Abstract
While advances in therapeutic approaches have resulted in improved survival rates for women diagnosed with breast cancer, subsets of these survivors develop persistent psychoneurological symptoms (fatigue, depression/anxiety, cognitive dysfunction) that compromise their quality of life. The biological basis for these persistent symptoms is unclear, but could reflect the acquisition of soma-wide chromosomal instability following the multiple biological/psychological exposures associated with the diagnosis/treatment of breast cancer. An essential first step toward testing this hypothesis is to determine if these cancer-related exposures are indeed associated with somatic chromosomal instability frequencies. Towards this end, we longitudinally studied 71 women (ages 23-71) with early-stage breast cancer and quantified their somatic chromosomal instability levels using a cytokinesis-blocked micronuclear/cytome assay at 4 timepoints: before chemotherapy (baseline); four weeks after chemotherapy initiation; six months after chemotherapy (at which time some women received radiotherapy); and one year following chemotherapy initiation. Overall, a significant change in instability frequencies was observed over time, with this change differing based on whether the women received radiotherapy (p=0.0052). Also, significantly higher instability values were observed one year after treatment initiation compared to baseline for the women who received: sequential taxotere/doxorubicin/cyclophosphamide (p<0.001) or taxotere/cyclophosphamide (p=0.014). Significant predictive associations for acquired micronuclear/cytome abnormality frequencies were also observed for race (p=0.0052), tumor type [luminal B tumors] (p=0.0053), and perceived stress levels (p=0.0129). The impact of perceived stress on micronuclear/cytome frequencies was detected across all visits, with the highest levels of stress being reported at baseline (p =0.0024). These findings suggest that the cancer-related exposome has an impact on both healthy somatic cells and tumor cells, and may lead to persistent chromosomal instability. In addition, stress was a significant predictor of chromosomal instability; thus, interventions that aim to reduce stress may reduce acquired soma-wide chromosomal instability for cancer survivors.
Introduction
Innovations in treatment approaches for women with early-stage breast cancer, such as the use of taxanes and anthracylines in sequential, standard or dose dense regimens (along with radiotherapy and/or hormonal antagonists), have resulted in improvements in survival rates [1,2]. Unfortunately, these improvements in survival rates have been accompanied by reports from many survivors of the acquisition and persistence of adverse side effects, which include (but are not limited to): anxiety [3], depression [3], fatigue [4], cognitive dysfunction [5], sleep disturbance [6], and pain [7,8], which we categorize as “psychoneurological symptoms” (PNS). For some survivors these side effects are short-term and limited to the treatment phase. However, long-term side effects can persist for years after treatment and result in reduced quality of life for cancer survivors [9–11]. The biological basis underlying the genesis of these long-term adverse side effects is not known, with no clear consensus regarding a biological mediator(s) yet emerging [12–15]. Furthermore, it is not known if these acquired PNS are associated with the treatments, the perceived stress that accompanies a diagnosis of cancer and its treatment, the cancer itself, or a combination of exposures to biological and psychological factors.
Given that the acquired biological alteration(s) leading to the development and persistence of these adverse side effects must provide a means to be “remembered” or retained for months/years, we have hypothesized that these PNS arise, at least in part, from acquired somatic genetic or epigenetic alterations [16]. One such potential genetic alteration is somatic chromosomal instability. Chromosomal instability is defined as “a constitutional gain or loss of whole chromosomes or fractions of chromosomes” [17]. Soma-wide chromosomal instability could result in the development of a clonal population(s) of cells having a genetic imbalance. Furthermore, increased non-clonal chromosomal instability could alter the function of somatic cells through aberrant gene expression. Evidence supporting a contributing role for acquired somatic chromosomal instability in neurological and psychological conditions has been noted in other health conditions similar to those experienced by breast cancer survivors and includes (but is not limited to) studies of people with chronic pain [18–23], age-related cognitive decline [24], mild cognitive dysfunction [25], fatigue [18], and stress exposure [26]. Furthermore, telomere shortening, which has consistently been shown to lead to increased frequencies of chromosomal instability, has been associated with the development of several PNS (reviewed in [27,28]); however, additional studies are warranted to further evaluate these potential biological relationships.
An efficient means for quantifying acquired chromosomal instability associated with exposures is the cytokinesis-block micronucleus (CBMN) assay [29]. Briefly, a micronucleus/micronuclei (MN) is/are a small chromatin-containing structure(s) that is juxtaposed to the main nucleus or daughter binucleates following the completion of mitosis (Fig 1). MN can arise as a result of numerical chromosomal abnormalities (e.g. whole chromosomal lagging or malsegregation at mitosis) or from structural chromosomal abnormalities (e.g. the failure of an acentric fragment or dicentric chromosome to segregate at mitosis)[30]. This relatively high-throughput methodology allows for the assessment of a large numbers of cells, thereby enhancing one’s ability to accurately quantify even low levels of chromosomal instability. In addition, this technique is not readily influenced by technical artifact (e.g., it allows for minimization of in vitro growth selection since only one round of cell division is completed in vitro). Moreover, this method overcomes several of the challenges experienced when scoring exposure-related chromosomal instability using conventional metaphase chromosomal studies, such as artifactual chromosome “loss” due to cell breakage at harvesting or slide making.
Fig 1. Examples of giemsa stained binucleates with MN.
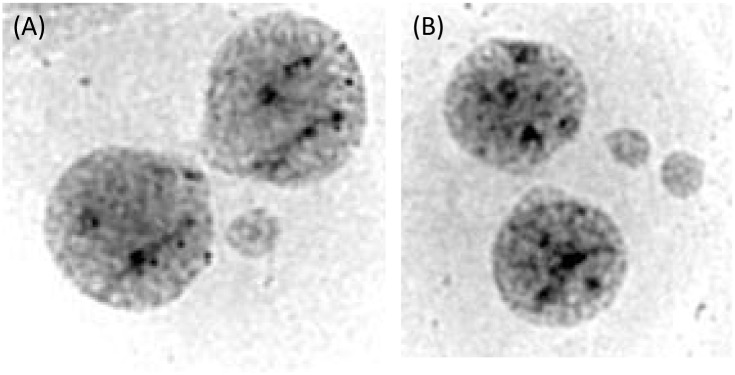
Mitotic cells were blocked at cytokinesis (but did complete karyokinesis) to result in binucleates. In the figure to the left (A) a single micronucleus is present. In the right figure (B) 2 micronuclei have been excluded from the parental cell.
One of the most effective means for assessing the short-term and long-term impact of cancer therapy on normal cells is to longitudinally evaluate patients and compare baseline biological values to those measured during treatment and into recovery. Thus, we initiated a longitudinal study to test the hypothesis that attributes of the tumor cells, exposure to chemotherapy, radiation, and/or perceived stress, lead to an increased frequency of somatic cell chromosomal instability that persists beyond the time of treatment for women diagnosed with breast cancer. Determining if these exposures lead to acquired chromosomal instability is a necessary first step for assessing its potential role in the acquisition/persistence of PNS associated with breast cancer and its treatment.
Materials and Methods
Ethics Statement
Human subjects research was approved by the Virginia Commonwealth University IRB (protocol number HM 13194). Written documentation of informed consent was obtained from all research participants.
Study Participant Ascertainment and Specimen Collection
A total of 77 women with early stage (I to IIIA) breast cancer, who ranged from 23 to 71 years of age, were ascertained through 5 regional cancer centers in Central Virginia. To identify potential study participants, each site had a study coordinator screen patients for eligibility. The eligibility criteria were: (1) an age of 21 years or older; (2) a diagnosis of early stage breast cancer with a scheduled visit to receive chemotherapy; and (3) female gender (males were excluded since too few male participants were available for study). Exclusion criteria were a history of: (1) a previous cancer, or chemotherapy; (2) a diagnosis of dementia; (3) active psychosis; or (4) immune-related diagnoses (e. g. multiple sclerosis; systemic lupus erythematosus). After providing informed consent (VCU IRB #HM 13194), participants were enrolled and the first study visit was scheduled prior to the initiation of chemotherapy. The four time points for evaluation in this longitudinal study were: (1) Visit 1 (baseline), which occurred prior to chemotherapy (but following surgery for the majority of participants); (2) Visit 2, which was scheduled prior to the fourth cycle of chemotherapy; (3) Visit 3, which was scheduled approximately 6 months following the initiation of chemotherapy, at which time a subset of women received radiotherapy; and (4) Visit 4; which was scheduled approximately 1 year following the initiation of chemotherapy. A peripheral blood sample was collected at each visit by venipuncture or through an existing access device and transported to the cytogenetics laboratory. The specimens were coded prior to their delivery to the lab to ensure that the cytogeneticists were unaware of the clinical history or therapy status of each participant at the time of sample processing and evaluation.
Chromosome Instability Methodology
Scoring of Micronuclei, Buds, and Bridges
Chromosomal instability levels were quantified for each specimen using the cytokinesis-block micronucleus (CBMN) and cytome assay [29]. Leukocytes, which were isolated using Histopaque-1077 (Sigma), were established in culture according to standard techniques [31]. Briefly, following their mitogenic stimulation using phytohemaglutinin (PHA), lymphocytes were arrested at cytokinesis by adding cytochalasin B to the cells 44 hours after the cultures were initiated. The cells were harvested 72 hours after culture initiation and slides prepared (2 per specimen) as described previously [32].
Micronuclei, buds, and/or bridges were visualized following giemsa staining (4% Harleco Giemsa solution) and identified according to the criteria established by Fenech [29, 33]. The proportion of abnormalities was calculated by adding the values obtained from two replicate scores (1000 binucleates/mononucleates were evaluated from each of two slides for a total of 2000 binucleates/mononucleates per study participant). The total number of binucleates and mononucleates with abnormalities was then divided by the total number of cells scored to obtain the frequency of chromosomal instability.
Scoring of Nuclear Division Cytotoxicity Index
Given that breast cancer treatments might also contribute to differences in nuclear proliferation/viability, the nuclear division cytotoxicity index (NDCI) was determined using the scoring and calculation criteria of Eastmond and Tucker [34], as adapted by Fenech [31]. Briefly, the NDCI was calculated using the following formula:
where Ap = the number of apoptotic cells; Nec = the number of necrotic cells; M1; M2; M3; and M4 = the number of cells having 1, 2, 3, or 4 nuclei, respectively; and N = total number of cells scored (viable as well as non-viable).
Perceived Stress Assessment
The degree to which individuals perceived their lives to be stressful was measured using the Perceived Stress Scale (PSS; V.9/21/09) [35]. This 10-question self-report measure indicates how often individuals found their lives to be unpredictable, uncontrollable, and overloaded in the past month. Each question has five possible Likert scale responses (never, almost never, sometimes, fairly often, and very often). Of the 10 total responses on the PSS, 6 were “negative” responses and were given the following numerical value: never = 0; almost never = 1; sometimes = 2; fairly often = 3; and very often = 4. The remaining 4 positive items in the PSS were scored in a reversed format according to standard approaches (i.e. never = 4…very often = 0) [36, 37]. All 10 response values were then summed to calculate the participants’ total perceived stress score, with higher scores indicating higher levels of perceived stress [35].
Recognition of Treatments and Breast Tumor Characteristics
Medical records were used to determine the type and duration of the chemotherapy regimens, as well as breast tumor attributes of patients. Medical records also revealed if the participants received radiotherapy in addition to chemotherapy. Based on pathology reports, the tumors were categorized as either: (1) luminal A (human epidermal growth factor receptor 2 [HER2] -; estrogen receptor and/or progesterone receptor +; no information was available about Ki67 values); (2) luminal B (HER2+; estrogen receptor and/or progesterone receptor +; no information was available about Ki67 values); (3) HER2 positive (HER2+; estrogen receptor -; progesterone receptor -); or (4) triple negative (HER2-; estrogen receptor -; progesterone receptor -) [38–42].
Demographic, Lifestyle, and Other Health Information
Demographic information for the study participants was collected by self-report using a questionnaire format. Demographic variables evaluated included age, race, and income. Similarly, lifestyle factors [nutritional practices (eating vegetables/fruit); smoking status, and alcohol consumption], and menopausal status (pre- or post-) were evaluated by self-report. However, medical records were used to determine body mass index.
Statistical analysis
Descriptive statistics of MN/cytome frequencies were computed for the demographic, tumor characteristic, treatments, and stress variables noted above. All data were examined graphically to determine their distributional properties. It was anticipated that the MN/cytome frequencies would follow a log-normal distribution, as is typically seen with MN frequency data [43]. However, because of the relatively high number of MN in this population, the MN frequencies were approximately normally distributed. Demographic, tumor characteristics and treatment variables in Tables 1–3 were compared using chi-square tests for categorical data and a two-sample t-test for continuous data when looking for differences in Race (African American vs. Caucasian).
Table 1. Demographic, health and lifestyle findings in study participants receiving chemotherapy for breast cancer.
| Demographic Variables 1 | African American N = 22 | Caucasian N = 49 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TAC | TC | TCH | AA Total | TAC | TC | TCH | C. Total | Study Total | |
| 10 (45%) | 6 (27%) | 6 (27%) | 22 (31%) | 29 (59%) | 15 (31%) | 5 (10%) | 49 (69%) | 71 (100%) | |
| Income 1 | |||||||||
| Less than $30,000 | 5 (23%) | 4 (18%) | 3 (14%) | 12 (55%) | 6 (12%) | 1 (2%) | 0 | 7 (14%) | 19 (27%) |
| $30,000-$59,999 | 3 (14%) | 2 (9%) | 3 (14%) | 8 (36%) | 6 (12%) | 1 (2%) | 0 | 7 (14%) | 15 (21%) |
| $60,000-$89,999 | 1 (5%) | 0 | 0 | 1 (5%) | 6 (12%) | 7 (14%) | 3 (6%) | 16 (33%) | 17 (24%) |
| $90,000+ | 1 (5%) | 0 | 0 | 1 (5%) | 11 (22%) | 6 (12%) | 2 (4%) | 19 (39%) | 20 (28%) |
| Age 2 | |||||||||
| 44.0 (3.4) | 47.0 (2.5) | 50.5 (2.9) | 46.6 (1.9) | 52.2 (1.7) | 57.1 (2.6) | 50.2 (6.4) | 53.5 (1.5) | 51.3 (1.2) | |
| Menopausal Status 1 | |||||||||
| Pre- or Peri- | 7 (32%) | 3 (14%) | 2 (9%) | 12 (55%) | 11 (22%) | 5 (10%) | 3 (6%) | 19 (39%) | 31 (44%) |
| Post- | 3 (14%) | 3 (14%) | 4 (18%) | 10 (45%) | 18 (37%) | 10 (20%) | 2 (4%) | 30 (61%) | 40 (56%) |
| BMI 2 | |||||||||
| 32.0 (2.7) | 29.8 (2.5) | 35.6 (6.3) | 32.4 (2.1) | 30.1 (1.5) | 26.8 (0.9) | 37.3 (4.7) | 29.8 (1.1) | 30.6 (1.0) | |
| Nutrition 2 , 3 | |||||||||
| 2.6 (0.18) | 2.2 (0.21) | 2.4 (0.25) | 2.4 (0.12) | 2.7 (0.11) | 2.9 (0.13) | 2.7 (0.30) | 2.8 (0.08) | 2.7 (0.07) | |
| Smoking Status 1 | |||||||||
| Yes | 2 (9%) | 3 (14%) | 2 (9%) | 7 (32%) | 4 (8%) | 2 (4%) | 1 (2%) | 7 (12%) | 14 (20%) |
| No | 8 (36%) | 3 (14%) | 4 (18%) | 15 (68%) | 25 (51%) | 13 (27%) | 4 (8%) | 42 (88%) | 57 (80%) |
| Ethanol Use 1 | |||||||||
| Yes | 3 (14%) | 1 (5%) | 2 (9%) | 6 (27%) | 19 (39%) | 11 (22%) | 4 (8%) | 34 (69%) | 40 (56%) |
| No | 7 (32%) | 5 (23%) | 4 (18%) | 16 (73%) | 10 (20%) | 4 (8%) | 1 (2%) | 15 (31%) | 31 (44%) |
1Frequency (percentage of study participants for the category)
2Mean (standard error)
3Nutritional assessments for the participants’ intake of fruits and vegetables
Table 3. Study participant treatments.
| African American N = 22 | Caucasian N = 49 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TAC | TC | TCH | AA Total | TAC | TC | TCH | C. Total | Study Total | |
| Treatment 1 | 10 (45%) | 6 (27%) | 6 (27%) | 22 (%) | 29 (59%) | 15 (31%) | 5 (10%) | 49 (%) | 71 (100%) |
| Surgery 1 | |||||||||
| Biopsy | 3 (14%) | 0 | 0 | 3 (14%) | 2 (4%) | 0 | 0 | 2 (4%) | 5 (7%) |
| Lumpectomy | 4 (18%) | 0 | 1 (5%) | 5 (23%) | 8 (16%) | 6 (12%) | 1 (2%) | 15 (31%) | 20 (28%) |
| Segmental | 1 (5%) | 5 (23%) | 3 (14%) | 9 (41%) | 1 (2%) | 5 (10%) | 0 | 6 (13%) | 15 (23%) |
| Simple | 2 (9%) | 1 (5%) | 2 (9%) | 5 (23%) | 18 (37%) | 3 (6%) | 4 (8%) | 25 (52%) | 30 (42%) |
| Neoadjuvant 1 | |||||||||
| Yes | 3 (14%) | 0 | 1 (5%) | 4 (18%) | 2 (4%) | 1 (2%) | 0 | 3 (6%) | 7 (10%) |
| No | 7 (32%) | 6 (27%) | 5 (23%) | 18 (82%) | 27 (55%) | 14 (29%) | 5 (10%) | 46 (94%) | 64 (90%) |
| Radiotherapy 1 | |||||||||
| Yes | 9 (41%) | 5 (23%) | 5 (23%) | 19 (86%) | 23 (47%) | 12 (24%) | 1 (2%) | 36 (73%) | 55 (77%) |
| No | 1 (5%) | 1 (5%) | 1 (5%) | 3 (14%) | 6 (12%) | 3 (6%) | 4 (8%) | 13 (27%) | 16 (23%) |
1Frequency (percentage of study participants for the category)
Using the model building approach proposed by Hosmer and Lemeshow [44], a mixed effects linear model [45] was fit to determine the best subset of predictors of MN/cytome frequencies. In the first stage of the model building process, a base model was selected that represented the design of the data collection and of the timing of the treatments (chemotherapy and radiation). Fixed effects included visit (baseline, mid-chemo, six months and 1 year post-chemo), chemotherapy regimen, visit by chemotherapy interaction (chemotherapy was administered only during visit 2), radiation (Yes/No); visit by radiation interaction (radiation was only administered proximal to visit 3), and a random effect for study participant. In the second stage, each potential predictor was fit individually with the base model and, if the p-value was 0.25 or less, that predictor was used in the next stage. Potential predictors included demographic variables (age, race, BMI, income, menopausal status, alcohol consumption, and lifestyle nutrition), tumor characteristic variables (grade, stage. luminal A, luminal B, triple negative & HER 2 positive status), surgery, and PSS. In the third stage, all potential regressors (p≤0.25) were put into a multiple variable model. This initial model was further refined by sequentially removing variables from the model with the highest p-values (backward stepwise) until all remaining factors had a p-value of 0.05 or less. At this stage, all pairwise interactions were added. Again using a backward stepwise approach, all interaction terms with p-values greater than 0.05 were removed. This model was considered the final prediction model. The SAS v9.4 and JMP v11.1 statistical packages were used for these analyses (SAS 9.3 and JMP 11.1: SAS Institute Inc., SAS Campus Drive, Cary, North Carolina 27513).
Results
A total of 77 women were recruited for this study, with only 3 women (all Caucasians; ages 48, 62, and 66) failing to complete the multiple visits required for this longitudinal investigation, which resulted in a 96% retention rate. Two of the 3 women who did not complete the study elected to withdraw due to feeling “overwhelmed”. The third woman, who developed osteomyelitis after visit 2, no longer met the eligibility criteria and thus was excluded from the study. Data and specimen collections were completed for visits 1 (baseline) and 2 (mid-chemo) for all 74 retained study participants. However, MN/cytome abnormality frequencies were not successful at baseline or visit 2 for one woman, reducing the sample size to 73 participants. At the time of data analysis in this ongoing study, 64 of the 73 participants had completed visits 1, 2, and 3 (visit 3 occurs 6 months post the initiation of chemo), and 50 women had completed visits 1, 2, 3 and 4 (visit 4 occurs 1 year after chemo initiation). MN/cytome abnormality frequencies were successfully obtained for all visit 3 and 4 specimens.
Chemotherapy and Radiation Treatments
Three primary types of chemotherapy regimens were administered to the study participants. These were categorized as: (1) TAC, which included women who received sequential administration of doxorubicin (Adriamycin), cyclophosphamide (Cytoxan), and docetaxel (Taxotere); (2) TC, which included women who received docetaxel (Taxotere) and cyclophsophamide (Cytoxan); or (3) TCH, which included women who received docetaxel (Taxotere), carboplatin (Paraplatin), and trastuzumab (Herceptin). Of the 73 women fully participating in this study, the majority (n = 39) of participants received a TAC chemotherapy regimen. A total of 21 participants received TC treatment, and 11 women received TCH treatment. Two study participants received a cyclophosphamide, methotrexate and 5-fluorouracil treatment. Given the small number of women receiving this latter treatment, these two participants were excluded from the statistical analysis. Thus, comparisons of exposure factors influencing peripheral blood cell chromosomal instability frequencies were completed for a total of 71 women.
Of these 71 women, a total of 55 participants received radiotherapy (77%), including 32 of the 39 women assigned to the TAC regimen, 17 of the 21 assigned to the TC regimen, and 6 of the 11 women assigned to the TCH regimen.
Demographic and health information
Demographic data for the 71 participants receiving the most common types of treatment regimens is shown in Table 1. These participants included 22 African American and 49 Caucasian women. Two women (1 in the African American cohort and 1 in the Caucasian cohort) self-reported that they were of Hispanic ethnicity. However, given the small number of women having a Hispanic heritage, this sub-group was not analyzed separately.
The average age of the study participants was 51.3 years, with a significant difference in age being observed between the African American (mean = 46.6 years, s.e. = 1.9 years) and Caucasian (mean = 53.5 years, s.e. = 1.5 years) sub-groups (p = 0.0079) (Table 1). Annualized income levels also varied between the racial groups, with the Caucasian patients having significantly higher incomes than the African American women (p<0.0001). As expected, the majority of women (56%) were post-menopausal with 44% being either pre- or peri-menopausal (Table 1). No significant difference in menopausal status was detected between the African American and Caucasian study participants, but (as expected due to the younger age of the African American women) a trend was observed toward more pre- or peri-menopausal women in the African American cohort (p = 0.2163). Comparisons of lifestyle/exposure histories showed that significantly more Caucasian women reported consuming alcohol [69% (34/49)] when compared to African American women [27% (6/22)](p = 0.0016)(Table 1). None of the 14 women who reported smoking met the criteria of a heavy smoker (≥ 30 cigarettes per day), as suggested by Fenech et al. [46]. Thus, smoking was not included in the stepwise prediction model.
Tumor Characteristics
Due to the inclusion criteria established for this study, all of the study participants had early stage breast cancer (stages I to IIIA). The participants’ tumor characteristics and their pre-chemotherapy treatments are summarized in Tables 2 and 3, respectively. While the proportion of African American women having grade 3 tumors (41%) tended to be lower than the proportion for Caucasian women (59%), the grade distributions of the tumors were not significantly different between races (p = 0.153). Similarly, while no significant difference in the overall proportion of tumors subtypes was observed between the racial sub-groups (p = 0.5698), there was a trend toward a higher proportion of triple negative or HER2+ tumors and a lower proportion of luminal A tumors in the African American women (36% triple negative tumors; 18% HER2+; 36% luminal A tumors) compared to the Caucasian women (24% triple negative tumors; 4% HER2+; 61% luminal A tumors; (Table 2). A significant difference in the surgical approaches used for African American compared to Caucasian women was observed (p = 0.0135), but no differences in adjuvant/neoadjuvant status (p = 0.1305), chemotherapy (chemotherapy p = 0.2011) or radiotherapy (p = 0.213) regimens were observed between the racial groups (Table 3).
Table 2. Study participants’ tumor characteristics.
| African American N = 22 | Caucasian N = 49 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TAC | TC | TCH | AA Total | TAC | TC | TCH | C. Total | Study Total | |
| Tumor Characteristic 1 | 10 (46%) | 6 (27%) | 6 (27%) | 22 (31%) | 29 (59%) | 15 (31%) | 5 (10%) | 49 (69%) | 71 (100%) |
| Luminal A 1 | |||||||||
| Yes | 6 (27%) | 2 (9%) | 0 | 8 (36%) | 21 (43%) | 9 (18%) | 0 | 30 (61%) | 38 (54%) |
| No | 4 (18%) | 4 (18%) | 6 (27%) | 14 (64%) | 8 (16%) | 6 (12%) | 5 (10%) | 19 (39%) | 33 (46%) |
| Luminal B 1 | |||||||||
| Yes | 0 | 0 | 2 (9%) | 2 (9%) | 2 (4%) | 0 | 3 (6%) | 5 (10%) | 7 (10%) |
| No | 10 (45%) | 6 (27%) | 4 (18%) | 20 (91%) | 27 (55%) | 15 (31%) | 2 (4%) | 44 (90%) | 64 (90%) |
| Triple negative 1 | |||||||||
| Yes | 3 (14%) | 4 (18%) | 1 (5) | 8 (36%) | 6 (1%2) | 6 (12%) | 0 | 12 (24%) | 20 (28%) |
| No | 7 (32%) | 2 (9%) | 5 (23%) | 14 (64%) | 23 (47%) | 9 (18%) | 5 (10%) | 37 (76%) | 51 (72%) |
| HER2+, ER- & PR- 1 | |||||||||
| Yes | 1 (5%) | 0 | 3 (14%) | 4 (18%) | 0 | 0 | 2 (4) | 2 (4%) | 6 (8%) |
| No | 9 (41%) | 6 (27%) | 3 (14%) | 18 (82%) | 29 (59%) | 15 (31%) | 3 (6%) | 47 (96%) | 65 (92%) |
| Grade 1 | |||||||||
| 1 | 1 (5%) | 0 | 0 | 1 (5%) | 2 (4%) | 2 (4%) | 0 | 4 (8%) | 5 (7%) |
| 2 | 5 (23%) | 3 (14%) | 4 (18%) | 12 (55%) | 12 (25%) | 4 (8%) | 0 | 16 (33%) | 28 (39%) |
| 3 | 4 (18%) | 3 (14%) | 2 (9%) | 9 (41%) | 15 (31%) | 9 (18%) | 5 (10%) | 29 (59%) | 38 (54%) |
| Stage 1 | |||||||||
| I | 1 (5%) | 4 (18%) | 0 | 5 (23%) | 5 (10%) | 8 (16%) | 2 (4%) | 15 (31%) | 20 (28%) |
| IIA | 6 (27%) | 2 (9%) | 3 (14%) | 11 (50%) | 11 (22%) | 6 (12%) | 1 (2%) | 18 (37%) | 29 (41%) |
| IIB | 3 (14%) | 0 | 3 (14%) | 6 (27%) | 5 (10%) | 1 (2%) | 2 (4%) | 8 (16%) | 14 (20%) |
| IIIA | 0 | 0 | 0 | 0 | 8 (16%) | 0 | 0 | 8 (16%) | 8 (11%) |
1Frequency (percentage of study participants for the category)
Perceived Stress Scores (PSS)
An assessment of total PSS showed a higher value at baseline for the total group of study participants (pooled across all women) (Table 4) (p = 0.0024). The proportion of women reporting “high/severe” total PSS values (as defined in the DASS21-stress subscale; [47] ranged from 20% at baseline to 8% 1 year following the initiation of chemotherapy (Table 4). A total of 6 women (2 African American women and 4 Caucasian women) reported high perceived stress levels across more than one visit (range of 2 to 4 visits), with the remaining high PSS values being associated with a single time point (either visit 1, 2, 3, or 4).
Table 4. Perceived Stress Levels Reported by Women Treated for Breast Cancer.
| Total Perceived Stress (PSS-10) 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal (0–15) | Mild to Moderate (16–22) | High/Severe (23–40) | |||||||
| Visit | N 2 | Mean | Std Err | N 2 | Mean | Std Err | N 2 | Mean | Std Err |
| 1 | 32 | 10.06 | 0.74 | 25 | 19.00 | 0.44 | 14 | 28.43 | 1.46 |
| 2 | 38 | 8.84 | 0.65 | 21 | 18.56 | 0.43 | 11 | 26.55 | 0.72 |
| 3 | 38 | 8.82 | 0.72 | 18 | 19.22 | 0.50 | 8 | 28.00 | 1.51 |
| 4 | 30 | 8.23 | 0.94 | 15 | 18.20 | 0.45 | 4 | 26.25 | 1.80 |
1The DASS21 descriptors/PSS-10 correlate values are based on Andreou, et al., 2011.
2N = Number of women providing values for this PSS-10 score category. PSS-10 values were not available for one women at visit 2 (case 2076) and one woman at visit 4 (case 2005).
NDCI values
To determine if there might be differential levels of cellular proliferation/viability following exposure to the diagnosis and treatment of breast cancer, NDCI values were evaluated (Table 5). No significant differences in NDCI values were observed with visits, chemotherapy or radiotherapy.
Table 5. Nuclear division cytotoxicity index (NDCI) values in lymphocytes.
| Visit | Chemotherapy Regimen | |||||
|---|---|---|---|---|---|---|
| TAC | TC | TCH | ||||
| Mean | Std Err | Mean | Std Err | Mean | Std Err | |
| 1 | 1.89 | 0.05 | 1.88 | 0.09 | 1.88 | 0.08 |
| 2 | 1.93 | 0.05 | 1.94 | 0.07 | 1.83 | 0.07 |
| 3 | 2.03 | 0.05 | 2.01 | 0.12 | 1.84 | 0.17 |
| 4 | 1.86 | 0.06 | 1.87 | 0.03 | 1.87 | 0.03 |
Model Fitting to Identify Factors Predictive of MN/Cytome frequencies
To determine if any of the treatment, health, stress, or demographic variables were associated with MN/cytome abnormality frequencies, the data were fit to a mixed effects linear model. In the second stage, each potential predictor was fit individually with the base model and, if the p-value was 0.25 or less, that predictor was used in the next stage. Potential predictors fit included: age (p = 0.9599), race (p = 0.0652), BMI (p = 0.2719), income (p = 0.3728), menopausal status (p = 0.4964), alcohol consumption (p = 0.0816), lifestyle nutrition (p = 0.3062), grade (p = 0.7563), stage (p = 0.2981), luminal A (p = 0.9807), luminal B (p = 0.0594), triple negative (p = 0.1792), HER 2 positive status (p = 0.6460), surgery (p = 0.3042), and Perceived Stress Scale (PSS)(p = 0.1088).
The final model is reported in Table 6 and showed significant effects attributable to a subset of items in the base model, as well as effects for race (p = 0.0061), luminal B tumors (p = 0.0053) and PSS (p = 0.0129). When assessing the effect of chemotherapies on somatic chromosomal instability levels, at the mid-chemo time point (scheduled prior to the fourth treatment [visit 2]) the women who received TAC chemotherapy showed the greatest increase in MN/cytome abnormality frequencies when compared to baseline values (p = 0.0329) (Fig 2). Also, the mean MN/cytome abnormality frequency was noted to be significantly lower at baseline than at visit 4 for the women who received TAC (p<0.001) or TC (p = 0.014) regimens, but not the TCH treatment (p = 0.0884). An impact of radiotherapy on MN/cytome abnormality frequencies was demonstrated by a significant increase in chromosomal instability levels one year post the initiation of chemo [visit 4] compared to baseline [visit 1] in the women receiving radiotherapy (p<0.0001)(Fig 3). Overall, a significant change in chromosomal instability frequencies was observed over time, with this change differing based on whether the women received radiotherapy (Radiation by Visit; p = 0.0052)(Table 6).
Table 6. Mixed effects linear model fitting assessment of predictive associations of variables with peripheral blood micronuclei/cytome abnormality frequencies 1 , 2 .
| Variable | NDF | DFD | F Ratio | p-value |
|---|---|---|---|---|
| Visit | 3 | 175.1 | 12.7033 | <.0001 |
| Chemo | 2 | 63.58 | 2.1494 | 0.1249 |
| Radiation | 1 | 65.05 | 7.3550 | 0.0085 |
| Chemo by Visit | 6 | 174.1 | 0.9863 | 0.4361 |
| Radiation by Visit | 3 | 175.7 | 4.3921 | 0.0052 |
| Race | 1 | 61.48 | 8.0619 | 0.0061 |
| Luminal B | 1 | 67.08 | 8.3236 | 0.0053 |
| PSS | 1 | 145.6 | 6.3448 | 0.0129 |
1The base model, which was determined by the study design, was: MN frequency = Visit + Chemotherapy (3 types) + Radiation therapy + Visit by Chemotherapy* + Visit by Radiation therapy* with the study subject being a random effect
2Variables having predictive p values greater than 0.25 in the initial stepwise model included: Age (p = 0.9599); BMI (p = 0.2719); Income (p = 0.3728); Menopausal status (p = 0.4964); Alcohol consumption (p = 0.0816); Tumor grade (p = 0.7563); Tumor stage (p = 0.2981); Luminal A (p = 0.9807); HER2 positive (p = 0.6360); and Surgery (p = 0.3092)
*The visit by chemo and visit by radiation interaction variables allowed these values to differ across time points.
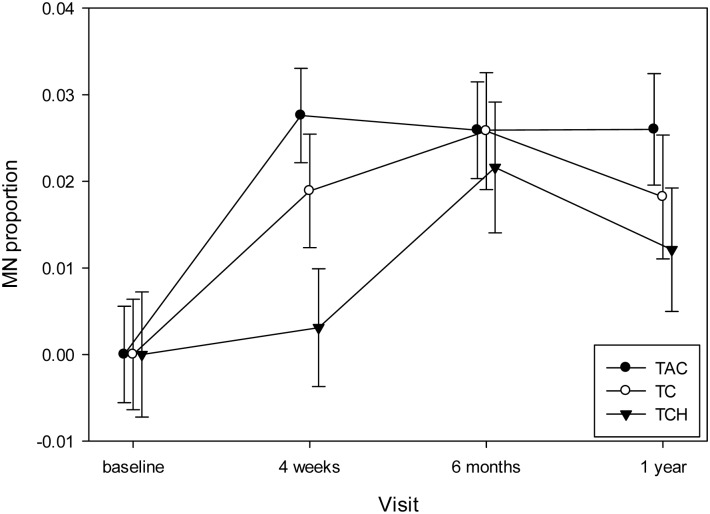
Fig 2. Changes in MN/cytome abnormality frequencies over the 1 year follow-up period based on the women’s chemotherapy regimen.
When compared to baseline values, the frequencies of acquired chromosomal instability values were significantly higher for at least 1-year following the initiation of treatment (visit 4) for the women who received the TAC (p<0.0001) or TC (p = 0.014) regimens, but not the TCH treatment (p = 0.0884). TAC = black circles; TC = white circles; TCH = black triangles.
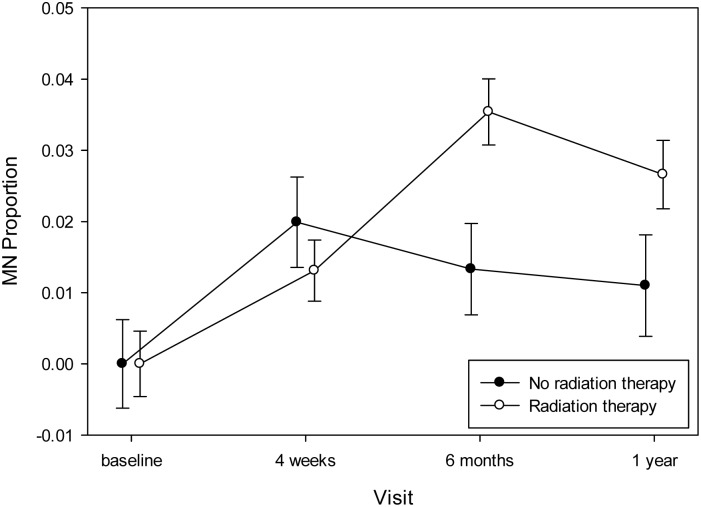
Fig 3. Changes in MN/cytome abnormality frequencies associated with radiotherapy.
At the baseline and 4 weeks (mid-chemo) time points, no significant differences in the chromosomal instability frequencies were observed between the women who did (white circles) or did not (black circles) receive radiation therapy. However, a statistically significantly increase in MN/cytome abnormality frequencies was observed at six months (compared to baseline) for the group of women receiving radiotherapy versus the group not receiving radiation (see Table 6). Also, for the subset of women who received radiotherapy, this increase in chromosomal instability values persisted at the 1 year time point (p<0.0001).
Acquired somatic cell chromosomal instability was also observed to be significantly impacted by factors other than those directly attributable to therapy. The influence of perceived stress on MN/cytome frequencies was detected for the total data set (across all visits), and was not differentially associated with a specific diagnostic, therapeutic or recovery time point in this longitudinal study (Fig 4). Tumor characteristics (specifically luminal B tumors) were also shown to be predictive of MN/cytome abnormality frequencies. Given that the tumor characteristics determine the type of therapy regimen received by the study subjects, it is feasible that this predictive relationship is confounded by the impact of chemotherapy and/or radiation. However, the retention of a consistent significant association for tumor characteristic variables and MN/cytome abnormality frequencies, following the stepwise removal of the potentially related therapy variables, suggest the presence of a separate effect [distinct from the effect(s) related to therapies] for the tumor sub-types on peripheral blood MN/cytome abnormality frequencies. Lastly, race was also observed to be predictive of chromosomal instability frequencies (p = 0.0061). Specifically, MN/cytome values were significantly lower in the African American participants (mean = 0.059; SE = 0.0043) compared to the Caucasian participants (mean = 0.071; SE = 0.0031). One might conjecture that this association is confounded by other influences (e. g. age differences between the racial sub-groups; tumor attribute variation between races). However, as noted above, the consistent recognition of an association between MN/cytome abnormality frequencies and race following the stepwise removal of other potential confounding variables (including age and tumor characteristics) supports the presence of a separate, differential impact of race on chromosomal instability frequencies following treatment for breast cancer.
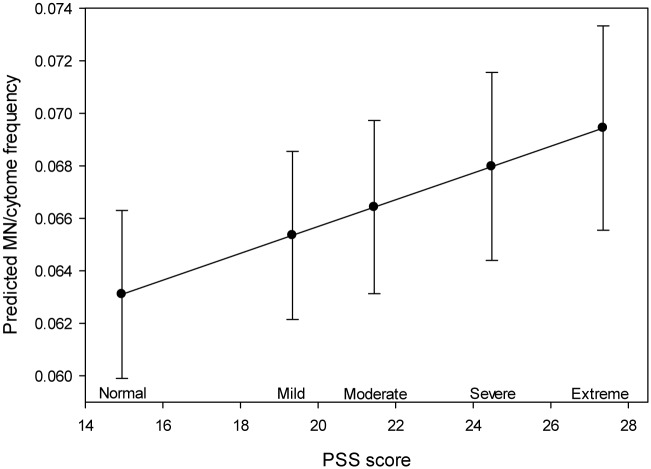
Fig 4. Increased MN/cytome abnormality frequencies were significantly associated with higher levels of perceived stress.
The results of the linear mixed effects model are illustrated in this figure, which shows the increasing trend line of the MN/cytome frequency with increasing PSS (p = 0.0129). The PSS categories proposed by Andreou, et al., 2011 are shown on the X axis (a subset of PSS range); the MN/cytome frequencies (predicted values from linear mixed effects model) are shown on the Y axis.
Discussion and Conclusions
By evaluating MN/cytome frequencies using a longitudinal study design we identified exposure-related, tumor-related, and intrinsic factors that were predictive of acquired chromosomal instability levels in lymphocytes. These predictive factors included: (1) the time interval during the course of diagnosis, treatment, and recovery (e.g. visit); (2) the type of chemotherapy received; (3) whether radiotherapy was received; (4) the woman’s perceived stress level; (5) tumor attributes; and (6) race.
Visit
The MN/cytome frequencies were lowest at baseline values, with the values observed 1 year after the initiation of therapy being higher than baseline values. The highest frequencies of chromosomal instability were observed for visits 2 and 3, which coincide with the mid-cycle of chemotherapy administration (100% of women) and radiotherapy administration (77.5% of women), respectively. This visit-related observation is not surprising given that both of these types of treatment have been shown to be genotoxic and/or have the potential to induce DNA/chromosomal damage [48].
Chemotherapy
The lymphocytes from most women showed increases in chromosomal instability during the administration of chemotherapy. Each of the three chemotherapeutic regimens administered to the study participants included the drug docetaxel (Taxotere). Given that this drug causes microtubule depolymerization, one could anticipate that it might contribute to the acquisition of chromosomal abnormalities, especially aneuploidy [49]. Of the three chemotherapeutic regimens administered to women in this study, the frequency of MN/cytome abnormalities was highest for the TAC regimens at the mid-cycle time (visit 2). Doxorubicin is the likely contributory factor to this observed effect since this is the only drug that differs between the TC and TAC regimens. Doxorubicin is a member of the anthracyclin class of compounds. Although this drug is widely used, the mechanisms for its effectiveness in preventing/reducing cancer cell proliferation are not fully known, with some investigators proposing that it induces cell apoptosis or senescence by inhibiting topoisomerase II, while others cite roles for DNA adduct formation, oxidative stress, ceramide overproduction and/or torsion-induced nucleosome destabilization as the causal means for this drug’s efficacy [50–53]. Although the mechanisms of action for doxyrubicin remain controversial, this drug has consistently been shown to induce chromosomal instability (Table 7), with telomere dysfunction also being associated with this increase in acquired chromosomal abnormality frequencies [54–56]. Thus, the results of our longitudinal in vivo study are in agreement with the in vitro findings of several other investigators and the in vivo results of the few other groups reporting frequencies following treatment in humans (Table 7) [57–67].
Table 7. Summary of previous findings related to cancer/cancer treatment in which MN frequencies were evaluated in peripheral blood.
| Topic | References |
|---|---|
| Breast Cancer Effects | |
| Higher MN frequencies at baseline for women with breast cancer prior to treatment irrespective of smoking and aging | Santos et al., 2010 [57] |
| Significant differences in MN frequencies at baseline between patients with breast cancer and controls | Cardinale et al. 2012 [58] |
| Chemotherapy Effects: | |
| In vitro study showing variation in response to doxyrubicin-induced MN between lymphocyte cell lines; Polymorphism in the GSTP1 gene was associated with an increased MN frequency | Ramos et al., 2011 [59] |
| In vitro study showing a significant increase in MN frequencies in breast cancer cell lines following exposure to doxyrubicin; use of both methoxyamine and doxorubicin significantly increased levels of MN frequencies (combination higher than single exposure values) | Guerreiro et al., 2013 [60] |
| In vivo study showing MN frequencies in lymphocytes and buccal mucosa cells from children with malignant tumors (various types) increased significantly following chemotherapy | Minicucci et al., 2008 [61] |
| In vivo longitudinal study (1 year) showing MN frequencies increased significantly after one cycle of chemotherapy and remained increased for 2 months after the completion of chemotherapy (Cisplatin); MN frequencies also correlated with the cumulative dose of Cisplatin and with Cisplatin-induced nephrotoxicity | Elsendoorn et al., 2001 [62] |
| Radiation Effects: | |
| In vivo study showing MN frequencies increased significantly during radiation and further increased immediately following the completion of radiation; MN frequencies not influenced by age. | Jagetia et al., 2001 [63] |
| Higher MN frequencies during radiotherapy; while decreases in frequency were observed 6 months and one year after radiotherapy, MN frequencies did not return to pre-therapy levels for the majority of patients; older subjects (ages 75–80; also had more advanced tumors) showed higher MN frequencies than younger subjects (ages 57–70). | Gamulin et al., 2010 [64] |
| In vivo studies showing radiation-induced MN frequencies were correlated with dose and quality of occupational, medical, accidental, or therapy-related radiation. | Vral et al., 2011 [65] |
| In vitro study showing significant increase in MN frequencies in cells exposed to ionizing radiation, with the difference in frequency responses of patients with breast cancer compared to controls exceeding the differences observed at baseline. | Cardinale et al., 2012 [58] |
| Chemotherapy and Radiation Effects: | |
| In vivo study showing an increased frequency following radiation alone and following radiation in combination with chemotherapy regardless of age or smoking. | Milosevic-Djordjevic et al., 2011 [66] |
| In vivo longitudinal study (18 months) showing MN frequencies increased after radiation (20 patients received radiation) but not after chemotherapy (only 9 out of 20 patients received and were assessed after chemotherapy); MN frequencies were not significantly correlated with tumor size, receptor status, nodal status, family history of cancer, age, or smoking habits. | Aristei et al., 2009 [67] |
The women receiving the TAC regimen also had higher chromosomal instability values than the women receiving the TCH regimen, the latter of which includes carboplatin and trastuzumab. Carboplatin is an alkylating agent that causes cross-linking of guanine bases in DNA, which in turn, leads to supercoiling of DNA, thereby compromising DNA separation, replication and division [68]. Trastuzumab, which is a targeted monoclonal antibody that binds to the HER2 receptor, has been associated with multiple influences on cells that prohibit their proliferation, including (but not limited to), the arrest of cells in the G1 phase of the cell cycle, disruption of signaling cascades (PI3K), suppression of angiogenesis, and the facilitation of immune cell-mediated cytotoxicity [69–71].
The total body clearance for these chemotherapeutic drugs (TC, TAC, and TCH regimens) has been shown to be influenced by BMI and age [72–75]. However, our study results showed no impact of BMI or age on MN/cytome frequencies. Thus, one can infer that the persistence of somatic chromosomal instability (at least 1 year following chemotherapy treatment) reflects the presence of cells/clonal cell lines that originated at the time of exposure to these drugs. Alternatively, the long-term increased MN/cytome frequencies may reflect additional influences beyond direct temporal exposure to the chemotherapeutic regimens since the drug exposure for the TC and TAC regimens was completed by visit 3 (women receiving the TCH regimen continue to receive trastuzumab for up to one year). Also, our observation of persistence of acquired chromosomal instability frequencies in lymphocytes for at least one year following cancer treatment underscores the potential impact that these therapeutic regimens have on all types of somatic cells (healthy and cancerous).
Radiotherapy
Akin to the impact of chemotherapy, radiotherapy was also identified to have a significant predictive influence on the women’s acquired peripheral blood chromosomal instability frequencies. As with Doxorubicin, radiation exposure has been consistently associated with increased levels of chromosomal instability in human cells, primarily through in vitro study designs (Table 7).
Perceived Stress
In addition to the treatment-related exposures, women diagnosed with breast cancer experience considerable stress. While no significant differences in stress levels were reported across the 4 visits assessed over the one year interval evaluated in this study, levels at baseline (visit 1) tended to be highest and those at visit 4 tended to be lower than visits 2 and 3. This same pattern in perceived stress levels was also observed in 227 women with breast cancer who were evaluated by Wu, et al. [76] as part of a 1 year longitudinal study to assess physiological stress responses.
To our knowledge, this is the first report of a significant predictive positive association between perceived stress levels and acquired chromosomal instability in lymphocytes from women longitudinally studied following their treatment for breast cancer. York et al. [26] were the first to show a significant association between stress and an increased rate of peripheral blood MN/cytome abnormality frequencies through their studies of identical twins who were discordant for exposure to childhood sexual abuse. In agreement with these observations, Morath, et al. [77] recently showed that DNA breakage occurred at an increased frequency in people with post-traumatic stress disorder. Analogous to these human studies, research using a mouse model has shown high stress to be associated with an increased susceptibility to the mutagenic effects of radiation and drug exposure, leading to increased levels of chromosomal aberrations and MN in mice with higher stress [78]. Therefore, our observation of a predictive relationship between perceived stress related to breast cancer and its treatment is in agreement with the few previous reports assessing relationships between stress and chromosomal/genome instability.
While there is a paucity of studies investigating associations between stress and acquired chromosomal instability frequencies in women with breast cancer (or any type of cancer), several investigators have reported an association between stress and telomere shortening [27, 79]; especially chronic stress [80]. Given that telomere shortening has also consistently been observed to result in increased frequencies of acquired chromosomal abnormalities, it follows that stress may also be associated with chromosomal instability.
Race
An unexpected finding in this study was the significant predictive association of race on MN/cytome abnormality frequencies, with the Caucasian study participants having higher overall instability frequencies than the African American participants. While our step-wise statistical approach suggests this observation is an independent association, given the inter-relatedness of human epidemiology variables, one cannot fully rule out the possibility that it may be confounded by other factors, such as age or tumor characteristics. For example, the Caucasian women participating in this study were significantly older than the African American participants. This difference is consistent with data from the American Cancer Society, which shows that African American women develop more aggressive forms of breast cancer at younger ages and show a poorer prognosis than Caucasians. Given that increasing age has consistently been shown to be positively correlated with MN frequencies in healthy women [30, 81], it is possible that the age of the Caucasian participants contributed to their observed increased rate of MN/cytome abnormalities. We also observed differences in annualized income levels and alcohol consumption rates between the racial groups in our study, with the Caucasian women having significantly higher incomes and alcohol consumption practices than the African American women. Therefore, given the inter-related nature of the data, the basis for the association between race and MN/cytome abnormality frequencies in this study is not clear, but warrants further study.
There is a paucity of information available regarding MN frequencies in healthy subjects from different racial groups [82] and to our knowledge, there are no previous reports of lymphocyte MN/cytome frequency differences of racially diverse women treated for breast cancer. However, the observation that spontaneous somatic mosaicism involving large (greater than 2 megabases) autosomal alterations occurs more frequently with age (consistent with observations of spontaneously occurring MN in healthy people), but less frequently in people of African ancestry compared to European ancestry, suggests that there may be differences in acquired somatic cell chromosomal instability levels that are related to race [83]. It is interesting to note that African American women have been described to have significantly lower levels of dietary-related oxidative DNA damage than white women, suggesting that there may also be race-related differences in the cellular processes involved in oxidative DNA damage recognition/repair [84]. Moreover, ethnic differences have been detected for chemotherapy drug metabolism and inflammatory responses to cancer treatment [85], both of which might contribute to the acquisition of somatic cell chromosomal instability.
Tumor characteristics
Another aspect of breast cancer that has been associated with racial differences is the proportion of breast cancer tumor sub-groups in African American compared to Caucasian populations [86]. We observed a trend toward a higher proportion of HER2 positive (18%) tumors in the African American participants in our study compared to those noted in the Caucasian participants (4%). This trend is consistent with the pattern reported by Carey, et al [86] who studied 496 women from North Carolina. Thus, the breast tumors in our participants appear to be representative of those seen in larger populations. The treatment regimen for each study participant was determined, in large part, by her breast tumor characteristics. Therefore, if treatments are related to chromosomal instability levels, one might also anticipate detecting the presence of a relationship between tumor characteristics and acquired chromosomal changes. However, our stepwise reduction model showed a predictive influence of tumor characteristics on MN/cytome abnormality frequencies for the subset of women having luminal B tumors beyond that of treatment. Differential response in the gene expression patterns of luminal compared to basal epithelial cells following exposure to chemotherapeutic agents has been reported [87] and it is feasible that the gene expression differences could impact acquired chromosomal instability levels in healthy normal tissues. Another observation that supports that conjecture that the actual tumors (not just treatments) can influence acquired chromosomal instability levels in peripheral blood comes from reports of consistently higher pre-treatment MN frequencies in women and men with a variety of cancers, especially when compared to age-matched controls without cancer [30, 81].
Age Effect
We did not detect a predictive association between age and MN/cytome abnormality frequencies in our women receiving treatment for breast cancer. Other investigators have also failed to detect an age effect for MN frequencies in patients with cancer, or specifically with breast cancer, despite the clear association between age and MN frequencies that has been observed in healthy adults [30, 81]. This lack of a clear, independent influence of chronological age on peripheral blood cell MN frequencies in women with breast cancer could reflect the narrow age range of the participants sampled in this study (and other studies of women with breast cancer). Alternatively, the influence(s) of factors related to breast cancer development may override or “mask” the effects of age.
Other factors
In addition to the exposure-related factors evaluated in this study, the frequency of acquired chromosomal instability in lymphocytes following treatment for cancer is likely to reflect germ-line genetic make-up differences between people, which are factors that were not evaluated in this study. For example, pharmocogeneticists have shown differential metabolism of chemotherapy drugs based on the variant form(s) of alleles that an individual carries for several genes (e. g. CYP2D6, ESR1, ESR2, etc), including genes involved in DNA repair (FANCD2; MTHFR, RB1) [88–91]. Furthermore, in healthy people, baseline levels of micronuclei have been shown to be influenced by heritable genetic (as well as environmental) factors, including polymorhisms of genes involved in DNA repair [92, 93].
Conclusions
In summary, we determined that women treated for breast cancer showed higher lymphocyte chromosomal instability frequencies during and after treatment than at baseline, with these increased frequencies persisting for at least one year following treatment. Of particular interest was the recognition of stress as a contributor to increased levels of chromosomal instability, since this factor has the potential to be modulated (reduced) through intervention. Given that stress-related chromosomal instability has been shown to persist (and even accumulate) for years [26], one could speculate that these somatic chromosomal changes could lead to gene expression imbalances that could contribute to the development/persistence of long-term side effects associated with treatment for cancer. Alternatively, the increases in peripheral blood MN/cytome abnormality frequencies may be simply correlative in nature rather than mechanistically related, with the frequency of chromosomal instability in healthy cells serving as a “chronicle” or biomarker to document that biologically relevant soma-wide changes have occurred that could lead to PNS. Regardless of the direct or indirect role played by acquired chromosomal instability, this MN/cytome test warrants further study to determine if it can be used as a potential biomarker for the future development of an algorithm to identify individuals most “at risk” for acquiring persistent adverse side effects associated with cancer and to determine if interventions to reduce stress could have a positive health outcome on women with increased levels of chromosomal instability following their treatment for breast cancer.
Supporting Information
(XLSX)
Acknowledgments
We thank Yu-Jiun Chen (assisted with culture set-ups, harvests, and scoring of NDCI) and Xiaoyan Deng (database management and assistance with data analysis/development of tables) for their contributions to this research. We also thank Jennifer DeWitt for her clerical assistance in preparing this manuscript.
Data Availability
All relevant data are available in a supporting information file.
Funding Statement
This research was supported by Award Number R01NR012667 from the National Institute of Nursing Research, National Institutes of Health (multi-PIs: DL CJC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. American Cancer Society breast cancer facts and figures 2013–2014. Atlanta: American Cancer Society, Inc; 2013. [Google Scholar]
- 2. Swain SM, Tang G, Geyer CE Jr, Rastogi P, Atkins JN, Donnellan PP, et al. Definitive results of a phase III adjuvant trial comparing three chemotherapy regimens in women with operable, node-positive breast cancer: the NSABP B-38 trial. J Clin Oncol. 2013. September 10;31(26):3197–204. 10.1200/JCO.2012.48.1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Badger T, Segrin C, Dorros SM, Meek P, Lopez AM. Depression and anxiety in women with breast cancer and their partners. Nurs Res. 2007. Jan-Feb;56(1):44–53. . [DOI] [PubMed] [Google Scholar]
- 4. Berger AM, Wielgus K, Hertzog M, Fischer P, Farr L. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support Care Cancer. 2010. January;18(1):105–14. 10.1007/s00520-009-0636-0 . [DOI] [PubMed] [Google Scholar]
- 5. Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011. July;12(7):703–8. 10.1016/S1470-2045(10)70294-1 . [DOI] [PubMed] [Google Scholar]
- 6. Lee K, Cho M, Miaskowski C, Dodd M. Impaired sleep and rhythms in persons with cancer. Sleep Med Rev. 2004. June;8(3):199–212. Review. . [DOI] [PubMed] [Google Scholar]
- 7. Utne I, Miaskowski C, Bjordal K, Paul SM, Rustoen T. The relationships between mood disturbances and pain, hope, and quality of life in hospitalized cancer patients with pain on regularly scheduled opioid analgesic. J Palliat Med. 2010. March;13(3):311–8. 10.1089/jpm.2009.0294 . [DOI] [PubMed] [Google Scholar]
- 8. Valeberg BT, Rustøen T, Bjordal K, Hanestad BR, Paul S, Miaskowski C. Self-reported prevalence, etiology, and characteristics of pain in oncology outpatients. Eur J Pain. 2008. July;12(5):582–90. . [DOI] [PubMed] [Google Scholar]
- 9. Braybrooke JP, Mimoun S, Zarca D, Elia D, Pinder B, Lloyd AJ, et al. Patients' experiences following breast cancer treatment: an exploratory survey of personal and work experiences of breast cancer patients from three European countries. Eur J Cancer Care (Engl). 2014. July 23 10.1111/ecc.12222 . [DOI] [PubMed] [Google Scholar]
- 10. Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013. Nov-Dec;63(6):419–37. 10.3322/caac.21204 . [DOI] [PubMed] [Google Scholar]
- 11. Koppelmans V, de Ruiter MB, van der Lijn F, Boogerd W, Seynaeve C, van der Lugt A, et al. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat. 2012. April;132(3):1099–106. 10.1007/s10549-011-1888-1 . [DOI] [PubMed] [Google Scholar]
- 12. Fumaz CR, Gonzalez-Garcia M, Borras X, Muñoz-Moreno JA, Perez-Alvarez N, Mothe B, et al. Psychological stress is associated with high levels of IL-6 in HIV-1 infected individuals on effective combined antiretroviral treatment. Brain Behav Immun. 2012. May;26(4):568–72. 10.1016/j.bbi.2012.01.001 . [DOI] [PubMed] [Google Scholar]
- 13. Lee BN, Dantzer R, Langley KE, Bennett GJ, Dougherty PM, Dunn AJ, et al. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11(5):279–92. Review. . [DOI] [PubMed] [Google Scholar]
- 14. Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003. June 1;97(11):2919–25. . [DOI] [PubMed] [Google Scholar]
- 15. Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001. March;15(1):7–24. Review. . [DOI] [PubMed] [Google Scholar]
- 16. Lyon D, Elmore L, Aboalela N, Merrill-Schools J, McCain N, Starkweather A, et al. Potential epigenetic mechanism(s) associated with the persistence of psychoneurological symptoms in women receiving chemotherapy for breast cancer: a hypothesis. Biol Res Nurs. 2014. April;16(2):160–74. 10.1177/1099800413483545 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McClelland SE, Burrell RA, Swanton C. Chromosomal instability: a composite phenotype that influences sensitivity to chemotherapy. Cell Cycle. 2009. October 15;8(20):3262–6. . [DOI] [PubMed] [Google Scholar]
- 18. Menzies V, Lyon DE, Archer KJ, Zhou Q, Brumelle J, Jones KH, Gao G, York TP, Jackson-Cook C. Epigenetic alterations and an increased frequency of micronuclei in women with fibromyalgia. Nurs Res Pract. 2013;2013:795784 10.1155/2013/795784 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Castellanos MV, Hernández JM, Ramos L, Belén González M, Gutiérrez NC, Leone PE, et al. Chromosomal abnormalities are related to location and grade of osteoarthritis. Osteoarthritis Cartilage. 2004. December;12(12):982–5. . [DOI] [PubMed] [Google Scholar]
- 20. Dahlén A, Broberg K, Domanski HA, Toksvig-Larsen S, Lindstrand A, Mandahl N, Mertens F. Analysis of the distribution and frequency of trisomy 7 in vivo in synovia from patients with osteoarthritis and pigmented villonodular synovitis. Cancer Genet Cytogenet. 2001. November;131(1):19–24. . [DOI] [PubMed] [Google Scholar]
- 21. Kinne RW, Liehr T, Beensen V, Kunisch E, Zimmermann T, Holland H, et al. Mosaic chromosomal aberrations in synovial fibroblasts of patients with rheumatoid arthritis, osteoarthritis, and other inflammatory joint diseases. Arthritis Res. 2001;3(5):319–30. ; PMCID: PMC64845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mertens F, Pålsson E, Lindstrand A, Toksvig-Larsen S, Knuutila S, Larramendy ML, et al. Evidence of somatic mutations in osteoarthritis. Hum Genet. 1996. December;98(6):651–6. . [DOI] [PubMed] [Google Scholar]
- 23. Broberg K, Höglund M, Lindstrand A, Toksvig-Larsen S, Mandahl N, Mertens F. Polyclonal expansion of cells with trisomy 7 in synovia from patients with osteoarthritis. Cytogenet Cell Genet. 1998;83(1–2):30–4. . [DOI] [PubMed] [Google Scholar]
- 24. Faggioli F, Vijg J, Montagna C. Chromosomal aneuploidy in the aging brain. Mech Ageing Dev. 2011. August;132(8–9):429–36. 10.1016/j.mad.2011.04.008 Review. ; PMCID: PMC3168579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee SL, Thomas P, Hecker J, Faunt J, Fenech M. Chromosomal DNA damage measured using the cytokinesis-block micronucleus cytome assay is significantly associated with cognitive impairment in South Australians. Environ Mol Mutagen. 2015. January;56(1):32–40. 10.1002/em.21890 . [DOI] [PubMed] [Google Scholar]
- 26. York TP, Brumelle J, Juusola J, Kendler KS, Eaves LJ, Amstadter AB, et al. Increased frequency of micronuclei in adults with a history of childhood sexual abuse: a discordant monozygotic twin study. PLoS One. 2013;8(1):e55337 10.1371/journal.pone.0055337 ; PMCID: PMC3559336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, Epel ES. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology. 2013. September;38(9):1835–42. 10.1016/j.psyneuen.2013.03.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Starkweather AR, Alhaeeri AA, Montpetit A, Brumelle J, Filler K, Montpetit M, et al. An integrative review of factors associated with telomere length and implications for biobehavioral research. Nurs Res. 2014. Jan-Feb;63(1):36–50. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protoc. 2007;2(5):1084–104. . [DOI] [PubMed] [Google Scholar]
- 30. Kirsch-Volders M, Plas G, Elhajouji A, Lukamowicz M, Gonzalez L, Vande Loock K, Decordier I. The in vitro MN assay in 2011: origin and fate, biological significance, protocols, high throughput methodologies and toxicological relevance. Arch Toxicol. 2011. August;85(8):873–899. 10.1007/s00204-011-0691-4 [DOI] [PubMed] [Google Scholar]
- 31. Fenech M (2000) The in vitro micronucleus technique. Mutation research 455:81–95. [DOI] [PubMed] [Google Scholar]
- 32. Leach NT, Jackson-Cook C (2001) The application of spectral karyotyping (SKY) and fluorescent in situ hybridization (FISH) technology to determine the chromosomal content(s) of micronuclei. Mutation research 495: 11–19. [DOI] [PubMed] [Google Scholar]
- 33. Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, et al. (2003) HUMN project: detailed description of the scoring criteria for the cytokinesis block micronucleus assay using isolated human lymphocyte cultures. Mutation Research 534: 65–75. [DOI] [PubMed] [Google Scholar]
- 34. Eastmond DA, Tucker JD (1989) Identification of aneuploidy-inducing agents using cytokinesis-blocked human lymphocytes and an antikinetochore antibody. Environmental and Molecular Mutagenesis 13: 34–43. [DOI] [PubMed] [Google Scholar]
- 35. Cohen S, Kamarck T, Mermelstein R: A global measure of perceived stress. J Health Soc Behav 1983; 24:385–396. . [PubMed] [Google Scholar]
- 36. Innes KE, Selfe TK, Brown CJ, Rose KM, Thompson-Heisterman A. The effects of meditation on perceived stress and related indices of psychological status and sympathetic activation in persons with Alzheimer's disease and their caregivers: a pilot study. Evid Based Complement Alternat Med. 2012. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abut YC, Kitapcioglu D, Erkalp K, Toprak N, Boztepe A, Sivrikaya U, et al. Job burnout in 159 anesthesiology trainees. Saudi J Anaesth. 2012. January;6(1):46–51. 10.4103/1658-354X.93059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000. August 17;406(6797):747–52. . [DOI] [PubMed] [Google Scholar]
- 39. Hu Z, Fan C, Oh DS, Marron JS, He X, Qagish BF, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006. April 27;7:96 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fan C, Oh DS, Wessels L, Weigelt B, Nuyten DS, Nobel AB, van’t Veer LJ, Perou CM. Concordance among gene-expression-based predictors for breast cancer. N Engl J Med. 2006. August 10; 355(6):560–9. . [DOI] [PubMed] [Google Scholar]
- 41. Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010. April 1;28(10):1684–91. 10.1200/JCO.2009.24.9284 [DOI] [PubMed] [Google Scholar]
- 42. Carey LA. Molecular intrinsic subtypes of breast cancer In: UpToDate. Hayes DF, Dizon DS (eds.). Waltham, MA: UpToDate, 2013. [Google Scholar]
- 43. Ceppi M, Gallo F, Bonassi S. Study design and statistical analysis of data in human population studies with the micronucleus assay. Mutagenesis. 2011. January;26(1):247–52. 10.1093/mutage/geq060 Review. . [DOI] [PubMed] [Google Scholar]
- 44. Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed John Wiley and Sons; 2000. [Google Scholar]
- 45. Brown H, Prescott R. Applied Mixed Models in Medicine. 2nd ed John Wiley and Sons; 2006. [Google Scholar]
- 46. Fenech M, Holland N, Zeiger E, Chang WP, Burgaz S, Thomas P, et al. The HUMN and HUMNxL international collaboration projects on human micronucleus assays in lymphocytes and buccal cells—past, present and future. Mutagenesis. 2011. January;26(1):239–45. 10.1093/mutage/geq051 Review. . [DOI] [PubMed] [Google Scholar]
- 47. Andreou E, Alexopoulos EC, Lionis C, Varvogli L, Gnardellis C, Chrousos GP, Darviri C. Perceived Stress Scale: reliability and validity study in Greece. Int J Environ Res Public Health. 2011. August;8(8):3287–98. 10.3390/ijerph8083287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ahire V, Mishra KP, Kulkarni GR. On the mechanism of cellular toxicity in breast cancer by ionizing radiation and chemotherapeutic drugs. J Environ Pathol Toxicol Oncol. 2014;33(1):69–82. . [DOI] [PubMed] [Google Scholar]
- 49. Rohena CC, Mooberry SL. Recent progress with microtubule stabilizers: new compounds, binding modes and cellular activities. Nat Prod Rep. 2014. March;31(3):335–55. 10.1039/c3np70092e . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999. April 1;57: 727–741. . [DOI] [PubMed] [Google Scholar]
- 51. Senchenkov A, Litvak DA, Cabot MC. Targeting ceramide metabolism—a strategy for overcoming drug resistance. J Natl Cancer Inst. 2001. March 7;93(5): 347–57. . [DOI] [PubMed] [Google Scholar]
- 52. Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004. June;56(2): 185–229. . [DOI] [PubMed] [Google Scholar]
- 53. Yang F, Teves SS, Kemp CJ, Henikoff S. Doxorubicin, DNA torsion, and chromatin dynamics. Biochim Biophys Acta. 2014. January;1845(1): 84–9. 10.1016/j.bbcan.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Elmore LW, Rehder CjW, Xu D, McChesney PA, Jackson-Cook CK, Gewirtz DA, Holt SE. Adriamycin-induced senescence in breast tumor cells involves functional p53 and telomere dysfunction. J Biol Chem. 2002. September 20;277(38): 35509–15. . [DOI] [PubMed] [Google Scholar]
- 55. Khan F1, Sherwani AF, Afzal M. Chromosomal aberration and micronucleus studies of two topoisomerase (II) targeting anthracyclines. J Environ Biol. 2009. May;30(3):409–12. . [PubMed] [Google Scholar]
- 56. Li P, Hou M, Lou F, Björkholm M, Xu D. Telomere dysfunction induced by chemotherapeutic agents and radiation in normal human cells. Int J Biochem Cell Biol. 2012;44(9):1531–40. 10.1016/j.biocel.2012.06.020 [DOI] [PubMed] [Google Scholar]
- 57. Santos RA, Teixeira AC, Mayorano MB, Carrara HH, Andrade JM, Takahashi CS. Basal levels of DNA damage detected by micronuclei and comet assays in untreated breast cancer patients and healthy women. Clin Exp Med. 2010;10(2):87–92. 10.1007/s10238-009-0079-4 . [DOI] [PubMed] [Google Scholar]
- 58. Cardinale F, Bruzzi P, Bolognesi C. Role of micronucleus test in predicting breast cancer susceptibility: a systematic review and meta-analysis. Br J Cancer. 2012. February 14;106(4):780–90. 10.1038/bjc.2011.567 Review. ; PMCID: PMC3324300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ramos DL, Gaspar JF, Pingarilho M, Gil OM, Fernandes AS, Rueff J, Oliveira NG. Genotoxic effects of doxorubicin in cultured human lymphocytes with different glutathione S-transferase genotypes. Mutat Res. 2011. September 18;724(1–2):28–34. 10.1016/j.mrgentox.2011.04.013 . [DOI] [PubMed] [Google Scholar]
- 60. Guerreiro PS, Fernandes AS, Costa JG, Castro M, Miranda JP, Oliveira NG. Differential effects of methoxyamine on doxorubicin cytotoxicity and genotoxicity in MDA-MB-231 human breast cancer cells. Mutat Res. 2013. October 9;757(2):140–7. 10.1016/j.mrgentox.2013.08.003 . [DOI] [PubMed] [Google Scholar]
- 61. Minicucci EM, Ribeiro DA, de Camargo B, Costa MC, Ribeiro LR, Favero Salvadori DM. DNA damage in lymphocytes and buccal mucosa cells of children with malignant tumours undergoing chemotherapy. Clin Exp Med. 2008. June;8(2):79–85. 10.1007/s10238-008-0161-3 . [DOI] [PubMed] [Google Scholar]
- 62. Elsendoorn TJ, Weijl NI, Mithoe S, Zwinderman AH, Van Dam F, De Zwart FA, et al. Chemotherapy-induced chromosomal damage in peripheral blood lymphocytes of cancer patients supplemented with antioxidants or placebo. Mutat Res. 2001. November 15;498(1–2):145–58. [DOI] [PubMed] [Google Scholar]
- 63. Jagetia GC, Jayakrishnan A, Fernandes D, Vidyasagar MS. Evaluation of micronuclei frequency in the cultured peripheral blood lymphocytes of cancer patients before and after radiation treatment. Mutat Res. 2001. April 5;491(1–2):9–16. . [DOI] [PubMed] [Google Scholar]
- 64. Gamulin M, Garaj-Vrhovac V, Kopjar N, Ramić S, Viculin T, Juretić A, Grgić M. DNA and cytogenetic damage in white blood cells of postmenopausal breast cancer patients treated with radiotherapy. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2010;45(3):292–304. 10.1080/10934520903467881 . [DOI] [PubMed] [Google Scholar]
- 65. Vral A, Fenech M, Thierens H. The micronucleus assay as a biological dosimeter of in vivo ionising radiation exposure. Mutagenesis. 2011. January;26(1):11–7. 10.1093/mutage/geq078 Review. . [DOI] [PubMed] [Google Scholar]
- 66. Milosevic-Djordjevic O, Stosic I, Vuckovic M, Grujicic D, Marinkovic D. Baseline and therapy-induced chromosome damages in peripheral blood lymphocytes of breast cancer patients assessed by the micronucleus assay. J BUON. 2011. Jul-Sep;16(3):437–43. . [PubMed] [Google Scholar]
- 67. Aristei C, Stracci F, Guerrieri P, Anselmo P, Armellini R, Rulli A, et al. Frequency of sister chromatid exchanges and micronuclei monitored over time in patients with early-stage breast cancer: results of an observational study. Cancer Genet Cytogenet. 2009. July;192(1):24–9. 10.1016/j.cancergencyto.2009.02.019 . [DOI] [PubMed] [Google Scholar]
- 68. Knox RJ, Friedlos F, Lydall DA, Roberts JJ: Mechanism of cytotoxicity of anticancer platinum drugs: evidence that cis-diamminedichloroplatinum(II) and cis-diammine-(1,1-cyclobutanedicarboxylato)platinum(II) differ only in the kinetics of their interaction with DNA. Cancer Res. 1986. April;46(4 Pt 2):1972–9. . [PubMed] [Google Scholar]
- 69. Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol. 2012. June;2:62 10.3389/fonc.2012.00062 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007. July;357(1):39–51. . [DOI] [PubMed] [Google Scholar]
- 71. Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med 2000;6:443–6. [DOI] [PubMed] [Google Scholar]
- 72. Powis G, Reece P, Ahmann DL, Ingle JN. Effect of body weight on the pharmacokinetics of cyclophosphamide in breast cancer patients. Cancer Chemother Pharmacol. 1987;20(3):219–22. . [DOI] [PubMed] [Google Scholar]
- 73. Clarke SJ, Rivory LP. Clinical pharmacokinetics of docetaxel. Clin Pharmacokinet. 1999. February; 32(2):99–114. . [DOI] [PubMed] [Google Scholar]
- 74. Nagar S. Pharmacokinetics of anti-cancer drugs used in breast cancer chemotherapy. Adv Esp Med Biol. 2010;678:124–32. . [DOI] [PubMed] [Google Scholar]
- 75. Lien EA, Søiland H, Lundgren S, Aas T, Steen VM, Mellgren G, Gjerde J. Serum concentrations of tamoxifen and its metabolites increase with age during steady-state treatment. Breast Cancer Res Treat. 2013. September;141(2):243–9. 10.1007/s10549-013-2677-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wu SM, Yang HC, Thayer JF, Andersen BL. Association of the physiological stress response with depressive symptoms in patients with breast cancer. Psychosom Med. 2014. May;76(4):252–6. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Morath J, Moreno-Villanueva M, Hamuni G, Kolassa S, Ruf-Leuschner M, Schauer M, et al. Effects of psychotherapy on DNA strand break accumulation originating from traumatic stress. Psychother Psychosom. 2014;83(5):289–97. 10.1159/000362739 . [DOI] [PubMed] [Google Scholar]
- 78. Sacharczuk M, Jaszczak K, Sadowski B. Cytogenetic comparison of the sensitivity to mutagens in mice selected for high (HA) and low (LA) swim stress-induced analgesia. Mutat Res. 2003. February 5;535(1):95–102. . [DOI] [PubMed] [Google Scholar]
- 79. Surtees PG, Wainwright NW, Pooley KA, Luben RN, Khaw KT, Easton DF, Dunning AM. Life stress, emotional health, and mean telomere length in the European Prospective Investigation into Cancer (EPIC)-Norfolk population study. J Gerontol A Biol Sci Med Sci. 2011. November;66(11):1152–62. 10.1093/gerona/glr112 . [DOI] [PubMed] [Google Scholar]
- 80. Quinlan J, Tu MT, Langlois EV, Kapoor M, Ziegler D, Fahmi H, Zunzunegui MV. Protocol for a systematic review of the association between chronic stress during the life course and telomere length. Syst Rev. 2014. April 30;3:40 10.1186/2046-4053-3-40 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fenech M, Bonassi S. The effect of age, gender, diet and lifestyle on DNA damage measured using micronucleus frequency in human peripheral blood lymphocytes. Mutagenesis. 2011. January;26(1):43–9. 10.1093/mutage/geq050 Review. . [DOI] [PubMed] [Google Scholar]
- 82. Huen K, Gunn L, Duramad P, Jeng M, Scalf R, Holland N. Application of a geographic information system to explore associations between air pollution and micronucleus frequencies in African American children and adults. Environ Mol Mutagen. 2006. May;47(4):236–46. . [DOI] [PubMed] [Google Scholar]
- 83. Machiela MJ, Zhou W, Sampson JN, Dean MC, Jacobs KB, Black A, et al. Characterization of large structural genetic mosaicism in human autosomes. Am J Hum Genet. 2015. March;96(3):487–97. 10.1016/j.ajhg.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Watters JL, Satia JA, Kupper LL, Swenberg JA, Schroeder JC, Switzer BR. Associations of antioxidant nutrients and oxidative DNA damage in healthy African-American and White adults. Cancer Epidemiol Biomarkers Prev. 2007. July;16(7):1428–36. . [DOI] [PubMed] [Google Scholar]
- 85. Phan VH, Moore MM, McLachlan AJ, Piquette-Miller M, Xu H, Clarke SJ. Ethnic differences in drug metabolism and toxicity from chemotherapy. Expert Opin Drug Metab Toxicol. 2009. March;5(3):243–57. 10.1517/17425250902800153 [DOI] [PubMed] [Google Scholar]
- 86. Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006. June 7;295(21):2492–502. . [DOI] [PubMed] [Google Scholar]
- 87. Troester MA, Hoadley KA, Sørlie T, Herbert BS, Børresen-Dale AL, Lønning PE, et al. Cell-type-specific responses to chemotherapeutics in breast cancer. Cancer Res. 2004. June 15;64(12):4218–26. . [DOI] [PubMed] [Google Scholar]
- 88. Province MA, Klein TE. International Tamoxifen Pharmacogenomics Consortium. Re: CYP2D6 genotyping and the use of tamoxifen in breast cancer. J Natl Cancer Inst. 2014. February;106(2):djt379 10.1093/jnci/djt379 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Westbrook K, Stearns V. Pharmacogenomics of breast cancer therapy: an update. Pharmacol Ther. 2013. July;139(1):1–11. 10.1016/j.pharmthera.2013.03.001 Review. ; PMCID: PMC3660522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ingle JN. Pharmacogenomics of endocrine therapy in breast cancer. J Hum Genet. 2013. June;58(6):306–12. 10.1038/jhg.2013.35 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Robinson TJ, Liu JC, Vizeacoumar F, Sun T, Maclean N, Egan SE, et al. RB1 status in triple negative breast cancer cells dictates response to radiation treatment and selective therapeutic drugs. PLoS One. 2013. November;8(11):e78641 10.1371/journal.pone.0078641 eCollection 2013. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jones KH, York TP, Juusola J, Ferreira-Gonzalez A, Maes HH, Jackson-Cook C. Genetic and environmental influences on spontaneous micronuclei frequencies in children and adults: a twin study. Mutagenesis. 2011. November;26(6):745–52. 10.1093/mutage/ger042 ; PMCID: PMC3198889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dhillon VS, Thomas P, Iarmarcovai G, Kirsch-Volders M, Bonassi S, Fenech M. Genetic polymorphisms of genes involved in DNA repair and metabolism influence micronucleus frequencies in human peripheral blood lymphocytes. Mutagenesis. 2011. January;26(1):33–42. 10.1093/mutage/geq076 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are available in a supporting information file.