Abstract
Proteases are an expanding class of drugs that hold great promise. The U.S. FDA (Food and Drug Administration) has approved 12 protease therapies, and a number of next generation or completely new proteases are in clinical development. Although they are a well-recognized class of targets for inhibitors, proteases themselves have not typically been considered as a drug class despite their application in the clinic over the last several decades; initially as plasma fractions and later as purified products. Although the predominant use of proteases has been in treating cardiovascular disease, they are also emerging as useful agents in the treatment of sepsis, digestive disorders, inflammation, cystic fibrosis, retinal disorders, psoriasis and other diseases. In the present review, we outline the history of proteases as therapeutics, provide an overview of their current clinical application, and describe several approaches to improve and expand their clinical application. Undoubtedly, our ability to harness proteolysis for disease treatment will increase with our understanding of protease biology and the molecular mechanisms responsible. New technologies for rationally engineering proteases, as well as improved delivery options, will expand greatly the potential applications of these enzymes. The recognition that proteases are, in fact, an established class of safe and efficacious drugs will stimulate investigation of additional therapeutic applications for these enzymes. Proteases therefore have a bright future as a distinct therapeutic class with diverse clinical applications.
Keywords: procoagulant, protease therapy, protein degradation, protein engineering, trypsin fold
PROTEASES ARE UBIQUITOUS IN BIOLOGY
Sequencing of the human genome revealed that more than 2% of our genes encode proteases, suggesting that these enzymes possess functions more complex than the simple digestive role that they are often assumed to play [1]. For example, proteases regulate growth factors, cytokines, chemokines and cellular receptors, both through activation and inactivation leading to downstream intracellular signalling and gene regulation. For most proteases, is it unclear how many physiologically relevant substrates they have, how active a given protease is within particular tissues in the human body, and how these characteristics differ in disease. Up-regulation of proteolysis is associated commonly with different types of cancer and is linked to tumour metastasis, invasion and growth [2]. Dysregulated proteolysis is also a feature of various inflammatory, and other, diseases. Prior to the use of high-throughput proteomic analyses it was assumed that most human proteases had thousands of substrates, but now a more accurate view is that most of them have 100 or fewer substrates, which are profoundly determined by spatial and temporal factors. Considerable effort is required to resolve the detailed activities of proteases in vivo and this is currently being catalogued by a number of high-throughput genomic and proteomic approaches whose findings will be use ful and necessary to guide further therapeutic development [3]. Many of the current therapeutic proteases have only a few physiological substrates. This small number of physiological substrates enables their clinical application. Restricting, or altogether redesigning, the selectivity of proteases to minimize the number of substrates they engage in vivo is also becoming increasingly possible for the creation of new therapeutic proteases.
Understanding protease biology is complicated, because the potential substrate repertoire is the entire human proteome. Proteases are encoded by more than 550 human genes [4–6]. Comparing this number against popular drug targets, there are 518 kinase genes [7], 950 GPCR (G-protein-coupled receptor) genes [8] and 107 phosphatase genes [9]. The most abundant human protease genes are metalloproteases followed by serine, cysteine, threonine and aspartyl family members [4]. Of these proteases, almost 100 are thought to be inactive on the basis of the absence of key catalytic residues; the roles of these non-proteolytic proteins are for the most part unclear. An exception is HGF (hepatocyte growth factor), which has a trypsin fold but lacks any of the three catalytic residues typical of a protease of this type, that has a clear role in cell signalling and proliferation [10]. Several HGF-like proteins are found in our genome and inactive trypsins are found in abundance in the insect genomes [11]. Protease-dependent processes include digestion, protein processing, homoeostasis, development, apoptosis, protein turnover, immunity, complement activation, blood coagulation and many others. The roles of proteases are therefore numerous and their activities occur both intra- and extra-cellullarly. Regulation of proteolytic activity is an important factor in the application of proteases as therapeutics.
Proteases are tightly regulated at multiple levels. First, transcription, translation, and often extensive post-translational modification are tissue specific. Once produced as proteins, proteases may be localized to specific organs, tissues and subcellular locations through protein–protein interactions, or circulate in the vascular or lymphatic systems. To control the spatial and temporal location of protease activity, nearly all proteases are made initially as zymogen precursors that have very low to undetectable levels of catalytic activity. Such zymogens must be activated by proteolytic processing and hence are often found in the pathways involving sequential protease activation. Proteolytic activity is regulated further by protease inhibitors that modulate the effective concentration of active enzyme [12]. Several of these inhibitors, such as SPINK5 (serine peptidase inhibitor, Kazal type 5), contain multiple inhibitory domains that differentially regulate the activity of a variety of proteases and have the potential for therapeutic application on their own [13]. The abundance and effectiveness of endogenous inhibitors plays a dominant role in the application of proteases as therapeutics.
Perhaps the most important lesson that can be learned from current protease therapeutics is the difficulty in overcoming these protective mechanisms to achieve therapeutic benefit while minimizing side effects. For example, the half-life of t-PA (tissue-type plasminogen activator) in its active form is on the order of minutes, due to its rapid clearance by endogenous inhibitors, yet levels of t-PA that are too high can lead to haemorrhage and other bleeding problems. Specificity of proteases is highly variable and ranges from promiscuous degradation to limited proteolysis of a select subsets of proteins. Proteases may also share substrates, and their signalling pathways can link disparate biological processes [14]. The numerous ways to affect their activity, and the resulting broad and dynamic range of catalytic efficiencies, provide important mechanisms that affect the pharmacokinetics and pharmacodynamics of therapeutic proteases.
The U.S. FDA (Food and Drug Administration) has approved a variety of proteases for therapeutic application. As shown in Table 1, the twelve available protease drugs include treatments for haemophilia, stroke, AMI (acute myocardial infarction), sepsis, traumatic bleeding, muscle spasms and digestive disorders. Zenpep® is the most recent FDA-approved digestive aid that contains a number of proteolytic enzymes; however, many similar formulations are currently on the market [15]. Each therapeutic protease carries out its function by cleaving proteins to either activate or inactivate them. The first approved proteases were extracted from natural sources. For example, urokinase was initially extracted from urine, bovine thrombin was purified from bovine blood and FIX (Factor IX) was derived from the plasma of human donors [16]. The application of these therapeutics both led to the recognition of their pathogen-associated risks, including hepatitis C and AIDS, and spurred the implementation of improved safety measures that now apply to many other biological-based therapies. Proteases are preferably generated recombinantly using non-human sources to minimize such contamination. Currently used production hosts include CHO (Chinese hamster ovary) cells, the Gram-negative bacteria Escherichia coli and the Gram-positive bacteria Clostridium botulinum. All currently marketed proteases are serine proteases with the exception of the bacterial botulinum neurotoxins, which are zinc metalloproteases.
Table 1.
FDA-approved protease drugs
| Usage | Protease | Indications | Source of protein | Target protein or pathway | Type of protease | Year approved by FDA |
|---|---|---|---|---|---|---|
| Thrombolysis | Urokinase (u-PA) | Thrombus, catheter clearing | Extracted from urine or from primary kidney cell culture |
Converts plasminogen into plasmin |
Serine | 1978 |
| t-PA (alteplase, Activase®) | AMI, stroke, catheter clearing | Recombinant in CHO cells | Plasminogen activator | Serine | 1987 (AMI) | |
| 1996 (stroke) | ||||||
| 2002 (catheter clearing) | ||||||
| Reteplase (Retevase) | AMI | Recombinant in E. coli | Plasminogen activator | Serine | 1996 | |
| TNK-tPA (tenecteplase, Metalyse®) |
Myocardial infarction | Recombinant in CHO cells | Plasminogen activator | Serine | 2000 | |
| Procoagulant | FIX | Haemophilia B | Human plasma | FX activator | Serine | 1990 |
| FIX (BeneFIX®) | Haemophilia B | Recombinant in CHO cells | FX activator | Serine | 1997 | |
| FVIIa (NovoSeven®) | Haemophilia A and B | Recombinant in BHK cells | FX and FIX activator | Serine | 1999 | |
| Topical thrombin in bandages | Bleeding | Bovine | Fibrinogen activator | Serine | 2006 | |
| Thrombin (Recothrom®) | Bleeding | Recombinant in CHO cells | Fibrinogen activator | Serine | 2008 | |
| Sepsis | Activated protein C, (drotrecogin alfa, Xigris®) |
Sepsis, septic shock | Recombinant in human cell line |
Plasminogen activator | Serine | 2001 |
| Neuromuscular | Botulinum toxin A (Botox®) | Various muscle spasms | Bacterial (C. botulinum) | Syntaxin and SNAP-25 deactivator |
Zinc | 1989 |
| Botulinum toxin B (Myobloc) | Cervical dystonia | Bacterial (C. botulinum) | Synaptobrevin deactivator |
Zinc | 2000 | |
| Digestion | Zenpep® (pancrelipase) | Exocrine Pancreatic Insufficiency | Porcine pancreatic extract | Aids digestion of protein | Serine | 2009 |
BHK, baby hamster kidney.
The first thrombolytic drug: u-PA (urokinase-type plasminogen activator)
The first FDA-approved purified protease drug u-PA (urokinase) ushered in the era of enzyme-based thrombolytic therapy and provided a welcome alternative to the surgical removal of emboli, which had a limited effectiveness at restoring patency to occluded vessels. u-PA, derived from primary neonatal kidney cell culture, was approved for clinical application in 1978 and remains in use for its ability to dissolve blood clots in blood vessels and intravenous catheters. It achieves this function by converting endogenous plasminogen into its active form, plasmin, by specific cleavage at a single position in the molecule (Figure 1). Clot lysis occurs in two phases. In the first phase, activation of plasminogen occurs on the surface of the fibrin clot [17]. In the second, the fibrin that is degraded by plasmin exposes additional binding sites for both plasminogen and t-PA thus leading to amplification of the clot breakdown. The size of the clot and its geometry can therefore affect significantly its rate of dissolution [18]. In addition, the composition of the nascent fibrin ends is modulated by TAFI (thrombin-activatable fibrinolysis inhibitor), a carboxypeptidase whose action reduces the binding of plasminogen [19]. Cell-surface association of u-PA occurs through one of several mechanisms, including association with its glycolipid-anchored receptor uPAR (u-PA receptor), which has been reported to enhance the rate of plasminogen activation [20,21]. u-PA consists of three domains: a serine protease domain, a kringle domain and an EGF (epidermal growth factor)-like domain. None of these domains are involved in u-PA binding to fibrin and it has a low affinity for fibrin relative to other fibrinolytic agents. u-PA requires localized administration to sites containing thrombi to focus its activity and minimize the adverse side affects. u-PA has been associated with the degradation of extracellular matrix proteins in tumour cell invasion and metastasis, thereby implicating this protease as a target for both cancer treatment and diagnostics [22]. u-PA continues to find utility in the clinic, such as for catheter clearing, due to its relatively low cost compared with newer agents with similar properties [23].
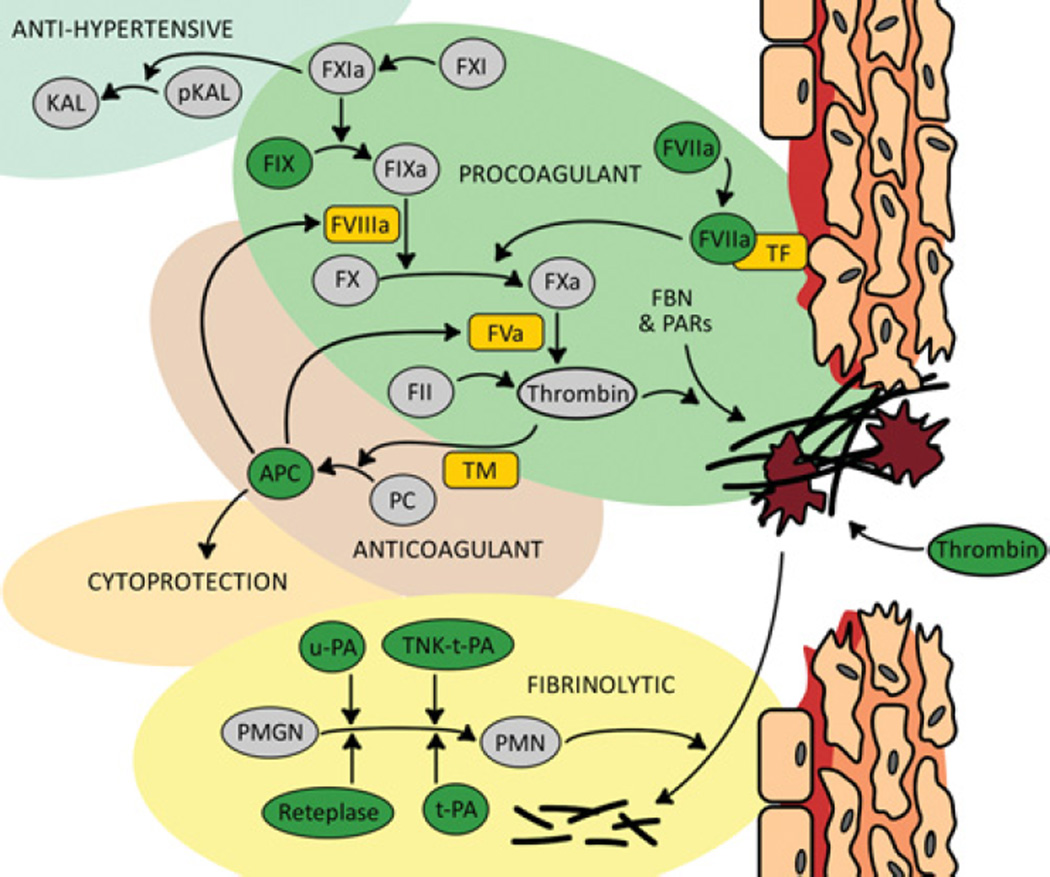
Figure 1. Protease therapeutics applied successfully for procoagulant and fibrinolytic indications.
Proteolytic cascades are responsible for the formation and dissolution of blood clots and therefore they can be used individually for therapeutic benefit. Eight proteases (shown in dark green circles) have been approved for clinical use. The use of proteases for benefits outside of clotting has begun to emerge from a deeper understanding of protease biology and better delivery technologies. For example, the anticoagulant APC may be modified for its anti-inflammatory effect whereas tissue prekallikrein (pKAL) is a target for gene therapy owing to its anti-hypertensive potential. Protein cofactors are represented by rectangles with rounded edges. FBN, fibronectin; FII, prothrombin; KAL, kallikrein; PC, protein C; PMGN, plasminogen; PMN, plasmin; TNKase, tenecteplase.
Attacking heart attacks and stoke: t-PA and streptokinase
The second protease developed to treat thrombotic disease was t-PA [24]. Full-length t-PA has five domains: the fibronectin finger domain, a growth factor domain, two kringle domains and a catalytic serine protease domain. Like u-PA, t-PA cleaves plasminogen into its active form plasmin, which then degrades the fibrin meshwork of blood clots. However, unlike u-PA, t-PA binds specifically to fibrin (mediated by the fibronectin finger and EGF2 domains) and this binding greatly stimulates plasminogen activation [25]. Consequently, plasminogen activation by t-PA occurs preferentially at sites of fibrin deposition (i.e. thrombi). In contrast with u-PA and streptokinase therefore, intravenous administration of t-PA results in local, rather than systemic, fibrinolysis.
Alteplase, marketed as Activase® (Genentech), was the first t-PA molecule to reach the clinic. It was first approved by the FDA in 1987 to treat AMI, then for treating stroke in 1996 and then for catheter clearing in 2002. The clinical manifestations of thrombi depends on their location in the body. Thrombi in coronary arteries can lead to myocardial infarction, whereas those in the arteries of the brain can cause stroke. In the extremities, peripheral arterial occlusion causes pain, muscle atrophy and, in severe cases, ischaemia requiring amputation. Alternatively, deep vein thrombosis can result in pulmonary embolism. Although distinct in their locations, their common mechanism of generation and similar composition provides a basis for therapeutic intervention. Recombinant t-PA is useful clinically for the treatment of each of these conditions because it can be administered systemically to produce local fibrinolysis and clot dissolution. However, poor pharmacokinetics requires t-PA to be administered as a bolus followed by a constant infusion. Active t-PA is inactivated rapidly by endogenous inhibitors, primarily PAI-1 (plasminogen activator inhibitor-1), leading to a biological half-life of only 6 min [26]. t-PA is also cleared by recognition of its high mannose carbohydrate moiety. Given the success of t-PA, considerable effort was spent in improving its properties. Second-generation products have been approved and others are along the pathway to approval.
Two variants of t-PA are currently in use, reteplase and tenecteplase, in addition to the first-generation product alteplase. Differences in the underlying composition of these two agents and their design highlight the variability in development pathways available to protease-based therapeutics. Reteplase (Retavase®; Boehringer Mannheim) is a truncated form of t-PA approved for the thrombolytic treatment of AMI. The N-terminal fibronect in finger, the EGF domain and the first kringle domains are deleted in reteplase compared with the native t-PA and these changes reduce the affinity of the protease for fibrin as well as the rate of clearance, thus increasing its half-life in plasma. This permits the administration of reteplase as a double bolus rather than as an infusion, reducing the time and expense of administration. The molecule lacks glycosylation because it is expressed recombinantly in E. coli. The effectiveness of reteplase has been demonstrated in several clinical trials [27,28]. Taken together these studies show that reteplase is not inferior in efficacy to the first-generation t-PA and can be administered more conveniently. Tenecteplase (TNKase®, TNK-tPA; Genentech) was approved by the FDA in 2000 and improved upon the first-generation agent by engineering the protease to avoid inactivation by endogenous inhibitors using site-directed mutagenesis and manipulating its carbohydrate content to improve its circulating half-life [29].
Three sites of the protease were mutated: a substitution of an asparagine residue for Thr103 (T103N), a substitution of a glutamine residue for Asn117 (N117Q) and alanine substitutions for residues 296–299. The T103N mutation creates a new N-linked glycosylation site to improve protein solubility and extend the proteases circulation half-life, the N117Q mutation eliminates a high mannose glycosylation site to reduce the clearance rate, and the changes at residues 296–299 limit interaction with PAI-1 [30]. Compared with the first-generation t-PA molecule, none of the associated protein domains are missing, so fibrin selectivity of the protease remains high thus reducing plasmin activation at non-preferred sites and the concomitant risk of intracerebral haemorrhage [31]. Tenecteplase has a half-life of 18 min in humans and the drug has been tested extensively in clinical trials [32–34]. In contrast with alteplase and retevase, tenectplase proved efficacious during clinical trials when administered as a single bolus; tenecteplase therefore allows a simpler and more convenient dosing than the other two t-PAs. Despite the practical advantages of second-generation t-PA molecules, alteplase remains widely used due to its established and near equivalent efficacy and its lower cost. The two engineering strategies used to generate the two approved t-PA variants, modification of binding properties and resistance to endogenous inhibitors by truncation or mutagenesis, will probably prove useful in bringing other proteases to the clinic.
In an indirect route to protease therapy, stimulation of endogenous protease activity by protein cofactors can be used for therapeutic effect. Streptokinase is a protease activator produced by β-haemolytic streptococci that has been approved as a way to activate plasminogen into an active protease to enable its further self-activation to plasmin [35]. The mechanism of action of streptokinase does not involve the canonical proteolytic activation of the zymogen, but involves forming a binary complex with plasminogen inducing a conformational change into an active protease. As streptokinase is not of human origin, circulating antibodies to the protein are typically present in plasma from previous streptococcal infections. Streptokinase must therefore be administered with a large initial dose to overcome these antibodies. The half-life of streptokinase is approx. 80 min after the clearance of the antibodies [36]. Because streptokinase may deplete circulating plasminogen after a few hours, its optimal use requires multiple administrations and intermittent treatment with heparin, an anticoagulant. Streptokinase causes several adverse reactions that u-PA and the various forms of t-PA do not. The initial preparations of streptokinase were associated with a very high incidence of antigenicity and severe allergic reactions. The current purified formulations are relatively free of such pyrogens, but the protein itself is still antigenic. Streptokinase may induce the formation of additional antibodies making subsequent treatment with this drug difficult, if not impossible.
The first haemophilia drug: FIX
FIX is essential for efficient blood clotting and maintenance of normal haemostasis (Figure 1). In the initial response to injury, cell-based TF (tissue factor) is exposed to the bloodstream and this initiates a cascade of events that starts with formation of a complex between TF and FVII (Factor VII) or FVIIa. The TF– FVIIa complex then activates the zymogens of two additional coagulation proteases, FIX and FX (Factor X). The active form of FIX (Factor IXa) stimulates a small release of thrombin, priming platelets and activating FVIII (Factor VIII). These initial events provide the system for rapid amplification leading to the formation of a blood clot, as the activated FIXa–FVIIIa complex binds to the platelet surface activating FX more efficiently to yield a burst of thrombin formation [37]. Thrombin then converts fibrinogen into fibrin, which forms the meshwork of a blood clot.
Several proteins of the coagulation cascade, as well as relatively complex mixtures of coagulation proteins, are approved for the treatment of bleeding disorders. Three coagulation proteins, FVIII, FIX and FVII, are used as factor replacement therapy for haemophilia A, B and C respectively. FVIII is a large protein that when activated to FVIIIa is a cofactor of FIXa, whereas FVII and FIX are serine protease zymogens. Early treatments for haemophilia relied on plasma extracts to replace missing coagulation factors [38] and numerous plasma-derived biologicals containing proteases are approved currently. Epidemics such as AIDS and hepatitis C were devastating for haemophiliacs due to the biological source of the agents used to treat them, which provided a strong impetus for the development of safer recombinant versions of coagulation factors. This has improved the overall safety of biological therapeutics in general. Although the risk of contamination by human viruses and prions from plasma-derived proteases has been reduced greatly with improved screening and viral inactivation techniques [39], recombinant technology is now the preferred method of protease production.
The FIX protease has been used successfully as a replacement therapy for haemophilia B patients to treat acute bleeding episodes and bleeding during surgical procedures. More recently, FIX has been used prophylactically to mitigate some of the chronic complications of this disease [40]. FIX collected as a plasma fraction, either complexed with FII, FVII and FX or purified, is marketed by several manufacturers. Examples of such products include AlphaNine® (Grifols Biologicals), Konyne® (Bayer), Mononine® (CSL Behring), Prolifnine® (Grifols Biologicals) and Proplex T® (Baxter).Inhumans, FIX is synthesized in hepatocytes as a single-chain zymogen that is modified extensively post-translationally before it is released into the blood. Since the potent procoagulant activity of FIX is dependent upon these post-translational modifications, the drug cannot be produced in bacteria and requires the use of eukaryotic cell cultures. FIX contains a Gla (γ-carboxyglutamic acid) domain at its N-terminus and its modification is essential for binding to activated platelet membranes [41]. Many other clotting factors are similarly modified. In this vitamin K-dependent process, an additional carboxy group is added on to glutamate residues allowing the modified proteins to bind to negatively charged membrane surfaces in the presence of calcium. Proper γ-carboxylation is also important for the efficient secretion of FIX from its recombinant production hosts. Two EGF-like domains and an activation peptide separate the serine protease domain from the cell surface and contribute to substrate and cofactor binding. The molecule also contains β-hydroxyaspartic acid residues that are important in calcium binding within the EGF domains [42,43]. The activation peptide is removed when FIX is activated to FIXa leaving two chains, one containing the Gla and EGF domains and the other containing the serine protease domain; the two chains are linked covalently by a disulfide bridge [44,45]. Recombinant FIX produced in CHO cells, marketed as BeneFIX® (Wyeth), was approved by the FDA in 1997. As this protease is administered in an inactive zymogen state, it is not rapidly cleared by interaction with endogenous inhibitors.
Although it has been very successful in treating acute bleeding episodes, FIX replacement therapy remains challenging for chronic prophylactic use because of the enzyme’s relatively short half-life in circulation. FIX is cleared from the blood with a terminal half-life in humans of approx. 18 h; consequently, two to three injections a week of FIX are required to mitigate effectively bleeding tendencies in haemophilia B patients [46]. A complication of FIX replacement therapy is the development over time of antibodies against it due to the required multiple and continued dosing. Anti-FIX antibodies are known as inhibitors since they were observed prior to their identification as antibodies, and they result in prolonged bleeding times once they arise [47]. Inhibitors arise in approx. 2.5% of patients, most commonly in patients with severe haemophilia who have extremely low endogenous FIX levels [48]. These patients do not respond well to immune tolerance therapy, which involves periods of sustained dosing, a procedure that has proved more successful in haemophilia A patients with antibodies against FVIII. To combat this complication, knowledge of the coagulations system has been used to promote enhanced haemostasis using the extrinsic pathway and FVIIa, which is known as bypass therapy [49].
Expanding indications from haemophilia to bleeding: FVIIa
Recombinant FVIIa, NovoSeven® (Novo Nordisk), is the first recombinant product available for the treatment of haemophilia patients that have neutralizing antibodies (i.e. inhibitors) towards FVIII or FIX [50]. FVIIa is currently generating significant interest for trauma care and for uncontrolled haemorrhage and coagulopathy [51]. Other indications for FVIIa therapy include patients with bleeding tendencies from liver disease [52], thrombocytopenia [53], qualitative platelet dysfunction [54] and for patients with normal coagulation systems undergoing extensive surgery [55] or who have experienced major trauma and haemorrhage [56]. As mentioned above, development of antibody-based inhibitors is one of the most serious limitations of haemophilia A and B replacement therapy and renders conventional replacement therapy ineffective. Recombinant coagulation FVIIa has been demonstrated to be a safe and efficacious alternative procoagulant therapy for patients with inhibitors in several clinical trials [55,57,58]. The drug is approved in the U.S.A. for use in haemophilia patients with inhibitors and in Europe for additional indications such as FVII deficiency and Glanzmann’s thrombasthenia.
FVIIa initiates coagulation and accelerates clot formation though its interaction with TF (Figure 1). Because FVIIa plays such a crucial role in the initiation of blood coagulation, genetic defects that reduce dramatically its expression or function are extremely rare. Typical zymogen activation of trypsin-like proteases involves the burial of a nascent N-terminus into the core of the protein leading to the stabilization of a highly active conformation of the protease. However, the propensity of the N-terminus in free FVIIa to become buried is low, thus rendering the activated protein zymogen-like and its proteolytic activity dependent upon binding to TF. Complexation induces structural rearrangement involving both the active site, a key structural helix, as well as a loop in the extended substrate binding pocket of FVIIa [59,60]. Some structures of the active enzyme indicate that activation may also involve conformational changes in the oxyanion hole, which is responsible for stabilization of the transition state during catalysis [61]. Like FIX, FVIIa is γ-carboxylated; it has a Gla domain that is required for membrane interaction and two EGF domains. Both the protease and the EGF domains are involved in the recognition of TF as defined clearly by the crystal structure of their complex [62]. Because FVIIa promotes coagulation by binding exposed TF and/or activated platelets at the site of injury, its effects are local and systemic effects are thought to be minimal, a feature that is vital for the success of current and future versions of this therapeutic protease.
FVIIa belongs to a subset of proteases whose active form presents a non-canonical active-site cleft that allow them to evade interaction with endogenous inhibitors, thus imparting a prolonged half-life. For these proteases to express their full catalytic activity they must interact with specific cofactors or substrates. Following activation into its two-chain form, FVIIa displays poor activity towards substrates and inhibitors until it forms a complex with TF [63]. In turn, this allows FVIIa to evade interaction with inhibitors and results in a longer half-life of approx. 3 h, which is considerably longer than most other homologous proteases that are administered in an active form. Distorted active-site architectures are also observed in the complement cascade where several of the proteases circulate in the bloodstream as an active form that is resistant to inhibition. Current protein engineering efforts aim to improve the activity of FVIIa while maintaining these favourable pharmacokinetic properties. Our understanding and application of FVIIa would almost certainly be enhanced significantly by the solution of the structure of the inhibitor-free protease, either by crystallography or NMR, to guide future developments.
Surgical sealant: thrombin
Thrombin, a pivotal component of the coagulation cascade, converts fibrinogen into fibrin monomers that then multimerize to form stable blood clots (Figure 1) [64]. Thrombin binds and activates other substrates including FVIII, FV (Factor V) and PAR (protease-activated receptor)-1 and, in severe trauma, PAR-4 [65]. In complex with TM (thrombomodulin), thrombin elicits anticoagulant effects by activating protein C. As will be discussed below, this dual nature of thrombin has been exploited in protein engineering studies to produce anticoagulant molecules that lack the procoagulant functionality and have potential clinical application [66]. Prothrombin, the zymogen of thrombin contains an N-terminal Gla domain, two kringle domains and a serine protease domain [67]. Proteolytic activation of prothrombin by FXa bound to FVa removes the calcium- and membrane-binding Gla domain, thus allowing thrombin to diffuse locally near sites of its activation. Bovine thrombin, combined with fibrin and sometimes collagen, has been used in various formulations to treat bleeding from surgery or trauma. For example, bandages containing both bovine thrombin and bovine fibrin (such as D-Stat Dry) have been approved as medical devices to control surface bleeding. Despite the fact that proteins of bovine origin may result in the development of allergic reactions, they offer the advantage of being relatively inexpensive to manufacture. In 2008 the FDA approved topical recombinant human thrombin marketed as Recothrom® (Zymogenetics) to help stop small blood vessels from bleeding after surgery. Recombinant human thrombin was found to be as effective as bovine thrombin while being less immunogenic [68,69]. Procoagulant proteases, formulated into bandages with or without their natural substrates, offer significant potential for the treatment of traumatic injury.
Tackling sepsis: APC (activated protein C)
Sepsis represents a spectrum of severe diseases that result from serious infection and the protective response to microbial invasion. In the U.S.A. as many individuals die annually from sepsis as from acute myocardial infarction; consequently, there is considerable need for therapeutics to combat this disease. Among the myriad effects resulting from sepsis is the high prevalence of disseminated intravascular coagulation. Reduced levels of APC during sepsis suggests this protease as a potential drug that could alleviate this hypercoagulable state and permit medical intervention via additional treatments [70]. APC is a plasma serine protease involved in both blood coagulation and inflammation [71,72]. The protein C zymogen is composed of two peptide chains joined by a disulfide bond. The N-terminal light chain contains two EGF domains and an N-terminal Gla domain [73]. Protein C is activated by the thrombin–TM complex [74]. APC cleaves FVa and FVIIIa in the presence of the cofactor protein S, thus removing their cofactor function, and down-regulates the magnitude of the coagulation response [75]. Both the anticoagulant and anti-inflammatory properties of APC are relevant therapeutically; however, the pleiotropic effects of APC have complicated its clinical use.
Anti-inflammatory and anti-apoptotic functions of APC are mediated by its interactions with the EPCR (endothelial protein C receptor) [76]. Much of the cytoprotective effect is attributable to the interaction of APC with the EPCR and the subsequent cleavage of PAR-1 [77]. Downstream signalling through PAR-1 alters the gene expression profile towards the anti-inflammatory and anti-apoptotic pathways [78]. However, this effect is paradoxical to the procoagulant signalling through PAR-1 resulting from its activation by thrombin [79]. Downstream signalling through APC can function independently of PAR-1, such as through the ApoE (apolipoprotein E) receptor [80] and leucocyte integrins β1 and β3 [81]. Much work remains to be done to fully elucidate the role of these pathways in the biology of APC.
Recombinant human APC [drotrecogin alfa, Xigris® (Eli Lilly)] was approved in 2001 for the treatment of severe sepsis. There was considerable disagreement during the FDA deliberations, yet approval was given for the use of APC in patients with sepsis at high risk of death as indicated by multiple organ failures [82]. The observed efficacy of (activated) drotrecogin alfa is principally on the basis of the results of a single randomized double-blind placebo-controlled multicentre study [83]. Despite its noted benefits, APC remains controversial and studies have questioned its therapeutic benefit [84,85]. At issue are the classification criteria for patients responsive to treatment with APC. Patients with higher scores in the APACHE II (Acute Physiology and Chronic Health Evaluation II) system tend to respond better to APC [86]. However, the excessive anticoagulant abilities of APC are significant regardless of patient classification and an increased prevalence of serious bleeding events and patient death has been documented for APC-based therapy [87,88]. Absence of alternative strategies and prior success in APC therapeutic development warrants modification of the protease through protein engineering to reduce its anticoagulant properties, while maintaining its cytoprotective benefits to minimize these unwanted side effects.
Taming toxins for therapy: botulinum toxins A and B
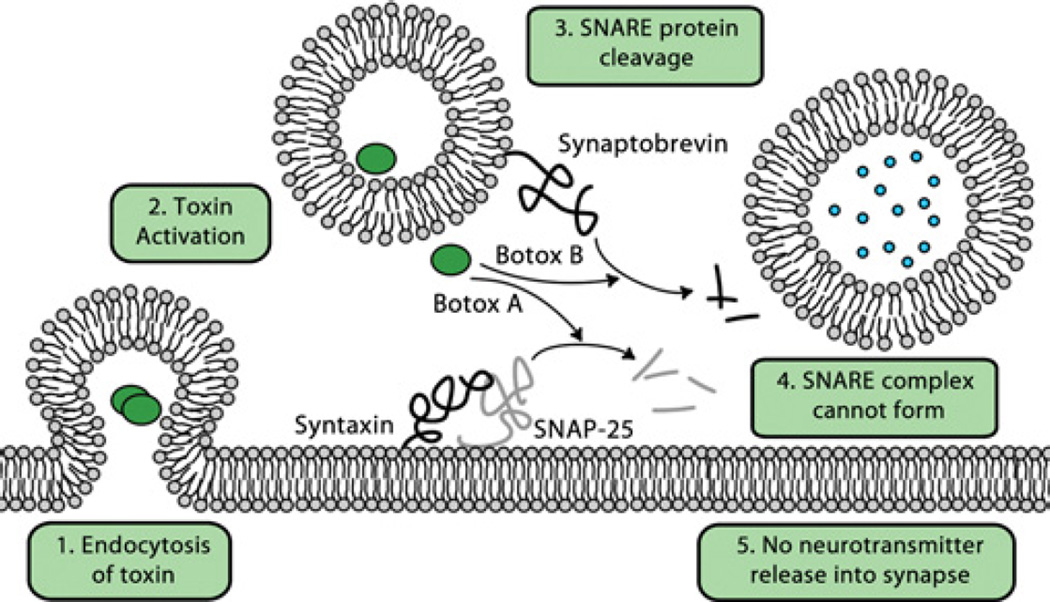
Botulinum toxin is a neurotoxin produced by C. botulinum, a Gram-positive spore-forming obligate anaerobe found in soil [89]. Botulinum toxin occurs as seven antigenically distinct types designated as A–G, which are defined by a lack of antibody cross-neutralization. Other strains of clostridia have also been shown to produce botulinum toxin. Of the seven known serotypes, type A botulinum toxin has the longest duration of action, and thus is most appropriate for cosmetic and medical uses; however, the reason for the differences in its properties is unknown [90]. Although botulinum toxin is cleared rapidly, its effects can last for months [91]. Botulinum toxin disrupts neurotransmission by inhibiting the release of acetylcholine at the presynaptic cholinergic nerve terminals of the peripheral nervous system and at the ganglionic nerve terminals of the autonomic nervous system (Figure 2). The heavy chain of botulinum toxin mediates the binding of the toxin to the neuronal cell membrane at the nerve terminus, and the toxin is then internalized by receptor-mediated endocytosis. The light chain then undergoes ATP-and pH-dependent translocation into the cytosol where, as a zinc-dependent endoprotease, it cleaves and inactivates specific components of the SNAP (soluble N-ethylmaleimide-sensitive factor-attachment protein)–SNARE (SNAP receptor) complex. Although all botulinum toxin serotypes inhibit acetylcholine release, the intracellular substrate of botulinum toxin varies by serotype: botulinum toxin types A, C and E cleave SNAP-25; types B, D, F and G cleave synaptobrevin; and type C cleaves syntaxin.
Figure 2. Mechanism of botulinum toxin action.
Endocytosis leads to activation of the toxin and separation into its heavy and light chains. The light-chain protease transports to the cytoplasm where it degrades one or more proteins involved in SNARE-mediated vesicle transport. The downstream effect of these proteolytic events is a diminished release of acetylcholine and neurotransmission. Botulinum toxin type A (Botox A) and botulinum toxin type B (Botox B) have different substrates in the synaptic fusion complex. Botox A cleaves synaptosomal-associated protein of 25 kDa (SNAP-25), whereas Botox B inactivates synaptobrevin, and both are key members of the SNARE complex. An animated version of this Figure is available at http://www.BiochemJ.org/bj/435/bj4350001add.htm
In 1989, the FDA approved botulinum neurotoxin A Botox® (Allergan) for the treatment of strabismus and blepharospasm associated with dystonia, which involve muscular contractions around the eye [92]. Subsequently, various other disorders related to muscle physiology were explored and in 2002 the FDA approved botulinum toxin type A for cosmetic use to treat glabellar lines, also known as frown lines. In 2000, botulinum toxin type B was approved for the treatment of cervical dystonia. Botulinum toxin type A is in clinical trials for numerous additional indications, including pain, muscle spasticity, overactive bladder, alopecia areata, benign prostatic hyperplasia and headaches, whereas type B is in clinical trials for spasticity due to cerebral palsy and cervical dystonia [93]. Besides the development of botulinum toxin for more indications, other efforts are aiming to reduce immunogenicity of the material, developing less invasive delivery methods and improving stability of the drug [94,95].
Proteases as digestive aids
Virtually all patients with cystic fibrosis suffer from severe intestinal malabsorption that is due mainly to a deficiency in pancreatic enzymes. Abnormal levels of bile salts, bicarbonate deficiency and other factors contribute to the problem. Effective treatments therefore should allow a normal to high-fat diet, control symptoms such as pancreatic lesion and achieve normal nutrition. Pancreatic enzyme replacement therapy involving a defined mixture of proteases, lipases and amylases can be used to achieve normal or near-normal absorption in most people with cystic fibrosis. Zenpep® (Eurand), or pancrelipase, is a porcine-derived pancreatic enzyme product that was recently approved for cystic fibrosis patients and that has been shown to improve both fat and nitrogen uptake in patients [96]. It is one of many such digestive protease preparations that have been approved and marketed [97].
Non-FDA approved uses of proteases: wound debridement
Debridement involves the removal of dead and damaged tissue from wounds in order to assist in their healing. Much of the material is protein and proteolytic enzymes therefore offer the opportunity to speed degradation of this material and hence the recovery process. For a number of years, papain, a cysteine protease from the papaya fruit, was used without regulation for wound debridement. In 2008, the therapeutic use of papain was brought under regulation by the FDA and removed from sale for this purpose following reports of adverse effects. It should be noted that papain is a common agent for meat tenderization, highlighting its broad and potentially excessive potency. Recent research has explored new ways of more properly administering papain for wound debridement [98]. Other proteases including collagenase, bromelain, trypsin and thermolysin have been suggested for the debridement of wounds and burns [99].
EXPANDING THE PROTEASE PHARMACOPEIA
As shown in Table 2, several protease drugs are in development with the potential to improve upon the currently approved therapeutics. These include FVII, FIX and t-PA protein-based biologicals, and the use of gene therapy to deliver FIX. In addition to the proteases in clinical trials that represent potential improvements on currently marketed therapies, novel protease therapies are also being evaluated as summarized in Table 3. Each protease drug is discussed below and the key points of their development described.
Table 2.
Improved protease therapies in clinical development
| Protease | Developer | Description | Development stage |
|---|---|---|---|
| Desmoteplase | Lundbeck | Recombinant bat t-PA, rDSP | Phase III |
| FIX–Fc | Biogen Idec | Fusion of an Fc fragment of IgG with FIX | Phase III |
| FVIIa analogue, NN1731 | Novo Nordisk | Fast-acting FVIIaV158D/E296V/M298Q–FVIIa | Phase II |
| GlycoPEGylated FVIIa, NN7128 | Novo Nordisk | Long-acting FVIIa derivative | Phase II |
| FIX | St. Jude Children’s Research Hospital | Adeno-associated viral vector expressing human FIX (scAAV2/8-LP1-hFIXco) |
Phase I/II |
| GlycoPEGylated FIX, NN7999 | Novo Nordisk | Long-acting FIX derivative | Phase I |
| FIX–albumin fusion | CSL Behring | FIX fused with albumin | Phase I |
| GlycoPEGylated FVIIa, NN7129 | Novo Nordisk | Subcutaneous long-acting FVIIa derivative | Phase I |
| BAY 86–6150 | Bayer Schering Pharma/Maxygen | Recombinant FVIIa variant, developed with gene shuffling technology |
Phase I |
| FIX | Children’s Hospital of Philadelphia | Adeno-associated viral vector expressing human FIX (AAV2-hFIX) |
Phase I |
| FVIIa analogue | Pfizer/Catalyst Biosciences | Engineered FVIIa | Preclinical |
| PEGylated FIX | Baxter International | Long-acting rFIX derivative | Preclinical |
| rFVIIa–albumin fusion | CSL Behring | FVIIa fused with albumin | Preclinical |
| FVIIa–CTP, MOD-5023 | Prolor Biotech | Long-acting FVIIa analogue | Preclinical |
| FXa analogue | Pfizer/Children’s Hospital of Philadelphia | Engineered FXa | Preclinical |
| Engineered FIX and other proteases | Bayer Schering Pharma/Direvo | Therapase technology, proteases with altered specificity and inhibitor resistance |
Preclinical |
Table 3.
Novel protease therapies in clinical and preclinical development
| Protease | Developer | Technology description | Disease indication | Development stage |
|---|---|---|---|---|
| Liprotamase, protease from the fungus Aspergillus melleus |
Eli Lilly | A component of Trizytek™ (digestive enzymes: lipase, protease and amylase mixture) |
Cystic fibrosis with exocrine pancreatic insufficiency |
Phase III/NDA (New Drug Application) |
| Microplasmin | ThromboGenics NV | Recombinant microplasmin injected into the vitreous humour of the eye |
Vitreomacular adhesion | Phase III |
| Glutamine-specific cysteine protease (EP-B2) and a proline-specific prolylendopeptidase (PEP), ALV003 |
Alvine Phamraceuticals | Recombinant proteases to digest gluten |
Coeliac disease | Phase II |
| Microplasmin | ThromboGenics NV | Recombinant microplasmin | Acute peripheral arterial occlusion, deep vein thrombosis |
Phase II |
| Plasmin | Talecris Biotherapeutics | Plasma-derived plasmin | Peripheral arterial occlusion | Phase I |
| Recombinant plasmin | Talecris Biotherapeutics | Recombinant plasmin | Peripheral arterial occlusion | Preclinical |
| Recombinant plasmin (BLX-155) | Biolex | Recombinant plasmin | Acute peripheral arterial disease, deep vein thrombosis and haemodialysis |
Preclinical |
| Recombinant human lysosomal protease, HTI-501 |
Halozyme | Recombinant FVIIa variant, developed with gene shuffling technology |
Medical and cosmetic dermatological applications |
Preclinical |
| Kallikrein | Wanxing Pharmaceutical | Recombinant human kallikrein-1 | Thrombosis, peripheral vascular disease, cerebrovascular ischaemia |
Preclinical |
| Calpain 3 | Généthon | Gene therapy; adenoviral vectors containing caplain 3 |
Replacement therapy, calpainopathy (a limbgirdle muscular dystophy) |
Discovery |
| Penzyme | Zymetech | Proteases that digest outer layers of skin |
Psoriasis, eczema and dermatitis, cosmetic dermatological use |
Marketed for topical use, not FDA-approved |
New thrombolytics and anticoagulants
t-PA variants
Although thrombolytic therapy has been adopted widely as a frontline strategy in the treatment of AMI and stroke, significant need still exists for thrombolytic agents with improved pharmacokinetics and pharmacodynamics. Multiple approaches have been, and continue to be, applied in developing next-generation t-PAs, while other efforts are focusing on developing formulations of plasmin, the protease produced by t-PA activity in vivo. Approaches to extending the half-life of t-PA and other proteases via engineering, include fusions with other proteins, mutations, deletions and post-translational modifications. The redesign of t-PA has been reviewed extensively and, as described above, provided second-generation therapeutic agents [100,101]. The success of t-PA in its therapeutic application relies, in part, upon its requirement for the selective binding to fibrin to express any significant activity, thus limiting the systemic generation of plasmin and focusing the activity to sites of clotting. Building upon this concept, agents with an even higher affinity for fibrin have been sought. For example desmoteplase, a plasminogen activator from the saliva of Desmodus rotundus, is more specific for fibrin than human t-PA [102]. In a preclinical model, desmoteplase was twice as potent as alteplase with a shorter lysis time and lower reocclusion rate of blood vessels. Desmoteplase appears more resistant to proteolysis by plasmin, the product of its action, and this may improve its activity. An initial Phase II trial also showed better efficacy than alteplase with a comparable safety profile [103]; however, a second Phase II trial [DIAS-2 (Desmoteplase In Acute Ischemic Stroke 2)] did not reproduce this positive outcome. Alternatively, the therapeutic efficacy of t-PA molecules is being enhanced by lowering its affinity for endogenous inhibitors via rational design as demonstrated in tenecteplase. Newer variants include duteplase [104] and monteplase [105]. Notwithstanding the success of t-PA therapeutics, it appears that alternative directions may be necessary for optimal thrombolytic therapy. Contributions from the architecture of the fibrin clot, as well as the TAFI-mediated abrogation of clot dissolution, may be key parameters that influence these outcomes and should be examined further. As proteases often function within cascades of proteolytic activation, agents either upstream or downstream within the target pathway can be used for therapeutic benefit. For t-PA, a natural shortcut focuses directly on plasmin administration.
Plasmin from both plasma-derived and recombinant sources is being developed for the treatment of vascular occlusion. Prior to emergence of t-PA and u-PA, plasmin received significant attention and was tested in several animal models. These early results demonstrated effectiveness of this protease when administered locally to sites of thrombi, with systemic administration being observed to be ineffective [107]. The rapid inactivation of plasmin is mediated by the serpin α2-antiplasmin. Plasmin was abandoned in these early years for two reasons. First, the utility of systemic dosing afforded by u-PA and then t-PA appeared clearly advantageous. Secondly, plasmin administration required catheterization, which as a technique has only recently has become amenable for its routine use in the clinic. Given the recognized bleeding risks associated with systemic administration, there is an increased interest in more directed therapies. In this approach, plasmin is administered near sites of the thrombus where it binds and is shielded from α2-antiplasmin [108]. Several domain deletion variants of plasmin are being tested that contain either a protease and single kringle domain or a protease domain alone. Truncation of these domains does not remove the ability of the protease to bind fibrin. but instead reduces the rate of association with α2-antiplasmin. Production of the full-length form of the protease requires it to be maintained at lower pH owing to its autolysis, and production schemes for other variants have harnessed this intrinsic property. For example, recombinant microplasmin lacks all five kringle domains that result from the complete autolytic breakdown. Despite the impressive biochemical data obtained with plasmin and its variants in vitro, the early clinical trials have not reflected this potential [109]. However, plasmin and its derivatives may find use in other settings. For example, microplasmin is in development for enzymatic vitreous disruption and the enzyme appears to be more effective than surgical separation of the vitreous cortex from the retina, which is of relevance to the treatment of retinal detachment and vitreous haemorrhage.
Snake-derived proteases and inhibitors
Novel proteins that modulate haemostasis will continue to expand the pharmacopeia of proteases. A protease inhibitor hirudin, from the medicinal leech Hirudo medicinalis, has been used widely for restricting the activity of thrombin and engineered extensively for improved therapeutic properties [110]. Snake venoms and other pathogens that modify the haemostatic system, such as fleas and ticks, contain a wealth of proteases and protease inhibitors that have therapeutic potential [111]. Moreover, some of the unique features by which these novel proteases achieve their function can be applied to improve the properties of recombinant human proteins. In particular, glycosylation at sites around the active-site cleft of proteases offers the potential to prolong half-life in vivo by restricting inhibition by serpins. Notably, such glycosylation does not appear to restrict the interaction with the target substrates or their catalytic turnover, suggesting evolutionary fine-tuning that can be mimicked within the laboratory.
Ancrod is a defibrinogenating protease purified from the venom of Calloselasma that was given FDA fast-track status in 2005. Its expression in Pichia pastoris has been demonstrated as an effective alternative to the production of the material from live snakes [112]. The active form of the protease has a remarkable half-life of 4–5 h due to its heavy glycosylation. Ancrod cleaves and inactivates most of the circulating plasma fibrinogen pool. One of the breakdown products of fibrinogen, desAA-Fibrin, acts as cofactor for t-PA-induced plasminogen activation and hence ancrod is profibrinolytic. An important consequence of ancrod treatment is its ability to decrease blood viscosity by removing fibrinogen, a significant component of blood, from circulation. Increased blood flow is desirable because it associates with less intense pain and improves limb mobility in patients with peripheral arterial occlusion, thus enabling physical therapy. However, efficacy of ancrod was not demonstrated in a Phase III trial of 500 subjects for treatment of acute ischaemic stroke, despite reductions in fibrinogen levels, and its application as a therapeutic is being re-evaluated [113]. Similarly, alfimeprase (Bayer), a 23 kDa zinc-containing metalloprotease with similar fibrinolytic function from Agkistrodon contortrix, has been in development despite its failure to show efficacy in two Phase III clinical trials in 2006 [114,115]. As most snake venoms contain multiple proteolytic enzymes, other possibilities for novel proteases with therapeutic applicability exist, perhaps to be used in combinations more reflective of the venoms from which they derive.
Bacterially derived protease activators
Novel protease activators may also be obtained from pathogenic bacteria. Staphylokinase is a 136-amino-acid protein produced by certain strains of Staphylococcus aureus, which was shown more than 40 years ago to have profibrinolytic properties. The mechanism of activation of plasminogen by staphylokinase bears similarities to that of streptokinase. Staphylokinase forms a 1:1 stoichiometric complex with plasminogen, but requires conversion into staphylokinase–plasmin whereupon it becomes an extremely potent plasminogen activator [116]. Notably, the initial complex is not recognized by endogenous inhibitors and this allows the effects of the complex to be localized to sites of proteolytic activity and sustained for longer periods of time.
Despite strong in vitro results, initial tests using staphylokinase in dogs were discouraging and the protease showed limited thrombolytic potency and bleeding. Interest in the development of staphylokinase as a thrombolytic agent diminished during the 1990s, yet recently it was shown these studies were misleading because dogs are particularly sensitive to systemic fibrinolytic activation. In a randomized study compared with alteplase in patients with AMI, recombinant staphylokinase was shown to be at least as potent and significantly more fibrin selective than t-PA [117]. However, as a heterologous protein staphylokinase induces antibody formation and resistance to its repeated administration. Protein engineering has targeted the immunodominant epitopes of the molecule to minimize this limitation. Results from preclinical studies suggest that a second-generation staphylokinase may have the potential to provide an even better efficacy and safety profile and, most importantly, reduced its immunogenic response compared with earlier versions of staphylokinase as well as to some other earlier established thrombolytics.
Improved digestion
Oral delivery of combinations of proteases and other digestive enzymes for the treatment of pancreatic insufficiency applies to the treatment of cystic fibrosis, cancer and a number of other diseases. Replacement enzymes are derived currently from porcine pancreas extracts. However, patients can develop allergies to such extracts and thus recombinant human enzymes are desirable for treating these conditions. Trizytek™ (Eli Lilly) (formerly Altus-135) is a mixture of recombinant digestive enzymes being developed currently. Trizytek™ comprises a lipase, amylase and an alkaline elastolytic protease from the fungus Aspergillus melleus [118]. A Phase III clinical trial of Trizytek™ has been completed and the patients showed a statistically significant improvement in the coefficient of absorbed nitrogen, indicating that the protease component of the mixture was improving protein digestion.
Proteases to digest peptides in gluten may eventually be used to treat coeliac disease, which is caused by gluten hypersensitivity resulting in inflammation of the intestine [119]. A glutamine-specific cysteine endoprotease, EP-B2, and a proline-specific prolyl zinc-dependent endopeptidase, PEP (prolyl endopeptidase), are currently being developed in combination for oral therapy. These enzymes hydrolyse the immunotoxic gliadin peptides produced when gluten is hydrolysed by normal digestive enzymes. Gliadin peptides that are rich in proline and glutamine residues result from gluten digestion, and a 33-mer gliadin peptide is reported to be responsible for initiating the inflammatory cascade in coeliac disease patients [120]. PEP is expressed by various bacteria including Lactobacillus helveticus [121], which is used in cheese making, and EP-B2 is expressed in barley [122]. Combination of these proteases has been tested in a Phase I clinical trial and found to be safe in healthy volunteers.
Cosmeceuticals and dermatology
Recombinant human lysosomal proteinase is being developed for dermatological use. The cysteine protease cleaves collagen, but because it normally acts in the lysosome that has a pH of approx. 4.8, the enzyme has reduced activity at pH 7.4. Thus one use of this protease is to deliver it by injection in a low-pH buffer. As the pH at the injection site gradually re-equilibrates to physiological pH, the activity decreases thus minimizing any deleterious activities. Human lysosomal proteinase is currently in development for remodelling scar tissue. Penzyme is a mixture of trypsin and chymotrypsin obtained from the gastrointestinal tract of north Atlantic cod and digests the outer layers of the skin for the treatment of psoriasis, among other dermatological conditions, and shows promise.
CHALLENGES AND SOLUTIONS FOR THE FUTURE
Protein engineering
Proteases can be engineered to modify their half-lives through altering their interactions with other macromolecules and minimizing their antigenicity. The half-life for proteases can be improved greatly by their administration as inactive zymogens, which are larger and incapable of being removed by endogenous inhibitors. However, the extent of in vivo zymogen activation is limited and would require the active protease to be extremely potent. As observed with snake venom proteases, glycosylation can be used to sterically hinder the protease, yet this has not been tested rigorously by engineering other enzymes and the limited surface area of a protease suggests potential challenges. Protein engineering may be used to reduce the antigenicity of non-human therapeutic proteases or exogenous protein-based protease activators, such as streptokinase and staphylokinase, because the patient’s immune system does not recognize the missing protease as self [123]. Extending the therapeutic window for protease activity may produce undesirable effects due to the many physiological roles of proteases and other possible unintended consequences. For example, the t-PA variant lanetoplase was designed to have a longer half-life that would have improved clinical utility by reducing dosing frequency. However, lanetoplase was associated with severe intracranial haemorrhage, probably due to unregulated thrombolysis [124]. A comprehensive understanding of the multiple biological roles of proteases at both physiological and pharmacological levels is therefore an important component for advancing their therapeutic use. Protein engineering to sharpen and focus protease activity to only one or a few targets or sites is one promising avenue for future research.
Engineering of proteases to improve clinical utility will require a deeper understanding of their interactions with protease inhibitors. Serpins are especially significant in this respect given their abundance and strength of interaction with their target proteases using their ‘suicide-inhibition’ mechanism that destroys both the serpin and the protease in the creation of a covalent complex [125]. Coagulation, inflammation and complement are controlled by the serpins α1-antithrombin, α1-antitrypsin and C1-inhibitor respectively [126]. In contrast, PAI-1 plays a key role in the modulation of angiogenesis and affects both wound healing and tumour growth [127]. Serpin–protease complexes are rapidly cleared from circulation by the liver where they are bound and endocytosed by LDLR (low-density lipoprotein receptor-related) proteins [128,129]. Ligand binding by LDLR proteins is pH-dependent such that the complex dissociates in the lowered pH environment of endosomal compartments, which allows the LDLRs to be recycled back to the cell surface [130]. The abundance of inhibitors and their receptors for uptake after complex formation makes bypassing inhibition a key priority when translating the in vitro properties of proteases into in vivo and therapeutic settings. Although these restrictions can be achieved by mutagenesis of specific amino acids near the active site of the protease, they are difficult to find as they must not impinge upon the catalytic activity of the protease or negatively influence its interaction with its desired substrate. Another attractive approach used successfully with t-PA involves the disruption of this interaction via molecular modelling and rational design thus creating inhibitor-resistant proteases [30]. As many therapeutic proteases are members of the trypsin fold, they share similar sites amenable to engineering that alter their interaction with macromolecules (Figure 3). The following three examples of trypsin-like therapeutic proteases, thrombin, coagulation FVIIa and APC, are all in various degrees of development and illustrate key mutagenesis strategies.
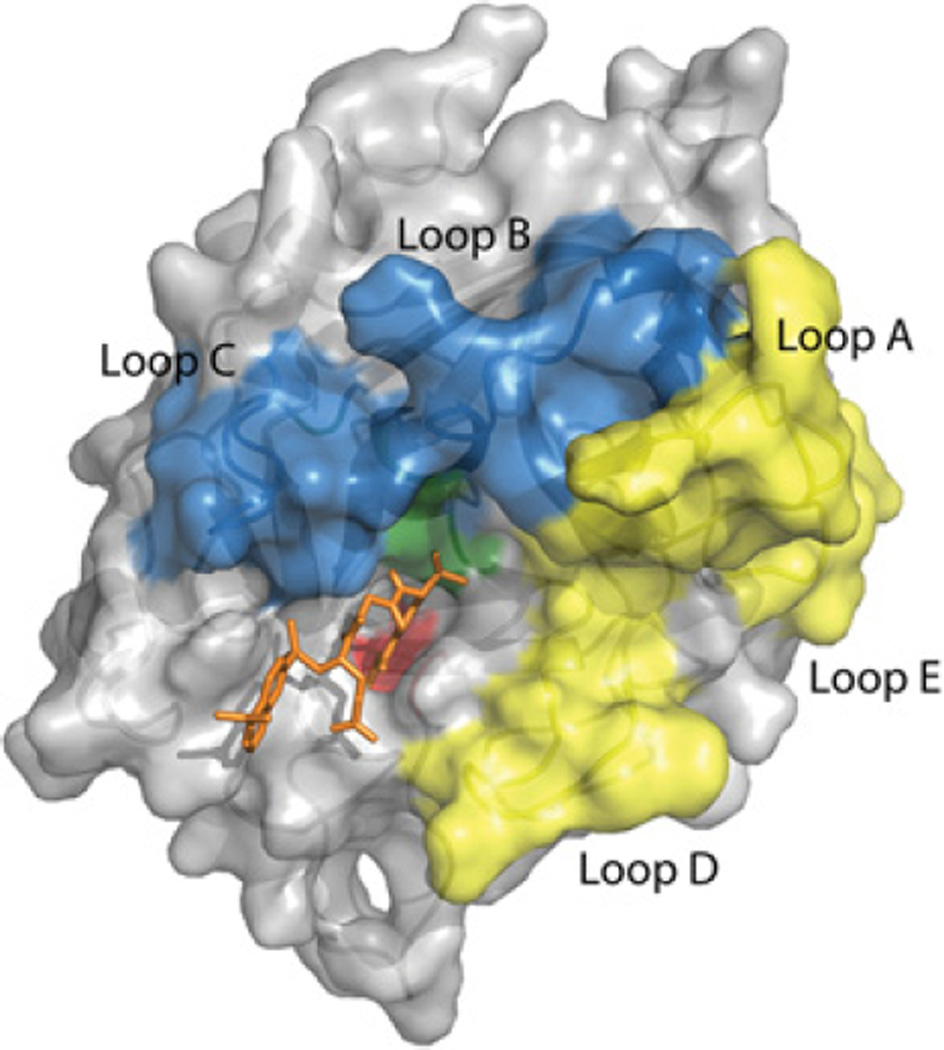
Figure 3. The trypsin fold is the most common protease fold found in the genomes of higher organisms [186] and is the most commonly scaffold of existing therapeutic proteases.
Shown is the structure of the protease domain t-PA, which like other members of the family, contains two six-stranded Greek key β-barrels lying on top of and perpendicular to one another with the active-site cleft between them [187]. Eight loops surround the catalytic triad (green) and the primary specificity pocket (red) of which five have been particularly useful targets for protein engineering. The loops above the active-site cleft near the catalytic triad (blue) make direct contacts with the substrate near the scissile bond and have been manipulated widely [188]. For reference a tripeptide inhibitor is shown in orange. The positions outside of the active-site cleft (yellow) have been engineered to restrict the interaction with macromolecular substrates and inhibitors [189]. For example, four successive alanine residue substitutions in Loop A were used to restrict the interaction of t-PA with PAI-1 [190]. Alterations within the active-site cleft have also been made to modify the properties of the protease. In thrombin such changes have been used to shift the protein to an anticoagulant with limited procoagulant functionality [64,191]. The presence or absence of protein domains attached to the protease domain can be engineered for improved therapeutic efficacy. The loops are labelled and numbered according to [192]: Loop A, residues 34–41; Loop B, residues 56–64; Loop C, residues 94–103; Loop D, residues 143–151; and Loop E, residues 74–80 in the chymotrypsin numbering system.
Thrombin has opposing functions in haemostasis whose selective reduction can be designed for therapeutic benefit [64]. As a procoagulant, it cleaves and activates fibrinogen and protease activated receptors. When bound to TM, thrombin activates protein C whose functions are anticoagulant. Shifting the balance between these two outcomes through protein engineering of thrombin has been successful and has provided many structural and biochemical insights into the molecular mechanisms of clotting factor proteases. Initial studies defined the sites of interaction with procoagulant substrates through mutagenesis and demonstrated that these interactions could be restricted [131]. However, such changes did not sufficiently shift the balance towards generation of an anticoagulant protease. The reason for these difficulties stems, in part, from the highly allosteric nature of thrombin whose structure and catalytic properties differ based upon the presence or absence of its substrates and cofactors.
At least three forms of thrombin have been reported to exist in solution and it has been proposed that re-distribution of the relative populations of these forms is directly linked to the resulting anticoagulant potency [132,133]. Transition between forms involves the binding of the monovalent cation Na+, an abundant ion in the bloodstream, producing a high activity fast form, which is procoagulant and acts upon fibrinogen and other physiological substrates. Coagulation agents FVIIa, FIXa and FXa, as well as APC, are similarly Na+-activated enzymes yet it is unclear how the structural transitions and allosteric mechanisms in thrombin are related to these proteases and their affinity towards the cation is considerable lower [134]. In the absence of Na+, thrombin interconverts slowly between two states, with one exhibiting slow catalytic turnover behaviour, whereas the other (far less abundant) form is inactive. Forcing thrombin to adopt these alternate states through mutagenesis produces molecules whose activity requires TM binding, which produces anticoagulant properties due to a severely restricted procoagulant activity. A variety of mutations have been shown to mediate this outcome due to the highly co-operative nature of this allosteric mechanism [132,135,136]. Preliminary studies have shown Na+-dependent allosteric activation is required to build a minimal variant of FXa, starting from a bacterial trypsin, implicating allostery as a fundamental component underlying coagulation factor protease function [137,138]. The W217A/E217A double mutant of thrombin has shown promise in a primate model of thrombosis [139]. The anticoagulant effect of the protease is heightened by its ability to act as a GPIb (glycoprotein 1b) antagonist and thus restrict platelet aggregation [140]. In contrast with thrombin, where selective reduction of activity is the goal, FVIIa requires fine-tuning to increase its activity while maintaining its underlying resistance to inhibition.
FVIIa is the focus of a number of studies that aim to increase its half-life owing to the large potential market for this protease [141,142]. Some strategies aim to stabilize FVIIa in the circulation by fusing it with larger proteins [143]. Genetic fusion with albumin to therapeutic proteins has been used successfully with cytokines, insulin and growth hormone [144–147]. Preclinical studies in rats of albumin fused to FVIIa indicates that the half-life of FVIIa could be substantially extended in vivo, and this could significantly reduce the overall dosing frequency required for a therapeutic benefit. Other strategies include N-glycan PEGylation and formulation with PEGylated liposomes [148,149]. As Ca2+ stimulates both the activity of FVIIa and its interaction with TF, mutations with a protease that mimic this effect may also prove useful [150]. Two FVIIa analogues are worth noting given the differences in their design strategy. The first involves increasing the catalytic activity of the protease, whereas the second focuses upon improving targeted activation.
An increased efficacy of FVIIa is sought via gains in intrinsic activity or localization. Both approaches offer the potential to induce blood clot formation more rapidly and create thrombi that are more robust and resistant to fibrinolysis. NN1731 (vatreptacog alfa) is a triple mutant (V158D/E296V/M298Q) of FVIIa in development by Novo Nordisk [151]. Mutations introduced in the molecule are found in the serine protease domain, and the region between the EGF and protease domain that stabilizes the activated form of the protease in a state that is similar to the structural transition induced by TF [152]. These mutations stabilize burial of the N-terminus of the protease that is pivotal for the expression of its catalytic activity and the engineered form is approx. 30-fold more effective in the activation of FX in both solution and on the surface of activated platelets [151,152] As expected, stabilization of the active site of FVIIa into a more conventional trypsin-like state is associated with an increased susceptibility to inhibition, both by small molecule and macromolecular inhibitors. NN1731 is in a Phase II clinical trial to treat haemophilia A or B patients who have developed inhibitors. Enhanced localization to sites where activity of the therapeutic protease affords another mechanism for enhancing the in vivo efficacy of FVIIa. One of the key limitations of FVIIa results from its low affinity for platelet membranes, which has been documented to be much higher in other Gla domain containing proteases and suggests an effect that could be transplanted. Structure–function studies of the Gla domain of FVIIa have successfully created proteases with enhanced binding characteristics and improved procoagulant properties. For example, a mutant of FVIIa bearing five substitutions in the Gla domain has been documented as presenting more than a 150-fold increase in affinity for membranes and an approx. 40-fold gain in procoagulant activity [153]. A variant of this mutant, BAY 86–6150 (Bayer), has recently completed a Phase I clinical trial for Bayer under the name BAY7. Gains in potency sought for FVIIa contrast with the selective reduction targeted in APC.
Therapeutic proteases need not rely solely on their proteolytic activity to achieve beneficial outcomes. The utility of APC, if controversial, has been demonstrated yet gains appear probable if the anticoagulant and cytoprotective effects can be tailored appropriately [154]. Considerable effort has been extended in engineering forms of APC to understand how its activities are mediated separately [155,156]. Two alternate strategies have been applied for their potential application in vivo. In the first, the overall activities of the protease are increased in an attempt to reduce the amount of protease administered, thus limiting the extent of the anticoagulant effect. In the second, the anticoagulant activity is abrogated leaving only the cytoprotective effect. Improving the pharmacokinetic properties of APC may accrue from limiting the extent of inhibition by endogenous inhibitors and, in particular, the serpin α1-antitrypsin that is present in blood at high concentration (150–350 mg/dl in serum) [157,158]. Active-site-cleft mutagenesis of APC showed an increased half-life and a reduction in the rate of inactivation in human plasma of 4–6-fold. Neutralization of the positively charged cluster within the protease domain restricted its interaction with FVa, while maintaining cytoprotective effects [159]. Moreover, critical residues that disrupt the interaction with PAR-1 have been defined that eliminate the cytoprotective signalling of APC without affecting its anticoagulant activity [160]. It has been shown that calcium plays a role in both the activation of protein C and the anticoagulant activity upon activation. Building upon this observation, stabilization of the calcium binding loop of APC by the addition of an engineered disulfide bond enhanced its activation and eliminated its anticoagulant activities [161]. However, the desirable anti-inflammatory activities were also reduced by these mutations highlighting the often co-operative roles for individual amino acids within the protease domain. Finally, the anticoagulant activity of APC can be reduced by mutations with the Gla domain while still allowing the construct to bind to endothelial cells [162]. APC variants that have anti-inflammatory properties, but entirely lack anticoagulant properties, may find utility in a number of inflammatory disease indications such as multiple sclerosis, ARDS (acute respiratory distress syndrome) and rheumatoid arthritis [163]. Considerable work remains to better define the non-anticoagulant mechanisms of APC to improve its clinical application.
Delivering biologicals
In general protein drugs are usually delivered by injection because of their size and sensitivity to denaturation. The route of protease drug delivery depends on the disease indication and location of the protease target. Most proteases are delivered intravenously as thrombolytic and coagulation events take place in blood. Thrombin may be administered topically to stop bleeding locally. No clinically used proteases act intracellularly, except for botulinum toxin that is delivered by injection into muscle and has a natural mechanism of endocytosis for cellular entry. Digestive enzymes are delivered orally due to their action in the digestive tract and the fact that they are resistant to the low pH of the stomach. For systemic applications minimally invasive delivery approaches would be desirable and, in the case of haemophilia, constitutive replacement of these proteins would be preferable to injections. We can anticipate more improvements using engineered proteases and new delivery options such as gene and cell therapy [164]. New routes of delivery could expand the safety and clinical utility of protease therapies. For example, the most common adverse event of APC therapy is bleeding. Studies of APC in a endotoxin-induced pulmonary inflammation murine model suggest that APC inhalation might be a strategy to improve lung function, while avoiding the risk of bleeding. [165]. Much like the return of plasmin, on the basis of technological gains in catheterization these improved methods of delivery may allow proteases to be used successfully in a variety of additional settings.
Gene therapy for constitutive replacement of proteases
The ability to deliver proteases continuously and intracellularly would expand greatly the potential clinical applications of numerous proteases. The short half-life of proteases is generally a disadvantage for their therapeutic application because it requires the protease be dosed frequently. For certain disease indications, sustained delivery via gene therapy would be desirable. Adenoviral vectors expressing FVII and FIX have been tested in rodents [166–169]. Gene therapy using adenoviral vectors to deliver F9, the gene that encodes human FIX, is now in a Phase I clinical trial [170,171]. In this study, an adenoviral vector containing F9 will be injected into the liver. Instead of correcting a deficiency of a protease, gene therapy to enhance production of tissue kallikrein is being explored to treat the hypertension and renal injury that results from chronic kidney disease and end-stage renal failure. Kallikrein is present in tissues involved in blood pressure regulation and cardiovascular function, and it cleaves kininogen to produce the nine-residue peptide bradykinin [172]. Studies in a rat model of chronic kidney failure, using recombinant adenovirus to deliver the gene that encodes human tissue kallikrein, showed that kallikrein attenuated hypertension and renal dysfunction, and protected the kidney from morphological changes [173]. The natural protective mechanisms that resist unwanted proteolysis support protease-based gene therapies.
Gene therapy for protease replacement has additional potential applications for indications that are less well recognized for the key contributions made by proteolytic enzymes. Low levels of expression of calpain 3 causes limb-girdle muscular dystrophy type 2A and replacement of this calcium-dependent cysteine protease using gene therapy is a potential treatment for this genetic disorder. Benefits of this strategy have been observed in a mouse model that include improved muscle contractility [174]. Lastly, LINCL (late infantile neuronal ceroid lipofuscinosis) is a lysosomal storage disorder caused by a deficiency of TPP-I (tripeptidyl peptidase-I). Transgenic mouse studies that replaced TPP-I, encoded by the TPP1 gene, demonstrated a therapeutic benefit [175,176]. Gene therapy for protease replacement therapy is a promising strategy moving forward.
Proteases can be engaged to act therapeutically in the treatment of cancer. Several groups are exploring the applications of proteases involved in apoptosis delivered using gene therapy to selectively kill cancer cells [177–179]. Activation of certain caspases, which are cysteine proteases, is one such approach. Using an expression system for caspase 8, in a model system containing the oncogenic human telomerase reverse transcriptase gene, the inhibition of tumours in mice has been observed [180]. Later experiments indicated that caspase 6, a downstream protease of caspase 8, was superior in its ability to induce apoptosis in two different malignant glioma cell lines [181]. The addition of caspase combinations to cancer cell lines may illicit synergistic results [182]. More recently, small molecule approaches to caspase activation have been demonstrated [183]. In this work, high-throughput screening was used to identify agents capable of activating directly procaspase 3 and 6, and that were subsequently capable of promoting cell death. Other apoptotic machinery has also been investigated for use as selective killing agents. For example, granzyme B, a serine protease typically found in the secretory vesicles of natural killer cells, is suggested as an effector domain for immunotoxins that has been actively pursued [184]. Such strategies may become more robust following additional advances in our ability to selectively deliver molecules into the cytosol of target cells.
THE OUTLOOK FOR PROTEASE THERAPEUTICS
The therapeutic use of proteases over the past several decades has provided clinical results that clearly suggest a bright future for their expanded use. When administered in their active form, proteases can have a biological half-life on the scale of minutes and this can be extended by several hours using one of several approaches. Protease engineering has been, and will continue to be, used successfully to modify their properties. The therapeutic benefits of protease drugs need not solely arise from their primary proteolytic functions and they can be applied in situations where they are not normally involved. Importantly, proteases can be administered in conjunction with conventional small molecule therapies. Similarly, proteases can be formulated with other proteins and not destroy them before administration to the patient. From a design standpoint, both human and non-human sources of proteases can be antigenic and this response depends on factors based on the composition of the protease itself, length of time exposed in circulation and both the number and amount of doses required. Proteases that are designed to have a diminished reactivity with endogenous inhibitors is a promising strategy to optimize the therapeutic benefits of proteases, yet their effects must be balanced against the potential for unwanted proteolysis and undesirable consequences. Lastly, topical administration or strategic localization of activity by interaction with cofactors or cell surfaces can avoid some of the key issues in therapeutic application of proteases.
As proteases play key roles in physiology and pathophysiology, many opportunities are available to exploit the use of proteases as therapeutics. Protease engineering and new approaches to protein delivery offer the ability to enhance or alter protease activity to expand clinical utility of proteases. In the long-term, gene therapy will probably become an option for protease replacement therapy. Recent protease drugs are replacing human- or animal-derived proteins with recombinant proteins that reduce the risk of infection from human or zoonotic pathogens, and reduce immunogenicity to non-human proteins. Puente et al. [6] have reported 53 hereditary diseases that are caused by mutations in protease genes which lead to loss-of-function. Although these diseases are rare, the recognition that they are caused by lack of protease function or low levels of expression may present new opportunities for protease replacement therapies for some of these orphan diseases akin to the use of glycosidases [185]. With further advances in our understanding of protease functions and properties coupled with improvements in protein engineering, we can expect that many more protease therapeutics will gain regulatory approval and make significant contributions in healthcare in the near-term.
ACKNOWLEDGEMENT
We thank Cammy Deluca–Flaherty for help with production of the Tables.
Glossary
Abbreviations used
- AMI
acute myocardial infarction
- APC
activated protein C
- CHO
Chinese hamster ovary
- EGF
epidermal growth factor
- EPCR
endothelial protein C receptor
- FDA
Food and Drug Administration
- FV
Factor V
- FVII
Factor VII
- FVIII
Factor VIII
- FIX
Factor IX
- FX
Factor X
- Gla
γ-carboxyglutamic acid
- HGF
hepatocyte growth factor
- LDLR
low-density lipoprotein receptor-related
- LINCL
late infantile neuronal ceroid lipofuscinosis
- PAI-1
plasminogen activator inhibitor-1
- PAR
protease-activated receptor
- PEP
prolyl endopeptidase
- SNAP
soluble N-ethylmaleimide-sensitive factor-attachment protein
- SNARE
SNAP receptor
- TAFI
thrombin-activatable fibrinolysis inhibitor
- t-PA
tissue-type plasminogen activator
- TF
tissue factor
- TM
thrombomodulin
- TPP-I
tripeptidyl peptidase-I
- u-PA
urokinase-type plasminogen activator
REFERENCES
- 1.Rodriguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim. Biophys. Acta. 2010;1803:39–54. doi: 10.1016/j.bbamcr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Duffy MJ, McGowan PM, Gallagher WM. Cancer invasion and metastasis: changing views. J. Pathol. 2008;214:283–293. doi: 10.1002/path.2282. [DOI] [PubMed] [Google Scholar]
- 3.Overall CM, Blobel CP. In search of partners: linking extracellular proteases to substrates. Nat. Rev. Mol. Cell Biol. 2007;8:245–257. doi: 10.1038/nrm2120. [DOI] [PubMed] [Google Scholar]
- 4.Puente XS, Sánchez LM, Gutiérrez-Fernández A, Velasco G, López-Otín C. A genomic view of the complexity of mammalian proteolytic systems. Biochem. Soc. Trans. 2005;33:331–334. doi: 10.1042/BST0330331. [DOI] [PubMed] [Google Scholar]
- 5.Puente XS, Lopez-Otin C. A genomic analysis of rat proteases and protease inhibitors. Genome Res. 2004;14:609–622. doi: 10.1101/gr.1946304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puente XS, Sánchez LM, Overall CM, López-Otín C. Human and mouse proteases: a comparative genomic approach. Nat. Rev. Genet. 2003;4:544–558. doi: 10.1038/nrg1111. [DOI] [PubMed] [Google Scholar]
- 7.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 8.Takeda S, Kadowaki S, Haga T, Takaesu H, Mitaku S. Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett. 2002;520:97–101. doi: 10.1016/s0014-5793(02)02775-8. [DOI] [PubMed] [Google Scholar]
- 9.Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Hanna JA, Bordeaux J, Rimm DL, Agarwal S. The function, proteolytic processing, and histopathology of Met in cancer. Adv. Cancer Res. 2009;103:1–23. doi: 10.1016/S0065-230X(09)03001-2. [DOI] [PubMed] [Google Scholar]
- 11.Bergstrom FC, Reynolds S, Johnstone M, Pike RN, Buckle AM, Kemp DJ, Fischer K, Blom AM. Scabies mite inactivated serine protease paralogs inhibit the human complement system. J. Immunol. 2009;182:7809–7817. doi: 10.4049/jimmunol.0804205. [DOI] [PubMed] [Google Scholar]
- 12.Rawlings ND, Tolle DP, Barrett AJ. Evolutionary families of peptidase inhibitors. Biochem. J. 2004;378:705–716. doi: 10.1042/BJ20031825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott CJ, Taggart CC. Biologic protease inhibitors as novel therapeutic agents. Biochimie. 2010;92:1681–1688. doi: 10.1016/j.biochi.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Overall CM, Dean RA. Degradomics: systems biology of the protease web. Pleiotropic roles of MMPs in cancer. Cancer Metastasis Rev. 2006;25:69–75. doi: 10.1007/s10555-006-7890-0. [DOI] [PubMed] [Google Scholar]
- 15.Baker SS, Borowitz D, Duffy L, Fitzpatrick L, Gyamfi J, Baker RD. Pancreatic enzyme therapy and clinical outcomes in patients with cystic fibrosis. J. Pediatr. 2005;146:189–193. doi: 10.1016/j.jpeds.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Williams JR. The fibrinolytic activity of urine. Br. J. Exp. Pathol. 1951;32:530–537. [PMC free article] [PubMed] [Google Scholar]
- 17.Medved L, Nieuwenhuizen W. Molecular mechanisms of initiation of fibrinolysis by fibrin. Thromb. Haemostasis. 2003;89:409–419. [PubMed] [Google Scholar]
- 18.Weisel JW. Structure of fibrin: impact on clot stability. J. Thromb. Haemostasis. 2007;5:116–124. doi: 10.1111/j.1538-7836.2007.02504.x. [DOI] [PubMed] [Google Scholar]
- 19.Mosnier LO, Bouma BN. Regulation of fibrinolysis by thrombin activatable fibrinolysis inhibitor, an unstable carboxypeptidase B that unites the pathways of coagulation and fibrinolysis. Arterioscler. Thromb. Vasc. Biol. 2006;26:2445–2453. doi: 10.1161/01.ATV.0000244680.14653.9a. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Gong Y, Grella DK, Castellino FJ, Miles LA. Endogenous plasmin converts Glu-plasminogen to Lys-plasminogen on the monocytoid cell surface. J. Thromb. Haemostasis. 2003;1:1264–1270. doi: 10.1046/j.1538-7836.2003.00155.x. [DOI] [PubMed] [Google Scholar]
- 21.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat. Rev. Mol. Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 22.Dass K, Ahmad A, Azmi AS, Sarkar SH, Sarkar FH. Evolving role of uPA/uPAR system in human cancers. Cancer Treat. Rev. 2008;34:122–136. doi: 10.1016/j.ctrv.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Dillon PW, Jones GR, Bagnall-Reeb HA, Buckley JD, Wiener ES, Haase GM. Prophylactic urokinase in the management of long-term venous access devices in children: a Children’s Oncology Group study. J. Clin. Oncol. 2004;22:2718–2723. doi: 10.1200/JCO.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Rijken DC, Lijnen HR. New insights into the molecular mechanisms of the fibrinolytic system. J Thromb. Haemostasis. 2009;7:4–13. doi: 10.1111/j.1538-7836.2008.03220.x. [DOI] [PubMed] [Google Scholar]
- 25.Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol. Chem. 1982;257:2912–2919. [PubMed] [Google Scholar]
- 26.Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell. Mol. Life Sci. 2000;57:25–40. doi: 10.1007/s000180050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bode C, Smalling RW, Berg G, Burnett C, Lorch G, Kalbfleisch JM, Chernoff R, Christie LG, Feldman RL, Seals AA, Weaver WD. Randomized comparison of coronary thrombolysis achieved with double-bolus reteplase (recombinant plasminogen activator) and front-loaded, accelerated alteplase (recombinant tissue plasminogen activator) in patients with acute myocardial infarction. Circulation. 1996;94:891–898. doi: 10.1161/01.cir.94.5.891. [DOI] [PubMed] [Google Scholar]
- 28.The Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO III) investigators. A comparison of reteplase with alteplase for acute myocardial infarction. N. Engl. J. Med. 1997;337:1118–1123. doi: 10.1056/NEJM199710163371603. [DOI] [PubMed] [Google Scholar]
- 29.Semba CP, Sugimoto K, Razavi MK. Alteplase and tenecteplase: applications in the peripheral circulation. Tech. Vasc. Interv. Radiol. 2001;4:99–106. doi: 10.1016/s1089-2516(01)90003-4. [DOI] [PubMed] [Google Scholar]
- 30.Madison EL, Goldsmith EJ, Gerard RD, Gething MJ, Sambrook JF. Serpin-resistant mutants of human tissue-type plasminogen activator. Nature. 1989;339:721–724. doi: 10.1038/339721a0. [DOI] [PubMed] [Google Scholar]
- 31.Smalling RW. Molecular biology of plasminogen activators: what are the clinical implications of drug design? Am. J. Cardiol. 1996;78:2–7. doi: 10.1016/s0002-9149(96)00736-9. [DOI] [PubMed] [Google Scholar]
- 32.van de Werf F, Cannon CP, Luyten A, Houbracken K, McCabe CH, Berioli S, Bluhmki E, Sarelin H, Wang-Clow F, Fox NL, Braunwald E. Safety assessment of single-bolus administration of TNK tissue-plasminogen activator in acute myocardial infarction: the ASSENT-1 trial. The ASSENT-1 Investigators. Am. Heart J. 1999;137:786–791. doi: 10.1016/s0002-8703(99)70400-x. [DOI] [PubMed] [Google Scholar]
- 33.Cannon CP, Gibson CM, McCabe CH, Adgey AA, Schweiger MJ, Sequeira RF, Grollier G, Giugliano RP, Frey M, Mueller HS. TNK-tissue plasminogen activator compared with front-loaded alteplase in acute myocardial infarction: results of the TIMI 10B trial. Circulation. 1998;98:2805–2814. doi: 10.1161/01.cir.98.25.2805. [DOI] [PubMed] [Google Scholar]
- 34.van de Werf F, Adgey J, Ardissino D, Armstrong PW, Aylward P, Barbash G, Betriu A, Binbrek AS, Califf R, Diaz R. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354:716–722. doi: 10.1016/s0140-6736(99)07403-6. [DOI] [PubMed] [Google Scholar]
- 35.Kunamneni A, Abdelghani TT, Ellaiah P. Streptokinase: the drug of choice for thrombolytic therapy. J. Thromb. Thrombolysis. 2007;23:9–23. doi: 10.1007/s11239-006-9011-x. [DOI] [PubMed] [Google Scholar]
- 36.Ueshima S, Matsuo O. Development of new fibrinolytic agents. Curr. Pharm. Des. 2006;12:849–857. doi: 10.2174/138161206776056065. [DOI] [PubMed] [Google Scholar]
- 37.Howard EL, Becker KC, Rusconi CP, Becker RC. Factor IXa inhibitors as novel anticoagulants. Arterioscler. Thromb. Vasc. Biol. 27:722–727. doi: 10.1161/01.ATV.0000259363.91070.f1. [DOI] [PubMed] [Google Scholar]
- 38.Eley RC, Green AA, McKhann CF. The use of a blood coagulant from the human placenta in the treatment of haemophilia. 1936;1936;J. Pediatr.8:13. [Google Scholar]
- 39.Pipe SW, Kaufman RJ. A chamber of hope for haemophilia. Nat. Biotechnol. 2000;18:264–265. doi: 10.1038/73690. [DOI] [PubMed] [Google Scholar]
- 40.Monahan PE, Di Paola J. Recombinant Factor IX for clinical and research use. Semin. Thromb. Hemostasis. 2010;36:498–509. doi: 10.1055/s-0030-1255444. [DOI] [PubMed] [Google Scholar]
- 41.Stafford DW. The vitamin K cycle. J. Thromb. Haemostasis. 2005;3:1873–1878. doi: 10.1111/j.1538-7836.2005.01419.x. [DOI] [PubMed] [Google Scholar]
- 42.Di Scipio Rg, Davie EW. Characterization of protein S, a γ-carboxyglutamic acid containing protein from bovine and human plasma. Biochemistry. 1979;18:899–904. doi: 10.1021/bi00572a026. [DOI] [PubMed] [Google Scholar]
- 43.McMullen BA, Fujikawa K, Kisiel W. The occurrence of β-hydroxyaspartic acid in the vitamin K-dependent blood coagulation zymogens. Biochem. Biophys. Res. Commun. 1983;115:8–14. doi: 10.1016/0006-291x(83)90961-0. [DOI] [PubMed] [Google Scholar]
- 44.Di Scipio RG, Kurachi K, Davie EW. Activation of human Factor IX (Christmas factor) J. Clin. Invest. 1978;61:1528–1538. doi: 10.1172/JCI109073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurachi K, Davie EW. Isolation and characterization of a cDNA coding for human Factor IX. Proc. Natl. Acad. Sci. U.S.A. 1982;79:6461–6464. doi: 10.1073/pnas.79.21.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gavino ACP. A 5-month-old male with an isolated prolonged partial thromboplastin time. Lab. Med. 2007;38:218–221. [Google Scholar]
- 47.Oldenburg J, Pavlova A. Genetic risk factors for inhibitors to factors VIII and IX. Haemophilia. 12:15–22. doi: 10.1111/j.1365-2516.2006.01361.x. [DOI] [PubMed] [Google Scholar]
- 48.Ljung R, Petrini P, Tengborn L, Sjo¨rin E. Haemophilia B mutations in Sweden: a population-based study of mutational heterogeneity. Br. J. Haematol. 2001;113:81–86. doi: 10.1046/j.1365-2141.2001.02759.x. [DOI] [PubMed] [Google Scholar]
- 49.Mannucci PM. Back to the future: a recent history of haemophilia treatment. Haemophilia. 2008;14:10–18. doi: 10.1111/j.1365-2516.2008.01708.x. [DOI] [PubMed] [Google Scholar]
- 50.McVey JH, Boswell E, Mumford AD, Kemball-Cook G, Tuddenham EG. Factor VII deficiency and the FVII mutation database. Hum. Mutat. 2001;17:3–17. doi: 10.1002/1098-1004(2001)17:1<3::AID-HUMU2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 51.D’Angelo MR, Dutton RP. Management of trauma-induced coagulopathy: trends and practices. AANA J. 2010;78:35–40. [PubMed] [Google Scholar]
- 52.Franchini M, Montagnana M, Targher G, Zaffanello M, Lippi G. The use of recombinant Factor VIIa in liver diseases. Blood Coagul. Fibrinolysis. 2008;19:341–348. doi: 10.1097/MBC.0b013e32830496a7. [DOI] [PubMed] [Google Scholar]
- 53.Lisman T, Moschatsis S, Adelmeijer J, Nieuwenhuis HK, de Groot PG. Recombinant Factor VIIa enhances deposition of platelets with congenital or acquired α IIb β 3 deficiency to endothelial cell matrix and collagen under conditions of flow via tissue factor-independent thrombin generation. Blood. 2003;101:1864–1870. doi: 10.1182/blood-2002-09-2761. [DOI] [PubMed] [Google Scholar]
- 54.Laurian Y. Treatment of bleeding in patients with platelet disorders: is there a place for recombinant Factor VIIa? Pathophysiol. Haemostasis Thromb. 2002;32:37–40. doi: 10.1159/000057300. [DOI] [PubMed] [Google Scholar]
- 55.Friederich PW, Henny CP, Messelink EJ, Geerdink MG, Keller T, Kurth KH, Bu¨ller HR, Levi M. Effect of recombinant activated Factor VII on perioperative blood loss in patients undergoing retropubic prostatectomy: a double-blind placebo-controlled randomised trial. Lancet. 2003;361:201–205. doi: 10.1016/S0140-6736(03)12268-4. [DOI] [PubMed] [Google Scholar]
- 56.Woodruff SI, Dougherty AL, Dye JL, Mohrle CR, Galarneau MR. Use of recombinant Factor VIIA for control of combat-related haemorrhage. Emerg. Med. J. 2010;27:121–124. doi: 10.1136/emj.2008.060657. [DOI] [PubMed] [Google Scholar]
- 57.Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, Axelsen M, Kluger Y. Recombinant Factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J. Trauma. 2005;59:8–15. doi: 10.1097/01.ta.0000171453.37949.b7. [DOI] [PubMed] [Google Scholar]
- 58.Shapiro AD, Gilchrist GS, Hoots WK, Cooper HA, Gastineau DA. Prospective, randomised trial of two doses of rFVIIa (NovoSeven) in haemophilia patients with inhibitors undergoing surgery. Thromb. Haemostasis. 1998;80:773–778. [PubMed] [Google Scholar]
- 59.Kemball-Cook G, Johnson DJ, Tuddenham EG, Harlos K. Crystal structure of active site-inhibited human coagulation Factor VIIa (des-Gla) J. Struct. Biol. 1999;127:213–223. doi: 10.1006/jsbi.1999.4158. [DOI] [PubMed] [Google Scholar]
- 60.Rand KD, Andersen MD, Olsen OH, Jørgensen TJ, Ostergaard H, Jensen ON, Stennicke HR, Persson E. The origins of enhanced activity in Factor VIIa analogs and the interplay between key allosteric sites revealed by hydrogen exchange mass spectrometry. J. Bio. Chem. 2008;283:13378–13387. doi: 10.1074/jbc.M709716200. [DOI] [PubMed] [Google Scholar]
- 61.Bajaj SP, Schmidt AE, Agah S, Bajaj MS, Padmanabhan K. High resolution structures of p-aminobenzamidine- and benzamidine-VIIa/soluble tissue factor: unpredicted conformation of the 192–193 peptide bond and mapping of Ca2+, Mg2+, Na+, and Zn2+ sites in Factor VIIa. J. Bio. Chem. 2006;281:24873–24888. doi: 10.1074/jbc.M509971200. [DOI] [PubMed] [Google Scholar]
- 62.Banner DW, D’Arcy A, Che`ne C, Winkler FK, Guha A, Konigsberg WH, Nemerson Y, Kirchhofer D. The crystal structure of the complex of blood coagulation Factor VIIa with soluble tissue factor. Nature. 1996;380:41–46. doi: 10.1038/380041a0. [DOI] [PubMed] [Google Scholar]
- 63.Sichler K, Banner DW, D’Arcy A, Hopfner KP, Huber R, Bode W, Kresse GB, Kopetzki E, Brandstetter H. Crystal structures of uninhibited Factor VIIa link its cofactor and substrate-assisted activation to specific interactions. J. Mol. Biol. 2002;322:591–603. doi: 10.1016/s0022-2836(02)00747-7. [DOI] [PubMed] [Google Scholar]
- 64.Di Cera E. Thrombin. Mol. Aspects Med. 2008;29:203–254. doi: 10.1016/j.mam.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coughlin SR. Protease-activated receptors in haemostasis, thrombosis and vascular biology. J. Thromb. Haemostasis. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 66.Di Cera E. Thrombin as procoagulant and anticoagulant. J. Thromb. Haemostasis. 2007;5:196–202. doi: 10.1111/j.1538-7836.2007.02485.x. [DOI] [PubMed] [Google Scholar]
- 67.van de Locht A, Stubbs MT, Bauer M, Bode W. Crystallographic evidence that the F2 kringle catalytic domain linker of prothrombin does not cover the fibrinogen recognition exosite. J. Bio. Chem. 1996;271:3413–3416. doi: 10.1074/jbc.271.7.3413. [DOI] [PubMed] [Google Scholar]
- 68.Chapman WC, Singla N, Genyk Y, McNeil JW, Renkens KL, Jr, Reynolds TC, Murphy A, Weaver F. A Phase 3, randomized, double-blind comparative study of the efficacy and safety of topical recombinant human thrombin and bovine thrombin in surgical haemostasis. J. Am. Coll. Surg. 2007;3205:256–265. doi: 10.1016/j.jamcollsurg.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 69.Bowman LJ, Anderson CD, Chapman WC. Topical recombinant human thrombin in surgical haemostasis. Semin. Thromb. Hemostasis. 2010;36:477–484. doi: 10.1055/s-0030-1255441. [DOI] [PubMed] [Google Scholar]
- 70.Yan SB, Helterbrand JD, Hartman DL, Wright TJ, Bernard GR. Low levels of protein C are associated with poor outcome in severe sepsis. Chest. 2001;120:915–922. doi: 10.1378/chest.120.3.915. [DOI] [PubMed] [Google Scholar]
- 71.Esmon CT. Inflammation and the activated protein C anticoagulant pathway. Semin. Thromb. Hemostasis. 2006;32:49–60. doi: 10.1055/s-2006-939554. [DOI] [PubMed] [Google Scholar]
- 72.Esmon CT, Fukudome K, Mather T, Bode W, Regan LM, Stearns-Kurosawa DJ, Kurosawa S. Inflammation, sepsis, and coagulation. Haematologica. 1999;84:254–259. [PubMed] [Google Scholar]
- 73.Mather T, Oganessyan V, Hof P, Huber R, Foundling S, Esmon C, Bode W. The 2.8 Å crystal structure of Gla-domainless activated protein C. EMBO J. 1996;15:6822–6831. [PMC free article] [PubMed] [Google Scholar]
- 74.Esmon CT. The roles of protein C and thrombomodulin in the regulation of blood coagulation. J. Bio. Chem. 1989;264:4743–4746. [PubMed] [Google Scholar]
- 75.Kisiel W. Human plasma protein C: isolation, characterization, and mechanism of activation by α-thrombin. J. Clin. Invest. 1979;64:761–769. doi: 10.1172/JCI109521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Esmon CT. The endothelial protein C receptor. Curr. Opin. Hematol. 2006;13:382–385. doi: 10.1097/01.moh.0000239712.93662.35. [DOI] [PubMed] [Google Scholar]
- 77.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 78.Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein C defines new mechanisms modulating inflammation and apoptosis. J. Bio. Chem. 2001;276:11199–11203. doi: 10.1074/jbc.C100017200. 2001. [DOI] [PubMed] [Google Scholar]
- 79.Nakanishi-Matsui M, Zheng YW, Sulciner DJ, Weiss EJ, Ludeman MJ, Coughlin SR. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404:609–613. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- 80.Yang XV, Banerjee Y, Fernández JA, Deguchi H, Xu X, Mosnier LO, Urbanus RT, de Groot PG, White-Adams TC, McCarty OJ, Griffin JH. Activated protein C ligation of ApoER2 (LRP8) causes Dab1-dependent signaling in U937 cells. Proc. Natl. Acad. Sci. U.S.A. 2009;106:274–279. doi: 10.1073/pnas.0807594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elphick GF, Sarangi PP, Hyun YM, Hollenbaugh JA, Ayala A, Biffl WL, Chung HL, Rezaie AR, McGrath JL, Topham DJ, et al. Recombinant human activated protein C inhibits integrin-mediated neutrophil migration. Blood. 2009;113:4078–4085. doi: 10.1182/blood-2008-09-180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dellinger RP. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis x. Proc. Natl. Acad. Sci. Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 84.Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, Franc¸ois B, Guy JS, Bru¨ckmann M, Rea-Neto A, et al. Drotrecogin α (activated) for adults with severe sepsis and a low risk of death. N. Engl. J. Med. 2005;353:1332–1341. doi: 10.1056/NEJMoa050935. [DOI] [PubMed] [Google Scholar]
- 85.Nilsson G, Hojgard S, Berntorp E. Treatment of the critically ill patient with protein C: is it worth the cost? Thromb. Res. 2010;125:494–500. doi: 10.1016/j.thromres.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 86.Toussaint S, Gerlach H. Activated protein C for sepsis. N. Engl. J. Med. 2009;361:2646–2652. doi: 10.1056/NEJMct0808063. [DOI] [PubMed] [Google Scholar]
- 87.Bernard GR, Ely EW, Wright TJ, Fraiz J, Stasek JE, Jr, Russell JA, Mayers I, Rosenfeld BA, Morris PE, Yan SB, Helterbrand JD. Safety and dose relationship of recombinant human activated protein C for coagulopathy in severe sepsis. Crit. Care Med. 2001;29:2051–2059. doi: 10.1097/00003246-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 88.Gentry CA, Gross KB, Sud B, Drevets DA. Adverse outcomes associated with the use of drotrecogin alfa (activated) in patients with severe sepsis and baseline bleeding precautions. Crit. Care Med. 2009;37:19–25. doi: 10.1097/CCM.0b013e318192843b. [DOI] [PubMed] [Google Scholar]
- 89.Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000;80:717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- 90.Keller JE. Recovery from botulinum neurotoxin poisoning in vivo. Neuroscience. 2006;139:629–637. doi: 10.1016/j.neuroscience.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 91.Ravichandran E, Gong Y, Al Saleem FH, Ancharski DM, Joshi SG, Simpson LL. An initial assessment of the systemic pharmacokinetics of botulinum toxin. J. Pharmacol. Exp. Ther. 2006;318:1343–1351. doi: 10.1124/jpet.106.104661. [DOI] [PubMed] [Google Scholar]
- 92.Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology. 1980;87:1044–1049. doi: 10.1016/s0161-6420(80)35127-0. [DOI] [PubMed] [Google Scholar]
- 93.Truong DD, Stenner A, Reichel G. Current clinical applications of botulinum toxin. Curr. Pharm. Des. 2009;15:3671–3680. doi: 10.2174/138161209789271843. [DOI] [PubMed] [Google Scholar]
- 94.Dressler D. Botulinum toxin drugs: future developments. J. Neural Transm. 2008;115:575–577. doi: 10.1007/s00702-007-0863-9. [DOI] [PubMed] [Google Scholar]
- 95.Chen S, Barbieri JT. Engineering botulinum neurotoxin to extend therapeutic intervention. Proc. Natl. Acad. Sci. U.S.A. 2009;106:9180–9184. doi: 10.1073/pnas.0903111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wooldridge JL, Heubi JE, Amaro-Galvez R, Boas SR, Blake KV, Nasr SZ, Chatfield B, McColley SA, Woo MS, Hardy KA, et al. EUR-1008 pancreatic enzyme replacement is safe and effective in patients with cystic fibrosis and pancreatic insufficiency. J. Cystic Fibrosis. 2009;8:405–417. doi: 10.1016/j.jcf.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 97.Littlewood JM, Wolfe SP, Conway SP. Diagnosis and treatment of intestinal malabsorption in cystic fibrosis. Pediatr. Pulmonol. 2006;41:35–49. doi: 10.1002/ppul.20286. [DOI] [PubMed] [Google Scholar]
- 98.Ramundo J, Gray M. Enzymatic wound debridement. J. Wound Ostomy Continence Nurs. 2008;35:273–280. doi: 10.1097/01.WON.0000319125.21854.78. [DOI] [PubMed] [Google Scholar]
- 99.Klasen HJ. A review on the nonoperative removal of necrotic tissue from burn wounds. Burns. 2000;26:207–222. doi: 10.1016/s0305-4179(99)00117-5. [DOI] [PubMed] [Google Scholar]
- 100.Keyt BA, Paoni NF, Bennett WF. In: Site-directed mutagenesis of tissue-type plasminogen activator. In Protein Engineering: Principles and Practice. Cleland JL, Craik CS, editors. New York: Wiley-Liss; 1996. pp. 435–466. [Google Scholar]
- 101.van de Werf FJ. The ideal fibrinolytic: can drug design improve clinical results? Eur. Heart J. 1999;20:1452–1458. doi: 10.1053/euhj.1999.1659. [DOI] [PubMed] [Google Scholar]
- 102.Liberatore GT, Samson A, Bladin C, Schleuning WD, Medcalf RL. Vampire bat salivary plasminogen activator (desmoteplase): a unique fibrinolytic enzyme that does not promote neurodegeneration. Stroke. 2003;34:537–543. doi: 10.1161/01.str.0000049764.49162.76. [DOI] [PubMed] [Google Scholar]
- 103.Tebbe U, Bramlage P, Graf A, Lechleitner P, Bode C, Riess FC, Clemens N, Al-Rawi Y, Konstantinides S, Goldhaber SZ. Desmoteplase in acute massive pulmonary thromboembolism. Thromb. Haemostasis. 2009;101:557–562. [PubMed] [Google Scholar]
- 104.Malcolm AD, Keltai M, Walsh MJ. ESPRIT: a European study of the prevention of reocclusion after initial thrombolysis with duteplase in acute myocardial infarction. Eur. Heart J. 1996;17:1522–1531. doi: 10.1093/oxfordjournals.eurheartj.a014716. [DOI] [PubMed] [Google Scholar]
- 105.Inoue T, Nishiki R, Kageyama M, Node K. Therapeutic potential of monteplase in acute myocardial infarction. Am. J. Cardiovasc. Drugs. 2005;5:225–231. doi: 10.2165/00129784-200505040-00002. [DOI] [PubMed] [Google Scholar]
- 106.Monrad ES. Thrombolysis: the need for a critical review. J. Am. Coll. Cardiol. 1991;18:1573–1578. doi: 10.1016/0735-1097(91)90692-3. [DOI] [PubMed] [Google Scholar]
- 107.Ambrus JL, Ambrus CM, Back N, Sokal JE, Collins GL. Clinical and experimental studies on fibrinolytic enzymes. Ann. N.Y. Acad. Sci. 1957;68:97–136. doi: 10.1111/j.1749-6632.1957.tb42616.x. [DOI] [PubMed] [Google Scholar]
- 108.Marder VJ, Landskroner K, Novokhatny V, Zimmerman TP, Kong M, Kanouse JJ, Jesmok G. Plasmin induces local thrombolysis without causing haemorrhage: a comparison with tissue plasminogen activator in the rabbit. Thromb. Haemostasis. 2001;86:739–745. [PubMed] [Google Scholar]
- 109.Marder VJ, Novokhatny V. Direct fibrinolytic agents: biochemical attributes, preclinical foundation and clinical potential. J. Thromb. Haemostasis. 2010;8:433–444. doi: 10.1111/j.1538-7836.2009.03701.x. [DOI] [PubMed] [Google Scholar]
- 110.Di Nisio M, Middeldorp S, Buller HR. Direct thrombin inhibitors. N. Engl. J. Med. 2005;353:1028–1040. doi: 10.1056/NEJMra044440. [DOI] [PubMed] [Google Scholar]
- 111.Koh CY, Kini RM. Molecular diversity of anticoagulants from haematophagous animals. Thromb. Haemostasis. 2009;102:437–453. doi: 10.1160/TH09-04-0221. [DOI] [PubMed] [Google Scholar]
- 112.Yu X, Xia X, Fang H, Zhou C, Chen H. Expression and purification of ancrod, an anticoagulant drug, in Pichia pastoris . Protein Expression Purif. 2007;55:257–261. doi: 10.1016/j.pep.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 113.Levy DE, del Zoppo GJ, Demaerschalk BM, Demchuk AM, Diener HC, Howard G, Kaste M, Pancioli AM, Ringelstein EB, Spatareanu C, Wasiewski WW. Ancrod in acute ischemic stroke: results of 500 subjects beginning treatment within 6 hours of stroke onset in the ancrod stroke program. Stroke. 2009;40:3796–3803. doi: 10.1161/STROKEAHA.109.565119. [DOI] [PubMed] [Google Scholar]
- 114.Randolph A, Chamberlain SH, Chu HL, Retzios AD, Markland FS, Jr, Masiarz FR. Amino acid sequence of fibrolase, a direct-acting fibrinolytic enzyme from Agkistrodon contortrix contortrix venom. Protein Sci. 1992;1:590–600. doi: 10.1002/pro.5560010505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shah AR, Scher L. Drug evaluation: alfimeprase, a plasminogen-independent thrombolytic. IDrugs. 2007;10:329–335. [PubMed] [Google Scholar]
- 116.Jespers L, Vanwetswinkel S, Lijnen HR, van Herzeele N, van Hoef B, Demarsin E, Collen D, De Maeyer M. Structural and functional basis of plasminogen activation by staphylokinase. Thromb. Haemostasis. 1999;81:479–485. [PubMed] [Google Scholar]
- 117.Vanderschueren S, Barrios L, Kerdsinchai P, Van Den Heuvel P, Hermans L, Vrolix M, De Man F, Benit E, Muyldermans L, Collen D. A randomized trial of recombinant staphylokinase versus alteplase for coronary artery patency in acute myocardial infarction. Circulation. 1995;92:2044–2049. doi: 10.1161/01.cir.92.8.2044. [DOI] [PubMed] [Google Scholar]
- 118.Luisetti M, Piccioni PD, Dyne K, Donnini M, Bulgheroni A, Pasturenzi L, Donnetta AM, Peona V. Some properties of the alkaline proteinase from Aspergillus melleus . Int. J. Tissue React. 1991;13:187–192. [PubMed] [Google Scholar]
- 119.Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat. Rev. Immunol. 2002;2:647–655. doi: 10.1038/nri885. [DOI] [PubMed] [Google Scholar]
- 120.Shan L, Molberg Ø, Parrot I, Hausch F, Filiz F, Gray GM, Sollid LM, Khosla C. Structural basis for gluten intolerance in celiac sprue. Science. 2002;297:2275–2279. doi: 10.1126/science.1074129. [DOI] [PubMed] [Google Scholar]
- 121.Chen YS, Christensen JE, Broadbent JR, Steele JL. Identification and characterization of Lactobacillus helveticusPepO2, an endopeptidase with post-proline specificity. Appl. Environ. Microbiol. 2003;69:1276–1282. doi: 10.1128/AEM.69.2.1276-1282.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vora H, McIntire J, Kumar P, Deshpande M, Khosla C. A scaleable manufacturing process for pro-EP-B2, a cysteine protease from barley indicated for celiac sprue. Biotech. Bioeng. 2007;98:177–185. doi: 10.1002/bit.21423. [DOI] [PubMed] [Google Scholar]
- 123.Lollar P. Mapping factor VIII inhibitor epitopes using hybrid human/porcine factor VIII molecules. Haematologica. 2000;85:26–28. [PubMed] [Google Scholar]
- 124.Bhana N, Spencer CM. Lanoteplase. BioDrugs. 2000;13:217–224. doi: 10.2165/00063030-200013030-00006. [DOI] [PubMed] [Google Scholar]
- 125.Huntington JA, Read RJ, Carrell RW. Structure of a serpin-protease complex shows inhibition by deformation. Nature. 2000;407:923–926. doi: 10.1038/35038119. [DOI] [PubMed] [Google Scholar]
- 126.Lomas DA, Evans DL, Finch JT, Carrell RW. The mechanism of Z α1-antitrypsin accumulation in the liver. Nature. 1992;357:605–607. doi: 10.1038/357605a0. [DOI] [PubMed] [Google Scholar]
- 127.Higgins PJ, Ryan MP, Providence KM. Induced expression of p52(PAI-1) in normal rat kidney cells by the microfilament-disrupting agent cytochalasin D. J. Cell Physiol. 1994;159:187–195. doi: 10.1002/jcp.1041590123. [DOI] [PubMed] [Google Scholar]
- 128.Cao C, Lawrence DA, Li Y, von Arnim CA, Herz J, Su EJ, Makarova A, Hyman BT, Strickland DK, Zhang L. Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J. 2006;25:1860–1870. doi: 10.1038/sj.emboj.7601082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jensen JK, Dolmer K, Gettins PG. Specificity of binding of the low density lipoprotein receptor-related protein to different conformational states of the clade E serpins plasminogen activator inhibitor-1 and proteinase nexin-1. J. Bio. Chem. 2009;284:17989–17997. doi: 10.1074/jbc.M109.009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guimond S, Turnbull JE. Proteoglycans make the grade-ient. Mol. Cell. 2004;16:159–160. doi: 10.1016/j.molcel.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 131.Gibbs CS, Coutre´ SE, Tsiang M, Li WX, Jain AK, Dunn KE, Law VS, Mao CT, Matsumura SY, Mejza SJ. Conversion of thrombin into an anticoagulant by protein engineering. Nature. 1995;378:413–416. doi: 10.1038/378413a0. [DOI] [PubMed] [Google Scholar]
- 132.Bah A, Carrell CJ, Chen Z, Gandhi PS, Di Cera E. Stabilization of the E* form turns thrombin into an anticoagulant. J. Biol. Chem. 284:20034–20040. doi: 10.1074/jbc.M109.012344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bah A, Garvey LC, Ge J, Di Cera E. Rapid kinetics of Na+ binding to thrombin. J. Bio. Chem. 2006;281:40049–40056. doi: 10.1074/jbc.M608600200. [DOI] [PubMed] [Google Scholar]
- 134.Page MJ, Di Cera E. Role of Na+ and K+ in enzyme function. Physiol. Rev. 2006;86:1049–1092. doi: 10.1152/physrev.00008.2006. [DOI] [PubMed] [Google Scholar]
- 135.Niu W, Bush-Pelc LA, Bah A, Gandhi PS, Di Cera E. Mutant N143P reveals how Na+ activates thrombin. J. Bio. Chem. 2009;284:36175–36185. doi: 10.1074/jbc.M109.069500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Marino F, Pelc LA, Vogt A, Gandhi PS, Di Cera E. Engineering thrombin for selective specificity toward protein C and PAR1. J. Bio. Chem. 2010;285:19145–19152. doi: 10.1074/jbc.M110.119875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Page MJ, Carrell CJ, Di Cera E. Engineering protein allostery: 1.05 A resolution structure and enzymatic properties of a Na+-activated trypsin. J. Mol. Biol. 2008;378:666–672. doi: 10.1016/j.jmb.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Page MJ, Di Cera E. Combinatorial enzyme design probes allostery and cooperativity in the trypsin fold. J. Mol. Biol. 2010;399:306–319. doi: 10.1016/j.jmb.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gruber A, Cantwell AM, Di Cera E, Hanson SR. The thrombin mutant W215A/E217A shows safe and potent anticoagulant and antithrombotic effects in vivo . J. Bio. Chem. 2002;277:27581–27584. doi: 10.1074/jbc.C200237200. [DOI] [PubMed] [Google Scholar]
- 140.Berny MA, White TC, Tucker EI, Bush-Pelc LA, Di Cera E, Gruber A, McCarty OJ. Thrombin mutant W215A/E217A acts as a platelet GPIb antagonist. Arterioscler. Thromb. Vasc. Biol. 2008;28:329–334. doi: 10.1161/ATVBAHA.107.156273. [DOI] [PubMed] [Google Scholar]
- 141.Kenet G, Lubetsky A, Luboshitz J, Martinowitz U. A new approach to treatment of bleeding episodes in young haemophilia patients: a single bolus megadose of recombinant activated factor VII (NovoSeven) J. Thromb. Haemostasis. 2003;1:450–455. doi: 10.1046/j.1538-7836.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- 142.Persson E, Bolt G, Steenstrup TD, Ezban M. Recombinant coagulation factor VIIa: from molecular to clinical aspects of a versatile haemostatic agent. Thromb. Res. 2010;125:483–489. doi: 10.1016/j.thromres.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 143.Weimer T, Wormsbächer W, Kronthaler U, Lang W, Liebing U, Schulte S. Prolonged in vivo half-life of factor VIIa by fusion to albumin. Thromb. Haemostasis. 2008;99:659–667. doi: 10.1160/TH07-08-0525. [DOI] [PubMed] [Google Scholar]
- 144.Chuang VT, Kragh-Hansen U, Otagiri M. Pharmaceutical strategies utilizing recombinant human serum albumin. Pharm. Res. 2002;19:569–577. doi: 10.1023/a:1015396825274. [DOI] [PubMed] [Google Scholar]
- 145.Duttaroy A, Kanakaraj P, Osborn BL, Schneider H, Pickeral OK, Chen C, Zhang G, Kaithamana S, Singh M, Schulingkamp R, et al. Development of a long-acting insulin analog using albumin fusion technology. Diabetes. 2005;54:251–258. doi: 10.2337/diabetes.54.1.251. [DOI] [PubMed] [Google Scholar]
- 146.Sung C, Nardelli B, LaFleur DW, Blatter E, Corcoran M, Olsen HS, Birse CE, Pickeral OK, Zhang J, Shah D, et al. An IFN-β-albumin fusion protein that displays improved pharmacokinetic and pharmacodynamic properties in nonhuman primates. J. Interferon Cytokine Res. 2003;23:25–36. doi: 10.1089/10799900360520423. [DOI] [PubMed] [Google Scholar]
- 147.Osborn BL, Sekut L, Corcoran M, Poortman C, Sturm B, Chen G, Mather D, Lin HL, Parry TJ. Albutropin: a growth hormone-albumin fusion with improved pharmacokinetics and pharmacodynamics in rats and monkeys. Eur. J. Pharmacol. 2002;456:149–158. doi: 10.1016/s0014-2999(02)02644-4. [DOI] [PubMed] [Google Scholar]
- 148.Sen P, Ghosh S, Ezban M, Pendurthi UR, Vijaya Mohan Rao L. Effect of glycoPEGylation on factor VIIa binding and internalization. Haemophilia. 2001;16:339–348. doi: 10.1111/j.1365-2516.2009.02121.x. [DOI] [PubMed] [Google Scholar]
- 149.Yatuv R, Dayan I, Carmel-Goren L, Robinson M, Aviv I, Goldenberg-Furmanov M, Baru M. Enhancement of factor VIIa haemostatic efficacy by formulation with PEGylated liposomes. Haemophilia. 2008;14:476–483. doi: 10.1111/j.1365-2516.2008.01741.x. [DOI] [PubMed] [Google Scholar]
- 150.Neuenschwander PF, Morrissey JH. Roles of the membrane-interactive regions of factor VIIa and tissue factor. The factor VIIa Gla domain is dispensable for binding to tissue factor but important for activation of factor X. J. Bio. Chem. 1994;269:8007–8013. [PubMed] [Google Scholar]
- 151.Allen GA, Persson E, Campbell RA, Ezban M, Hedner U, Wolberg AS. A variant of recombinant factor VIIa with enhanced procoagulant and antifibrinolytic activities in an in vitro model of haemophilia. Arterioscler. Thromb. Vasc. Biol. 2007;27:683–689. doi: 10.1161/01.ATV.0000257204.82396.2b. [DOI] [PubMed] [Google Scholar]
- 152.Persson E, Kjalke M, Olsen OH. Rational design of coagulation factor VIIa variants with substantially increased intrinsic activity. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13583–13588. doi: 10.1073/pnas.241339498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Harvey SB, Stone MD, Martinez MB, Nelsestuen GL. Mutagenesis of the γ-carboxyglutamic acid domain of human factor VII to generate maximum enhancement of the membrane contact site. J. Bio. Chem. 2003;278:8363–8369. doi: 10.1074/jbc.M211629200. [DOI] [PubMed] [Google Scholar]
- 154.Sarangi PP, Lee HW, Kim M. Activated protein C action in inflammation. Br. J. Haematol. 2010;148:817–833. doi: 10.1111/j.1365-2141.2009.08020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Weiler H, Kerschen E. Modulation of sepsis outcome with variants of activated protein C. J. Thromb. Haemostasis. 2009;7:127–131. doi: 10.1111/j.1538-7836.2009.03377.x. [DOI] [PubMed] [Google Scholar]
- 156.Loubele ST, Spronk HM, Ten Cate H. Activated protein C: a promising drug with multiple effects? Mini Rev. Med. Chem. 2009;9:620–626. doi: 10.2174/138955709788167547. [DOI] [PubMed] [Google Scholar]
- 157.Berg DT, Gerlitz B, Shang J, Smith T, Santa P, Richardson MA, Kurz KD, Grinnell BW, Mace K, Jones BE. Engineering the proteolytic specificity of activated protein C improves its pharmacological properties. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4423–4428. doi: 10.1073/pnas.0736918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brantly ML, Wittes JT, Vogelmeier CF, Hubbard RC, Fells GA, Crystal RG. Use of a highly purified α1-antitrypsin standard to establish ranges for the common normal and deficient α 1-antitrypsin phenotypes. Chest. 1991;100:703–708. doi: 10.1378/chest.100.3.703. [DOI] [PubMed] [Google Scholar]
- 159.Mosnier LO, Yang XV, Griffin JH. Activated protein C mutant with minimal anticoagulant activity, normal cytoprotective activity, and preservation of thrombin activable fibrinolysis inhibitor-dependent cytoprotective functions. J. Bio. Chem. 2007;282:33022–33033. doi: 10.1074/jbc.M705824200. [DOI] [PubMed] [Google Scholar]
- 160.Yang L. Identification of a specific exosite on activated protein C for interaction with protease-activated receptor 1. J. Bio. Chem. 2007;282:25493–25500. doi: 10.1074/jbc.M702131200. [DOI] [PubMed] [Google Scholar]
- 161.Bae JS, Yang L, Manithody C, Rezaie AR. Engineering a disulfide bond to stabilize the calcium-binding loop of activated protein C eliminates its anticoagulant but not its protective signaling properties. J. Bio. Chem. 2007;282:9251–9259. doi: 10.1074/jbc.M610547200. [DOI] [PubMed] [Google Scholar]
- 162.Harmon S, Preston RJ, Ni Ainle F, Johnson JA, Cunningham MS, Smith OP, White B, O’Donnell JS. Dissociation of activated protein C functions by elimination of protein S cofactor enhancement. J. Bio. Chem. 2008;283:30531–30539. doi: 10.1074/jbc.M802338200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Han MH, Hwang SI, Roy DB, Lundgren DH, Price JV, Ousman SS, Fernald GH, Gerlitz B, Robinson WH, Baranzini SE, et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451:1076–1081. doi: 10.1038/nature06559. [DOI] [PubMed] [Google Scholar]
- 164.Murphy SL, High KA. Gene therapy for haemophilia. Br. J. Haematol. 2008;140:479–487. doi: 10.1111/j.1365-2141.2007.06942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Slofstra SH, Groot AP, Maris NA, Reitsma PH, Cate HT, Spek CA. Inhalation of activated protein C inhibits endotoxin-induced pulmonary inflammation in mice independent of neutrophil recruitment. Br. J. Pharmacol. 2006;149:740–746. doi: 10.1038/sj.bjp.0706915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Margaritis P, Arruda VR, Aljamali M, Camire RM, Schlachterman A, High KA. Novel therapeutic approach for haemophilia using gene delivery of an engineered secreted activated Factor VII. J. Clin. Invest. 2004;113:1025–1031. doi: 10.1172/JCI20106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Margaritis P, High KA. Advances in gene therapy using factor VIIa in haemophilia. Semin. Hematol. 2006;43:S101–S104. doi: 10.1053/j.seminhematol.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 168.Schneider H, Mühle C, Douar AM, Waddington S, Jiang QJ, von der Mark K, Coutelle C, Rascher W. Sustained delivery of therapeutic concentrations of human clotting factor IX: a comparison of adenoviral and AAV vectors administered in utero . J. Gene Med. 2002;4:46–53. doi: 10.1002/jgm.233. [DOI] [PubMed] [Google Scholar]
- 169.Graham T, McIntosh J, Work LM, Nathwani A, Baker AH. Performance of AAV8 vectors expressing human factor IX from a hepatic-selective promoter following intravenous injection into rats. Genet. Vaccines Ther. 2008;6:9. doi: 10.1186/1479-0556-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, Ragni MV, Manno CS, Sommer J, Jiang H, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 171.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, et al. Successful transduction of liver in haemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 172.Rocha M, Silva M. Bradykinin; mechanism of its release by trypsin and kallikrein. Arch. Int. Pharmacodyn. Ther. 1951;88:271–282. [PubMed] [Google Scholar]
- 173.Tu L, Xu X, Wan H, Zhou C, Deng J, Xu G, Xiao X, Chen Y, Edin ML, Voltz JW, et al. Delivery of recombinant adeno-associated virus-mediated human tissue kallikrein for therapy of chronic renal failure in rats. Hum. Gene Ther. 2008;19:318–330. doi: 10.1089/hum.2007.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bartoli M, Roudaut C, Martin S, Fougerousse F, Suel L, Poupiot J, Gicquel E, Noulet F, Danos O, Richard I. Safety and efficacy of AAV-mediated calpain 3gene transfer in a mouse model of limb-girdle muscular dystrophy type 2A. Mol. Ther. 2006;13:250–259. doi: 10.1016/j.ymthe.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 175.Sleat DE, El-Banna M, Sohar I, Kim KH, Dobrenis K, Walkley SU, Lobel P. Residual levels of tripeptidyl-peptidase I activity dramatically ameliorate disease in late-infantile neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 2008;94:222–233. doi: 10.1016/j.ymgme.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sondhi D, Peterson DA, Edelstein AM, del Fierro K, Hackett NR, Crystal RG. Survival advantage of neonatal CNS gene transfer for late infantile neuronal ceroid lipofuscinosis. Exp. Neurol. 2008;213:18–27. doi: 10.1016/j.expneurol.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Muzio M, Chinnaiyan AM, Kischkel FC, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 178.Lu Y, Wu LQ, Wang SG, Lv ZH, Han B. Caspase-3 gene transfected with LIGHT gene: can it be used for therapy of human hepatocellular carcinoma? Clin. Chem. Lab. Med. 2008;46:470–474. doi: 10.1515/CCLM.2008.094. [DOI] [PubMed] [Google Scholar]
- 179.Komata T, Kondo Y, Kanzawa T, Hirohata S, Koga S, Sumiyoshi H, Srinivasula SM, Barna BP, Germano IM, Takakura M, et al. Treatment of malignant glioma cells with the transfer of constitutively active caspase-6 using the human telomerase catalytic subunit (human telomerase reverse transcriptase) gene promoter. Cancer Res. 2001;61:5796–5802. [PubMed] [Google Scholar]
- 180.Komata T, Kondo Y, Kanzawa T, Ito H, Hirohata S, Koga S, Sumiyoshi H, Takakura M, Inoue M, Barna BP, et al. Caspase-8 gene therapy using the human telomerase reverse transcriptase promoter for malignant glioma cells. Hum. Gene Ther. 2002;13:1015–1025. doi: 10.1089/104303402753812421. [DOI] [PubMed] [Google Scholar]
- 181.Takeuchi H, Kanzawa T, Kondo Y, Komata T, Hirohata S, Kyo S, Kondo S. Combination of caspase transfer using the human telomerase reverse transcriptase promoter and conventional therapies for malignant glioma cells. Int. J. Oncol. 2004;25:57–63. [PubMed] [Google Scholar]
- 182.Zhang X, Turner C, Godbey WT. Comparison of caspase genes for the induction of apoptosis following gene delivery. Mol. Biotechnol. 2009;41:236–246. doi: 10.1007/s12033-008-9133-9. [DOI] [PubMed] [Google Scholar]
- 183.Wolan DW, Zorn JA, Gray DC, Wells JA. Small-molecule activators of a proenzyme. Science. 2009;326:853–858. doi: 10.1126/science.1177585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kurschus FC, Jenne DE. Delivery and therapeutic potential of human granzyme B. Immunol. Rev. 2010;235:159–171. doi: 10.1111/j.0105-2896.2010.00894.x. [DOI] [PubMed] [Google Scholar]
- 185.Kakkis ED, Muenzer J, Tiller GE, Waber L, Belmont J, Passage M, Izykowski B, Phillips J, Doroshow R, Walot I, et al. Enzyme-replacement therapy in mucopolysaccharidosis I. N. Engl. J. Med. 2001;344:182–188. doi: 10.1056/NEJM200101183440304. [DOI] [PubMed] [Google Scholar]
- 186.Page MJ, Di Cera E. Evolution of peptidase diversity. J. Bio. Chem. 2008;283:30010–30014. doi: 10.1074/jbc.M804650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Renatus M, Engh RA, Stubbs MT, Huber R, Fischer S, Kohnert U, Bode W. Lysine 156 promotes the anomalous proenzyme activity of tPA: X-ray crystal structure of single-chain human tPA. EMBO J. 1997;16:4797–4805. doi: 10.1093/emboj/16.16.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Paoni NF, Chow AM, Peña LC, Keyt BA, Zoller MJ, Bennett WF. Making tissue-type plasminogen activator more fibrin specific. Protein Eng. 1993;6:529–534. doi: 10.1093/protein/6.5.529. [DOI] [PubMed] [Google Scholar]
- 189.Page MJ, Macgillivray RT, Di Cera E. Determinants of specificity in coagulation proteases. J. Thromb. Haemostasis. 2005;3:2401–2408. doi: 10.1111/j.1538-7836.2005.01456.x. [DOI] [PubMed] [Google Scholar]
- 190.Eastman D, Wurm FM, van Reis R, Higgins DL. A region of tissue plasminogen activator that affects plasminogen activation differentially with various fibrin(ogen)-related stimulators. Biochemistry. 1992;31:419–422. doi: 10.1021/bi00117a016. [DOI] [PubMed] [Google Scholar]
- 191.Carter WJ, Myles T, Gibbs CS, Leung LL, Huntington JA. Crystal structure of anticoagulant thrombin variant E217K provides insights into thrombin allostery. J. Bio. Chem. 2004;279:26387–26394. doi: 10.1074/jbc.M402364200. [DOI] [PubMed] [Google Scholar]
- 192.Perona JJ, Craik CS. Evolutionary divergence of substrate specificity within the chymotrypsin-like serine protease fold. J. Bio. Chem. 1997;272:29987–29990. doi: 10.1074/jbc.272.48.29987. [DOI] [PubMed] [Google Scholar]