Abstract
Objective
Initiation of antiretroviral therapy (ART) during tuberculosis (TB) treatment improves survival in TB-HIV co-infected patients. In patients with CD4+ counts <50cells/mm3, there is a substantial clinical and survival benefit of early ART initiation. The purpose of this study was to assess the costs and cost effectiveness of starting ART at various time points during TB treatment in patients with CD4+ counts ≥50cells/mm3.
Methods
In the SAPiT trial, 642 HIV-TB co-infected patients were randomized to three arms, either receiving ART within 4 weeks of starting TB treatment (early treatment arm; Arm-1), after the intensive phase of TB treatment (late treatment arm; Arm-2), or after completing TB treatment (sequential arm; Arm-3). Direct healthcare costs were measured from a provider perspective using a micro-costing approach. The incremental cost per death averted was calculated using the trial outcomes.
Results
For patients with CD4+ count≥50cells/mm3, median monthly variable costs per patient were $116, $113 and $102 in Arms-1, -2 and -3, respectively. There were 12 deaths in 177 patients in Arm-1, 8 deaths in 180 patients in the Arm-2 and 19 deaths in 172 patients in Arm-3. While the costs were lower in Arm-3, it had a substantially higher mortality rate. The incremental cost per death averted associated with moving from Arm-3 to Arm-2 was $4199. There was no difference in mortality between Arm-1 and Arm-2, but Arm-1 was slightly more expensive.
Conclusions
Initiation of ART after the completion of the intensive phase of TB treatment is cost effective for patients with CD4+ counts≥50cells/mm3.
Introduction
Tuberculosis (TB) is a leading cause of death among human immunodeficiency virus (HIV) infected patients.1 In Africa, 46% of -TB patients are HIV-positive 2 and in South Africa TB-HIV comorbidity is estimated at 73%3.
Initiation of antiretroviral therapy (ART) during TB treatment improved survival in TB HIV co-infected patients.4 Whilst severely immunosuppressed patients (CD4+ count <50cells/mm3) have better survival with early initiation of ART, the timing of ART initiation during TB treatment in patients with higher CD4+ counts is less clear.5,6
Based partly on the Starting Antiretroviral Therapy at Three Points in TB (SAPiT) study,4 World Health Organisation7 and South African guidelines8 recommended in 2010 that TB-HIV co-infected patients receive ART within 8 weeks of commencing TB treatment. In 2012, both guidelines were updated recommending that all HIV-positive TB patients initiate ART immediately, irrespective of CD4+ count.9,10
Given the extent of the HIV-epidemic in South Africa,11 the large number of HIV- TB co-infected patients eligible for ART, the budgetary implications of these changes in ART treatment guidelines are far-reaching.
Information on the cost of starting HIV treatment during TB treatment is vital for budgeting in countries where scale-up of early ART and TB care is required due to large numbers of co-infected patients. Extensive research has been done on the cost and cost-effectiveness of ART, ART provision, monitoring strategies, and regimen choices in sub-Saharan Africa.12–21 However, no studies were found that examined the comparative costs or cost-effectiveness of different timing of ART initiation in TBtherapy for co-infected patients.22
Integration of HIV and TB services has the potential to save money through shared utilization of resources, such as monitoring and evaluation, avoidance of duplicate testing, medicine procurement, laboratory equipment, infrastructure and human resources.23–25 Starting ART during TB treatment could also increase cost, as ART is provided to more patients and may require additional resources, such as infrastructure and staff training.23
Effectiveness and cost are both important considerations when determining the value of starting ART during TB treatment, especially in resource limited settings where efficient allocation of health care resources is necessary. While cost analysis methodologies quantify resources used for health interventions, thus enabling budgeting and planning, they do not inform about the overall value of interventions in terms of years of life saved. Cost-effectiveness analysis weighs up both the costs and effectiveness of starting ART during TB treatment. Given the important budgetary implications of changes in ART eligibility for high-burden countries, consideration of costs may be of benefit in guiding the timing of ART.
The purpose of this study is to assess the costs and cost-effectiveness of initiating ART with TB treatment (early treatment), at the end of the intensive phase of TB treatment (late treatment) or upon completion of TB treatment (sequential treatment), for adult patients co-infected with TB and HIV with CD4 counts ≥50cells/mm3.
Methods
The SAPiT trial, conducted between 2005 and 2010, was a randomized, open-label, controlled clinical trial in patients co-infected with TB and HIV with CD4+ counts<500cells/mm3. The study design, ART and TB regimens and eligibility criteria have been described elsewhere.4,6 Patients were recruited at a municipal TB outpatient clinic where TB treatment was provided. HIV treatment was provided at an outpatient research clinic co-located with the TB outpatient clinic but in a different area with a different clinical team. Patients were randomized to three arms: initiate ART within 4 weeks following the initiation of TB treatment (Early treatment: Arm-1), within the first 4 weeks of the continuation phase of TB treatment (Late treatment: Arm-2) or after the completion of TB therapy (Sequential treatment: Arm-3). Each patient was followed for 18 months. Patient characteristics are described elsewhere. 4,6
All patients gave written informed consent. The trial was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (E107/05) and the South African Medicines Control Council (20060137).
Data collection
Variable financial costs were estimated using a micro-costing approach from randomization onwards. The costs included were ART and non-ART medication costs, laboratory test costs, radiographs, outpatient consultations and hospitalization costs. Resource utilization was captured at the patient-level. Table 1 summarizes unit costs in US dollars ($). The average exchange rate for 15 December 2009 was used (7.47 South African Rands per US Dollar, www.xe.com).
Table 1.
Unit costs of inputs and resource utilization by treatment arm
| Unit costs of inputs in US $ | Unit cost | ||
|---|---|---|---|
| Antiretroviral therapy | |||
| Cost of first line ART regimen (per patient per month): didanosine, lamivudine, efavirenz | 47.17 | ||
| Laboratory tests (cost per test) | |||
| CD4+ count | 8.51 | ||
| HIV viral load | 40.16 | ||
| Chest radiograph | 11.91 | ||
| Liver function test | 39.41 | ||
| Full blood count | 6.47 | ||
| Urine & Electrolytes | 19.33 | ||
| Visit costs | |||
| Average cost of unscheduled consultation | 23.68 | ||
| Cost per missed visit (cost of tracing)3 | 6.16 | ||
|
Hospitalizations | |||
| Cost per hospital day (level 2 hospital) | 45.65 | ||
| Costs in different arms |
Arm-1 (N=214) |
Arm-2 (N= 215) |
Arm-3 (N=213) |
| Median monthly cost of second line ART (for patients on second-line) | 73.45 | 85.27 | 85.27 |
| Outpatient visit costs 1 | |||
| Average staff cost per ART consultation (clinician) | 20.79 | 20.10 | 20.10 |
| Average staff cost per ART consultation (professional nurse)2 | 16.49 | 16.49 | 16.49 |
| Average staff cost per tuberculosis only consultation (clinician) | 13.93 | 14.01 | 14.01 |
| Total cost of scheduled ART consultations over 18 months | 393.57 | 386.08 | 386.08 |
| Hospitalizations | |||
| Average “other costs” per hospital admission3 | 120.12 | 108.81 | 146.08 |
| Resource utilization | |||
| Number of patients with: | n (%) | n (%) | n (%) |
| More than one missed visit | 29 (13.6%) | 41 (19.1%) | 47 (22.1%) |
| More than one unscheduled consultation in first 3 months of tuberculosis treatment. | 57 (26.6%) | 13 (6.1%) | 4 (1.9%) |
| Switched to second line ART regimen | 10 (4.7%) | 8 (3.7%) | 7 (3.3%) |
| Did not initiate ART | 15 (7.0%) | 52 (24.2%) | 77 (36.1%) |
| Multidrug resistant tuberculosis | 13 (6.1%) | 8 (3.7%) | 9 (4.2%) |
| Immune reconstitution syndrome | 43 (20.1%) | 18 (8.4%) | 20 (9.4%) |
| Serious adverse events | 52 (24.3%) | 43 (20.0%) | 62 (29.1%) |
| Hospitalization | 40 (18.7%) | 30 (14.0%) | 46 (21.6%) |
| More than 5 days hospitalized | 26 (12.2%) | 21 (9.8%) | 36 (16.9%) |
| Total number of | N | N | N |
| Hospital days | 869 | 519 | 1241 |
| Patient months | 3019 | 2836 | 2703 |
| Median (IQR) | Median (IQR) | Median (IQR) | |
| Number of unscheduled consultations | 1 (1–3) | 1 (1–2) | 1 (1–3) |
| Time to ART regimen switch (months) | 8.5 (3.5–10.9) | 11.8 (9.1–12.5) | 8.2 (5.3,9.0) |
| Length of tuberculosis treatment (days) | 204 (196–253) | 202 (194–253) | 198 (182–252) |
| Number of CD4+ count tests | 5 (4 – 6) | 6 (4 – 6) | 5 (3–6) |
| Number of viral load tests | 4.5 (3 – 5) | 5 (3–5) | 4 (2–5) |
| Rate (per 100 person years) | |||
| Rate of hospitalization | 18.2 | 15.5 | 23.0 |
ART: Antiretroviral treatment
All costs in 2009 US dollars
The staff cost includes the cost of all categories of staff that deal directly with the patient (reception, nursing staff, clinicians and counsellors). The cost of a visit is determined by the length of the visit as well as the salary of the type of staff member conducting the consultation.
Professional nurses saw all stable patients for scheduled consultations from the eighth month of ART treatment onwards. Clinicians saw patients at regular scheduled intervals, following CD4+ count and viral load tests.
Includes the cost of transportation to hospital, CAPRISA staff cost of admission and follow-up while in hospital.
Source of safety laboratory test prices: National Health Laboratory Services (2009)
Calculation of costs
The cost of voluntary counseling and testing, screening and baseline consultations, TB diagnostics, TB treatment, capital costs, fixed costs and overhead costs were excluded as they were common to all patients and did not vary by study arm. The decision to exclude these costs was guided by principles provided by Drummond et al (1997).26 Total cost in this study is thus an underestimation of the true cost. Excluding the costs of TB treatment was appropriate as there was no statistically significant difference in the length of TB treatment, type of treatment and incidence of multi drug resistant (MDR) TB between arms.
Medication
Medication use, including start and stop dates, was documented in study records. ART doses were specified, and for other medications standard doses were assumed. Provincial ART tender prices (valid to December 2010) were used to cost ART. Private sector prices were used for enteric-coated didanosine (Videx EC®), as this was not available in the public sector. Public sector prices obtained from facility-level requisitions in February 2009 (where available) or private sector prices (obtained from the Mediscor PBM product database) were used for non-ART medications.
Laboratory tests
CD4+ count and viral load tests were recorded in patient files. Electronic results for safety laboratory tests were obtainable from February 2007to the end of the study. Only 347 patients (285 with CD4+ count>=50) had electronic laboratory data for the entire study period. This laboratory cost sample included a disproportionately low number of patients who did not initiate ART (12% in the sample, 22% overall), and a disproportionally high number who experienced immune reconstitution syndrome (IRIS) (18% in the sample, 13% overall). To overcome the problem of missing data, while addressing sources of potential bias, conditional mean imputation by category of patient was used to impute laboratory test costs for the remaining 296 patients.
The categories used in the imputation were defined by arm, presence of IRIS, ART initiation and CD4 category (CD4+ counts<50cells/mm3 or CD4+ counts>50cells/mm3). Three outliers (with laboratory costs of greater than USD 1339) were excluded from the process of imputation in order to reduce potential bias. These patients remained in the overall patient sample, as these laboratory costs did not appear to be erroneous. The cost calculated for each subgroup was then applied to all patients with those characteristics who did not have laboratory data recorded.
The 2009 public sector price (charged by the National Health Laboratory Service) was used for all laboratory tests . The costs of documented radiographs were included. To make the results of this analysis more generalizable to primary health care settings where ART and TB treatment are usually provided all tertiary level laboratory tests were excluded from the calculation of laboratory test cost.
Labor and overhead costs
Outpatient consultations (number and type) were recorded in patient files. Interviews were conducted with a sample of staff to determine the time taken for different types of clinical consultations. These time estimates were multiplied by the average hourly public sector salary per staff type to determine staff costs per consultation. Department of Public Service and Administration 2009 Salary data (with effect 1 April 2009) was obtained from KZN Department of Health directly. Salaries included all benefits, such as pension and leave. Where specific roles did not exist within the public sector, CAPRISA salaries were used. The cost per different type of consultation was calculated.
Hospital costs
Dates of hospital admission and discharge were recorded in patient files. Records were reviewed to determine resource use during hospitalization, including procedures performed, use of intravenous fluids and blood products and level of hospital and ward admitted to. Data on medications prescribed and laboratory tests performed in hospital were incomplete and were not used, instead prices per inpatient day and procedures performed in a public sector hospital from the 2009 Uniform Patient Fee Schedule (UPFS)27 were used. These prices are flat fees charged to patients with medical insurance who make use of public sector hospitals and are calculated to cover the estimated cost of consumables (with the exception of some high cost theatre and ward consumables), medication, hospital overheads, cost of support and medical staff. The prices exclude discharge medication, medication not on the essential drug list, anaesthetic and laboratory tests. Blood products were charged at the South African National Blood Service rate to public sector patients (SANBS State patient price list, 2009, www.sanbs,gov.za). Hospital costs include the cost of transportation to hospital and the cost of staff time spent referring patients. Several simplifying assumptions were made; for example, ward admitted to was inferred from information available, blood transfusions were assumed to have consisted of two units, costs of procedures and intravenous drugs were not included, and patients admitted to high care were assumed to have spent 50% of the stay in high care, the rest in a general ward. Assumptions made are expected to bias hospitalization costs downward. In the sensitivity analysis, the cost of procedures and intravenous drugs were included in hospitalization cost.
Analysis
Direct healthcare costs were measured from a provider perspective. Each resource used by each patient was multiplied by its unit cost and summed to determine the total cost per patient and per arm. The median cost per patient per month was calculated by dividing the total cost per patient by the number of months of follow-up. Discounting was not used, as the treatment spanned a short period and the timing of costs and benefits was similar across arms. To adjust for inflation 2009 prices were used throughout.
The outcome measure was all-cause mortality at 18 months using Kaplan-Meier methods. A simple patient-level micro costing model was developed in OpenOffice Calc (version 3.2) to combine data and calculate total variable cost per patient and patient month. The incremental cost per death averted was calculated over 18 months, by calculating the ratio of incremental total variable cost to incremental number of deaths averted between arms.26 The three treatment options were compared based on total cost and number of deaths associated with the treatment option. Any dominated options (treatment options with higher cost and lower effectiveness than the next alternative) were eliminated. Fisher’s exact test was used with categorical data, and Wilcoxon two-sample test or Kruskal-Wallis test with continuous data. Data were analysed in SAS (version 9.2).
One-way sensitivity analysis was used to test the implications of lower ART, lower viral load and laboratory test costs and alternative costs per inpatient day on the cost per patient month. Scenario analysis was conducted to evaluate the impact of several key assumptions on the model. The cost of laboratory tests received particular attention as these were inflated due to the frequency and comprehensiveness of laboratory safety monitoring within a clinical trial setting.
Some data were missing in non-ART medications and in detailed hospitalization data (procedures, use of intravenous fluids, ward). Where duration and dosage information was missing, standard duration and dosage for the indication was assumed. Non-ART medication costs were small and even if incorrectly estimated, would not impact overall results. Data used were from December 2009. A previous publication was based on earlier incomplete interim data.4
Results
The SAPiT trial enrolled 642 patients and demonstrated a 56% reduction in mortality (hazard ratio:0.44, 95% confidence interval (CI):0.25–0.79,p=0.003) among patients initiating ART during TB treatment compared to after the completion of TB treatment.4 No difference in mortality was found between patients randomized to Arm-1 and Arm-2 (incidence rate ratio=0.96, 95%CI:0.44–2.10), except in patients with CD4+ counts<50cells/mm3.6 Mortality rates in patients with CD4+ counts ≥50cells/mm3 was 5.6/100 person years (95%CI:2.9–9.8) in Arm-1; 3.8/100 person years (95%CI:1.7–7.6) in Arm-2, and 10.0/100 person years (95%CI:6.4–15.7) in Arm-3.4,6 The mortality benefit was pronounced in patients with CD4+ counts<50 cells/mm3 and cost effectiveness arguments were not relevant in this subgroup. Consideration of cost-effectiveness is appropriate in choosing the optimal strategy for patients with CD4+ counts≥50cells/mm3. While all results are for patients with CD4+ counts≥50cells/mm3, the sub-group analysis of costs per arm in patients with <50 cells/mm3 is presented in Table 3. Loss to follow-up was 8.9%, 11.6% and 13.1% in the three treatment arms.
Table 3.
Variable cost per patient (over 18 months and per month) in 2009 US$
| Arm-1 N = 214 |
Arm-2 N = 215 |
Arm-3 N = 213 |
All 3 arms p- value |
Arm 1 vs 2 |
Arm 2 vs 3 |
Arm 1 vs 3 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median costs over 18 months | Median | IQR | Median | IQR | Median | IQR | ||||
| All patients | ||||||||||
| Antiretroviral therapy | 742.88 | 471–889 | 611.78 | 0–699 | 349.59 | 0–481 | <0.001 | <0.001 | <0.001 | <0.001 |
| Other medications | 3.20 | 0–13 | 4.74 | 0–13 | 5.61 | 0–12 | 0.612 | |||
| All investigations1 | 698.75 | 638 – 784 | 766.57 | 461 – 851 | 761.17 | 350 – 838 | 0.139 | |||
| CD4+ count and viral load | 227.55 | 163 – 252 | 243.37 | 155 – 252 | 203.21 | 106 – 252 | 0.042 | 0.530 | 0.110 | 0.010 |
| Safety laboratory tests | 436.07 | 395–522 | 515.08 | 255–558 | 481.54 | 216–550 | 0.484 | |||
| X-rays | 47.66 | 36 – 48 | 35.74 | 24 – 48 | 35.74 | 24 – 48 | 0.008 | 0.009 | 0.900 | 0.006 |
| Outpatient consultation2 | 399.37 | 352 – 423 | 399.48 | 191 – 423 | 383.50 | 100 – 407 | <0.001 | 0.041 | 0.014 | <0.001 |
| Hospitalization (in patients who were hospitalized) | 578.20 | 265 – 1360 | 643.70 | 297 – 1309 | 1 057.59 | 348 – 2318 | 0.218 | |||
| Total variable cost over 18 months | 1930.71 | 1752–2108 | 1856.88 | 1030–2021 | 1659.09 | 658–1834 | <0.001 | 0.003 | <0.001 | <0.001 |
| Median cost per patient month | ||||||||||
| Antiretroviral therapy | 42.67 | 42 – 51 | 35.07 | 0 – 40 | 20.08 | 0–28 | <0.001 | <0.001 | <0.001 | <0.001 |
| All investigations2 | 44.19 | 39–63 | 50.11 | 44–74 | 49.04 | 45–67 | <0.001 | <0.001 | 0.730 | <0.001 |
| Outpatient consultation cost1 | 24.65 | 23 – 29 | 23.71 | 23 – 27 | 22.23 | 21 – 25 | <0.001 | 0.001 | <0.001 | <0.001 |
| Total variable cost per patient month | 118.47 | 110–168 | 114.21 | 106–149 | 102.98 | 92–134 | <0.001 | 0.013 | <0.001 | <0.001 |
| Patients with CD4+ count < 50 cells/mm3 | ||||||||||
| Antiretroviral therapy | 742.88 | 699 – 889 | 655.48 | 393 – 699 | 209.79 | 0 – 481 | <0.001 | <0.001 | <0.001 | <0.001 |
| Other medications | 6.84 | 1–20 | 8.73 | 2–20 | 6.84 | 0–9 | 0.356 | |||
| All investigations | 861.78 | 728–901 | 866.62 | 741–969 | 620.64 | 330–829 | <0.001 | 0.748 | 0.002 | <0.001 |
| CD4+ count and viral load | 251.89 | 203 – 252 | 251.89 | 203 – 252 | 154.54 | 57 – 252 | 0.002 | 0.840 | 0.004 | 0.001 |
| Safety laboratory tests | 597.97 | 474–602 | 648.35 | 453–648 | 399.53 | 238–550 | <0.001 | 0.615 | 0.002 | <0.001 |
| X-rays | 47.66 | 36 – 60 | 35.74 | 36 – 48 | 35.74 | 24 – 48 | 0.026 | 0.060 | 0.369 | 0.011 |
| Outpatient consultation | 430.57 | 399 – 465 | 401.61 | 339 – 445 | 322.14 | 81 – 397 | <0.001 | 0.092 | 0.005 | <0.001 |
| Hospitalization (in patients who were hospitalized) | 615.82 | 513–1347 | 594.52 | 230–1187 | 643.90 | 371–1239 | 0.825 | |||
| Total variable cost over 18 months | 2128.45 | 1967–2512 | 1965.25 | 1785–2254 | 1697.16 | 751–1930 | <0.001 | 0.025 | 0.008 | <0.001 |
| Median cost per patient month | ||||||||||
| Antiretroviral therapy | 42.67 | 42–51 | 37.58 | 30 – 40 | 15.83 | 0 – 27 | <0.001 | <0.001 | <0.001 | <0.001 |
| All investigations | 50.97 | 48–67 | 55.62 | 46–89 | 58.24 | 47–79 | 0.696 | |||
| Outpatient consultation cost | 25.79 | 24 – 28 | 25.16 | 23 – 29 | 22.27 | 18 – 24 | <0.001 | 0.201 | 0.001 | <0.001 |
| Total variable cost per patient month | 132.34 | 119–165 | 127.38 | 111–164 | 112.25 | 99–197 | 0.126 | |||
| Patients with CD4+ count > 50 cells/mm3 | ||||||||||
| Antiretroviral therapy | 742.88 | 380 – 889 | 611.78 | 0 – 699 | 349.59 | 0 – 481 | <0.001 | <0.001 | <0.001 | <0.001 |
| Other medications | 2.54 | 0–12 | 3.30 | 0–13 | 4.25 | 0–12 | 0.390 | |||
| All investigations1 | 686.94 | 634–741 | 758.06 | 379–819 | 773.61 | 350–840 | 0.014 | 0.005 | 0.385 | 0.026 |
| CD4+ count and viral load | 203.21 | 155 – 252 | 211.73 | 106 – 252 | 207.47 | 106–252 | 0.496 | |||
| Safety laboratory tests | 436.07 | 382–448 | 507.04 | 181–519 | 490.57 | 209–550 | 0.049 | 0.035 | 0.173 | 0.059 |
| X-rays | 47.66 | 36 – 48 | 35.74 | 24 – 48 | 35.74 | 24 – 48 | 0.085 | |||
| Outpatient consultation | 399.37 | 296 – 423 | 397.34 | 143 – 411 | 383.50 | 106 – 407 | 0.002 | 0.149 | 0.158 | <0.001 |
| Hospitalization (in patients who were hospitalized) | 563.97 | 240–1366 | 815.41 | 298–1309 | 1294.80 | 348–2318 | 0.126 | |||
| Total variable cost over 18 months | 1882.14 | 1470–2036 | 1839.79 | 746–1987 | 1656.80 | 552–1812 | <0.001 | 0.014 | 0.005 | <0.001 |
| Median cost per patient month | ||||||||||
| Antiretroviral therapy | 42.67 | 42 – 51 | 35.01 | 0 – 40 | 21.89 | 0 – 27 | <0.001 | <0.001 | <0.001 | <0.001 |
| All investigations | 42.53 | 39–63 | 48.46 | 44–73 | 48.61 | 45–62 | <0.001 | <0.001 | 0.713 | <0.001 |
| Outpatient consultation cost | 24.25 | 23 – 30 | 23.47 | 23 – 27 | 22.23 | 21 – 25 | <0.001 | 0.003 | <0.001 | <0.001 |
| Total variable cost per patient month | 116.20 | 108–167 | 113.09 | 106–144 | 101.81 | 90–126 | <0.001 | 0.038 | <0.001 | <0.001 |
IQR:Interquartile range
This include the cost of CD4+ count, viral load testing, X-rays and all other laboratory tests. It includes staff cost, the price paid for investigations and consumables cost.
This is the median consultation cost per patient for all scheduled tuberculosis and antiretroviral therapy consultations attended and all unscheduled consultations. It includes staff cost and consumables cost.
Note: Only the major components of median cost per patient month are shown in the table, for ease of reading
The median monthly variable cost per patient was $116 (Arm-1), $113 (Arm-2) and $102 (Arm-3), p<0.001. Arm-1 and Arm-2 had similar costs and mortality . Although costs in Arm-3 were the lowest, this arm had the highest mortality. Incremental cost per death averted in Arm-2, compared to Arm-3, was $4199 (Figure 1). Switching from sequential ART (ART offered at the end of TB treatment) to offering ART at the end of the intensive phase of TB treatment amounted to a cost of $4199 per death avoided. Arm-1 has slightly higher mortality than Arm-2, while Arm-1 is also marginally more expensive. This means that Arm-1 is dominated by Arm-2, and Arm-2 is the better choice both in terms of cost and mortality outcomes.
Figure 1.
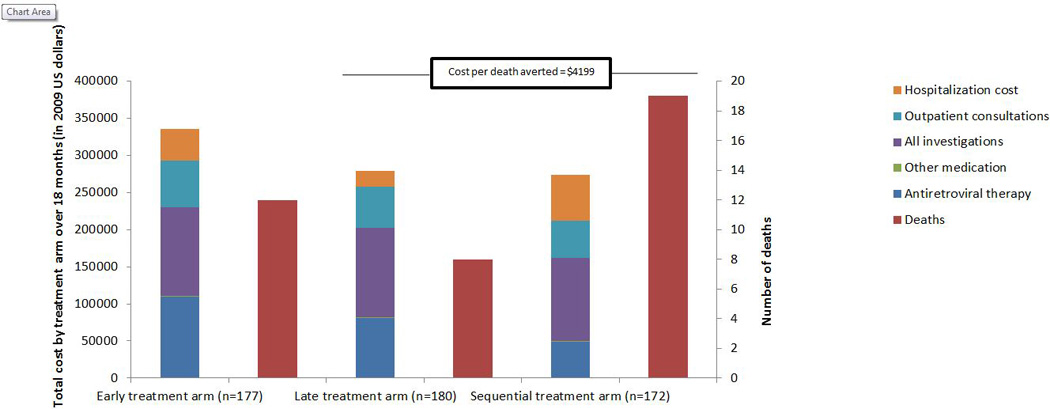
The costs of treating co-infected patients comprise four components: drugs, laboratory testing, outpatient care and hospitalization. Laboratory investigations, driven largely by research protocol safety requirements, contributed the largest proportion of variable cost in all three treatment arms (35.5% in Arm-1, 43.0% in Arm-2 and 10.7% in Arm-3. CD4+ count and viral load tests contributed between 10.6% and 12.2% to total variable costs, depending on arm. The number of CD4+ count and viral load tests performed was higher in Arm-2 than in the other two arms. Spending on laboratory tests was significantly higher in Arm-2 than in Arm-1 (p=0.005). Baseline CD4+ count <50cells/mm3 and presence of IRIS significantly increased spending on laboratory tests (p<0.01). Spending on laboratory testing was higher for patients who did not initiate ART due to abnormalities in safety laboratory results since these patients required repeated testing. The cost of ART was the second largest contributor to total variable costs in Arm-1 (32.4%) and Arm-2 (28.7%), while it contributed only 17.6% to total costs in Arm-3. Outpatient consultations made up between 18.3 1nd 19.7% of total costs across the arms. Hospitalization cost was the third largest cost in Arm-3 (Table 2).
Table 2.
Percentage (and total spending) of total variable cost spent on different categories (Patients with CD4+ count ≥ 50 cells/mm3)
| Arm-1 N = 177 |
Arm-2 N = 180 |
Arm-3 N = 172 |
Total N = 529 |
|
|---|---|---|---|---|
| Antiretroviral therapy | 32.4% ($109,224) | 28.7%($80,452) | 17.6%($48,423) | 26.7%($238,098) |
| Other medication | 0.4%($1,351) | 0.6%($1,544) | 0.6%($1,739) | 0.5%($4,634) |
| All investigations | 35.5%($119,456) | 43.0%($120,577) | 40.7%($111,808) | 39.4%($351,837) |
| CD4+ count and viral load | 10.6%($35,731) | 12.2%($34,115) | 11.4%($31,207) | 11.3%($101,054) |
| Safety laboratory tests | 22.7%($76,433) | 28.3%($79,558) | 26.9%($73,953) | 25.8%($229,945) |
| X-rays | 2.2%($7,292) | 2.5%($6,898) | 2.4%($6,648) | 2.3%($20,838) |
| Outpatient consultations | 18.6%($62,527) | 19.7%($55,374) | 18.3%($50,418) | 18.9%($168,319) |
| Hospitalization cost | 12.9%($43,291) | 7.7%($21,710) | 22.4%($61,453) | 14.2%($126,454) |
The median cost of consultations was similar across arms. The only difference in length of consultations was for the ART initiation visit, which was longer in Arm-1.
Hospitalizations comprised 12.9%, 7.7% and 22.4% of the total variable costs for Arms-1,-2, and-3, respectively. Both mean and median costs per patient hospitalized were highest in Arm-3. The median cost per patient hospitalized was $564 in Arm-1, $815 in Arm-2 and $1295 in Arm-3. The mean cost of hospitalization per patient hospitalized was much higher: $1603 in Arm-1, $1086 in Arm-2 and $2195 in Arm-3. The costs of hospitalization were more variable than other costs.
Most hospital admissions (89.6%, 120/134) were to a general ward, 9.7% (13/134) were to high care, and 0.7% (1/134) to intensive care; 62.7% (84/134) were admitted to secondary level hospitals. Procedures were recorded for 60.4% (81/134) of hospitalizations: these were procedures not requiring an operating theatre (54.5%, 54/99), radiological (20.2%, 20/99), minor surgical (20.2%, 20/99) and major surgical (5.1%, 5/99) procedures. The most common procedures performed were lumbar puncture, CT scan, administration of intravenous fluids for rehydration, radiograph and ultrasound. The median cost per procedure was $20. General ward costs accounted for 78% of total hospital costs.
The costs in Arm-2 were similar to costs in Arm-1 and patient survival was similar. Patients in the late treatment arm had the lowest number of hospitalizations, serious adverse events and IRIS; which are recognized cost drivers in the provision of TB and HIV services.
Over 18 months, Arm-1 was the most expensive ($1882), followed by Arm-2 ($1840) and Arm-3 ($657, p<0.001). The lower cost in Arm-3 was driven by shorter duration of ART provision, which lowered the cost of ART. The median cost of ART in Arm-3 was less than half the cost of ART in Arm1.
Sensitivity analyses showed that the median cost per patient month is not affected by changes in several key assumptions and changes in key prices (Table 4). While some scenarios resulted in much lower cost estimates, the trends between the arms remained similar. Costs per patient month were most sensitive to changes in ART prices and costs of investigations. Changes in the price of viral load testing made little difference. The difference in costs between Arms-1 and-2 was small when ART prices were reduced, but both strategies were still more expensive than Arm-3. The magnitude of the cost differences between the strategies did not change when the costs of laboratory testing were reduced. New ART tender prices have been negotiated since starting this study. The median cost per patient month was slightly lower when using these prices (Table 4).
Table 4.
Sensitivity Analysis: Median (Interquartile range) variable cost per patient month in US $, in patients with CD4 count ≥ 50
| Parameter(s) altered in sensitivity analysis | Arm-1 N = 177 |
Arm-2 N = 180 |
Arm-3 N = 172 |
|---|---|---|---|
| Scenario 1 (base case): The mean estimate of clinician time per activity was used. The lower estimated cost per missed visit was used ($6). Procedures and intravenous treatment were not included in hospital cost | 116.20 (108 – 168) | 113.09(106 – 144) | 101.81 (90 – 126) |
| Scenario 2: The lowest estimate of clinician time per activity was used. The higher estimated cost per missed visit was used ($7.50). Hospitalization cost: Intravenous treatment duration was assumed to be the lesser of 5 days or total length of stay; procedures were included | 114.55 (106 – 171) | 111.19 (103 – 145) | 100.10 (90 – 126) |
| Scenario 3: The highest estimate of clinician time per activity was used. The higher estimated cost per missed visit was used ($7.50). Hospitalization cost: Intravenous treatment duration was assumed to be the lesser of 7 days or total length of stay; procedures were included | 117.03 (110 – 173) | 114.90 (107 – 148) | 103.42 (92 – 128) |
| 2012 National Department of Health ART tender prices were used for ART costs | 88.05 (82 – 141) | 91.41 (84 – 121) | 86.24 (78 – 111) |
| Lowest ART prices available in developing countries in 2011 were used for ART costs | 85.47 (80 – 141) | 89.17 (82 – 120) | 84.67 (77 – 109) |
| Hospital cost was estimated as $88 per day | 116.20 (108 – 176) | 113.40 (106 – 147) | 101.81 (90 – 129) |
| Viral load prices were reduced by 25% | 113.74 (105 – 166) | 110.71 (103 141) | 98.56 (87 – 123) |
| Viral load prices were reduced by 35% | 112.59 (104 – 165) | 109.63 (102 139) | 97.29 (86 – 122) |
| Frequency of CD4, VL reduced to SA national treatment guidelines | 109.72 (101 – 158) | 106.57 (99 – 128) | 90.93 (81 – 115) |
| Frequency of CD4, VL and safety laboratory tests reduced to SA national treatment guidelines | 87.37 (79 – 101) | 77.47 (69 – 86) | 60.85 (50 – 76) |
ART: Antiretroviral therapy
Discussion
The timing of ART initiation during TB care has implications for quality and cost of health service provision. In patients with CD4+ counts <50cells/mm3, the survival benefit of starting ART early during TB treatment is clear5,6 and outweighs the higher cost. Starting ART during TB treatment results in substantial increases in survival at moderate cost. However, in patients with CD4+ counts ≥50cells/mm3, the best time to initiate ART during TB treatment is less clear as survival was similar among patients initiated on ART in Arms-1 and-2. Cost-analysis could be used to decide between early and late initiation of ART during TB treatment in these patients.
In countries like South Africa where a cost-effectiveness threshold has not been established, GDP per capita is used to decide the cost-effectiveness of interventions. Interventions costing between one and three times annual GDP per capita are considered cost-effective. The cost per death averted in moving from sequential to late integrated treatment was estimated at $4199. This is below annual GDP per capita in South Africa in 2009 ($5758 at 2009 prices). As the cost per death averted is lower that the annual GDP per capita, late integration of ART into TB treatment is cost-effective for South Africa.
Our cost analysis suggests that late initiation of ART during TB treatment (Arm-2) is the optimal strategy for patients with CD4+ ≥50cells/mm3, especially in resource constrained settings.
Cost of the various options
The largest driver of costs in all three arms was laboratory investigations, which were done routinely every 6 months, and when toxicity was suspected. Some of the tests are done routinely in a care setting, and some were specific to the research setting. Safety tests were repeated until safety parameters returned to normal. These costs will be lower in routine settings, with less frequent testing. The differences in ART and investigations costs are likely to shrink as duration on ART increases and ART prices decrease over time.
The cost of hospitalization per patient hospitalized was lowest in Arm-1, higher in Arm-2and much higher in Arm-3. Hospitalization contributed 22.4% of the total cost in Arm-3, due to the larger number of patients hospitalized and longer duration of hospital stays among patients in this group. While outpatient costs in Arms 1 and 2 are higher, these arms reduce the burden on the hospital system and associated costs. The Department of Health should be expecting to spend approximately $1086 per patient hospitalized, if a strategy of starting ART after the intensive phase of TB treatment adopted.
The difference in cost between Arms-1 and-2 is small, but given the large numbers of TB-HIV co-infected patients in South Africa, the choice of late over early initiation of ART could lead to substantial savings.
Based on a coverage rate of 70% and an estimated 270 000 HIV-TB co-infected people in South Africa28, an estimated difference in cost of $3.11 per month between Arm-1 and Arm-2 equates to a total saving of more than $7 million per annum in South Africa through selecting late integration of ART rather than early integration. This assumes that all patients had drug susceptible pulmonary TB.
Comparison of costs to other settings
The costs in this study were higher than published reports from HIV-only treatment programs.15,16 This is due to inclusion of hospitalization costs in our analysis. Some differences are country-specific, as hospital and labor costs are relatively high in South Africa. Importantly, our cost estimates were in a clinical trial context, with more frequent visits (monthly) and laboratory monitoring and more staff time per patient than in routine care settings.26 We endeavored to provide enough detail on resource utilization and various cost scenarios in our sensitivity analysis to enable generalizability of our findings to other countries. Though the total cost estimate in this clinical trial is higher than in routine care settings, the relative difference in price between the three treatment strategies is likely to translate to other settings.
A recent study by the Clinton Health Access Initiative (CHAI) found that the total cost of treating HIV in South African facilities was $682 per annum, considerably higher than the average for four other African countries, which was $200 per annum.12 This equates to $1023 for 18 months, lower than the cost estimated in this study. However, the CHAI study did not include hospital costs, or TB-related costs which were included in this analysis. The cost of providing HIV-treatment in PEPFAR-supported programs in 43 clinics in Botswana, Ethiopia, Nigeria, Uganda, and Vietnam was $880 per annum, or $1320 over 18 months. These estimates did not include costs of hospitalization and management of TB.16
Costing of a single ART clinic in Haiti concluded that co-treatment of TB and HIV involved a small increase in cost and physician time compared with treating HIV alone, and suggested integration of TB and ART as a way to conserve physician time.29
Study limitations
Our patient population was ambulant and relatively healthy. The costs might not be generalizable to populations with higher morbidity or patients with non-pulmonary TB. The most important limitation is the short follow-up, of 18 months. ART monthly costs decline after 12 months.30,31 The short duration of the study therefore likely inflated median monthly cost. Monthly ART costs are likely to be similar in all arms at later time points since all patients will be receiving ART, leading to the shrinkage of cost differences associated with ART provision between the arms.
The study was done at one site only, but the costs are generalizable to other outpatient sites. Hospitalization costs may be underestimated as only average medication cost while hospitalized was included. If patients required atypical and expensive medications not included on the Essential Medicines List applicable in the public sector, these were not included.
The small number of patients with IRIS in the laboratory sample may have led to inaccurate estimates of laboratory costs for these patients. While laboratory costs were the biggest cost driver, a major limitation of our study is that these results are not readily generalizable to a clinical care setting as most of the laboratory tests were research related safety assessments.
New technologies have been adopted in South Africa for TB diagnosis and resistance testing since this clinical trial. The introduction of Xpert MTB/RIF will alter the costs estimates.
Conclusion
Current WHO recommendations call for initiating ART in all TB-HIV co-infected patients irrespective of CD4+ count. As more co-infected patients initiate ART, data on the cost effectiveness of initiating ART during TB therapy becomes important. This study provides health system utilization and cost data associated with starting ART during TB treatment with implications for health policy makers and funders.
The SAPiT4,6 CAMELIA,32 and ACTG5 trials have shown that starting ART during TB treatment saves lives and this analysis shows that starting ART during TB treatment is cost-effective. Late initiation of ART during TB and HIV treatment for patients with CD4+ counts≥50cells/mm3 is the most cost-effective. Cost-effectiveness is only one consideration in starting ART in HIV-TB co-infected patients. The efficacy of the intervention and practical challenges of implementation of integrated services, especially in resource limited settings, should also be considered.
In sub-Saharan Africa, where public sector hospitals are overburdened, the reduction of the burden on a struggling hospital system by starting ART during TB therapy needs to be emphasized.
Acknowledgements
Sources of funding: The US President's Emergency Plan for AIDS Relief (PEPfAR) funded the care of participants in the SAPiT trial, while the Global Fund to fight AIDS, Tuberculosis and Malaria funded the cost of the medications.
CAPRISA was established as part of the Comprehensive International Program of Research on AIDS (CIPRA) (grant # AI51794) from the US National Institutes of Health. The research infrastructure to conduct this trial, including the data management, laboratory and pharmacy cores were established through the CIPRA grant. We acknowledge funding for this study and patient care from the United States President’s Emergency Plan for AIDS Relief (PEPFAR). We gratefully acknowledge the participants in the study. In addition, we wish to acknowledge Dr Jessica Trusler (BARC SA) for supplying laboratory test prices, and Ms Madelein Bester (Mediscor PBM (Pty) Limited) for assistance with medicine prices and Mrs Cheryl Baxter for reviewing the manuscript.
KN, ACG and ND planned the study. KN and ND implemented the study. AG assisted with cost analysis and was a co-investigator in the SAPiT study. ACG wrote the initial draft of the paper and was a co-investigator in the SAPiT study. KN and TL did the analysis. KN provided oversight on collection of all cost data. SG assisted in providing resource utilization data and cost estimates. SAK planned and was the PI of the SAPiT study. All authors participated in the writing and editing of the paper.
Footnotes
Conflicts of Interest: None
References
- 1.Mukadi YD, Maher D, Harries A. Tuberculosis case fatality rates in high HIV prevalence populations in sub-Saharan Africa. AIDS. 2001;15:143–152. doi: 10.1097/00002030-200101260-00002. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global tuberculosis report 2012. Geneva: World Health Organization; 2012. [Google Scholar]
- 3.Department of Health. Global AIDS Response Progress Report 2012. Pretoria: 2012. [Google Scholar]
- 4.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010 Feb 25;362(8):697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Havlir DV, Kendall MA, Ive P, et al. Timing of Antiretroviral Therapy for HIV-1 Infection and Tuberculosis. N Engl J Med. 2011;365(16):1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of Antiretroviral Therapy with Tuberculosis Treatment. N Engl J Med. 2011;365(16):1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Treatment of tuberculosis guidelines. 4 ed. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 8.Department of Health. Clinical guidelines for the management of HIV and AIDS in adults and adolescents. Pretoria: Department of Health, Republic of South Africa; 2010. [Google Scholar]
- 9.Department of Health. National Strategic Plan on HIV, STIs and TB 2012–2016. Pretoria: National Department of Health; 2012. [Google Scholar]
- 10.World Health Organization. WHO policy on collaborative TB/HIV activities: guidelines for national programmes and other stakeholders. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 11.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global report: UNAIDS report on the global AIDS epidemic, 2010. Geneva: UNAIDS; 2010. [Google Scholar]
- 12.Boseley S. Aids breakthrough as study says treatment should cost less. The Guardian. 2012 Jul 20; 2012. [Google Scholar]
- 13.Cleary SM, McIntyre D, Boulle A. The cost-effectiveness of antiretroviral treatment in Khayelitsha, South Africa - a primary data analysis. Cost Eff Resour Alloc. 2006;4 doi: 10.1186/1478-7547-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badri M, Maartens G, Mandalia S, et al. Cost-Effectiveness of Highly Active Antiretroviral Therapy in South Africa. PLoS Med. 2005;3(1):e4. doi: 10.1371/journal.pmed.0030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stover J, Bollinger L, Avila C. Estimating the Impact and Cost of the WHO 2010 Recommendations for Antiretroviral Therapy. AIDS Res Treat. 2011;2011:738271. doi: 10.1155/2011/738271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menzies NA, Berruti AA, Berzon R, et al. The cost of providing comprehensive HIV treatment in PEPFAR-supported programs. AIDS. 2011;25(14):1753–1760. doi: 10.1097/QAD.0b013e3283463eec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bendavid E, Grant P, Talbot A, Owens DK, Zolopa A. Cost-effectiveness of antiretroviral regimens in the World Health Organization's treatment guidelines: a South African analysis. AIDS. 2011;25:211–220. doi: 10.1097/QAD.0b013e328340fdf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badri M, Cleary S, Maartens G, et al. When to initiate highly active antiretroviral therapy in sub-Saharan Africa? A South African cost-effectiveness study. Antivir Ther. 2006;11(1):63–72. [PubMed] [Google Scholar]
- 19.Walensky RP, Wolf L, Wood R, et al. When to start Antiretroviral therapy in resource limited settings. Annals of Internal Medicine. 2009;151:157–166. doi: 10.7326/0003-4819-151-3-200908040-00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bendavid E, Wood R, Katzenstein DA, Bayoumi AM, Owens DK. Expanding antiretroviral options in resource-limited settings--a cost-effectiveness analysis. J Acquir Immune Defic Syndr. 2009;52(1):106–113. doi: 10.1097/QAI.0b013e3181a4f9c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bendavid E, Young SD, Katzenstein DA, Bayoumi AM, Sanders GD, Owens DK. Cost-effectiveness of HIV monitoring strategies in resource-limited settings: a southern African analysis. Archives of internal medicine. 2008;168(17):1910–1918. doi: 10.1001/archinternmed.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweeney S, Obure CD, Maier CB, Greener R, Dehne K, Vassall A. Costs and efficiency of integrating HIV/AIDS services with other health services: a systematic review of evidence and experience. Sex Transm Infect. 2012;88:85–89. doi: 10.1136/sextrans-2011-050199. [DOI] [PubMed] [Google Scholar]
- 23.Legido-Quigley H, Montgomery CM, Khan P, Fakoya A, Getahun H, D GA. Integrating tuberculosis and HIV services in low- and middle- income countries: a systematic review. Montreux, Switzerland: World Health Organization; 2010. [DOI] [PubMed] [Google Scholar]
- 24.Maher D. Re-thinking global health sector efforts for HIV and tuberculosis epidemic control: promoting integration of programme activities within a strengthened health system. BMC Public Health. 2010;10(1):394. doi: 10.1186/1471-2458-10-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loveday M, Zweigenthal V. TB and HIV integration: obstacles and possible solutions to implementation in South Africa. Trop Med Int Health. 2011;16(4):431–438. doi: 10.1111/j.1365-3156.2010.02721.x. [DOI] [PubMed] [Google Scholar]
- 26.Drummond MF, O'Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. 2 ed. Oxford: Oxford Medical Publications: Oxford University Press; 1997. [Google Scholar]
- 27.User Guide - Uniform patent fee schedule for paying patients attending public hospitals 2009. Pretoria, South Africa: 2009. UPFS Tariff committee National Department of Health. [Google Scholar]
- 28.World Health Organisation. Global Tuberculosis Report 2013. Geneva: World Health Organisation; 2013. [Google Scholar]
- 29.Koenig SP, Riviere C, Leger P, et al. The Cost of Antiretroviral Therapy in Haiti. Cost Eff Resour Alloc. 2008 Feb 14;6(3) doi: 10.1186/1478-7547-6-3. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harling G, Wood R. The evolving cost of HIV in South Africa: Changes in Health Care Cost with Duration on Antiretroviral Therapy for Public Sector Patients. J Acquir Immune Defic Syndr. 2007 Jul 3;45(3) doi: 10.1097/QAI.0b013e3180691115. 2007. [DOI] [PubMed] [Google Scholar]
- 31.Leisegang R, Cleary S, Hislop M, et al. Early and Late Direct Costs in a Southern African Antiretroviral Treatment Programme: A Retrospective Cohort Analysis. PLoS Med. 2009 Dec 12;6(12) doi: 10.1371/journal.pmed.1000189. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanc F-X, Sok T, Laureillard D, et al. Earlier versus Later Start of Antiretroviral Therapy in HIV-Infected Adults with Tuberculosis. N Engl J Med. 2011;365(16):1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]


