Abstract
Background
CD4+ T cells, the principal target in acute SIV and HIV infection, are crucial for the establishment and dissemination of HIV infection in mucosal tissues. Studies indicate that α4β7 CD4+ T cells are preferentially infected by HIV in vitro and during acute SIV infection. The integrin α4β7 is thought to promote HIV capture by target cells; however, the role of integrin α4β7 in HIV transmission remains controversial. In this study, we characterized immune phenotypes of human cervical T cells and examined HIV preference in integrin α4β7+ CD4+ T cells. In vitro all-trans retinoic acid differentiated peripheral CD4+ T cells (at-RA differentiated cells) were included as a comparison.
Results
In both peripheral and cervical cells, the majority of HIV p24+ cells were activated CD4+ T cells expressing integrin α4β7. Among infected at-RA differentiated cells, the frequency of CCR5 expression was higher in HIV p24+ cells than in HIV p24- cells; no such difference was observed in cervical cells. Neither the cyclic hexapeptide CWLDVC nor a monoclonal antibody against integrin α4β7 blocked HIV attachment or gp120 binding to target cells regardless of the presence of CD4, indicating that integrin α4β7 did not facilitate HIV capture by target cells.
Conclusion
Integrin α4β7 expression increases HIV susceptibility, but the mechanism is not through promoting HIV binding to target cells.
Introduction
CD4+ T cells are the principal target in acute SIV and HIV infection and are crucial for the establishment and dissemination of HIV infection in mucosal tissues (reviewed in (1)). Although the subject remains controversial, CD4+ T cells expressing high levels of integrin α4β7 (α4β7 CD4+ T cells) are preferentially infected by HIV in vitro (2) and during acute SIV infection (3, 4). In the gut, these cells are predominantly resting CD4+ T cells (CD25-CD69-HLADR-Ki67-) with a central memory phenotype (CD28+CCR7+CD45RA-) (3). Additionally, α4β7 CD4+ T cell subsets include most Th17 CD4+ T cells, and are significantly depleted during acute SIV infection (4). HIV preference for α4β7 CD4+ T cells in vitro has been characterized using all-trans retinoic acid (at RA)-differentiated α4β7 CD4+ T cells (2, 5-7). RA produces by dendritic cells in gut associated lymphoid organs and plays a crucial role in lymphocyte differentiation and homing (8, 9). Integrin α4β7, significantly induced by RA, has been associated with preferential trafficking to the intestine, which is attributed to its interaction with the mucosal addressin cell adhesion molecule-2 (MAdCAM) (8, 10-13). Higher levels of intracellular HIV p24 signal were found in α4β7high CD4+ T cells, which also have high levels of CCR5 and are metabolically active (Ki67+) compared to α4β7low/negative CD4+ T cells (2). Additionally, HIV replication in α4β7high CD4+ T cells was higher than in α4β7low/negative CD4+ T cells (2). These two T cell subsets were enriched by negatively selected using anti-CCR7 (for α4β7high) or anti-CCR5 (for α4β7low/negative) antibodies based on the observation that β7high CD4+ T cells are CCR5 high and CCR7 low.(2). However the markers on HIV p24+ cells in these subsets are not characterized. The role of integrin α4β7 in HIV susceptibility remains controversial as peripheral CCR6+CD4+ T cells, which exhibit a Th17 profile, are highly permissive to HIV infection regardless of their expression of integrin β (6, 14). Additionally, anti-α4β7 integrin antibody cannot block HIV infection by transmitted/founder and chronic subtype C virus (7).
Analysis of cervical cells collected by cytobrush from female sex workers (FSWs) indicate that the majority of Th17 cells express α4β7 as well as CCR5, and that Th17 cervical cells are depleted in HIV+ FSWs compared to HIV- FSWs (15). However, immunological markers of HIV-susceptible cervical CD4+ T cells have not been characterized. Because identification of immunological parameters of target cells in cervical mucosa that are highly susceptible to HIV will likely provide insights contributing to development of preventative agents, we examined the immunological markers for HIV preference in atRA-differentiated peripheral CD4+ T cells and cervical cells. Our results indicate that integrin α4β7 may be an important immunological parameter for HIV preference in activated CD4+ T cells; however, it is unlikely to play a role in facilitating HIV capture.
Materials and methods
Cell isolation
CD4+ T cells were isolated from PBMCs from healthy donors by negative selection using CD4+ T cells isolation kit II from Miltenyi Biotec followed by activation with atRA (10 nM), IL-2 and immobilized anti-CD3 Ab (1 μg/mL) for 5 days to generate atRA differentiated CD4 cells (atRA-CD4 cells). In brief, 6-well plates were coated with 1 μg/mL anti-CD3 Ab in PBS at 1 mL/well for 1h at 37°C. After washing with PBS, CD4+ T cells were seeded at 1×107 cells per well in 2 mL medium supplemented with IL-2 (50 IU/mL) and atRA (10 nM) and were cultured for 5 days. Human B cell line RPMI 8866 was purchased from Sigma-Aldrich.
Cervical tissues without gross pathology were obtained from women with a median age of 47.2 (IQR 45-56) undergoing therapeutic hysterectomy. The protocol including informed consent and human subjects protection received IRB approval from Rutgers-New Jersey Medical School. Cervical cells were isolated from the mucosal epithelium and underlying stroma by collagenase IV digestion according to the protocol of Grivel and Margolis (16). Briefly, the mucosal epithelium and the underlying stroma were separated from the muscular tissue. Cervical tissues were cut into 2 mm3 pieces and digested with collagenase IV at 37°C for 90 min. After passing through a 70 μm cell strainer, the cells were collected by centrifugation and washed with PBS. Isolated cervical cells were cultured overnight at 37°C in a CO2 incubator to allow epithelial cells to attach. Non-adherent cells were collected for FACS analysis and HIV infection assay. The percentages of live cells from tissues following digestion were determined by using the live/dead zombie UV fixable viability kit (BioLegend). Overall, approximately 90-94% of live cells within the cervical CD45+CD3+CD4+ T cell population.
Immunological phenotyping by FACS analysis
Cervical cells or atRA-CD4 cells were first blocked in PBS with 2% FBS and 1% human serum (blocking buffer) for 20 min at 4°C. Cells were stained with fluorochrome-conjugated antibodies against various surface markers (see Supplementary Table 1) in blocking buffer for 30 min at 4°C. Cells were washed twice and then analyzed on a BD LSR II. Results were analyzed with FlowJo (Tree Star, OR).
For atRA-CD4 cells, the expression of integrin α4β7, CD38, CCR5, CCR7, and CD45RA was analyzed on live CD4+ T cells. Isotype-matched control Abs stained samples were included as the negative control for these immunological markers (Supplementary Figure 1). To determine immune phenotypes of cervical cells, the lymphocyte population in cervical cells was identified according to their light-scattering properties on a forward-scatter (FSC) versus side-scatter (SSC) histogram. Leukocytes were further identified by the expression of CD45. CD4+ T cells among leukocytes were gated based on the expression of CD3 and CD4. The expression of integrin α4β7, CD38, CCR5, CCR7, and CD45RA was further analyzed in CD4+ T cells.
HIV-1 infection
Cervical cells or atRA-CD4 cells were infected with CCR5-using virus HIV-1BaL (Advanced Biotechnologies Inc., MD) of HIV-1SF162 for 2 h at a multiplicity of infection (MOI) of 0.05-0.1. After washing off unbound virus and culturing for 5 days, cells were stained for surface markers and then fixed with 2% paraformaldehyde in PBS at room temperature for 20 min. Cells were then permeabilized with BD Cytoperm™ permeabilization buffer and incubated on ice for 10 min. After washing once with Cytoperm buffer, cells were stained with PE-conjugated anti-HIV-1 p24 Ab or isotype matched control Ab at room temperature for 30 min. After washing, the cells were fixed with 2% paraformaldehyde in PBS at room temperature for 20 min. Expression of cell surface markers on HIVp24+ T cells was analyzed by FACS analysis as described above. When AZT or TAK779, an inhibitor for reverse transcriptase or HIV entry, respectively, was included in the assay, cells were pretreated with inhibitors for one hr prior to HIV exposure for 2 h. After washing off unbound virus, inhibitors were added back to the culture. In addition to FACS analysis, HIV production was also monitored by HIV p24 ELISA (Leidos Biomedical Research, Inc) as described previously (17) or HIV p24 AlphaLISA kits (Perkin Elmer).
Flow cytometry binding assay
Monomer gp120 was conjugated with PE by Lighting-Link™ R-PE conjugation kit (Innova Biosciences). PHA-activated PBLs at 2×105 cells per sample were incubated with 20 μM PE-conjugated gp120 monomer at 4°C for 1 h in the presence of binding buffer containing PBS with 100 mM CaCl2 and 1 mM MnCl2. After washing off unbound gp120-PE, PHA-activated PBLs were stained with mouse anti-human CD8 Ab to identify different subsets of T cells (CD4+ and CD8+). Ab against CD4 was not used to avoid any potential competition with gp120 for CD4 binding. Data were acquired with a BD Accuri™ C6 flow cytometer.
Cell adhesion assay
PHA-activated PBMCs were labeled with Vybrant® DiO (Invitrogen, Life Technologies) per manufacturer's protocol. After washing 3 times with warm serum-free RPMI-1640 medium, the labeled cells were re-suspended at 1 × 106 cells/mL in binding buffer (50 mM Tris pH7.5, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2). Nunc-Immuno™ 96-well plates coated with 5 μg/mL VCAM-1 in PBS at 100 μL/well for 30 min at room temperature. Control wells were coated with PBS alone. All wells were blocked with 1% bovine serum albumin (BSA) for 60 min before incubation with DiO-labeled cells (100 μL) at 37°C for 1 h. For cell adhesion inhibition, the cells were first incubated with integrin α4β7 binding peptide or control peptide for 30 min at 37°C, then added to the VCAM-1 coated plate. After binding, unbound cells were removed and the wells were washed 3 times. Cells bound to the bottom of the plate were lysed by addition of 100 μL/well binding buffer containing 1% Triton X-100 and incubated for 20 min at room temperature before reading the fluorescence in each well on a Stratagene MX3005P (Agilent).
Statistical analysis
Differences between data sets were analyzed by two-tailed Student's t test or Wilcoxon signed-rank tests (SAS Version 9.2; SAS Inc, Cary, NC USA). P<0.05 was considered significant.
Results
In atRA-CD4 cells, α4β7+ subsets were activated but were not CCR5 high
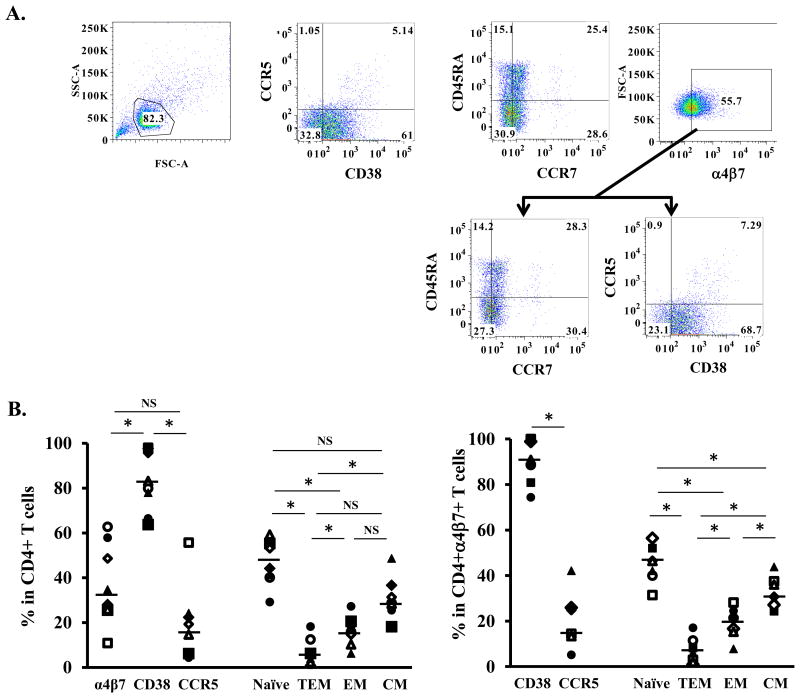
Studies on the susceptibility of human α4β7 CD4+ T cells to HIV have been primarily performed using atRA in vitro differentiated α4β7 CD4+ T cells (2, 5-7). Results have not been consistent. We first examined the immune phenotypes of atRA-CD4 cells by FACS analysis. Based on the gating strategy shown in Fig. 1A, surface expression of integrin α4β7, CD38 (activation marker), CCR5 (HIV co-receptor) and markers representing different CD4+ T cell subsets were analyzed (Fig. 1B, left panel). The majority of atRA-CD4 cells were CD38+ activated cells (median 81.5%, IQR 75.0-96.2%). atRA significantly increased the α4β7+ CD4+ T cell population (median 31.4%, IQR 25.7-50.9%) compared to freshly isolated CD4+ T cells in PBMCs (median 13.1%, IQR 13.0-16.9%) or 17.9% in PBMCs (Supplement Figure 2). Approximately 14.6% of atRA-CD4 cells were CCR5+ (median 14.6%, IQR 5.8-20.8%). Analysis of different CD4+ T cell subsets revealed that 44.3% (median, IQR 40.7-54.2%) of total CD4+ T cells were naïve, 3.9% (median, IQR 1.6-9.3%) were terminal effector memory (TEM) cells, 15.8% (median, IQR 12.4-19.5) were effector memory (EM) cells, and 28.7% (median, IQR 27.0-33.4%) were central memory (CM) cells (Fig. 1B, left panel).
Figure 1. Immunological phenotypes of atRA-differentiated CD4+ cells.
Primary CD4+ T cells were isolated and then differentiated in the presence of atRA and anti-CD3 antibodies as described in Materials and Methods. (A) Gating strategy and analysis of cell surface expression of CD38, CCR5, CCR7, CD45RA, and integrin α4β7. T cell subsets were analyzed based on the following phenotypes: naïve (naïve cells, CD45RA+CCR7+), TEM (terminal effector memory cells, CD45RA+CCR7-), EM (effector memory cells, CD45RA-CCR7-) and CM (central memory cells, CD45RA-CCR7+). (B) Summary of percentages of cells expressing integrin α4β7, CD38 (activation marker), and CCR5 (HIV co-receptor) as well as different T cell subsets among total CD4+ population (left panel) and CD4+α4β7+ population (right panel) from 8 donors. Horizontal lines indicate the medians of the results from 8 donors. * indicates statistically significant difference (p ≤ 0.05, two-tailed Wilcoxon signed-rank test) between compared groups. NS, no significant difference (p>0.05).
Further analysis of the immunological phenotype of the α4β7+ CD4+ population indicated that 90% (median, IQR 86.7-99.0%) of cells were activated, and only 14.4% (median, IQR 13.4-25.4%) of cells expressed CCR5 (Fig. 1B, right penal). The distribution of different CD4+ T cell subsets among α4β7+CD4+ cells was similar to that in total CD4+ T cells: naïve cells 44.3% (median, IQR 38.0-47.9%), TEM 4.6% (median, IQR 1.8-9.0%), EM 19.0% (median, IQR 15.6-22.5), and CM 29.0% (median, IQR 27.0-36.5%) of α4β7+CD4+ T cells (Fig. 1B, right panel).
In atRA-CD4 cells, HIV preferentially infected activated cells expressing α4β7
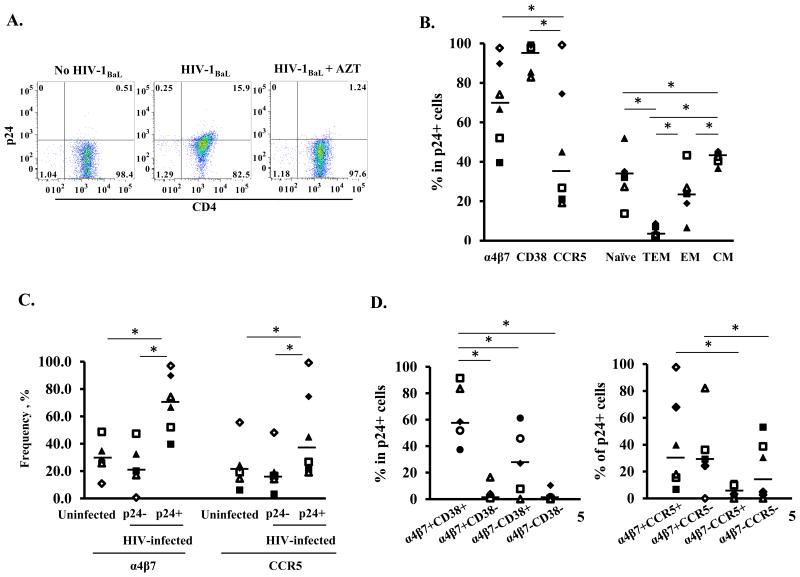
We then determined the immunological phenotypes of atRA-CD4 cells that were preferentially infected by HIV. Depending on donors, 7.8% to 27.1% of total atRA-CD4 cells were HIV p24 positive. The HIV p24 signal was significantly decreased in cells in the presence of a reverse transcriptase inhibitor, AZT (Fig. 2A), indicating that the HIV p24 signal was from productive HIV infection rather than from non-specific virus binding.
Figure 2. Immunological phenotypes of atRA-differentiated CD4+ T cells that are preferentially infected by HIV.
(A) atRA-differentiated CD4+ cells were infected by HIV-1BaL and HIV infection was monitored by FACS analysis with intracellular staining of HIV-1 Gag protein p24 at day 5 after infection. Cells were treated with the reverse transcriptase inhibitor AZT (10 μM) (right panel) to confirm the signal was from de novo synthesis of p24 rather than from HIV capture. (B) Percentage of cells expressing surface markers (integrin α4β7, CD38, CCR5) and of T cell subsets in HIV p24+ cells. (C) The frequency of cells expressing integrin α4β7 or CCR5 in HIV p24+ and HIVp24- populations in HIV-infected cells. Uninfected cells are included as a comparison. * P<0.05, two-tailed Wilcoxon signed-rank test. (D) Among HIV p24+ T cells, summary of analysis of co-expression of α4β7 and CD38 (left panel) and of α4β7 and CCR5 (right panel) from 6 donors. *p<0.05, two-tailed Wilcoxon signed-rank test.
Among 6 donors (Fig 2B), HIV infection of atRA-CD4 cells was primarily found in activated cells (CD38+, median 97.8%, IQR 85.4-99.1%). The percentages of HIV p24+ cells expressing integrin α4β7 and CCR5 varied depending on donors. Overall, 70.4% (median, IQR 55.7-85.8%) of HIV p24+ cells expressed integrin α4β7; however, only 35.8% (median, IQR 22.4-67.1%) were CCR5 positive. At day 5 post-infection, there was no significant difference in the percentages of integrin α4β7 in at-RA-differentiated CD4+ T cells with or without HIV infection (Supplementary Table 2). Analysis of T cell subsets in HIV p24+ cells revealed that 32.1% (median, IQR 27.3-35.1%) were naïve cells and 42.6% (median, IQR 40.4-43.4%) were CM. There were 23.8% EM (median, IQR 18.9-26.8%) and 4.8% TEM (median, IQR 3.3-7.1%) cells within HIV p24+ populations (Fig. 2B).
Interestingly, when the degree of cell surface integrin α4β7 or CCR5 on HIV p24+ cells was compared to that in HIV p24- cells or uninfected cells, higher frequencies of integrin α4β7 or CCR5 were found in HIV p24+ cells. The fraction of α4β7+ cells in uninfected cells was 26.9% (median, IQR 25.6-33.0%), in HIV p24- cells, 19.7% (median, IQR 17.6-29.3%), in HIV p24+ cells, 70.5% (median, IQR 55.7-85.8%). Similarly, the expression level of α4β7+ (mean fluorescence intensity, MFI) in HIV p24+ cells (median 380, IQR 193-636.8) was higher than that in HIV p24- cells (median174, IQR 142.5-213.8). The fraction of CCR5+ cells in uninfected cells was 20.8% (median, IQR 15.8-23.7%), in HIV p24- cells, 16.8% (median, IQR 14.8-18.7%), in HIV p24+ cells, 35.8% (median, IQR 22.4-67.1%). This distribution suggests that HIV preferentially replicated in activated cells or in cells with high levels of integrin α4β7 or CCR5 (Fig. 2C). We further analyzed co-expression of α4β7, CD38, and CCR5 in p24+ populations (Fig. 2D). HIV p24+ cells were found primarily in α4β7+CD38+ cells (median 58.5%, IQR 51.8-83.5%) followed by α4β7-CD38+ cells (median 27.0%, IQR 7.7-45.8%). Less than 3% of HIV p24+ cells were found in CD38- populations regardless of their expression of α4β7 (α4β7+CD38-, median 1.0%, IQR 0.7-4.3%; α4β7-CD38-, median 1.4%, IQR 0.2-1.4%). In the analysis of integrin α4β7 and CCR5 co-expression on HIV p24+ cells, we found that the HIV p24 signal was higher on integrin α4β7+ cells than on α4β7-, cells regardless of the presence of CCR5. In HIV p24+ populations, α4β7+CCR5+ cells were 28.8% (median, IQR 16.0-60.9%); α4β7+CCR5- cells, were 27.4% (median, IQR 24.6-34.4%); α4β7+CCR5- cells were 3.6% (median, IQR 0.1-8.4%), and α4β7-CCR5- cells were 17.6%, IQR 3.2-36.6% (Fig. 2D, right panel).
CCR5+CD4+ T cells are preferentially depleted during HIV and SIV infection, although other studies have demonstrated that SIV infects both CCR5+ and CCR5- CD4+ T cell subsets during acute infection (4, 7, 18-22). We found that the frequency of CCR5 on HIV p24+ cells was moderate compared to integrin α4β7 and CD38. Similar CCR5 staining results were obtained when two different anti-CCR5 antibodies were used (Supplementary Figure 3). Because TAK779, an HIV entry inhibitor targeting CCR5 blocked HIV infection of at-RA CD4+ T cells (Supplementary Figure 4), we concluded that CCR5 was present but the level was below our detection limit in this study.
Immunological phenotypes of α4β7+ cervical CD4+ T cells
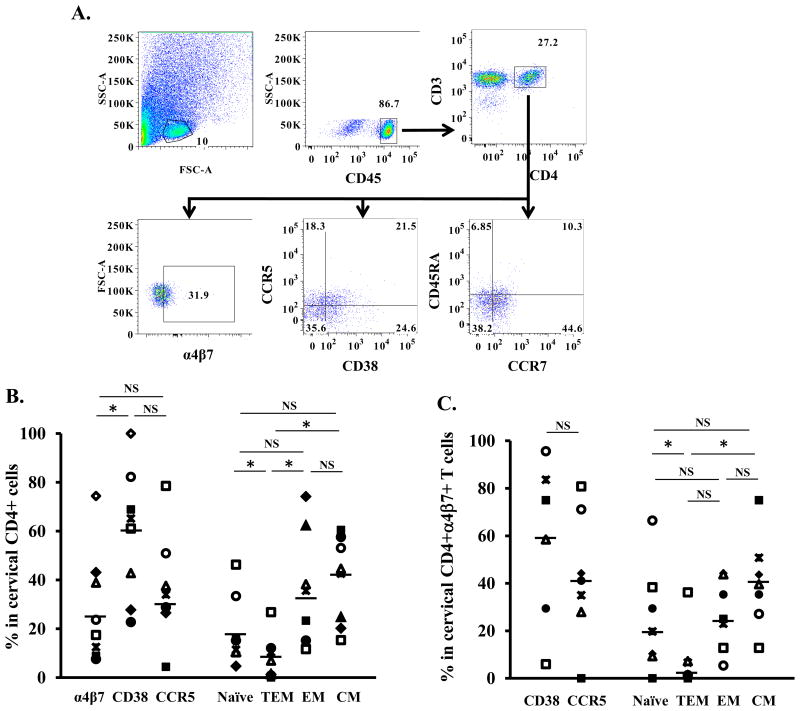
HIV preference in cervical CD4+ T cells expressing integrin α4β7 has not been reported. Analysis of lymphocyte populations in cervical cells (Fig. 3A) revealed that among CD45+CD3+CD4+ T cells, the cell surface levels of integrin α4β7 (median 23.7%, IQR 12.5-38.9%), CD38 (median 65.2%, IQR 42.8-71.2%), and CCR5 (median 34%, IQR 27.7-44.3%) varied by donor (Fig. 3B). Memory cells accounted for the majority of the cervical CD4+ T cell subsets, which comprised 42.6% (median, IQR 23.8-46.7%) CM, 35.3% (median, IQR 20.7-44.3%) EM, and 4.2% (median, IRQ 1.2-9.2%) TEM. Naïve cells represented 12.6% (median, IQR 10.8-20.6%) of the cervical CD4+ T cells (Fig. 3B). Analysis of cells co-expressing α4β7+, CD38 and CCR5 indicated 58.8% (median, IQR 43.9-79.3%) were α4β7+CD38+ and 41.1% (median, IQR 31.5-57.7%) were α4β7+CCR5+. The distribution of T cell subsets among cervical CD4+α4β7+ cells was similar to that in total cervical CD4+ T cells with memory cells as the dominant populations: naïve cells, median 19.7%, IQR 9.8-33.9%; TEM, median 1.9%, IQR 0.7-6.9%; EM, median 25.0%, IQR 17.9-39.6, and CM, median 39.6%, IQR 31.2-47.2% (Fig. 3C).
Figure 3. Immunological phenotypes of cervical CD4+ T cells.
Single-cell suspensions from cervical tissues without gross pathology from women undergoing therapeutic hysterectomy were prepared as described in materials and methods. (A) Gating strategy and FACS analysis of cell surface markers in samples from one donor. The lymphocyte populations were identified according to their light-scattering properties. Leukocytes were further identified by the expression of CD45. CD4+ T cells among leukocytes were gated as CD3+CD4+ cells. Expression of integrin α4β7, CD38, CCR5, CCR7, and CD45RA on the cervical CD4+ T cells was then analyzed. (B) Summary of percentage of CD4+ cervical cells expressing specific markers and T cell subsets from 9 donors. (C). Frequencies of cervical CD4+α4β7+ T cells expressing specific markers and T cell subsets from 9 donors. * p<0.05, statistically significant difference, two-tailed Wilcoxon signed-rank test. NS, no significant difference (p>0.05).
In cervical CD4+ T cells, HIV preferentially infects α4β7+ CD38+ cells
Depending on donors, HIV p24+ populations comprised 4.3% to 36.8% of CD3+CD45+CD4+ cervical cells. Among HIV p24+ cervical CD4+ T cells, 70.1% (median, IQR 41.8-80.7%) expressed integrin α4β7, 72.6% (median, IQR 56.3-95.6%) expressed CD38 activation marker, and 38.2% (median, IQR 21.2-50.6%) expressed CCR5. At day 5 post-infection, there was no significant difference in the percentages of integrin α4β7 in CD45+CD3+CD4+ T cell populations with or without HIV infection (Supplementary Table 2). Memory cells (CD45RA-) comprised 50.0% (median, IQR 26.4-61.6%) of HIV p24+ cells (Fig. 4A). Higher frequencies of integrin α4β7 were found in HIV p24+ cells than HIV p24- cells or uninfected cells. Among uninfected cells, 22.0% (median, IQR 15.9-38.5%) expressed α4β7; in HIV p24- cells, 14.8% (median, IQR 5.8-27.6%) expressed α4β7; in HIV p24+ cells, 74.4% (median, IQR 54.5-81.1%) expressed α4β7. The expression level of integrin α4β7 (MFI) in HIV p24+ cells (median 336.5, IQR 313.3-486.5) was also higher than that in HIV p24- cells (median 240.5, IQR 217-321). Although CD38+CD4 T cells seemed to be preferentially infected by HIV, the difference in the frequency of CD38+ CD4+ T cells between HIV p24+ (median 80.1%, IQR 64.5-96.3%.) and HIV p24- cells (median 64.2%, IQR 50.3-66.9%) was not significant. These results suggest that HIV preferentially replicate in integrin α4β7-expressing cervical CD4+ T cells and possibly in activated cells (Fig. 4B). However, unlike the results obtained in HIV-infected atRA-differentiated cells (Fig. 2C), there was no difference in percentages of CCR5+ cells in HIV p24+ (median 39.3%, IQR 37.2-54.4%) and HIV p24- (median 31.0%, IQR 28.1-37.8%) populations.
Figure 4. Immunological phenotypes of cervical CD4+ T cells that are preferentially infected by HIV.
Cervical cells were exposed to HIV-1BaL for 2 h. After washing off unbound virus, infected cells were cultured for 5 days before FACS analysis. (A) Summary of HIV preference in cervical CD4+ T cells from 7 donors. The p24+ cells were back-gated onto various cell markers including integrin α4β7, CD38, CCR5, and CD45RA. (B) Frequencies of cells expressing integrin α4β7, CD38, or CCR5 in HIV p24+ and HIVp24- populations in HIV-infected cells. Uninfected cells are included as a comparison. *p<0.05, statistically significant difference, two-tailed Wilcoxon-sign rank test; NS, not significant. (C) Frequencies of HIV p24+CD4+ T cells that were co-expressed α4β7 and CD38 (left panel) or of α4β7 and CCCR5 (right panel).
As was found in atRA-differentiated cells, among cervical cells, 73.4% (median, IQR 61.8-91.7%) of α4β7+CD38+ cells expressed HIVp24; whereas, 21.1% (median, IQR 8.3-20.8%) of α4β7-CD38+ cells expressed HIVp24 (Fig. 4C). In contrast to findings in atRA-differentiated cells, the difference in percentage of HIV p24+ cells between α4β7+ and α4β7- in CCR5+ or CCR5- subpopulations was not significant (Fig. 4C, right panel).
Integrin α4β7 does not promote HIV binding to atRA-differentiated CD4+ T cells or cervical cells
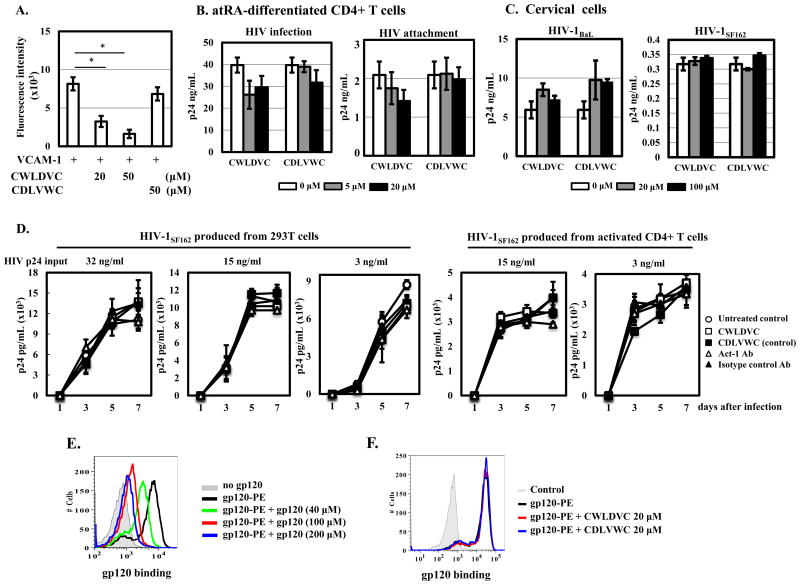
Integrin α4β7 has been suggested to facilitate HIV binding to cells (2). A cyclic hexapeptide Cys-Trp-Leu-Asp-Val-Cys (CWLDVC), which inhibits the binding of integrin α4β7 to its natural ligands, prevents binding of HIV-1 gp120 to α4β7 on atRA-treated CD8+ T cells (5). To assess the role of integrin α4β7 in promoting HIV infection, we first demonstrated that the CWLDVC peptide but not control peptide abolished the binding of integrin α4β7 to VCAM-1 on activated PBMCs (Fig. 5A), which was consistent with previous findings (23). However, in at-RA CD4 cells, the CWLDVC or control peptide did not block HIV infection or HIV attachment (Fig. 5B). The CWLDVC peptide did not block HIV binding to a human B cell line RPMI-8866 that does not have CD4 but expresses integrin α4β7 constitutively (data not shown). Similarly, neither peptide affected attachment of HIV-1BaL or of HIV-1SF162 (which has high α4β7-reactivity) (24), to cervical cells (Fig. 5C).
Figure 5. Integrin α4β7 does not promote HIV binding to atRA-differentiated CD4+ T cells or cervical cells.
(A) Effect of integrin α4β7 binding peptide CWLDVC or control peptide (CDLVWC) on VCAM-1 mediated cell adhesion was determined as described in materials and methods. (B) atRA-differentiated CD4+ cells or (C) cervical cells were pretreated with integrin α4β7 binding peptide or control peptide at the indicated concentrations for 1 h at 37°C prior to HIV exposure for 2 h. For HIV infection, peptides were added back after washing off unbound virus and viral production in the culture media was determined by p24 ELISA at day 7 after infection. For HIV attachment, cells were exposed to HIV for 2 h at 4°C in the presence of peptides. After washing with ice-cold PBS, the cells were pelleted and lysed in PBS with 1% Triton X-100. Cell-associated HIV was determined by p24 ELISA. (D) HIV-1SF162 was produced from either 293T cells by transfection or from PHA-activated CD4+ T cells. PHA-activated CD4+ T cells (1×105 cells per well in a 96-well plate) were pre-treated with integrin α4β7 binding peptide (100 μM), control peptide (100 μM), Act-1 Ab (5 μg/ml) or isotype control Ab (5 μg/ml) for 1 h before exposure to different concentrations of HIV-1SF162 from 293T cells or activated CD4+T cells. After 2 h incubation, cells were washed and peptides or antibodies were added back. HIV replication was monitored at different time points by measuring HIV p24 in media using HIVp24 alphaLISA kits. (E) Binding specificity of HIV gp120 to PHA-activated PBLs. Cells were incubated with gp120-PE (20 μM) at 4°C for 1 h in the presence of unconjugated gp120 at indicated concentrations followed by FACS analysis. (F) Effect of integrin α4β7 binding peptide on HIV gp120 binding to CD4+ T cells. PHA-activated PBLs were incubated with the CWLDVC peptide or the control peptide (CDLVWC) for 1 h at 4°C before addition of HIV gp120-PE at 20 μM in the presence of peptides and bivalent ions (100 mM CaCl2 and 1 mM MnCl2 in PBS) for an additional hour. HIV gp120 biding to target cells was then assessed by FACS analysis. *p<0.05, statistically significant difference, two-tailed Student's t test. Results were representatives of three independent experiments from different donors.
Parrish et al have shown that the integrin α4β7 specific Act-1 Ab promotes infection by HIV reporter virus pseudotyped with HIV-1JR-FL or HIV-1SF162 compared to infected cells without antibody treatment, although it appears to inhibit infection by highly diluted replication competent virus HIV-1SF162 but not HIV-1YU2 or other subtype C strains (7). We assessed the effect of α4β7-binding peptide or Act-1 Ab on HIV infection by different titers of HIV-1SF162 that were prepared from 293T cells (by transfection) or from PHA-activated CD4+ T cells. Neither α4β7-binding peptide nor Act-1 Ab blocked HIV replication regardless of the sources or titrations of viruses (Fig. 5C). Although Act-1 Ab slightly inhibited infection by diluted HIV-1SF162 (3 ng HIV p24 input), the reduction of HIV replication was not significant compared to untreated control or samples with isotype control Ab. Additionally, α4β7-binding peptide or Act-1 Ab had no effect on attachment of diluted HIV-1SF162 (data not shown).
The V2 loop of HIV-1BaL gp120 contains an α4β7-binding motif, LDI, can serve as an alternative minimal epitope for α4 integrin (5). Because HIV-1BaL was used in the infection studies (Fig. 2 and Fig. 4), we assessed the effect of α4β7-binding peptide on the binding of HIV-1BaL gp120 to PHA-activated PBLs that expressed significant levels of α4β7 integrin (Supplementary Figure 5). The binding of gp120-PE to target cells was competed off by non-conjugated gp120, indicating its specificity (Fig. 5E) but not by the CWLDVC peptide (Fig. 5F).
Arthos et al have shown that, in the presence of CD4 blocking mAb, a number of α4-specific and β7-specific Abs including Act-1, a mAb specific for α4β7 heterodimers (10), block the binding of gp120 to atRA-treated PBMCs (5). We examined CD4-independent gp120 binding to α4β7 integrin. A broadly neutralizing antibody VRC01 that binds to the CD4-binding site of gp120 (25) effectively blocked HIV-1 gp120 binding to CD4+ cells in a dose-dependent manner, whereas Act-1 mAb had no effect (Supplementary Figure 6A). Neither the α4β7-binding peptide CWLDVC (Supplementary Figure 6B) nor Act-1 mAb (Supplementary Figure 6C) modulated the effect of VRC01 on gp120 binding to CD4+ cells. Similar results were observed when the mean fluorescence intensity (MFI) was analyzed in these samples (Supplementary Figure 6D). Thus, despite the fact that HIV infection was primarily found in α4β7+CD38+ CD4+ T cells in both atRA-differentiated and cervical cells, integrin α4β7 does not appear to play an active role in facilitating HIV gp120 binding to target cells.
Discussion
In our study, although HIV infection could be found in both integrin α4β7+ and α4β7- cells, the frequency of integrin α4β7+ was higher in HIV p24+ cells than in HIV p24- cells regardless of the source of cells. The majority of HIV p24+ cells co-expressed integrin α4β7 and CD38. We conclude that integrin α4β7 may serve as an immunological marker for HIV preference in activated CD4+ T cell populations.
Our findings did not support the view that integrin α4β7 plays a role in HIV preference by promoting HIV capture (2, 26). In our study, neither α4β7 binding peptide (CWLDVC) nor the Act-1 monoclonal antibody, both of which target integrin α4β7, inhibited HIV infection, attachment or HIV gp120 binding to various cell types expressing integrin α4β7. The α4β7 binding peptide (CWLDVC) does, however, block the binding of PBMCs to VCAM-1. Additionally, the presence of blocking peptides or Act-1 mAb did not affect HIV gp120 binding regardless of the presence of CD4. In agreement with our findings, anti-integrin α4 antibody (natalizumab) does not block infection of atRA-treated PBMCs by several HIV-1 strains, including two with the LDI/V tripeptide binding motif in the V2 region (27). Parrish et al demonstrated Act-1 antibody inhibited replication of SF162 Env molecular clone (NL4-3-SF162) at a very low multiplicity of infection after infection for 6 days, whereas Act-1 antibody promoted SF162 infection in a single-cycle infection (7). Nevertheless, they showed that Act-1 Ab did not inhibit HIV infection by early transmitted/founder virus or chronic subtype C virus (7). Similar results were observed using HIV-1 subtype A viruses, suggesting that integrin α4β7 is unlikely to play a role in facilitating HIV attachment or in selecting specific genotypes (28).
The immunological role of integrin α4β7 in HIV susceptibility has been controversial. In atRA-CD4 cells, the majority of α4β7+ cells (90%) were activated (Fig. 1B, right panel), whereas approximately 58% of α4β7+CD4+ cervical T cells expressed CD38 activation marker (Fig. 1C), indicating that immunological profiles between atRA-CD4 cells and cervical CD4+T cells were different. The frequencies of α4β7+ cells in peripheral and mucosal cells (e.g., approximately 30% in the total CD4+ T cell population) did not predict the frequencies that were infected by HIV. In infection analysis, around 70% of cells were HIV p24+. These results suggest that it is important to measure immunological markers in HIV-infected cells at the viral protein or DNA/RNA levels. Nevertheless, our results demonstrated in activated peripheral and cervical CD4+ T cell populations that the majority of HIV+p24 cells expressed integrin α4β7. Using CD3/CD28-activated CD4+ T cells, Monteiro et al found that cells expressing CCR6, a marker for memory cells, exhibit higher levels of HIV production and of integrated HIV DNAs than CCR6 negative cells regardless of the presence of integrin β7 (6, 14). Interestingly, in that study, in two out of three donors, the highest levels of integrated HIV DNA were found in cells co-expressing CCR6 and integrin β7. Additionally, in vitro Th17-enriched CCR6+ cells, preferentially infected by HIV, expressed higher levels of integrin α4β7, CD4, and CXCR4 but not CCR5 and CCR5 ligands than CCR6- cells (29). Frequencies of Th17 with or without CCR6 in highly HIV susceptible cervical CD4+ T cell subsets co-expressing integrin α4β7 and CD38 remain to be determined.
Monterio et al demonstrated that CCR6 memory CD4+ T cells express high CCR5 and produce Th17 cells; however, integrin β7 is not a marker for IL-17 producing CD4+ T cells (6, 14). Although we did not characterize expression of CCR6 and IL-17 in our study, co-expression of IL-17 and integrin α4β7 has been reported in human cervical CD4+ T cells from female sex workers (15) and in PBMCs from rhesus macaques (4). CD4+ T cells expressing CCR6 or integrin α4β7high express higher levels of CCR5, which have been suggested to contribute to high HIV susceptibility (2, 6). Interestingly, enhancement of HIV permissiveness in memory CCR6+ CD4+ T cells was found when cells were exposed to HIV with VSV envelopes that were independent of CCR5 coreceptor (6), suggesting that CCR5 does not contribute to high susceptibility to HIV infection of CCR6+ T cells through its HIV co-receptor function. We have found inconsistent results using Act-1 Ab to distinguish integrin α4β7high vs integrin α4β7low/negative among different donors. Other studies have used anti-integrin β7 antibody to demonstrate that integrin α4β7high cells are CCR5+; however, the α4β7low cells are mainly CCR5- (2). Both CCR5+ and CCR5- populations have been reported in integrin α4β7high memory CD4+ T cells, and the frequency of integrin α4β7high memory CD4+ T cells, but not the frequency of CCR5+ cells, correlates with susceptibility to rectal SIV infection (30). Interestingly, in atRA-differentiated cells, but not in cervical cells, a higher frequency of CCR5 was found in HIV p24+ cells than in HIV p24- cells. It is possible that cervical cells are more heterogeneous compared to purified at-RA differentiated cells. Our results indicated that cell surface expression of CCR5 could not predict HIV preference despite CCR5-dependence of HIV infection, indicating that FACS analysis may not have sufficient sensitivity to assess HIV preference.
We concluded that HIV preferentially infected activated peripheral and cervical CD4+ T expressing integrin α4β7, which, however, did not play a role in promoting HIV attachment. Future studies on the underlying mechanism of high HIV susceptibility of cells that co-express integrin α4β7 and CD38 may provide insights germane to developing a better strategy for HIV prevention.
Supplementary Material
Acknowledgments
We thank William Honnen and Abraham Pinter for providing HIV gp120 recombinant proteins and for helpful discussions. We also thank Sukhwinder Singh at Rutgers-NJMS Flow Cytometry Core Facility for his technical assistance. This work is supported by NIH grants R01AI081559 (to TLC) and R01AI110372 (to TLC and ZHP).
Sources of Funding: NIH: R01AI081559 and R01AI110372; The authors declare that they have no competing interests.
References
- 1.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011 Feb 18;62:127–39. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 2.Cicala C, Martinelli E, McNally JP, et al. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc Natl Acad Sci U S A. 2009 Dec 8;106(49):20877–82. doi: 10.1073/pnas.0911796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kader M, Bixler S, Roederer M, Veazey R, Mattapallil JJ. CD4 T cell subsets in the mucosa are CD28+Ki-67-HLA-DR-CD69+ but show differential infection based on alpha4beta7 receptor expression during acute SIV infection. J Med Primatol. 2009 Oct;38(Suppl 1):24–31. doi: 10.1111/j.1600-0684.2009.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kader M, Wang X, Piatak M, et al. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009 Sep;2(5):439–49. doi: 10.1038/mi.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arthos J, Cicala C, Martinelli E, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008 Mar;9(3):301–9. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 6.Monteiro P, Gosselin A, Wacleche VS, et al. Memory CCR6+CD4+ T cells are preferential targets for productive HIV type 1 infection regardless of their expression of integrin beta7. J Immunol. 2011 Apr 15;186(8):4618–30. doi: 10.4049/jimmunol.1004151. [DOI] [PubMed] [Google Scholar]
- 7.Parrish NF, Wilen CB, Banks LB, et al. Transmitted/founder and chronic subtype C HIV-1 use CD4 and CCR5 receptors with equal efficiency and are not inhibited by blocking the integrin alpha4beta7. PLoS Pathog. 2012;8(5):e1002686. doi: 10.1371/journal.ppat.1002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004 Oct;21(4):527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Iwata M. The roles of retinoic acid in lymphocyte differentiation. Seminars in immunology. 2009 Feb;21(1):1. doi: 10.1016/j.smim.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Schweighoffer T, Tanaka Y, Tidswell M, et al. Selective expression of integrin alpha 4 beta 7 on a subset of human CD4+ memory T cells with Hallmarks of gut-trophism. J Immunol. 1993 Jul 15;151(2):717–29. [PubMed] [Google Scholar]
- 11.Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazarovits AI, Tidswell M. Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol. 1994 Jul 15;153(2):517–28. [PubMed] [Google Scholar]
- 12.Rott LS, Briskin MJ, Andrew DP, Berg EL, Butcher EC. A fundamental subdivision of circulating lymphocytes defined by adhesion to mucosal addressin cell adhesion molecule-1. Comparison with vascular cell adhesion molecule-1 and correlation with beta 7 integrins and memory differentiation. J Immunol. 1996 May 15;156(10):3727–36. [PubMed] [Google Scholar]
- 13.Agace WW. Tissue-tropic effector T cells: generation and targeting opportunities. Nat Rev Immunol. 2006 Sep;6(9):682–92. doi: 10.1038/nri1869. [DOI] [PubMed] [Google Scholar]
- 14.Gosselin A, Monteiro P, Chomont N, et al. Peripheral blood CCR4+CCR6+ and CXCR3+CCR6+CD4+ T cells are highly permissive to HIV-1 infection. J Immunol. 2010 Feb 1;184(3):1604–16. doi: 10.4049/jimmunol.0903058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKinnon LR, Nyanga B, Chege D, et al. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol. 2011 Dec 1;187(11):6032–42. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- 16.Grivel JC, Margolis L. Use of human tissue explants to study human infectious agents. Nat Protoc. 2009;4(2):256–69. doi: 10.1038/nprot.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tasker C, Ding J, Schmolke M, Rivera-Medina A, Garcia-Sastre A, Chang TL. 17beta-estradiol protects primary macrophages against HIV infection through induction of interferon-alpha. Viral immunology. 2014 May;27(4):140–50. doi: 10.1089/vim.2013.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004 Sep 20;200(6):749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005 Apr 28;434(7037):1093–7. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 20.Krzysiek R, Rudent A, Bouchet-Delbos L, et al. Preferential and persistent depletion of CCR5+ T-helper lymphocytes with nonlymphoid homing potential despite early treatment of primary HIV infection. Blood. 2001 Nov 15;98(10):3169–71. doi: 10.1182/blood.v98.10.3169. [DOI] [PubMed] [Google Scholar]
- 21.Veazey RS, Mansfield KG, Tham IC, et al. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J Virol. 2000 Dec;74(23):11001–7. doi: 10.1128/jvi.74.23.11001-11007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veazey RS, Marx PA, Lackner AA. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. J Infect Dis. 2003 Mar 1;187(5):769–76. doi: 10.1086/368386. [DOI] [PubMed] [Google Scholar]
- 23.Vanderslice P, Ren K, Revelle JK, et al. A cyclic hexapeptide is a potent antagonist of alpha 4 integrins. J Immunol. 1997 Feb 15;158(4):1710–8. [PubMed] [Google Scholar]
- 24.Nawaz F, Cicala C, Van Ryk D, et al. The genotype of early-transmitting HIV gp120s promotes alpha (4) beta(7)-reactivity, revealing alpha (4) beta(7) +/CD4+ T cells as key targets in mucosal transmission. PLoS Pathog. 2011 Feb;7(2):e1001301. doi: 10.1371/journal.ppat.1001301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou T, Georgiev I, Wu X, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010 Aug 13;329(5993):811–7. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cicala C, Arthos J, Fauci AS. HIV-1 envelope, integrins and co-receptor use in mucosal transmission of HIV. Journal of translational medicine. 2011;9(Suppl 1):S2. doi: 10.1186/1479-5876-9-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pauls E, Ballana E, Moncunill G, et al. Evaluation of the anti-HIV activity of natalizumab, an antibody against integrin alpha4. Aids. 2009 Jan 14;23(2):266–8. doi: 10.1097/qad.0b013e328320a7f8. [DOI] [PubMed] [Google Scholar]
- 28.Etemad B, Gonzalez OA, McDonough S, Pena-Cruz V, Sagar M. Early infection HIV-1 envelope V1-V2 genotypes do not enhance binding or replication in cells expressing high levels of alpha4beta7 integrin. J Acquir Immune Defic Syndr. 2013 Nov 1;64(3):249–53. doi: 10.1097/QAI.0b013e3182a06ddd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez Y, Tuen M, Shen G, et al. Preferential HIV infection of CCR6+ Th17 cells is associated with higher levels of virus receptor expression and lack of CCR5 ligands. J Virol. 2013 Oct;87(19):10843–54. doi: 10.1128/JVI.01838-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinelli E, Veglia F, Goode D, et al. The frequency of alpha(4)beta(7)(high) memory CD4(+) T cells correlates with susceptibility to rectal simian immunodeficiency virus infection. J Acquir Immune Defic Syndr. 2013 Dec 1;64(4):325–31. doi: 10.1097/QAI.0b013e31829f6e1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.