Abstract
Efficient sensory processing of the environment is a critical function for any organism to survive and is accomplished by having neurons adapt their responses to stimuli based on behavioral context in part through neuromodulators such as serotonin. We have recently shown that one critical function of the serotonergic system in weakly electric fish is to enhance sensory pyramidal neuron responses within the electrosensory lateral line lobe to stimuli caused by same sex conspecifics, thereby enhancing their perception. This enhancement is accomplished by making pyramidal neurons more excitable through downregulation of potassium channels. However, the nature of the 5-HT receptors that mediate this effect is not known. Here we show that the 5-HT2 receptor antagonist ketanserin can effectively block the effects of 5-HT on pyramidal neuron excitability in vitro. Indeed, serotonin application subsequent to ketanserin application did not cause any significant changes in neuron excitability and responses to current injection. We further show that ketanserin applied in vivo can block the effects of serotonin on behavioral responses. Thus, our results strongly suggest that the previously observed effects of serotonin on sensory processing within ELL and their consequences for behavior are mediated by 5-HT2 receptors.
Keywords: neural coding, weakly electric fish, neuromodulation, neuroethology
Introduction
Efficient and adaptive processing of behaviorally relevant sensory stimuli is essential for survival and can be accomplished by dynamically regulating sensory neuron tuning to these stimuli (Abbott, 2005, Wark et al., 2007). Such regulation is, in part, implemented by neuromodulators such as serotonin (5-HT). There are massive serotonergic projections from the Raphe nuclei to almost every part of the vertebrate brain, including sensory areas such as thalamus and cortex (see Berger et al., 2009 for review). Indeed, 5-HT has been shown to significantly alter processing across several sensory systems and is thought to impose dynamic filters whose properties are tied to environmental events and/or the brain’s internal state rather than the detailed information about the ongoing stimulus (Hurley et al., 2004).
Weakly electric fish generate an electric field around their body through the electric organ discharge (EOD) and can sense amplitude modulations of this field through an array of electroreceptors scattered on their skin surface (Turner et al., 1999, Chacron et al., 2011). These electroreceptors make synaptic contact onto pyramidal neurons within the electrosensory lateral line lobe (ELL) (Bell and Maler, 2005), whose physiology has been well characterized both in vitro and in vivo (see (Maler, 2009) for review). The ELL is composed of four parallel maps of the body surface; three of which (centro-medial: CMS, centro-lateral: CLS, and lateral: LS) receive identical tuberous electroreceptor input (Carr et al., 1982, Heiligenberg and Dye, 1982, Shumway, 1989a, b). These maps have been shown to mediate different behaviors (Metzner and Juranek, 1997): in particular, the lateral map is involved in the processing of communication signals (Shumway, 1989a, Krahe et al., 2008, Marsat et al., 2009, Marsat and Maler, 2010) associated with aggressive behavior (Zakon et al., 2002, Hupe et al., 2008). Previous studies have shown that the tuning of pyramidal neurons within the maps is intrinsic (Mehaffey et al., 2008b) and originates in part from membrane conductances (Ellis et al., 2007b, Ellis et al., 2008, Krahe et al., 2008).
Pyramidal cells also receive large amounts of neuromodulatory input (Maler et al., 1981, Johnston et al., 1990, Ellis et al., 2007a, Deemyad et al., 2011) (see (Marquez et al., 2013) for review). In particular, serotonergic innervation is densest in the lateral segment and sparsest in the centro-medial segment (Deemyad et al., 2011). Recent studies have shown that the effects of 5-HT on ELL pyramidal neurons in vivo is to render them more excitable, thereby increasing their responses to stimuli associated with same sex conspecifics (Deemyad et al., 2013). Remarkably, similar effects were observed in vitro, where it was shown that 5-HT increases pyramidal neuron excitability by downregulating both small conductance calcium activated (SK) and M potassium channels (Deemyad et al., 2011). However, the nature of the 5-HT receptors that mediate this effect is not known. There are seven different families of 5-HT receptors which can be broken down further into up to 14 different subtypes, all of which are metabotropic except for 5-HT3 receptors which are ionotropic. Based on previous studies showing that 5-HT2 receptors downregulate potassium channels in other systems (Barnes and Sharp, 1999, Hoyer et al., 2002), we tested whether the 5-HT2 receptor antagonist ketanserin (ket) can block the effects of 5-HT on ELL pyramidal neuron excitability in vitro and behaviorally at the organismal level.
Methods
Animals
The weakly electric fish Apteronotus leptorhynchus was used exclusively in this study. Animals were obtained from tropical fish suppliers and were acclimated to laboratory conditions according to published guidelines (Hitschfeld et al., 2009). All procedures were approved by McGill University’s animal care committee.
Preparation of slices
Slices were prepared as done previously (Mathieson and Maler, 1988, Ellis et al., 2007a, Deemyad et al., 2011, Deemyad et al., 2012). In preparation for surgery, fish were initially anaesthetized by placing them in a pH buffered MS-222 solution. Fish were then quickly transferred into a holding chamber with the head firmly secured to a breathing apparatus where they were respired via a mouth tube with a well-oxygenated solution containing pH buffered MS-222. Soft tissue covering the skull was removed using a scalpel and the skull itself was removed using forceps. Incisions severing the afferents and spinal cord were made and the brain was removed. Finally the brain was blocked at roughly a 45° angle to provide an optimal angle to slice hindbrain ELL. The hindbrain block was then placed onto the vibratome platform and fixed with super glue. The platform was then covered with an agarose solution (15%). 400 μm ELL slices were cut by a microtome and transferred immediately to a well infused ACSF solution where they were allowed to rest for a minimum of one hour before being transferred into a recording chamber with a constant ACSF flow rate of 4 ml/min. The ACSF was composed of (in mM): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 18 NaHCO3, 2.4 CaCl2, and 11 d-glucose) and was superfused with carbogen (95% O2, 5% CO2).
In vitro pharmacology
Both 5-HT and ket were obtained from Tocris and were dissolved in ACSF using concentrations of 1 mM and 100 μM, respectively. Previous studies have shown that 5-HT at 1 mM concentration will significantly alter LS pyramidal neuron activity (Deemyad et al., 2011) while ket will be most selective for 5-HT2A receptors at a concentration of 100 μM (Janssen et al., 2002). Both drugs were applied to the ELL Dorsal Molecular Layer (DML) using a PicoSpritzer III (Parker Hannifin, Cleveland, OH) using custom made electrodes with a tip diameter of 1- to 2-μm (Ellis et al., 2007a, Mehaffey et al., 2008a, Deemyad et al., 2011, Deemyad et al., 2012). PicoSpritzer pressure was 14 PSI with an ejection time of 140 ms. All slices were oriented so that pharmacological agents applied would, through the natural ACSF flow of the recording chamber, also pass over the ventral molecular and pyramidal cell layers (Ellis et al., 2007a, Deemyad et al., 2011, Deemyad et al., 2012). We used a double-barrel electrode that was advanced over the molecular layer above the recording electrode. One barrel was filled with ket while the other was filled with 5-HT. We first obtained control data from each cell as described below. In some experiments, 5-HT was then applied in order to quantify its effects on pyramidal neuron excitability. In other experiments, we first applied ket and quantified its effects on pyramidal neuron excitability before applying 5-HT.
Recording
Sharp intracellular recordings from pyramidal neurons were obtained using previously published techniques (Ellis et al., 2007a, Deemyad et al., 2011, Deemyad et al., 2012). Glass sharp intracellular electrodes were pulled using a P-97 filament puller (Sutter Instruments) and were filled with a 2M KAC solution. These electrodes had resistances between 70–110 MΩ. Upon entering the cell with an electrode, we injected a baseline current (typically around −0.5 nA) in order to keep the cell just under firing threshold. The recorded membrane potential difference was amplified using an Axoclamp 900A while data was recorded with a Digidata 1440A and Clampex 10.3 (Molecular Devices, Sunnyvale, California) using a 10 kHz sampling rate. Previous studies have shown that 5-HT expression is graded across the ELL segments (Deemyad et al., 2011). In particular, weak expression was found in CMS while LS displayed the strongest levels of expression. Neurophysiological studies performed in vitro have shown that 5-HT did not have any significant effect on CMS pyramidal neuron activity, had weak effects on CLS pyramidal neuron activity, and had the strongest effects on LS neuron activity (Deemyad et al., 2011). As we wanted to test whether ket would block the effects of 5-HT, we thus focused on recording from LS pyramidal neurons where the effects of 5-HT were strongest.
Experiment protocols and data analysis
The spiking activities of pyramidal neurons were obtained during a current step of 0.5 nA from the baseline holding current that lasted 2 sec. These were quantified by the mean firing rate (i.e. the number of spikes per unit time). Further, ISI sequences were computed as the time intervals between consecutive spikes, and ISI probability densities were generated with 1 ms binwidth. The coefficient of variation (CV) was computed as the standard deviation to mean ratio of the ISI probability density. The burst fraction was defined as the proportion of ISIs that are less than 10 ms, which correspond to the range of ISIs that will give rise to dendritic failure (Oswald et al., 2004). This value also corresponds to the trough in the bimodal ISI distribution as plotted on a log-linear scale (Turner et al. 1994) which is frequently taken as the threshold to segregate bursts from isolated spikes (Chacron et al., 2001, Doiron et al., 2003, Avila Akerberg and Chacron, 2011, Khosravi-Hashemi et al., 2011, Khosravi-Hashemi and Chacron, 2012, 2014).
Average action potential waveforms were generated in order to measure the afterhyperpolarization (AHP) following each action potential. The medium component of the AHP was measured as the differences between the baseline membrane potential value before a spike (i.e. the average membrane potential at 10–15 ms before the peak of the spike) and the membrane potential value measured at 10–40 ms after the action potential peak (Faber and Sah, 2002, Koyama and Appel, 2006). We only considered action potentials that were “isolated” (i.e. that were not immediately preceded or followed by other action potentials) in order to estimate the full length of the AHP.
We used a set of 10 current steps each lasting 1 sec and ranging from 0.05–0.5 nA above the baseline holding current. We then measured the mean firing rate during each step. The mean firing rate was then plotted as a function of the current value minus the baseline value in order to obtain the f–I curve. The gain was determined from the slope of the best linear fit to the f–I curve (Mehaffey et al., 2005).
Behavior
The procedures were the same as those used in a previous study (Deemyad et al., 2013). Briefly, 0.1–0.5 mg of Tubocurarine (Sigma, St. Louis, MO) was injected intramuscularly to immobilize the fish. The fish was then transferred to a recording tank and respirated via a mouth tube at a flow rate of 10 ml/min. To expose the hindbrain for recording, we first locally anaesthetized the skin on the skull by applying 2% Lidocaine. To stabilize the head, a metal post was glued to the exposed area of the skull, rostral to the opening. We drilled two small holes of ~2 mm2 over the cerebelli and the ELL areas, caudal to the T0 vein on each side of the brain. Drug application electrodes were two-barrel KG-33 glass micropipettes (OD 1.5 mm, Garner Glass Co., Claremont, CA, USA) pulled by a micropipette puller (David Kopf Instruments, Inc., Tujunga, CA) to a fine tip and subsequently broken to attain a tip diameter of ~10 μm as done previously (Chacron and Bastian, 2008, Toporikova and Chacron, 2009). The two barrels were used for separate or simultaneous application of ket (100 μM) and 5-HT (1 mM) in both hemispheres of the ELL using a PicoSpritzer III (Parker Hannifin, Cleveland, OH). EOD frequency changes due to a 4 Hz amplitude modulation (AM) were recorded previous to drug application (control), to ket alone as well as to a subsequent 5-HT application. After 45 min, behavioral responses were recorded to a sole application of 5-HT to prove the correct positioning of the electrodes. Note that previous studies have shown that saline applied in this manner does not significantly alter behavioral responses (Deemyad et al., 2013).
Statistics and presentation of data
As previous studies have reported significant heterogeneities within the ELL pyramidal cell population (see (Maler, 2009) for review), we normalized all quantities with respect to control values in order to minimize the effects of such heterogeneities before performing statistical comparisons as done previously (Deemyad et al., 2011, Deemyad et al., 2012, Deemyad et al., 2013). However, we report un-normalized population-averages (mean ± SEM) in the results throughout. Error bars in the figures represent ±1 SEM. All statistical tests consisted of one-way ANOVAs with Tukey post-hoc HSD to allow for comparison between all groups.
Results
Ket blocks the effects of 5-HT on pyramidal neuron firing in vitro
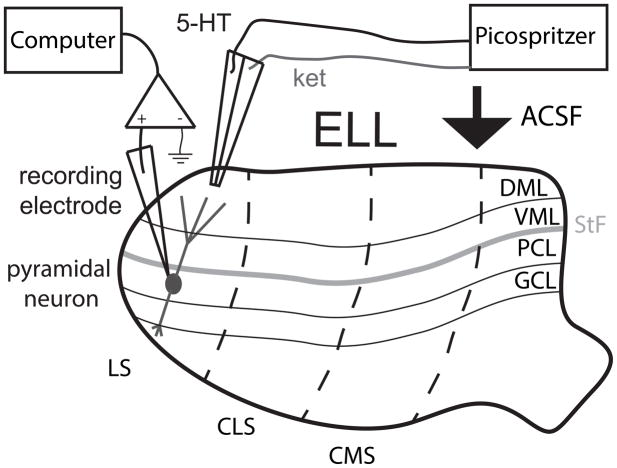
We first tested whether ket would block the effects of 5-HT on ELL pyramidal neuron excitability in vitro. To do so, we used the setup depicted in Fig. 1.
Figure 1.
Schematic of a typical ELL slice as it would appear in a recording chamber. The tractus stratum fibrosum (StF) is readily apparent in vitro and presents a landmark located just above the pyramidal cell layer (PCL). The slice is optimally placed for the artificial cerebrospinal fluid (ACSF) to flow over the molecular layer of the ELL (Dorsal Molecular Layer, DML and Ventral Molecular Layer, VML) as shown by the arrow. Accordingly, picospritzer pipettes were placed in the DML to allow for both ket and 5-HT diffusion across DML, VML, StF, PCL. GCL: granule cell layer.
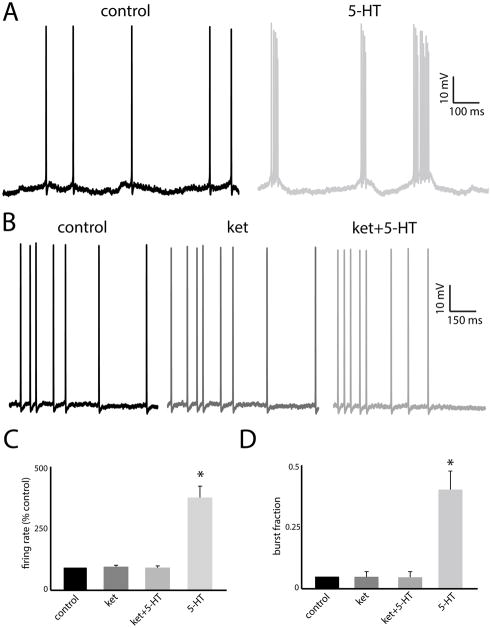
Previous studies have shown that 5-HT application will increase pyramidal neuron excitability in LS, causing increased burst firing (i.e. an increased tendency to fire packets of action potentials followed by quiescence) (Deemyad et al., 2011). Thus, we first tested whether ket would block the effects of 5-HT on increased burst firing. Fig. 2A shows the recorded transmembrane potential from a typical LS pyramidal neuron under control conditions (left) and after 5-HT application (right). It can be seen that 5-HT has a large effect on pyramidal neuron excitability by promoting the firing of packets of action potentials followed by quiescence (i.e. “bursts”), confirming previous results (Deemyad et al., 2011). In contrast, Fig. 2B shows the recorded transmembrane potential from a typical LS pyramidal neuron under control (left), after ket application (center), and after ket+5-HT application (right). Comparing all traces revealed that: 1) ket did not have any noticeable effects on pyramidal neuron excitability by itself; and 2) 5-HT applied after ket did not cause a transition to burst firing. Rather, 5-HT applied after ket did not have any noticeable effects on pyramidal neuron excitability.
Figure 2.
A: Example voltage traces from a pyramidal neuron under control (left) and after 5-HT application (right). Note the transition from tonic to burst firing. B: Example voltage traces from a pyramidal neuron under control (left), after ket application (center), and after ket+5-HT application (right). Note the similarity between the traces. C: Population-averaged firing rates under all conditions. D: Population-averaged burst fractions under all conditions. “*” indicates that the 5-HT group is significantly different from all others at the p=0.05 level using a one-way ANOVA with Tukey post-hoc highly significant difference (HSD) (n=7). All other comparisons between group pairs were not significant (p>0.1).
We quantified these data by computing the mean firing rate as well as the burst fraction (i.e. the fraction of interspike intervals less than 10 ms). 5-HT caused a large and significant increase in both firing rate (control: 5.11 ± 0.95 Hz; 5-HT: 12.45 ± 1.42 Hz; Fig. 2C) and burst fraction (control: 0.06 ± 0.06 Hz; 5-HT: 0.37 ± 0.12 Hz; Fig. 2D). In contrast, neither ket nor ket+5-HT caused any significant change in either firing rate (control: 5.96 ± 1.65 Hz; ket: 5.45 ± 1.30 Hz; ket+5-HT: 5.42 ± 1.62 Hz; Fig. 2C) or burst fraction (control: 0.07 ± 0.02; ket: 0.06 ± 0.01; ket+5-HT: 0.07 ± 0.03; Fig. 2D). Thus, these data show that ket can block the effects of 5-HT on pyramidal neuron excitability.
Ket blocks the effects of 5-HT on pyramidal neuron AHP in vitro
We further tested that ket can indeed block the effects of 5-HT by quantifying the AHP after each spike. To do so, we computed the average spike shape under each condition. Fig. 3A shows the average spike shapes under control (black) and after 5-HT application (gray). Confirming previous results (Deemyad et al., 2011), 5-HT increases pyramidal neuron excitability by decreasing the AHP after each spike (Fig. 3A, compare black and gray traces). In contrast, the average spike shapes obtained under control (Fig. 3B, black), after ket application (Fig. 3B, dark gray), and after ket+5-HT application (Fig. 3B, light gray) were similar to one-another. We then quantified the AHP as done previously. While 5-HT caused a large reduction (control: 3.12 ± 0.72 mV; 5-HT: 1.62 ± 0.43 mV), neither ket nor ket+5-HT caused significant changes in the AHP (control: 3.45 ± 0.58 mV; ket: 3.50 ± 0.62 mV; ket+5-HT: 3.45 ± 0.64 mV; Fig. 3C).
Figure 3.
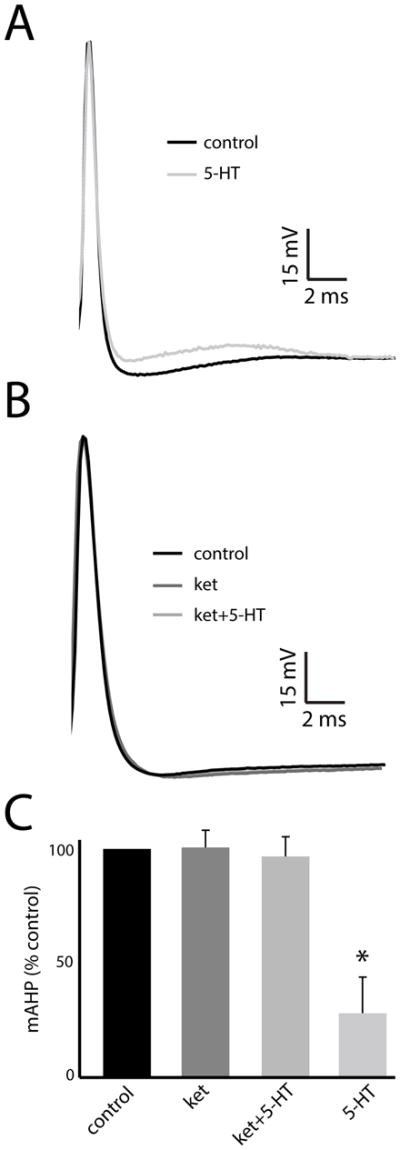
A: Average spike shapes under control (black) and after 5-HT application (light gray). B: Average spike shapes under control (black), after ket application (dark gray), and after ket+5-HT application (medium gray). C: Population-averaged AHP values under all conditions. “*” indicates that the 5-HT group is significantly different from all others at the p=0.05 level using a one-way ANOVA with Tukey post-hoc HSD (n=7). All other comparisons between group pairs were not significant (p>0.15).
Ket blocks the effects of 5-HT on pyramidal neuron responses to current injection
We next tested whether ket would block the effects of 5-HT on pyramidal neuron responses to different current injections. To do so, we injected different current steps and computed the firing rate as a function of current (i.e. “f–I”) curve. Fig. 4A shows recorded voltage traces at different holding currents for control, after ket application, after ket+5-HT application, and after 5-HT application. It can be seen that the frequency of firing increases at the same rate for control, ket, and ket+5-HT but instead increases at a larger rate for 5-HT. We quantified these changes by computing the slope of the f–I curve (i.e. the gain) (Fig. 4B). While 5-HT significantly increased the gain (control: 0.12 ± 0.02 kHz/nA; 5-HT: 0.23 ± 0.04 kHz/nA), neither ket nor ket+5-HT caused any significant changes in gain (control: 0.13 ± 0.02 kHz/nA; ket: 0.11 ± 0.03 kHz/nA; ket+5-HT: 0.13 ± 0.02 kHz/nA; Fig. 4C).
Figure 4.
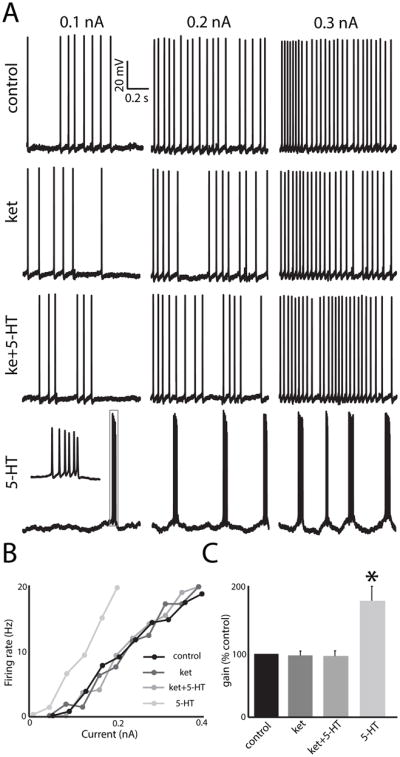
A: Example responses from an example pyramidal neuron to different levels of current injection under control, after ket application, after ket+5-HT application, and after 5-HT application. B: f–I curves from this same neuron under control (black), after ket application (dark gray), and after ket+5-HT application (medium gray), and after 5-HT application (light gray). C: Population-averaged gain values. “*” indicates that the 5-HT group is significantly different from all others at the p=0.05 level using a one-way ANOVA with Tukey post-hoc HSD (n=7). All other comparisons between group pairs were not significant (p>0.21).
Ket blocks the effects of 5-HT behavioral responses
We next tested whether ket blocks the effects of 5-HT injection on behavioral responses to electrosensory stimuli. In particular, we focused on two behaviors: the jamming avoidance response (JAR) as well as the production of electrocommunication stimuli called “chirps” that occur during agonistic situations (Zakon et al., 2002, Hupe et al., 2008). The JAR is a complex behavior by which the animal shifts its EOD frequency in order to increase the frequency content of jamming stimuli that occur when two conspecifics are near to one another, thereby shifting it away from the frequency range of other stimuli such as prey (Heiligenberg, 1991) and is frequently used as a measure of perception in weakly electric fish (Carlson and Kawasaki, 2006). The rate at which chirps are emitted was used as a control as previous studies have shown that 5-HT injection will reduce chirp production (Maler and Ellis, 1987, Smith and Combs, 2008, Deemyad et al., 2013).
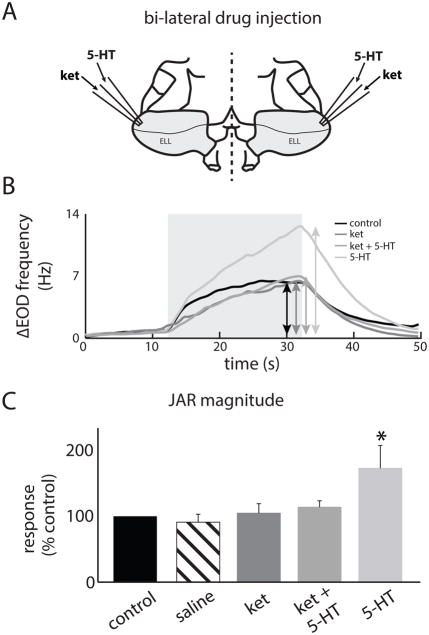
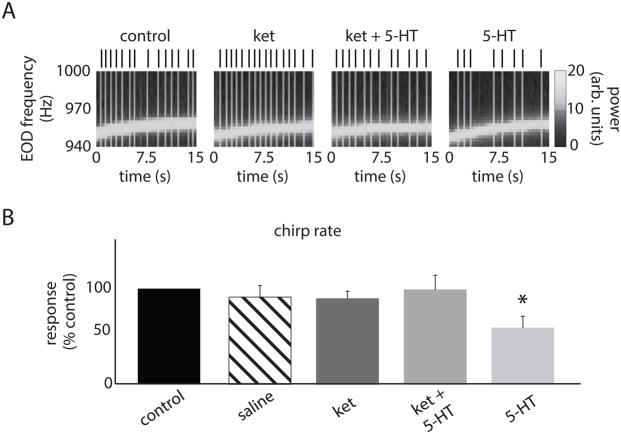
We recorded behavioral responses of immobilized animals and injected all compounds bilaterally in the ELL (Fig. 5A). This was done in order to ensure that any changes in behavior resulting from drug injection were solely due to altered ELL pyramidal cell output rather than the drug affecting another brain area (Deemyad et al., 2013). We found that 5-HT application significantly increased the JAR magnitude (control: 7.02 ± 1.34 Hz; 5-HT: 11.65 ± 2.36 Hz; Figs. 5B,C). However, ket application, which by itself did not significantly alter the JAR, did block the effects of subsequent 5-HT application (i.e. ket+5-HT) on the JAR (ket: 7.23 ± 2.36 Hz; ket+5-HT: 8.1 ± 1.23 Hz; Figs. 5B,C). Injection of saline alone did not have any significant effect on the JAR magnitude (saline: 6.63 ± 1.56 Hz, Fig. 5C). We counted the number of chirps using the EOD spectrogram as done previously (Hupe and Lewis, 2008) (Fig. 6A). While 5-HT application reduced the number of chirps per unit time, ket application by itself did not significantly alter chirp rate but blocked the effects of subsequent application of 5-HT (Fig. 6A). These effects were confirmed across our dataset (control: 0.51 ± 0.18 Hz; ket: 0.48 ± 0.18 Hz; ket+5-HT: 0.43 ± 0.14 Hz; 5-HT: 0.23 ± 0.13 Hz; Fig. 6B). Injecting saline alone did not have any significant effect on the chirp rate (saline: 0.49 ± 0.17 Hz, Fig. 5C). We thus conclude that ket can block the effects of 5-HT on JAR and chirp rate when applied in ELL.
Figure 5.
A: Schematic of the experimental setup used for bilateral exogenous ket and 5-HT application in the ELL. Two electrodes containing the drugs were placed in each ELL (Materials and Methods). B: EOD frequency from an example specimen as a function of time before (black) and after drug application (ket: dark gray, ket+5-HT: medium gray, 5-HT: light gray). The double-head arrows indicate the frequency excursion while the light gray square shows the time period during which the stimulus was applied. C: JAR magnitude before application (black), after saline application (striped), after ket application (dark gray), after ket+5-HT application (medium gray), and after 5-HT application (light gray). “*” indicates that the 5-HT group is significantly different from all others at the p=0.05 level using a one-way ANOVA with Tukey post-hoc HSD (n=7). All other comparisons between group pairs were not significant (p>0.8).
Figure 6.
A: EOD spectrogram (i.e., EOD power spectrum as a function of time) with the vertical bands corresponding to the black vertical bars representing chirps before injection (outside left), after ket injection (left), after ket+5-HT injection (right), and after 5-HT injection (outside right). B: Chirp rate before injection (black), after saline injection (striped), after ket injection (dark gray), after ket+5-HT injection (medium gray), and after 5-HT injection (light gray). “*” indicates that the 5-HT group is significantly different from all others at the p=0.05 level using a one-way ANOVA with Tukey post-hoc HSD (n=7). All other comparisons between group pairs were not significant (p>0.8).
Discussion
Summary of results
We have investigated the nature of the receptors that mediate the previously observed effects of 5-HT on the excitability of ELL pyramidal cells and the resulting effects on electrosensory behaviors. First, using an in vitro preparation, we found that the 5-HT2 antagonist ket blocked the effects of 5-HT on pyramidal cell excitability using several measures. Specifically, while 5-HT gave rise to significant increases in firing rate and burst fraction through a decreased AHP, prior ket application blocked this effect as there was no significant change in either of firing rate, burst fraction, or the AHP. Further, while 5-HT application significantly increased the gain (i.e. the slope of the f–I curve), prior application of ket blocked this effect as it did not lead to significant increases in gain. Importantly, ket application did not significantly alter firing rate, burst fraction, AHP, or gain. Next, using an in vivo preparation, we investigated the effects of 5-HT and ket application within the ELLs on two electrosensory behaviors, namely chirp production and the JAR. While 5-HT application significantly decreased chirp rate and increased the JAR, prior ket application blocked this effect as 5-HT application then did not lead to any significant changes in chirp rate or JAR.
5-HT receptors present in ELL and their effects on potassium channels
Previous studies have found evidence that 5-HT can increase neural excitability by inhibiting potassium channels (Araneda and Andrade, 1991, Fagni et al., 1992, Tanaka and Nishizuka, 1994). In particular, there is strong evidence that activation of 5-HT2 receptors will inhibit potassium channels (see (D’Adamo et al., 2013) for review) whereas activation of 5-HT1 receptors will instead activate these (Kelly et al., 1991, Raymond et al., 2001). Previous studies have found that 5-HT increases ELL pyramidal cell excitability by inhibiting potassium conductances (Deemyad et al., 2011), which is similar to decreases in AHP seen in other systems (Araneda and Andrade, 1991). Our results showing that the 5-HT2 antagonist ket can block the effects of 5-HT on ELL pyramidal cell excitability and the resulting changes in behavior thus strongly support the hypothesis that these effects are mediated by 5-HT2 receptors.
Previous results have found that ket is a selective antagonist for 5-HT2 with greater affinity for 5HT2A/2C receptors in weakly electric fish (Allee et al., 2008). The similarity between the effects of 5-HT agonist and antagonists used in weakly electric fish and in mammals (Smith and Combs, 2008), together with the fact that the anatomy of the 5-HT system is remarkably conserved across vertebrates (Parent, 1981), thus make it likely that the effects of 5-HT on ELL pyramidal cell activity are mediated by 5-HT2A/C receptors. Further evidence for this hypothesis comes from the fact that, although previous studies have shown that the 5-HT1A receptor antagonist WAY-100635 can affect behavioral responses in A. leptorhynchus when injected systemically (Smith and Combs, 2008), our preliminary data indicates that this same compound does not block the effects of 5-HT when injected into the ELLs (data not shown). It is therefore unlikely that the effects of 5-HT on ELL pyramidal neuron excitability are mediated by 5-HT1A receptors.
The hypothesis that 5HT2A/C receptors mediate the effects of 5-HT on ELL pyramidal neuron activity is interesting as previous studies have found that 5-HT increased ELL pyramidal cell excitability by inhibiting two different potassium channels: SK and M currents (Deemyad et al., 2011). While SK currents are activated through increases in the intracellular calcium concentration (Adelman et al., 2012), M currents are instead voltage-gated (Yue and Yaari, 2004, Drion et al., 2010). In ELL pyramidal cells, previous studies have found that SK channels were activated following an action potential (i.e. suprathreshold) and lead to an afterhyperpolarization (Ellis et al., 2007b, Toporikova and Chacron, 2009, Deemyad et al., 2011, Deemyad et al., 2012). In contrast, M currents are instead activated at membrane voltages below those necessary to elicit action potential firing (i.e. subthreshold) (Deemyad et al., 2011, Deemyad et al., 2012). A previous study has found that, despite having similar effects on the AHP and ELL pyramidal cell excitability, these currents had opposite effects on frequency tuning (Deemyad et al., 2012). Intrinsic membrane conductances are major determinants of the frequency tuning of ELL pyramidal cells (Ellis et al., 2007a, Ellis et al., 2007b, Krahe et al., 2008, Mehaffey et al., 2008c, Toporikova and Chacron, 2009) and their activation/inhibition by neuromodulators is thus expected to have major effects on their responses to sensory input (Marquez et al., 2013). We thus propose that 5-HT can either increase or decrease ELL pyramidal cell responses to sensory input by inhibiting either SK or M currents through either of 5-HT2A or 5-HT2C receptors. A recent study conducted in vivo has shown that the function of the serotonergic system on sensory processing by ELL pyramidal cells is to selectively enhance their responses to stimuli associated with same sex conspecifics (Deemyad et al., 2013). While these results showed that the effects of 5-HT only enhanced responses, it is important to note that they were obtained through either exogenous 5-HT application or endogenous release via raphe nuclei stimulation. Further studies using more natural behavioral contexts are needed to test whether 5-HT could either enhance or decrease ELL pyramidal cell responses to sensory input.
We note that previous studies have shown that 5-HT expression is graded across the ELL segments in A. leptorhynchus; expression was weakest in the centromedial segment (CMS), intermediate in the centrolateral segment (CLS), and strongest in the lateral segment (L) (Deemyad et al., 2011). In vitro electrophysiological results show excellent correlation with expression across segments as 5-HT did not significantly alter CMS pyramidal cell activity, significantly increased CLS pyramidal cell excitability, and strongly increased LS pyramidal cell excitability (Deemyad et al., 2011). Previous studies have shown that, under control conditions, CMS neurons respond most strongly to stimuli causing a JAR (Shumway, 1989a, Krahe et al., 2008) and the CMS is required for JAR behavior under control conditions (Metzner and Juranek, 1997). However, the results of Deemyad et al. (2011), taken together with the fact that 5-HT injected into ELL will increase LS neuron responses to stimuli eliciting the JAR (Deemyad et al., 2013), provide strong evidence against the hypothesis that CMS neurons contribute to the observed increase in JAR magnitude caused by 5-HT. Rather, we speculate that the increase in JAR behavior is caused by the increased LS neural responses to stimuli eliciting this behavior.
Effects of 5-HT on behavior
Previous studies have shown that 5-HT has significant influences on behavioral responses. In particular, elevated levels of 5-HT have been associated with decreased aggression and social dominance across species (Larson and Summers, 2001, Perreault et al., 2003, Sperry et al., 2003). In weakly electric fish, exogenous 5-HT systemic application decreases aggressivity as measured by the production of electro-communication signals called “chirps” that are produced during aggressive behavioral contexts (Maler and Ellis, 1987, Allee et al., 2008, Smith and Combs, 2008, Zubizarreta et al., 2012, Deemyad et al., 2013) as well as simultaneously increase the perception of low frequency signals generated by same sex conspecifics (Deemyad et al., 2013). Different types of 5-HT receptors have been previously shown to mediate decreases in aggressivity. Indeed, previous studies using the same species of weakly electric fish as our own have shown that activation of 5-HT1A receptor agonists/antagonists increased/decreased chirp production while 5-HT2A receptor agonists/antagonists instead decreased/increased chirp production (Smith and Combs, 2008). Qualitatively similar results were obtained in another species using measures of social dominance (Allee et al., 2008). While our results showing that the 5-HT2A/C receptor antagonist ket has no effect on chirp production appear to contradict the results of Smith and Combs (2008) at first glance, it is important to note that our injections of ket were localized to the ELLs whereas their injections were instead systemic. 5-HT receptors are located throughout the brain of weakly electric fish (Johnston et al., 1990) and, in particular, are present in brain areas controlling chirp production (Telgkamp et al., 2007). Future studies should focus on understanding how different behavioral contexts activate 5-HT receptors across different brain areas from sensory to motor in order to give rise to changes in perception and behavioral responses to stimuli arising from these contexts.
Conclusion
Our results have shown that ket can block the effects of 5-HT of ELL pyramidal cell excitability and the resulting changes in behavior. While the similarity between the effects of 5-HT agonists/antagonists in weakly electric fish and mammals and the remarkable conservation of the 5-HT system across vertebrates lend strong support to our hypothesis that 5-HT2 receptors mediate the effects of 5-HT, it is important to realize that 5-HT receptors have not yet been cloned in A. leptorhynchus. Cloning of these receptors followed by in situ hybridization/immunohistochemistry is necessary to fully establish the nature of the 5-HT receptors present in ELL as well as whether they display functional homology with their mammalian counterparts.
Acknowledgments
This research was supported by the Canadian Institutes of Health Research, the Fonds de Recherche du Québec Nature et technologies, and the Canada Research Chairs (M.J.C.).
List of abbreviations
- 5-HT
serotonin
- AHP
afterhyperpolarization
- ANOVA
analysis of variance
- DML
dorsal molecular layer
- ELL
electrosensory lateral line lobe
- GCL
granule cell layer
- HSD
highly significant difference
- JAR
jamming avoidance response
- PCL
pyramidal cell layer
- SEM
standard error of the mean
- StF
stratum tractus fibrosum
- VML
ventral molecular layer
- ket
ketanserin
References
- Abbott LF. Where are the Switches on This Thing? In: Van Hemmen JL, Sejnowski TJ, editors. 23 Problems in Systems Neuroscience. New York: Oxford; 2005. pp. 423–431. [Google Scholar]
- Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annual review of physiology. 2012;74:245–269. doi: 10.1146/annurev-physiol-020911-153336. [DOI] [PubMed] [Google Scholar]
- Allee SJ, Markham MR, Salazar VL, Stoddard PK. Opposing actions of 5HT1A and 5HT2-like serotonin receptors on modulations of the electric signal waveform in the electric fish Brachyhypopomus pinnicaudatus. Horm Behav. 2008;53:481–488. doi: 10.1016/j.yhbeh.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Avila Akerberg O, Chacron MJ. In vivo conditions influence the coding of stimulus features by bursts of action potentials. Journal of Computational Neuroscience. 2011;31:369–383. doi: 10.1007/s10827-011-0313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bell CC, Maler L. Springer handbook of auditory research: electroreception. New York: Springer; 2005. Central neuroanatomy of electrosensory systems in fish. [Google Scholar]
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annual review of medicine. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BA, Kawasaki M. Ambiguous Encoding of Stimuli by Primary Sensory Afferents Causes a Lack of Independence in the Perception of Multiple Stimulus Attributes. J Neurosci. 2006;26:9173–9183. doi: 10.1523/JNEUROSCI.1513-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CE, Maler L, Sas E. Peripheral organization and central projections of the electrosensory organs in gymnotiform fish. Journal of Comparative Neurology. 1982;211:139–153. doi: 10.1002/cne.902110204. [DOI] [PubMed] [Google Scholar]
- Chacron MJ, Bastian J. Population coding by electrosensory neurons. J Neurophysiol. 2008;99:1825–1835. doi: 10.1152/jn.01266.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacron MJ, Longtin A, Maler L. Simple models of bursting and non-bursting electroreceptors. Neurocomputing. 2001;38:129–139. [Google Scholar]
- Chacron MJ, Longtin A, Maler L. Efficient computation via sparse coding in electrosensory neural networks. Current Opinion in Neurobiology. 2011;21:752–760. doi: 10.1016/j.conb.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Adamo MC, Servettini I, Guglielmi L, Di Matteo V, Di Maio R, Di Giovanni G, Pessia M. 5-HT2 receptors-mediated modulation of voltage-gated K+ channels and neurophysiopathological correlates. Exp Brain Res. 2013;230:453–462. doi: 10.1007/s00221-013-3555-8. [DOI] [PubMed] [Google Scholar]
- Deemyad T, Kroeger J, Chacron MJ. Sub- and suprathreshold adaptation currents have opposite effects on frequency tuning. J Physiol (lond) 2012;590:4839–4858. doi: 10.1113/jphysiol.2012.234401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deemyad T, Maler L, Chacron MJ. Inhibition of SK and M channel mediated currents by 5-HT enables parallel processing by bursts and isolated spikes. J Neurophysiol. 2011;105:1276–1294. doi: 10.1152/jn.00792.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deemyad T, Metzen MG, Pan Y, Chacron MJ. Serotonin selectively enhances perception and sensory neural responses to stimuli generated by same-sex conspecifics. PNAS. 2013;110:19609–19614. doi: 10.1073/pnas.1314008110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doiron B, Chacron MJ, Maler L, Longtin A, Bastian J. Inhibitory feedback required for network oscillatory responses to communication but not prey stimuli. Nature. 2003;421:539–543. doi: 10.1038/nature01360. [DOI] [PubMed] [Google Scholar]
- Drion G, Bonjean M, Waroux O, Scuvee-Moreau J, Liegeois JF, Sejnowski TJ, Sepulchre R, Seutin V. M-type channels selectively control bursting in rat dopaminergic neurons. Eur J Neurosci. 2010;31:827–835. doi: 10.1111/j.1460-9568.2010.07107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis LD, Krahe R, Bourque CW, Dunn RJ, Chacron MJ. Muscarinic receptors control frequency tuning through the downregulation of an A-type potassium current. J Neurophysiol. 2007a;98:1526–1537. doi: 10.1152/jn.00564.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis LD, Maler L, Dunn RJ. Differential distribution of SK channel subtypes in the brain of the weakly electric fish Apteronotus leptorhynchus. Journal of Comparative Neurology. 2008;507:1964–1978. doi: 10.1002/cne.21597. [DOI] [PubMed] [Google Scholar]
- Ellis LD, Mehaffey WH, Harvey-Girard E, Turner RW, Maler L, Dunn RJ. SK channels provide a novel mechanism for the control of frequency tuning in electrosensory neurons. J Neurosci. 2007b;27:9491–9502. doi: 10.1523/JNEUROSCI.1106-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ESL, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci. 2002;22:1618–1628. doi: 10.1523/JNEUROSCI.22-05-01618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagni L, Dumuis A, Sebben M, Bockaert J. The 5-HT4 receptor subtype inhibits K+ current in colliculi neurones via activation of a cyclic AMP-dependent protein kinase. British journal of pharmacology. 1992;105:973–979. doi: 10.1111/j.1476-5381.1992.tb09087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiligenberg W. Neural Nets in Electric Fish. Cambridge MA: MIT Press; 1991. [Google Scholar]
- Heiligenberg W, Dye J. Labelling of electrosensory afferents in a gymnotid fish by intracellular injection of HRP: The mystery of multiple maps. Journal of Comparative Physiology A-Sensory Neural & Behavioral Physiology. 1982;148:287–296. [Google Scholar]
- Hitschfeld ÉM, Stamper SA, Vonderschen K, Fortune ES, Chacron MJ. Effects of Restraint and Immobilization on Electrosensory Behaviors of Weakly Electric Fish. ILAR Journal. 2009;50:361–372. doi: 10.1093/ilar.50.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacology, biochemistry, and behavior. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Hupe GJ, Lewis JE. Electrocommunication signals in free swimming brown ghost knifefish, Apteronotus leptorhynchus. J Exp Biol. 2008;211:1657–1667. doi: 10.1242/jeb.013516. [DOI] [PubMed] [Google Scholar]
- Hupe GJ, Lewis JE, Benda J. The effect of difference frequency on electrocommunication: chirp production and encoding in a species of weakly electric fish, Apteronotus leptorhynchus. J Physiol Paris. 2008;102:164–172. doi: 10.1016/j.jphysparis.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol. 2004;14:488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Janssen P, Prins NH, Meulemans AL, Lefebvre RA. Smooth muscle 5-HT2A receptors mediating contraction of porcine isolated proximal stomach strips. British journal of pharmacology. 2002;137:1217–1224. doi: 10.1038/sj.bjp.0704992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SA, Maler L, Tinner B. The distribution of serotonin in the brain of Apteronotus leptorhynchus: an immunohistochemical study. Journal of chemical neuroanatomy. 1990;3:429–465. [PubMed] [Google Scholar]
- Kelly JS, Larkman P, Penington NJ, Rainnie DG, McAllister-Williams H, Hodgkiss J. Serotonin receptor heterogeneity and the role of potassium channels in neuronal excitability. Advances in experimental medicine and biology. 1991;287:177–191. doi: 10.1007/978-1-4684-5907-4_15. [DOI] [PubMed] [Google Scholar]
- Khosravi-Hashemi N, Chacron MJ. Bursts and isolated spikes code for opposite movement directions in midbrain electrosensory neurons. PLoS One. 2012;7:e40339. doi: 10.1371/journal.pone.0040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi-Hashemi N, Chacron MJ. Motion processing across multiple topographic maps in the electrosensory system. Physiological Reports. 2014;2:e00253. doi: 10.1002/phy2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi-Hashemi N, Fortune ES, Chacron MJ. Coding Movement Direction by Burst Firing in Electrosensory Neurons. J Neurophysiol. 2011;106:1954–1968. doi: 10.1152/jn.00116.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Appel SB. Characterization of M-current in ventral tegmental area dopamine neurons. J Neurophysiol. 2006;96:535–543. doi: 10.1152/jn.00574.2005. [DOI] [PubMed] [Google Scholar]
- Krahe R, Bastian J, Chacron MJ. Temporal processing across multiple topographic maps in the electrosensory system. J Neurophysiol. 2008;100:852–867. doi: 10.1152/jn.90300.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson ET, Summers CH. Serotonin reverses dominant social status. Behav Brain Res. 2001;121:95–102. doi: 10.1016/s0166-4328(00)00393-4. [DOI] [PubMed] [Google Scholar]
- Maler L. Receptive field organization across multiple electrosensory maps. I. Columnar organization and estimation of receptive field size. Journal of Comparative Neurology. 2009;516:376–393. doi: 10.1002/cne.22124. [DOI] [PubMed] [Google Scholar]
- Maler L, Collins M, Mathieson WB. The distribution of acetylcholinesterase and choline acetyl transferase in the cerebellum and posterior lateral line lobe of weakly electric fish (Gymnotidae) Brain research. 1981;226:320–325. doi: 10.1016/0006-8993(81)91106-9. [DOI] [PubMed] [Google Scholar]
- Maler L, Ellis WG. Inter-male aggressive signals in weakly electric fish are modulated by monoamines. Behav Brain Res. 1987;25:75–81. doi: 10.1016/0166-4328(87)90046-5. [DOI] [PubMed] [Google Scholar]
- Marquez BT, Krahe R, Chacron MJ. Neuromodulation of early electrosensory processing in gymnotiform weakly electric fish. J Exp Biol. 2013;216:2442–2450. doi: 10.1242/jeb.082370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsat G, Maler L. Neural heterogeneity and efficient population codes for communication signals. J Neurophysiol. 2010;104:2543–2555. doi: 10.1152/jn.00256.2010. [DOI] [PubMed] [Google Scholar]
- Marsat G, Proville RD, Maler L. Transient signals trigger synchronous bursts in an identified population of neurons. J Neurophysiol. 2009;102:714–723. doi: 10.1152/jn.91366.2008. [DOI] [PubMed] [Google Scholar]
- Mathieson WB, Maler L. Morphological and electrophysiological properties of a novel in vitro preparation: the electrosensory lateral line lobe brain slice. Journal of Comparative Physiology A. 1988;163:489–506. doi: 10.1007/BF00604903. [DOI] [PubMed] [Google Scholar]
- Mehaffey WH, Doiron B, Maler L, Turner RW. Deterministic multiplicative gain control with active dendrites. J Neurosci. 2005;25:9968–9977. doi: 10.1523/JNEUROSCI.2682-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehaffey WH, Ellis LD, Krahe R, Dunn RJ, Chacron MJ. Ionic and Neuromodulatory Regulation of Burst Discharge Controls Frequency Tuning. J Physiol (Paris) 2008a;102:195–208. doi: 10.1016/j.jphysparis.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehaffey WH, Maler L, Turner RW. Intrinsic frequency tuning in ELL pyramidal cells varies across electrosensory maps. J Neurophysiol. 2008b;99:2641–2655. doi: 10.1152/jn.00028.2008. [DOI] [PubMed] [Google Scholar]
- Mehaffey WH, Maler L, Turner RW. Intrinsic frequency tuning in ELL pyramidal cells varies across electrosensory maps. J Neurophysiol. 2008c;99:2641–2655. doi: 10.1152/jn.00028.2008. [DOI] [PubMed] [Google Scholar]
- Metzner W, Juranek J. A sensory brain map for each behavior? PNAS. 1997;94:14798–14803. doi: 10.1073/pnas.94.26.14798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald AMM, Chacron MJ, Doiron B, Bastian J, Maler L. Parallel Processing of Sensory Input by Bursts and Isolated Spikes. J Neurosci. 2004;24:4351–4362. doi: 10.1523/JNEUROSCI.0459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A. Comparative anatomy of the serotoninergic systems. J Physiol (Paris) 1981;77:147–156. [PubMed] [Google Scholar]
- Perreault HA, Semsar K, Godwin J. Fluoxetine treatment decreases territorial aggression in a coral reef fish. Physiology & behavior. 2003;79:719–724. doi: 10.1016/s0031-9384(03)00211-7. [DOI] [PubMed] [Google Scholar]
- Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacology & therapeutics. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- Shumway C. Multiple electrosensory maps in the medulla of weakly electric Gymnotiform fish. I. Physiological differences. J Neurosci. 1989a;9:4388–4399. doi: 10.1523/JNEUROSCI.09-12-04388.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway C. Multiple electrosensory maps in the medulla of weakly electric Gymnotiform fish. II. Anatomical differences. J Neurosci. 1989b;9:4400–4415. doi: 10.1523/JNEUROSCI.09-12-04400.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GT, Combs N. Serotonergic activation of 5HT1A and 5HT2 receptors modulates sexually dimorphic communication signals in the weakly electric fish Apteronotus leptorhynchus. Horm Behav. 2008;54:69–82. doi: 10.1016/j.yhbeh.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Sperry TS, Thompson CK, Wingfield JC. Effects of acute treatment with 8-OH-DPAT and fluoxetine on aggressive behaviour in male song sparrows (Melospiza melodia morphna) Journal of neuroendocrinology. 2003;15:150–160. doi: 10.1046/j.1365-2826.2003.00968.x. [DOI] [PubMed] [Google Scholar]
- Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signalling. Annual Review of Neuroscience. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- Telgkamp P, Combs N, Smith GT. Serotonin in a diencephalic nucleus controlling communication in an electric fish: sexual dimorphism and relationship to indicators of dominance. Developmental neurobiology. 2007;67:339–354. doi: 10.1002/dneu.20356. [DOI] [PubMed] [Google Scholar]
- Toporikova N, Chacron MJ. Dendritic SK channels gate information processing in vivo by regulating an intrinsic bursting mechanism seen in vitro. J Neurophysiol. 2009;102:2273–2287. doi: 10.1152/jn.00282.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RW, Maler L, Burrows M. Electroreception and electrocommunication. J Exp Biol. 1999;202:1167–1458. [Google Scholar]
- Wark B, Lundstrom BN, Fairhall A. Sensory adaptation. Current Opinion in Neurobiology. 2007;17:423–429. doi: 10.1016/j.conb.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue C, Yaari Y. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J Neurosci. 2004;24:4614–4624. doi: 10.1523/JNEUROSCI.0765-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakon HH, Oestreich J, Tallarovic S, Triefenbach F. EOD modulations of brown ghost electric fish: JARs, chirps, rises, and dips. J Physiol (Paris) 2002;96:451–458. doi: 10.1016/S0928-4257(03)00012-3. [DOI] [PubMed] [Google Scholar]
- Zubizarreta L, Perrone R, Stoddard PK, Costa G, Silva AC. Differential serotonergic modulation of two types of aggression in weakly electric fish. Frontiers in behavioral neuroscience. 2012;6:77. doi: 10.3389/fnbeh.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]