Summary
Patient-specific primers from 10 children/adolescents with Burkitt leukaemia (BL) ± central nervous system disease who were treated with French-British-American/Lymphome Malins de Burkitt 96 C1 plus rituximab were developed from diagnostic blood/bone marrow. Minimal residual disease (MRD) was assessed by real-time polymerase chain reaction at the end of induction (EOI) and consolidation (EOC). Seventy percent (7/10) and 71% (5/7) were MRD-positive at EOI and EOC, respectively, with no disease recurrences. MRD after induction and consolidation did not predict relapse and subsequent therapy appeared to eliminate MRD. Thus, assessing MRD at a later time point is warranted in future trials to determine its clinical significance.
Keywords: children, CNS, leukaemia
Introduction
We previously reported the feasibility of detecting minimal residual disease (MRD) in children and adolescents with intermediate-risk (Stage III/IV) mature B-cell non-Hodgkin lymphoma (NHL) treated with French-British-American/Lymphome Malins de Burkitt 96 (FAB/LMB96) B4 modified chemotherapy and rituximab (Shiramizu, et al 2011). Children and adolescents with advanced B-NHL that is either mature B-cell leukaemia (bone marrow, BM ≥ 25% blasts) and/or with central nervous system (CNS) involvement have high-risk disease with higher risk of relapse/progression than intermediate-risk patients. Both the Berlin-Frankfurt-Münster (BFM) and FAB international cooperative studies have unsuccessfully attempted to reduce therapy in this high-risk patient population (Cairo, et al 2007, Cairo, et al 2012, Woessmann, et al 2005). Because children and adolescents with recurrent or refractory disease have poor salvage and survival rates (<20%), one strategy to improve outcomes in childhood B-NHL, in addition to newer targeted therapies, may lie in early identification of patients at risk for relapse by detection of MRD during, or at the end of, therapy (Cairo, et al 2007, Cairo, et al 2012). This pilot study report highlights the potential significance of choosing the optimal time point to assess MRD and reports a quality control (QC) validation of the assay across two reference laboratories.
Patients and methods
General
The Children’s Oncology Group (COG) ANHL01P1 (clinicaltrials.gov NCT00057811) study investigated the addition of rituximab to the FAB/LMB96 C1 chemotherapy backbone (Goldman, et al 2014), which was open to all COG centres. The protocol was approved by each respective institutional review board (IRB). Staging classification utilized the NHL St. Jude Staging (Murphy 1980). Parents or patients over 18 years of age signed an IRB-approved informed consent before study enrollment in accordance with the Declaration of Helsinki, which included a separate informed consent process for participation in the optional MRD substudy.
Eligibility and evaluation
Patients under 30 years of age with newly diagnosed de-novo mature B-cell lymphoma classified by the Revised European-American Lymphoma and World Health Organization criteria were eligible (Swerdlow, et al 2008). CD20-positive immunohistochemistry was required for study eligibility with central pathology review to confirm diagnosis. Group C risk was defined as patients with BM ≥ 25% blasts and/or CNS disease (Cairo, et al 2007, Cairo, et al 2012). CNS disease was defined as any cerebral spinal fluid blasts found on diagnostic lumbar puncture (prior to intrathecal therapy administration) and/or isolated intracerebral mass, cranial nerve palsy, clinical spinal cord compression and/or parameningeal extension (Cairo, et al 2007).
Treatment
Chemotherapy and Immunotherapy
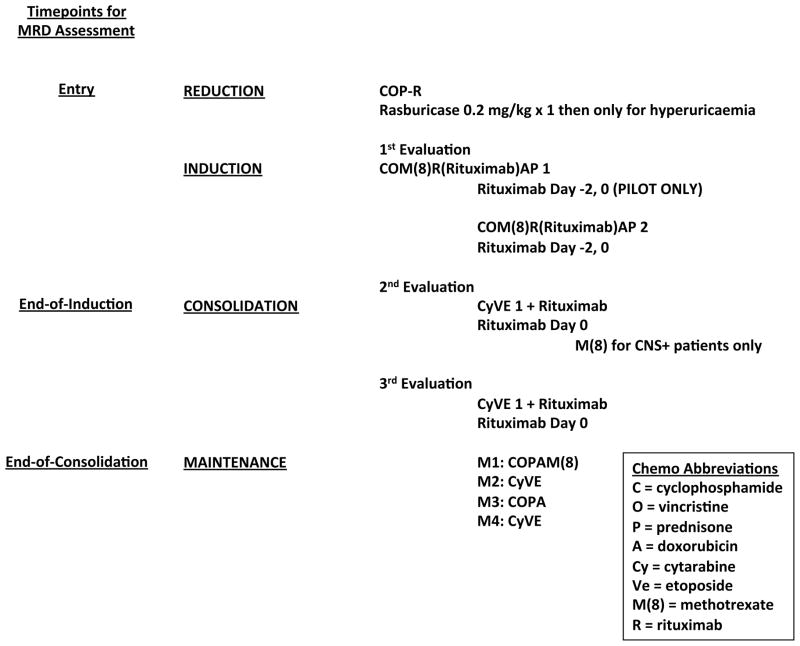
Details of the chemotherapy with rituximab were recently published for the entire trial including the subset of patients analysed for MRD (Goldman, et al 2013, Goldman, et al 2014). Figure 1 shows two MRD assessment time points in relation to the different blocks of chemo/immunotherapy, end of induction (EOI) and end of consolidation (EOC) (Goldman, et al 2013). By EOC, patients had received 2 (CNS−) or 3 (CNS+) cycles with high dose methotrexate (8 g/m2) and multiagent intensive cycles of chemotherapy including fractionated alkylators and high dose cytarabine with 6 infusions of rituximab (Goldman, et al 2014). Following EOC, patients received four maintenance cycles of chemotherapy without rituximab (Cairo, et al 2007, Goldman, et al 2014).
Figure 1. Minimal Residual Disease (MRD) Timepoints.
The timepoints at which blood and/or bone marrow were obtained with respect to the treatment schema.
Measurement of MRD
Peripheral blood (PB) and BM DNA from diagnosis, EOI and EOC were extracted using DNA isolation kits (Qiagen, Valencia, CA) and assessed by ultraviolet spectrophotometry and HBB control polymerase chain reaction (PCR) for quality. Minimal disease was assessed as previously described (Shiramizu, et al 2011). Briefly, diagnostic specimens were initially screened for IGHV family usage with primer pools: IGHV1/IGHV2; IGHV3/IGHV4; IGHV5, IGHV6/IGHV7 (Agsalda, et al 2009), combined with consensus 3′-primers: LJH and VLJH. Semi-nested real-time PCR with resulting melt curves were analysed (Agsalda, et al 2009, Shiramizu, et al 2011). From these results, the unique IGHV family usage was identified and then used to analyse follow-up specimens.
MRD assays are incorporated in an ongoing international randomized prospective study to determine if addition of rituximab to the FAB C1 chemotherapy backbone significantly improves event-free survival (EFS) in a larger cohort of patients tested by two international MRD reference laboratories that using different assays. This provided an opportunity to complete a QC validation between the two reference laboratories in Europe (Clinica di Oncoematologia Pediatrica, University of Padua, Padua, Italy) and the United States (University of Hawaii Tropical Medicine Medical Microbiology & Pharmacology, Honolulu, Hawaii, USA). QC specimens were provided by the European Reference Laboratory and analysed by both sites using their respective MRD assays (Mussolin, et al 2007, Shiramizu, et al 2011). The assay used by the European Reference Laboratory detects chromosomal t(8;14) by long-distance PCR (Mussolin, et al 2007). QC specimens provided by the European laboratory included a standard that was used in their assay of a specimen with known chromosomal t(8;14) as well as investigational tumour and follow-up specimens.
Results
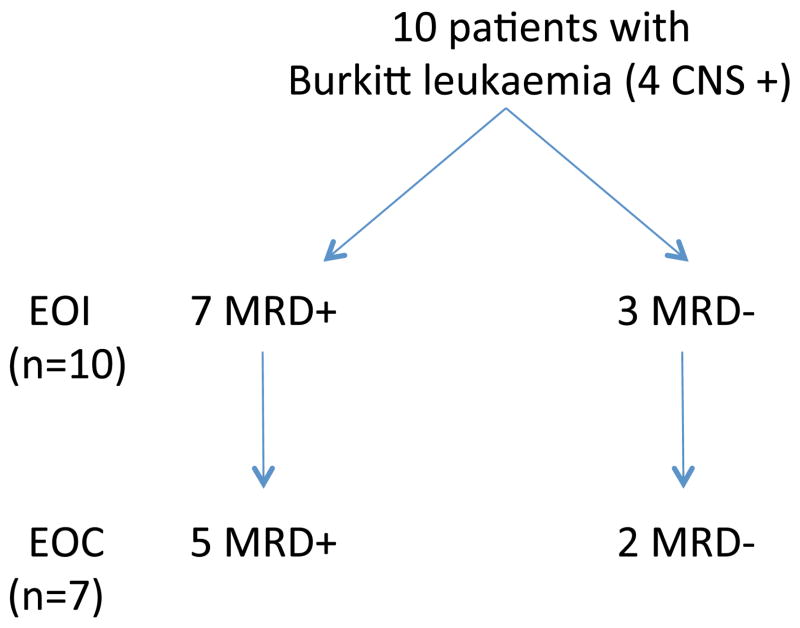
Ten evaluable patients with Burkitt leukaemia (BL) were included in this pilot study of the total of 40 patients enrolled in the ANHL01P1 clinical trial. All 10 patients were evaluable using the MRD assay. Four patients had concomitant CNS disease. As anticipated, all of these patients had molecular-positive PB and/or BM from their diagnostic specimens, which identified unique primer pairs for each patient. These unique primer pairs were then used to assess MRD on follow-up specimens. At EOI, 7/10 patients had measurable MRD (Fig. 2). At EOC, 5/7 submitted patient samples were MRD-positive (Fig. 2). There were no recurrences (3-year overall survival 100%) among the 10 group C patients for whom MRD specimens were submitted. Similarly, the 3-year overall EFS of all patients on study (n=40) was 90% (Goldman, et al 2014). In the 3 patients who were MRD-negative at EOI, 2 patients had specimens available at EOC and these were also negative (Fig. 2).
Figure 2. Minimal Residual Disease (MRD) Assay Time Points and MRD Melt Curves.
Minimal residual disease (MRD) time point at diagnosis, 7 of 10 patients were MRD-positive at end-of-induction (EOI). Of 7 patients who had specimens available for analysis at end-of-consolidation (EOC), 5 were MRD-positive.
The QC assessment of the MRD assay from the COG MRD Reference Laboratory using specimens from the European Reference Laboratory demonstrated MRD-positivity for each specimen, whereas the results from the European Reference Laboratory showed one follow-up specimen to be MRD-negative. In addition to the uniqueness of each respective assay, the discordance in these results could be due to differences in sensitivities between the two assays.
Discussion
These results demonstrate for the first time that children and adolescents with BL with or without CNS disease have measurable residual disease in PB after intensive induction and consolidation including rituximab. Even though approximately 70% of evaluable patients remained MRD-positive after induction and consolidation, none of these patients relapsed. Although the number of patients is small (n=10), these time points did not predict relapse, which is different from our recent findings in children and adolescents with mature B-NHL (including BL, diffuse large B cell lymphoma and primary mediastinal B-cell lymphoma) with intermediate risk disease, in which MRD had predictive value (Shiramizu, et al 2011). We believe that this assay truly measures minimal disease burden for several reasons, including our ability to identify unique IGHV primer pairs for each patient, and because patients who became MRD-negative early, remained undetectable at a later time point. The 5 patients who remained MRD-positive after induction and consolidation were eventually long-term disease-free survivors after the four additional cycles of lower dose monthly maintenance and no further immunotherapy which were administered to all Group C patients in the study as previously reported (Cairo, et al 2007, Goldman, et al 2014). A later time point to measure MRD, such as end-of-therapy evaluation should be investigated in future trials. The COG is currently participating in an international randomized prospective study to determine if the addition of rituximab to this FAB C1 chemotherapy backbone significantly improves EFS in a larger cohort of patients. That study includes an MRD substudy that will provide the foundational data for future strategies on the clinical utility of MRD in these patients using the respective MRD assays for European and COG institutions. The MRD assay using long-distance PCR at the European sites will assess the chromosomal translocation t(8;14)(q24;q32), which previously demonstrated that MRD-positive status correlated with poor outcome (Mussolin, et al 2007). Both the assay in this current report (sensitivity of 10−5) (Agsalda, et al 2009) and the assay used by the European Reference Laboratory (sensitivity of 10−3 to 10−4) (Mussolin, et al 2007, Mussolin, et al 2011) have been validated and will be compared in the ongoing international randomized prospective study. The ongoing clinical trial was recently amended to add a later time point for MRD assessment based on the current report and ongoing analyses of the data. Neither of the assays are quantitative which could be a future objective in improving the assays. The 10 patient samples in the current study were only assayed with the assay in the US laboratory because the European assay required diagnostic tumour tissue, which was not available from the 10 patients in the current study.
Our finding of residual disease in the PB of the majority of patients immediately prior to maintenance therapy raises the possibility that the kinetics of cure in Group C BL patients may require all four maintenance cycles to eliminate minimal disease burden. The previous FAB 96 international study, which randomly eliminated 3 maintenance cycles with simultaneous reduction of cytarabine and etoposide, was halted early due to poor outcome in the reduced therapy arm (Cairo, et al 2007). In the ongoing international trial, the last two maintenance cycles have been empirically eliminated in Group C patients (regardless of their immunotherapy randomization). MRD testing could potentially assess future patients with regard to the need for maintenance cycles. The value of MRD testing during and at completion of childhood BL therapy has the potential to make an impact on improving prognosis.
Acknowledgments
The authors would like to acknowledge Angelo Rosolen, MD, PhD, a leader in childhood and adolescent NHL MRD studies who passed away during the past year.
Supported by: Division of Cancer Treatment, National Cancer Institute, and National Institutes of Health, Department of Health and Human Services (COG) (CA98543-09 and CA98413-09), Pediatric Cancer Research Foundation (M.S.C), U54MD007584 (B.S.), Thrasher Research Fund (B.S.), St. Baldrick’s Foundation (B.S.)
Footnotes
Presented in part at the American Society of Hematology, December 2011, San Diego, CA and the American Society of Clinical Oncology, June 2012, Chicago, IL.
Author contributions
BS designed and performed the research, analysed the results and wrote the paper; SG designed the research, analysed the results and wrote the paper; LS analysed the data and critically reviewed the paper; MA performed the research, analysed the data and critically reviewed the paper; PG analysed the data and critically reviewed the paper; SLP analysed the data and critically reviewed the paper; KF analysed the data and critically reviewed the paper; WS analysed the data and critically reviewed the paper; JRA analysed the data and critically reviewed the paper; TGG analysed the data and critically reviewed the paper; HW analysed the data and critically reviewed the paper; LH analysed the data and critically reviewed the paper; MB analysed the data and critically reviewed the paper; LM designed the quality assurance experiments, analysed the data and critically reviewed the paper; MSC designed and analysed the data and wrote the paper.
Competing interests: The authors have no competing interests.
References
- Agsalda M, Kusao I, Troelstrup D, Shiramizu B. Screening for Residual Disease in Pediatric Burkitt Lymphoma Using Consensus Primer Pools. Adv Hematol. 2009:412163. doi: 10.1155/2009/412163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo MS, Gerrard M, Sposto R, Auperin A, Pinkerton CR, Michon J, Weston C, Perkins SL, Raphael M, McCarthy K, Patte C. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109:2736–2743. doi: 10.1182/blood-2006-07-036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo MS, Sposto R, Gerrard M, Auperin A, Goldman SC, Harrison L, Pinkerton R, Raphael M, McCarthy K, Perkins SL, Patte C. Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (>/= 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell non-Hodgkin’s lymphoma: results of the FAB LMB 96 study. J Clin Oncol. 2012;30:387–393. doi: 10.1200/JCO.2010.33.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S, Smith L, Anderson JR, Perkins S, Harrison L, Geyer MB, Gross TG, Weinstein H, Bergeron S, Shiramizu B, Sanger W, Barth M, Zhi J, Cairo MS. Rituximab and FAB/LMB 96 chemotherapy in children with Stage III/IV B-cell non-Hodgkin lymphoma: a Children’s Oncology Group report. Leukemia. 2013;27:1174–1177. doi: 10.1038/leu.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S, Smith L, Galardy P, Perkins SL, Frazer JK, Sanger W, Anderson JR, Gross TG, Weinstein H, Harrison L, Shiramizu B, Barth M, Cairo MS. Rituximab with chemotherapy in children and adolescents with central nervous system and/or bone marrow-positive Burkitt lymphoma/leukaemia: a Children’s Oncology Group Report. Br J Haematol. 2014;167:394–401. doi: 10.1111/bjh.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin’s lymphomas: dissimilarities from lymphomas in adults. Semin Oncol. 1980;7:332–339. [PubMed] [Google Scholar]
- Mussolin L, Pillon M, Conter V, Piglione M, Lo Nigro L, Pierani P, Micalizzi C, Buffardi S, Basso G, Zanesco L, Rosolen A. Prognostic role of minimal residual disease in mature B-cell acute lymphoblastic leukemia of childhood. J Clin Oncol. 2007;25:5254–5261. doi: 10.1200/JCO.2007.11.3159. [DOI] [PubMed] [Google Scholar]
- Mussolin L, Pillon M, d’Amore ES, Conter V, Piglione M, Lo Nigro L, Garaventa A, Buffardi S, Arico M, Rosolen A. Minimal disseminated disease in high-risk Burkitt’s lymphoma identifies patients with different prognosis. J Clin Oncol. 2011;29:1779–1784. doi: 10.1200/JCO.2010.32.8161. [DOI] [PubMed] [Google Scholar]
- Shiramizu B, Goldman S, Kusao I, Agsalda M, Lynch J, Smith L, Harrison L, Morris E, Gross TG, Sanger W, Perkins S, Cairo MS. Minimal disease assessment in the treatment of children and adolescents with intermediate-risk (Stage III/IV) B-cell non-Hodgkin lymphoma: a children’s oncology group report. Br J Haematol. 2011;153:758–763. doi: 10.1111/j.1365-2141.2011.08681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon: 2008. [Google Scholar]
- Woessmann W, Seidemann K, Mann G, Zimmermann M, Burkhardt B, Oschlies I, Ludwig WD, Klingebiel T, Graf N, Gruhn B, Juergens H, Niggli F, Parwaresch R, Gadner H, Riehm H, Schrappe M, Reiter A. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM Group Study NHL-BFM95. Blood. 2005;105:948–958. doi: 10.1182/blood-2004-03-0973. [DOI] [PubMed] [Google Scholar]