Abstract
The first and last occurrences of hexapod families in the fossil record are compiled from publications up to end-2009. The major features of these data are compared with those of previous datasets (1993 and 1994). About a third of families (>400) are new to the fossil record since 1994, over half of the earlier, existing families have experienced changes in their known stratigraphic range and only about ten percent have unchanged ranges. Despite these significant additions to knowledge, the broad pattern of described richness through time remains similar, with described richness increasing steadily through geological history and a shift in dominant taxa, from Palaeoptera and Polyneoptera to Paraneoptera and Holometabola, after the Palaeozoic. However, after detrending, described richness is not well correlated with the earlier datasets, indicating significant changes in shorter-term patterns. There is reduced Palaeozoic richness, peaking at a different time, and a less pronounced Permian decline. A pronounced Triassic peak and decline is shown, and the plateau from the mid Early Cretaceous to the end of the period remains, albeit at substantially higher richness compared to earlier datasets. Origination and extinction rates are broadly similar to before, with a broad decline in both through time but episodic peaks, including end-Permian turnover. Origination more consistently exceeds extinction compared to previous datasets and exceptions are mainly in the Palaeozoic. These changes suggest that some inferences about causal mechanisms in insect macroevolution are likely to differ as well.
Introduction
A key contribution of palaeontology to the study of the diversity of life has been the elucidation of macroevolutionary patterns and processes through deep time, with fossils providing the only direct temporal evidence of how life has responded to a variety of biotic and abiotic forces [1–4]. If there are general rules underlying macroevolutionary responses to these forces, studying the past may also inform the future. Palaeontology can therefore, potentially, provide important information on the future progression of the extinction crisis facing the biosphere today, and its likely consequences [1,5].
In addition to such strategic questions, palaeontological data can help solve many basic questions of perennial interest. Comprising over 50% of described species [6], hexapods (insects and their close relatives such as springtails) form a major component of almost all continental ecosystems. An explanation of how and why this group has come to so dominate terrestrial biodiversity is a major challenge in macroevolutionary biology.
Palaeodiversity data are typically compiled in the form of taxonomic databases of fossils that provide either temporal ranges or discrete occurrence data. Commonly, criticisms of such databases focus around the integrity of the data and its resilience to the addition of further information [7]. Substantial additional knowledge, both taxonomic and stratigraphic, of the fossil records of tetrapods [8] and all marine animal families [9], has nonetheless yielded very similar variation in originations and extinctions though time. This supports the notion that broad biological signals can be seen through the statistical noise of an imperfect fossil record, providing the error is randomly distributed [10]. However, the effect of additional data on macroevolutionary patterns has not been tested for the majority of continental groups. This is important because many terrestrial taxa, such as insects, preserved primarily in exceptional conditions (Lagerstätten taxa) are likely to have substantially incomplete fossil records where the potential for change is much greater.
Using data on the temporal ranges of families, Labandeira [11] and Labandeira and Sepkoski [12] considered that, apart from the Late–end-Permian extinction, no other mass extinction event known from other groups appears to have had any major impact on insects at the family level. In addition, a steady increase of insect family-level richness began in the Triassic and was attributable not to particularly high levels of origination, but to consistently low extinction–noticeably lower than that in the Palaeozoic. The rise of angiosperms during the Cretaceous apparently did not cause any increase in family diversity in insects and may even have caused some decline in richness into the Late Cretaceous. However, Labandeira and Sepkoski [12] noted that much of the variation around this long term trend of increasing richness could be linked to specific rich fossil deposits (Lagerstätten) or stages where insect-bearing fossil deposits are poorly known and so were cautious with any such interpretations. Jarzembowski and Ross [13], using data based on but slightly updated from Ross and Jarzembowksi [14], highlighted four major insect origination events; during the Permo-Carboniferous, Early Jurassic, Early Cretaceous and the Eocene. They concurred with Labandeira and Sepkoski [12] that today’s exceptionally high insect diversity is the result of low extinction levels and sustained origination but disagreed that insects were essentially immune to mass extinction after the end-Permian event. Highlighting in particular an apparent decline in family richness seen in the Upper Cretaceous record, they suggest a causal link to the radiation of angiosperms. Additionally, Ross et al. [15] noted the increase in counts of origination and extinction in the Cretaceous as evidence of ecological turnover associated with angiosperms.
The field of palaeoentomology has expanded rapidly in the last two decades, with large increases in the number of active researchers and consequent publication output [16], as well important changes in taxonomy (e.g. the resurrection of the order Cnemidolestodea [17]), the dating of fossil deposits (e.g. the recognition of the mid-Cretaceous age of Burmese amber; see [18,19]) and the exploration of newly discovered insect-bearing formations globally (e.g. the Eocene amber deposits of India [20]).
To take account of these developments, in the first instance, a new dataset of the temporal ranges of hexapod families, compiled from literature (about 2,500 papers) published up to the end of 2009, is compared with that of Ross and Jarzembowski [14] (data from literature published up to the end of 1991) and Labandeira [11] by documenting changes and additions to the data. Then richness time series derived from these datasets are compared to assess any change in the signal provided by the fossil record in light of additional data. Although many recent studies of fossil richness through time have been derived from sample-based occurrence data (e.g. [1]), which facilitates the elimination of biases, studies of the face-value record are still valuable from a comparative perspective: they will, for example, help in the understanding of sampling-based artefacts by comparison with the geological record (e.g. [21,22]). In addition, taxonomic range data still retain considerable utility for the dating of phylogenies. There have to-date been no sample-standardized studies of fossil insect richness, and, although constraints on the completeness and comparability of data may limit what can currently be achieved, we expect that future studies will attempt them. A breakdown of the new data show which main groups of hexapods make a dominant contribution to the signal through time. From the first and last occurrence data, rates of origination and extinction can be calculated per stage indicating the timing of major radiation and extinction events as well as long-term trends and the relative importance of these to hexapod family richness.
Methods
Changes and additions to the hexapod fossil record
We quantified the amount of change in the new dataset (NEW) relative to the fossil insect family datasets presented by Ross and Jarzembowski [14] (downloaded from www.fossilrecord.net 2012-10-05) and Labandeira [11] (referred to herein as FR2 and LAB, respectively). The NEW dataset is dated according to the International Commission on Stratigraphy stages [23]. First, each family in NEW was categorised in the following ways with respect to FR2 and LAB: ‘no change’, ‘new in list’ and ‘range change’. The first of these is self-explanatory with respect to LAB, which, like NEW, presents data at stage resolution. However, FR2 presents data at both epoch and stage level, and no change for a family where data in FR2 were given at epoch or period level represents a case where the data in NEW confirm it was indeed present throughout that epoch or period. ‘New in list’ can refer to newly described families, those brought out of synonymy or Recent families which now have a fossil record. ‘Range change’, used only for comparison with FR2, involves a change in the recorded stratigraphic range of a family, whether an extension or contraction from the finding of new specimens but also includes improved resolution or revised dating of deposits from which previously known specimens occur (i.e. the deposit is now dated to a different stage). Since most of the LAB data is resolved to stage level and so is more directly comparable with the new data, range change was subdivided into three categories: contraction, extension and shift. A contraction is any situation where the NEW range has fewer stages than recorded in LAB, while an extension is any family where the new range covers a greater number of stages. This does not distinguish between whether the first and/or last occurrence has changed to create the contraction or extension and can also include instances where the NEW range has no overlap with that in LAB, e.g. the palaeodictyopteran family Hanidae, P1(Artinskian) in LAB but C2(Gzhelian)–P1(Sakmarian) in the new dataset. Shifts represent when the NEW range for a family covers a different set but the same total number of stages.
Derivation of richness time series from origination and extinction data
Before describing how various time series can be derived from first and last occurrence data, it is worth defining the four classes of taxa which can be counted in a time interval [24] (Fig 1).
Fig 1. Four classes of taxa recorded in an interval using first and last occurrence data.
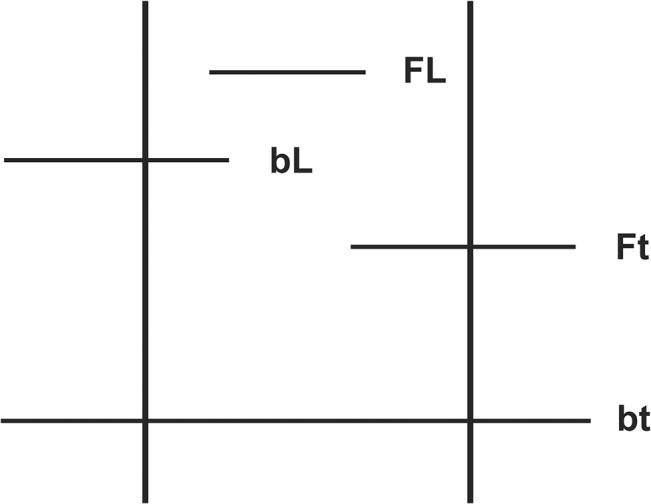
After Foote [24]. The horizontal axis represents time progressing from left to right. The vertical lines represent the start (left) and end (right) of a specified time interval of interest. Horizontal lines represent the temporal ranges of four types of taxa of interest: FL originates and goes extinct within the interval, bL originates before and becomes extinct within the interval, Ft originates within and continues beyond the interval and bt originates before and continues after the interval.
Some taxa (bt: bottom, top) originate before the time interval in question and have their last occurrence sometime after it, thus crossing the bottom and top boundaries. Some taxa (bL: bottom, Last) originate before the interval and have their last occurrence within it. Others (Ft: First, top) first appear in the interval and range beyond it. Finally, still others (FL: First, Last–also known as single-interval taxa) appear to originate and go extinct entirely within the interval, never crossing either the bottom or top boundaries. The term ‘single-interval taxon’ is preferable to the commonly used term ‘singleton’ when describing such taxa (as unfortunately done in e.g. [24–26]) as the word is already in common usage in ecology for taxa represented by one specimen [27,28].
Two commonly-used counting methods exist for deriving diversity time series from first and last occurrence data–range through (RT) and boundary crossers (BC), and a third employed here, minimum assumption (MIN) [22,28]. These are applied to NEW and LAB data, while with the FR2 data only RT is used but under two assumptions–FR2+ and FR2–, explained below.
RT is the classic method of counting a taxon as present in every stage between and including its first and last occurrences in the fossil record (or up to the present day if still extant), as well as those which originate and go extinct within the same time interval (known as single-interval taxa or FL in the notation given above), used, for example, by Sepkoski [9], Labandeira and Sepkoski [12] and Jarzembowski and Ross [13]. This is the sum total of taxa observed and inferred to exist within a time interval and can be written as RT = bt+Ft+bL+FL. For FR2, inconsistent stratigraphic resolution makes it necessary to use maximum and minimum assumptions of the ranges given when comparing with datasets at stage level. FR2+, then, is based on the assumption that the family originates in the first stage of the interval in which lies its first appearance and goes extinct in the last stage of the interval containing its last appearance, while FR2– assumes the origination in the last stage of the interval of first appearance and extinction in the first stage of the interval of last appearance [5]. Consequently, any family which is recorded at epoch or period level but in only one interval is removed from the FR2– series.
The BC series are made up of only those taxa which range between two or more time intervals, i.e. excluding single-interval taxa (FL). However, they are not simply RT minus FL. Rather, BC series represent the number of taxa crossing the bottom boundary into the interval, thereby tying diversity to a single point in time (the boundary) and not adding that diversity to events which occur cumulatively within the interval. It can be written as BC = bt+bL. By restricting the richness count to taxa which cross a single point in time, the data record an actual faunal cohort rather than the accumulation of taxa which exist throughout an interval. The specific advantage of this is that it is immune to changes in interval length, while it might be expected that longer intervals will accumulate more taxa than shorter ones, thereby inflating the richness measurement for that observation point. BC series have found use in some more recent palaeodiversity studies [29–31] and have been advocated within the palaeoentomological community more recently by Ponomarenko and Dmitriev [32]. As these are values for interval boundaries, in order to make possible the comparison with data within intervals (placed at stage-midpoint) the geometric mean of the bottom and top boundaries of each interval are used for analyses, i.e.
where BC 1 and BC 2 are the number of bottom and top boundary crossers of a given interval, respectively. Possible drawbacks of excluding single-interval taxa are that it excludes some true biological variation; may increase taxonomic bias by virtue of eliminating particular types of organism from the data; and the data then cease to represent all described variation, which is one of their chief merits.
The MIN series is derived from only the first, last and single-interval taxa, without filling in ranges. Like RT, this is a summation of events within a stage and can be written as MIN = Ft+FL+bL. This is the most conservative of the three as it makes the minimum assumption of what has actually been recorded in each stage and is more directly related to sampling proxies such as formation or collection counts [22]. It can be viewed as a subset of sampled-in-bin counts (counting only taxa which have actually been recorded in a time bin, rather than merely inferred to have existed at that time). Of course, it is a highly truncated version of true sampled-in-bin counts as the original purpose of the dataset was to record only first and last occurrences.
To quantify the similarity between the new and previous datasets, untransformed RT data from FR2, LAB and NEW are assessed using Spearman’s rank correlation because, even when logged, the data were skewed, breaking parametric assumptions. The normal associated probabilities are not reported because autocorrelations in the data invalidate them. Bootstrap estimates for significance of correlations, which reduce the necessary set of assumptions about the data, are instead calculated using the boot.ci function from the boot library in R to re-sample the original data 9999 times, each time recalculating the correlation coefficient, to generate a bootstrapped distribution of the test statistic which indicates the extent of uncertainty in it. Confidence intervals at the 95% and 99% level are calculated using the bca (bias corrected and accelerated or BCa) method due to Efron [33], which corrects for the bias (the difference between the mean of the bootstrap replicates and the true correlation) and asymmetry of the bootstrap distribution [33]. Where the confidence intervals do not bracket zero, the correlation can be said to be significantly different from zero. Note that in the case of “dependent” data such as time-series, bootstrapping may still not accurately estimate confidence intervals around statistical parameters, so, whilst likely more valid than the standard p-values, should still be treated with caution. Correlations were also explored for two detrended versions of each time series: first differencing explores the changes between successive time steps (stages), whilst generalized differencing (first differencing of the residuals from linear regression) quantifies the successive changes after removing the overall long term trend. Differences were calculated using the statistical programming language R [34]. All correlations are on data from the Serpukhovian (top of Early Carboniferous, stage midpoint 323.2Ma) to Piacenzian (top of the Pliocene, stage midpoint ~3.1Ma), as this is the range for which there is a reasonable fossil record of hexapods (i.e. including the long period of almost no record before the Carboniferous would increase all of the coefficients simply from a lack of data).
Calculating origination and extinction rates
The rates of origination and extinction employed here are Foote’s [24] estimated per-capita rates, and respectively. They are derived as follows:
where N t is the total number of taxa crossing the top boundary out of the interval (i.e. bt+Ft), N b is the total number crossing the bottom boundary into it (i.e. bt+bL) and N bt is the number of taxa crossing both the bottom and top boundary. The advantage of using these over counts of events within an interval is that they are robust to variation in interval duration, disregard single-interval taxa (which are prone to disproportionately distort the signal) and are independent of each other as they are derived from numbers of taxa passing into and out of intervals rather than the addition of events taking place within them. Due to inconsistent stratigraphic resolution, this is not attempted for the FR2 data.
Results
Changes in the data
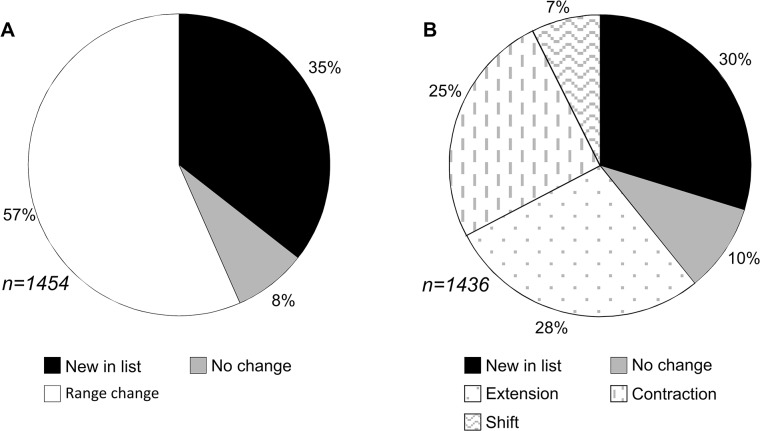
The NEW dataset (S1 Appendix and S1 Dataset) contains a total of 1454 families of Hexapoda, of which 1436 are Insecta. In comparison to FR2, a substantial amount of change has left only 8% of families with the same ranges as recorded in 1993; 35% are new to the record, and well over half have a change in the recorded range (Fig 2A). The picture is broadly similar when compared to LAB (Fig 2B), with 10% remaining unchanged and 30% new. The majority of the range changes are made up of roughly equal amounts of extensions and contractions, and only 7% of the total representing a shift in range. Although the NEW dataset has a higher total number of families (1454) than either FR2 (1087 in [14]) or LAB (1272; 1276 if including ‘uncertain’ families), 230 and 263 families listed in FR2 and LAB, respectively, are included in NEW within other families (as synonyms or subfamilies), or no longer have a fossil record, due mostly to taxonomic revisions.
Fig 2. Proportions of changes in new data for family stratigraphic range compared with previous datasets.
Richness series from new and previous datasets
The richness time series of all three datasets show broad similarities in long-term trends of increasing richness and the synchronicity (or nearly so) of several pulses (Fig 3) but some differences are worth noting.
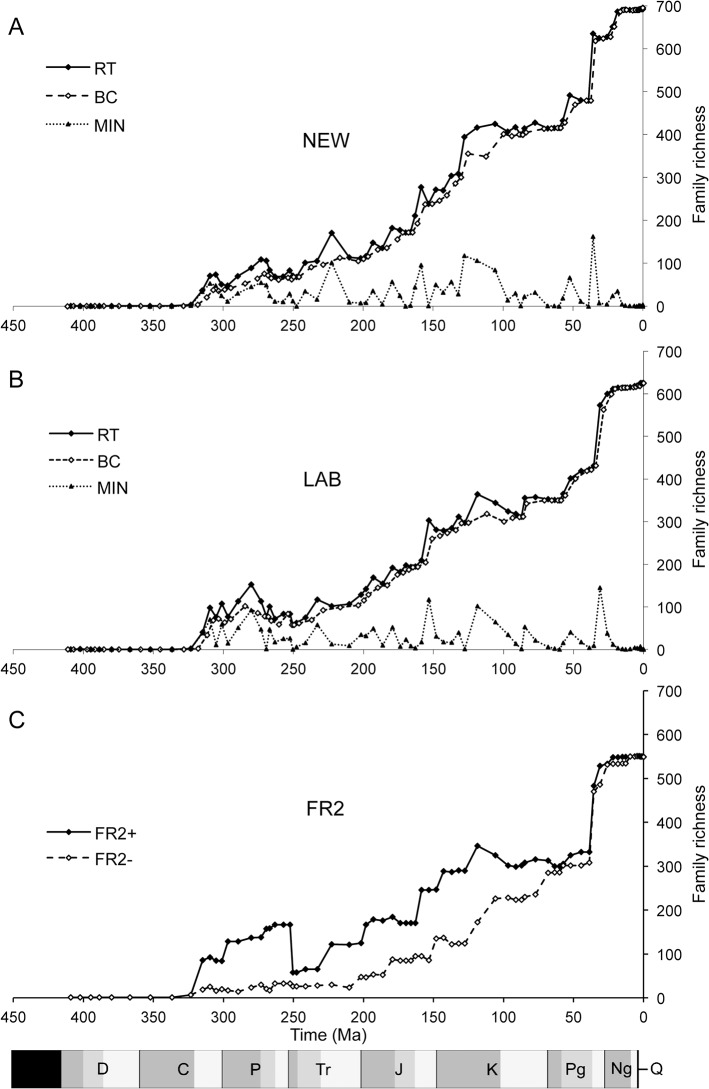
Fig 3. Family richness of insects through time.
Richness time series derived from (A) NEW data, presented here, (B) LAB data from Labandeira [11] and (C) FR2 data from Ross and Jarzembowski [14]. RT = range through, i.e. all taxa ranging anywhere into an interval, with maximum (+) and minimum (–) assumptions for FR2, plotted at stage-midpoints. BC = boundary crossers, i.e. taxa crossing interval boundaries, plotted at stage boundaries. MIN = minimum richness, representing firm occurrences within stages (i.e. first, last and single-interval taxa records).
For the Palaeozoic, the RT series from NEW and LAB are more similar to each other than to FR2+. However, the NEW series shows consistently lower richness than LAB and the two main peaks are offset by one stage, reaching a maximum of 109 families by NEW RT (Kungurian: 273 Ma) and 153 by LAB RT (Artinskian: 280 Ma) (Fig 3). FR2+ shows a gradual and steady increase in richness through the Palaeozoic with a dramatic drop at the end-Permian (~250 Ma), after reaching a maximum of 168 families (Fig 3). FR2– shows no such increase and decline but rather remains conspicuously flat through until the Late Triassic at around 210 Ma (Norian). This is also not mirrored by LAB RT and NEW RT, which show slightly less sharp declines from the Early–Middle Permian towards the end-Permian, when a small increase is seen in the final stage (Changhsingian, data point at 252 Ma). The BC series in NEW and LAB mirror the peaks and troughs of the RT curves but they are less pronounced (Fig 3).
In the Triassic (251–200 Ma) all three datasets show a marked increase in richness, with the largest increase in the Carnian (223 Ma) for FR2+ (up to 123 families) and NEW (171 families) and in the Ladinian (233 Ma) for LAB (117 families) (Fig 3). The NEW RT series show the most pronounced Triassic peak followed by an apparent crash in richness, mirrored in the NEW MIN series but NEW BC shows a smooth increase with only a slight decrease after the Carnian, reflecting that many of the records are single-interval taxa.
The Jurassic (200–146 Ma) continues the long-term increase in described richness (Fig 4). The NEW RT series shows a distinct, four-pulsed increase at 193 (Sinemurian), 179 (Toarcian), 158 (Oxfordian), and 148 Ma (Tithonian); the first three are followed by drops in richness, although this is not reflected in the BC series which shows an uninterrupted, fairly smooth increase. An almost identical pattern is seen in LAB RT while FR2+ shows two distinct increases followed by plateaus.
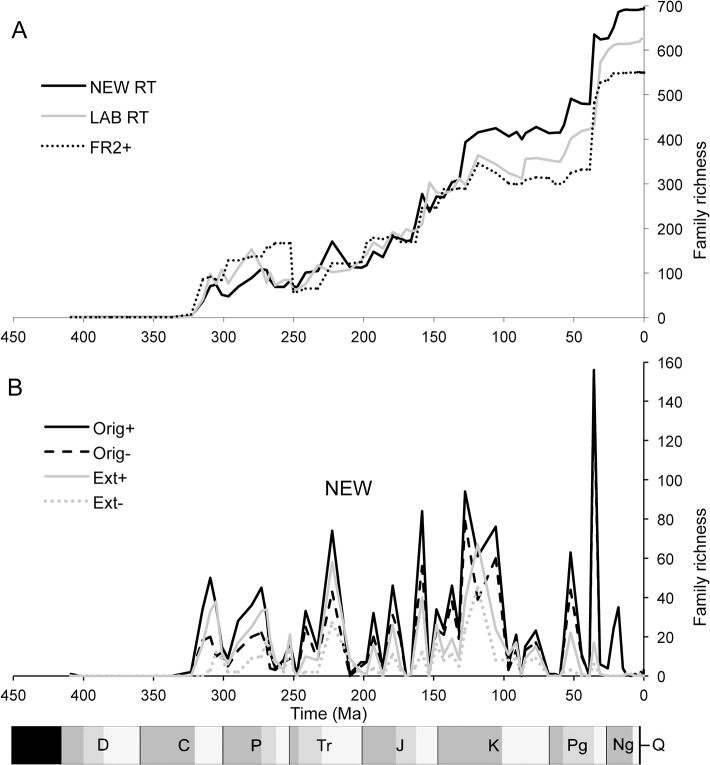
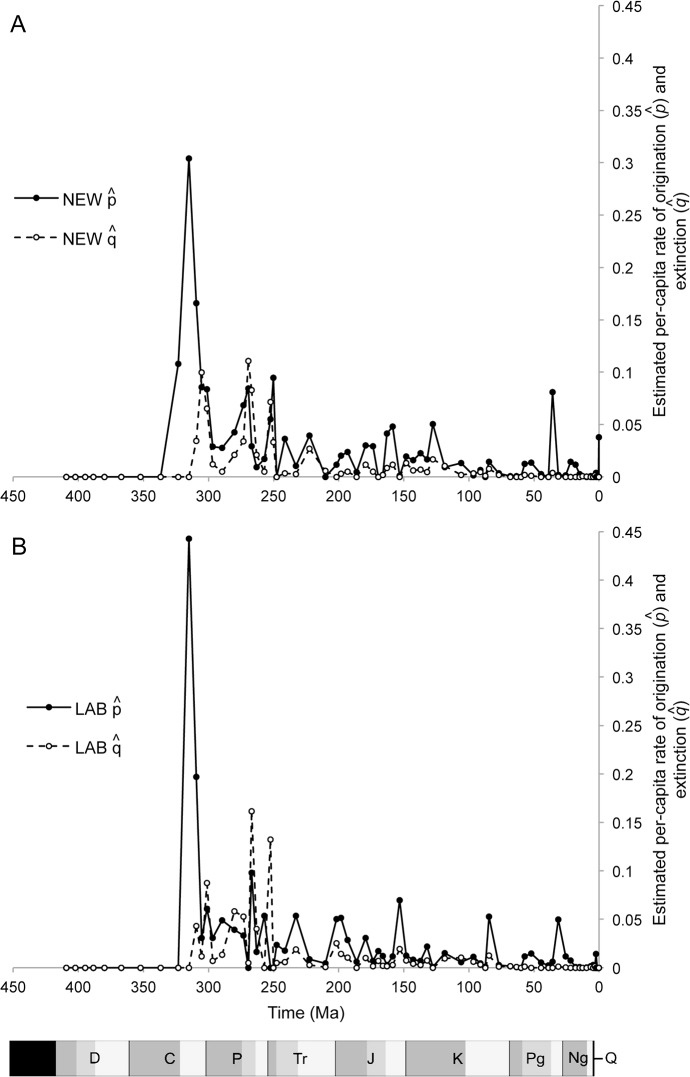
Fig 4. Richness and rates of origination and extinction in fossil hexapods.
(A) Range through time series for NEW, LAB and FR2. (B) Origination (Orig) and extinction (Ext) counts, both including (+) and excluding (–) single interval taxa, from NEW.
During the Early Cretaceous (146–100 Ma) a more rapid rise is seen, most steeply in NEW RT. LAB and FR2 are similar in then showing a pronounced and sustained drop in richness after their synchronous peaks in the Aptian (point at 119 Ma) in both RT and BC series while the NEW RT series continues to increase, albeit at a decelerated rate until it plateaus across a similar range of stages as LAB and FR2. This plateau is accompanied by very low values in the NEW MIN series. No marked drop in richness is apparent at or near the Cretaceous/Palaeogene boundary (65.5 Ma).
The NEW RT series averages 15% and 26% higher across the Cretaceous and Tertiary compared with LAB and FR2, respectively ending with maxima of 695 (NEW), 549 (FR2) and 625 (LAB) families. All three show the most rapid increase in richness in the entire fossil record through the Tertiary with very little deviation between RT (or +) and BC (or−) series.
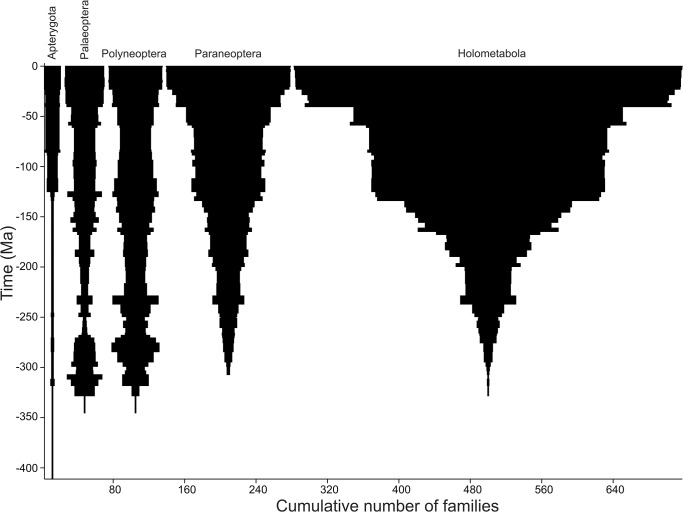
The major taxa dominating richness in NEW (RT) varied at different times (Fig 5). The earliest known hexapod families are in the ‘Apterygota’. These contribute very little to hexapod fossil richness in the long term. The Carboniferous and Permian peaks and subsequent declines are seen only in the Palaeoptera and Polyneoptera. Paraneoptera and Holometabola had originated before the Permian peak but show no sign of any decline towards the end-Permian, rather a slow but steady increase in richness (Fig 5). The Late Triassic peak seen in the RT (but not BC) series is apparent in all groups except Apterygota. Except for occasional pulses of increased richness, which are synchronous with the other three major contributing groups, Palaeoptera show very slow and steady growth in richness, only attaining their previous Palaeozoic richness in the Palaeogene/Neogene from ~60 Ma onwards. A broadly similar pattern is seen in Polyneoptera. Paraneoptera, however, continue their steady increase from the Palaeozoic and show a pronounced increase during the Early Cretaceous (Berriasian–Albian), between ~150 and 100 Ma (Fig 5). This then levels out until they enter a phase of rapid expansion starting in the Palaeogene, from ~65 Ma onwards. The Holometabola enter a more rapid phase of expansion earlier than the Paraneoptera, starting in the Early Jurassic (Sinemurian, from ~193 Ma onwards). They show a pronounced jump in richness at 128 Ma (Barremian), being the largest contributing group to the rapid rise in richness during the Early Cretaceous seen in the NEW RT series. This is followed by a long plateau and then the most rapid expansion phase seen in the entire hexapod fossil record from the lower Eocene (52.2 Ma) onwards (Fig 5).
Fig 5. Spindle diagram showing NEW range-through family richness in major constituent hexapod groups through time.
Generated using PAST [69].
Both the FR2+ and LAB RT series are highly correlated (i.e. strongly co-vary) with NEW RT (Table 1), with all values of Spearman’s rho greater than 0.95 and significant at the 99% confidence limit. This decreases substantially with both first and generalised differencing (Table 1), and correlations between NEW RT and LAB RT lose significance, whilst those between NEW RT and FR2+ retain their significance.
Table 1. Spearman rank correlations between richness time series using raw values and after first differencing and generalised differencing.
| LAB RT | LAB BC | FR2 + | FR2 – | LAB | LAB | |
|---|---|---|---|---|---|---|
| Raw values | ||||||
| NEW RT | .976** | .956** | ||||
| NEW BC | .982** | .979** | ||||
| NEW | .277 | |||||
| NEW | .559** | |||||
| First difference | ||||||
| NEW RT | .183 | .367* | ||||
| NEW BC | .331* | .135 | ||||
| NEW | -.070 | |||||
| NEW | -.028 | |||||
| Generalized difference | ||||||
| NEW RT | .241 | .442** | ||||
| NEW BC | .273 | .111 | ||||
| NEW | .191 | |||||
| NEW | .375* |
NEW = new fossil hexapod family richness data presented here, LAB = insect family richness data from Labandeira [11]
FR2 = hexapod family richness data from Ross and Jarzembowski [14], RT = range through, BC = boundary crossers
+ = maximum assumption of richness and
− = minimum assumption of richness for FR2
= per capita origination rate and = per capita extinction rate. Significance assessed using bootstrapping.
* = significant at 95% confidence limit
** = significant at 99% confidence limit.
Calculated origination and extinction rates
First and last occurrences occur episodically throughout the fossil record of insects (Fig 4B), with an apparent synchrony between origination and extinction through time with origination outstripping extinction. The modal origination occurs in the Palaeogene with large peaks in the Triassic, Late Jurassic and Early Cretaceous. Modal extinction occurs in the Early Cretaceous with large peaks in the late Carboniferous, Permian, Triassic, later Jurassic and Early Cretaceous. Per capita rates of origination and extinction ( and , respectively; Fig 6), however, show distinctly different profiles in the Palaeozoic and post-Palaeozoic (boundary at 251 Ma) in both NEW and LAB data. Greater variance is seen in the Palaeozoic for both rates in both datasets as well as the highest values reached in each. As for raw counts, per capita origination rates stay robustly higher than extinction from the Triassic onwards and both show long term declines towards the present. There are some notable differences between NEW and LAB: the timing and size of Permian origination peaks differs; there is no Late Cretaceous origination peak; the Carboniferous extinction peak is more pronounced, and those in the Permian less pronounced, not exceeding originations by much. As a result, Spearman rank correlations of these rates between NEW and LAB show no significant relationship in origination rates, while the extinction rates are positively correlated in the raw and generalised differenced time series but retain no relationship after first differencing (Table 1). In general, origination rates seem to more consistently exceed extinction rates.
Fig 6. Estimated per-capita rates of origination and extinction .
Rates are from (A) NEW insect family data and (B) Labandeira [11].
Discussion
Changes in the data
The robustness of described richness through time in the insects, to new discoveries over fifteen years (eighteen years from FR2 data up to end 1991), was tested by compiling a new dataset of fossil hexapod family-richness from literature published up to the end of 2009. Only ten percent of families in the new data remain unchanged over that time, with about 60% of families having different stratigraphic ranges, and 30% of families being completely new to the fossil record. For scientists interested in the details of individual fossil families, for example for dating phylogenies above family level (e.g. [35,36]), the current dataset represents a substantial improvement over previous datasets available. The implication is that the previous fossil insect datasets now have largely historical interest only and should not be used for future macroevolutionary research. Studies based on them ideally require re-assessment. For example, in an analysis based on Labandeira’s [11] data, Yang [37] suggested that differences in origination rates account for the higher diversity of Holometabola compared to Paraneoptera. However, Nicholson et al. [38], using the present data set, found the converse: origination rates between these groups is not significantly different, but extinction rates are significantly reduced in Holometabola compared to Paraneoptera.
While the change in ranges from FR2 in the NEW data (Fig 2A) can be attributed largely to improvement in the stratigraphic resolution of family ranges to stages, the differences from LAB (Fig 2B) require more subtle explanation. Extensions of known ranges in fossil families are to be expected, with continued exploration of fossil sites and descriptions of new finds likely to turn up new first or last occurrences, such as the high rate of discovery in Mesozoic deposits of China (e.g. see [39]). The high proportion of range contractions (25%) seems at first unexpected but can be ascribed to differences in the dates for fossil deposits used (e.g. the Karabastau Formation, Kazakhstan: Kimmeridgian in LAB but Oxfordian in NEW) and extensive changes in taxonomy reducing the number of fossils included in some families, such as in a recent review of termites by Engel et al. [40] wherein several fossil taxa, previously attributed to extant families, were reassigned, thus contracting the known range of some families and removing the Hodotermitidae from the fossil record altogether.
The rate of discovery of new fossil hexapods seems disproportionately concentrated in the Cretaceous, with high numbers of publications on the extensive Jiulongshan and Yixian formations in China [39], continued interest in the Crato Formation in Brazil [41], a new supply of Burmese amber [18,42] and abundant new amber deposits in France [43] and Spain [44], although new material continues to be found across almost the entire temporal range of hexapods [6,16,45]. There are an estimated 1067 extant hexapod families (data compiled from the relevant sections of [46]), implying that ~370 extant families (35%) are not yet known from the fossil record and could in principle be found in future. This sets a broad potential upper limit to the height of the richness curve, indicating substantial, but not excessive, potential for future discovery at the family level. The majority of these (196 families) are from the Holometabola. However, in terms of proportion of extant families represented in the fossil record, Holometabola have the most coverage with ~69% represented, followed closely by Polyneoptera (65%), Paraneoptera (64%) and Palaeoptera (58%). Only 33% of extant Apterygota families have a fossil record, perhaps a result of their small size, habitat requirements, and lack of wings [6].
Other informative ways of assessing the potential for future discovery, beyond the scope of the present study, would be to construct taxon vs. specimen accumulation curves to observe if the number of taxa described through time has asymptoted (e.g. [47–49]), or by quantifying the gaps in the record implied by phylogenies (e.g. [47,50,51]). Although some data pertinent to the former (dates of description of extinct families) are present in the current data, one would additionally need to compile the date at which extant families were first described from the fossil record, which is not normally the date of the taxon’s first description.
Changes in the richness series
Despite major changes to the ranges of insect families over fifteen years of discovery, changes to the pattern of described richness through time derived from those data seem less extensive. Correlations between the time series of the new and previous datasets show that the broad pattern of rise in discovered taxa through time is very similar to that previously described. The generally steady rise in richness through time suggests support for the previous conclusion [12] that no strong logistic limits to family richness have yet been met. However, some of the Cenozoic rise may be attributable to the Pull-of-the-Recent [52] whereby the ranges of extant taxa are pulled forward, accentuating the richness rise nearer the present. Sampling may also have been strongly affected by the abundance of suitable deposits, such as Baltic amber and compression deposits such as the Green River and Florissant formations, which coincide with the Eocene rise [53]. These issues will be examined in future papers.
Other important features preserved in the NEW richness series include evidence for a mass extinction at the end-Permian. The Permian drop in richness is however less abrupt than in FR2. This effect is probably due to the improved temporal resolution from epoch to stage, which pulls the ranges of taxa in FR2 forward to the end of the Permian. At stage level resolution, many of these families are instead seen to have last occurrences before the end Permian. In turn, the asynchronicity in extinction may be genuine, but probably is partly an artefact of an incomplete record (the Signor-Lipps effect [54]) which tends to drag extinctions backwards in time. The major turnover in dominant taxa (Fig 5) accompanying the Permian to Triassic interval is strongly reminiscent of the end-Permian extinction in many other taxa (e.g. [55]). In the hexapod case there was a replacement of the Palaeozoic fauna of mainly Palaeoptera and Polyneoptera by a fauna dominated by Paraneoptera and Holometabola, which appear to have suffered little reduction in their richness [13,53]. Studies on the coherence of these different faunas would be useful (see [56]).
Despite the evidence for an end-Permian extinction, the NEW richness data leave no evidence of an end-Cretaceous extinction, in common with previous data [15,53]. Given the known widespread ecosystem impacts of this event, it is difficult to imagine that insects were completely unaffected but extinction may have occurred below the family level. Some genus-level data provide some support for this [13], as do some studies of trophic interactions [57], but others suggest a weaker extinction in insects than in other taxa [58].
Although all datasets show an increase in richness in the Triassic, a subsequent drop is suggested by the NEW RT series (Fig 4A). Many non-insect taxa apparently experienced a mass extinction at the end-Triassic [59,60] but there has never been good evidence for this in insects. However, the drop is lost in the NEW BC series (Fig 3A), indicating that it is due primarily to abundance of single interval taxa and hence may be an artefact of sampling bias. Indeed the total number of extinctions detected at the end-Triassic boundary is close to zero, indicating that it would be premature to suggest an insect extinction then (Fig 4B).
Surprisingly, the overall level of richness in the NEW data is not always higher than the older data. This is mostly the case in the Palaeozoic, where there was an historical tendency by early workers such as Handlirsch and Tillyard to oversplit taxa, while revisions have decreased the number of valid families. Additionally, and perhaps more importantly, of the 324 families in the new data with ranges in the Palaeozoic, 28% of them represent contractions with respect to LAB. This suggests a specific effect of taxonomy on apparent richness that may be important for other researchers.
The correlations between the differenced time series for the new and old data, although positive, are much less strong than for the raw time series, suggesting moderate differences in the shorter term variation in richness from stage to stage. This is potentially important when assessing the drivers behind diversity change, as time series are generally detrended to remove spurious correlations, and it is the short term variation around the long term trends that are analysed (e.g. [5,61]). The Palaeozoic contains much of the discordance between the series (Fig 4A), with FR2 and NEW having very different shapes while the richness peaks of LAB and NEW are offset from each other. Declines seen in both LAB and FR2 during the Early–mid-Cretaceous (~120–85 Ma) are not shared by NEW, which shows more of a plateau. This plateau could simply be a result of the relative paucity in insect-preserving localities in the Upper Cretaceous, however in an analysis of plant-associated insect families Labandeira [62] found that this plateau coincides with a period of transition from gymnosperm-associated families to angiosperm-associated families, with extinctions in the former approximately matching the level of originations in the latter. Future analyses of occurrence-based data, subsampled to remove the effects sampling bias, will help to elucidate the relative importance of these alternative (but not mutually exclusive) explanations.
Patterns of origination and extinction
Labandeira [53] picks out five major periods of originations in the insects and four major extinctions. Of the originations, all are still found in the NEW origination series (Fig 4), namely in order, the Late Carboniferous (Bashkirian–Moscovian–first appearance of winged insects and colonization of forested ecosystems); Early Permian (peaking in the Kungurian–colonization of wider environments and the rise of Paraneoptera and Holometabola); Late Jurassic (Oxfordian–radiation of communities on advanced seed plants); Early Cretaceous (peaking in the Barremian–radiations in decomposer and freshwater systems); and the Eocene (Priabonian–suspected to be a sampling artefact that may represent earlier radiations that are poorly sampled). The main addition to this description in the NEW data is the higher peak in the Triassic, which Labandeira [53] attributes to a rebound from the Permian extinction.
In terms of extinctions, the Late Carboniferous peak is attributed by Labandeira [53] to changes in plant communities and trophic structure. The Permian extinction is high in absolute numbers of extinctions but lower in per capita rates (cf. Fig 4 and Fig 6) and is generally attributed to high continentality and hot dry climates on land [63]. In addition, there were substantial extinctions in the Late Jurassic (attributed to competitive turnover during the simultaneous radiation; [53]) and the Early Cretaceous (attributed to competitive turnover of taxa adapting to new environments, including angiosperms; see [13,15]). The NEW series add to this a large peak in extinctions in the Triassic, as seen for originations. As discussed above, this may represent the detection of the more general end-Triassic mass extinction, although it may also be an artefact of sampling bias.
In general the high agreement between the timing of originations and extinctions in NEW and FR2 is consistent with the findings of similar studies on other taxa [8,9], suggesting that the great potential for change in the insect fossil record has not translated into major changes in pattern. Some previous authors [9] have interpreted this as encouragement that incomplete and partially erroneous data can preserve broad generalizations about the history of life [10]. However, recent experiences with alternative ways of compiling the data suggest that other issues with the data can remain important in correctly describing and interpreting them [29,64,65].
In general there is high synchronicity between the origination and extinction series (Fig 4B), which is the pattern expected if one biologically depends on the other. This pattern is also expected if they are both simply artefacts of sampling, hence determined by the availability of insect-bearing deposits. The pattern is not simply due to the abundance of single interval taxa (Fig 4B), suggesting perhaps some biological signal in the data.
Originations mostly exceed extinctions across intervals, explaining the consistent rise in family level diversity through time, as well as high extant richness [4,15,66]. In terms of rates, the decline from the Palaeozoic to Mesozoic and Cenozoic is the most obvious feature, in common with other family and genus level analyses [60,64]. Explanations for this include lineage sorting, density-dependent processes and the fact that higher taxa are disproportionately described for older groups [64]. Some of the peaks are different in height in the NEW data compared to LAB (Fig 6); a result of taxonomic changes and shifts in the dating of deposits. The Late Cretaceous (Santonian: 85 Ma) LAB origination peak is not seen in NEW, probably from range extensions pulling more first occurrences back to Lower Cretaceous deposits.
An important question now is to what extent the updated richness, origination and extinction series reflect geological and sampling biases, and thus what features may remain once such biases are removed. Previous work on the marine invertebrate record suggests that many features of the face-value fossil record can remain preserved after sample-standardization (e.g. [67]), although diversity curves may become flatter due to the elimination of the Pull-of-the-Recent [65]. In addition, short term patterns after detrending may be altered, potentially altering macroevolutionary inference [64,68]. Because hexapods generally require exceptional preservation conditions, it is possible that sampling and other biases are a more prominent influence on the face-value record, although considerable biological signal may be retained. Occurrence data of community samples will likely help solve this. However, the range data here are likely to retain value in the dating of phylogenetic trees, since one can be more confident about the completeness of the family ranges, and they are easy to update.
In summary, a new compilation of the fossil ranges of insect families shows changes in the ranges of a high proportion of families, and significant changes in described short term richness and some origination and extinction patterns, but little change in broad temporal patterns. Representing an additional 15 years of data in a rapidly expanding field compared with previously available compendia [11,14], it is hoped that this new dataset will form the basis for future work on elucidating the evolutionary history of the hexapods.
Supporting Information
(XLSX)
Acknowledgments
DBN was supported by a NERC studentship and by the Natural History Museum and National Museums Scotland. We thank the many authors who sent us reprints of their work, and two anonymous reviewers for their helpful comments on the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
DBN was supported by a Natural Environment Research Council studentship grant NE/G524236/1. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Alroy J. The shifting balance of diversity among major marine animal groups. Science. 2010; 329: 1191–1194. 10.1126/science.1189910 [DOI] [PubMed] [Google Scholar]
- 2. Benson RBJ, Mannion PD. Multi-variate models are essential for understanding vertebrate diversification in deep time. Biol Lett. 2012; 8: 127–130. 10.1098/rsbl.2011.0460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ezard THG, Aze T, Pearson PN, Purvis A. Interplay between changing climate and species’ ecology drives macroevolutionary dynamics. Science. 2011; 332: 349–351. 10.1126/science.1203060 [DOI] [PubMed] [Google Scholar]
- 4. Mayhew PJ. Why are there so many insect species? Perspectives from fossils and phylogenies. Biol Rev. 2007; 82: 425–454. 10.1111/j.1469-185X.2007.00018.x [DOI] [PubMed] [Google Scholar]
- 5. Mayhew PJ, Jenkins GB, Benton TG. A long-term association between global temperature and biodiversity, origination and extinction in the fossil record. Proc R Soc B Biol Sci. 2008; 275: 47–53. 10.1098/rspb.2007.1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grimaldi DA, Engel MS. Evolution of the Insects. Cambridge University Press; 2005. [Google Scholar]
- 7. Benton MJ. The history of life: large databases in palaeontology In: Harper DAT, editor. Numerical palaeobiology: Computer-based modelling and analysis of fossils and their distributions, pp. 249–283. Chichester and New York: John Wiley & Sons; 1991. pp. 249–283. [Google Scholar]
- 8. Maxwell WD, Benton MJ. Historical tests of the absolute completeness of the fossil record of tetrapods. Paleobiology. 1990; 16: 322–335. [Google Scholar]
- 9. Sepkoski JJ Jr. Ten years in the library: new data confirm paleontological patterns. Paleobiology. 1993; 19: 43–51. [DOI] [PubMed] [Google Scholar]
- 10. Adrain JM, Westrop SR. An empirical assessment of taxic paleobiology. Science. 2000; 289: 110–112. 10.1126/science.289.5476.110 [DOI] [PubMed] [Google Scholar]
- 11. Labandeira CC. A compendium of fossil insect families. Milwaukee Public Museum Contrib Biol Geol. 1994; 88: 1–71. [Google Scholar]
- 12. Labandeira CC, Sepkoski JJ Jr. Insect diversity in the fossil record. Science. 1993; 261: 310–315. 10.1126/science.11536548 [DOI] [PubMed] [Google Scholar]
- 13. Jarzembowski EA, Ross AJ. Insect origination and extinction in the Phanerozoic. Geol Soc London Spec Publ. 1996; 102: 65–78. 10.1144/GSL.SP.1996.001.01.05 [DOI] [Google Scholar]
- 14. Ross AJ, Jarzembowski EA. Arthropoda (Hexapoda; Insecta) In: Benton MJ, editor. The Fossil Record 2. London: Chapman and Hall; 1993. pp. 363–426. [Google Scholar]
- 15. Ross AJ, Jarzembowski EA, Brooks SJ. The Cretaceous and Cenozoic record of insects (Hexapoda) with regard to global change In: Culver SJ, Rawson PF, editors. Biotic Response to Global Change, the Last 145 Million Years. Cambridge University Press; 2000. pp. 288–302. 10.1017/CBO9780511535505.020 [DOI] [Google Scholar]
- 16.Ross AJ. The development of Palaeoentomology over the past 25 years. In: 5th International Conference on Fossil Insects, 4th World Congress on Amber Inclusions, 4th international Meeting on Continental Palaeoarthropodology, Beijing, Program & Abstract; 2010. pp. 90–91.
- 17. Béthoux O. Cnemidolestodea (Insecta): an ancient order reinstated. J Syst Palaeontol. 2005; 3: 403–408. 10.1017/S147720190500163X [DOI] [Google Scholar]
- 18. Ross AJ, Mellish C, York P, Crighton B. Burmese Amber In: Penny D, editor. Biodiversity of fossils in amber from the major world deposits. Siri Scientific Press; 2010. pp. 208–235. [Google Scholar]
- 19. Shi G, Grimaldi DA, Harlow GE, Wang J, Wang J, Yang M, et al. Age constraint on Burmese amber based on U–Pb dating of zircons. Cretac Res. 2012; 37: 155–163. 10.1016/j.cretres.2012.03.014 [DOI] [Google Scholar]
- 20. Rust J, Singh H, Rana RS, McCann T, Singh L, Anderson K, et al. Biogeographic and evolutionary implications of a diverse paleobiota in amber from the early Eocene of India. Proc Natl Acad Sci USA. 2010; 107: 18360–18365. 10.1073/pnas.1007407107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peters SE. Geologic constraints on the macroevolutionary history of marine animals. Proc Natl Acad Sci USA. 2005; 102: 12326–12331. 10.1073/pnas.0502616102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peters SE, Foote M. Biodiversity in the Phanerozoic: a reinterpretation. Paleobiology. 2001; 27: 583–601. [DOI] [Google Scholar]
- 23. Ogg JG, Ogg G, Gradstein FM. The Concise Geologic Time Scale. Cambridge University Press; 2008. 10.1017/S0016756809006207 [DOI] [Google Scholar]
- 24. Foote M. Origination and extinction components of taxonomic diversity: general problems. Paleobiology. 2000; 26: 74–102. 10.1666/0094-8373(2000)26[74:OAECOT]2.0.CO;2 [DOI] [Google Scholar]
- 25. Alroy J. New methods for quantifying macroevolutionary patterns and processes. Paleobiology. 2000; 26: 707–733. [DOI] [Google Scholar]
- 26. Fitzgerald PC, Carlson SJ. Examining the latitudinal diversity gradient in Paleozoic terebratulide brachiopods: should singleton data be removed? Paleobiology. 2006; 32: 367–386. 10.1666/05029.1 [DOI] [Google Scholar]
- 27. Preston FW. The commonness, and rarity, of species. Ecology. 1948; 29: 254–283. 10.2307/1930989 [DOI] [Google Scholar]
- 28. Alroy J. Fair sampling of taxonomic richness and unbiased estimation of origination and extinction rates. Paleontol Soc Pap. 2010; 16: 55–80. [Google Scholar]
- 29. Alroy J. Successive approximations of diversity curves: Ten more years in the library. Geology. 2000; 28: 1023–1026. [DOI] [Google Scholar]
- 30. Alroy J, Marshall CR, Bambach RK, Bezusko K, Foote M, Fürsich FT, et al. Effects of sampling standardization on estimates of Phanerozoic marine diversification. Proc Natl Acad Sci USA. 2001; 98: 6261–6266. 10.1073/pnas.111144698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bambach RK. Energetics in the global marine fauna: A connection between terrestrial diversification and change in the marine biosphere. Geobios. 1999; 32: 131–144. 10.1016/S0016-6995(99)80025-4 [DOI] [Google Scholar]
- 32. Ponomarenko AG, Dmitriev VY. Diversity curves revisited. Paleontol. J. 2009; 43: 226–229. 10.1134/S0031030109020154 [DOI] [Google Scholar]
- 33. Efron B. Better bootstrap confidence intervals. J Am Stat Assoc. 1987; 82: 171–185. 10.2307/2289144 [DOI] [Google Scholar]
- 34. R Core Team. R: A Language and Environment for Statistical Computing. Vienna Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 35. Davis RB, Nicholson DB, Saunders ELR, Mayhew PJ. Fossil gaps inferred from phylogenies alter the apparent nature of diversification in dragonflies and their relatives. BMC Evol Biol. 2011; 11 10.1186/1471-2148-11-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rainford JL, Hofreiter M, Nicholson DB, Mayhew PJ. Phylogenetic distribution of extant richness suggests metamorphosis is a key innovation driving diversification in insects. PLoS One. 2014; 9: e109085 10.1371/journal.pone.0109085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang AS. Modularity, evolvability, and adaptive radiations: a comparison of the hemi- and holometabolous insects. Evol Dev. 2001; 3: 59–72. 10.1046/j.1525-142x.2001.003002059.x [DOI] [PubMed] [Google Scholar]
- 38. Nicholson DB, Ross AJ, Mayhew PJ. Fossil evidence for key innovations in the evolution of insect diversity. Proc R Soc B Biol Sci. 2014; 281 10.1098/rspb.2014.1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ren D, Shih C-K, Gao T-P, Yao Y-Z, Zhao Y-Y. Silent Stories–Insect Fossil Treasures from Dinosaur Era of the Northeastern China. Beijing: Science Press; 2010. [Google Scholar]
- 40. Engel MS, Grimaldi DA, Krishna K. Termites (Isoptera): their phylogeny, classification, and rise to ecological dominance. Am Museum Novit. 2009; 3650: 1–27. 10.1206/651.1 [DOI] [Google Scholar]
- 41. Martill DM, Bechly G, Heads SW. Appendix: species list for the Crato Formation In: Martill DM, Bechly G, Loveridge RF, editors. The Crato Fossil Beds of Brazil: Window into an Ancient World. Cambridge University Press; 2007. pp. 582–607. [Google Scholar]
- 42. Grimaldi DA, Engel MS, Nascimbene PC. Fossiliferous Cretaceous amber from Myanmar (Burma): Its rediscovery, biotic diversity, and paleontological significance. Am Museum Novit. 2002; 3361: 1–71. [DOI] [Google Scholar]
- 43. Perrichot V, Néraudeau D. Foreword: Cretaceous ambers from southwestern France: geology, taphonomy, and palaeontology. Geodiversitas. 2009; 31: 7–11. 10.5252/g2009n1a1 [DOI] [Google Scholar]
- 44. Delclòs X, Arillo A, Peñalver E, Barrón E, Soriano C, López Del Valle R, et al. Fossiliferous amber deposits from the Cretaceous (Albian) of Spain. Comptes Rendus Palevol. 2007; 6: 135–149. 10.1016/j.crpv.2006.09.003 [DOI] [Google Scholar]
- 45. Nel A, Roques P, Nel P, Prokin AA, Bourgoin T, Prokop J, et al. The earliest known holometabolous insects. Nature. 2013; 503: 257–261. 10.1038/nature12629 [DOI] [PubMed] [Google Scholar]
- 46. Resh VH, Cardé RT. Encyclopedia of Insects. 2nd ed. Elsevier Academic Press; 2009. [Google Scholar]
- 47. Smith AB. Intrinsic versus extrinsic biases in the fossil record: contrasting the fossil record of echinoids in the Triassic and early Jurassic using sampling data, phylogenetic analysis, and molecular clocks. Paleobiology. 2007; 33: 310–323. 10.1666/06073.1 [DOI] [Google Scholar]
- 48. Puchalski SS, Eernisse DJ, Johnson CC. The effect of sampling bias on the fossil record of chitons (Mollusca, Polyplacophora). Am Malacol Bull. 2008; 95: 87–95. 10.4003/0740-2783-25.1.87 [DOI] [Google Scholar]
- 49. Bernard EL, Ruta M, Tarver JE, Benton MJ. The fossil record of early tetrapods: Worker effort and the end-Permian mass extinction. Acta Palaeontol Pol. 2010; 55: 229–239. 10.4202/app.2009.0025 [DOI] [Google Scholar]
- 50. Wills MA. How good is the fossil record of arthropods? An assessment using the stratigraphic congruence of cladograms. Geol J. 2001; 36: 187–210. 10.002/gj.882 [DOI] [Google Scholar]
- 51. Ksepka DT, Boyd CA. Quantifying historical trends in the completeness of the fossil record and the contributing factors: an example using Aves. Paleobiology. 2012; 38: 112–125. 10.5061/dryad.k7t00 [DOI] [Google Scholar]
- 52. Jablonski D, Roy K, Valentine JW, Price RM, Anderson PS. The impact of the pull of the recent on the history of marine diversity. Science. 2003; 300: 1133–1135. 10.1126/science.1083246 [DOI] [PubMed] [Google Scholar]
- 53. Labandeira CC. The fossil record of insect extinction: new approaches and future directions. Am Entomol. 2005; 51: 14–29. 10.1093/ae/51.1.14 [DOI] [Google Scholar]
- 54. Signor PW, Lipps JH. Sampling bias, gradual extinction patterns and catastrophes in the fossil record. Geol Soc Am Spec Pap. 1982; 190: 291–296. [Google Scholar]
- 55. Brusatte SL, Benton MJ, Ruta M, Lloyd GT. 2008. Superiority, competition, and opportunism in the evolutionary radiation of dinosaurs. Science. 2008; 321: 1485–1488. 10.1126/science.1161833 [DOI] [PubMed] [Google Scholar]
- 56. Alroy J. Are Sepkoski’s evolutionary faunas dynamically coherent? Evol Ecol Res. 2004; 6: 1–32. [Google Scholar]
- 57. Labandeira CC, Johnson KR, Wilf P. Impact of the terminal Cretaceous event on plant-insect associations. Proc Natl Acad Sci USA. 2002; 99: 2061–2066. 10.1073/pnas.042492999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wappler T, Currano ED, Wilf P, Rust J, Labandeira CC. No post-Cretaceous ecosystem depression in European forests? Rich insect-feeding damage on diverse middle Palaeocene plants, Menat, France. Proc R Soc B Biol Sci. 2009; 276: 4271–4277. 10.1098/rspb.2009.1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Raup DM, Sepkoski JJ Jr. Mass extinctions in the marine fossil record. Science. 1982; 215: 1501–1503. 10.1126/science.215.4539.1501 [DOI] [PubMed] [Google Scholar]
- 60. Benton MJ. Diversification and extinction in the history of life. Science. 1995; 268: 52–58. 10.1126/science.7701342 [DOI] [PubMed] [Google Scholar]
- 61. Hannisdal B, Peters SE. 2011. Phanerozoic Earth system evolution and marine biodiversity. Science. 2011; 334: 1121–1124. 10.1126/science.1210695 [DOI] [PubMed] [Google Scholar]
- 62.Labandeira C. Why Did Terrestrial Insect Diversity Not Increase During the Angiosperm Radiation? Mid-Mesozoic, Plant-Associated Insect Lineages Harbor Clues. In: Pontarotti P, editor. Evolutionary Biology: Genome Evolution, Speciation, Coevolution and Origin of Life; 2014. pp. 261–299. 10.1007/978-3-319-07623-2_13 [DOI]
- 63. Benton MJ. When life nearly died: the greatest mass extinction of all time London, New York: Thames & Hudson; 2003. [Google Scholar]
- 64. Alroy J. Dynamics of origination and extinction in the marine fossil record. Proc Natl Acad Sci USA. 2008; 105: 11536–11542. 10.1073/pnas.0802597105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Alroy J, Aberhan M, Bottjer DJ, Foote M, Fürsich FT, Harries PJ, et al. Phanerozoic trends in the global diversity of marine invertebrates. Science. 2008; 321: 97–100. 10.1126/science.1156963 [DOI] [PubMed] [Google Scholar]
- 66. Mayhew PJ. Shifts in hexapod diversification and what Haldane could have said. Proc R Soc B Biol Sci. 2002;269:969–974. 10.1098/rspb.2002.1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Benton MJ. The Red Queen and the Court Jester: species diversity and the role of biotic and abiotic factors through time. Science. 2009;323:728–732. 10.1126/science.1157719 [DOI] [PubMed] [Google Scholar]
- 68. Mayhew PJ, Bell MA, Benton TG, McGowan AJ. Biodiversity tracks temperature over time. Proc Natl Acad Sci USA. 2012;109:15141–15145. 10.1073/pnas.1200844109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4:1–9. [Google Scholar]
- 70. Kaddumi HF. Amber of Jordan, the Oldest Prehistoric Insects in Fossilized Resin. Publications of the Eternal River Museum of Natural History, Amman; 2005. [Google Scholar]
- 71. Lubbock JW. Notes on the Thysanura. Part IV. Transactions of the Linnean Society of London. 1871;27:277–297. [Google Scholar]
- 72. Delclòs X, Arillo A, Peñalver E, Barrón E, Soriano C, López-Del-Valle R, et al. Fossiliferous amber deposits from the Cretaceous (Albian) of Spain. Comptes Rendus Palevol. 2007;6(1–2):135–149. [Google Scholar]
- 73. Christiansen K, Pike EM. Cretaceous Collembola (Arthropoda, Hexapoda) from the Upper Cretaceous of Canada. Cretaceous Research. 2002;23(2):165–188. [Google Scholar]
- 74. Riek EF. An entomobryid collembolan (Hexapoda: Collembola) from the Lower Permian of Southern Africa. Paleontologica Africana. 1976;19:141–143. [Google Scholar]
- 75. Christiansen K, Nascimbene P. Collembola (Arthropoda, Hexapoda) from the mid Cretaceous of Myanmar (Burma). Cretaceous Research. 2006;27:318–363. [Google Scholar]
- 76. Ross AJ, York PV. A catalogue of the type and figured specimens of Hexapoda from the Rhynie chert (early Devonian) at The Natural History Museum, London, UK. Transactions of the Royal Society of Edinburgh: Earth Sciences. 2004;94(4):319–395. [Google Scholar]
- 77. McKellar RC, Wolfe AP, Tappert R, Muehlenbachs K. Correlation of Grassy Lake and Cedar Lake ambers using infrared spectroscopy, stable isotopes, and palaeoentomology. Canadian Journal of Earth Sciences. 2008;45(9):1061–1082. [Google Scholar]
- 78. Börner C. Zur Systematik der Hexapoden. Zoologischer Anzeiger. 1904;27:511–533. [Google Scholar]
- 79. Weitschat W, Wichard W. Atlas of plants and animals in Baltic amber Verlag Dr. Friedrich Pfeil, Mnchen; 2002. [Google Scholar]
- 80. Poinar GO. Life in amber. Stanford University Press, Standford, California; 1992. [Google Scholar]
- 81. Wilson HM, Martill DM. A new japygid dipluran from the Lower Cretaceous of Brazil. Palaeontology. 2001;44(5):1025–1031. [Google Scholar]
- 82. Kukalová-Peck J. New Carboniferous Diplura, Monura, and Thysanura, the hexapod ground plan, and the role of thoracic side lobes in the origin of wings (Insecta). Canadian Journal of Geology. 1987;65:2327–2345. [Google Scholar]
- 83. Engel MS. A new Lower Permian bristletail from the Wellington Formation in Kansas (Archaeognatha: Dasyleptidae). Transactions of the Kansas Academy of Science. 2009;112(1/2):40–44. [Google Scholar]
- 84. Rasnitsyn AP. Taxonomy and morphology of Dasyleptus Brongniart, 1885, with description of a new species (Insecta: Machilida: Dasyleptidae). Russian Entomological Journal. 2000;8[for 1999](3):145–154. [Google Scholar]
- 85. Rasnitsyn AP, Ross AJ. A preliminary list of arthropod families present in the Burmese amber collection at The Natural History Museum, London. Bulletin of The Natural History Museum, Geology Series. 2000;56(1):21–24. [Google Scholar]
- 86. Sturm H, Poinar GO. Cretaceomachilis libanensis, the oldest known bristle-tail of the family Meinertellidae (Machiloidea, Archaeognatha, Insecta) from the Lebanese amber. Deutsche entomologische Zeitschrift. 1998;45(1):43–48. [Google Scholar]
- 87. Bitsch J, Nel A. Morphology and classification of the extinct Archaeognatha and related taxa (Hexapoda). Annales de la Société entomologique de France. 1999;35(1):17–29. [Google Scholar]
- 88. Sinitshenkova ND. A review of Triassic mayflies, with a description of new species from western Siberia and Ukraine (Ephemerida = Ephemeroptera). Paleontological Journal. 2000;34(Suppl. 3):S275–S283. [Google Scholar]
- 89. Kluge NJ. A new suborder of Thysanura for the Carboniferous insect originally described as larva of Bojophlebia, with comments on characters of the orders Thysanura and Ephemeroptera. Zoosystematica Rossica. 1996;4(1):71–75. [Google Scholar]
- 90. Engel MS. A note of the relic silverfish Tricholepidion gertschi (Zygentoma). Transactions of the Kansas Academy of Science. 2006;109(3/4):236–238. [Google Scholar]
- 91. Rasnitsyn AP. Subclass Lepismatona Latreille, 1804. The wingless insects (= Thysanura Latreille 1796, s.l.) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 69–74. [Google Scholar]
- 92. Staniczek AH, Bechly G. 11.2 Apterygota: primarily wingless insects In: Martill DM, Bechly G, Loveridge RF, editors. The Crato Fossil Beds of Brazil: Window into an Ancient World. Cambridge University Press; 2007. p. 149–154. [Google Scholar]
- 93. Mendes LF, Poinar GO. A new fossil Nicoletiidae (Zygentoma, "Apterygota") in Dominican amber. Proceedings of the Entomological Society of Washington. 2004;106(1):102–109. [Google Scholar]
- 94. Novokshonov VG. New fossil insects (Insecta: Grylloblattida, ordinis incertis) from the Lower Permian of the middle Urals. Paleontological Journal. 2000;34(5):513–518. [Google Scholar]
- 95. Béthoux O, Nel A, Lapeyrie J, Gand G. New data on Paleozoic grylloblattid insects (Neoptera). Journal of Paleontology. 2005;79(1):125–138. [Google Scholar]
- 96. Carpenter FM. Superclass Hexapoda. In: Treatise on Invertebrate Paleontology, Part R, Arthropoda 4 (3&4) Boulder, C. O. and Lawrence, K. A.: Geological Society of America and University of Kansas Press; 1992. p. xxi + 655. [Google Scholar]
- 97. Hong YC. Hebeigramma nom. nov., a new name for Mesogramma Hong, 1984 (Caloneurodea) from the Lower Cretaceous of Hebei Province, China. Geological Bulletin of China. 2003;22(9):686–687. [Google Scholar]
- 98. Rasnitsyn AP. 2.2.1.2.1.2. Order Caloneurida Handlirsch, 1906 (= Caloneurodea Martynov, 1938) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 106–108. [Google Scholar]
- 99. Rasnitsyn AP. 2.2 Subclass Scarabaeona Laicharting, 1781. The winged insects (= Pterygota Lang, 1888) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 75–82. [Google Scholar]
- 100. Brauckmann C, Hahn G. Ein neuer Insektenfund aus dem Westfalium von Ibbenbüren (Westdeutschland). Paläontologische Zeitschrift. 1980;54(3–4):301–312. [Google Scholar]
- 101. Novokshonov VG. New fossil insects (Insecta: Grylloblattida, Caloneurodea, Hypoperlida?, ordinis incertis) from the Kungurian beds of the middle Urals. Paleontological Journal. 1998;32(4):362–368. [Google Scholar]
- 102. Novokshonov VG. New fossil insects (Insecta: Hypoperlida, Panorpida, ordinis incertis) from the Chekarda locality. Paleontological Journal. 1999;33(1):52–56. [Google Scholar]
- 103. Beckemeyer RJ. The Permian insect fossils of Elmo, Kansas. The Kansas School Naturalist. 2000;46(1):1–16. [Google Scholar]
- 104. Sinitshenkova ND. 2.2.1.2.3 Superorder Dictyoneuridea Handlirsch, 1906 (= Palaeodictyopteroidea) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 115–124. [Google Scholar]
- 105. Simon E. Essai d’une classification des Opiliones Mecostethi. Annales de la Société entomologique de Belgique. 1879;22:183–241. [Google Scholar]
- 106. Béthoux O, Nel A, Lapeyrie J. The extinct order Caloneurodea (Insecta: Pterygota: Panorthoptera): wing venation, systematics and phylogenetic relationships. Annales zoologici. 2004;54(2):289–318. [Google Scholar]
- 107. White RD. A type catalog of fossil invertebrates (Arthropoda: Hexapoda) in the Yale Peabody Museum. Postilla. 1995;209:1–55. [Google Scholar]
- 108. Sinitshenkova ND, Marchal-Papier F, Grauvogel-Stamm L, Gall JC. The Ephemeridea (Insecta) from the Grès à Voltzia (early Middle Triassic) of the Vosges (NE France). Paläontologische Zeitschrift. 2005;79(3):377–397. [Google Scholar]
- 109.Hyatt A, Arms JM. Guides for Science-Teaching, no. 8: Insecta. Boston; 1890.
- 110. McCafferty WP. Chapter 2. Ephemeroptera In: Grimaldi DA, editor. Insects from the Santana Formation, Lower Cretaceous, of Brazil. vol. 195 Bulletin of the American Museum of Natural History; 1990. p. 25–50. [Google Scholar]
- 111. McCafferty WP. Higher classification of the burrowing mayflies (Ephemeroptera: Scapphodonta). Entomological News. 2004;115:84–92. [Google Scholar]
- 112. Kluge NJ. The phylogenetic system of Ephemeroptera Kluwer Academic Publishers, The Netherlands; 2004. [Google Scholar]
- 113. Sinitshenkova ND. The first fossil prosopistomatid mayfly from Burmese amber (Ephemeroptera; Prosopistomatidae). Bulletin of The Natural History Museum, Geology Series. 2000;56(1):25–28. [Google Scholar]
- 114. Godunko RJ, Kłonowska-Olejnik M. The first fossil representative of the genus Analetris Edmunds, 1972 (Insecta: Ephemeroptera: Acanthametropodidae) from the Eocene Baltic amber. Annales zoologici. 2006;56(4):785–790. [Google Scholar]
- 115. Hubbard MD. Ephemeroptera. Fossilium Catalogus 1: Animalia. 1987;129:1–99. [Google Scholar]
- 116. McCafferty WP. Toward a phylogenetic classification of the Ephemeroptera (Insecta): a commentary on systematics. Annals of the Entomological Society of America. 1991;84(4):343–360. [Google Scholar]
- 117. Godunko RJ, Neumann C, Krzeminski W. Fossil mayfly collections of the Museum für Naturkunde, Humboldt University, Berlin. II. Redescription of Baltameletus oligocaenicus Demoulin, 1968 with notes on Ameletidae McCafferty, 1991 (Insecta: Ephemeroptera) from the Eocene Baltic amber. Annales zoologici. 2008;58(1):105–114. [Google Scholar]
- 118. Wichard W, Gröhn C, Seredszus F. Aquatic insects in Baltic amber, Wasserinsketen in Baltischen Bernstein. Verlag Kessel; 2009. [Google Scholar]
- 119. Sinitshenkova ND. New Jersey amber mayflies: the first North American Mesozoic members of the order (Insecta; Ephemeroptera In: Grimaldi DA, editor. Studies on Fossils in Amber, with Particular Reference to the Cretaceous of New Jersey. Backhuys Publishers, Leiden, The Netherlands; 2000. p. 111–125. [Google Scholar]
- 120. McCafferty WP, Santiago-Blay JA. A new Cretaceous mayfly from Burmese amber (Ephemeroptera: Australiphemeridae). Entomological News. 2009;119(5):492–496. [Google Scholar]
- 121. Kluge NJ. New data on mayflies (Ephemeroptera) from Mesozoic and Cenozoic resins. Paleontological Journal. 1994;27(1A):35–49. [Google Scholar]
- 122. McCafferty WP. Discovery and analysis of the oldest mayflies (Insecta, Ephemeroptera) known from amber. Bulletin de la Société d’Histoire Naturelle de Toulouse. 1997;133:77–82. [Google Scholar]
- 123. Kluge NJ, Godunko RJ, Krzeminski W. A new mayfly family (Insecta: Ephemeroptera) from Eocene Baltic amber. Annales zoologici. 2006;56(1):181–185. [Google Scholar]
- 124. Zamboni JC. Contribution to the knowledge of the aquatic paleoentomofauna from Santana Formation (Araripe Basin, Lower Cretaceous, northeast Brazil) with description of new taxa. Acta Geologica Leopoldensia. 2001;24(52/53):129–135. [Google Scholar]
- 125. Staniczek AH. 11.4 Ephemeroptera: mayflies In: The Crato Fossil Beds of Brazil: Window into an Ancient World. Cambridge University Press; 2007. p. 163–184. [Google Scholar]
- 126. Pescador ML, Richard BA, Hubbard MD, Staniczek AH. Evolution of Baetiscidae (Ephemeroptera): current state of knowledge of the family. Aquatic Insects. 2009;31(Supplement 1):137–147. [Google Scholar]
- 127. Kukalová-Peck J. Ephemeroid wing venation based upon new gigantic Carboniferous mayflies and basic morphology, phylogeny and metamorphosis of pterygote insects (Insecta, Ephemerida). Canadian Journal of Zoology. 1985;63:933–955. [Google Scholar]
- 128. Wootton RJ, Kukalová-Peck J. Flight adaptations in Palaeozoic Palaeoptera (Insecta). Biological Review. 2000;75:129–167. [DOI] [PubMed] [Google Scholar]
- 129. Sinitshenkova ND. Main ecological events in aquatic insects history. Acta zoologica cracoviensia. 2003;46(suppl.—Fossil Insects):381–392. [Google Scholar]
- 130. Sinitshenkova ND. New late Mesozoic mayflies from the Shar-Teeg locality, Mongolia (Insecta, Ephemerida = Ephemeroptera). Paleontological Journal. 2002;36(3):270–276. [Google Scholar]
- 131. Zhang JF, Kluge NJ. Jurassic larvae of mayflies (Ephemeroptera) from the Daohugou Formation in Inner Mongolia, China. Oriental Insects. 2007;41:351–366. [Google Scholar]
- 132. Jacobus LM, McCafferty WP. Revision of Ephemerellidae genera (Ephemeroptera). Transactions of the American Entomological Society. 2008;134(1/2):185–274. [Google Scholar]
- 133. Huang JD, Ren D, Sun JH. Progress in the study of Ephemeroptera (mayfly) fossils. Acta Zootaxonomica Sinica. 2007;32(2):391–404. [Google Scholar]
- 134. Kluge NJ, Sinitshenkova ND. 2.2.1.1.1.3 Order Ephemerida Latreille, 1810. The true mayflies (= Ephemeroptera Hyatt et Arms, 1891 (s.l.); = Euephemeroptera Kluge, 2000) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 89–97. [Google Scholar]
- 135. Peñalver E, Martínez-Delclòs X, Arillo A. Yacimientos con insectos fósiles en España. Revista Española de Paleontología. 1999;14(2):231–245. [Google Scholar]
- 136. Ren D, Gao KQ, Guo ZG, Ji S, Tan JJ, Song Z. Stratigraphic division of the Jurassic in the Daohugou area, Ningcheng, Inner Mongolia. Geological Bulletin of China. 2002;21(8–9):584–591. In Chinese with English summary. [Google Scholar]
- 137. Ogden TH, Gattolliat JL, Sartori M, Staniczek AH, Soldán T, Whiting MF. Towards a new paradigm in mayfly phylogeny (Ephemeroptera): combined analysis of morphological and molecular data. Systematic Entomology. 2009;34(4):616–634. [Google Scholar]
- 138. Lewis SE. Two new species of fossil mayflies (Ephemeroptera: Neoephemeridae and Siphlonuridae) from the Ruby River Basin (Oligocene) of southwestern Montana. Proceedings of the Entomological Society of Washington. 1977;79(4):583–587. [Google Scholar]
- 139. Godunko RJ, Krzeminski W. New fossil findings of the mayfly genera Balticobaetisca Staniczek & Bechly, 2002 (Ephemeroptera: Baetiscidae) and Borinquena Traver, 1938 (Leptophlebiidae: Atalophlebiinae). Aquatic Insects. 2009;31(Supplement 1):125–136. [Google Scholar]
- 140. Godunko RJ, Neumann C. Fossil mayfly collections of the Museum für Naturkunde, Humboldt University Berlin. I. Electroletus soldani gen. and sp. nov. (Ephemeroptera: Ameletidae) from the Eocene Baltic amber. Annales zoologici. 2006;56(1):175–180. [Google Scholar]
- 141. Novokshonov VG. New insects (Insecta) from the Lower Permian of Chekarda (Central Urals). Paleontological Journal. 1994;27 [for 1993](1A):172–178. [Google Scholar]
- 142. Novokshonov VG, Aristov DS. New and little-known Permian insects (Insecta: Grylloblattida; Orthoptera) from the Chekarda locality, Central Ural Mountains. Paleontological Journal. 2002;36(6):644–649. [Google Scholar]
- 143. Rasnitsyn AP. 2.2.1.1.1.2. Order Syntonopterida Handlirsch, 1911 In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 88–89. [Google Scholar]
- 144. Kinzelbach R, Lutz H. Eine neue Eintagsfliege Misthodotes stapfi n. sp. aus dem Rotliegenden des Nahe-Gebietes (Ephemeroptera: Permoplectoptera: Misthodotidae). Paläontologische Zeitschrift. 1984;58(3/4):247–253. [Google Scholar]
- 145. Sinitshenkova ND. A new mayfly species of the extant genus Neoephemera from the Eocene of North America (Insecta: Ephemerida = Ephemeroptera: Neoephemeridae. Paleontological Journal. 1999;33(4):403–405. [Google Scholar]
- 146. Jacobus LM, McCafferty WP. Reevaluation of the phylogeny of the Ephemeroptera infraorder Pannota (Furcatergalia), with adjustments to higher classification. Transactions of the American Entomological Society. 2006;132(1/2):81–90. [Google Scholar]
- 147. Whalley PES, Jarzembowski EA. Fossil insects from the Lithographic Limestone of Montsech (late Jurassic-early Cretaceous), Lerida Province, Spain. Bulletin of the British Museum (Natural History), Geology. 1985;38(5):381–412. [Google Scholar]
- 148.Rowland JM. The late Paleozoic insect assemblage at Carrizo Arroyo, New Mexico p.1-7. In: Lucas SG, Estep JW, Williamson TE, Morgan GS, editors. New Mexico’s Fossil Record 1. vol. Bulletin 11. New Mexico Museum of Natural History and Science, Albuquerque; 1997.
- 149. Zhou CF, Peters JG. The nymph of Siphluriscus chinensis and additional imaginal description: a living mayfly with Jurassic origins (Siphluriscidae new family: Ephemeroptera). Florida Entomologist. 2003;86(3):345–352. [Google Scholar]
- 150. Zhang JF. New mayfly nymphs from the Jurassic of northern and northeastern China (Insecta: Ephemeroptera). Paleontological Journal. 2006;40(5):553–559. [Google Scholar]
- 151. Lin QB, Huang DY. New Middle Jurassic mayflies (Insecta: Ephemeroptera: Siphlonuridae) from Inner Mongolia, China. Annales zoologici. 2008;58(3):521–527. [Google Scholar]
- 152. Garrouste R, Nel A, Gand G. New fossil arthropods (Notostraca and Insecta: Syntonopterida) in the continental Middle Permian of Provence (Bas-Argens Basin, France). Comptes Rendus Palevol. 2009;8(1):49–57. [Google Scholar]
- 153. Krzeminski W, Lombardo C. New fossil Ephemeroptera and Coleoptera from the Ladinian (Middle Triassic) of Canton Ticino (Switzerland). Rivista Italiana di Paleontologia e Stratigrafia. 2001;107(1):69–78. [Google Scholar]
- 154. Sinitshenkova ND. New Mesozoic mayflies (Ephemerida) from Mongolia. Paleontological Journal. 1989;23(3):26–37. [Google Scholar]
- 155. Handlirsch A. Revision der paläozoischen Insekten. Denkschriften der Kaiserlichen Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Klasse. 1919;96:511–592. [Google Scholar]
- 156.Rasnitsyn AP, Aristov DS, Gorokhov AV, Rowland JM, Sinitshenkova ND. Important new insect fossils from Carrizo Arroyo and the Permo-Carboniferous faunal boundary. In: Lucas SG, Zeigler KE, editors. Carboniferous-Permian Transition at Carrizo Arroyo, Central New Mexico. New Mexico Museum of Natural History and Science, Albuquerque, Bulletin 25; 2004. p. 215–246.
- 157. van Dijk DE, Geertsema H. Permian insects from the Beaufort Group of Natal, South Africa. Annals of the Natal Museum. 1999;40:137–171. [Google Scholar]
- 158. Shcherbakov DE, Makarkin VN, Aristov DS, Vasilenko DV. Permian insects from the Russky Island, South Pimorye. Russian Entomological Journal. 2009;18(1):7–16. [Google Scholar]
- 159. Tasch P, Zimmerman JR. The Asthenohymen-Delopterum bed—A new Leonardian insect horizon in the Wellington of Kansas and Oklahoma. Journal of Paleontology. 1962;36:1319–1333. [Google Scholar]
- 160. Beckemeyer RJ, Engel MS. An enigmatic new genus of biarmohymenid from the early Permian Wellington Formation of Noble County, Oklahoma (Palaeodictyopterida: Diaphanopterodea). Transactions of the Kansas Academy of Science. 2009;112(1/2):103–108. [Google Scholar]
- 161. Béthoux O, Nel A. Revision of Diaphanoptera species and new diagnosis of Diaphanopteridae (Palaeoptera: Diaphanopterodea). Journal of Paleontology. 2003;77(5):1016–1020. [Google Scholar]
- 162. Zajíc J, Štamberg S. Selected important fossiliferous horizons of the Boskovice Basin in the light of the new zoopaleontological data. Acta Musei Reginaehradecensis Series A, Scientiae naturales. 2004;30:5–14. [Google Scholar]
- 163. Carpenter FM. Studies on Carboniferous insects from Commentry, France: Part V. The genus Diaphanoptera and the order Diaphanopterodea. Psyche. 1963;70:240–256. [Google Scholar]
- 164. Rohdendorf BB. Fundamentals of Paleontology Volume 9: Arthropoda, Tracheata, Chelicerta Orlov YA, editor. Smithsonian Institution Libraries and The National Science Foundation, Washington D.C.; 1991. [Google Scholar]
- 165. Sinitshenkova ND. The peculiar “dipterous" insects from the Permian of Arkhangelsk district and Urals (Hypoperlida, Eukulojidae). Bulletin of Moscow Society of Naturalists, Biological Series. 1981;56(3):116–126. In Russian. [Google Scholar]
- 166. Béthoux O, Nel A, Lapeyrie J, Gand G, Galtier J. New Martynoviidae from the Permian of Southern France (Lodève basin) (Insecta: Palaeoptera: Diaphanopterodea). Geobios. 2003;36:131–139. [Google Scholar]
- 167. Brauckmann C, Herd KJ. Insekten-Funde aus dem Westfalium D (Ober-Karbon) des Piesberges bei Osnabrück (Deutschland). Teil 1: Palaeoptera. Osnabrücker naturwissenschafliche Mitteilungen. 2003;28 (for 2002):27–69. [Google Scholar]
- 168. Kukalová-Peck J, Sinitshenkova ND. The wing venation and systematics of Lower Permian Diaphanopterodea from the Ural Mountains, Russia (Insecta: Paleoptera). Canadian Journal of Zoology. 1992;70:229–235. [Google Scholar]
- 169. Kukalová-Peck J, Brauckmann C. Wing folding in pterygote insects, and the oldest Diaphanopterodea from the early Late Carboniferous of West Germany. Canadian Journal of Zoology. 1990;68:1104–1111. [Google Scholar]
- 170. Carpenter FM. 14A Insecta In: Shabica CW, Hay AA, editors. Richardson’s Guide to The Fossil Fauna of Mazon Creek. Northeastern Illinois University; 1997. p. 184–193. [Google Scholar]
- 171. Prokop J, Nel A. Systematic position of Triplosoba, hitherto the oldest mayfly, from Upper Carboniferous of Commentry in Central France (Insecta: Palaeodictyopterida). Systematic Entomology. 2009;34(4):610–615. [Google Scholar]
- 172. Brongniart C. Les insectes fossiles des terrains primaires. Coup d’oeil rapide sur la faune entomologique des terrains paléozoïques. Bulletin de la Société des amis des sciences naturelles de Rouen. 1885;1885:50–68. [Google Scholar]
- 173. Kukalová-Peck J. Megasecoptera from the Lower Permian of Moravia. Psyche. 1975;82:1–19. [Google Scholar]
- 174. Carpenter FM. A megasecopteran from Upper Carboniferous strata in Spain. Psyche. 1963;70:44–49. [Google Scholar]
- 175. Brauckmann C. Notiz über Insekten-Reste aus dem Ober-Karbon in Spanien. Jahresberichte des Naturwissenschaften Vereins in Wuppertal. 1993;46:115–119. [Google Scholar]
- 176. Sinitshenkova ND. A new family Aykhalidae from the Upper Palaeozoic of Yakutia Sakha (Insecta: Mischopteridae = Megasecoptera). Paleontological Journal. 1994;27(1993)(1A):131–134. [Google Scholar]
- 177. Brauckmann C, Schöllmann L, Sippel W. Die fossilen Insekten, Spinnentiere und Eurypteriden von Hagen-Vorhalle. Geologie und Paläontologie in Westfalen. 2003;59:5–89. [Google Scholar]
- 178. Zessin W. Zwei neue Insektenreste (Megasecoptera, Odonatoptera) aus dem Westfalium D (Oberkarbon) des Piesberges bei Osnabrück, Deutschland. Virgo, Mitteilungsblatt des Entomologischen Vereins Mecklenburg. 2006;9(1):37–45. [Google Scholar]
- 179. Nelson CR, Tidwell WD. Brodioptera stricklani n. sp. (Megasecoptera: Brodiopteridae), a new fossil insect from the Upper Manning Canyon Shale Formation, Utah (Lowermost Namurian B). Psyche. 1987;94:309–316. [Google Scholar]
- 180. Brauckmann C. Ein neuer Inseckten-Rest (Megasecoptera) aus dem Ober-Karbon von Osnabrück. Osnabrücker naturwissenschafliche Mitteilungen. 1991;17:25–32. [Google Scholar]
- 181. Béthoux O, Galtier J, Nel A. Earliest evidence of insect endophytic oviposition. Palaios. 2004;19(4):408–413. [Google Scholar]
- 182. Carpenter FM. Studies on Carboniferous insects from Commentry, France: Part II. The Megasecoptera. Journal of Paleontology. 1951;25(3):336–355. [Google Scholar]
- 183. Béthoux O, McBride J, Maul C. Surface laser scanning of fossil insect wings. Palaeontology. 2004;47(1):13–19. [Google Scholar]
- 184. Labandeira CC. 1.3.9 Rise and Diversification of Insects In: Briggs DEG, Crowther PR, editors. Palaeobiology II. Blackwell Science, London; 2001. p. 82–88. [Google Scholar]
- 185. Prokop J, Ren D. New significant fossil insects from the Upper Carboniferous of Ningxia in northern China (Palaeodictyoptera, Archaeorthoptera). European Journal of Entomology. 2007;104(2):267–275. [Google Scholar]
- 186. Béthoux O, Nel A. Révision de Protagrion audouini Brongniart, 1893, du Carbonifère supérieur (Palaeoptera). Bulletin de la Société entomologique de France. 2003;108(3):237–244. [Google Scholar]
- 187. Hong YC. New fossil genera and species of Shanxi Formation in Xishan of Taiyuan. Entomotaxonomia. 1985;7(2):83–91. [Google Scholar]
- 188. Rasnitsyn AP, Sukatsheva ID, Aristov DS. Permian insects of the Vorkuta Group in the Pechora Basin, and their stratigraphic implications. Paleontological Journal. 2005;39(4):404–416. [Google Scholar]
- 189. Pinto ID. Sphecorydaloides lucchesei a new Carboniferous megasecopteran Insecta from Argentina. Pesquisas. 1994;21(2):85–89. [Google Scholar]
- 190.Pinto ID, Adami-Rodrigues K. A revision of South American Paleozoic insects. In: Scoggin M, editor. AMBA projects AM/PFICM98/1.99: Proceedings of the First International Palaeoentomological Conference, Moscow 1998; 1999. p. 117–124.
- 191. Novokshonov VG. New insects (Insecta: Hypoperlida, Mischopterida, Jurinida) from the Lower Permian of the Middle Urals. Paleontological Journal. 1998;32(1):46–53. [Google Scholar]
- 192. Pinto ID. Carboniferous insects from Argentina III Familia Xenopteridae Pinto, nov. Ordo Megasecoptera. Pesquisas. 1986;18:23–29. [Google Scholar]
- 193.Goldenberg CF. Fauna Saraepontana fossilis. Die fossilen Thiere aus der Steinkohlenformation von Saarbrücken, Part II. Möllinger: Saarbrücken; 1877.
- 194. Prokop J, Nel A. A new genus and species of Homoiopteridae from the Upper Carboniferous of the Intra-Sudetic Basin, Czech Republic (Insecta: Palaeodictyoptera). European Journal of Entomology. 2004;101(4):583–589. [Google Scholar]
- 195. Brauckmann C, Koch L, Kemper M. Spinnentiere (Arachnida) und Insekten aus den Vorhalle-Schichten (Namurium B; Ober-Karbon) von Hagen-Vorhalle (West Deutschland). Geologie und Paläontologie in Westfalen. 1985;3:1–131. [Google Scholar]
- 196. Béthoux O, Nel A, Schneider JW, Gand G. Lodetiella magnifica nov. gen. and nov. sp. (Insecta: Palaeodictyoptera; Permian), an extreme situation in wing morphology of palaeopterous insects. Geobios. 2007;40(2):181–189. [Google Scholar]
- 197. Özdikmen H. Some nomenclatural changes for Blattodea and Dictyoneurida (= Palaeodictyoptera). Munis Entomology and Zoology. 2008;3(2):745–748. [Google Scholar]
- 198. Gorjunova RV. New Carboniferous bryozoans of the Gobi Altai. Sovmestnaya Sovetsko-Mongolskaya Paleontologischeskaya Ekspeditsiya Trudy. 1988;33:10–23. [Google Scholar]
- 199. Sharov AG, Sinitshenkova ND. Novyye Paleodictyoptera s territorii SSSR. [New Palaeodictyoptera from the USSR.]. Paleontologicheskii Zhurnal. 1977;1977:48–63. [Google Scholar]
- 200. Prokop J, Smith R, Jarzembowski EA, Nel A. New homoiopterids from the Late Carboniferous of England (Insecta: Palaeodictyoptera). Comptes Rendus Palevol. 2006;5(7):867–873. [Google Scholar]
- 201. Handlirsch A. Revision of American Paleozoic insects. Proceedings of the United States National Museum. 1906;29:661–820. [Google Scholar]
- 202. Kukalová J. Revisional study of the order Palaeodictyoptera in the Upper Carboniferous Shales of Commentry, France. Part I. Psyche. 1969;76:163–215. [Google Scholar]
- 203. Kukalová J. Revisional study of the order Palaeodictyoptera in the Upper Carboniferous Shales of Commentry, France. Part II. Psyche. 1969;76:349–486. [Google Scholar]
- 204. Bolton H. A Monograph of the Fossil Insects of the British Coal Measures. Part I. Palaeontographical Society, London; 1921. [Google Scholar]
- 205. Jell PA. The fossil insects of Australia. Memoirs of the Queensland Museum. 2004;50(1):1–124. [Google Scholar]
- 206. Brauckmann C, Schneider J. Ein unter-karbonisches Insekt aus dem Raum Bitterfeld/Delitzsch (Pterygota, Arnsbergium, Deutschland). Neues Jahrbuch für Geologie und Paläontologie, Monatshefte. 1996;1996(1):17–30. [Google Scholar]
- 207. Laurentiaux-Vieira F, Laurentiaux D. Paleodictyoptere nouveau du Namurien belge. Annales de la Société Géologique du Nord. 1986;105:187–193. [Google Scholar]
- 208. Sinitshenkova ND. A new species of the Tchirkovaeidae (Insecta, Dictyoneurida) from the Upper Carboniferous of the Tunguska Basin. Paleontological Journal. 1981;15(1):121–123. [Google Scholar]
- 209. Brodsky AK. The evolution of insect flight Oxford University Press; 1994. [Google Scholar]
- 210. Gutiérrez PR, Muzón J, Limarino CO. The earliest late Carboniferous winged insect (Insecta, Protodonata) from Argentina: geographical and stratigraphical location. Ameghiniana. 2000;37(3):375–378. [Google Scholar]
- 211. Fabricius JC. Entomologicae Systematica, v. 2. Hafniae; 1793. [Google Scholar]
- 212. Fleck G, Nel A, Bechly G, Martínez-Delclòs X. Revision and phylogenetic affinities of the Jurassic Steleopteridae Handlirsch, 1906 (Odonata: Zygoptera). Insect Systematics & Evolution. 2001;32:285–305. [Google Scholar]
- 213. Nel A, Paicheler JC. Les Odonata fossiles: état actuel des connaissances. Deuxième partie: Les Petaluridae et Cordulegastridae fossiles (Odonata, Anisoptera, Petaluroidea). Nouvelle Revue dEntomologie. 1992;9(4):305–323. [Google Scholar]
- 214. Fleck G, Nel A. Revision of the Mesozoic family Aeschnidiidae (Odonata: Anisoptera). Zoologica. 2003;153:1–172. [Google Scholar]
- 215. Nel A, Martínez-Delclòs X, Escuillié F, Brisac P. Les Aeshnidae fossils: Etat actuel des connaissances (Odonata, Anisoptera). Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 1994;194(2/3):143–186. [Google Scholar]
- 216. Nel A, Bechly G, Jarzembowski EA, Martínez-Delclòs X. A revision of the fossil petalurid dragonflies (Insecta: Odonata: Anisoptera: Petalurida). Paleontologica Lombarda Nuova serie. 1998;10:1–68. [Google Scholar]
- 217. Bechly G, Nel A, Martínez-Delclòs X, Jarzembowski EA, Coram R, Martill D, et al. A revision and phylogenetic study of Mesozoic Aeshnoptera, with description of numerous new taxa (Insecta: Odonata: Anisoptera). Neue Paläontologische Abhandlungen. 2001;4:1–219. [Google Scholar]
- 218. Bechly G, Ueda K. The first fossil record and first New World record for the dragonfly clade Chlorogomphida (Insecta: Odonata: Anisoptera: Araripechlorogomphidae n. fam.) from the Crato Limestone (Lower Cretaceous, Brazil). Stuttgarter Beiträge zur Naturkunde Serie B (Geologie und Paläontologie). 2002;328:1–11. [Google Scholar]
- 219. Bechly G. 11.5 Odonata: damselflies and dragonflies In: Martill DM, Bechly G, Loveridge RF, editors. The Crato Fossil Beds of Brazil: Window into an Ancient World. Cambridge University Press; 2007. p. 184–222. [Google Scholar]
- 220. Bechly G. Morphologische Untersuchungen am Flügelgeäder der rezenten Libellen und deren Stammgruppenvertreter (Insecta: Pterygota: Odonata), unter besonderer Berücksichtigung der Phylogenetischen Systematik und des Grundplanes der Odonata. Petalura, Special Volume. 1996;2:1–402. [Google Scholar]
- 221. Fleck G, Nel A, Bechly G, Delclòs X, Jarzembowski EA, Coram RA. New Lower Cretaceous ‘libelluloid’ dragonflies (Insecta: Odonata: Cavilabiata) with notes about estimated divergence dates for this group. Palaeodiversity. 2008;1:19–36. [Google Scholar]
- 222. Bechly G. New fossil dragonflies from the Lower Cretaceous Crato Formation of northeast Brazil (Insecta: Odonata. Stuttgarter Beiträge zur Naturkunde Serie B (Geologie und Paläontologie). 1998;264:1–66. [Google Scholar]
- 223. Jarzembowski EA. Chapter 10. Arthropods 2: Insects p.149–160. In: Swift A, Martill DM, editors. Fossils of the Rhaetian Penarth Group. The Palaeontological Association, London; 1999. [Google Scholar]
- 224. Sukatsheva ID, Rasnitsyn AP. Jurassic insects (Insecta) from the Sai-Sagul locality (Kyrgyzstan, Southern Fergana). Paleontological Journal. 2004;38(2):182–186. [Google Scholar]
- 225. Nel A, Paicheler JC, Henrotay M. Les “Anisozygoptera” fossiles. Phylogénie et classification (Odonata). Martinia. 1993;3:1–311. [Google Scholar]
- 226. Petrulevicius JF, Nel A. Austroperilestidae, a new family of damselflies from early Eocene of Argentina (Insecta: Odonata). Phylogenetic relationships within Odonata. Journal of Paleontology. 2005;79(4):658–662. [Google Scholar]
- 227. Nel A, Papier F, Grauvogel-Stamm L, Gall JC. Voltzialestes triasicus gen. nov., sp. nov., le premier Odonata Protozygoptera du Trias inférieur des Vosges (France). Paleontologica Lombarda Nuova serie. 1996;5:25–36. [Google Scholar]
- 228. Nel A, Gand G, Garric J, Lapeyrie J. The first recorded protozygopteran insects from the Upper Permian of France. Palaeontology. 1999;42(1):83–97. [Google Scholar]
- 229. Jarzembowski EA, Nel A. The earliest damselfly-like insect and the origin of modern dragonflies (Insecta: Odonatoptera: Protozygoptera). Proceedings of the Geologists’ Association. 2002;113:165–169. [Google Scholar]
- 230. Zessin W. Überblick über die paläozoischen Libellen (Insecta, Odonatoptera). Virgo, Mitteilungsblatt des Entomologischen Vereins Mecklenburg. 2008;11(1):5–32. [Google Scholar]
- 231. Gentilini G. Fossil damselflies and dragonflies from the Eocene of Monte Bolca, Italy (Insecta: Odonata). Studi e Ricerche sui Giaimenti Terziari di Bolca. 2002;9:7–22. [Google Scholar]
- 232. Fleck G, Waller A, Serafin J, Nel A. The oldest Calopterygidae in the Eocene Baltic amber (Odonata: Zygoptera). Zootaxa. 2009;1985:52–56. [Google Scholar]
- 233. Nel A, Bechly G, Delclòs X, Huang DY. New and poorly known Mesozoic damseldragonflies (Odonata: Isophlebioidea: Campterophlebiidae, Isophlebiidae). Palaeodiversity. 2009;2:209–232. [Google Scholar]
- 234. Huguet A, Nel A, Martínez-Delclòs X, Bechly G, Martins-Neto R. Preliminary phylogenetic analysis of the Protanisoptera (Insecta: Odonatoptera). Geobios. 2002;35(5):537–560. [Google Scholar]
- 235. Petrulevicius JF, Nel A. First Cordulephyidae dragonfly in America: A new genus and species from the Paleogene of Argentina (Insecta: Odonata). Comptes Rendus Palevol. 2009;8(4):385–388. [Google Scholar]
- 236. Bechly G. A new fossil dragonfly (Anisoptera: Corduliidae) from the Paleocene Fur Formation (Mo clay) of Denmark. Stuttgarter Beiträge zur Naturkunde Serie B (Geologie und Paläontologie). 2005;358:1–7. [Google Scholar]
- 237. Jarzembowski EA, Martínez-Delclòs X, Bechly G, Nel A, Coram R, Escuillié F. The Mesozoic non-calopterygoid Zygoptera: description of new genera and species from the Lower Cretaceous of England and Brazil and their phylogenetic significance (Odonata, Zygoptera, Coenagrionoidea, Hemiphlebioidea, Lestoidea). Cretaceous Research. 1998;19(3–4):403–444. [Google Scholar]
- 238. Coram RA, Nel A. A new petalurid dragonfly from the Lower Cretaceous of southern England (Odonata: Petalurida:? Cretapetaluridae). Palaeodiversity. 2009;2:205–208. [Google Scholar]
- 239. Nel A, Bechly G. The third petalurid dragonfly from the Lower Cretaceous of Brazil (Odonata: Cretapetaluridae). Annales zoologici. 2009;59(3):281–285. [Google Scholar]
- 240. Bechly G. New fossil odonates from the Upper Triassic of Italy, with a redescription of Italophlebia gervasuttii Whalley, and a reclassification of Triassic dragonflies (Insecta: Odonata). Rivista del Museo Civico di Scienze Naturali “Enrico Caffi”. 1997;19:31–70. [Google Scholar]
- 241. Nel A, Huang DY. First Chinese Cymatophlebiidae from the Middle Jurassic of Inner Mongolia (Odonata: Anisoptera: Aeshnoptera). Palaeodiversity. 2009;2:199–204. [Google Scholar]
- 242. Rust J, Petrulevicius JF, Nel A. The first damselflies from the lowermost Eocene of Denmark, with a description of a new subfamily (Odonata, Zygoptera: Dysagrionidae). Paleontology. 2008;51(3):709–713. [Google Scholar]
- 243. Nel A, Arillo A. The first Baltic amber dysagrionine damselfly (Odonata: Zygoptera: Thaumatoneuridae: Dysagrioninae). Annales de la Société entomologique de France (Nouvelle série). 2006;42(2):179–182. [Google Scholar]
- 244. Nel A, Néraudeau D, Perrichot V, Girard V, Gomez B. A new dragonfly family from the Upper Cretaceous of France. Acta Palaeontologica Polonica. 2008;53(1):165–168. [Google Scholar]
- 245. Pritykina LN. Two new dragonflies from the Lower Cretaceous deposits of west Mongolia (Anisoptera: Sonidae fam. nov., Corduliidae). Odonatologica. 1986;15(2):169–184. [Google Scholar]
- 246. Nel A, Paicheler JC. Les Odonata fossiles: état actuel des connaissances. Huitième partie: Les Calopterygoidea fossiles (Odonata, Zygoptera). Bulletin de la Société entomologique de France. 1993;97(4):381–396. [Google Scholar]
- 247. Petrulevicius JF, Nel A, Rust J, Bechly G, Kohls D. New Paleogene Epallagidae (Insecta: Odonata) recorded in North America and Europe. Biogeographic implications. Alavesia. 2007;1:15–25. [Google Scholar]
- 248. Fleck G, Bechly G, Martínez-Delclòs X, Jarzembowski E, Coram R, Nel A. Phylogeny and classification of the Stenophlebioptera (Odonata: Epiproctophora). Annales de la Société entomologique de France (Nouvelle série). 2003;39(1):55–93. [Google Scholar]
- 249.Bridges CA. Catalogue of the family-group, genus-group and species-group names of the Odonata of the world (3rd edition). Urbana, Illinois, USA; 1994.
- 250. Jarzembowski EA. Early Cretaceous zygopteroids of southern England, with the description of Cretacoenagrion alleni gen. nov., spec. nov. (Zygoptera: Coenagrionidae; “Anisozygoptera”: Tarsophlebiidae, Euthemistidae). Odonatologica. 1990;19(1):27–37. [Google Scholar]
- 251. Petrulevicius JF, Nel A. Frenguelliidae, a new family of dragonflies from the earliest Eocene of Argentina (Insecta: Odonata): phylogenetic relationships within Odonata. Journal of Natural History. 2003;37(24):2909–2917. [Google Scholar]
- 252. Petrulevicius JF, Nel A. Enigmatic and little known Odonata (Insecta) from the Paleogene of Patagonia and northwest Argentina. Annales de la Société entomologique de France (Nouvelle série). 2007;43(3):341–347. [Google Scholar]
- 253. Prokop J, Fikacek M. An annotated list of early Oligocene insect fauna from Seifhennersdorf (Saxony, Germany). Acta Musei Nationalis Pragae, Series B Historia Naturalis. 2007;63(2–4):209–217. [Google Scholar]
- 254. Schlüter T. Fossil insects in Gondwana—localities and palaeodiversity trends. Acta zoologicacracoviensia. 2003;46(suppl.- Fossil Insects):345–371. [Google Scholar]
- 255. Bechly G, Nel A, Martínez-Delclòs X, Fleck G. Four new dragonflies from the Upper Jurassic of Germany and the Lower Cretaceous of Mongolia (Anisoptera: Hemeroscopidae, Sonidae, and Proterogomphidae fam. nov.). Odonatologica. 1998;27(2):149–187. [Google Scholar]
- 256. Ren D, Liu JY, Cheng XD. A new hemeroscopid dragonfly from the Lower Cretaceous of Northeast China (Odonata: Hemeroscopidae). Acta Entomologica Sinica. 2003;46(5):622–628. [Google Scholar]
- 257. Vasilenko DV. New damselflies (Odonata: Synlestidae, Hemiphlebiidae) from the Mesozoic Transbaikalian locality of Chernovski Kopi. Paleontological Journal. 2005;39(3):280–283. [Google Scholar]
- 258. Zalessky G. Two new Permian dragonfly-like insects of the order Permodonata [in Russian]. Doklady Akademii Nauk SSSR. 1955;104(4):630–633. [Google Scholar]
- 259. Ansorge J. Heterophlebia buckmani (Brodie 1845) (Odonata: "Anisozygoptera")–das erste Insekt aus dem untertoarcischen Posidonienschiefer von Holzmaden (Wüttemberg, SW Deutschland). Stuttgarter Beiträge zur Naturkunde Serie B (Geologie und Paläontologie). 1999;275:1–9. [Google Scholar]
- 260. Bechly G, Wichard W. Damselfly and dragonfly nymphs in Eocene Baltic amber (Insecta: Odonata), with aspects of their palaeobiology. Palaeodiversity. 2008;1:37–73. [Google Scholar]
- 261. Nel A, Petrulevicius JF, Jarzembowski EA. New fossil Odonata from the European Cenozoic (Insecta: Odonata: Thaumatoneuridae, Aeshnidae,? Idionychidae, Libellulidae). Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 2005;235(3):343–380. [Google Scholar]
- 262. Pritykina LN. Isophlebiid dragonflies from the late Mesozoic of eastern Transbaikalia (Odonata: Isophlebiidae). Paleontological Journal. 2006;40(6):636–645. [Google Scholar]
- 263. Bybee SM, Ogden TH, Branham MA, Whiting MF. Molecules, morphology and fossils: a comprehensive approach to odonate phylogeny and the evolution of the odonate wing. Cladistics. 2008;24:477–514. [DOI] [PubMed] [Google Scholar]
- 264.Bechly G. Juracordulia schiemenzi gen. et. sp. nov., Eine neue Libelle aus den
- 265. Solnhofener Plattenkalken (Insecta: Odonata: Anisoptera). Archaeopteryx. 1998;16:29–36. [Google Scholar]
- 266. Nel A, Bechly G, Martínez-Delclòs X, Fleck G. A new family of Anisoptera from the Upper Jurassic of Karatau in Kazakhstan (Insecta: Odonata: Juragomphidae n. fam.). Stuttgarter Beiträge zur Naturkunde Serie B (Geologie und Paläontologie). 2001;314:1–9. [Google Scholar]
- 267. Huang DY, Nel A. Oldest ‘libelluloid’ dragonfly from the Middle Jurassic of China (Odonata: Anisoptera: Cavilabiata). Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 2007;246(1):63–68. [Google Scholar]
- 268. Nel A, Béthoux O, Bechly G, Martínez-Delclòs X, Papier F. The Permo-Triassic Odonatoptera of the “Protodonate” grade (Insecta: Odonatoptera). Annales de la Société entomologique de France (Nouvelle série). 2001;37(4):501–525. [Google Scholar]
- 269. Petrulevicius JF, Nel A. A new damselfly family from the Upper Palaeocene of Argentina. Palaeontology. 2004;47(1):109–116. [Google Scholar]
- 270. Nel A, Paicheler JC. Les Lestoidea (Odonata, Zygoptera) fossils: un inventaire critique. Annales de Paléontologie. 1994;80(1):1–59. [Google Scholar]
- 271. Huang DY, Nel A, Lin QB. A new genus and species of aeshnopteran dragonfly from the Lower Cretaceous of China. Cretaceous Research. 2003;24(2):141–147. [Google Scholar]
- 272. Etter W, Kuhn O. An articulated dragonfly (Insecta, Odonata) from the Upper Liassic Posidonia Shale of northern Switzerland. Palaeontology. 2000;43(5):967–977. [Google Scholar]
- 273. Nel A, Petrulevicius JF. A new genus and species of damsel-dragonfy from the early Liassic of Germany (Odonata, Liassophlebiidae). Bulletin de la Société entomologique de France. 2005;110(2):185–188. [Google Scholar]
- 274. Nel A, Paicheler JC. Les Libelluloidea autres ue Libellulidae fossils. Un inventaire critique (Odonata, Corduliidae, Macromiidae, Synthemistidae, Chlorogomphidae et Mesophlebiidae). Nouvelle Revue d’Entomologie. 1994;11(4):321–334. [Google Scholar]
- 275. Fleck G, Nel A, Martínez-Delclòs X. The oldest record of libellulid dragonflies from the Upper Cretaceous of Kazakhstan (Insecta: Odonata, Anisoptera). Cretaceous Research. 1999;20:655–658. [Google Scholar]
- 276. Bechly G. Two new fossil dragonfly species (Insecta: Odonata: Anisoptera: Araripegomphidae and Lindeniidae) from the Crato Limestone (Lower Cretaceous, Brazil). Stuttgarter Beiträge zur Naturkunde Serie B (Geologie und Paläontologie). 2000;296:1–16. [Google Scholar]
- 277. Azar D, Nel A. First Baltic amber megapodagrionid damselfly (Odonata: Zygoptera). Annales de la Société entomologique de France (Nouvelle série). 2008;44(4):451–457. [Google Scholar]
- 278. Bechly G. Description of a new species of Nannogomphus (Insecta: Odonata: Nannogomphidae) from the Upper Jurassic Solenhofen Limestone in Germany. Stuttgarter Beiträge zur Naturkunde Serie B (Geologie und Paläontologie). 2003;339:1–6. [Google Scholar]
- 279. Lin QB, Huang DY, Nel A. A new family of Cavilabiata from the Lower Cretaceous Yixian Formation, China (Odonata: Anisoptera). Zootaxa. 2007;1469:59–64. [Google Scholar]
- 280. Carle FL. Evolution, taxonomy, and biogeography of ancient Gondwanian libelluloides, with comments on anisopteroid evolution and phylogenetic systematics (Anisoptera: Libelluloidea). Odonatologica. 1995;24(4):383–424. [Google Scholar]
- 281. Petrulevicius JF, Nel A, Muzón J. A new libelluloid family from the upper Palaeocene of Argentina. Palaeontology. 1999;42(4):677–682. [Google Scholar]
- 282. Petrulevicius JF, Nel A. New palaeomacromiid dragonflies from the upper Palaeocene of Argentina. Palaeontology. 2002;45(4):751–758. [Google Scholar]
- 283. Nel A. A new Odonata family from the Jurassic of Central Asia (Odonata: Epiproctophora). Journal of Natural History. 2009;43(1–2):57–64. [Google Scholar]
- 284. Bechly G. A re-description of “Stenophlebia” casta (Insecta: Odonata: Parastenophlebiidae n. fam.) from the Upper Jurassic Solenhofen Limestone in Germany. Stuttgarter Beiträge zur Naturkunde Serie B (Geologie und Paläontologie). 2005;359:1–12. [Google Scholar]
- 285. Vasilenko DV, Rasnitsyn AP. Fossil ovipositions of dragonflies: review and interpretation. Paleontological Journal. 2007;41(11):1156–1161. [Google Scholar]
- 286. Petrulevicius JF, Nel A. Oldest petalurid dragonfly (Insecta: Odonata): a Lower Cretaceous specimen from south Patagonia, Argentina. Cretaceous Research. 2003;24(1):31–34. [Google Scholar]
- 287. Nel A. Piroutetia liasina Meunier, 1907, Insecte du Lias de France, espèce-type des Piroutetiidae nov. fam. (Odonatoptera, Meganeurina). Bulletin du Muséum National d’Histoire Naturelle, Série 4, Section C. 1989;11(1):15–19. [Google Scholar]
- 288. Wappler T, Petrulevicius JF. Priscalestidae, a new damselfly family (Odonata: Lestinoidea) from the Middle Eocene Eckfeld maar of Germany. Alavesia. 2007;1:69–73. [Google Scholar]
- 289. Nel A, Bechly G, Martínez-Delclòs X. A new fossil dragonfly from the Upper Jurassic in Germany [Odonata, Anisoptera, Protolindeniidae]. Revue française d’Entomologie. 2001;23(4):257–261. [Google Scholar]
- 290. Nel A, Petrulevicius JF, Martínez-Delclòs X. New Mesozoic Protomyrmeleontidae (Insecta: Odonatoptera: Archizygoptera) from Asia with a new phylogenetic analysis. Journal of Systematic Palaeontology. 2005;3(2):187–201. [Google Scholar]
- 291. Nel A, Jarzembowski EA. New protomyrmeleontid dragonflies from the Lower Cretaceous of southern England (Insecta, Odonata, Archizygoptera). Cretaceous Research. 1998;19(3–4):393–402. [Google Scholar]
- 292. Lin QB, Zhang S, Huang DY. Fuxiaeschna hsiufnia gen. nov., spec. nov., a new Lower Cretaceous dragonfly from northwestern China (Aeshnoptera: Rudiaeschnidae). Odonatologica. 2004;33(4):437–442. [Google Scholar]
- 293. Nel A, Gand G, Fleck G, Béthoux O, Lapeyrie J, Garric J. Saxonagrion minutus nov. gen. et sp., the oldest damselfly from the Upper Permian of France (Odonatoptera, Panodonata, Saxonagrionidae nov. fam.). Geobios. 1999;32(6):883–888. [Google Scholar]
- 294. Zessin W. Eine unwahrscheinliche Erfolgsbilanz: die Evolution der Libellen. Virgo, Mitteilungsblatt des Entomologischen Vereins Mecklenburg. 2005;8(1):54–66. [Google Scholar]
- 295. Nel A, Petrulevicius JF, Gentilini G, Martínez-Delclòs X. Phylogenetic analysis of the Cenozoic family Sieblosiidae (Insecta: Odonata), with description of new taxa from Russia, Italy and France. Geobios. 2005;38(2):219–233. [Google Scholar]
- 296. Nel A, Marie V, Schmeißner S. Revision of the lower Mesozoic dragonfly family Triassolestidae Tillyard, 1918 (Odonata: Epiproctophora). Annales de Paléontologie. 2002;88:189–214. [Google Scholar]
- 297. Fleck G, Bechly G, Martínez-Delclòs X, Jarzembowski EA, Nel A. A revision of the Upper Jurassic-Lower Cretaceous dragonfly family Tarsophlebiidae, with a discussion on the phylogenetic positions of the Tarsophlebiidae and Sieblosiidae (Insecta, Odonatoptera, Panodonata). Geodiversitas. 2004;26(1):33–60. [Google Scholar]
- 298. Huang DY, Nel A. The first Chinese Tarsophlebiidae from the Lower Cretaceous Yixian Formation, with morphological and phylogenetic implications (Odonatoptera: Panodonata). Cretaceous Research. 2009;30(2):429–433. [Google Scholar]
- 299. Martins-Neto RG, Gallego OF, Zavattieri AM. A new Triassic insect fauna from Cerro Bayo, Potrerillos (Mendoza Province, Argentina) with descriptions of new taxa (Insecta: Blattoptera and Coleoptera). Alcheringa. 2007;31(2):199–213. [Google Scholar]
- 300. Jarzembowski EA, Nel A. New fossil dragonflies from the Lower Cretaceous of SE England and the phylogeny of the superfamily Libelluloidea (Insecta: Odonata). Cretaceous Research. 1996;17:67–85. [Google Scholar]
- 301. Bechly G. New fossil damselflies from Baltic amber, with description of a new species, a redescription of Litheuphaea carpenteri Fraser, and a discussion on the phylogeny of Epallagidae (Zygoptera: Caloptera). International Journal of Odonatology. 1998;1(1):33–63. [Google Scholar]
- 302.Brongniart C. Recherches pour servir à l’histoire des insectes fossiles des temps primaires, précédées d’une étude sur la nervation des ailes des insectes. Thèse présentée à la Fauclté des Sciences de Paris. 1893;821:495.
- 303. Nel A, Huguet A. Revision of the enigmatic Upper Carboniferous insect Campyloptera eatoni Brongniart, 1893 (Insecta: Odonatoptera). Organisms Diversity & Evolution. 2002;2(4):313–318. [Google Scholar]
- 304. Nel A, Fleck G, Garrouste R, Gand G, Lapeyrie J, Bybee SM, et al. Revision of Permo-Carboniferous griffenflies (Insecta: Odonatoptera: Meganisoptera) based upon new species and redescription of selected poorly known taxa from Eurasia. Palaeontographica Abteilung A. 2009. September;289(4–6):89–121. [Google Scholar]
- 305. Nel A, Gand G, Garric J. A new family of Odonatoptera from the continental Upper Permian: The Lapeyriidae (Lodève Basin, France). Geobios. 1999;32(1):63–72. [Google Scholar]
- 306. Béthoux O. The insect fauna from the Permian of Lodève (Hérault, France): state of the art and perspectives. Journal of Iberian Geology. 2008;34(1):109–113. [Google Scholar]
- 307. Ren D, Nel A, Prokop J. New early griffenfly, Sinomeganeura huangheensis from the late Carboniferous of northern China (Meganisoptera: Meganeuridae). Insect Systematics & Evolution. 2008;39:223–229. [Google Scholar]
- 308. Rehn AC. Phylogenetic analysis of higher-level relationships of Odonata. Systematic Entomology. 2003;28(2):181–239. [Google Scholar]
- 309. Martynov AV. Über eine neue Ordnung der fossilen Insekten, Miomoptera nov. Zoologischer Anzeiger. 1927;72:99–109. [Google Scholar]
- 310. Rasnitsyn AP. 2.2.1.3.1. Superorder Palaeomanteidea Handlirsch, 1906. Order Palaeomanteida Handlirsch, 1906 (= Miomoptera Martynov, 1927) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 161–164. [Google Scholar]
- 311. Rasnitsyn AP. On skimming hypothesis of the insect flight origin. Acta zoologica cracoviensia. 2003;46(suppl.- Fossil Insects):85–88. [Google Scholar]
- 312. Prokop J, Nel A. An enigmatic Palaeozoic stem-group: Paoliida, designation of new taxa from the Upper Carboniferous of the Czech Republic (Insecta: Paoliidae, Katerinkidae fam. n.). African Invertebrates. 2007;48(1):77–86. [Google Scholar]
- 313.Brunner von Wattenwyl K. Prodromus der europäischen orthoptèren. Engelmann, Leipzig; 1882.
- 314. Vršanskyý P. Phyloblatta grimaldii sp. nov.—a new Triassic cockroach (Insecta: Blattaria) from Virginia. Entomological Problems. 2003;32(1–2):51–53. [Google Scholar]
- 315. Shcherbakov DE. Madygen, Triassic Lagerstätte number one, before and after Sharov. Alavesia. 2008;2:113–124. [Google Scholar]
- 316. Martins-Neto RG, Mancuso AC, Gallego OF. The Triassic insect fauna from Argentina. Blattoptera from Los Rastros Formation (Bermejo Basin) La Rioja province. Ameghiniana. 2005;42(4):705–723. [Google Scholar]
- 317. Liang JH, Ren D, Ye QP, Liu M, Meng XM. The fossil Blattaria of China: a review of present knowledge. Acta Zootaxonomica Sinica. 2006;31(1):102–108. [Google Scholar]
- 318. Lin QB. Mesozoic insects from Zhejiang and Anhui provinces, China In: of Geology NI, Palaeontology, editors. Division and correlation of the Mesozoic volcano-sedimentary formations in the provinces of Zhejiang and Anhui. Science Press, Beijing; 1980. p. 211–234. [Google Scholar]
- 319. Lin QB. Cretaceous insects of China. Cretaceous Research. 1994;15:305–316. [Google Scholar]
- 320. Li XH, Chen S, Cao K, Chen YH, Xu BL, Ji Y. Paleosols of the Mid-Cretaceous: a report from Zhejiang and Fujian, SE China. Earth Science Frontiers. 2009;16(5):63–70. [Google Scholar]
- 321. Bechly G. 11.8 ‘Blattaria’: cockroaches and roachoids In: Martill DM, Bechly G, Loveridge RF, editors. The Crato Fossil Beds of Brazil: Window into an Ancient World. Cambridge University Press; 2007. p. 239–249. [Google Scholar]
- 322. Béthoux O, Klass KD, Schneider JW. Tackling the Protoblattoidea problem: Revision of Protoblattinopsis stubblefieldi (Dictyoptera; late Carboniferous). European Journal of Entomology. 2009;106:145–152. [Google Scholar]
- 323. Hörnschemeyer T, Stapf H. Review of Blattinopsidae (Protorthoptera) with description of new species from the Lower Permian of Niedermoschel (Germany). Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 2001;221(3):81–132. [Google Scholar]
- 324. Rasnitsyn AP. 2.2.1.2.1.1. Order Blattinopseida Bolton, 1925 In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p.106. [Google Scholar]
- 325. Béthoux O, Nel A. Venation pattern and revision of Orthoptera sensu nov. and sister groups. Phylogeny of Palaeozoic and Mesozoic Orthoptera sensu nov. Zootaxa. 2002;96:1–88. [Google Scholar]
- 326. Cifuentes-Ruiz P, Vršanskyý P, Vega FJ, Cevallos-Ferriz SRS, González-Soriano E, Delgado de Jesús CR. Campanian terrestrial arthropods from the Cerro del Pueblo Formation, Difunta Group in northeastern Mexico. Geologica Carpathica. 2006;57(5):347–354. [Google Scholar]
- 327. Vršanskyý P. Decreasing variability—from the Carboniferous to the present! (Validated on independent lineages of Blattaria). Paleontological Journal. 2000;34(Suppl. 3):S374–S379. [Google Scholar]
- 328. Vršanskyý P, Ansorge J. Lower Jurassic cockroaches (Insecta: Blattaria) from Germany and England. African Invertebrates. 2007;48(1):103–126. [Google Scholar]
- 329. Vršanskyý P, Vishniakova VN, Rasnitsyn AP. 2.2.2.1.1. Order Blattida Latreille, 1810. The cockroaches (= Blattodea Brunner von Wattenvill, 1882) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 263–270. [Google Scholar]
- 330. Vršanskyý P, Ansorge J. New Lower Cretaceous polyphagid cockroaches from Spain (Blattaria, Polyphagidae, Vitisminae subfam. nov.). Cretaceous Research. 2001;22(2):157–162. [Google Scholar]
- 331. Handlirsch A. Neue Untersuchungen über die fossilen Insekten mit Ergänzungen und Nachträgen sowie Ausblicken auf phylogenetische, palaeogeographische und allgemein biologische Probleme. I Teil. Annalen des Naturhistorischen Museums in Wien. 1937;48:1–140. [Google Scholar]
- 332. Clifford E, Coram RA, Jarzembowski EA, Ross AJ. A supplement to the insect fauna from the Purbeck Group of Dorset. Proceedings of the Dorset Natural History and Archaeological Society. 1994;115:143–146. [Google Scholar]
- 333. Vršanskyý P. Albian cockroaches (Insecta, Blattida) from French amber of Archingeay. Geodiversitas. 2009;31(1):73–98. [Google Scholar]
- 334. Liang JH, Vršanskyý P, Ren D, Shih CK. A new Jurassic carnivorous cockroach (Insecta, Blattaria, Raphidiomimidae) from the Inner Mongolia in China. Zootaxa. 2009;1974:17–30. [Google Scholar]
- 335.Ross AJ. The cockroaches (Blattodea) of the Purbeck Limestone Group and Wealden Supergroup (Lower Cretaceous) of southern England p.59-60. In: 2nd International Congress on Palaeoentomology, Krakow, abstract volume; 2001.
- 336. Vršanskyý P, Liang JH, Ren D. Advanced morphology and behaviour of extinct earwig-like cockroaches (Blattida: Fuziidae fam. nov.). Geologica Carpathica. 2009;60(6):449–462. [Google Scholar]
- 337. Vršanskyý P. Origin and the early evolution of mantises. AMBA projekty. 2002;6(1):1–16. [Google Scholar]
- 338. Vršanskyý P. Mesozoic relative of the common synanthropic German cockroach (Blattodea). Deutsche entomologische Zeitschrift. 2008;55(2):215–221. [Google Scholar]
- 339. Jarzembowski EA, Schneider JW. The stratigraphical potential of blattodean insects from the late Carboniferous of southern Britain. Geological Magazine. 2007;144(3):449–456. [Google Scholar]
- 340. Li Z, Hong YC, Yang D. A new Middle Triassic genus and species of Mylacridae (Blattodea) from China. Zootaxa. 2007;1660:53–59. [Google Scholar]
- 341. Schneider J. Die Blattodea (Insecta) des Paläzoikums, Teil 1: Systematik, Ökologie und Biostratigraphie. Freiberger Forschungenshefte, Reihe C. 1983;382:106–145. [Google Scholar]
- 342. Hong YC. The discovery of Late Palaeozoic insecta in Shanxi Province. Geological Review. 1980;26(2):89–95. [Google Scholar]
- 343. Zhang ZL, Sun KQ, Yin JR. Sedimentology and sequence stratigraphy of the Shanxi Formation (Lower Permian) in the northwestern Ordos Basin, China: an alternative sequence model for fluvial strata. Sedimentary Geology. 1997;112:123–136. [Google Scholar]
- 344. Hong YC. Establishment of fossil entomofaunas and their evolutionary succession in north China. Entomologia Sinica. 1998;5(4):283–300. [Google Scholar]
- 345. Vršanskyý P. New blattarians and a review of dictyopteran assemblages from the Lower Cretaceous of Mongolia. Acta Palaeontologica Polonica. 2008;53(1):129–136. [Google Scholar]
- 346.Schneider JW, Lucas SG, Rowland JM. The Blattida (Insecta) fauna of Carrizo Arroyo, New Mexico—biostratigraphic link between marine and nonmarine Pennsylvanian/Permian boundary profiles. In: Lucas SG, Zeigler KE, editors. Carboniferous-Permian Transition at Carrizo Arroyo, Central New Mexico. vol. Bulletin 25. New Mexico Museum of Natural History and Science, Albuquerque; 2004. p. 247–261.
- 347. Schneider J. Die Blattodea (Insecta) des Palaozoikums Teil II: Morphogenese der Flugelstrukturen und Phylogenie. Freiberger Forschungenshefte, Reihe C. 1984;391:5–34. [Google Scholar]
- 348. Vršanskyý P. Jumping cockroaches (Blattaria, Skokidae fam. n.) from the late Jurassic of Karatau in Kazakhstan. Biologia. 2007;62(5):588–592. [Google Scholar]
- 349.Schneider JW, Werneburg R. Insect biostratigraphy of the Euramerican continental late Pennsylvanian and early Permian. In: Lucas SG, Cassinis G, Schneider JW, editors. Non-Marine Permian Biostratigraphy and Biochronology. Geological Society of London Special Publications 265; 2006. p. 325–336.
- 350. Papier F, Nel A. Les Subioblattidae (Blattodea, Insecta) du Trias d’Asie Centrale. Paläontologische Zeitschrift. 2001;74(4):533–542. [Google Scholar]
- 351. Gorokhov AV. New and little known orthopteroid insects (Polyneoptera) from fossil resins: Communication 1. Paleontological Journal. 2006;40(6):646–654. [Google Scholar]
- 352. Chen SX, Tan JJ. A new family of Coleoptera from the Lower Cretaceous of Kansu. Acta Entomologica Sinica. 1973;16(2):169–178. [Google Scholar]
- 353. Vršanskyý P. Umenocoleoidea—an amazing lineage of aberrant insects (Insecta, Blattaria). AMBA projekty. 2003;7(1):1–32. [Google Scholar]
- 354. Perrichot V, Néraudeau D, Nel A, de Ploëg G. A reassessment of the Cretaceous amber deposits from France and their palaeontological significance. African Invertebrates. 2007;48(1):213–227. [Google Scholar]
- 355. Beckemeyer RJ. Ligogramma wichita, a new species of Caloneurodea (Polyneoptera: Orthopterida) from the Lower Permian Wellington Formation of Noble County, Oklahoma. Journal of the Kansas Entomological Society. 2009;82(4):300–304. [Google Scholar]
- 356. Aristov DS. A new family of the order Grylloblattida (Insecta) from the Middle Permian of Russia. Paleontological Journal. 2009;43(2):178–182. [Google Scholar]
- 357. Béthoux O, Nel A, Lapeyrie J, Gand G. The Permostridulidae fam. n. (Panorthoptera), a new enigmatic insect family from the Upper Permian of France. European Journal of Entomology. 2003;100:581–585. [Google Scholar]
- 358. Martins-Neto RG, Gallego OF, Brauckmann C, Cruz JL. A review of the South American Palaeozoic entomofauna part I: the Ischnoneuroidea and Cacurgoidea, with description of new taxa. African Invertebrates. 2007;48(1):87–101. [Google Scholar]
- 359. Béthoux O. Revision and phylogenetic affinities of the lobeattid species bronsoni Dana, 1864 and silvatica Laurentiaux & Laurentiaux-Vieira, 1980 (Pennsylvanian; Archaeorthoptera). Arthropod Systematics & Phylogeny. 2008;66(2):145–163. [Google Scholar]
- 360. Béthoux O, Nel A. Some Palaeozoic ‘Protorthoptera’ are ‘ancestral’ orthopteroids: major wing braces as clues to a new split among the ‘Protorthoptera’ (Insecta). Journal of Systematic Palaeontology. 2005;2 [for 2004](4):285–309. [Google Scholar]
- 361. Béthoux O, Nel A, Galtier J, Lapeyrie J, Gand G. A new species of Tococladidae Carpenter, 1966 from the Permian of France (Insecta: Archaeorthoptera). Geobios. 2003;36(3):275–283. [Google Scholar]
- 362. Rasnitsyn AP. 2.2.2. Infraclass Gryllones Laicharting, 1781. The Grylloneans (= Polyneoptera Martynov, 1938) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 254–262. [Google Scholar]
- 363. Martins-Neto RG. Estágio atual da paleoartropodologia brasileira: Hexápodes, Miriápodes, Crustáceos (Isopoda, Decapoda, Eucrustacea e Copepoda) e quelicerados. Arquivos do Museu Nacional, Rio de Janeiro. 2005;63(3):471–494. [Google Scholar]
- 364. Béthoux O. Ordinal assignment of the genus Tococladus Carpenter 1996 (Insecta: Archaeorthoptera). Alavesia. 2007;1:3. [Google Scholar]
- 365. Rasnitsyn AP. 2.2.1.2.2. Superorder Hypoperlidea Martynov, 1928. Order Hypoperlida Martynov, 1928 In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 111–115. [Google Scholar]
- 366.de Geer C. Mémoires pour servir à l’histoire des insectes, v. 3. Stockholm; 1773.
- 367. Engel MS, Haas F. Family-group names for earwigs (Dermaptera). American Museum Novitates. 2007;3567:1–20. [Google Scholar]
- 368. Haas F. 11.6 Dermaptera: earwigs In: Martill DM, Bechly G, Loveridge RF, editors. The Crato Fossil Beds of Brazil: Window into an Ancient World. Cambridge University Press; 2007. p. 222–234. [Google Scholar]
- 369. Wappler T, Engel MS, Haas F. The earwigs (Dermaptera: Forficulidae) from the middle Eocene Eckfeld maar, Germany. Polskie Pismo Entomologiczne. 2005;74(3):227–250. [Google Scholar]
- 370. Zhang JF. The most primitive fossil earwigs (Archidermaptera, Dermaptera, Insecta) from the Upper Jurassic of Nei Mongol Autonomous Region, northeastern China. Acta Micropalaeontologica Sinica. 2002;19(4):348–362. [Google Scholar]
- 371. Spahr U. Ergänzungen und Berichtigungen zu R. Keilbach’s Bibliographie und Liste der Bernsteinfossilien–“Apterygota”. Stuttgarter Beiträge zur Naturkunde Serie B (Geologie und Paläontologie). 1990;166:1–23. [Google Scholar]
- 372. Engel MS, Grimaldi DA. A primitive earwig in Cretaceous amber from Myanmar (Dermaptera: Pygidicranidae). Journal of Paleontology. 2004;78(5):1018–1023. [Google Scholar]
- 373. Engel MS. The earwigs of Kansas, with a key to genera north of Mexico (Insecta: Dermaptera). Transactions of the Kansas Academy of Science. 2003;106(3/4):115–123. [Google Scholar]
- 374. Kusnezov NJ. A new species of Embia Latr. from the Crimea (Neuroptera, Embiodea) (preliminary description). Revue Russe d’Entomologie. 1903;3(3–4):208–210. [Google Scholar]
- 375. Engel MS, Grimaldi DA. The earliest webspinners (Insecta: Embiodea). American Museum Novitates. 2006;3514:1–15. [Google Scholar]
- 376. Zhang JF. New Miocene species of Bibionidae (Insecta: Diptera) with discussion on taxonomic position of Clothonopsis miocenica . Acta Palaeontologica Sinica. 1993;32(2):141–150. [Google Scholar]
- 377. Ross ES. EMBIA Contributions to the biosystematics of the insect order Embiidina Part 5: A review of the family Anisembiidae with descriptions of new taxa. Occasional Papers of the California Academy of Sciences. 2003;154:1–123. [Google Scholar]
- 378. Spahr U. Ergänzungen und Berichtigungen zu R. Keilbachs Bibliographie und Liste der Bernsteinfossilien—Klasse Insecta. (Ausgenommen: “Apterygota”, Hemipteroidea, Coleoptera, Hymenoptera, Mecopteroidea). Stuttgarter Beiträge zur Naturkunde Serie B (Geologie und Paläontologie). 1992;182:1–102. [Google Scholar]
- 379. Handlirsch A. Die fossilen Insekten und die Phylogenie der rezenten Formen. Ein Handbuch für Paläontologen und Zoologen. Engelmann, Leipzig; 1906–1908. [Google Scholar]
- 380. Huang DY, Nel A. Oldest webspinners from the Middle Jurassic of Inner Mongolia, China (Insecta: Embiodea). Zoological Journal of the Linnean Society. 2009;156(4):889–895. [Google Scholar]
- 381.Brues CT, Melander AL. Key to the families of North American insects. An introduction to the classification of insects. Boston and Pullman; 1915.
- 382. Storozhenko SY. Classification of the order Grylloblattida (Insecta), with description of new taxa. Far Eastern Entomologist. 1997;42:1–20. [Google Scholar]
- 383. Kukalová J. Permian insects of Moravia Part II—Liomopteridea. Sborník Geologickyých Ved, Paleontologie, P. 1964;3:39–118. [Google Scholar]
- 384. Aristov DS, Bashkuev AS. New insects (Insecta: Mecoptera, Grylloblattida) from the Middle Permian Chepanikha Locality, Udmurtiya. Paleontological Journal. 2008;42(2):159–165. [Google Scholar]
- 385. Aristov DS. The fauna of grylloblattid insects (Grylloblattida) from the end of the late Permian to the first half of the Triassic. Paleontological Journal. 2004;38(5):514–521. [Google Scholar]
- 386. Aristov DS. The fauna of grylloblattid insects (Grylloblattida) of the Lower Permian locality of Tshekarda. Paleontological Journal. 2004;38(Suppl. 2):S80–S145. [Google Scholar]
- 387. Storozhenko SY. New and little known Mesozoic Grylloblattids. Paleontological Journal. 1988;22(4):45–52. [Google Scholar]
- 388. Aristov DS, Novokshonov VG, Pan’kov NN. Taxonomy of the fossil grylloblattid nymphs (Insecta: Grylloblattida). Paleontological Journal. 2006;40(1):79–89. [Google Scholar]
- 389. Storozhenko SY. New Permian and Mesozoic insects (Insecta, Grylloblattida: Blattogryllidae, Geinitziidae) from Asia. Paleontological Journal. 1990;24(4):53–61. [Google Scholar]
- 390. Aristov DS. New Grylloblattida (Insecta) from the Middle and Upper Permian of the Russia. Far Eastern Entomologist. 2008;188:1–7. [Google Scholar]
- 391. Aristov DS, Prevec R, Mostovski MB. New and poorly known grylloblattids (Insecta: Grylloblattida) from the Lopingian of the Lebombo Basin, South Africa. African Invertebrates. 2009;50(2):279–286. [Google Scholar]
- 392. Aristov DS. Grylloblattids of the family Chaulioditidae (= Tomiidae syn. nov.) (Insecta: Grylloblattida) from the Upper Permian of the Orenburg Region. Paleontological Journal. 2004;38(Suppl. 2):S146–S149. [Google Scholar]
- 393. Beckemeyer RJ. Raaschiidae (Grylloblattida: Protoperlina), a new insect family from the Lower Permian Wellington Formation of Noble County, Oklahoma. Journal of the Kansas Entomological Society. 2004;77(3):215–221. [Google Scholar]
- 394. Storozhenko SY. New Upper Carboniferous grylloblattids (Insecta, Grylloblattida) from Siberia. Far Eastern Entomologist. 1996;26:18–20. [Google Scholar]
- 395. Storozhenko SY. 2.2.2.2.1. Order Grylloblattida Walker, 1914 (= Notoptera Crampton, 1915, = Grylloblattodea Brues et Melander, 1932, +Protorthoptera Handlirsch, 1906, = Paraplecoptera Martynov, 1925, +Protoperlaria Tillyard, 1928) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 278–281. [Google Scholar]
- 396. Carpenter FM. The Lower Permian insects of Kansas. Part 10. The order Protorthoptera: The family Liomopteridae and its relatives. Proceedings of the American Academy of Arts and Sciences. 1950;78(4):187–219. [Google Scholar]
- 397. Béthoux O. Emptying the Paleozoic wastebasket for insects: member of a Carboniferous ‘protorthopterous family’ assigned to natural groups. Alavesia. 2007;1:41–48. [Google Scholar]
- 398. Rasnitsyn AP, Aristov DS. Two new insects from the Upper Permian (Tatarian) of Belmont, New South Wales, Australia (Insecta: Hypoperlida: Anthracoptilidae = Permarrhaphidae; Grylloblattida: Sylvaphlebiidae). Paleontological Journal. 2004;38(Suppl. 2):S158–S163. [Google Scholar]
- 399. Aristov DS. Review of the stratigraphic distribution of Permian Grylloblattida (Insecta), with descriptions of new taxa. Paleontological Journal. 2009;43(6):643–651. [Google Scholar]
- 400. Aristov DS, Wappler T, Rasnitsyn AP. New and little-known grylloblattids of the family Geinitziidae (Insecta: Grylloblattida) from the Triassic and Jurassic of Europe, Asia, and South Africa. Paleontological Journal. 2009;43(4):418–424. [Google Scholar]
- 401. Storozhenko SY. New Triassic grylloblattids from Kirghizia. Spixiana. 1994;17(1):27–35. [Google Scholar]
- 402. Aristov DS. New Grylloblattids (Insecta: Grylloblattida) from the Triassic of eastern Europe, eastern Kazakhstan and Mongolia. Paleontological Journal. 2005;39(2):173–177. [Google Scholar]
- 403. Storozhenko SY. Permian fossil insects of north-east Europe: new and little-known Ideliidae (Insecta, Plecopteroidea, Grylloblattida). Entomologica Fennica. 1992;3(1):21–39. [Google Scholar]
- 404. Aristov DS, Rasnitsyn AP. The family Tillyardembiidae Zalessky, 1938 and the system of the plecopteroid insects. Russian Entomological Journal. 2009;18(4):257–264. [Google Scholar]
- 405. Huang DY, Nel A. A new Middle Jurassic “grylloblattodean” family from China (Insecta: Juraperlidae fam. n.). European Journal of Entomology. 2007;104(4):837–840. [Google Scholar]
- 406. Aristov DS. New Grylloblattida (Insecta) from Kargala locality (Russia; Middle Permian). Far Eastern Entomologist. 2009;192:1–8. [Google Scholar]
- 407. Cairncross B, Anderson JM, Anderson HM. Palaeoecology of the Triassic Molteno Formation, Karoo Basin, South Africa sedimentological and palaeontological evidence. South African Journal of Geology. 1995;98(4):452–478. [Google Scholar]
- 408. Storozhenko SY. A new family of Triassic grylloblattids from Central Asia. Spixiana. 1992;15(1):67–73. [Google Scholar]
- 409. Aristov DS. New grylloblattids of the family Megakhosaridae (Insecta: Grylloblattida) from the Permian of Russia. Paleontological Journal. 2008;42(3):269–272. [Google Scholar]
- 410. Storozhenko SY. New Mesozoic grylloblattid insects (Grylloblattida) from Central Asia. Paleontological Journal. 1992;26(1):85–95. [Google Scholar]
- 411. Storozhenko SY. New Triassic Mesorthopteridae. Spixiana. 1996;19(1):115–127. [Google Scholar]
- 412. Ansorge J. Insekten aus dem oberen Lias von Grimmen (Vorpommern, Norddeutschland). Neue Paläontologische Abhandlungen. 1996;2:1–132. [Google Scholar]
- 413. Huang DY, Nel A, Petrulevicius JF. New evolutionary evidence of Grylloblattida from the Middle Jurassic of Inner Mongolia, north-east China (Insecta, Polyneoptera). Zoological Journal of the Linnean Society. 2008;152(1):17–24. [Google Scholar]
- 414. Peng DC, Hong YC, Zhang ZJ. Namurian insects (Diaphanopterodea) from Qilianshan Mountains, China. Geological Bulletin of China. 2005;24(3):219–234. [Google Scholar]
- 415. Béthoux O, Beckemeyer RJ. New and rare insect species from the Wellington Formation (Orthoptera, Grylloblattodea; Lower Permian, USA). Alavesia. 2007;1:49–61. [Google Scholar]
- 416. Storozhenko SY, Novokshonov VG. Revision of the Permian family Sojanoraphidiidae (Grylloblattida). Russian Entomological Journal. 1994;3(3–4):37–39. [Google Scholar]
- 417. Aristov DS. A new family of early Permian grylloblattids (Insecta: Grylloblattida) from Ural Mountains. Far Eastern Entomologist. 2000;85:1–4. [Google Scholar]
- 418. Aristov DS. New insects of the order Grylloblattida (Insecta) from the Lower Permian of the middle Urals. Paleontological Journal. 2000;34(5):519–521. [Google Scholar]
- 419. Storozhenko SY, Vršanskyý P. New fossil family of the Order Grylloblattida (Insecta: Plecopteroidea) from Asia. Far Eastern Entomologist. 1995;19:1–4. [Google Scholar]
- 420.Brullé GA. IV. Classe Insectes. p63-345. In: Expédition Scientifique de Morée. Section des sciences physiques. Tome III. I. Partie. Zoologie. Deuxiéme Section. Des animaux articulés. Lavrault, Paris; 1832.
- 421. Engel MS, Grimaldi DA, Krishna K. Primitive termites from the early Cretaceous of Asia (Isoptera). Stuttgarter Beiträge zur Naturkunde Serie B (Geologie und Paläontologie). 2007;371:1–32. [Google Scholar]
- 422. Bordy EM, Bumby AJ, Catuneanu O, Eriksson PG. Possible trace fossils of putative termite origin in the Lower Jurassic (Karoo Supergroup) of South Africa and Lesotho. South African Journal of Science. 2009;105:356–362. [Google Scholar]
- 423. Bechly G. 11.9 Isoptera: termites In: Martill DM, Bechly G, Loveridge RF, editors. The Crato Fossil Beds of Brazil: Window into an Ancient World. Cambridge University Press; 2007. p. 249–262. [Google Scholar]
- 424. Grimaldi DA, Engel MS, Krishna K. The species of Isoptera (Insecta) from the early Cretaceous Crato Formation: a revision. American Museum Novitates. 2008;3626:1–30. [Google Scholar]
- 425. Krishna K, Grimaldi DA. The first Cretaceous Rhinotermitidae (Isooptera): a new species, genus, and subfamily in Burmese amber. American Museum Novitates. 2003;3390:1–10. [Google Scholar]
- 426. Poinar GO. Description of an early Cretaceous termite (Isoptera: Kalotermitidae) and its associated intestinal protozoa, with comments on their co-evolution. Parasites & Vectors. 2009;2(2):17pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427. Martins-Neto RG, Pesenti M. The first fossil Termitidae (Isoptera) from the Oligocene of South America: the Entre-Córregos Formation of the Aiuruoca Basin, Minas Gerais, Brazil. Journal of the Entomological Research Society. 2006;8(3):63–68. [Google Scholar]
- 428. Engel MS, Grimaldi DA, Krishna K. A synopsis of Baltic amber termites (Isoptera). Stuttgarter Beiträge zur Naturkunde Serie B (Geologie und Paläontologie). 2007;372:1–20. [Google Scholar]
- 429.Burmeister HCC. Handbuch der Entomologie, 2 volumes. Reimer, Berlin; 1838–1839.
- 430. Ware JL, Litman J, Klass KD, Spearman LA. Relationships among the major lineages of Dictyoptera: the effect of outgroup selection on dictyopteran tree topology. Systematic Entomology. 2008;33(3):429–450. [Google Scholar]
- 431. Svenson GJ, Whiting MF. Reconstructing the origins of praying mantises (Dictyoptera, Mantodea): the roles of Gondwanan vicariance and morphological convergence. Cladistics. 2009;25(5):468–514. [DOI] [PubMed] [Google Scholar]
- 432. Grimaldi DA. A revision of Cretaceous mantises and their relationships, including new taxa (Insecta: Dictyoptera: Mantodea). American Museum Novitates. 2003;3412:1–47. [Google Scholar]
- 433. Vršanskyý P. Central ocellus of extinct cockroaches (Blattida: Caloblattinidae). Zootaxa. 2008;1958:41–50. [Google Scholar]
- 434. Gratshev VG, Zherikhin VV. New fossil mantids (Insecta, Mantida). Paleontological Journal. 1994;27 (for 1993)(1A):148–165. [Google Scholar]
- 435. Zherikhin VV. 2.2.2.1.3. Order Mantida Latreille, 1802. The Mantises (= Mantodea Burmeister, 1838) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 273–276. [Google Scholar]
- 436. Vršanskyý P. Jantarimantis nom. nov. and Jantarimantidae nom. nov., new replacement names for the genus Archimantis Vršanskyý, 2002, and the family Archimantidae Vršanskyý, 2002 (Insecta, Mantodea). AMBA projekty. 2002;6(2):1. [Google Scholar]
- 437. Vršanskyý P. Lower Cretaceous cockroaches and mantids (Insecta: Blattaria, Mantodea) from the Sharin-Gol in Mongolia. Entomological Problems. 2005;35(2):163–167. [Google Scholar]
- 438. Nel A, Roy R. Revision of the fossil mantid and ephemerid species described by Piton from the Palaeocene of Menat (France) (Mantodea: Chaeteessidae, Mantidae; Ensifera: Tettigonioidea). European Journal of Entomology. 1996;93:223–234. [Google Scholar]
- 439. Zompro O. Inter- and intra-ordinal relationships of the Mantophasmatodea, with comments on the phylogeny of polyneopteran orders (Insecta: Polyneoptera). Mitteilungen aus dem Geologisch-Paläontologischen Institut der Universität Hamburg. 2005;89:85–116. [Google Scholar]
- 440. Grimaldi DA. 11.7 Mantodea: praying mantises. In: Martill DM, Bechly G, Loveridge RF, editors. The Crato Fossil Beds of Brazil: Window into an Ancient World. Cambridge University Press; 2007. p. 234–238. [Google Scholar]
- 441. Béthoux O, Wieland F. Evidence for Carboniferous origin of the order Mantodea (Insecta: Dictyoptera) gained from forewing morphology. Zoological Journal of the Linnean Society. 2009;156:79–113. [Google Scholar]
- 442. Klass KD, Zompro O, Kristensen NP, Adis J. Mantophasmatodea: A new insect order with extant members in the afrotropics. Science. 2002;296(5572):1456–1459. [DOI] [PubMed] [Google Scholar]
- 443. Arillo A, Engel MS. Rock crawlers in Baltic amber (Notoptera: Mantophasmatodea). American Museum Novitates. 2006;3539:1–10. [Google Scholar]
- 444. Damgaard J, Klass KD, Picker MD, Buder G. Phylogeny of the heelwalkers (Insecta: Mantophasmatodea) based on mtDNA sequences, with evidence for additional taxa in South Africa. Molecular Phylogenetics and Evolution. 2008;47(2):433–462. [DOI] [PubMed] [Google Scholar]
- 445. Huang DY, Nel A, Zompro O, Waller A. Mantophasmatodea now in the Jurassic. Naturwissenschaften. 2008;95:947–952. 10.1007/s00114-008-0412-x [DOI] [PubMed] [Google Scholar]
- 446.Olivier GA. Encyclodpédie méthodique. Dictionnaire des insectes, v. 5. Pankouke, Paris; 1789.
- 447. Heads SW, Martins-Neto RG. 11.11 Orthopterida: grasshoppers, crickets, locusts and stick insects In: Martill DM, Bechly G, Loveridge RF, editors. The Crato Fossil Beds of Brazil: Window into an Ancient World. Cambridge University Press; 2007. p. 265–283. [Google Scholar]
- 448. Selden PA, Penney D. A fossil spider (Araneae: Pisauridae) of Eocene age from Horsefly, British Columbia, Canada. Contributions to Natural History. 2009;12:1269–1282. [Google Scholar]
- 449. Gorokhov AV. New fossil orthopterans of the families Adumbratomorphidae fam. n., Pruvostitidae and Proparagryllacrididae (Orthoptera, Ensifera) from Permian and Triassic deposits of the USSR [in Russian]. Vestnik zoologii. 1987;1987(4):20–28. [Google Scholar]
- 450. Gorokhov AV. System and evolution of the suborder Ensifere (Orthoptera), Part 1. Trudy Paleontologicheskogo instituta Akademiia nauk SSSR. 1995;260(1):1–224. [Google Scholar]
- 451. Martins-Neto RG. New Orthoptera Stenopelmatoidea and Hagloidea (Ensifera) from the Santana Formation (Lower Cretaceous, northeast Brazil) with description of new taxa. Gaea. 2007;3(1):3–8. [Google Scholar]
- 452. Martins-Neto RG. Araripelocustidae, Fam. N. uma nova família de gafanhotos (Insecta, Caelifera) da formação Santana Cretáceo Inferior do nordeste do Brasil. Revista Brasileira de Entomologia. 1995;39(2):311–319. [Google Scholar]
- 453. Gorokhov AV. Mesozoic crickets (Orthoptera, Grylloidea) of Asia. Paleontological Journal. 1985;19(2):56–66. [Google Scholar]
- 454. Gorokhov AV, Jarzembowski EA, Coram RA. Grasshoppers and crickets (Insecta: Orthoptera) from the Lower Cretaceous of southern England. Cretaceous Research. 2006;27(5):641–662. [Google Scholar]
- 455. Martins-Neto RG, Tassi LV. The Orthoptera (Ensifera) from the Santana Formation (early Cretaceous, northeast Brazil): A statistical and paleoecological approach, with description of new taxa. Zootaxa. 2009;2080:21–37. [Google Scholar]
- 456. Gorokhov AV. Review of Triassic Orthoptera with descriptions of new and little known taxa: Part 1. Paleontological Journal. 2005;39(2):68–76. [Google Scholar]
- 457. Shcherbakov DE. Insect recovery after the Permian/Triassic crisis. Alavesia. 2008;2:125–131. [Google Scholar]
- 458. Martins-Neto RG. Review of some Insecta from Mesozoic and Cenozoic Brazilian deposits, with descriptions of new taxa. Acta Geologica Leopoldensia. 2001;24(52/53):115–124. [Google Scholar]
- 459. Martins-Neto RG. Systematics of the Caelifera (Insecta Orthopteroida) from the Santana Formation, Araripe Basin (Lower Cretaceous, northeast Brazil). Acta zoologica cracoviensia. 2003;46(suppl.—Fossil Insects):205–228. [Google Scholar]
- 460. Gorokhov AV. New data on Triassic Orthoptera from Middle Asia. Zoosystematica Rossica. 1994;3(1):53–54. [Google Scholar]
- 461. Gorokhov AV. Review of Triassic Orthoptera with descriptions of new and little known taxa: Part 2. Paleontological Journal. 2005;39(3):272–279. [Google Scholar]
- 462. Poinar GO, Gorokhov AV, Buckley R. Longioculus burmensis, n. gen., n. sp. (Orthoptera: Elcanidae) in Burmese amber. Proceedings of the Entomological Society of Washington. 2007;109(3):649–655. [Google Scholar]
- 463. Pérez-Gelabert DE, Rowell CHF. Further investigations of Hispaniolan eumastacoid grasshoppers (Espagnolinae: Episactidae: Orthoptera). Journal of Orthoptera Research. 2006;15(2):241–249. [Google Scholar]
- 464. Pérez DE, Hierro B, Dominici GO, Otte D. New eumastacid greasshopper taxa (Orthoptera: Eumastacidae: Episactinae) from Hispaniola, including a fossil new genus and species from Dominican amber. Journal of Orthoptera Research. 1997;6:139–151. [Google Scholar]
- 465. Heads SW. The first fossil Proscopiidae (Insecta, Orthoptera, Eumastacoidea) with comments on the historical biogeography and evolution of the family. Palaeontology. 2008;51(2):499–507. [Google Scholar]
- 466. Gorokhov AV. Triassic grasshoppers of the superfamily Hagloidea (Orthoptera). Trudy Zoologicheskogo Instituta. 1986;143:65–100. In Russian. [Google Scholar]
- 467. Marchal-Papier F, Nel A, Grauvogel-Stamm L. Nouveaux Orthoptères (Ensifera, Insecta) du Trias des Vosges (France). Acta Geologica Hispanica. 2000;35(1–2):5–18. [Google Scholar]
- 468. Gorokhov AV. Classification and Phylogeny of Grasshoppers (Gryllida = Orthoptera, Tettigonioidea) [in Russian] In: Ponomarenko AG, editor. The Cretaceous Biocenotic Crisis and the Evolution of Insects. Nauka, Moscow; 1988. p. 145–190. [Google Scholar]
- 469. Kevan DKM, Wighton DC. Paleocene orthopteroids from south-central Alberta, Canada. Canadian Journal of Earth Sciences. 1981;18(12):1824–1837. [Google Scholar]
- 470. Gorokhov AV. New fossil Orthopterans of the families Bintoniellidae, Mesoedischiidae fam. n. and Pseudelcanidae fam. n. (Orthoptera, Ensifera) from Permian and Triassic deposits of the USSR [in Russian]. Vestnik zoologii. 1987;1987(1):18–23. [Google Scholar]
- 471. Béthoux O, Nel A. New data on Tcholmanvissiidae (Orthoptera; Permian). Journal of Orthoptera Research. 2002;11(2):223–235. [Google Scholar]
- 472. Béthoux O. Cladotypic taxonomy applied: titanopterans are orthopterans. Arthropod Systematics & Phylogeny. 2007;65(2):135–156. [Google Scholar]
- 473. Gorokhov AV. The first representative of the suborder Mesotitanina from the Paleozoic and notes on the system and evolution of the order Titanoptera (Insecta: Polyneoptera). Paleontological Journal. 2007;41(6):621–625. [Google Scholar]
- 474. Heads SW. New pygmy grasshoppers in Miocene amber from the Dominican Republic (Orthoptera: Tetrigidae). Denisia. 2009;26:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475. Martins-Neto RG. Complementos ao estudo sobre os Ensifera (Insecta, Orthopteroida) da Formação Santana, Cretáceo Inferior do nordeste do Brasil. Revista Brasileira de Entomologia. 1995;39(2):321–345. [Google Scholar]
- 476. Prokop J, Nel A, Hoch I. Discovery of the oldest known Pterygota in the Lower Carboniferous of the Upper Silesian Basin in the Czech Republic (Insecta: Archaeorthoptera). Geobios. 2005;38(3):383–387. [Google Scholar]
- 477. Béthoux O, Nel A, Gand G, Lapeyrie J, Galtier J. Discovery of the genus Iasvia Zalessky, 1934 in the Upper Permian of France (Lodève basin) (Orthoptera, Ensifera, Oedischiidae). Geobios. 2002;35(3):293–302. [Google Scholar]
- 478. Gorokhov AV. Phasmomimidae: are they Orthoptera or Phasmatoptera? Paleontological Journal. 2000;34(3):295–300. [Google Scholar]
- 479. Gorokhov AV. On the classification of fossil grasshoppers of the superfamily Phasmomimoidea (Orthoptera) with descriptions of new taxa [in Russian]. Trudy Paleontologicheskogo instituta Akademiia nauk SSSR. 1988;178:32–44. [Google Scholar]
- 480. Koçak AO, Kemal M. Replacement names among the genus and family group taxa in Orthoptera. Centre for Entomological Studies Ankara, Miscellaneous Papers. 2008;141:1–5. [Google Scholar]
- 481. Özdikmen H. New subfamily and genus names for Ferganiinae Gorochov, 1987 and Fergania Sharov, 1968 (Orthoptera). Munis Entomology and Zoology. 2008;3(2):731–732. [Google Scholar]
- 482. Lin QB, Huang DY. Revision of “Parahagla lamina” Lin, 1982 and two new species of Aboilus (Orthoptera: Prophalangopsidae) from the Early-Middle Jurassic of northwest China. Progress in Natural Science. 2006;16(Special Issue):303–307. [Google Scholar]
- 483. Gorokhov AV, Rasnitsyn AP. 2.2.2.3. Superorder Gryllidea Laicharting, 1781 (= Orthopteroidea Handlirsch, 1903) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 293–303. [Google Scholar]
- 484. Perrichot V, Néraudeau D, Azar D, Menier JJ, Nel A. A new genus and species of fossil mole cricket in the Lower Cretaceous amber of Charente-Maritime, SW France (Insecta: Orthoptera: Gryllotalpidae). Cretaceous Research. 2002;23(3):307–314. [Google Scholar]
- 485. Zherikhin VV. 3.2 Ecological history of the terrestrial insects In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p.331–388. [Google Scholar]
- 486. Béthoux O, Nel A, Lapeyrie J, Gand G, Galtier J. Raphogla rubra gen. n., sp. n., the oldest representative of the clade of modern Ensifera (Orthoptera: Tettigoniidea, Gryllidae). European Journal of Entomology. 2002;99(1):111–116. [Google Scholar]
- 487. Gorokhov AV. On the system and evolution of the order Orthoptera. Zoologicheskii Zhurnal. 1995;74(10):39–45. [Google Scholar]
- 488. Heads SW. A new pygmy mole cricket in Cretaceous amber from Burma (Orthoptera: Tridactylidae). Denisia. 2009;26:75–82. [DOI] [PubMed] [Google Scholar]
- 489. Papier F, Nel A, Grauvogel-Stamm L, Gall JC. La plus ancienne sauterelle Tettigoniidae, Orthoptera (Trias, NE France): mimétisme ou exaptation? Paläontologische Zeitschrift. 1997;71(1/2):71–77. [Google Scholar]
- 490. Gall JC, Grauvogel-Stamm L. The early Middle Triassic ‘Grès à Voltzia’ Formation of eastern France: a model of environmental refugium. Comptes Rendus Palevol. 2005;4(6–7):637–652. [Google Scholar]
- 491. Zessin W. Thueringoedischia trostheiedi nov. gen. et nov. sp. (Insecta, Orthoptera) aus dem unteren Rotliegenden von Thüringen. Veröffentlichungen Naturkundemuseum Erfurt.1997;16:172–183. [Google Scholar]
- 492. Gorokhov AV. Grasshoppers of the superfamily Hagloidea (Orthoptera) from the Lower and Middle Jurassic [in Russian]. Paleontologicheskii Zhurnal. 1988;1988(2):54–66. [Google Scholar]
- 493. Brunner von Wattenwyl K. Prodromus der europäischen orthoptèren. Révision du sytème des Orthoptères. Annali del Museo civico di storia naturale di Genoa. 1893;2(13):1–230. [Google Scholar]
- 494. Tilgner EH. The fossil record of Phasmida (Insecta: Neoptera). Insect Systematics & Evolution. 2001;31(4):473–480. [Google Scholar]
- 495. Ansorge J. Zur systematischen Position von Schesslitziella haupti Kuhn 1952 (Insecta: Phasmatodea) aus dem Oberen Lias von Nordfranken (Deutschland). Paläontologische Zeitschrift. 1996;70(3/4):475–479. [Google Scholar]
- 496. Zompro O. The Phasmatodea and Raptophasma n. gen., Orthoptera incertae sedis, in Baltic amber (Insecta: Orthoptera). Mitteilungen aus dem Geologisch-Paläontologischen Institut der Universität Hamburg. 2001;85:229–261. [Google Scholar]
- 497. Poinar G, Poinar R. The amber forest, a reconstruction of a vanished world Princeton University Press; 1999. [Google Scholar]
- 498. Nel A, Marchal-Papier F, Béthoux O, Gall JC. A “stick insect-like” from the Triassic of the Vosges (France) (“pre-Tertiary Phasmatodea”). Annales de la Société entomologique de France (Nouvelle série). 2004;40(1):31–36. [Google Scholar]
- 499. Solórzano Kraemer MM. Systematic, palaeoecology, and palaeobiogeography of the insect fauna from the Mexican amber. Palaeontographica Abteilung A. 2007;282(1–6):1–133. [Google Scholar]
- 500. Wedmann S, Bradler S, Rust J. The first fossil leaf insect: 47 million years of specialized cryptic morphology and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(2):565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501. Sinitshenkova ND. Istoricheskoe razvitie vesnyanok [Historical development of stoneflies]. Trudy Paleontologicheskogo instituta Akademiia nauk SSSR. 1987;221:1–143. In Russian. [Google Scholar]
- 502. Rasnitsyn AP, Zherikhin VV. 4.1 Impression Fossils In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 437–444. [Google Scholar]
- 503. Sinitshenkova ND. 2.2.2.2.2. Order Perlida Latreille, 1810. The Stoneflies (= Plecoptera Burmeister, 1839) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 281–287. [Google Scholar]
- 504. Ansorge J. Dobbertiniopteryx capniomimus gen. et sp. nov.—die erste Steinfliege (Insecta: Plecoptera) aus dem Europäischen Jura. Paläontologische Zeitschrift. 1993;67(3/4):287–292. [Google Scholar]
- 505. Liu YS, Sinitshenkova ND, Ren D. A revision of the Jurassic stonefly genera Dobbertiniopteryx Ansorge and Karanemoura Sinitshenkova (Insecta: Plecoptera), with the description of new species from the Daohugou locality, China. Paleontological Journal. 2009;43(2):183–190. [Google Scholar]
- 506. Sinitshenkova ND. New Mesozoic stoneflies from Asia. Paleontological Journal. 1990;24(3):62–70. [Google Scholar]
- 507. van Dijk DE, Geertsema H. A new genus of Permian Plecoptera (Afroperla) from KwaZulu-Natal, South Africa. African Entomology. 2004;12(2):268–270. [Google Scholar]
- 508. Sinitshenkova ND. New upper Mesozoic stoneflies from central Transbaikalia (Insecta, Perlida = Plecoptera). Paleontological Journal. 1998;32(2):167–173. [Google Scholar]
- 509. Lin QB. Late Triassic insect fauna from Toksun, Xinjiang. Acta Palaeontologica Sinica. 1992;31(3):313–335. In Chinese, English summary. [Google Scholar]
- 510. Liu YS, Ren D. Progress in the study of Plecoptera fossils. Acta Zootaxonomica Sinica. 2006;31(4):758–768. [Google Scholar]
- 511. Liu YS, Ren D, Sinitshenkova ND, Shih CK. Three new stoneflies (Insecta: Plecoptera) from the Yixian Formation of Liaoning, China. Acta geologica sinica. 2008;82(2):249–256. [Google Scholar]
- 512. Liu YS, Ren D, Sinitshenkova ND, Shih CK. A new Middle Jurassic stonefly from Daohugou, Inner Mongolia, China (Insecta: Plecoptera). Annales zoologici. 2006;56(3):549–554. [Google Scholar]
- 513. Sinitshenkova ND. New stoneflies of the family Palaeonemouridae from the Upper Permian of Udmurtiya and the Orenburg Region (Insecta: Perlida = Plecoptera). Paleontological Journal. 2004;38(Suppl. 2):S164–S172. [Google Scholar]
- 514. Liu YS, Ren D. Two new Jurassic stoneflies (Insecta: Plecoptera) from Daohugou, Inner Mongolia, China. Progress in Natural Science. 2008;18:1039–1042. [Google Scholar]
- 515. Sinitshenkova ND. New upper Mesozoic stone flies from Yakutia (Insecta: Perlida = Plecoptera). Paleontological Journal. 1992;26(3):43–55. [Google Scholar]
- 516. Martins-Neto RG, Gallego OF, Zavattieri A. The Triassic insect fauna from Argentina: Coleoptera, Hemiptera and Orthoptera from the Potrerillos Formation, south of cerro Cacheuta, Cuyana basin. Alavesia. 2008;2:47–58. [Google Scholar]
- 517. Liu YS, Sinitshenkova ND, Ren D. A new genus and species of stonefly (Insecta: Plecoptera) from the Yixian Formation, Liaoning Province, China. Cretaceous Research. 2007;28(2):322–326. [Google Scholar]
- 518. Shcherbakov DE. 2.2.2.2.3 Order Forficulida Latreille, 1810. The earwigs and protelytropterans (= Dermaptera DeGeer, 1773 +Protelytroptera Tillyard, 1931) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 288–291. [Google Scholar]
- 519. Brauckmann C, Herd KJ. Insekten-Funde aus dem Westfalium D (Ober-Karbon) des Piesberges bei Osnabrück (Deutschland). Teil 2: Neoptera. Osnabrücker naturwissenschafliche Mitteilungen. 2006;30/31 (for 2005):19–65. [Google Scholar]
- 520. Rasnitsyn AP. 2.2.2.0.1. Order Eoblattida Handlirsch, 1906 (= Cacurgida Handlirsch, 1906, = Protoblattodea Handlirsch, 1906) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 256–260. [Google Scholar]
- 521. Novokshonov VG. New taxa of fossil insects from the Lower Permian of the middle Urals. Paleontological Journal. 1997;31(4):383–388. [Google Scholar]
- 522. Handlirsch A. New Paleozoic insects from the vicinity of Mazon Creek, Illinois. American Journal of Science (series 4). 1911;31:297–326. [Google Scholar]
- 523. Ross AJ, Nicholson DB, Jarzembowski EA. Boltonocostidae nom. nov. (Insecta, Hypoperlida), a replacement name for Orthocostidae Bolton, 1912. Bulletin of Zoological Nomenclature. 2013;70(4):291–292. [Google Scholar]
- 524. Bolton H. Insect-remains from the Midland and South-Eastern Coal Measures. Quarterly Journal of the Geological Society. 1912;68(3):310–323. [Google Scholar]
- 525. Carpenter FM. Substitute names for some extinct genera of fossil insects. Psyche. 1986;92[for 1985]:575–583. [Google Scholar]
- 526. Carpenter FM. Studies of North American Carboniferous insects. 8. New Palaeodictyoptera from Kansas, U.S.A. Psyche. 1992;99(2/3):141–146. [Google Scholar]
- 527. Novokshonov VG, Ivanov VV, Aristov DS. New insects from the late Permian of the Ural Mountains. Paleontological Journal. 2002;36(2):157–160. [Google Scholar]
- 528. Novokshonov VG. New and little-known representatives of the family Hypoperlidae (Insecta: Hypoperlida). Paleontological Journal. 2001;35(1):40–44. [Google Scholar]
- 529. Pinto ID. Rigattoptera ornellasae n. g., n. sp., a new fossil insect from the Carboniferous of Argentina. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte. 1996;1996(1):43–47. [Google Scholar]
- 530. Novokshonov VG. New enigmatic insects (Insecta: Hypoperlidea?: Sojanoperidae) from the Upper Permian of northern Russia. Paleontological Journal. 2002;36(1):48–49. [Google Scholar]
- 531. Silvestri F. Descrizione di un nuovo ordine di Insetti. Bollettino del Laboratio di zoologia generale e agraria della R scuola superiore di agricultura in Portici. 1913;7:193–209. [Google Scholar]
- 532. Engel MS. A new apterous Zorotypus in Miocene amber from the Dominican Republic (Zoraptera: Zorotypidae). Acta Entomolgica Slovenica. 2008;16(2):127–136. [Google Scholar]
- 533. Novokshonov VG, Aristov DS. New taxa of hypoperlids (Insecta: Hypoperlida) from the Upper Permian of the Arkhangelsk Region. Paleontological Journal. 2004;38(1):60–66. [Google Scholar]
- 534. Brauckmann C. Ausgewählte Arthropoden: Insecta, Arachnida, Xiphosura, Eurypterida, “Myriapoda”, Arthropleurida und Trilobita. Courier Forschungsinstitut Senckenberg. 2005;254:87–102. [Google Scholar]
- 535. Béthoux O. Revision of Cacurgus Handlirsch, 1911, a basal Pennsylvanian Archaeorthoptera (Insecta: Neoptera). Bulletin of the Peabody Museum of Natural History. 2006;47(1–2):29–35. [Google Scholar]
- 536. Pinto ID, Pinto de Ornellas LP. Substitute names for the extinct Insecta families Narkemocacurgidae Pinto & Ornellas, 1978 and Cacurgonarkemidae Pinto, 1990. Pesquisas. 1991;18(1):93. [Google Scholar]
- 537. Zhang XW, Ren D, Pang H, Shih CK. Late Mesozoic chresmodids with forewing from Inner Mongolia, China (Polyneoptera: Archaeorthoptera). Acta geologica sinica (English Edition). 2009;84(1):38–46. [Google Scholar]
- 538. Delclòs X, Nel A, Bechly G, Dunlop JA, Engel MS, Heads SW. The enigmatic Mesozoic insect taxon Chresmodidae (Polyneoptera): New palaeobiological and phylogenetic data, with the description of a new species from the Lower Cretaceous of Brazil. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 2008;247(3):353–381. [Google Scholar]
- 539. Béthoux O, Briggs DEG. How Gerarus lost its head: stem-group Orthoptera and Paraneoptera revisited. Systematic Entomology. 2008;33(3):529–547. [Google Scholar]
- 540. Zessin W. Ploetzgerarus krempieni n. gen. et sp. eine neue Geraride (Insecta: Panorthoptera: Geraridae) aus dem Oberkarbon (Stephanium C) von Plötz bei Halle (Deutschland). Virgo, Mitteilungsblatt des Entomologischen Vereins Mecklenburg. 2009;12:22–29. [Google Scholar]
- 541.MacLeay WS. Annulosa Javanica, or an attempt to illustrate the natural affinities and analogies of the insects collected in Java by Thomas Horsfield, M.D. F.L. & G.S. and deposited by him in the museum of the honourable East-India Company. Number 1. Kingsbury, Parbury and Allen, London; 1825.
- 542. Brauckmann C, Arillo A, Ortuño VM. A new Geraridae (Insecta, hemipteroid stem assemblage) from the Upper Carboniferous of La Magdalena (León, northern Spain). Boletín Geológico y Minero. 2001;112(2):57–62. [Google Scholar]
- 543. Béthoux O, Schneider JW. Description of a hind wing of a new basal Archaeorthoptera (Mazon Creek, IL; Pennsylvanian). Alavesia. 2009;3:81–85. [Google Scholar]
- 544. Béthoux O. Protophasma dumasii Brogniart, 1879, a link between Orthoptera and the ‘dictyopterid’ orders? Journal of Orthoptera Research. 2003;12(1):57–62. [Google Scholar]
- 545. Rasnitsyn AP, Krassilov VA. The first documented occurrence of phyllophagy in pre-Cretaceous insects: Leaf tissues in the gut of Upper Jurassic insects from southern Kazakhstan. Paleontological Journal. 2000;34(3):301–309. [Google Scholar]
- 546. Beckemeyer RJ. Artinska ovata (Sellards) 1909 and Paraprisca fragilis (Sellards) 1909 (Insecta: Polyneoptera: Lemmatophoridae) newly reported from the Lower Permian of Noble County, Oklahoma, with notes on Wellington Formation Lemmatophoridae. Transactions of the Kansas Academy of Science. 2009;112(1/2):45–56. [Google Scholar]
- 547. Aristov DS. New grylloblattids of the family Lemmatophoridae (Insecta: Grylloblattida) from the Permian of Russia. Paleontological Journal. 2009;43(3):272–276. [Google Scholar]
- 548.Hörnschemeyer T. Fossil insects from the Lower Permian of Nierdermoschel (Germany). In: Scoggin M, editor. AMBA projects AM/PFICM98/1.99: Proceedings of the First International Palaeoentomological Conference, Moscow 1998; 1999. p. 57–60.
- 549. Novokshonov VG, Rasnitsyn AP. A new enigmatic group of insects (Psocidea, Tshekarcephalidae) from Tshekarda (Lower Permian of the middle Urals. Paleontological Journal. 2000;34(Suppl. 3):S284–S287. [Google Scholar]
- 550. Aristov DS, Rasnitsyn AP. Position and taxonomy of the Permian fossil insect family Permembiidae (Insecta: Palaeomanteida = Miomoptera). Russian Entomological Journal. 2008;17(4):327–334. [Google Scholar]
- 551. Martynov AV. On a new Permian order of orthopteroid insects, Glosselytrodea [In Russian]. Izvestiya akademii nauk SSSR, otdelenie matematicheskikh i estestvennykh nauk. 1938;1938:187–206. [Google Scholar]
- 552. Béthoux O, Nel A, Gand G, Lapeyrie J. Surijoka lutevensis nov. sp.: the first Glosselytrodea (Insecta) from the Upper Permian of France (Lodève Basin). Geobios. 2001;34(4):405–413. [Google Scholar]
- 553. Ponomarenko AG. New alderflies (Megaloptera: Parasialidae) and glosselytrodeans (Glosselytrodea: Glosselytridae) from the Permian of Mongolia. Paleontological Journal. 2000;34(Suppl. 3):S309–S311. [Google Scholar]
- 554. Rasnitsyn AP. 2.2.1.3.3.4. Order Jurinida M. Zalessky, 1928 (= Glosselytrodea Martynov, 1938) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 189–192. [Google Scholar]
- 555. Hong YC. Discovery of the fossil glosselytrods (Insecta: Glosselytrodea) from Shaanxi, China. Acta Entomolgica Sinica. 2007;50(3):271–280. [Google Scholar]
- 556. Huang DY, Nel A, Lin QB, Dong FB. The first Glosselytrodea (Insecta) from the latest Middle Permian of Anhui Province, China. Bulletin de la Société entomologique de France. 2007;112(2):179–182. [Google Scholar]
- 557. Béthoux O, Beattie RG, Nel A. Wing venation and relationships of the order Glosselytrodea (Insecta). Alcheringa. 2007;31(3):285–296. [Google Scholar]
- 558. Beckemeyer RJ, Hall JD. Permopanorpa inaequalis Tillyard, 1926 (Insecta: Holometabola: Panorpida: Permopanorpidae): A fossil mecopteroid newly reported for the Lower Permian Wellington Formation of Noble County, Oklahoma. Transactions of the Kansas Academy of Science. 2007;110(1/2):23–29. [Google Scholar]
- 559. Hong YC. First discovery of Midtriassic order Miomoptera (Insecta) in China. Geological Bulletin of China. 2009;28(1):11–15. [Google Scholar]
- 560. Novokshonov VG, Zhuzhgova LV. Discussion of the system and phylogeny of the order Palaeomanteida (= Miomoptera) with description of new representatives of the genus Permosialis Mart. from the late Permian of Kirov Region and Triassic of Kyrgyzstan. Paleontological Journal. 2004;38(Suppl. 2):S173–S184. [Google Scholar]
- 561.Linnaeus C. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis [10th edition, revised]. Salviae, Holmiae [Stockholm], Sweden; 1758.
- 562. Andersen NM. Water striders from the Paleogene of Denmark with a review of the fossil record and evolution of semiaquatic bugs (Hemiptera, Gerromorpha). Biologiske Skrifter. 1998;50:1–157. [Google Scholar]
- 563. Andersen NM, Grimaldi DA. A fossil water measurer from the mid-Cretaceous Burmese amber (Hemiptera: Gerromorpha: Hydrometridae). Insect Systematics & Evolution. 2001;32(4):381–392. [Google Scholar]
- 564. Damgaard J. Evolution of the semi-aquatic bugs (Hemiptera: Heteroptera: Gerromorpha) with a re-interpretation of the fossil record. Acta Entomologica Musei Nationalis Pragae. 2008;48(2):251–268. [Google Scholar]
- 565. Shcherbakov DE. The earliest find of Tropiduchidae (Homoptera: Auchenorrhyncha), representing a new tribe, from the Eocene of Green River, USA, with notes on the fossil record of higher Fulgoroidea. Russian Entomological Journal. 2006;15(3):315–322. [Google Scholar]
- 566. Wappler T. Die Insekten aus dem Mittel-Eozän des Eckfelder Maares, Vulkaneifel. Mainzer Naturwissenschafliches Archiv, Beiheft. 2003;27:1–234. [Google Scholar]
- 567. Szwedo J. Achilidae from the Eocene Baltic amber. Bulletin of Insectology. 2008;61(1):109–110. [Google Scholar]
- 568. Koteja J, Poinar GO. A new family, genus, and species of scale insect (Hemiptera: Coccinea: Kukaspididae, new family) from Cretaceous Alaskan amber. Proceedings of the Entomological Society of Washington. 2001;103(2):356–363. [Google Scholar]
- 569. Koteja J. Scale insects (Hemiptera: Coccinea) from Cretaceous Myanmar (Burmese) amber. Journal of Systematic Palaeontology. 2004;2(2):109–114. [Google Scholar]
- 570. Shcherbakov DE. The most primitive whiteflies (Hemiptera; Aleyrodidae; Bernaeinae subfam. nov.) from the Mesozoic of Asia and Burmese amber, with an overview of Burmese amber hemipterans. Bulletin of The Natural History Museum, Geology Series. 2000;56(1):29–37. [Google Scholar]
- 571. Yao YZ, Cai WZ, Ren D. New Jurassic fossil true bugs of Pachymeridiidae (Hemiptera: Pentatomomorpha) from northeast China. Acta geologica sinica (English Edition). 2008;82(1):35–47. [Google Scholar]
- 572. Shcherbakov DE, Popov YA. 2.2.1.2.5. Superorder Cimicidea Laicharting, 1781 Order Hemiptera Linné, 1758. The bugs, cicadas, plantlice, scale insects, etc. (= Cimicida Laicharting, 1781, = Homoptera Leach, 1815 + Heteroptera Latreille, 1810) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 143–157. [Google Scholar]
- 573. Klimaszewski SM. New psyllids from the Baltic amber (Insecta: Homoptera, Aphalaridae). Mitteilungen aus dem Geologisch-Paläontologischen Institut der Universität Hamburg. 1997;80:151–171. [Google Scholar]
- 574. Popov YA. A new notion on the heteropterofauna (Insecta: Hemiptera: Heteroptera) from the Pliocene of Willershausen. Paläontologische Zeitschrift. 2007;81(4):429–439. [Google Scholar]
- 575. Wang Y, Ren D, Liang JH, Liu YS, Wang ZH. The fossil Homoptera of China: a review of present knowledge. Acta Zootaxonomica Sinica. 2006;31(2):294–303. [Google Scholar]
- 576. Popov YA, Bechly G. 11.15 Heteroptera: bugs In: Martill DM, Bechly G, Loveridge RF, editors. The Crato Fossil Beds of Brazil: Window into an Ancient World. Cambridge University Press; 2007. p. 317–328. [Google Scholar]
- 577. Popov YA, Dolling WR, Whalley PES. British Upper Triassic and Lower Jurassic Heteroptera and Coleorrhyncha (Insecta: Hemiptera). Genus. 1994;5(4):307–347. [Google Scholar]
- 578. Engel MS. The dustywings in Cretaceous Burmese amber (Insecta: Neuroptera: Coniopterygidae). Journal of Systematic Palaeontology. 2004;2(2):133–136. [Google Scholar]
- 579. Ansorge J. Insects from the lower Toarcian of middle Europe and England. Acta zoological cracoviensia. 2003;46(suppl.- Fossil Insects):291–310. [Google Scholar]
- 580. Koteja J. Xylococcidae and related groups (Hemiptera: Coccinea) from Baltic amber. Prace Muzeum Ziemi. 2008;49:19–56. [Google Scholar]
- 581. Szwedo J. 11.13 Fulgoromorpha: planthoppers In: Martill DM, Bechly G, Loveridge RF, editors. The Crato Fossil Beds of Brazil: Window into an Ancient World. Cambridge University Press; 2007. p. 297–313. [Google Scholar]
- 582. Shcherbakov DE. Extinct four-winged precoccids and the ancestry of scale insects and aphids (Hemiptera). Russian Entomological Journal. 2007;16(1):47–62. [Google Scholar]
- 583. Poinar GO, Brown AE. New Aphidoidea (Hemiptera: Sternorrhyncha) in Burmese amber. Proceedings of the Entomological Society of Washington. 2005;107(4):835–845. [Google Scholar]
- 584. Wegierek P. Cretaceous aphids of the Family Canadaphididae (Hemiptera, Aphidomorpha). Paleontologicheskii Zhurnal. 1991;1991(2):114–115. [Google Scholar]
- 585.Hamilton KGA. Chapter 6. Homoptera. In: Grimaldi DA, editor. Insects from the Santana Formation, Lower Cretaceous of Brazil. vol. 195. Bulletin of the American Museum of Natural History; 1990. p. 82–122.
- 586. Menon F, Heads SW, Szwedo J. 11.12 Cicadomorpha: cicadas and relatives In: Martill DM, Bechly G, Loveridge RF, editors. The Crato Fossil Beds of Brazil: Window into an Ancient World. Cambridge University Press; 2007. p. 283–297. [Google Scholar]
- 587. Shcherbakov DE. Mesozoic Velocipedinae (Nabidae s.l.) and Ceresopseidae (Reduvioidea), with notes on the phylogeny of Cimicomorpha (Heteroptera). Russian Entomological Journal. 2008;16 [for 2007](4):401–414. [Google Scholar]
- 588. Shcherbakov DE. Review of the fossil and extant genera of the cicada family Tettigarctidae (Hemiptera: Cicadoidea). Russian Entomological Journal. 2009;17 [for 2008](4):343–348. [Google Scholar]
- 589. Engel MS. A stem-group cimicid in mid-Cretaceous amber from Myanmar (Hemiptera: Cimicoidea). Alavesia. 2008;2:233–237. [Google Scholar]
- 590. Pérez-Gelabert DE. Arthropods of Hispaniola (Dominican Republic and Haiti): A checklist and bibliography. Zootaxa. 2008;1831:1–530. [DOI] [PubMed] [Google Scholar]
- 591.Szwedo J, Bourgoin T, Lefèbvre F. Fossil Planthoppers (Hemiptera: Fulgoromorpha) of the World. An annotated catalogue with notes on Hemiptera classification. Studio 1, Warsaw; 2004.
- 592. Koteja J. Advances in the study of fossil coccids (Hemiptera: Coccinea). Polskie Pismo Entomologiczne. 2000;69(2):187–218. [Google Scholar]
- 593. Shcherbakov DE, Wegierek P. Creaphididae, a new and the oldest aphid family from the Triassic of middle Asia. Psyche. 1991;98(1):81–85. [Google Scholar]
- 594. Hong YC, Zhang ZJ, Guo XR, Heie OE. A new species representing the oldest aphid (Hemiptera, Aphidomorpha) from the Middle Triassic of China. Journal of Paleontology. 2009;83(5):826–831. [Google Scholar]
- 595. Heie OE, Pike EM. New aphids in Cretaceous amber from Alberta (Insecta, Homoptera). The Canadian Entomologist. 1992;124(6):1027–1053. [Google Scholar]
- 596. Carvalho JCM. Mirídeos neotropicais, CCLIII: descriçĩes de bivis gêneros e espécies da tribo Orthotylni Van Duzee (Hemiptera). Revista Brasileira de Biologia. 1985;45(3):249–298. [Google Scholar]
- 597. Hong YC. Curvicubitidae fam. nov. (Lepidoptera? Insecta) from Middle Triassic of Shaanxi. Acta Palaeontologica Sinica. 1984;2(6):782–785. In Chinese with English summary. [Google Scholar]
- 598. Engel MS. 10. Arthropods in Mexican amber p.175-186. In: Bousquets JL, Morrone JJ, Ordóñez OY, Fernández IV, editors. Biodiversidad, taxonomía y biogeografía de artrópodos de México: hacia una síntesis de su conocimiento. Universidad Nacional Autónoma de México; 2004. p. xvi+660. [Google Scholar]
- 599. Szwedo J. First fossil record of Cedusini in the Eocene Baltic amber with notes on the tribe (Hemiptera: Fulgoromorpha: Derbidae). Russian Entomological Journal. 2006;15(3):327–333. [Google Scholar]
- 600. Wappler T, Ben-Dov Y. Preservation of armoured scale insects on angiosperm leaves from the Eocene of Germany. Acta Palaeontologica Polonica. 2008;53(4):627–634. [Google Scholar]
- 601. Szwedo J. A new tribe of Dictyopharidae planthoppers from Eocene Baltic amber (Hemiptera: Fulgoromorpha: Fulgoroidea), with a brief review of the fossil record of the family. Palaeodiversity. 2008;1:75–85. [Google Scholar]
- 602. Greenwood DR, Archibald SB, Mathewes RW, Moss PT. Fossil biotas from the Okanagan Highlands, southern British Columbia and northern Washington State: climates and ecosystems across an Eocene landscape. Canadian Journal of Earth Sciences. 2005;42(2):167–185. [Google Scholar]
- 603. Poinar GO, Milki R. Lebanese Amber: The Oldest Insect Ecosystem in Fossilized Resin. Oregon State University Press, Corvallis; 2001. [Google Scholar]
- 604. Zhang GX, Hong YC. A new family Drepanochaitophoridae (Homoptera: Aphidoidea) from Eocene Fushun amber of Liaoning Province, China. Entomologia Sinica. 1999;6(2):127–134. [Google Scholar]
- 605. Shcherbakov DE. On Permian and Triassic insect faunas in relation to biogeography and the Permian-Triassic crisis. Paleontological Journal. 2008;42(1):15–31. [Google Scholar]
- 606. Dmitriev VU, Zherikhin VV. Izmeneniya raznoobraziya semeistv nasekomykh po dannym metoda nakoplennykh poyavlenii In: Ponomarenko AG, editor. Melovoi biotsenoticheskii krizis i evolyutsiya nasekomykh [The Cretaceous biocenotic crisis and evolution of insects]. Nauka, Moscow; 1988. p. 208–215. [Google Scholar]
- 607. Martins-Neto RG, Gallego OF. Review of Dysmorphoptilidae Handlirsch (Hemiptera: Cicadomorpha) from the Argentinean Triassic, with description of a new subfamily, and a new species. Polskie Pismo Entomologiczne. 2006;75(2):185–197. [Google Scholar]
- 608. Perrichot V, Nel A, Guilbert E, Néraudeau D. Fossil Tingoidea (Heteroptera: Cimicomorpha) from French Cretaceous amber, including Tingidae and a new family, Ebboidae. Zootaxa. 2006;1203:57–68. [Google Scholar]
- 609. Koteja J. Scale insects (Homoptera, Coccinea) from Upper Cretaceous New Jersey amber In: Grimaldi DA, editor. Studies on fossils in amber, with particular reference to the Cretaceous of New Jersey. Backhuys Publishers, Leiden, The Netherlands; 2000. p. 147–229. [Google Scholar]
- 610. Koteja J, Azar D. Scale insects from Lower Cretaceous amber of Lebanon (Hemiptera: Sternorrhyncha: Coccinea). Alavesia. 2008;2:133–167. [Google Scholar]
- 611. Heie OE, Wegierek P. A list of fossil aphids (Homoptera: Aphidinea). Annals of the Upper Silesian Museum (Entomology). 1998;8–9:159–192. [Google Scholar]
- 612.Heie OE. Fossil aphids. A catalogue of fossil aphids, with comments on systematics and evolution. In: Evolution and biosystematics of aphids. Proceedings of the International Aphidological Symposium at Jablona, 5–11 April 1951. Polska Akademia Nauk, Instytut Zoologii, Warzawa; 1985. p. 101–131.
- 613. Azar D. Preservation and accumulation of biological inclusions in Lebanese amber and their significance. Comptes Rendus Palevol. 2007;6(1–2):151–156. [Google Scholar]
- 614. Szwedo J, Wappler T. New planthoppers (Hemiptera: Fulgoromorpha) from the Middle Eocene Messel Maar. Annales zoologici. 2006;56(3):555–566. [Google Scholar]
- 615. Bourgoin T, Szwedo J. The ‘cixiid-like’ fossil planthopper families. Bulletin of Insectology. 2008;61(1):107–108. [Google Scholar]
- 616. Szwedo J, Zyła D. New Fulgoridiidae genus from the Upper Jurassic Karatau deposits, Kazakhstan (Hemiptera: Fulgoromorpha: Fulgoroidea). Zootaxa. 2009;2281:40–52. [Google Scholar]
- 617. Hong YC. Granulidae, a new family of Homoptera from the Middle Triassic of Tongchuan, Shaanxi Province. Acta Zootaxonomica Sinica. 1980;5(1):63–70. In Chinese with English abstract. [Google Scholar]
- 618. Handlirsch A. Neue Untersuchungen über die fossilen Insekten. II. Teil. Annalen des Naturhistorischen Museums in Wien. 1939;49:1–240. [Google Scholar]
- 619. Popov YA, Shcherbakov DE. Mesozoic Peloridioidea and their ancestors. Geologica et Palaeontologica. 1991;25:215–235. [Google Scholar]
- 620. Heads SW. A new species of Yuripopovia (Coleorrhyncha: Progonocimicidae) from the early Cretaceous of the Isle of Wight. British Journal of Entomology and Natural History. 2008;21:247–253. [Google Scholar]
- 621. Wang B, Szwedo J, Zhang HC. Jurassic Progonocimicidae (Hemiptera) from China and phylogenetic evolution of Coleorrhyncha. Science in China Series D: Earth Sciences. 2009;52(12):1953–1961. [Google Scholar]
- 622. Evans JW. Palaeozoic and Mesozoic Hemiptera (Insecta). Australian Journal of Zoology. 1956;4(2):165–258. [Google Scholar]
- 623. Szwedo J. Paradise Lost? Cretaceous and Palaeogene diversification of planthoppers and leafhoppers. Bulletin of Insectology. 2008;61(1):111–112. [Google Scholar]
- 624. Bechly G, Wittmann M. Two new tropical bugs (Insecta: Heteroptera: Thaumastocoridae—Xylastodorinae and Hypsipterygidae) from Baltic amber. Stuttgarter Beiträge zur Naturkunde Serie B (Geologie und Paläontologie). 2000;289:1–11. [Google Scholar]
- 625. Zhang JF, Golub VB, Popov YA, Shcherbakov DE. Ignotingidae fam. nov. (Insecta: Heteroptera: Tingoidea), the earliest lace bugs from the upper Mesozoic of eastern China. Cretaceous Research. 2005;26(5):783–792. [Google Scholar]
- 626. Shcherbakov DE. Permian faunas of Homoptera (Hemiptera) in relation to phytogeography and the Permo-Triassic Crisis. Paleontological Journal. 2000;34 (suppl.-3):S251–S267. [Google Scholar]
- 627. Koteja J. Inka minuta gen. et sp. n. (Homoptera, Coccinea) from Upper Cretaceous Taymyrian amber. Annales zoologici. 1989;43(5):77–101. [Google Scholar]
- 628. Shcherbakov DE. The earliest leafhoppers (Hemiptera: Karajassidae n. fam.) from the Jurassic of Karatau. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte. 1992;1992(1):39–51. [Google Scholar]
- 629. Bourgoin T, Lefèbvre F. A new fossil Kinnaridae from Dominican amber (Hemiptera: Fulgoromorpha). Annales zoologici. 2002;52(4):583–585. [Google Scholar]
- 630. Popov YA. New peloridiids and heteropteran bugs Peloridiina (= Coleorhyncha) et Cimicina (= Heteroptera) [in Russian]. Transactions of the Joint Soviet-Mongolian Palaeontological Expedition. 1986;28:50–83. [Google Scholar]
- 631. Wegierek P, Peñalver E. Fossil representatives of the family Greenideidae (Hemiptera, Aphidoidea) from the Miocene of Europe. Geobios. 2002;35(6):745–757. [Google Scholar]
- 632.Polhemus JT. North American Mesozoic aquatic Heteroptera (Insecta, Naucoroidea, Nepoidea) from the Todilto Formation, New Mexico p.29-40. In: Lucas SG, editor. New Mexico’s Fossil Record 2. vol. Bulletin 16. New Mexico Museum of Natural History and Science, Albuquerque; 2000. p. 284.
- 633. Shcherbakov DE. New Mesozoic Homoptera. Sovmestnaya Sovetsko-Mongolskaya Paleontologischeskaya Ekspeditsiya Trudy. 1988;33:60–63. [Google Scholar]
- 634. Hamilton KGA. Lower Cretaceous Homoptera from the Koonwarra Fossil Bed in Australia, with a new superfamily and synopsis of Mesozoic Homoptera. Annales of the Entomological Society of America. 1992;85:423–430. [Google Scholar]
- 635. Perrichot V. Early Cretaceous amber from south-western France: insight into the Mesozoic litter fauna. Geologica Acta. 2004;2(1):9–22. [Google Scholar]
- 636. Popov YA. Klopy. Cimicina [Bugs. Cimicina]. Trudy Paleontologicheskogo institute Akademiia nauk SSSR. 1990;239:20–39. [Google Scholar]
- 637. Yao YZ, Cai WZ, Ren D. The fossil Heteroptera of China: a review of present knowledge. Acta Zootaxonomica Sinica. 2004;29:33–37. [Google Scholar]
- 638. Sinitshenkova ND. 3.3 Ecological history of the aquatic insects In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 388–426. [Google Scholar]
- 639. Shcherbakov DE. Mesozoic spider mimics Cretaceous Mimarachnidae fam. n. (Homoptera: Fulgoroidea). Russian Entomological Journal. 2007;16(3):259–264. [Google Scholar]
- 640. Szwedo J. Distributional and palaeoecological pattern of the Lower Cretaceous Mimarachnidae (Hemiptera: Fulgoromorpha). Entomologia Generalis. 2008;31(3):231–242. [Google Scholar]
- 641. Herczek A, Popov YA. Redescription of the oldest plant bugs from the Upper Jurassic of the southern Kazakhstan (Heteroptera: Cimicomorpha, Miridae). Annals of the Upper Silesian Museum (Entomology). 2001;10–11:121–128. [Google Scholar]
- 642. Szwedo J. Nymphs of a new family Neazoniidae fam. n. (Hemiptera: Fulgoromorpha: Fulgoroidea) from the Lower Cretaceous Lebanese amber. African Invertebrates. 2007;48(1):127–143. [Google Scholar]
- 643. Szwedo J. First discovery of Neazoniidae (Hemiptera: Fulgoromorpha) in the early Cretaceous Archingeay amber of SW France. Geodiversitas. 2009;31(1):105–116. [Google Scholar]
- 644. Ponomarenko AG. Fossil insects from the Tithonian “Solnhofener Plattenkalke” in the Museum of Natural History, Vienna. Annalen des Naturhistorischen Museums in Wien. 1985;87A:135–144. [Google Scholar]
- 645. Yao YZ, Cai WZ, Ren D. Pristinochterus gen. n. (Hemiptera: Ochteridae) from the Upper Mesozoic of northeastern China. European Journal of Entomology. 2007;104(4):827–835. [Google Scholar]
- 646. Ren D. Progress in the study of Mesozoic fossil insects during the last decade in China. Acta Entomologica Sinica. 2002;45(2):234–240. [Google Scholar]
- 647. Kania I, Wegierek P. Palaeoaphididae (Hemiptera, Sternorrhyncha) from Lower Cretaceous Baissa deposits. Morphology and classification. Monografie Faunistyczne. 2008;25:1–132. [Google Scholar]
- 648. Poinar GO, Buckley R. Palaeoleptus burmanicus n. gen., n. sp., an Early Cretaceous shore bug (Hemiptera: Palaeoleptidae n. fam.) in Burmese amber. Cretaceous Research. 2009;30(4):1000–1004. [Google Scholar]
- 649. Wang B, Zhang HC, Szwedo J. Jurassic Palaeontinidae from China and the higher systematics of Palaeontinoidea (Insecta: Hemiptera: Cicadomorpha). Palaeontology. 2009;52(1):53–64. [Google Scholar]
- 650. Wang B, Zhang HC, Fang Y. Some Jurassic Palaeontinidae (Insecta, Hemiptera) from Daohugou, Inner Mongolia, China. Palaeoworld. 2006;15(1):115–125. [Google Scholar]
- 651. Poinar GO, Brown AE. Remarks on Parvaverrucosa annulata (= Verrucosa annulata Poinar and Brown 2005) (Hemiptera: Sternorrhyncha: Aphidoidea). Proceedings of the Entomological Society of Washington. 2006;108(3):734–735. [Google Scholar]
- 652. Shcherbakov DE. An extraordinary new family of Cretaceous planthoppers (Homoptera: Fulgoroidea). Russian Entomological Journal. 2007;16(2):139–154. [Google Scholar]
- 653. Grimaldi DA, Engel MS. An unusual, primitive Piesmatidae (Insecta: Heteroptera) in Cretaceous amber from Myanmar (Burma). American Museum Novitates. 2008;3611:1–17. [Google Scholar]
- 654. Foldi I. Ground pearls: a generic revision of the Margarodidae sensu stricto (Hemiptera: Sternorrhyncha: Coccoidea). Annales de la Société entomologique de France (Nouvelle série). 2005;41(1):18–125. [Google Scholar]
- 655. Popov YA. Pavlostysia wunderlichi gen. nov. and sp. nov., the first fossil spider-web bug (Hemiptera: Heteroptera: Cimicomorpha: Plokiophilidae) from the Baltic Eocene amber. Acta Entomologica Musei Nationalis Pragae. 2008;48(2):497–502. [Google Scholar]
- 656. Popov YA. Jurassic bugs (Hemiptera: Heteroptera) from the Museum of Natural History in Vienna. Annalen des Naturhistorischen Museums in Wien. 1992;94A:7–14. [Google Scholar]
- 657. Zhang HC, Wang QF, Zhang JF. Some Jurassic homopteran insects from the Junggar basin, Xinjiang, China. Acta Palaeontologica Sinica. 2004;42 [for 2003](4):548–551. [Google Scholar]
- 658. Ren D, Yin JC, Dou WX. New planthoppers and froghoppers from the late Jurassic of northeast China (Homoptera: Auchenorrhyncha). Acta Zootaxonomica Sinica. 1998;23(3):281–287. [Google Scholar]
- 659. Bechly G, Szwedo J. 11.14 Coleorrhyncha: moss bugs In: Martill DM, Bechly G, Loveridge RF, editors. The Crato Fossil Beds of Brazil: Window into an Ancient World. Cambridge University Press; 2007. p. 313–317. [Google Scholar]
- 660. Geertsema H, van Dijk DE, van den Heever JA. Palaeozoic insects of southern Africa: a review. Palaeontologia africana. 2002;38:19–25. [Google Scholar]
- 661. Grimaldi DA. First amber fossils of the extinct family Protopsyllidiidae, and their phylogenetic significance among Hemiptera. Insect Systematics & Evolution. 2003;34(3):329–344. [Google Scholar]
- 662. López Ruf M, Pérez Goodwyn P, Martins-Neto RG. New Heteroptera (Insecta) from the Santana Formation, Lower Cretaceous (Northeastern Brazil), with description of a new family and new taxa of Naucoridae and Gelastocoridae. Gaea (Acta Geologica Leopoldensia). 2005;1(2):68–74. [Google Scholar]
- 663. Meyer HW. The Fossils of Florissant. Smithsonian Institution Press, Washington; 2003. [Google Scholar]
- 664. Yao YZ, Cai WZ, Ren D. Fossil flower bugs (Heteroptera: Cimicomorpha: Cimicoidea) from the late Jurassic of northeast China, including a new family, Vetanthocoridae. Zootaxa. 2006;1360:1–40. [Google Scholar]
- 665. Poinar GO, Poinar R. What Bugged the Dinosaurs? Insects, Disease, and Death in the Cretaceous. Princeton University Press; 2008. [Google Scholar]
- 666. Yao YZ, Cai WZ, Ren D, Shih CK. New fossil rhopalids (Heteroptera: Coreoidea) from the Middle Jurassic of Inner Mongolia, China. Zootaxa. 2006;1384:41–58. [Google Scholar]
- 667. Rust J. Biostratinomie von Insekten aus der Fur-Formation von Dänemark (Moler, oberes Paleozän / unteres Eozän). Paläontologische Zeitschrift. 1998;72(1/2):41–58. [Google Scholar]
- 668. Wegierek P. New species of Mesozoic aphids (Shaposhnikoviidae, Homoptera). Paleontological Journal. 1989;23(4):40–49. [Google Scholar]
- 669. Heie OE. Palaeontology and phylogeny In: Minks AK, Harrewijn P, editors. Aphids: their biology, natural enemies and control, Volume A Elsevier Academic Press, Amsterdam; 1987. p. 367–391. [Google Scholar]
- 670. Huang DY, Nel A. A new Middle Jurassic aphid family (Insecta: Hemiptera: Sternorrhyncha: Sinojuraphididae fam. nov.) from Inner Mongolia, China. Palaeontology. 2008;51(3):715–719. [Google Scholar]
- 671. Heie OE, Azar D. Two new species of aphids found in Lebanese amber and a revision of the family Tajmyraphididae Kononova, 1975 (Hemiptera: Sternorrhyncha). Annals of the Entomological Society of America. 2000;93(6):1222–1225. [Google Scholar]
- 672. Grimaldi DA, Engel MS. A termite bug in early Miocene amber of the Dominican Republic (Hemiptera: Termitaphididae). American Museum Novitates. 2008;3619:1–10. [Google Scholar]
- 673. Engel MS. A new termite bug in Miocene amber from the Dominican Republic (Hemiptera: Termitaphididae). ZooKeys. 2009;25:61–68. [Google Scholar]
- 674. Grazia J, Schuh RT, Wheeler WC. Phylogenetic relationships of family groups in Pentatomoidea based on morphology and DNA sequences (Insecta: Heteroptera). Cladistics. 2008;24(6):932–976. [DOI] [PubMed] [Google Scholar]
- 675. Golub VB, Popov YA. A remarkable fossil lace bug from Upper Cretaceous New Jersey amber (Heteroptera: Tingoidea, Vianaididae), with some phyogenetic commentary In: Grimaldi DA, editor. Studies on Fossils in Amber, with Particular Reference to the Cretaceous of New Jersey. Backhuys Publishers, Leiden, The Netherlands; 2000. p. 231–239. [Google Scholar]
- 676. Golub VB, Popov YA. A new species of Tingidae (Insecta: Hemiptera: Heteroptera) from the Lower Cretaceous of Transbaikalia. Paleontological Journal. 2008;42(1):86–89. [Google Scholar]
- 677.Heie OE. Aphids of the past (Hemiptera, Sternorrhyncha). In: Scoggin M, editor. AMBA projects AM/PFICM98/1.99: Proceedings of the First International Palaeoentomological Conference, Moscow 1998; 1999. p. 49–55.
- 678. Arillo A, Ortuño VM. Catalogue of fossil insect species described from Dominican amber (Miocene). Stuttgarter Beiträge zur Naturkunde Serie B (Geologie und Paläontologie). 2005;352:1–68. [Google Scholar]
- 679. Zhang JF, Sun B, Zhang X. Miocene insects and spiders from Shanwang, Shandong Science Press, Beijing; 1994. [Google Scholar]
- 680. Berger H, Heiss E, Kerzhner IM. Removal of homonymy between Urostylidae Dallas, 1851 (Insecta, Heteroptera) and Urostylidae Bütschli, 1889 (Ciliophora, Hypotrichia). Annalen des Naturhistorischen Museums in Wien. 2001;103B:301–302. [Google Scholar]
- 681.Bütschli O. Suctoria. In: Bronn HG, editor. Klassen und Ordnungen des Thier-Teichs, Band 1, Protozoa. Winter, Leipzig; 1889. p. 1842–1945.
- 682. Damgaard J. Phylogeny of the semiaquatic bugs (Hemiptera-Heteroptera, Gerromorpha). Insect Systematics & Evolution. 2008;39(4):431–460. [Google Scholar]
- 683. Golub VB, Popov YA. The new fossil genus of Vianaididae (Heteroptera: Tingoidea) from the Cretaceous amber of New Jersey; evolution of the family in the Late Cretaceous. Acta zoologica cracoviensia. 2003;46(suppl.- Fossil Insects):109–116. [Google Scholar]
- 684. Haeckel EHPA. Systematische Phylogenie. Entwurf eines natürlichen Systema der Organismen auf Grund ihrer Stammesgeschichte Zweiter Theil: Systematische Phylogenie der Wirbellosen Thiere (Invertebrata). Reimer, Berlin; 1896. [Google Scholar]
- 685. Dalgleish RC, Palma RL, Price RD, Smith VS. Fossil lice (Insecta: Phthiraptera) reconsidered. Systematic Entomology. 2006;31(4):648–651. [Google Scholar]
- 686. Kumar P. Antiquity of Phthiraptera: fossil evidence. Journal of the Palaeontological Society of India. 2004;49:159–168. [Google Scholar]
- 687. Smith VS, Dalgleish RC, Cruickshank RH. Fossil lice reconsidered erratum. Systematic Entomology. 2007;32(1):196. [Google Scholar]
- 688. Wappler T, Smith VS, Dalgleish RC. Scratching an ancient itch: an Eocene bird louse fossil. Proceedings of the Royal Society of London, B. 2004;271(Supplement 5):S255–S258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689. Mey E. Psittacobrosus bechsteini: ein neuer ausgestorbener Federling (Insecta, Phthiraptera, Amblycera) vom Dreifarbenara Ara tricolor (Psittaciiformes), nebst einer annotierten Übersicht über fossile und rezent ausgestorbene Tierläuse. Anzeiger des Vereins Thüringer Ornithologen. 2005;5:201–217. [Google Scholar]
- 690. Rasnitsyn AP. 2.2.1.2.4.1. Order Psocida Leach, 1815. The booklice (= Psocoptera Shipley, 1904 = Copeognatha Enderlein, 1903) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 128–131. [Google Scholar]
- 691. Azar D, Hajar L, Indary C, Nel A. Paramesopsocidae, a new Mesozoic psocid family (Insecta: Psocodea "Psocoptera": Psocomorpha). Annales de la Société entomologique de France (Nouvelle série). 2008;44(4):459–470. [Google Scholar]
- 692. Nel A, Prokop J, de Ploëg G, Millet J. New Psocoptera (Insecta) from the lowermost Eocene amber of Oise, France. Journal of Systematic Palaeontology. 2005;3(4):371–391. [Google Scholar]
- 693. Lienhard C, Smithers CN. Psocoptera (Insecta): World catalogue and bibliography. Instrumenta Biodiversitatis. 2002;5:xli+745. [Google Scholar]
- 694. Azar D, Nel A, Néraudeau D. A new Cretaceous psocodean family from the Charente-Maritime amber (France) (Insecta, Psocodea, Psocomorpha). Geodiversitas. 2009;31(1):117–127. [Google Scholar]
- 695. Baz A, Ortuño VM. Archaeatropidae, a new family of Psocoptera from the Cretaceous amber of Alava, northern Spain. Annals of the Entomological Society of America. 2000;93(3):367–373. [Google Scholar]
- 696. Perrichot V, Azar D, Néraudeau D, Nel A. New Psocoptera in the early Cretaceous amber of SW France and Lebanon (Insecta: Psocoptera: Trogiomorpha). Geological Magazine. 2003;140(6):669–683. [Google Scholar]
- 697. Azar D, Nel A. Four new Psocoptera from Lebanese amber (Insecta: Psocomorpha: Trogiomorpha). Annales de la Société entomologique de France (Nouvelle série). 2004;40(2):185–192. [Google Scholar]
- 698. Brasero N, Nel A, Michez D. Insects from the early Eocene amber of Oise (France): diversity and palaeontological significance. Denisia. 2009;26:41–52. [Google Scholar]
- 699. Huang DY, Nel A, Azar D, Nel P. Phylogenetic relationships of the Mesozoic paraneopteran family Archipsyllidae (Insecta: Psocodea). Geobios. 2008;41(4):461–464. [Google Scholar]
- 700. Hong YC. Amber insects of China. Scientific and Technological Publishing House, Beijing; 2002. [Google Scholar]
- 701. Nel A, Waller A. The first fossil Compsocidae from Cretaceous Burmese amber (Insecta, Psocoptera, Troctomorpha). Cretaceous Research. 2007;28(6):1039–1041. [Google Scholar]
- 702. Baz A, Ortuño VM. New genera and species of empheriids (Psocoptera: Empheriidae) from the Cretaceous amber of Alava, northern Spain. Cretaceous Research. 2001;22(5):575–584. [Google Scholar]
- 703. Engel MS, Perkovsky EE. Psocoptera (Insecta) in Eocene Rovno amber (Ukraine). Vestnik zoologii. 2006;40(2):175–179. [Google Scholar]
- 704. Grimaldi DA, Engel MS. Fossil Liposcelididae and the lice ages (Insecta: Psocodea). Proceedings of the Royal Society, B. 2006;273(1586):625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705. Peñalver E, Nel A, Martínez-Delclòs X. Insectos del Mioceno inferior de Ribesalbes (Castellón, Spain). Paleoptera y Neoptera poli- y paraneoptera. Treballs del Museu de Geología de Barcelona. 1996;5:15–95. [Google Scholar]
- 706.Krumbiegel G. Der Bitterfelder Bernstein (Succinit). Lausitzer und Mitteldeutsche Bergbau-Verwaltungsgesellschaft mbH; 1997.
- 707. Mockford EL. Species of Philotarsus from north and middle America and a new philotarsine genus from Mexicao, Guatemala, and the Greater Antilles (Psocoptera: Philotarsidae: Philotarsinae). Journal of the New York Entomological Society. 2007;114(3):108–139. [Google Scholar]
- 708. Ross AJ, York PV. A list of type and figured specimens of insects and other inclusions in Burmese amber. Bulletin of The Natural History Museum, Geology Series. 2000;56(1):11–20. [Google Scholar]
- 709. Grimaldi DA, Engel MS. Extralimital fossils of the “Gondwanan” family Sphaeropsocidae (Insecta: Psocodea). American Museum Novitates. 2006;3523:1–18. [Google Scholar]
- 710. Casasola González JA. Phylogenetic relationships of the genera of Epipsocetae (Psocoptera: Psocomorpha). Zootaxa. 2006;1194:1–32. [Google Scholar]
- 711. Haliday AH. An Epitome of the British genera, in the order Thysanoptera, with indications of a few species. Entomological Magazine. 1836;3:439–451. [Google Scholar]
- 712. Mound LA, Morris DC. The insect order Thysanoptera: classification versus systematics. Zootaxa. 2007;1668:395–411. [Google Scholar]
- 713. Grimaldi DA, Shmakov A, Fraser NC. Mesozoic thrips and early evolution of the order Thysanoptera (Insecta). Journal of Paleontology. 2004;78(5):941–952. [Google Scholar]
- 714. Shmakov AS. The oldest members of the families Aeolothripidae and Thripidae (Insecta: Thysanoptera) from the Lower Cretaceous of Transbaikalia. Paleontological Journal. 2009;43(4):428–432. [Google Scholar]
- 715. Schliephake G. Weitere neue Funde fossiler Fransenflügler aus dem Baltischen Bernstein (Insecta, Thysanoptera). Mitteilungen aus dem Geologisch-Paläontologischen Institut der Universität Hamburg. 2001;85:197–201. [Google Scholar]
- 716. Shmakov AS. The Jurassic thrips Liassothrips crassipes (Martynov, 1927) and its taxonomic position in the order Thysanoptera (Insecta). Paleontological Journal. 2008;42(1):47–52. [Google Scholar]
- 717. Nel P, Azar D, Nel A. A new ‘primitive’ family of thrips from early Cretaceous Lebanese amber (Insecta, Thysanoptera). Cretaceous Research. 2007;28(6):1033–1038. [Google Scholar]
- 718. Zherikhin VV. 2.2.1.2.4.3. Order Thripida Fallen, 1914. (= Thysanoptera Haliday, 1836) The thrips In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 133–143. [Google Scholar]
- 719. Beckemeyer RJ. A new species of the extinct family Lophioneuridae from the Lower Permian Wellington Formation of Noble County, Oklahoma. Journal of the Kansas Entomological Society. 2004;77(2):132–136. [Google Scholar]
- 720. Rasnitsyn AP, Zherikhin VV. First fossil chewing louse from the Lower Cretaceous of Baissa, Transbaikalia (Insecta, Pediculida = Phthiriaptera, Saurodectidae fam.n.). Russian Entomological Journal. 2000;8 [for 1999](4):253–255. [Google Scholar]
- 721. Tan JJ, Ren D, Shih CK. New beetles (Insecta: Coleoptera: Archostemata) from the late Mesozoic of North China. Annales zoologici. 2007;57(2):231–247. [Google Scholar]
- 722. Béthoux O. The earliest beetle identified. Journal of Paleontology. 2009;83(6):931–937. [Google Scholar]
- 723. Thomson CG. Skandinaviens Coleoptera, synoptiskt bearbetade, Vol. 1 Berlingska Boktryckeriet, Lund; 1859. [Google Scholar]
- 724. Perkovsky EE. The systematic position of the Lower Cretaceous beetle Mesecanus parvus (Coleoptera, Staphylinoidea) from Turga. Vestnik zoologii. 2001;35(4):79–81. [Google Scholar]
- 725. Kirejtshuk AG, Azar D. New taxa of beetles (Insecta, Coleoptera) from Lebanese amber with evolutionary and systematic comments. Alavesia. 2008;2:15–46. [Google Scholar]
- 726. Soriano C, Gratshev VG, Delclòs X. New early Cretaceous weevils (Insecta, Coleoptera, Curculionoidea) from El Montsec, Spain. Cretaceous Research. 2006;27(4):555–564. [Google Scholar]
- 727. Kubisz D. Fossil beetles (Coleoptera) from Baltic amber in the collection of the Museum of Natural History of ISEA in Kraków. Polskie Pismo Entomologiczne. 2000;69(2):225–230. [Google Scholar]
- 728. Beattie R. The geological setting and palaeoenvironmental and palaeoecological reconstructions of the Upper Permian insect beds at Belmont, New South Wales, Australia. African Invertebrates. 2007;48(1):41–57. [Google Scholar]
- 729. Zherikhin VV, Gratshev VG. Fossil curculionoid beetles (Coleoptera, Curculionoidea) from the Lower Cretaceous of northeastern Brazil. Paleontological Journal. 2004;38(5):528–537. [Google Scholar]
- 730.Legalov AA. Annotated checklist of Recent and fossil species of the family Belidae
- 731. Coleoptera from the world fauna. Amurian zoological journal. 2009;1(4):296–324. [Google Scholar]
- 732. Winkler JR. Berendtimiridae fam. n., a new family of fossil beetles from Baltic amber (Coleoptera, Cantharoidea). Mitteilungen der Münchner Entomologischen Gesellschaft. 1987;77:51–59. [Google Scholar]
- 733. Kupryjanowicz J. Arthropods in Baltic amber and their photographic record In: Kosmowska-Ceranowicz B, editor. The amber treasure trove. Oficyna Wydawnicza Sadyba, Warsaw; 2001. p. 19–72. [Google Scholar]
- 734. Zherikhin VV. Tertiary brachycerid weevils (Coleoptera: Brachyceridae) from the collections of Muséum Nationale d’Histoire Naturelle, Paris, with a review of other fossil Brachyceridae. Paleontological Journal. 2000;34(Suppl. 3):S333–S343. [Google Scholar]
- 735. Costa C, Vanin SA, Lawrence JF, Ide S, Branham MA. Review of the family Brachypsectridae (Coleoptera: Elateroidea). Annals of the Entomological Society of America. 2006;99(3):409–432. [Google Scholar]
- 736. Legalov AA. A review of fossil and recent species of the family Ithyceridae (Coleoptera) from the world fauna. Amurian zoological journal. 2009;1(2):117–131. [Google Scholar]
- 737. Bouchard P, Bousquet Y, Davies AE, Alonso-Zarazaga MA, Lawrence JF, Lyal CHC, et al. Family-group names in Coleoptera (Insecta). ZooKeys. 2011;88:1–972. 10.3897/zookeys.88.807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738. Jarzembowski EA. A provisional checklist of fossil insects from the Purbeck Beds of Dorset. Proceedings of the Dorset Natural History and Archaeological Society. 1992;114:175–179. [Google Scholar]
- 739. Ponomarenko AG. 2.2.1.3.2. Superorder Scarabaeidea Laicharting, 1781. Order Coleoptera Linné, 1758. The Beetles In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 164–176. [Google Scholar]
- 740. Tan JJ, Ren D. Two exceptionally well-preserved catiniids (Coleoptera: Archostemata: Catiniidae) from the late Mesozoic of northeastern China. Annals of the Entomological Society of America. 2007;100(5):666–672. [Google Scholar]
- 741. Zhang JF. The first find of chrysomelids (Insecta: Coleoptera: Chrysomeloidea) from Callovian-Oxfordian Daohugou biota of China. Geobios. 2005;38(6):865–871. [Google Scholar]
- 742. Ponomarenko AG. New Triassic beetles (Coleoptera) from northern European Russia. Paleontological Journal. 2008;42(6):600–606. [Google Scholar]
- 743. Chang HL, Kirejtshuk AG, Ren D, Shih CK. First fossil click beetles from the Middle Jurassic of Inner Mongolia, China (Coleoptera: Elateridae). Annales zoologici. 2009;59(1):7–14. [Google Scholar]
- 744. Kirejtshuk AG, Azar D, Beaver RA, Mandelshtam MY, Nel A. The most ancient bark beetle known: a new tribe, genus and species from Lebanese amber (Coleoptera, Curculionidae, Scolytinae). Systematic Entomology. 2009;34(1):101–112. [Google Scholar]
- 745. Kirejtshuk AG, Nel A. New beetles of the suborder Polyphaga from the lowermost Eocene French amber (Isecta: Coleoptera). Annales de la Société entomologique de France (Nouvelle série). 2008;44(4):419–442. [Google Scholar]
- 746. Böcher J. Palaeoentomology of the Kap København Formation, a Plio-Pleistocene sequence in Peary Land, North Greenland. Meddelelser om Grønland, Geoscience. 1995;33:1–82. [Google Scholar]
- 747. Ponomarenko AG. Two new species of Mesozoic dytiscoid beetles from Asia. Paleontological Journal. 1994;27 [for 1993](1A):182–191. [Google Scholar]
- 748. Wang B, Ponomarenko AG, Zhang HC. A new coptoclavid larva (Coleoptera: Adephaga: Dytiscoidea) from the Middle Jurassic of China, and its phylogenetic implication. Paleontological Journal. 2009;43(6):652–659. [Google Scholar]
- 749. Wolf-Schwenninger K, Schawaller W. 11.17 Coleoptera: beetles In: Martill DM, Bechly G, Loveridge RF, editors. The Crato Fossil Beds of Brazil: Window into an Ancient World. Cambridge University Press; 2007. p. 340–350. [Google Scholar]
- 750. Gratshev VG, Zherikhin VV. The fossil record of weevils and related beetle families (Coleoptera, Curculionoidea). Acta zoologica cracoviensia. 2003;46(suppl.–Fossil Insects):129–138. [Google Scholar]
- 751. Kirejtshuk AG, Azar D, Tafforeau P, Boistel R, Fernandez V. New beetles of Polyphaga (Coleoptera, Polyphaga) from Lower Cretaceous Lebanese amber. Denisia. 2009;26:119–130. [Google Scholar]
- 752. Hava J, Prokop J, Herrmann A. New fossil dermestid beetles (Coleoptera: Dermestidae) from the Baltic amber. Acta Societatis Zoologicae Bohemicae. 2006;69:281–287. [Google Scholar]
- 753. Britt BB, Scheetz RD, Dangerfield A. A suite of dermestid beetle traces on dinosaur bone from the Upper Jurassic Morrison Formation, Wyoming, USA. Ichnos. 2008;15(2):59–71. [Google Scholar]
- 754. Nazarenko VY, Perkovsky EE. A new genus and species of dryophthorid weevils (Coleoptera, Dryophthoridae: Stromboscerinae) from the Rovno amber. Paleontological Journal. 2009;43(9):1097–1100. [Google Scholar]
- 755. Martins-Neto RG, Gallego OF, Mancuso AC. The Triassic insect fauna from Argentina. Coleoptera from the Los Rastros Formation (Bermejo Basin), La Rioja Province. Ameghiniana. 2006;43(3):591–609. [Google Scholar]
- 756. Schmied H, Wappler T, Kolibác J. A new bark-gnawing beetle (Coleoptera, Trogossitidae) from the middle Eocene of Europe, with a checklist of fossil Trogossitidae. Zootaxa. 2009;1993:17–26. [Google Scholar]
- 757.Krell FT. Catalogue of fossil Scarabaeoidea (Coleoptera: Polyphaga) of the Mesozoic and Tertiary. Denver Museum of Nature and Science Technical Report 2007–8; 2007.
- 758. Prokop J, Nel A, Hájek J, Bubík M. First record of a fossil beetle (Coleoptera, Haliplidae) from the basal Paleocene flysch sediments in the Magura Unit (Outer Western Carpathians, Moravia). Geologica Carpathica. 2004;55(6):469–473. [Google Scholar]
- 759. Kirejtshuk AG, Poinar GO. Haplochelidae, a new family of Cretaceous beetles (Coleoptera: Myxophaga) from Burmese amber. Proceedings of the Entomological Society of Washington. 2006;108(1):155–164. [Google Scholar]
- 760. Wegrzynowicz P. Systematic position of the genus Tarrodacne Zhang, 1989 (Coleoptera: Helotidae non Erotylidae). Annales Zoologici. 2007;57(4):757–758. [Google Scholar]
- 761. Ponomarenko AG. Beetles. Scarabaeida (= Coleoptera). The Joint Soviet-Mongolian Palaeontological Expedition. 1986;28:84–105. [Google Scholar]
- 762. Poinar GO, Brown AE. Pantostictus burmanicus, a new genus and species of Cretaceous beetles (Coleoptera: Hydrophiloidea: Histeridae) in Burmese amber. Proceedings of the Entomological Society of Washington. 2009;111(1):38–46. [Google Scholar]
- 763. Ponomarenko AG. Ecological evolution of beetles. Acta zoologica cracoviensia. 2003;46(suppl.—Fossil Insects):319–328. [Google Scholar]
- 764.Hörnschemeyer T. Archostemata Kolbe, 1908. In: Beutel RG, editor. Morphology and systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim). Handbuch der Zoologie, 4: Arthropoda, 2. Insecta. Coleoptera, beetles, Part 38, Volume 1; 2005. p. 29–42.
- 765. Ponomarenko AG. New beetles from the Permian of European Russia. Paleontological Journal. 2000;34(Suppl. 3):S312–316. [Google Scholar]
- 766. Pütz A, Hernando C, Ribera I. A new genus of Limnichidae (Coleoptera) from Baltic amber. Insect Systematics & Evolution. 2004;35(3):323–334. [Google Scholar]
- 767. Hong YC. A new early Cretaceous beetle family—Magnocoleidae fam. nov. (Insecta: Coleoptera) in Hebei Province. Geoscience. 1998;12(1):40–49. [Google Scholar]
- 768. Engel MS. An Eocene ectoparasite of bees: The oldest definitive record of phoretic meloid triungulins (Coleoptera: Meloidae; Hymenoptera: Megachilidae). Acta zoologica cracoviensia. 2005;48B(1–2):43–48. [Google Scholar]
- 769. Liu M, Zhao YY, Ren D. Discovery of three new mordellids (Coleoptera, Tenebrionoidea) from the Yixian Formation of western Liaoning, China. Cretaceous Research. 2008;29(3):445–450. [Google Scholar]
- 770. Kirejtshuk AG, Nel A. New genera and species of Cucujiformia (Coleoptera, Polyphaga) from lowermost Eocene French amber. Denisia. 2009;26:103–118. [Google Scholar]
- 771. Soriano C. First record of the family Belidae (Insecta, Coleoptera) in amber. New genus and species from the uppermost Albian amber of France. Geodiversitas. 2009;31(1):99–104. [Google Scholar]
- 772. Legalov AA. Annotaed checklist of fossil and Recent species of the family Nemonychidae (Coleoptera from the world fauna). Amurian zoological journal. 2009;1(3):200–213. [Google Scholar]
- 773. Kirejtshuk AG. A current generic classification of sap beetles (Coleoptera, Nitidulidae). Zoosystematica Rossica. 2008;17(1):107–122. [Google Scholar]
- 774. Zherikhin VV, Gratshev VG. Obrieniidae, fam. nov., the oldest Mesozoic weevils (Coleoptera, Curculionoidea). Paleontological Journal. 1994;27(1A):50–69. [Google Scholar]
- 775. Kirejtshuk AG. Parandrexidae fam. nov., Jurassic beetles of the Infraorder Cucujiformia (Coleoptera, Polyphaga). Paleontological Journal. 1994;28(1):69–78. [Google Scholar]
- 776. Soriano C, Kirejtshuk AG, Delclòs X. The Mesozoic Laurasian family Parandrexidae (Insecta: Coleoptera), new species from the Lower Cretaceous of Spain. Comptes Rendus Palevol. 2006;5(6):779–784. [Google Scholar]
- 777. Ponomarenko AG, Mostovski MB. New beetles (Insecta: Coleoptera) from the late Permian of South Africa. African Invertebrates. 2005;46:253–260. [Google Scholar]
- 778. Lawrence JF, Archibald SB, Slipinski A. A new species of Prionoceridae from the Eocene of British Columbia. Annales zoologici. 2008;58(4):689–693. [Google Scholar]
- 779. Engel MS, Grimaldi DA. A jugular-horned beetle in Cretaceous amber from Myanmar (Coleoptera: Prostomidae). Alavesia. 2008;2:215–218. [Google Scholar]
- 780. Soriano C, Delclòs X, Ponomarenko AG. Beetle associations (Insecta: Coleoptera) from the Barremian (Lower Cretaceous) of Spain. Alavesia. 2007;1:81–88. [Google Scholar]
- 781. Alonso J, Arillo A, Barrón E, Corral JC, Grimalt J, López JF, et al. A new fossil resin with biological inclusions in Lower Cretaceous deposits from Álava (northern Spain, Basque-Cantabrian Basin). Journal of Paleontology. 2000;74(1):158–178. [Google Scholar]
- 782. Tan JJ, Ren D. Mesozoic archostematan fauna from China Science Press, Beijing; 2009. [Google Scholar]
- 783. Perrichot V, Nel A, Néraudeau D. Two new wedge-shaped beetles in Albo-Cenomanian ambers of France (Coleoptera: Ripiphoridae: Ripiphorinae). European Journal of Entomology. 2004;101(4):577–581. [Google Scholar]
- 784. Bellamy CL. Buprestidae (Coleoptera) from amber deposits: a brief review and family switch. Coleopterists Bulletin. 1995;42(2):175–177. [Google Scholar]
- 785. Kirejtshuk AG. A new genus and species of Sphaeriusidae (Coleoptera, Myxophaga) from Lower Cretaceous Burmese amber. Denisia. 2009;26:99–102. [Google Scholar]
- 786. Hörnschemeyer T, Stapf H. Die Insektentaphozönose von Niedermoschel (Asselian, unt. Perm; Deutschland). Terra Nostra Schriften der Alfred-Wegener-Stiftung. 1999;99/8:98. [Google Scholar]
- 787. Zherikhin VV. Podotryad Polyphaga [Suborder Polyphaga]. Trudy Paleontologicheskogo instituta Akademiia nauk SSSR. 1993;252:20–37. [Google Scholar]
- 788. Oberprieler RG, Marvaldi AE, Anderson RS. Weevils, weevils, weevils everywhere. Zootaxa. 2007;1668:419–520. [Google Scholar]
- 789. Grimaldi DA. A stalk-eyed ephydroid fly from the Eocene (Diptera: Ephydroidea: Camillidae). Proceedings of the Entomological Society of Washington. 2008;110(3):543–550. [Google Scholar]
- 790. Evenhuis NL. Catalogue of the fossil flies of the World. Backhuys, Leiden; 1994. [Google Scholar]
- 791. von Tschirnhaus M, Hoffeins C. Fossil flies in Baltic amber—insights in the diversity of Tertiary Acalyptratae (Diptera, Schizophora), with new morphological characters and a key based on 1,000 collected inclusions. Denisia. 2009;26:171–212. [Google Scholar]
- 792. Hauser M, Winterton SL. A new fossil genus of small-headed flies (Diptera: Acroceridae: Philopotinae) from Baltic amber. Annals of the Entomological Society of America. 2007;100(2):152–156. [Google Scholar]
- 793. Zlobin VV. The fossil limestone cyclorrhaphous Diptera Limestone of the Isle of Wight. International Journal of Dipterological Research. 2007;18(2):129–136. [Google Scholar]
- 794. Krzeminski W, Lukashevitch L. Ansorgiidae, a new family from the Upper Cretaceous of Kazakhstan (Diptera, Ptychopteromorpha). Acta zoologica cracoviensia. 1993;35:593–596. [Google Scholar]
- 795.Krzeminski W, Evenhuis NL. Review of Diptera palaeontological records. In: Papp L, Darvas B, editors. Contributions to a Manual of Palaearctic Diptera, Volume 1, General and applied Dipterology. Science Herald, Budapest; 2000. p. 535–564.
- 796. Michelsen V. Oldest authentic record of a fossil calyptrate fly (Diptera): a species of Anthomyiidae from early Coenozoic Baltic amber. Studia dipterologica. 2000;7(1):11–18. [Google Scholar]
- 797. Nagatomi A, Saigusa T, Nagatomi H, Lyneborg L. Apsilocephalidae, a new family of the orthorrhaphous Brachycera (Insecta, Diptera). Zoological Science. 1991;8:579–591. [Google Scholar]
- 798. Gaimari SD, Mostovski MB. Burmapsilocephala cockerelli, a new genus and species of Asiloidea (Diptera) from Burmese amber. Bulletin of The Natural History Museum, Geology Series. 2000;56(1):43–45. [Google Scholar]
- 799. Nagatomi A, Liu N. Apystomyiidae, a new family of Asiloidea (Diptera). Acta Zoologica Academiae Scientiarum Hungaricae. 1994;40:203–218. [Google Scholar]
- 800. Mostovski MB. Brachyceran assemblages (Insecta: Diptera) as indicators of terrestrial palaeoenvironments in the late Mesozoic. Palaeontologia africana. 2009;44:121–125. [Google Scholar]
- 801. Zhang KY, Li JH, Yang D, Ren D. A new species of Archirhagio Rohdendorf, 1938 from the Middle Jurassic of Inner Mongolia of China (Diptera: Archisargidae). Zootaxa. 2009;1984:61–65. [Google Scholar]
- 802. Nagatomi A, Yang D. A review of extinct Mesozoic genera and families of Brachycera (Insecta, Diptera, Orthorrhapha. Entomologist’s Monthly Magazine. 1998;134:95–192. [Google Scholar]
- 803. Dikow T. Phylogeny of Asilidae inferred from morphological characters of imagines (Insecta: Diptera: Brachycera: Asiloidea). Bulletin of the American Museum of Natural History. 2009;319:1–175. [Google Scholar]
- 804. Hong YC, Wang WL. Fossil insects from the Laiyang Basin, Shandong Province In: The Stratigraphy and Palaeontology of Laiyang Basin, Shandong Province: Shandong Bureau of Geology and Mineral Resources; 1990. p. 44–189. [Google Scholar]
- 805. Mostovski MB, Jarzembowski EA, Coram RA. Horseflies and anthericids (Diptera: Tabanidae, Athericidae) from the Lower Cretaceous of England and Transbaikalia. Paleontological Journal. 2003;37(2):162–169. [Google Scholar]
- 806. Zhang JF. First description of axymyiid fossils (Insecta: Diptera: Axymyiidae). Geobios. 2004;37(5):687–694. [Google Scholar]
- 807. Zhang JF, Lukashevitch ED. The oldest known net-winged midges (Insecta: Diptera: Blephariceridae) from the late Mesozoic of northeast China. Cretaceous Research. 2007;28(2):302–309. [Google Scholar]
- 808. Blagoderov V, Grimaldi DA. Fossil Sciaroidea (Diptera) in Cretaceous ambers, exclusive of Cecidomyiidae, Sciaridae, and Keroplatidae. American Museum Novitates. 2004;3433:1–76. [Google Scholar]
- 809. Evenhuis NL. Catalog of the Mythicomyiidae of the world (Insecta: Diptera. Bishop Museum Bulletin in Entomology. 2002;10:1–85. [Google Scholar]
- 810. Rognes K. The Calliphoridae (blowflies) (Diptera: Oestroidea) are not a monophyletic group. Cladistics. 1997;13:27–66. [DOI] [PubMed] [Google Scholar]
- 811. Grimaldi DA, Cumming J. Brachyceran Diptera in Cretaceous ambers and Mesozoic diversification of the Eremoneura. Bulletin of the American Museum of Natural History. 1999;239:1–124. [Google Scholar]
- 812. Jaschhof M. A neontologist’s review of two recently published articles on inclusions of Lestremiinae (Diptera: Cecidomyiidae) in Rovno amber. Paleontological Journal. 2007;41(1):103–106. [Google Scholar]
- 813. Mostovski MB, Ross AJ, Szadziewski R, Krzeminski W. Redescription of Simulidium priscum Westwood and Pseudosimulium humidum (Brodie) (Insecta: Diptera: Rhagionidae) from the Purbeck Limestone Group (Lower Cretaceous) of England. Journal of Systematic Palaeontology. 2003;1(1):59–64. [Google Scholar]
- 814. Borkent A. Upper and Lower Cretaceous biting midges (Ceratopogonidae: Diptera) from Hungarian and Austrian amber and the Koonwarra fossil bed of Australia. Stuttgarter Beiträge zur Naturkunde Serie B (Geologie und Paläontologie). 1997;249:1–10. [Google Scholar]
- 815. Borkent A. The frog-biting midges of the world (Corethrellidae: Diptera). Zootaxa. 2008;1804:1–456. [Google Scholar]
- 816. Grimaldi DA, Cumming JM, Arillo A. Chimeromyiidae, a new family of eremoneuran Diptera from the Cretaceous. Zootaxa. 2009;2078:34–54. [Google Scholar]
- 817. Blagoderov V, Grimaldi DA, Fraser NC. How time flies for flies: diverse Diptera from the Triassic of Virginia and early radiation of the order. American Museum Novitates. 2007;3572:1–39. [Google Scholar]
- 818. Stuke JH. Eine neue Blasenkopffliege der Gattung Palaeomyopa Meunier aus dem Baltischen Bernstein (Diptera: Conopidae). Studia dipterologica. 2003;10(1):91–96. [Google Scholar]
- 819. Mazzarolo LA, Amorim DS. Cratomyia macrorrhyncha, a Lower Cretaceous brachyceran fossil from the Santana Formation, Brazil, representing a new species, genus and family of the Stratiomyomorpha (Diptera). Insect Systematics & Evolution. 2000;31(1):91–102. [Google Scholar]
- 820. Willkommen J, Grimaldi DA. 11.20 Diptera: true flies, gnats and crane flies In: The Crato Fossil Beds of Brazil. Cambridge University Press; 2007. p. 369–387. [Google Scholar]
- 821. Martin SK. A new protorhyphid fly (Insecta: Diptera: Protorhyphidae) from the Lower Jurassic of the Perth Basin, western Australia. Alavesia. 2008;2:253–257. [Google Scholar]
- 822. Poinar GO, Zavortink TJ, Pike T, Johnston PA. Paleoculicis minutus (Diptera: Culicidae) n. gen., n. sp., from Cretaceous Canadian amber, with a summary of described fossil mosquitoes. Acta Geologica Hispanica. 2000;35(1–2):119–128. [Google Scholar]
- 823. Harbach RE. The Culicidae (Diptera): a review of taxonomy, classification and phylogeny. Zootaxa. 2007;1668:591–638. [Google Scholar]
- 824. Kirk-Spriggs AH. A reappraisal of the type fossil of Curtonotum gigas Théobald, 1937 (Diptera: Curtonotidae), a compression fossil of Early Oligocene age from Provence, France. Annals of the Eastern Cape Museums. 2007;6:13–20. [Google Scholar]
- 825. Haenni JP. Fossil Diptera in Baltic amber: the collection of the Muséum d’histoire naturelle Neuchâtel. Acta zoologica cracoviensia. 2003;46(suppl.- Fossil Insects):407–410. [Google Scholar]
- 826. Kotrba M. Prosphyracephala kerneggeri spec. nov.—a new stalk-eyed fly from Baltic amber. Spixiana. 2009;32(2):187–197. [Google Scholar]
- 827. Krzeminski W. The oldest Polyneura (Diptera) and their importance to the phylogeny of the group. Acta zoologica cracoviensia. 1992;35(1):45–52. [Google Scholar]
- 828. Lukashevitch ED. Mesozoic Dixidae (Insecta: Diptera) and systematic position of Dixamima Rohdendorf, 1964 and Rhaetomyia Rohdendorf, 1962. Paleontological Journal. 1996;30(1):46–51. [Google Scholar]
- 829. Krzeminska E, Blagoderov V, Krzeminski W. Elliidae, a new fossil family of the infraorder Axymyiomorpha (Diptera). Acta zoologica cracoviensia. 1993;35:581–591. [Google Scholar]
- 830. Blagoderov VA, Lukashevitch ED, Mostovski MB. 2.2.1.3.4.4. Order Diptera Linné, 1758. The true flies (= Muscida Laicharting, 1781) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 227–240. [Google Scholar]
- 831. Ren D, Guo ZG. A new genus and two new species of short-horned flies of Upper Jurassic from northeast China (Diptera: Eremochaetidae). Entomologia Sinica. 1995;2(4):300–307. [Google Scholar]
- 832. Zhang JF. Jurassic limoniid dipterans from China (Diptera: Limoniidae). Oriental Insects. 2006;40:115–126. [Google Scholar]
- 833. Krzeminski W, Krzeminska E, Papier F. Grauvogelia arzvilleriana sp. n. the oldest Diptera species (Lower/Middle Triassic of France). Acta zoologica cracoviensia. 1994;37(2):95–99. [Google Scholar]
- 834. Shcherbakov DE, Lukashevitch ED, Blagoderov VA. Triassic Diptera and initial radiation of the order. An International Journal of Dipterological Research. 1995;6(2):75–115. [Google Scholar]
- 835. Krzeminski W, Krzeminska E. Triassic Diptera: descriptions, revisions and phylogenetic relations. Acta zoologica cracoviensia. 2003;46(suppl.- Fossil Insects):153–184. [Google Scholar]
- 836. Lukashevitch ED, Huang DY, Lin QB. Rare families of lower Diptera (Hennigmatidae, Blephariceridae, Perissommatidae) from the Jurassic of China. Studia dipterologica. 2006;13(1):127–143. [Google Scholar]
- 837. Ansorge J, Krzeminski W. Revision of Mesorhyphus Handlirsch, Eoplecia Handlirsch and Heterorhyphus Bode (Diptera: Anisopodomorpha, Bibionomorpha) from the Upper Liassic of Germany. Paläontologische Zeitschrift. 1995;69(1/2):167–172. [Google Scholar]
- 838. Michelsen V. Hoffeinsmyiidae, a new extinct family of Schizophora (Diptera) from Baltic amber. Studia dipterologica. 2009;15 [for 2008](1/2):211–222. [Google Scholar]
- 839. Zhang JF. Some anisopodoids (Insecta: Diptera: Anisopodoidea) from the late Mesozoic deposits of northeast China. Cretaceous Research. 2007;28(2):281–288. [Google Scholar]
- 840. Peñalver E, Arillo A. A new species of the family Hybotidae in the Lower Cretaceous amber of El Caleyu (Asturias, Spain); Alavesia prietoi n. sp. Alavesia. 2007;1:63–68. [Google Scholar]
- 841. Krzeminski W. Triassic and Lower Jurassic stage of Diptera evolution. Mitteilungen der Schweizerischen entomologischen Gesellschaft. 1992;65:39–59. [Google Scholar]
- 842. Mostovski MB. New taxa of Ironomyiidae (Diptera, Phoromorpha) from the Cretaceous of Siberia and Mongolia [in Russian]. Paleontologicheskii Zhurnal. 1995;4:86–103. [Google Scholar]
- 843. Jarzembowski EA, Coram R. New fossil records from the Purbeck of Dorset and the Wealden of the Weald. Proceedings of the Dorset Natural History and Archaeological Society. 1996;1996:119–124. [Google Scholar]
- 844. Mostovski MB. On knowledge of fossil flies of the superfamily Archisargoidea (Diptera, Brachycera). Paleontological Journal. 1997;31(1):72–78. [Google Scholar]
- 845. Grimaldi DA, Triplehorn DM. Insects from the Upper Miocene Grubstake Formation of Alaska. American Museum Novitates. 2008;3612:1–19. [Google Scholar]
- 846. Zhang JF. Luanpingitidae—A new fossil insect family. Acta Palaeontologica Sinica. 1986;25(1):49–54. In Chinese, English summary. [Google Scholar]
- 847. Zhang ZQ. Diptera of China (Insecta): an annotated and indexed bibliography. Fauna of China. 2002;4:77–224. [Google Scholar]
- 848. Li TT, Ren D. A new fossil genus of Mesosciophilidae (Diptera, Nematocera) with two new species from the Middle Jurassic of Inner Mongolia, China. Progress in Natural Science. 2009;19(12):1837–1841. [Google Scholar]
- 849. Engel MS. A new interpretation of the oldest fossil bee (Hymenoptera: Apidae). American Museum Novitates. 2000;3296:1–11. [Google Scholar]
- 850. Whalley PES. The systematics and palaeogeography of the Lower Jurassic insects of Dorset, England. Bulletin of the British Museum (Natural History), Geology. 1985;39(3):107–189. [Google Scholar]
- 851. Lukashevitch ED. On the systematic position of Prodocidia (Diptera) from the Lower Lias of England. Paleontological Journal. 2000;34(Suppl. 3):S352–S354. [Google Scholar]
- 852. Lukashevitch ED. Ptychopteridae (Insecta: Diptera): History of its study and limits of the family. Paleontological Journal. 2008;42(1):66–74. [Google Scholar]
- 853. Blagoderov VA. Fungus gnats of the tribes Gnoristini and Leiini (Diptera, Mycetophilidae) from the early Cretaceous of Transbaikalia. Paleontological Journal. 1998;32(1):54–59. [Google Scholar]
- 854. Barraclough DA, McAlpine DK. Natalimyzidae, a new African family of acalyptrate flies (Diptera: Schizophora: Sciomyzoidea). African Invertebrates. 2006;47:117–134. [Google Scholar]
- 855. Wagner R, Hoffeins C, Hoffeins HW. A fossil nymphomyiid (Diptera) from the Baltic and Bitterfelder amber. Systematic Entomology. 2000;25(1):115–120. [Google Scholar]
- 856. Krzeminski W, Ansorge J. A new rhagionid fly from the Lower Jurassic of Germany (Diptera: Brachycera: Rhagionidae). Polskie Pismo Entomologiczne. 2005;74(3):369–372. [Google Scholar]
- 857. Coram RA, Jarzembowski EA, Mostovski MB. Two rare eremoneuran flies (Diptera: Empididae and Opetiidae) from the Purbeck Limestone Group. Paleontological Journal. 2000;34(Suppl. 3):S370–S373. [Google Scholar]
- 858.Mostovski MB. A brief review of brachycerous flies (Diptera, Brachycera) in the Mesozoic, with descriptions of some curious taxa. In: Scoggin M, editor. AMBA projects AM/PFICM98/1.99: Proceedings of the First International Palaeoentomological Conference, Moscow 1998; 1999. p. 103–110.
- 859.Blagoderov VA. New Bibionomorpha from the Triassic of Australia and Jurassic of Central Asia with notes on Paraxymyiidae Rohdendorf (Insecta, Diptera). In: Scoggin M, editor. AMBA projects AM/PFICM98/1.99: Proceedings of the First International Palaeoentomological Conference, Moscow 1998; 1999. p. 11–15.
- 860. Gentilini G, Korneyev VA, Kameneva EP. Fossil tephritoid flies (Diptera: Pallopteridae, Ulidiidae, Tephritidae) from the Upper Miocene of Monte Castellaro, Italy, and a review of fossil European Tephtitoids. Instrumenta Biodiversitatis. 2006;7:85–104. [Google Scholar]
- 861. Sabrosky CW, Thompson FC, Evenhuis NL. Family-group names in Diptera. Myia. 1999;10:1–576. [Google Scholar]
- 862. Coram RA, Jarzembowski EA. New fossil flies (Insecta: Diptera) from the Purbeck Limestone Group (Lower Cretaceous, Berriasian) of Dorset, UK. Cretaceous Research. 1999;20:853–861. [Google Scholar]
- 863. Ren D. Late Jurassic Brachycera from northeastern China. Acta Zootaxonomica Sinica. 1998;23(1):65–82. [Google Scholar]
- 864. Zhang KY, Yang D, Ren D. Notes on the extinct family Protapioceridae, with description of a new species from China (Insecta: Diptera: Asiloidea). Zootaxa. 2007;1530:27–32. [Google Scholar]
- 865. Zhang HC, Wang B, Fang Y. Evolution of insect diversity in the Jehol Biota. Science China Earth Sciences. 2010;53(12):1908–1917. [Google Scholar]
- 866. Zhang KY, Yang D, Ren D. The first Middle Jurassic Protobrachyceron Handlirsch fly (Diptera: Brachycera: Protobrachyceridae) from Inner Mongolia (China). Zootaxa. 2008;1879:61–64. [Google Scholar]
- 867. Zhang JF. New Mesozoic Protopleciidae (Insecta: Diptera: Nematocera) from China. Cretaceous Research. 2007;28(2):289–296. [Google Scholar]
- 868. Amorim DS. Catalogue of neotropical Diptera. Scatopsidae. Neotropical Diptera. 2008;4:1–17. [Google Scholar]
- 869. Jaschhof M, Didham RK. Rangomaramidae fam. nov. from New Zealand and implications for the phylogeny of the Sciaroidea (Diptera: Bibionomorpha). Studia Dipterologica Supplement. 2002;11:1–60. [Google Scholar]
- 870. Rindal DSAE. Phylogeny of the Mycetophiliformia, with proposal of the subfamilies Heterotrichinae, Ohakuneinae, and Chiletrichinae for the Rangomaramidae (Diptera, Bibionomorpha). Zootaxa. 2007;1535:1–92. [Google Scholar]
- 871. Chandler P. Heterotricha Loew and allied genera (Diptera: Sciaroidea): offshoots of the stem group of Mycetophilidae and or Sciaridae? Annales de la Société entomologique de France (Nouvelle série). 2002;38(1–2):101–144. [Google Scholar]
- 872. Krzeminski W, Krzeminska E. Rhaetaniidae, a new family of the Diptera from the Upper Triassic of Great Britain (Diptera: Nematocera). Annales zoologici. 2002;52(2):211–213. [Google Scholar]
- 873. Mostovski MB, Martínez-Delclòs X. New Nemestrinoidea (Diptera: Brachycera) from the Upper Jurassic-Lower Cretaceous of Eurasia, taxonomy and palaeobiology. Entomological Problems. 2000;31(2):137–148. [Google Scholar]
- 874. Wichard W, Weitschat W. Wasserinsketen im Bernstein—eine paläobiologische Studie. Entomologische Mitteilungen aus dem Löbbecke-Museum und Aquazoo. 1996;4:1–122. [Google Scholar]
- 875. Grimaldi DA, Amorim DS, Blagoderov V. The Mesozoic family Archizelmiridae (Diptera: Insecta). Journal of Paleontology. 2003;77(2):368–381. [Google Scholar]
- 876. Blagoderov VV, Martínez-Delclòs X. Two new fungus gnats (Insecta, Diptera, Mycetophilidae) from the Lower Cretaceous of Spain. Geobios. 2001;34(1):63–67. [Google Scholar]
- 877. Brooks DR, Evenhuis NL. Serendipidae Evenhuis, 1994 (Insecta: Diptera) and Serendipidae Brooks and Barriga, 1995 (Platyhelminthes: Eucestoda): proposed removal of homonymy. Journal of Parasitology. 1995;81(5):762 [PubMed] [Google Scholar]
- 878. Kalugina NS, Kovalev VG. Two winged insects from the Jurassic of Siberia [in Russian]. Nauka, Moscow; 1985. [Google Scholar]
- 879. Arillo A, Peñalver E, García-Gimeno V. First fossil Litoleptis (Diptera: Spaniidae) from the Lower Cretaceous amber of San Just (Teruel Province, Spain). Zootaxa. 2009;2026:33–39. [Google Scholar]
- 880. Marshall SA, Buck M, Skevington JH, Grimaldi D. A revision of the family Syringogastridae (Diptera: Diopsoidea). Zootaxa. 2009;1996:1–80. [Google Scholar]
- 881. Ansorge J, Krzeminski W. Lower Jurassic tanyderids (Diptera: Tanyderidae) from Germany. Studia dipterologica. 2002;9(1):21–29. [Google Scholar]
- 882. Grimaldi DA, Arillo A. The Tethepomyiidae, a new family of enigmatic Cretaceous Diptera. Alavesia. 2008;2:259–265. [Google Scholar]
- 883. Wagner R, Barták M, Borkent A, Courtney G, Goddeeris B, Haenni JP, et al. Global diversity of dipteran families (Insecta Diptera) in freshwater (excluding Simulidae, Culicidae, Chironomidae, Tipulidae and Tabanidae). Hydrobiologia. 2008;595(1):489–519. [Google Scholar]
- 884.Lukashevitch ED, Shcherbakov DE. A new Triassic family of Dipera from Australia. In: Scoggin M, editor. AMBA projects AM/PFICM98/1.99: Proceedings of the First International Palaeoentomological Conference, Moscow 1998; 1999. p. 81–89.
- 885. Krzeminska E, Krzeminski W, Dahl C. Monograph of fossil Trichoceridae (Diptera): over 180 million years of evolution Institute of Systematics and Evolution of Animals, Kraków; 2009. [Google Scholar]
- 886. Amorim DS, Grimaldi DA. Valeseguyidae, a new family of Diptera in the Scatopsoidea, with a new genus in Cretaceous amber from Myanmar. Systematic Entomology. 2006;31(3):508–516. [Google Scholar]
- 887. Krzeminski W, Ansorge J. On Protobrachyceron Handlirsch, 1920 (Diptera: Brachycera) from the Lower Jurassic of Germany. Polskie Pismo Entomologiczne. 2000;69(2):231–237. [Google Scholar]
- 888. Hong YC, Guo XR. Two new Middle Triassic genera and species of Mesopanorpodidae from the Shaanxi (Insecta, Mecoptera). Acta Zootaxonomica Sinica. 2003;28(4):715–720. [Google Scholar]
- 889. Engel MS, Grimaldi DA. The first Cretaceous sclerogibbid wasp (Hymenoptera: Sclerogibbidae). American Museum Novitates. 2006;3515:1–7. [Google Scholar]
- 890. Zhang HC, Rasnitsyn AP. Middle Jurassic Praeaulacidae (Insecta: Hymenoptera: Evanioidea) of Inner Mongolia and Kazakhstan. Journal of Systematic Palaeontology. 2008;6(4):463–487. [Google Scholar]
- 891. Engel MS. Two ensign wasps in Cretaceous amber from New Jersey and Myanmar (Hymenoptera: Evaniidae). Polskie Pismo Entomologiczne. 2006;75(3):443–454. [Google Scholar]
- 892. Jennings JT, Korgmann L. A new species of Pristaulacus Kieffer (Hymenoptera: Aulacidae) from Baltic amber. Insect Systematics & Evolution. 2009;40(2):201–207. [Google Scholar]
- 893. Lopez-Vaamonde C, Wikström N, Kjer KM, Weiblen GD, Rasplus JY, Machado CA, et al. Molecular dating and biogeography of fig-pollinating wasps. Molecular Phylogenetics and Evolution. 2009;52(3):715–726. 10.1016/j.ympev.2009.05.028 [DOI] [PubMed] [Google Scholar]
- 894. Ohl M. The first fossil representative of the wasp genus Dolichurus, with a review of fossil Ampulicidae (Hymenoptera: Apoidea). Journal of the Kansas Entomological Society. 2004;77(4):322–342. [Google Scholar]
- 895. Ortega-Blanco J, Rasnitsyn AP, Delclòs X. First record of anaxyelid woodwasps (Hymenoptera: Anaxyelidae) in Lower Cretaceous Spanish amber. Zootaxa. 2008;1937:39–50. [Google Scholar]
- 896. Rasnitsyn AP, Martínez-Delclòs X. Wasps (Insecta: Vespida = Hymenoptera) from the early Cretaceous of Spain. Acta Geologica Hispanica. 2000;35(1–2):65–95. [Google Scholar]
- 897. Engel MS. A monograph of the Baltic amber bees and evolution of the Apoidea (Hymenoptera). Bulletin of the American Museum of Natural History. 2001;259:1–192. [Google Scholar]
- 898. Rasnitsyn AP. Hymenoptera Apocrita of the Mesozoic. Trudy Paleontologicheskogo institute Akademiia nauk SSSR. 1975;174:1–191. [in Russian]. [Google Scholar]
- 899. Bennett DJ, Engel MS. A new moustache wasp in Dominican amber, with an account of apoid wasp evolution emphasizing Crabroninae (Hymenoptera: Crabronidae). American Museum Novitates. 2006;3529:1–10. [Google Scholar]
- 900. Rasnitsyn AP, Jarzembowski EA, Ross AJ. Wasps (Insecta: Vespida = Hymenoptera) from the Purbeck and Wealden (Lower Cretaceous) of southern England and their biostratigraphical and palaeoenvironmental significance. Cretaceous Research. 1998;19:329–391. [Google Scholar]
- 901. Pulawski WJ, Rasnitsyn AP, Brothers DJ, Archibald SB. New genera of Angarosphecinae: Cretosphecium from early Cretaceous of Mongolia and Eosphecium from early Eocene of Canada (Hymenoptera: Sphecidae). Journal of Hymenoptera Research. 2000;9(1):34–40. [Google Scholar]
- 902. Perkovsky EE, Rasnitsyn AP, Vlaskin AP, Taraschuk MV. A comparative analysis of the Baltic and Rovno amber arthropod faunas: representative samples. African Invertebrates. 2007;48(1):229–245. [Google Scholar]
- 903. Osten T. 11.8 Hymenoptera: bees, wasps and ants In: Martill DM, Bechly G, Loveridge RF, editors. The Crato Fossil Beds of Brazil: Window into an Ancient World. Cambridge University Press; 2007. p. 350–365. [Google Scholar]
- 904. Rasnitsyn AP, Kovalev OV. Gall wasps from the early Cretaceous of Transbaikalia (Hymenoptera, Cynipoidea, Archaeocynipidae fam. n.) [in Russian]. Vestnik zoologii. 1988;1988(1):18–21. [Google Scholar]
- 905. Nel A. New and poorly known Cenozoic sawflies of France (Hymenoptera, Tenthredinoidea, Pamphilioidea). Deutsche entomologische Zeitschrift. 2004;51(2):253–269. [Google Scholar]
- 906. Archibald SB, Cover SP, Moreau CS. Bulldog ants of the Eocene Okanagan Highlands and history of the subfamily (Hymenoptera: Formicidae: Myrmeciinae). Annals of the Entomological Society of America. 2006;99(3):487–523. [Google Scholar]
- 907. Engel MS, Grimaldi DA. Primitive new ants in Cretaceous amber from Myanmar, New Jersey, and Canada (Hymenoptera: Formicidae). American Museum Novitates. 2005;3485:1–23. [Google Scholar]
- 908. Rasnitsyn AP. 2.2.1.3.5. Superorder Vespida Laicharting, 1781. Order Hymenoptera Linné, 1758 (= Vespida Laicharting, 1781) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 242–254. [Google Scholar]
- 909. Perrichot V, Nel A. Eocene bethylid wasps from French amber (Hymenoptera: Bethylidae). Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 2008;248(1):91–101. [Google Scholar]
- 910. Brothers DJ, Rasnitsyn AP. Diversity of Hymenoptera and other insects in the late Cretaceous (Turonian) deposits at Orapa, Botswana: a preliminary review. African Entomology. 2003;11(2):221–226. [Google Scholar]
- 911. Perrichot V, Nel A, Quicke DLJ. New braconid wasps from French Cretaceous amber (Hymenoptera, Braconidae): synonymization with Eoichneumonidae and implications for the phylogeny of Ichneumonoidea. Zoologica Scripta. 2009;38(1):79–88. [Google Scholar]
- 912. Heraty JM, Darling DC. Fossil Eucharitidae and Perilampidae (Hymenoptera: Chalcidoidea) from Baltic amber. Zootaxa. 2009;2306:1–16. [Google Scholar]
- 913. Perrichot V. Long-tailed wasps (Hymenoptera: Megalyridae) from Cretaceous and Paleogene European amber. Paleontological Contributions. 2009;1:1–35. [Google Scholar]
- 914. Rasnitsyn AP. Hymenopterous insects (Insecta: Vespida) in the Upper Jurassic deposits of Shar Teg, SW Mongolia. Russian Entomological Journal. 2008;17(3):299–310. [Google Scholar]
- 915. Rasnitsyn AP, Ansorge J, Zessin W. New hymenopterous insects (Insecta: Hymenoptera) from the Lower Toarcian (Lower Jurassic) of Germany. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 2003;227(3):321–342. [Google Scholar]
- 916. Liu ZW, Engel MS, Grimaldi DA. Phylogeny and geological history of the cynipoid wasps (Hymenoptera: Cynipoidea). American Museum Novitates. 2007;3583:1–48. [Google Scholar]
- 917. Rasnitsyn AP, Zhang HC. A new family, Daohugoidae fam. n., of syricomorph hymenopteran (Hymenoptera = Vespida) from the Middle Jurassic of Daohugou in Inner Mongolia (China). Proceedings of the Russian Entomological Society. 2004;75(1):12–16. [Google Scholar]
- 918. Perrichot V, Nel A. A new belytine wasp in Cretaceous amber from France (Hymenoptera: Diapriidae). Alavesia. 2008;2:203–209. [Google Scholar]
- 919. Engel MS. An anteonine wasp in Cenomanian-Albian amber from Myanmar (Hymenoptera: Dryinidae). Journal of the Kansas Entomological Society. 2003;76(4):616–621. [Google Scholar]
- 920. Rasnitsyn AP. New early Cretaceous Embolemidae (Vespida = Hymenoptera: Chrysidoidea). Memoirs of the Entomological Society of Washington. 1996;17:183–187. [Google Scholar]
- 921. Simutnik SA, Perkovsky EE. A description of the encyrtid male (Hymenoptera, Chalcidoidea, Encyrtidae) with archaic structure of metasoma from Rovno amber. Vestnik zoologii. 2006;40(3):283–286. [Google Scholar]
- 922. Basibuyuk HH, Rasnitsyn AP, Fitton MG, Quicke DLJ. The limits of the family Evaniidae (Insecta: Hymenoptera) and a new genus from Lebanese amber. Insect Systematics & Evolution. 2002;33(1):23–34. [Google Scholar]
- 923. Hong YC. Atlas of Amber Insects of China. Scientific and Technological Publishing House, Henan; 2002. [Google Scholar]
- 924. Gibson GAP, Read J, Huber JT. Diversity, classification and higher relationships of Mymarommatoidea (Hymenoptera). Journal of Hymenoptera Research. 2007;16(1):51–146. [Google Scholar]
- 925. Engel MS, Grimaldi DA. New false fairy wasps in Cretaceous amber from New Jersey and Myanmar (Hymenoptera: Mymarommatoidea). Transactions of the Kansas Academy of Science. 2007;110(3/4):159–168. [Google Scholar]
- 926. Zhang JF, Rasnitsyn AP. Minute members of Baissinae (Insecta: Hymenoptera: Gasteruptiidae) from the upper Mesozoic of China and limits of the genus Manlaya Rasnitsyn, 1980. Cretaceous Research. 2004;25(6):797–805. [Google Scholar]
- 927. Engel MS, Archibald SB. An early Eocene bee (Hymenoptera: Halictidae) from Quilchena, British Columbia. The Canadian Entomologist. 2003;135:63–69. [Google Scholar]
- 928. Rasnitsyn AP, Zhang HC. Composition and age of the Daohugou hymenopteran (Insecta, Hymenoptera = Vespida) assemblage from Inner Mongolia, China. Palaeontology. 2004;47(6):1507–1517. [Google Scholar]
- 929. Kopylov DS. A new subfamily of ichneumonids from the Lower Cretaceous of Transbaikalia and Mongolia (Insecta: Hymenoptera: Ichneumonidae). Paleontological Journal. 2009;43(1):83–93. [Google Scholar]
- 930. Rasnitsyn AP, Brothers DJ. Two new hymenopteran fossils from the mid-Cretaceous of southern Africa (Hymenoptera: Jurapriidae, Evaniidae). African Invertebrates. 2007;48(1):193–202. [Google Scholar]
- 931. Rasnitsyn AP, Ansorge J, Zhang HC. Ancestry of the orussoid wasps, with description of three new genera and species of Karatavitidae (Hymenoptera = Vespida: Karatavitoidea stat. nov.). Insect Systematics & Evolution. 2006;37(2):179–190. [Google Scholar]
- 932. Rasnitsyn AP, Basibuyuk HH, Quicke DLJ. A basal chalcidoid (Insecta: Hymenoptera) from the earliest Cretaceous or latest Jurassic of Mongolia. Insect Systematics & Evolution. 2004;35(2):123–135. [Google Scholar]
- 933. Hong YC. Middle Jurassic Fossil Insects in North China. Geological Publishing House, Beijing; 1983. [Google Scholar]
- 934. Simon E. Étude sur les crustacés du sous-ordre des phyllopodes, I, revision des espécies Françaises. Annales de la Société Entomologique de France, Ser 6. 1886;6:393–460. [Google Scholar]
- 935. Rasnitsyn AP, Brothers DJ. New genera and species of Maimetshidae (Hymenoptera: Stephanoidea s.l.) from the Turonian of Botswana, with comments on the status of the family. African Invertebrates. 2009;50(1):191–204. [Google Scholar]
- 936. Michez D, de Meulemeester T, Rasmont P, Nel A, Patiny S. New fossil evidence of the early diversification of bees: Paleohabropoda oudardi from the French Paleocene (Hymenoptera, Apidae, Anthophorini). Zoologica Scripta. 2009;38(2):171–181. [Google Scholar]
- 937. Blank SM, Taeger A, Liston AD, Smith DR, Rasnitsyn AP, Shinohara A, et al. Studies toward a world catalog of Symphyta (Hymenoptera). Zootaxa. 2009;2254:1–96. [Google Scholar]
- 938. Michez D, Nel A, Menier JJ, Rasmont P. The oldest fossil of a melittid bee (Hymenoptera: Apiformes) from the early Eocene of Oise (France). Zoological Journal of the Linnean Society. 2007;150(4):701–709. [Google Scholar]
- 939. Poinar GO, Danforth BN. A fossil bee from early Cretaceous Burmese amber. Science. 2006;314:614 [DOI] [PubMed] [Google Scholar]
- 940. Poinar GO. Melittosphex (Hymenoptera: Melittosphecidae), a primitive bee and not a wasp. Palaeontology. 2009;52(2):483–484. [Google Scholar]
- 941. Brothers DJ. The first fossil Ephutini (Hymenoptera: Mutillidae), a new species of Ephuta Say from Dominican amber. Acta zoologica cracoviensia. 2003;46(suppl.–Fossil Insects):101–107. [Google Scholar]
- 942. Manley DG, Poinar GO. A new specimen of fossil Mutillidae (Hymenoptera) from Dominican amber. Proceedings of the Entomological Society of Washington. 2003;105(4):1069–1071. [Google Scholar]
- 943. Gumovsky AV. The status of some genera allied to Chrysonotomyia and Closterocerus (Hymenoptera: Eulophidae, Entedoninae), with description of a new species from Dominican amber. Phegea. 2001;29(4):125–141. [Google Scholar]
- 944. Vilhelmsen L. The old wasp and the tree: fossils, phylogeny and biogeography in the Orussidae (Insecta, Hymenoptera). Biological Journal of the Linnean Society. 2004;82(2):139–160. [Google Scholar]
- 945. Shih CK, Liu CX, Ren D. The earliest fossil record of pelecinid wasps (Insecta, Hymenoptera, Proctotrupoidea, Pelecinidae) from Inner Mongolia, China. Annals of the Entomological Society of America. 2009;102(1):20–38. [Google Scholar]
- 946. Naumann ID, Masner L. Parasitic wasps of the proctotrupoid complex: a new family from Australia and a key to world families (Hymenoptera: Proctotrupoidea sensu lato). Australian Journal of Zoology. 1985;33:761–783. [Google Scholar]
- 947. Johnson NF, Musetti L, Janzen JW. A new fossil species of the Australian endemic genus Peradenia Naumann & Masner (Hymenoptera: Proctotrupoidea, Peradeniidae) from Baltic amber. Insect Systematics & Evolution. 2001;32:191–194. [Google Scholar]
- 948. Rasnitsyn AP. Testing cladograms by fossil record: the ghost range test. Contributions to Zoology. 2000;69(4):251–258. [Google Scholar]
- 949. Engel MS, Grimaldi DA. The first Cretaceous spider wasp (Hymenoptera: Pompilidae). Journal of the Kansas Entomological Society. 2006;79(4):359–368. [Google Scholar]
- 950. Rasnitsyn AP. Ontology of evolution and methodology of taxonomy. Paleontological Journal. 2006;40(Suppl. 6):S679–S737. [Google Scholar]
- 951. Johnson NF, Musetti L, Manser L. The Cretaceous scelionid genus Proteroscelio Brues (Hymenoptera: Platygastroidea). American Museum Novitates. 2008;3603:1–7. [Google Scholar]
- 952. Engel MS. The wasp family Rhopalosomatidae in mid-Cretaceous amber from Myanmar (Hymenoptera: Vespoidea). Journal of the Kansas Entomological Society. 2008;81(3):168–174. [Google Scholar]
- 953. Bennett DJ, Engel MS. A primitive sapygid wasp in Burmese amber (Hymenoptera: Sapygidae). Acta zoologica cracoviensia. 2005;48B(1–2):1–9. [Google Scholar]
- 954. Engel MS, Grimaldi DA. Cretaceous Scolebythidae and the phylogeny of the family (Hymenoptera: Chrysidoidea). American Museum Novitates. 2007;3568:1–16. [Google Scholar]
- 955. Perkovsky EE, Zosimovich VY, Vlaskin AP. Rovno amber insects: first results of analysis. Russian Entomological Journal. 2003;12(2):119–126. [Google Scholar]
- 956. Fujiyama I. Two parasitic wasps from Aptian (Lower Cretaceous) Choshi amber, Chiba, Japan. Natural History Research. 1994;3(1):1–5. [Google Scholar]
- 957. Engel MS, Grimaldi DA. The first Mesozoic stephanid wasp (Hymenoptera: Stephanidae). Journal of Paleontology. 2004;78(6):1192–1197. [Google Scholar]
- 958. Engel MS, Grimaldi DA. Diversity and phylogeny of the Mesozoic wasp family Stigmaphronidae (Hymenoptera: Ceraphronoidea). Denisia. 2009;26:53–68. [Google Scholar]
- 959. Gibson GAP. Description of Leptoomus janzeni, n. gen. and n. sp. (Hymenoptera: Chalcidoidea) from Baltic amber, and discussion of its relationships and classification relative to Eupelmidae, Tanaostigmatidae and Encyrtidae. Zootaxa. 2008;1730:1–26. [Google Scholar]
- 960. Nyman T, Zinovjev AG, Vikberg V, Farrell BD. Molecular phylogeny of the sawfly subfamily Nematinae (Hymenoptera: Tenthredinidae). Systematic Entomology. 2006;31(4):569–583. [Google Scholar]
- 961. Gumovsky AV, Perkovsky EE. Taxonomic notes on Tetracampidae (Hymenoptera: Chalcidoidea) with description of a new fossil species of Dipricocampe from Rovno amber. Entomological Problems. 2005;35(2):123–130. [Google Scholar]
- 962. Engel MS, Ortega-Blanco J, Bennett DJ. A remarkable tiphiiform wasp in mid-Cretaceous amber from Myanmar (Hymenoptera: Tiphiidae). Transactions of the Kansas Academy of Science. 2009;112(1/2):1–6. [Google Scholar]
- 963. Huber JT. The gender and derivation of genus-group names in Mymaridae and Mymarommatidae (Hymenoptera). Acta Societatis Zoologicae Bohemicae. 2005;69:167–183. [Google Scholar]
- 964. Yoshimoto CM. Cretaceous chalcidoid fossils from Canadian amber. The Canadian Entomologist. 1975;107:499–528. [Google Scholar]
- 965. Nel A, Perrichot V, Néraudeau D. The oldest trigonalid wasp in the late Albian amber of Charente-Maritime (SW France) (Hymenoptera: Trigonalidae). Eclogae geologicae Helvetiae. 2003;96(3):503–508. [Google Scholar]
- 966. Brothers DJ, Rasnitsyn AP. A new genus and species of Euparagiinae from the late Cretaceous of southern Africa (Hymenoptera: Vespidae). Alavesia. 2008;2:73–76. [Google Scholar]
- 967. Engel MS. A new sawfly from the Triassic of Queensland, Australia (Hymenoptera: Xyelidae). Memoirs of the Queensland Museum. 2005;51(2):558. [Google Scholar]
- 968. Nel A, Petrulevicius JF, Henrotay M. New early Jurassic sawflies from Luxembourg: the oldest record of Tenthredinoidea (Hymenoptera: “Symphyta”). Acta Palaeontologica Polonica. 2004;49(2):283–288. [Google Scholar]
- 969. Gao TP, Ren D, Shih CK. The first Xyelotomidae (Hymenoptera) from the Middle Jurassic in China. Annals of the Entomological Society of America. 2009;102(4):588–596. [Google Scholar]
- 970. Rasnitsyn AP, Zhang H, Wang B. Bizarre fossil insects: web-spinning sawflies of the genus Ferganolyda (Vespida, Pamphilioidea) from the Middle Jurassic of Daohugou, Inner Mongolia, China. Palaeontology. 2006;49(4):907–916. [Google Scholar]
- 971. Kozlov MV. Paleontology of lepidopterans and problems in the phylogeny of the order Papilionida In: Ponomarenko AG, editor. The Cretaceous Biocoenotic Crisis in the Evolution of Insects. Nauka, Moscow; 1988. p. 16–69. [Google Scholar]
- 972. Peñalver E, Grimaldi DA. New data on Miocene butterflies in Dominican amber (Lepidoptera, Riodinidae and Nymphalidae) with the description of a new nymphalid. American Museum Novitates. 2006;3519:1–17. [Google Scholar]
- 973. Fernández-Rubio F. Fossil butterflies. Causes of their rarity and how they influence our knowledge of phylogeny and distribution of Zygaenini (Lepidoptera: Zygaenidae). Boletin de la SEA. 1999;26:521–532. [Google Scholar]
- 974. de Jong R. Estimating time and space in the evolution of the Lepidoptera. Tijdschrift voor Entomologie. 2007;150:319–346. [Google Scholar]
- 975. Lopez-Vaamonde C, Wikstrom N, Labandeira CC, Godfray HCJ, Goodman SJ, Cook JM. Fossil-calibrated molecular phylogenies reveal that leaf-mining moths radiated millions of years after their host plants. Journal of Evolutionary Biology. 2006;19(4):1314–1326. [DOI] [PubMed] [Google Scholar]
- 976. Krassilov VA. Mines and galls on fossil leaves from the late Cretaceous of southern Negev, Israel. African Invertebrates. 2007;48(1):13–22. [Google Scholar]
- 977. Skalski AW. The families Nepticulidae and Thyrididae in Baltic amber (Lepidoptera). Nota lepidopterologica Supplement. 1992;4:144–145. [Google Scholar]
- 978. Kozlov MV, Ivanov VD, Rasnitsyn AP. 2.2.1.3.4.3. Order Lepidoptera Linné, 1758. The butterflies and moths (= Papilionida Laicharting, 1781) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 220–227. [Google Scholar]
- 979. Bechly G. 11.21 Trichoptera and Lepidoptera: caddisflies and butterflies In: Martill DM, Bechly G, Loveridge RF, editors. The Crato Fossil Beds of Brazil: Window into an Ancient World. Cambridge University Press; 2007. p. 387–393. [Google Scholar]
- 980. Harris AC, Raine JI. A sclerite from a late Cretaceous moth (Insecta: Lepidoptera) from Rakaia Gorge, Canterbury, New Zealand. Journal of the Royal Society of New Zealand. 2002;32(3):457–462. [Google Scholar]
- 981. Kristensen NP, Skalski AW. Palaeontology and phylogeny In: Kristensen NP, editor. Handbuch der Zoology: Eine Naturgeschichte der Stämme des Tierreiches: Band IV: Arthopoda: Insecta: Tielband 35: Lepidoptera, Moths and Butterflies: Volume 1: Evolution, Systematics, and Biogeography Walter de Gruyter, Berlin; 1999. p. 7–25. [Google Scholar]
- 982. Grimaldi DA. The co-radiations of pollinating insects and angiosperms in the Cretaceous. Annals of the Missouri Botanical Garden. 1999;86(2):373–406. [Google Scholar]
- 983. Hall JP, Robbins RK, Harvey DJ. Extinction and biogeography in the Caribbean: new evidence from a fossil riodinid butterfly in Dominican amber. Proceedings of the Royal Society of London, B. 2004;271(1541):797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 984. Braby MF, Trueman JWH, Eastwood R. When and where did troidine butterflies (Lepidoptera: Papilionidae) evolve? Phylogenetic and biogeographic evidence suggests an origin in remnant Gondwana in the late Cretaceous. Invertebrate Systematics. 2005;19(2):113–143. [Google Scholar]
- 985. Gáll LF, Tiffney BH. A fossil noctuid moth egg from the late Cretaceous of eastern North America. Science. 1983;219(4584):507–509. [DOI] [PubMed] [Google Scholar]
- 986. Kvacek Z, Böhme M, Dvorák Z, Konzalová M, Mach K, Prokop J, et al. Early Miocene freshwater and swamp ecosystems of the Most Basin (northern Bohemia) with particular reference to the Bílina Mine section. Journal of the Czech Geological Society. 2004;49(1–2):1–40. [Google Scholar]
- 987. Sobczyk T, Kobbert MJ. Die Psychidae des baltischen Bernsteins. Nota lepidopterologica. 2009;32(1):13–22. [Google Scholar]
- 988. Labandeira CC. Paleobiology of middle Eocene plant-insect associations from the Pacific Northwest: A preliminary report. Rocky Mountain Geology. 2002;37:31–59. [Google Scholar]
- 989. Bonde N, Andersen S, Hald N, Jakobsen SL. Danekrae—Danmarks bedste fossiler. Gyldendal, Copenhagen; 2008. [Google Scholar]
- 990. Fernández-Rubio F, Nel A. Neurosymploca? oligocenica a new fossil species of Lepidoptera Zygaenoidea of the Oligocene of Céreste (Lubéron, France). Boletin de la SEA. 2000;27:7–16. [Google Scholar]
- 991. Packard AS. A new arrangement of the orders of insects. American Naturalist. 1886;20:808. [Google Scholar]
- 992. Novokshonov VG. 2.2.1.3.4.1. Order Panorpida Latreille, 1802. The Scorpionflies (= Mecoptera Packard, 1886, = Mecoptera Comstock et Comstock, 1895, +Neomecoptera Hinton, 1958, +Paratrichoptera Tillyard, 1919, +Paramecoptera Tillyard, 1919) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 194–199. [Google Scholar]
- 993. Rasnitsyn AP, Kozlov MV. A new group of fossil insects: Scorpionflies with the adaptations of bugs and butterflies [in Russian]. Doklady Akademii Nauk SSSR. 1990;310(4):973–976. [Google Scholar]
- 994. Labandeira CC, Kvacek J, Mostovski MM. Pollination drops, pollen, and insect pollination of Mesozoic gymnosperms. Taxon. 2007;56(3):663–695. [Google Scholar]
- 995. Ren D, Labandeira CC, Santiago-Blay JA, Rasnitsyn AP, Shih CK, Bashkuev A, et al. A probable pollination mode before angiosperms: Eurasian, long-proboscid scorpionflies. Science. 2009;326:840–847. 10.1126/science.1178338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 996. Novokshonov VG. The first mecopteroids (Insecta: Papilionidea = Mecopteroidea) and the origin of scorpionflies (Panorpida = Mecoptera), with description of a legless eruciform larva. Paleontological Journal. 2004;38(Suppl. 2):S204–S213. [Google Scholar]
- 997. Archibald SB. New Dinopanorpidae (Insecta: Mecoptera) from the Eocene Okanagan Highlands (British Columbia, Canada; Washington State, USA). Canadian Journal of Earth Sciences. 2005;42:119–136. [Google Scholar]
- 998. Krzeminski W. A revision of Eocene Bittacidae (Mecoptera) from Baltic amber, with the description of a new species. African Invertebrates. 2007;48(1):153–162. [Google Scholar]
- 999. Li YL, Ren D, Shih CK. Two Middle Jurassic hanging-flies (Insecta: Mecoptera: Bittacidae) from northeast China. Zootaxa. 2008;1929:38–46. [Google Scholar]
- 1000. Archibald SB. New Cimbrophlebiidae (Insecta: Mecoptera) from the Early Eocene at McAbee, British Columbia, Canada and Republic, Washington, USA. Zootaxa. 2009;2249:51–62. [Google Scholar]
- 1001. Archibald SB, Rasnitsyn AP, Akhmetiev MA. The ecology and distribution of Cenozoic Eomeropidae (Mecoptera), and a new species of Eomerope Cockerell from the early Eocene McAbee locality, British Columbia, Canada. Annals of the Entomological Society of America. 2005;98(4):503–514. [Google Scholar]
- 1002. Ren D, Shih CK. The first discovery of fossil eomeropids from China (Insecta, Mecoptera). Acta Zootaxonomica Sinica. 2005;30(2):275–280. [Google Scholar]
- 1003. Novokshonov VG. Permian scorpionflies (Insecta: Panorpida) of the families Kaltanidae, Permochoristidae and Robinjohniidae. Paleontological Journal. 1994;28(1):79–95. [Google Scholar]
- 1004. Hong YC, Li Z. Discovery of the oldest fossil Meropeidae (Insecta, Mecoptera) from Shaanxi, China. Acta Zootaxonomica Sinica. 2007;32(4):875–880. [Google Scholar]
- 1005. Hong YC. Mid Triassic new genera and species of Mesopanorpodidae (Insecta, Mecoptera) from Shaanxi, China. Acta Zootaxonomica Sinica. 2007;32(2):261–267. [Google Scholar]
- 1006. Sun JH, Ren D, Shih CK. Middle Jurassic Mesopanorpodidae from Daohugou, Inner Mongolia, China (Insecta, Mecoptera). Acta Zootaxonomica Sinica. 2007;32(4):865–874. [Google Scholar]
- 1007. Willmann R. Evolution und Phylogenetisches System der Mecoptera (Insecta: Holometabola). Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft. 1989;544:1–153. [Google Scholar]
- 1008. Beutel RG, Baum E. A longstanding entomological problem finally solved? Head morphology of Nannochorista (Mecoptera, Insecta) and possibly phylogenetic implications. Journal of Zoological Systematics and Evolutionary Research. 2008;46(4):346–367. [Google Scholar]
- 1009. Hong YC. Mid Triassic new genera and species of Orthophlebiidae and Neorthophlebiidae (Insecta, Mecoptera) from Shaanxi, China. Acta Zootaxonomica Sinica. 2009;34(3):423–427. [Google Scholar]
- 1010. Novokshonov VG. Some Mesozoic scorpionflies (Insecta: Panorpida = Mecoptera) of the families Mesopsychidae, Pseudopolycentropodidae, Bittacidae, and Permochoristidae. Paleontological Journal. 1997;31(1):65–71. [Google Scholar]
- 1011. Zhang ZJ, Lu LW, Jin YG, Fang XS, Hong YC. Discovery of fossil insects in the Tuodian Formation, central Yunnan. Geological Bulletin of China. 2003;22(6):452–455. [Google Scholar]
- 1012. Willmann R, Novokshonov V. Neue Mecopteren aus dem oberen Jura von Karatau (Kasachstan) (Insecta, Mecoptera: ‘Orthophlebiidae’). Paläontologische Zeitschrift. 1998;72(3/4):281–298. [Google Scholar]
- 1013.(Kasachstan) (Insecta, Mecoptera: ‘Orthophlebiidae’). Paläontologische Zeitschrift. 1998;72(3/4):281–298.
- 1014. Hong YC, Zhang ZJ. Reclassification of fossil Orthophlebiidae (Insecta: Mecoptera). Entomotaxonomia. 2007;29(1):26–36. [Google Scholar]
- 1015. Sun JH, Ren D, Huang JD. Current knowledge of research on Mecoptera fossils in China. Acta Zootaxonomica Sinica. 2007;32(4):881–889. [Google Scholar]
- 1016. Petrulevicius JF. A panorpid (Insecta: Mecoptera) from the Lower Eocene of Patagonia, Argentina. Journal of Paleontology. 2009;83(6):994–997. [Google Scholar]
- 1017. Novokshonov VG. Caddisflies of the genus Kamopanorpa (Trichoptera, Microptysmatidae) from the Kungurian of Chekarda (Perm District). Paleontological Journal. 1992;26(3):136–141. [Google Scholar]
- 1018. Willmann R. Mecoptera (Insecta, Holometabola). Fossilum Catalogus, Animalia. 1978;124:1–139. [Google Scholar]
- 1019. Novokshonov VG, Novokshonova EA. Okolpania favorabilis n. sp. (Planipennia; Neuroptera: Permithonidae) from the Lower Permian of Ural. Paläontologische Zeitschrift. 1997;71(1/2):89–90. [Google Scholar]
- 1020. Nel A, Roques P, Nel P, Prokop J, Steyer JS. The earliest holometabolous insect from the Carboniferous: a crucial innovation with delayed success (Insecta Protomeropina Protomeropidae). Annales de la Société entomologique de France (Nouvelle série). 2007;43(3):349–355. [Google Scholar]
- 1021. Sukatsheva ID, Beattie R, Mostovski MB. Permomerope natalensis sp. n. from the Lopingian of South Africa, and a redescription of the type species of Permomerope (Insecta: Trichoptera). African Invertebrates. 2007;48(2):245–251. [Google Scholar]
- 1022. Grimaldi D, Zhang JF, Fraser NC, Rasnitsyn AP. Revision of the bizarre Mesozoic scorpionflies in the Pseudopolycentropodidae (Mecopteroidea). Insect Systematics & Evolution. 2005;36(4):443–458. [Google Scholar]
- 1023. Sukatsheva ID, Novokshonov VG. A new family of scorpionflies from the Mesozoic of Yakutia (Insecta; Mecoptera, Sibirioithaumatidae fam. nov.). Paleontological Journal. 1998;32(6):596–597. [Google Scholar]
- 1024. Hong YC. First discovery of fossil Protomecoptera in the Tongchuan region, Shaanxi, China. Geological Bulletin of China. 2006;25(5):560–564. [Google Scholar]
- 1025.Latreille PA. Histoire Naturelle Générale et Particulièr des Crustacés et des Insectes, Tome 3. Dufart, Paris, France; 1802.
- 1026. Ponomarenko AG. 2.2.1.3.3. Superorder Myrmeleontidea Latreille, 1802 (= Neuropteroidea Handlirsch, 1903) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insect. Kluwer Academic Publishers, The Netherlands; 2002. p. 176–189. [Google Scholar]
- 1027. Wichard W, Chatterton C, Ross A. Corydasialidae fam. n. (Megaloptera) from Baltic amber. Insect Systematics & Evolution. 2005;36:279–283. [Google Scholar]
- 1028. Ansorge J. Dobbertinia reticulata Handlirsch 1920 from the Lower Jurassic of Dobbertin (Mecklenburg/Germany) the oldest representative of Sialidae (Megaloptera). Neues Jahrbuch für Geologie und Paläontologie, Monatshefte. 2001;2001(9):553–564. [Google Scholar]
- 1029. Engel MS. The alderflies of Kansas (Megaloptera: Sialidae). Transaction of the Kansas Academy of Science. 2004;107(3):119–125. [Google Scholar]
- 1030. Engel MS, Grimaldi DA. Diverse Neuropterida in Cretaceous amber, with particular reference to the paleofauna of Myanmar (Insecta). Nova Supplementa Entomologica. 2008;20:1–86. [Google Scholar]
- 1031. Ren D, Engel MS. Aetheogrammatidae, a new family of lacewings from the Mesozoic of China (Neuroptera: Myrmeleontiformia). Journal of the Kansas Entomological Society. 2008;81(3):161–167. [Google Scholar]
- 1032. Martins-Neto RG. The Santana Formation paleoentomofauna reviewed. Part I–Neuropteroida (Neuroptera and Raphidioptera): systematic and phylogeny, with description of new taxa. Acta Geologica Leopoldensia (São Leopoldo). 2002;25(55):35–66. [Google Scholar]
- 1033. Martins-Neto RG, Heads SW, Bechly G. 11.16 Neuropterida: snakeflies, dobsonflies and lacewings In: Martill DM, Bechly G, Loveridge RF, editors. The Crato Fossil Beds of Brazil: Window into an Ancient World. Cambridge University Press; 2007. p. 328–340. [Google Scholar]
- 1034. Ponomarenko AG, Shcherbakov DE. New lacewings (Neuroptera) from the terminal Permian and basal Triassic of Siberia. Paleontological Journal. 2004;38(Suppl. 2):S197–S203. [Google Scholar]
- 1035. Makarkin VN, Menon F. New species of the Mesochrysopidae (Insecta, Neuroptera) from the Crato Formation of Brazil (Lower Cretaceous), with taxonomic treatment of the family. Cretaceous Research. 2005;26(5):801–812. [Google Scholar]
- 1036. Ren D, Makarkin VN. Ascalochrysidae a new lacewing family from the Mesozoic of China (Insecta: Neuroptera: Chrysopoidea). Cretaceous Research. 2009;30(5):1217–1222. [Google Scholar]
- 1037. Martins-Neto RG, Vulcano MA. Neurópteros (Insecta, Planipennia) da Formação Santana (Cretáceo Inferior), Bacia do Araripe, Nordeste do Brasil. II—Superfamília Myrmeleontoidea. Revista Brasileira de Entomologia. 1989;33(2):367–402. [Google Scholar]
- 1038. Ponomarenko AG. Neuroptera (Insecta) from the Lower Cretaceous of Transbaykalia. Paleontological Journal. 1992;26(3):56–66. [Google Scholar]
- 1039. Makarkin VM. New psychopsoid Neuroptera from the Early Cretaceous of Baissa, Transbaikalia. Annales de la Société entomologique de France (Nouvelle série). 2010;46(1–2):254–261. [Google Scholar]
- 1040. Nel A, Delclòs X, Hutin A. Mesozoic chrysopid-like Planipennia: a phylogenetic approach (Insecta: Neuroptera). Annales de la Société entomologique de France (Nouvelle série). 2005;41(1):29–68. [Google Scholar]
- 1041. Engel MS, Grimaldi DA. The neuropterid fauna of Dominican and Mexican amber (Neuropterida: Megaloptera, Neuroptera). American Museum Novitates. 2007;3587:1–58. [Google Scholar]
- 1042. Makarkin VN, Archibald SB. Family affinity of the genus Palaeopsychops Andersen with description of a new species from the early Eocene of British Columbia, Canada (Neuroptera: Polystoechotidae). Annals of the Entomological Society of America. 2003;96(3):171–180. [Google Scholar]
- 1043. Ren D. A new lacewing family (Neuroptera) from the Middle Jurassic of Inner Mongolia, China. Entomologia Sinica. 2002;9(12):53–67. [Google Scholar]
- 1044. Jepson JE, Makarkin VN, Jarzembowski EA. New lacewings (Insecta: Neuroptera) from the Lower Cretaceous Wealden supergroup of southern England. Cretaceous Research. 2009;30(5):1325–1338. [Google Scholar]
- 1045. Andersen S. Silky lacewings (Neuroptera: Psychopsidae) from the Eocene-Paleocene transition of Denmark with a review of the fossil record and comments on phylogeny and zoogeography. Insect Systematics & Evolution. 2001;32:419–438. [Google Scholar]
- 1046. Makarkin VN, Ren D, Yang Q. Two new species of Kalligrammatidae (Neuroptera) from the Jurassic of China, with comments on venational homologies. Annals of the Entomological Society of America. 2009;102(6):964–969. [Google Scholar]
- 1047. Wedmann S, Makarkin VN. A new genus of Mantispidae (Insecta: Neuroptera) from the Eocene of Germany, with a review of the fossil record and palaeobiogeography of the family. Zoological Journal of the Linnean Society. 2007;149(4):701–716. [Google Scholar]
- 1048. Makarkin VN. Fossil Neuroptera of the Lower Cretaceous of Baisa, East Siberia. Part 6. Mesithonidae (Insecta). Neues Jahrbuch für Geologie und Paläontologie, Monatshefte. 1999;1999(12):705–712. [Google Scholar]
- 1049. Carpenter FM. A substitute name for the extinct genus Proberotha Riek (Neuroptera). Psyche. 1991;98(1):87. [Google Scholar]
- 1050. Menon F, Makarkin VN. New fossil lacewings and antlions (Insecta, Neuroptera) from the Lower Cretaceous Crato formation of Brazil. Palaeontology. 2008;51(1):149–162. [Google Scholar]
- 1051. Martins-Neto RG, Rodrigues VZ. New Neuroptera (Insecta, Osmylidae and Mesochrysopidae) from the Santana Formation, Lower Cretaceous of northeast Brazil. Gaea. 2009;5(1):15–20. [Google Scholar]
- 1052. Makarkin VN, Perkovsky EE. Rophalis relicta HAGEN (Neuroptera, Nevrorthidae) in the late Eocene Rovno amber, with a discussion of the taxonomic status of the genus. Denisia. 2009;26:137–144. [Google Scholar]
- 1053. Ren D, Engel MS. A split-footed lacewing and two epiosmylines from the Jurassic of China (Neuroptera). Annales zoologici. 2007;57(2):211–219. [Google Scholar]
- 1054. Ponomarenko AG. On some Neuroptera (Insecta) from Upper Jurassic Solnhofen Limestone. Annals of the Upper Silesian Museum (Entomology). 2003;12:87–100. [Google Scholar]
- 1055. Makarkin VN. Baissoleon cretaceus gen. and sp. nov. Fossil Neuroptera from the Lower Cretaceous of Baisa, East Siberia. 2. Nymphitidae. Annales de la Société Entomologique de France. 1990;26(1):125–126. [Google Scholar]
- 1056. Makarkin VN, Archibald SB. Substitute names for three genera of fossil Neuroptera, with taxonomic notes. Zootaxa. 2005;1054:15–23. [Google Scholar]
- 1057. Martins-Neto RG. Neurópteros (Insecta, Planipennia) da Formação Santana (Cretáceo Inferior), Bacia do Araripe, nordeste do Brasil. VII—Palaeoleontinae, nova subfamilia de Myrmeleontidae e descriçã de novos táxons. Revista Brasileira de Entomologia. 1992;36(4):803–815. [Google Scholar]
- 1058. Jepson JE, Penney D. Neuropteran (Insecta) palaeodiversity with predictions for the Cretaceous fauna of the Wealden. Palaeogeography, Palaeoclimatology, Palaeoecology. 2007;248(1–2):109–118. [Google Scholar]
- 1059. Whalley PES. Mesozoic Neuroptera and Raphidioptera (Insecta) in Britain. Bulletin of the British Museum (Natural History), Geology. 1988;44(1):45–63. [Google Scholar]
- 1060. Makarkin VN, Menon F. First record of fossil rapismatid-like Ithonidae (Insecta, Neuroptera) from the Lower Cretaceous Crato Formation of Brazil. Cretaceous Research. 2007;28(5):743–753. [Google Scholar]
- 1061. Ponomarenko AG. Upper Liassic neuropterans (Insecta) from Lower Saxony, Germany. Russian Entomological Journal. 1996;4(for 1995):73–89. [Google Scholar]
- 1062. Nel A, Garrouste R, Bechly G, Pohl B, Escuillié F. Rafaeliana, a replacement generic name for Rafaelia Nel et al., 2005 (Neuropterida). Bulletin de la Société entomologique de France. 2006;111(2):190. [Google Scholar]
- 1063. Nel A, Perrichot V, Azar D, Néraudeau D. New Rhachiberothidae (Insecta: Neuroptera) in early Cretaceous and early Eocene ambers from France and Lebanon. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 2005;235(1):51–85. [Google Scholar]
- 1064. Nel A, Menier JJ, Waller A, Hodebert G, de Ploëg G. New fossil spongilla-flies from the lowermost Eocene amber of France (Insecta, Neuroptera, Sisyridae). Geodiversitas. 2003;25(1):109–117. [Google Scholar]
- 1065. Makarkin VN. New Tertiary Neuroptera (Insecta) from the Russian Far East. Tertiary Research. 1998;18(3–4):77–83. [Google Scholar]
- 1066. Navás L. Notas sobre el orden de los Rafidiópteros (Ins.). Memorias de la Real Academia de Ciencias y Artes de Barcelona. 1916;12:507–513. [Google Scholar]
- 1067. Jepson JE, Jarzembowski EA. Two new species of snakefly (Insecta: Raphidioptera) from the Lower Cretaceous of England and Spain with a review of other fossil raphidiopterans from the Jurassic/Cretaceous transition. Alavesia. 2008;2:193–201. [Google Scholar]
- 1068. Engel MS. The smallest snakefly (Raphidioptera: Mesoraphidiidae): a new species in Cretaceous amber from Myanmar, with a catalog of fossil snakeflies. American Museum Novitates. 2002;3363:1–22. [Google Scholar]
- 1069. Aspöck U, Aspöck H. Two significant new snakeflies from Baltic amber, with discussion on autapomorphies of the order and its included taxa (Raphidioptera). Systematic Entomology. 2004;29(1):11–19. [Google Scholar]
- 1070. Perrichot V, Engel MS. Early Cretaceous snakefly larvae in amber from Lebanon, Myanmar, and France (Raphidioptera). American Museum Novitates. 2007;3598:1–11. [Google Scholar]
- 1071.Latreille PA. Familles naturelles du règne animal, exposées succinctement et dans un ordre analytique, avec l’indication de leurs genres. Paris; 1825.
- 1072. Whiting MF, Whiting AS, Hastriter MW, Dittmar K. A molecular phylogeny of fleas (Insecta: Siphonaptera): origins and host associations. Cladistics. 2008;24:1–31. [Google Scholar]
- 1073. Lewis RE, Grimaldi DA. A pulicid flea in Miocene amber from the Dominican Republic (Insecta: Siphonaptera: Pulicidae). American Museum Novitates. 1997;3205:1–9. [Google Scholar]
- 1074. Kirby W. Strepsiptera, a new order of Insects proposed and the characters of the order, with those of its genera, laid down. Transactions of the Linnean Society of London. 1815;11:86–123. [Google Scholar]
- 1075. Grimaldi DA, Kathirithamby J, Schawaroch V. Strepsiptera and triungula in Cretaceous amber. Insect Systematics & Evolution. 2005;36(1):1–20. [Google Scholar]
- 1076. Pohl H, Beutel RG, Kinzelbach R. Protoxenidae fam. nov. (Insecta, Strepsiptera) from Baltic amber—a ‘missing link’ in strepsipteran phylogeny. Zoologica Scripta. 2005;34(1):57–69. [Google Scholar]
- 1077. Pohl H. The oldest fossil strepsipteran larva (Insecta: Strepsiptera) from the Geisel Valley, Germany (Eocene). Insect Systematics & Evolution. 2009;40(4):333–347. [Google Scholar]
- 1078. Kirby W. An introduction to Entomology, volume 1 Longman, London; 1815. [Google Scholar]
- 1079. Sukatsheva ID. Yurskie rucheiniki yuzhnoi Sibiri [Jurassic caddisflies of Southern Siberia]. Trudy Paleontologicheskogo Instituta. 1985;211:115–119. In Russian. [Google Scholar]
- 1080. Ivanov VD, Sukatsheva ID. 2.2.1.3.4.2. Order Trichoptera Kirby, 1813. The caddisflies (= Phryganeida Latreille, 1810) In: Rasnitsyn AP, Quicke DLJ, editors. History of Insects. Kluwer Academic Publishers, The Netherlands; 2002. p. 199–220. [Google Scholar]
- 1081. Ivanov VD. Larvae of caddisflies (Insecta: Trichoptera) from the Mesozoic of Siberia. Paleontological Journal. 2006;40(2):178–189. [Google Scholar]
- 1082. Ponomarenko AG, Sukatsheva ID, Vasilenko DV. Some characteristics of the Trichoptera distribution in the Mesozoic of Eurasia (Insecta: Trichoptera. Paleontological Journal. 2009;43(3):282–295. [Google Scholar]
- 1083. Wichard W, Lüer C. Phylocentropus swolenskyi n. sp., eine Köcherfliege aus dem New Jersey Bernstein (Trichoptera, Dipseudopsidae). Mitteilungen aus dem Geologisch-Paläontologischen Institut der Universität Hamburg. 2003;87:162–169. [Google Scholar]
- 1084. Sukatsheva ID. New fossil caddis flies (Trichoptera) from the Shar-Teg locality in Mongolia. Paleontological Journal. 2000;34(Suppl. 3):S347–S351. [Google Scholar]
- 1085. Ansorge J. Revision of the “Trichoptera” described by Geinitz and Handlirsch from the Lower Toarcian of Dobbertin (Germany) based on new material. Nova Supplementa Entomologica. 2002;15:55–74. [Google Scholar]
- 1086. Ansorge J. Upper Liassic amphiesmenopterans (Trichoptera + Lepidoptera) from Germany—a review. Acta zoologica cracoviensia. 2003;46(suppl.- Fossil Insects):285–290. [Google Scholar]
- 1087. Sukatsheva ID, Jarzembowski EA. Fossil caddisflies (Insecta: Trichoptera) from the early Cretaceous of southern England II. Cretaceous Research. 2001;22(6):685–694. [Google Scholar]
- 1088. Melnitsky SI. A new caddisfly of the extinct genus Archaeotinodes (Insecta: Trichoptera: Ecnomidae) from the Baltic amber. Paleontological Journal. 2009;43(3):296–299. [Google Scholar]
- 1089. Ivanov VD, Melnitsky SI. The morphology of Dajella tenera (Trichoptera, Glossosomatidae): taxonomic status and evidence for the pheromone communication in the Mesozoic. Entomological Review. 2006;86(5):568–575. [Google Scholar]
- 1090. Novokshonov VG. Caddis flies (Insecta, Trichoptera, Microptysmatidae). Paleontological Journal. 1994;27(1A):90–102. [Google Scholar]
- 1091. Jarzembowski EA. Fossil caddisflies (Insecta: Trichoptera) from the early Cretaceous of southern England. Cretaceous Research. 1995;16:695–703. [Google Scholar]
- 1092. Hong YC, Li ZY. A new early Cretaceous family from Liupanshan, Ningxia, China (Insecta, Trichoptera). Acta Zootaxonomica Sinica. 2004;29(2):224–233. [Google Scholar]
- 1093. Wang MX, Zhao YY, Ren D. New fossil caddisfly from Middle Jurassic of Daohugou, Inner Mongolia, China (Trichoptera: Philopotamidae). Progress in Natural Science. 2009;19(10):1427–1431. [Google Scholar]
- 1094. Ivanov VD. A new family of caddis flies (Insecta, Trichoptera) from the Permian of the middle Urals. Paleontological Journal. 1992;26(4):36–41. [Google Scholar]
- 1095. Wichard W, Solórzano Kraemer MM, Luer C. First caddisfly species from Mexican amber (Insecta: Trichoptera. Zootaxa. 2006;1378:37–48. [Google Scholar]
- 1096. Nel A, Azar D, Martínez-Delclòs X, Makhoul E. A new Upper Cretaceous species of Chresmoda from Lebanon—a latest representative of Chresmodidae (Insecta: Polyneoptera inc. sed.): first record of homeotic mutations in the fossil record of insects. European Journal of Entomology. 2004;101(1):145–151. [Google Scholar]
- 1097. Rasnitsyn AP. Strashila incredibilis, a new enigmatic mecopteroid insect with possible siphonapteran affinities from the Upper Jurassic of Siberia. Psyche. 1993;99(4):323–333. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.