention cerebral hemodynamics are limited in scope.
In conclusion, our study shows that cerebral vascular impedance is decreased in pump twins, indicating a brain-sparing effect in these fetuses. Larger, multicenter studies correlating fetal cerebral hemodynamics in TRAP pump twins with ultimate neurodevelopmental outcomes are necessary to add to the current criteria for defining ideal candidates and optimal timing for fetal intervention. This information should be useful not only for management strategies, but also for counseling families with an affected pregnancy.
REFERENCES
- 1.Moore TR, Gale S, Benirschke K. Perinatal outcome of forty-nine pregnancies complicated by acardiac twinning. Am J Obstet Gynecol. 1990;163:907–912. doi: 10.1016/0002-9378(90)91094-s. [DOI] [PubMed] [Google Scholar]
- 2.Mohanty C, Mishra OP, Singh CP, Das BK, Singla PN. Acardiac anomaly spectrum. Teratology. 2000;62:356–359. doi: 10.1002/1096-9926(200011)62:5<356::AID-TERA10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 3.Wong AE, Sepulveda W. Acardiac anomaly: current issues in prenatal assessment and treatment. Prenat Diagn. 2005;25:796–806. doi: 10.1002/pd.1269. [DOI] [PubMed] [Google Scholar]
- 4.Osborn P, Gross TL, Shah JJ, Ma L. Prenatal diagnosis of fetal heart failure in twin reversed arterial perfusion syndrome. Prenat Diagn. 2000;20:615–617. doi: 10.1002/1097-0223(200008)20:8<615::aid-pd855>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.De Groot R, Van Den Wijngaard JP, Umur A, Beek JF, Nikkels PG, Van Gemert MJ. Modeling acardiac twin pregnancies. Ann N Y Acad Sci. 2007;1101:235–249. doi: 10.1196/annals.1389.023. [DOI] [PubMed] [Google Scholar]
- 6.Lee H, Wagner AJ, Sy E, Ball R, Feldstein VA, Goldstein RB, Farmer DL. Efficacy of radiofrequency ablation for twin-reversed arterial perfusion sequence. Am J Obstet Gynecol. 2007;196:459.e1–459.e4. doi: 10.1016/j.ajog.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 7.Mulcahy C, McAuliffe FM, Breathnach F, Geary M, Daly S, Higgins J, Hunter A, Morrison J, Burke G, Higgins S, Dicker P, Mahony R, Tully E, Malone F. Umbilical and fetal middle cerebral artery Doppler reference ranges in a twin population followed longitudinally from 24 to 38 weeks’ gestation. Ultrasound Obstet Gynecol. 2014;44:461–467. doi: 10.1002/uog.13302. [DOI] [PubMed] [Google Scholar]
- 8.Lee H, Bebbington M, Crombleholme TM. North American Fetal Therapy Network. The North American Fetal Therapy Network Registry data on outcomes of radiofrequency ablation for twin-reversed arterial perfusion sequence. Fetal Diagn Ther. 2013;33:224–229. doi: 10.1159/000343223. [DOI] [PubMed] [Google Scholar]
- 9.Byrne FA, Lee H, Kipps AK, Brook MM, Moon-Grady AJ. Echocardiographic risk stratification of fetuses with sacrococcygeal teratoma and twin-reversed arterial perfusion. Fetal Diagn Ther. 2011;30:280–288. doi: 10.1159/000330762. [DOI] [PubMed] [Google Scholar]
- 10.Rychik J, Ayres N, Cuneo B, Gotteiner N, Hornberger L, Spevak PJ, Van Der Veld M. American Society of Echocardiography guidelines and standards for performance of the fetal echocardiogram. J Am Soc Echocardiogr. 2004;17:803–810. doi: 10.1016/j.echo.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Huhta JC. Right ventricular function in the human fetus. J Perinat Med. 2001;29:381–389. doi: 10.1515/JPM.2001.054. [DOI] [PubMed] [Google Scholar]
- 12.Rand L, Lee H. Complicated monochorionic twin pregnancies: updates in fetal diagnosis and treatment. Clin Perinatol. 2009;36:417–430. x–xi. doi: 10.1016/j.clp.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Kinsel-Ziter ML, Cnota JF, Crombleholme TM, Michelfelder EC. Twin-reversed arterial perfusion sequence: pre- and postoperative cardiovascular findings in the ‘pump’ twin. Ultrasound Obstet Gynecol. 2009;34:550–555. doi: 10.1002/uog.6431. [DOI] [PubMed] [Google Scholar]
- 14.Turan OM, Turan S, Gungor S, Berg C, Moyano D, Gembruch U, Nicolaides KH, Harman CR, Baschat AA. Progression of Doppler abnormalities in intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;32:160–167. doi: 10.1002/uog.5386. [DOI] [PubMed] [Google Scholar]
- 15.Gramellini D, Folli MC, Raboni S, Vadora E, Merialdi A. Cerebral-umbilical Doppler ratio as a predictor of adverse perinatal outcome. Obstet Gynecol. 1992;79:416–420. doi: 10.1097/00006250-199203000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Donofrio MT, Bremer YA, Schieken RM, Gennings C, Morton LD, Eidem BW, Cetta F, Falkensammer CB, Huhta JC, Kleinman CS. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: the brain sparing effect. Pediatric Cardiol. 2003;24:436–443. doi: 10.1007/s00246-002-0404-0. [DOI] [PubMed] [Google Scholar]
- 17.Scherjon SA, Smolders-DeHaas H, Kok JH, Zondervan HA. The “brain-sparing” effect: antenatal cerebral Doppler findings in relation to neurologic outcome in very preterm infants. Am J Obstet Gynecol. 1993;169:169–175. doi: 10.1016/0002-9378(93)90156-d. [DOI] [PubMed] [Google Scholar]
- 18.Scherjon SA, Oosting H, Smolders-DeHaas H, Zondervan HA, Kok JH. Neurodevelopmental outcome at three years of age after fetal ‘brain-sparing’. Early Hum Dev. 1998;52:67–79. doi: 10.1016/s0378-3782(98)00004-8. [DOI] [PubMed] [Google Scholar]
- 19.Figueras F, Cruz-Martinez R, Sanz-Cortes M, Arranz A, Illa M, Botet F, Costas-Moragas C, Gratacos E. Neurobehavioral outcomes in preterm, growth-restricted infants with and without prenatal advanced signs of brain-sparing. Ultrasound Obstet Gynecol. 2011;38:288–294. doi: 10.1002/uog.9041. [DOI] [PubMed] [Google Scholar]
- 20.Szwast A, Tian Z, McCann M, Donaghue D, Rychik J. Vasoreactive response to maternal hyperoxygenation in the fetus with hypoplastic left heart syndrome. Circ Cardiovasc Imaging. 2010;3:172–178. doi: 10.1161/CIRCIMAGING.109.848432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams IA, Tarullo AR, Grieve PG, Wilpers A, Vignola EF, Myers MM, Fifer WP. Fetal cerebrovascular resistance and neonatal EEG predict 18-month neurodevelopmental outcome in infants with congenital heart disease. Ultrasound Obstet Gynecol. 2012;40:304–309. doi: 10.1002/uog.11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams IA, Fifer C, Jaeggi E, Levine JC, Michelfelder EC, Szwast AL. The association of fetal cerebrovascular resistance with early neurodevelopment in single ventricle congenital heart disease. Am Heart J. 2013;165:544.e1–550.e1. doi: 10.1016/j.ahj.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Lu M, Mussatto KA, Williams IA, Gustafson KE, Mital S, Pike N, Sood E, Mahle WT, Cooper DS, Dunbar-Masterson C, Krawczeski CD, Lewis A, Menon SC, Pemberton VL, Ravishankar C, Atz TW, Ohye RG, Gaynor JW Pediatric Heart Network Investigators. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation. 2012;125:2081–2091. doi: 10.1161/CIRCULATIONAHA.111.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goff DA, Shera DM, Tang S, Lavin NA, Durning SM, Nicolson SC, Montenegro LM, Rome JJ, Gaynor JW, Spray TL, Vossough A, Licht DJ. Risk factors for preoperative periventricular leukomalacia in term neonates with hypoplastic left heart syndrome are patient related. J Thorac Cardiovasc Surg. 2014;147:1312–1318. doi: 10.1016/j.jtcvs.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sethi V, Tabbutt S, Dimitropoulos A, Harris KC, Chau V, Poskitt K, Campbell A, Azakie A, Xu D, Barkovich AJ, Miller SP, McQuillen PS. Single-ventricle anatomy predicts delayed microstructural brain development. Pediatr Res. 2013;73:661–667. doi: 10.1038/pr.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jelin E, Hirose S, Rand L, Curran P, Feldstein V, Guevara-Gallardo S, Jelin A, Gonzales K, Goldstein R, Lee H. Perinatal outcome of conservative management versus fetal intervention for twin reversed arterial perfusion sequence with a small acardiac twin. Fetal Diagn Ther. 2010;27:138–141. doi: 10.1159/000295176. [DOI] [PubMed] [Google Scholar]
- 27.Berg C, Holst D, Mallmann MR, Gottschalk I, Gembruch U, Geipel A. Early vs late intervention in twin reversed arterial perfusion sequence. Ultrasound Obstet Gynecol. 2014;43:60–64. doi: 10.1002/uog.12578. [DOI] [PubMed] [Google Scholar]