Abstract
Assessing risk of colorectal adenoma at first-time colonoscopy that are of higher likelihood of developing advanced neoplasia during surveillance could help tailor first-line colorectal cancer screening. We developed prediction models for high-risk colorectal adenoma (at least one adenoma ≥1 cm, or with advanced histology, or ≥3 adenomas) among 4,881 asymptomatic white men and 17,970 women who underwent colonoscopy as their first-time screening for colorectal cancer in two prospective U.S. studies using logistic regressions. C-statistics and Hosmer-Lemeshow tests were used to evaluate discrimination and calibration. Ten-fold cross-validation was used for internal validation. A total of 330 (6.7%) men and 678 (3.8%) women were diagnosed with high-risk adenoma at first-time screening colonoscopy. The model for men included age, family history of colorectal cancer, BMI, smoking, sitting watching TV/VCR, regular aspirin/NSAID use, physical activity, and a joint term of multivitamin and alcohol. For women, the model included age, family history of colorectal cancer, BMI, smoking, alcohol, beef/pork/lamb as main dish, regular aspirin/NSAID, calcium, and oral contraceptive use. The C-statistic of the model for men was 0.67 and 0.60 for women (0.64 and 0.57 in cross-validation). Both models calibrated well. The predicted risk of high-risk adenoma for men in the top decile was 15.4% vs 1.8% for men in the bottom decile (Odds Ratio[OR]=9.41), and 6.6% vs 2.1% for women (OR=3.48). In summary, we developed and internally validated an absolute risk assessment tool for high-risk colorectal adenoma among the U.S. population that may provide guidance for first-time colorectal cancer screening.
Keywords: risk prediction, colorectal adenoma, colonoscopy, screening
INTRODUCTION
The decline in the incidence and mortality of colorectal cancer over the past two decades in the United States are largely attributable to colorectal cancer screening through prevention (identification and removal of polyps) and early detection.1 Current screening guideline recommends adults with average risk of colorectal cancer should start screening at age 50 and at age 40 in general for people with family history.2 Nevertheless, 35% of Americans aged 50 to 75 are not up-to-date with colorectal cancer screening.3 Despite a lack of consensus about a single best screening strategy, the demand for colonoscopy has surged in the past decade coincident with a decline in the use of sigmoidoscopy and fecal occult blood test. However, the majority of people who undergo colonoscopy do not harbor polyps.4, 5 Finally, substantial uncertainty exists about whether national colonoscopy capacity would be sufficient for more widespread use of colonoscopy as the primary screening test.6-9 Therefore, tailoring colorectal cancer screening by identifying high risk individuals who should be encouraged to get a colonoscopy is critical for the efficient use of healthcare resources.4, 10, 11
Risk assessment tools for colorectal cancer have been developed but the impact of previous endoscopy on subsequent cancer development was not fully assessed or was not designed to use in clinical settings because of requirement of periodic follow-up examinations.12-16 Alternatively, risk prediction for colorectal advanced adenoma may be more informative for screening and conservative for cancer prevention. According to the adenoma-carcinoma sequence,17 advanced adenomas (≥1cm in diameter or with tubulovillous or villous histologic features or high-grade/severe dysplasia), are the link between benign adenoma to colorectal cancer, and thus are considered as the primary target in screening endoscopies.4, 18-21 In addition, multiplicity of adenomas (≥3) were associated with 2-5 fold increased risk of subsequent detection of advanced adenomas and cancer, and similar to advanced adenoma, surveillance at 3 years is recommended once identified.22, 23
A few risk prediction tools for advanced adenoma/neoplasia have been established recently among Asian and European,24-28 but none were among U.S. adults or restricted exclusively to first screening colonoscopy to aid risk stratification of first-time screening; we therefore sought to develop a risk assessment tool for high-risk colorectal adenoma (advanced adenoma or ≥3 adenomas) that can be implemented in clinical/general settings through evaluating a comprehensive list of risk factors, among white men and women who underwent colonoscopy as their first routine screening for colorectal cancer.
MATERIAL AND METHODS
Study Population
We utilized two large ongoing prospective cohort studies; the Health Professionals Follow-up Study (HPFS), a cohort study of 51,529 US male health professionals aged 40-75 at enrollment in 1986, and the Nurses’ Health Study (NHS), a cohort study of 121,700 US female nurses aged 30-55 at enrollment in 1976. Participants have been mailed questionnaires at enrollment and 2 years thereafter to collect data on demographics, lifestyle factors, medical history, and disease outcomes and every 4 years to report update in dietary intake. The follow-up rates in both cohorts have been greater than 90%. The study protocol was approved by the institutional review boards of the Harvard School of Public Health and Brigham and Women's Hospital.
Between 1988 and 2002, both cohorts inquired whether the participants had a sigmoidoscopy or colonoscopy during the past 2 years and the indications for these procedures. Starting with the 2004 questionnaires, sigmoidoscopy and colonoscopy were assessed separately, and participants were also asked to provide information on when they had a sigmoidoscopy versus colonoscopy in any of the two-year period in the past, including procedures before 1986.
We included asymptomatic men and women who were initially free of cancer (except non-melanoma skin cancer), ulcerative colitis, and who had reported colonoscopy as their first-time screening endoscopy for colorectal cancer between 1988-2008 (HPFS) or 1986-2008 (NHS). Because most of the participants were white (90% in HPFS and 97% in NHS), we restricted our analysis to white men and women. Individuals who reported occult or visible blood, diarrhea, constipation, or abdominal pain as the indication(s) for the first colonoscopy were excluded.
Identification of cases and non-cases
On each biennial questionnaire, participants were asked whether polyps had been diagnosed in the past two years. When a diagnosis was reported, we obtained informed consent to acquire medical records and pathology reports. Investigators blinded to any exposure information reviewed all records and extracted data on histological type, anatomic location and size of the polyps. If more than one adenoma was diagnosed, the subject was classified according to the adenoma of the largest size and most advanced histological characteristics.
Men or women diagnosed with at least one adenoma ≥1 cm in diameter, or with advanced histology (tubulovillous or villous histologic features or high grade or severe dysplasia), or ≥3 adenomas at their first colonoscopy were considered as high-risk adenomas and cases in this analysis. Non-cases included those diagnosed with other adenomas or hyperplastic polyps, or free of polyps at their first colonoscopy.
Assessment of risk factors
While selecting the set of risk factors for evaluation, we thoroughly reviewed existing epidemiological evidence as well as the simplicity of each risk factor assessment to ensure that our tool can be implemented in clinical and general settings. For risk factors with periodic updates in our cohorts, we extracted the most updated information before the report of the first colonoscopy without taking advantages of multiple measures over time, which may better reflect long-term exposure but is more impractical to implement.
We considered the following established/potential risk factors for advanced adenoma, multiple adenomas, and colorectal cancer including age, personal history of diabetes, family history of colorectal cancer in first-degree relatives, regular use of aspirin or NSAIDs (nonsteroidal anti-inflammatory drugs), multivitamin use, body mass index (BMI, kg/m2), height (cm), calcium intake (mg/d) from supplement and food (total daily intake of milk, yogurt and calcium fortified orange juice multiplied by 300mg), supplemental vitamin D (IU/d), red meat intake in servings of beef/pork/lamb as a main dish, smoking in pack-years calculated as the product of packs of cigarettes smoked daily and the total years of smoking, alcohol intake (g/d) from total daily servings of beer, white wine, red wine and liquor, and sedentary behavior indicated by weekly hours of sitting watching TV/VCR. Physical activity level (low, moderate, high) was derived from weekly hours of brisk walking (≥3 miles/h) and time engaged in vigorous activities of ≥6 metabolic equivalents (running, jogging, bicycling, swimming, tennis, squash, racquetball and aerobics). We also evaluated oral contraceptive use, menopausal status and postmenopausal hormone use among women. The details of each risk factor assessment and validation were described previously.29-33 Due to the impracticality of using food frequency questionnaires to evaluate total dietary folate and vitamin D in clinical/general settings, we considered multivitamin use and supplemental vitamin D, which may capture part of the variations of the total intake of these two nutrients. Since the adverse effect of alcohol consumption on colorectal adenoma/cancer risk may be ameliorated by folic acid intake, we assessed the joint effect of alcohol and multivitamin use.34, 35 When participants had missing information on a certain risk factor, we carried information assessed from the previous questionnaire. Participants with missing information after carrying forward were excluded.
Statistical Analysis
For each risk factor, we first assessed its association with high-risk adenoma adjusting for 5-year age group by logistic regression. To assess dose-response, we compared the model fit between the models with continuous vs categorical risk factor using log likelihood ratio test. We tested for statistical interaction by adding a cross-product term of the two variables to a model along with the main effects and compared to the model with only main effects using a log likelihood ratio test.
Stepwise multiple logistic regressions with Pentry=0.15 and Pstay=0.20 were used to build the prediction model in men and women, respectively. Odds ratios (ORs) and 95% confidence intervals (CI) of high-risk colorectal adenoma were presented. Predicted risk of high-risk colorectal adenoma was calculated by exp(βo+∑βiXi)/(1+ exp(βo+∑βiXi)), where βo was the intercept, and βi was the regression coefficient for risk factor Xi.
Receiver-operating characteristic (ROC) curves were plotted for the final models as well as the models with age, and age and family history of colorectal cancer to evaluate the impact of adding modifiable risk factors to the current risk stratification criterion. C-statistics and age-adjusted C-statistics 36 which capture the predictive ability of the model without the impact of age were calculated to evaluate the discriminatory ability. Because of a limited sample size and overly pessimistic estimates of performance with large variability by split-sample method, as well as complex computation involved in bootstrapping, ten-fold cross-validation was performed to validate the models internally.37 We first randomly divided the data into 10 subsets, fitted the final model in 90% of the data (training set) and estimated the predictive risk of high-risk adenoma in the remaining 10% of the data (test set). We repeated this procedure for all the 10 subsets, and calculated the average C-statistics in the 10 test sets.
We assessed calibration by Hosmer-Lemeshow (HL) goodness-of-fit test comparing observed and predicted probabilities across deciles of predicted risk. PHL values greater than 0.05 indicate adequate calibration.
As sensitivity analyses, we examined whether using continuous instead of categorical variables and a unisex model would improve discrimination, respectively. All the statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
A total of 4,881 men and 17,970 women had completed colonoscopy as their first endoscopy for routine colorectal cancer screening by 2008, and 330 (6.7%) men and 678 (3.8%) women were diagnosed with high-risk adenoma (Table 1).
Table 1.
Age-standardized characteristics of participants at first-time screening colonoscopy, HPFS (1988-2008) and NHS (1986-2008)
| Men |
Women |
|||
|---|---|---|---|---|
| Characteristic | High-risk adenoma (n=330) | Non-cases (n=4551) | High-risk adenoma (n=678) | Non-cases (n=17292) |
| Age, yrs* | 65.6(7.3) | 63.1(8.1) | 65.0(7.3) | 64.2(7.2) |
| Personal history of diabetes, % | 8.0 | 6.5 | 7.0 | 7.0 |
| Family history of colorectal cancer, % | 20.6 | 15.7 | 28.6 | 22.0 |
| Height, cm | 179(6) | 179(7) | 164(6) | 164(6) |
| BMI, kg/m2 | 27.0(3.6) | 26.4(3.7) | 27.0(5.3) | 26.6(5.2) |
| Postmenopausal hormone use, % | ||||
| Never | 25.9 | 22.9 | ||
| Past | 28.9 | 35.4 | ||
| Current | 40.6 | 37.0 | ||
| Premenopausal, % | 4.6 | 4.8 | ||
| Former or current oral contraceptive user, % | 52.6 | 56.2 | ||
| Regular aspirin user , % | 35.7 | 39.7 | 36.8 | 39.9 |
| Regular NSAID user, % | 17.7 | 24.4 | 35.3 | 41.5 |
| Multivitamin | ||||
| Never, % | 25.0 | 18.4 | 2.9 | 3.0 |
| Past, % | 17.9 | 20.6 | 37.8 | 29.8 |
| Current,% | 57.1 | 61.0 | 59.2 | 67.2 |
| Years of use | 12.4(7.8) | 12.8(8.1) | 8.3(6.6) | 8.2(6.2) |
| Calcium intake, mg/d † | 450(437) | 510(465) | 845(598) | 911(608) |
| Vitamin D from supplement, IU/d | 192(235) | 214(248) | 238(253) | 288(275) |
| Beef/pork/lamb as main dish, servings/wk | 1.8(1.4) | 1.7(1.5) | 1.5(1.3) | 1.4(1.3) |
| Former or current smoker, % | 61.8 | 54.0 | 58.4 | 54.0 |
| Pack-years of smoking | 23.4(19.8) | 17.9(17.2) | 23.1(18.4) | 20.5(17.7) |
| Alcohol, g/d | 12.7(14.9) | 12.1(15.4) | 6.6(11.9) | 5.8(10.0) |
| Brisk walking, h/wk ‡ | 1.6(4.2) | 1.9(4.0) | 0.8(1.9) | 0.9(2.2) |
| Vigorous activity, h/wk ‡ | 1.4(2.3) | 2.0(3.6) | 1.1(4.0) | 0.9(2.3) |
| Physical activity‡ | ||||
| Low,% | 43.9 | 33.8 | 48.2 | 49.5 |
| Moderate,% | 28.7 | 32.6 | 37.7 | 34.9 |
| High,% | 27.3 | 33.6 | 14.1 | 15.5 |
| Sitting watching TV/VCR, h/d | 1.5(1.2) | 1.4(1.2) | 1.8(1.6) | 1.8(1.6) |
All values were calculated from the most recent questionnaire before the first colonoscopy, and other than age, have been directly standardized to age distribution of all the participants. Mean (SD) was presented for continuous variables.
Calcium intake was the sum of calcium intake from supplement and food. Calcium from food was derived by multiplying the total daily intake of milk, yogurt and fortified orange juice by 300mg.
Physical activity was defined by levels of brisk walking (walking speed ≥3 miles/hr) and vigorous activity (activities with ≥6 metabolic equivalents including running, jogging, bicycling, swimming, tennis, squash, racquetball and aerobics). Low: 0-4.9 hrs of brisk walking per week and no vigorous activity; moderate: 0-4.9 hrs of brisk walking and 0.1-2.9 hrs of vigorous activity; high: ≥5 hrs of brisk walking or ≥3 hrs of vigorous activity.
In the final risk model for men, factors positively associated with risk of high-risk adenoma included age, family history of colorectal cancer, BMI, pack-years of smoking and time spent sitting watching TV/VCR (Table 2). Factors associated with reduced risk included regular use of aspirin/NSAIDs and moderate to high levels of physical activity. The risk model also included a joint term of alcohol intake and multivitamin use, with the highest risk observed among men who never took multivitamin and consumed ≥30 g of alcohol per day compared to men who never took multivitamin and consumed <5 g of alcohol per day.
Table 2.
Risk prediction models for high-risk colorectal adenoma at first-time screening colonoscopy
| Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Case/Non-case (330/4551) | OR | (95% CI) | P* | Case/Non-case (678/17292) | OR | (95% CI) | P* |
| Age, yrs | ||||||||
| <55 | 17/639 | 1.00 | -- | <0.001 | 61/1550 | 1.00 | -- | 0.10 |
| 55-59 | 62/1195 | 2.00 | (1.15,3.47) | 105/3347 | 0.85 | (0.61,1.17) | ||
| 60-64 | 84/1019 | 3.08 | (1.80,5.29) | 172/4781 | 0.96 | (0.71,1.31) | ||
| 65-69 | 73/750 | 3.42 | (1.97,5.91) | 165/3882 | 1.13 | (0.83,1.54) | ||
| ≥70 | 94/948 | 3.64 | (2.13,6.23) | 175/3732 | 1.26 | (0.92,1.73) | ||
| Family history of colorectal cancer | 65/719 | 1.43 | (1.07,1.91) | 0.01 | 193/3814 | 1.41 | (1.18,1.67) | <0.001 |
| BMI, kg/m 2 † | ||||||||
| Quartile 1 | 54/1192 | 1.00 | -- | <0.001 | 149/4349 | 1.00 | -- | 0.14 |
| Quartile 2 | 90/1172 | 1.59 | (1.12,2.26) | 167/4338 | 1.14 | (0.91,1.43) | ||
| Quartile 3 | 87/1050 | 1.69 | (1.18,2.41) | 181/4330 | 1.23 | (0.99,1.54) | ||
| Quartile 4 | 99/1137 | 1.73 | (1.22,2.47) | 181/4275 | 1.29 | (1.03,1.61) | ||
| Aspirin (regular vs non-regular) | 121/1801 | 0.78 | (0.62,0.99) | 0.03 | 253/6901 | 0.88 | (0.75,1.04) | 0.15 |
| NSAIDs (regular vs non-regular) | 64/1110 | 0.70 | (0.53,0.93) | 0.02 | 238/7186 | 0.77 | (0.65,0.91) | 0.001 |
| Smoking, pack-years | ||||||||
| Never smoked | 121/2101 | 1.00 | -- | 0.008 | 283/7950 | 1.00 | -- | <0.001 |
| 1-4 | 36/646 | 0.91 | (0.62,1.35) | 61/1910 | 0.93 | (0.70,1.24) | ||
| 5-19 | 66/839 | 1.29 | (0.94,1.77) | 132/3380 | 1.11 | (0.90,1.37) | ||
| 20-39 | 69/674 | 1.46 | (1.06,2.01) | 122/2683 | 1.25 | (1.00,1.55) | ||
| ≥40 | 38/291 | 1.64 | (1.10,2.45) | 80/1369 | 1.50 | (1.15,1.95) | ||
| Physical activity‡ | ||||||||
| Low | 145/1534 | 1.00 | -- | 0.06 | ||||
| Moderate | 98/1486 | 0.81 | (0.62,1.07) | |||||
| High | 87/1531 | 0.70 | (0.53,0.94) | |||||
| Sitting watching TV/VCR, h/d | ||||||||
| <0.5 | 78/1267 | 1.00 | -- | 0.004 | ||||
| 0.5-<2 | 110/1831 | 1.03 | (0.76,1.39) | |||||
| ≥2 | 142/1453 | 1.49 | (1.11,1.99) | |||||
| Alcohol*Multivitamin | ||||||||
| <5 g/d, never used multivitamin | 25/392 | 1.00 | -- | 0.08 | ||||
| <5 g/d, ever used multivitamin | 104/1602 | 1.03 | (0.65,1.62) | |||||
| 5-29 g/d, never used multivitamin | 34/356 | 1.44 | (0.84,2.49) | |||||
| 5-29 g/d, ever used multivitamin | 121/1675 | 1.19 | (0.75,1.88) | |||||
| ≥30 g/d, never used multivitamin | 15/92 | 2.32 | (1.15,4.66) | |||||
| ≥30 g/d, ever used multivitamin | 31/434 | 1.02 | (0.59,1.79) | |||||
| Calcium, mg/d§ | ||||||||
| <300 | 139/2881 | 1.00 | -- | 0.07 | ||||
| 300-599 | 132/3173 | 0.88 | (0.69,1.13) | |||||
| ≥600 | 407/11238 | 0.80 | (0.66,0.98) | |||||
| Oral contraceptive (ever vs never) | 346/9728 | 0.89 | (0.76,1.05) | 0.03 | ||||
| Alcohol (≥30 vs <30 g/d) | 40/712 | 1.32 | (0.95,1.85) | 0.09 | ||||
| Beef/pork/lamb as main dish, servings | ||||||||
| <2/mo | 163/4506 | 1.00 | -- | 0.05 | ||||
| 2/mo-<2/wk | 286/7558 | 1.08 | (0.88,1.31) | |||||
| ≥2/wk | 229/5228 | 1.26 | (1.02,1.55) | |||||
P-value for stepwise selection.
BMI quartile cutoff points in men: 24.1, 25.9, 28.2 kg/m2; in women: 22.9, 25.8, 29.3 kg/m2.
Physical activity was defined by levels of brisk walking (walking speed ≥3 miles/hr) and vigorous activity (activities with ≥6 metabolic equivalents including running, jogging, bicycling, swimming, tennis, squash, racquetball and aerobics). Low: 0-4.9 hrs of brisk walking per week and no vigorous activity; moderate: 0-4.9 hrs of brisk walking and 0.1-2.9 hrs of vigorous activity; high: ≥5 hrs of brisk walking or ≥3 hrs of vigorous activity.
Calcium intake was the sum of calcium intake from supplement and food. Calcium from food was derived by multiplying the total daily intake of milk, yogurt and fortified orange juice by 300mg.
The risk model for women included the following factors that were positively associated with risk of high-risk adenoma: age, family history of colorectal cancer, BMI, smoking pack-years, alcohol intake, beef/pork/lamb as main dish; and factors that were inversely associated with risk of high-risk adenoma, including regular aspirin or NSAID use, calcium intake from supplement and food (milk, yogurt and orange juice), and oral contraceptive use (Table 2).
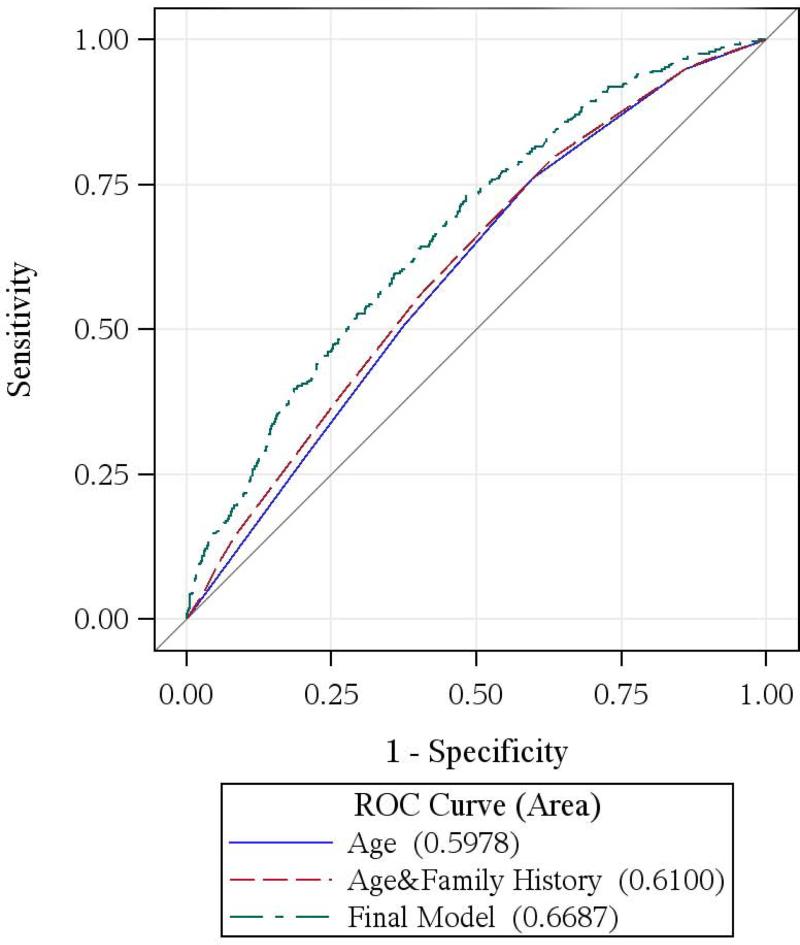
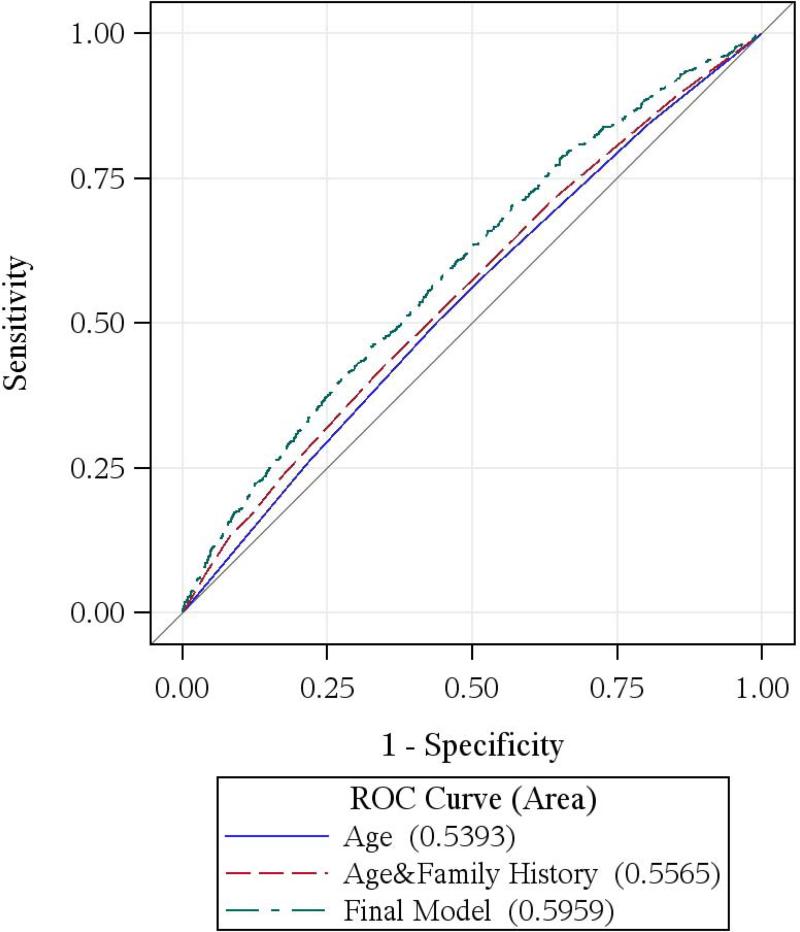
The C-statistic of the risk model for men was 0.67 (0.63 after adjusting for 5-year age group), and 0.60 (age-adjusted 0.59) for women (Table 3). C-statistics were not improved if continuous variables were used (0.65 for men and 0.59 for women) or when a unisex model with interactions was built combining data from men and women (0.64 for men and 0.59 for women).
Table 3.
C-statistics of the risk prediction models for high-risk colorectal adenoma compared with models with age and family history
| Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|
| Case/Non-case | Age+Family History |
Final Model |
P | Case/Non-case | Age+Family History |
Final Model |
P | |
| C | C | C | C | |||||
| Overall | 330/4551 | 0.61 | 0.67 | <0.001 | 678/17292 | 0.56 | 0.60 | <0.001 |
| By age | ||||||||
| <65 | 163/2853 | 0.61 | 0.69 | <0.001 | 338/9678 | 0.54 | 0.58 | 0.01 |
| ≥65 | 167/1698 | 0.52 | 0.60 | 0.01 | 340/7614 | 0.55 | 0.60 | 0.003 |
| Age-adjusted* | 330/4551 | 0.53 | 0.63 | <0.001 | 678/17292 | 0.53 | 0.59 | <0.001 |
| By type* | ||||||||
| ≥1cm in diameter | 242/4551 | 0.52 | 0.61 | <0.001 | 508/17292 | 0.53 | 0.59 | <0.001 |
| Advanced histology† | 150/4551 | 0.56 | 0.63 | 0.01 | 364/17292 | 0.54 | 0.60 | <0.001 |
| ≥3 adenomas | 126/4551 | 0.53 | 0.68 | <0.001 | 140/17292 | 0.55 | 0.65 | <0.001 |
| By location* | ||||||||
| Rectal | 68/4551 | 0.47 | 0.62 | 0.001 | 130/17292 | 0.53 | 0.59 | 0.03 |
| Distal | 178/4551 | 0.54 | 0.64 | <0.001 | 378/17292 | 0.53 | 0.61 | <0.001 |
| Proximal | 124/4551 | 0.54 | 0.65 | <0.001 | 226/17292 | 0.55 | 0.59 | 0.07 |
Adjusted for 5-year age groups.
With villous components (tubulovillous or villous), or with high-grade or severe dysplasia.
In 10-fold cross-validation, the average C-statistic across the 10 test sets was 0.64 for men, and 0.57 for women. The discriminatory power of the model was higher among men younger than 65 (C-statistic=0.69), and was less satisfactory among men aged 65 and above (C-statistic =0.60); but was similar for women aged less than 65 and 65 and above.
Significant improvement in C-statistics (overall, age-specific, age-adjusted overall and by type and location of high-risk adenoma) were observed in both men and women when our models were compared to the models with only age and family history (Table 3), suggesting the importance of adding modifiable lifestyle risk factors to the risk stratification of high-risk adenoma. ROC curves in Figure indicate that our models were also superior to models with age alone.
Figure.
ROC curves comparing models with age, age and family history, and the final prediction models of high-risk adenoma at first-time screening colonoscopy
A. Men
B. Women
In both men and women, our models were better at predicting ≥3 adenomas (C-statistic=0.68 for men and 0.65 for women), compared to large adenomas and adenomas of advanced histology (Table 3). The C-statistics were similar across sub-sites of the colorectum (i.e. proximal, distal and rectal high-risk adenomas).
Both models calibrated well across deciles of predicted risk (PHL =0.48 for men and 0.96 for women) (Table 4). The predicted risk of high-risk adenoma for men in the top decile was 15.4% compared to 1.8% for men in the bottom decile (OR=9.41; 95% CI 4.46-19.8). For women, the predicted risk in the top decile was 6.6% compared to 2.1% for women in the bottom decile (OR=3.48; 95% CI 2.38-5.08). The models also calibrated well among people under age 65 and aged 65 and above.
Table 4.
Calibration of risk prediction models for high-risk colorectal adenoma overall and by age across deciles of predicted risk
| Decile of predicted risk of high-risk colorectal adenoma |
PHL* | OR(95% CI)† | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||
| Men | ||||||||||||
| Overall (Case/Non-case=330/4551) | ||||||||||||
| Observed | 8 | 12 | 20 | 27 | 24 | 38 | 34 | 38 | 63 | 66 | ||
| Expected | 8.7 | 14.1 | 18.5 | 22.5 | 26.6 | 31.4 | 36.9 | 43.4 | 53.0 | 75.0 | ||
| Observed risk | 1.6% | 2.5% | 4.1% | 5.5% | 4.9% | 7.8% | 7.0% | 7.8% | 12.9% | 13.6% | ||
| Predicted Risk | 1.8% | 2.9% | 3.8% | 4.6% | 5.5% | 6.4% | 7.6% | 8.9% | 10.8% | 15.4% | 0.48 | 9.41 (4.46,19.8) |
| Age<65 (Case/Non-case=163/2853) | ||||||||||||
| Observed | 4 | 6 | 10 | 9 | 15 | 17 | 14 | 18 | 22 | 48 | ||
| Expected | 4.6 | 7.0 | 9.1 | 11.1 | 12.8 | 15.3 | 17.7 | 21.2 | 25.7 | 38.6 | ||
| Observed risk | 1.3% | 2.0% | 3.3% | 3.0% | 5.0% | 5.6% | 4.7% | 5.9% | 7.3% | 15.9% | ||
| Predicted Risk | 1.5% | 2.3% | 3.0% | 3.7% | 4.3% | 5.0% | 5.9% | 7.0% | 8.6% | 12.8% | 0.14 | 14.0 (4.99,39.4) |
| Age≥65 (Case/Non-case =167/1698) | ||||||||||||
| Observed | 6 | 12 | 16 | 18 | 14 | 16 | 16 | 20 | 15 | 34 | ||
| Expected | 6.8 | 9.2 | 11.0 | 12.8 | 14.6 | 16.3 | 18.5 | 20.8 | 23.9 | 33.2 | ||
| Observed risk | 3.2% | 6.4% | 8.6% | 9.7% | 7.5% | 8.6% | 8.5% | 10.8% | 8.1% | 18.2% | ||
| Predicted Risk | 3.6% | 4.9% | 5.9% | 6.9% | 7.8% | 8.7% | 9.8% | 11.2% | 12.8% | 17.7% | 0.28 | 6.67(2.73,16.3) |
| Women | ||||||||||||
| Overall (Case/Non-case o=678/17292) | ||||||||||||
| Observed | 36 | 44 | 48 | 64 | 62 | 72 | 67 | 78 | 88 | 119 | ||
| Expected | 37.6 | 45.6 | 51.2 | 56.1 | 61.0 | 66.1 | 72.0 | 79.7 | 90.7 | 118 | ||
| Observed risk | 2.0% | 2.5% | 2.7% | 3.6% | 3.5% | 4.0% | 3.7% | 4.3% | 4.9% | 6.6% | ||
| Predicted Risk | 2.1% | 2.5% | 2.8% | 3.1% | 3.4% | 3.7% | 4.0% | 4.4% | 5.0% | 6.6% | 0.96 | 3.48(2.38,5.08) |
| Age<65 (Case/Non-case=338/9678) | ||||||||||||
| Observed | 26 | 17 | 28 | 26 | 31 | 40 | 35 | 41 | 36 | 58 | ||
| Expected | 19.7 | 23.2 | 25.9 | 28.2 | 30.8 | 32.9 | 35.9 | 39.5 | 44.7 | 57.2 | ||
| Observed risk | 2.6% | 1.7% | 2.8% | 2.6% | 3.1% | 4.0% | 3.5% | 4.1% | 3.6% | 5.8% | ||
| Predicted risk | 2.0% | 2.3% | 2.6% | 2.8% | 3.1% | 3.3% | 3.6% | 3.9% | 4.5% | 5.7% | 0.48 | 2.32(1.45,3.71) |
| Age≥65 (Case/Non-case =340/7614) | ||||||||||||
| Observed | 17 | 24 | 23 | 33 | 29 | 31 | 44 | 40 | 40 | 59 | ||
| Expected | 20.0 | 23.8 | 26.3 | 28.5 | 30.7 | 33.0 | 36.0 | 39.5 | 44.6 | 57.6 | ||
| Observed risk | 2.1% | 3.0% | 2.9% | 4.2% | 3.6% | 3.9% | 5.5% | 5.0% | 5.0% | 7.4% | ||
| Predicted Risk | 2.5% | 3.0% | 3.3% | 3.6% | 3.8% | 4.2% | 4.5% | 5.0% | 5.6% | 7.2% | 0.85 | 3.66(2.12,6.34) |
PHL: P-value for Hosmer-Lemeshow goodness-of-fit test; P>0.05 indicates adequate fit.
OR of high-risk adenoma for the top decile compared with the bottom decile of predicted prevalence.
DISCUSSION
Using data of two large prospective cohort studies, we extensively evaluated established and potential risk factors for colorectal neoplasia to develop separate risk prediction models for high-risk adenoma among 22,851 asymptomatic white men and women who underwent colonoscopy as the first routine screening for colorectal cancer. The models performed adequately in terms of discriminatory accuracy and goodness-of-fit. To the best of our knowledge, this set of prediction models is the first comprehensive tool for risk assessment of high-risk adenoma among asymptomatic U.S. adults.
The unique strengths of our study are 1) the use of high-risk adenoma diagnosed at first-time screening colonoscopy as the primary study endpoint, which is not influenced by previous screening history, provide direct information to those who never had a colorectal cancer screening, and is more conservative and informative for cancer prevention as advanced adenomas are precursors of the majority of colorectal cancers and the target of screening colonoscopies, and multiple adenomas infer higher risk of detection of advanced adenoma at surveillance colonoscopy; 2) the estimation of absolute risk, a more useful parameter in clinical decision than relative risks alone; 3) the attempt to develop a simple risk calculator that could be conveniently used in general settings by taking into account the feasibility of each risk factor assessment; 4) the ability to consider a wide variety of prospectively collected data on established and suspected risk factors of colorectal neoplasia for model development; 5) the comparison to the models with only age and family history to evaluate the impact of adding modifiable risk factors to the current screening risk stratification criterion.
Previous models established in other western countries24, 27, 28 did not address the above issues at the same time. Most importantly, these models were developed in mixed population with first and surveillance colonoscopies. Risk of recurrent adenomas largely depends on pathologic findings in the first screening colonoscopy and could not help address whether the baseline screening colonoscopy should be initiated. As risk factors as well as strengths of associations may also vary for prevalent and recurrent adenomas, these models need to be improved. Discrimination of the model by Tao et al will be less optimal in discrimination (C-statistic 0.67) when restricted to first colonoscopies because previous colonoscopy and polyps history (relative risk [RR]=0.4 and 3.2, respectively) were the strongest risk factors included in the model.24 Moreover, established and prevalent risk factors, such as aspirin/NSAID use was not included in Kaminski et al28 and Betes et al.27
Gender-specific absolute risk prediction models for colorectal cancer have been developed utilizing two case-control studies and SEER data, and validated in an external population with modest C-statistics (0.61 for both men and women).13, 14 However, risk factors were assessed retrospectively, history of endoscopies were only accessed crudely up to previous 10 years with missing data, and a list of establish/potential risk factors were not evaluated in the model development (e.g. history of diabetes, 38 calcium intake,39 and height40).
The C-statistics for our models (0.67 for men and 0.60 for women) were modest but comparable to other cancer risk models, e.g. 0.58 to 0.63 for breast cancer,41-44 0.69 for lung cancer,45 0.59-0.60 for ovarian cancer. 41, 46
Almost all of the factors in our final risk models have been consistently reported as risk factors for colorectal adenoma and cancer.34, 35, 39, 47-55 Sedentary behaviors as indicated by TV viewing are less studied;56 however, independent of physical activity, prolonged TV watching has been associated with increased risk of obesity,57 a known risk factor for colorectal carcinogenesis.49 We built separate models for men and women because risk factors or the strengths of association for high-risk adenoma/colorectal cancer may vary by gender.49, 50, 54 Consistent with previous findings,10, 49 we found that influence of BMI and age were stronger in men than in women. For factors that emerged in the prediction model one sex but not the other may reflect a true etiologic association that is stronger in one group than the other (e.g., physical activity is a slighter stronger risk factor for men54); or our simplified definition of risk factors in one group may not fully capture long-term exposure variations as compared to the other group (e.g., calcium intake from supplement, milk, yogurt and fortified orange juice may capture less long-term variations of total calcium intake among men). Our models should be interpreted from the standpoint of clinical risk prediction not etiologic research.
Our study has limitations. The absolute risk of high-risk adenoma observed (6.7% in men and 3.8% in women) was comparable to other studies;4, 10 however, we were limited in power to fully assess the impact of extreme risk factors because health professionals are slightly more health-conscious than the general U.S. population (e.g., prevalence of current smoking was 4% in the HPFS compared to national average of 22%58). Additionally, our prediction was most applicable to white men and women, calibration in additional studies especially among other races were necessary before use in general settings. Finally, colonoscopy or sigmoidoscopy was self-reported. However, misclassification was unlikely as colonoscopy requires sedation.
In summary, we developed and evaluated a set of risk stratification tool that can be used to estimate current risk of high-risk colorectal adenoma and provide guidance regarding first-time colorectal cancer screening. Our models may encourage high-risk people to undertake colonoscopy, and will be particularly valuable in resource limited settings. Low-risk individuals should discuss screening alternatives (e.g.fecal occult blood test) with their physicians. The models could also help people target behaviors to lower their risk of colorectal neoplasia. A web/mobile based risk calculator/nomogram may increase the feasibility.
Acknowledgements
We thank participants and staff of the Health Professionals Follow-up Study and the Nurses’ Health Study for their valuable contributions and the state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Maine, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, Wyoming. We thank Mr. Scott G. Smith, from Department of Nutrition, Harvard School of Public Health, for his contribution in data management.
Grant sponsor: Supported by grants from the NIH (P01 CA87969 to Dr Meir Stampfer; P01 CA55075 and 1UM1 CA167552 to Dr Walter Willett; R01 CA137178 and K24 DK098311 to Dr Andrew Chan) and the Entertainment Industry Foundation through the National Colorectal Cancer Research Alliance. Dr Chan is a Damon Runyon Clinical Investigator.
REFERENCES
- 1.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D, Johnson CD, Levin TR, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Vital signs: colorectal cancer screening test use--United States, 2012. MMWR. Morbidity and mortality weekly report. 2013;62:881–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Regula J, Rupinski M, Kraszewska E, Polkowski M, Pachlewski J, Orlowska J, Nowacki MP, Butruk E. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. The New England journal of medicine. 2006;355:1863–72. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 5.Schroy PC, Coe A, Chen CA, O'Brien MJ, Heeren TC. Prevalence of Advanced Colorectal Neoplasia in White and Black Patients Undergoing Screening Colonoscopy in a Safety-Net Hospital. Annals of internal medicine. 2013;159:13–+. doi: 10.7326/0003-4819-159-1-201307020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vijan S, Inadomi J, Hayward RA, Hofer TP, Fendrick AM. Projections of demand and capacity for colonoscopy related to increasing rates of colorectal cancer screening in the United States. Alimentary pharmacology & therapeutics. 2004;20:507–15. doi: 10.1111/j.1365-2036.2004.01960.x. [DOI] [PubMed] [Google Scholar]
- 7.Seeff LC, Manninen DL, Dong FB, Chattopadhyay SK, Nadel MR, Tangka FK, Molinari NA. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology. 2004;127:1661–9. doi: 10.1053/j.gastro.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 8.Steinwachs D, Allen JD, Barlow WE, Duncan RP, Egede LE, Friedman LS, Keating NL, Kim P, Lave JR, Laveist TA, Ness RB, Optican RJ, et al. National Institutes of Health state-of-the-science conference statement: Enhancing use and quality of colorectal cancer screening. Annals of internal medicine. 2010;152:663–7. doi: 10.7326/0003-4819-152-10-201005180-00237. [DOI] [PubMed] [Google Scholar]
- 9.Ballew C, Lloyd BG, Miller SH. Capacity for colorectal cancer screening by colonoscopy, Montana, 2008. American journal of preventive medicine. 2009;36:329–32. doi: 10.1016/j.amepre.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Ferlitsch M, Reinhart K, Pramhas S, Wiener C, Gal O, Bannert C, Hassler M, Kozbial K, Dunkler D, Trauner M, Weiss W. Sex-specific prevalence of adenomas, advanced adenomas, and colorectal cancer in individuals undergoing screening colonoscopy. JAMA : the journal of the American Medical Association. 2011;306:1352–8. doi: 10.1001/jama.2011.1362. [DOI] [PubMed] [Google Scholar]
- 11.Brenner H, Hoffmeister M, Stegmaier C, Brenner G, Altenhofen L, Haug U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut. 2007;56:1585–9. doi: 10.1136/gut.2007.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colditz GA, Atwood KA, Emmons K, Monson RR, Willett WC, Trichopoulos D, Hunter DJ. Harvard report on cancer prevention volume 4: Harvard Cancer Risk Index. Risk Index Working Group, Harvard Center for Cancer Prevention. Cancer causes & control : CCC. 2000;11:477–88. doi: 10.1023/a:1008984432272. [DOI] [PubMed] [Google Scholar]
- 13.Freedman AN, Slattery ML, Ballard-Barbash R, Willis G, Cann BJ, Pee D, Gail MH, Pfeiffer RM. Colorectal cancer risk prediction tool for white men and women without known susceptibility. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:686–93. doi: 10.1200/JCO.2008.17.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park Y, Freedman AN, Gail MH, Pee D, Hollenbeck A, Schatzkin A, Pfeiffer RM. Validation of a colorectal cancer risk prediction model among white patients age 50 years and older. J Clin Oncol. 2009;27:694–8. doi: 10.1200/JCO.2008.17.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei EK, Colditz GA, Giovannucci EL, Fuchs CS, Rosner BA. Cumulative risk of colon cancer up to age 70 years by risk factor status using data from the Nurses’ Health Study. American journal of epidemiology. 2009;170:863–72. doi: 10.1093/aje/kwp210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Driver JA, Gaziano JM, Gelber RP, Lee IM, Buring JE, Kurth T. Development of a risk score for colorectal cancer in men. Am J Med. 2007;120:257–63. doi: 10.1016/j.amjmed.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 17.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 18.Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. The New England journal of medicine. 1992;326:658–62. doi: 10.1056/NEJM199203053261002. [DOI] [PubMed] [Google Scholar]
- 19.Winawer SJ, Zauber AG. The advanced adenoma as the primary target of screening. Gastrointest Endosc Clin N Am. 2002;12:1–9. v. doi: 10.1016/s1052-5157(03)00053-9. [DOI] [PubMed] [Google Scholar]
- 20.Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Using risk for advanced proximal colonic neoplasia to tailor endoscopic screening for colorectal cancer. Annals of internal medicine. 2003;139:959–65. doi: 10.7326/0003-4819-139-12-200312160-00005. [DOI] [PubMed] [Google Scholar]
- 21.Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D, Kirk L, Litin S, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124:544–60. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman DA, Weiss DG, Harford WV, Ahnen DJ, Provenzale D, Sontag SJ, Schnell TG, Chejfec G, Campbell DR, Kidao J, Bond JH, Nelson DB, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077–85. doi: 10.1053/j.gastro.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–57. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Tao S, Hoffmeister M, Brenner H. Development and validation of a scoring system to identify individuals at high risk for advanced colorectal neoplasms who should undergo colonoscopy screening. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12:478–85. doi: 10.1016/j.cgh.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 25.Yeoh KG, Ho KY, Chiu HM, Zhu F, Ching JY, Wu DC, Matsuda T, Byeon JS, Lee SK, Goh KL, Sollano J, Rerknimitr R, et al. The Asia-Pacific Colorectal Screening score: a validated tool that stratifies risk for colorectal advanced neoplasia in asymptomatic Asian subjects. Gut. 2011;60:1236–41. doi: 10.1136/gut.2010.221168. [DOI] [PubMed] [Google Scholar]
- 26.Cai QC, Yu ED, Xiao Y, Bai WY, Chen X, He LP, Yang YX, Zhou PH, Jiang XL, Xu HM, Fan H, Ge ZZ, et al. Derivation and Validation of a Prediction Rule for Estimating Advanced Colorectal Neoplasm Risk in Average-Risk Chinese. Am J Epidemiol. 2012;175:584–93. doi: 10.1093/aje/kwr337. [DOI] [PubMed] [Google Scholar]
- 27.Betes M, Munoz-Navas MA, Duque JM, Angos R, Macias E, Subtil JC, Herraiz M, De La Riva S, Delgado-Rodriguez M, Martinez-Gonzalez MA. Use of colonoscopy as a primary screening test for colorectal cancer in average risk people. The American journal of gastroenterology. 2003;98:2648–54. doi: 10.1111/j.1572-0241.2003.08771.x. [DOI] [PubMed] [Google Scholar]
- 28.Kaminski MF, Polkowski M, Kraszewska E, Rupinski M, Butruk E, Regula J. A score to estimate the likelihood of detecting advanced colorectal neoplasia at colonoscopy. Gut. 2014;63:1112–9. doi: 10.1136/gutjnl-2013-304965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, Hu FB. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. The American journal of clinical nutrition. 2011;94:1088–96. doi: 10.3945/ajcn.111.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. Journal of the American Dietetic Association. 1993;93:790–6. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 31.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. doi: 10.1093/oxfordjournals.aje.a116211. discussion 27-36. [DOI] [PubMed] [Google Scholar]
- 32.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, Ascherio A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–6. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. International journal of epidemiology. 1994;23:991–9. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 34.Giovannucci E, Rimm EB, Ascherio A, Stampfer MJ, Colditz GA, Willett WC. Alcohol, lowmethionine--low-folate diets, and risk of colon cancer in men. J Natl Cancer Inst. 1995;87:265–73. doi: 10.1093/jnci/87.4.265. [DOI] [PubMed] [Google Scholar]
- 35.Giovannucci E, Stampfer MJ, Colditz GA, Rimm EB, Trichopoulos D, Rosner BA, Speizer FE, Willett WC. Folate, methionine, and alcohol intake and risk of colorectal adenoma. J Natl Cancer Inst. 1993;85:875–84. doi: 10.1093/jnci/85.11.875. [DOI] [PubMed] [Google Scholar]
- 36.Rosner B, Glynn RJ. Power and sample size estimation for the Wilcoxon rank sum test with application to comparisons of C statistics from alternative prediction models. Biometrics. 2009;65:188–97. doi: 10.1111/j.1541-0420.2008.01062.x. [DOI] [PubMed] [Google Scholar]
- 37.Steyerberg EW, Harrell FE, Borsboom GJJM, Eijkemans MJC, Vergouwe Y, Habbema JDF. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. Journal of clinical epidemiology. 2001;54:774–81. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 38.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: A meta-analysis. Journal of the National Cancer Institute. 2005;97:1679–87. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 39.Cho E, Smith-Warner SA, Spiegelman D, Beeson WL, van den Brandt PA, Colditz GA, Folsom AR, Fraser GE, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. Journal of the National Cancer Institute. 2004;96:1015–22. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
- 40.Engeland A, Tretli S, Austad G, Bjorge T. Height and body mass index in relation to colorectal and gallbladder cancer in two million Norwegian men and women. Cancer Cause Control. 2005;16:987–96. doi: 10.1007/s10552-005-3638-3. [DOI] [PubMed] [Google Scholar]
- 41.Pfeiffer RM, Park Y, Kreimer AR, Lacey JV, Jr., Pee D, Greenlee RT, Buys SS, Hollenbeck A, Rosner B, Gail MH, Hartge P. Risk prediction for breast, endometrial, and ovarian cancer in white women aged 50 y or older: derivation and validation from population-based cohort studies. PLoS medicine. 2013;10:e1001492. doi: 10.1371/journal.pmed.1001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rockhill B, Spiegelman D, Byrne C, Hunter DJ, Colditz GA. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001;93:358–66. doi: 10.1093/jnci/93.5.358. [DOI] [PubMed] [Google Scholar]
- 43.Colditz GA, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses’ Health Study. American journal of epidemiology. 2000;152:950–64. doi: 10.1093/aje/152.10.950. [DOI] [PubMed] [Google Scholar]
- 44.Barlow WE, White E, Ballard-Barbash R, Vacek PM, Titus-Ernstoff L, Carney PA, Tice JA, Buist DS, Geller BM, Rosenberg R, Yankaskas BC, Kerlikowske K. Prospective breast cancer risk prediction model for women undergoing screening mammography. J Natl Cancer Inst. 2006;98:1204–14. doi: 10.1093/jnci/djj331. [DOI] [PubMed] [Google Scholar]
- 45.Cronin KA, Gail MH, Zou Z, Bach PB, Virtamo J, Albanes D. Validation of a model of lung cancer risk prediction among smokers. J Natl Cancer Inst. 2006;98:637–40. doi: 10.1093/jnci/djj163. [DOI] [PubMed] [Google Scholar]
- 46.Rosner BA, Colditz GA, Webb PM, Hankinson SE. Mathematical models of ovarian cancer incidence. Epidemiology. 2005;16:508–15. doi: 10.1097/01.ede.0000164557.81694.63. [DOI] [PubMed] [Google Scholar]
- 47.Cho E, Smith-Warner SA, Ritz J, van den Brandt PA, Colditz GA, Folsom AR, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Holmberg L, Kim DH, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Annals of internal medicine. 2004;140:603–13. doi: 10.7326/0003-4819-140-8-200404200-00007. [DOI] [PubMed] [Google Scholar]
- 48.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Annals of internal medicine. 1995;122:327–34. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 49.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:2533–47. doi: 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- 50.Bosetti C, Bravi F, Negri E, La Vecchia C. Oral contraceptives and colorectal cancer risk: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:489–98. doi: 10.1093/humupd/dmp017. [DOI] [PubMed] [Google Scholar]
- 51.Botteri E, Iodice S, Raimondi S, Maisonneuve P, Lowenfels AB. Cigarette smoking and adenomatous polyps: a meta-analysis. Gastroenterology. 2008;134:388–95. doi: 10.1053/j.gastro.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Chan AT, Giovannucci EL, Schernhammer ES, Colditz GA, Hunter DJ, Willett WC, Fuchs CS. A prospective study of aspirin use and the risk for colorectal adenoma. Annals of internal medicine. 2004;140:157–66. doi: 10.7326/0003-4819-140-3-200402030-00006. [DOI] [PubMed] [Google Scholar]
- 53.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA : the journal of the American Medical Association. 2005;294:914–23. doi: 10.1001/jama.294.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolin KY, Yan Y, Colditz GA. Physical activity and risk of colon adenoma: a meta-analysis. British journal of cancer. 2011;104:882–5. doi: 10.1038/sj.bjc.6606045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. British journal of cancer. 2009;100:611–6. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmid D, Leitzmann MF. Television viewing and time spent sedentary in relation to cancer risk: a meta-analysis. Journal of the National Cancer Institute. 2014:106. doi: 10.1093/jnci/dju098. [DOI] [PubMed] [Google Scholar]
- 57.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA : the journal of the American Medical Association. 2003;289:1785–91. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 58.Centers for Disease C, Prevention Vital signs: current cigarette smoking among adults aged >/=18 years--United States, 2005-2010. MMWR. Morbidity and mortality weekly report. 2011;60:1207–12. [PubMed] [Google Scholar]