Abstract
Objectives
Vulvar squamous cell carcinoma (vSCC) is a gynecologic malignancy diagnosed in nearly 4500 women in the U.S. each year. Current criteria for treatment planning provide inadequate assessment of aggressive vSCC cases, resulting in insufficient use of adjuvant treatments and high rates of vSCC recurrence. Perineural invasion (PNI) is a pathologic feature inconsistently included in the assessment of vSCC because its relevance to clinical outcomes in these women is not well defined. The purpose of this study was to determine the association between PNI and relevant clinical parameters such as recurrence.
Methods
103 cases of vSCC were evaluated for PNI using pathology report review and IHC dual-chromogen staining for S-100 and AE1/3. Medical records were reviewed for clinical and follow-up data. Data were analyzed using univariate and multivariate logistic regression statistical methods.
Results
Patients with vSCC containing PNI had a greater risk of cancer recurrence than those whose tumors did not contain PNI (OR = 2.8, p = 0.0290). There was no significant correlation between the presence of PNI and nodal involvement, stage, or lymph-vascular invasion (LVI). Tumors with PNI had greater depth of invasion (DOI) (p = 0.0047), however DOI was not associated with recurrence (p = 0.2220). When analyzed using a multivariable logistic regression model, PNI was an independent predictor of recurrence in vSCC (adjOR = 2.613, p = 0.045).
Conclusions
Perineural invasion is an independent indicator of risk for recurrence in vSCC. The association of PNI with increased risk for recurrence, independent of DOI, nodal involvement, LVI, or stage, should encourage practicing pathologists to thoroughly search for and report the presence of PNI in vSCC.
Keywords: vulva, perineural invasion, recurrence, adjuvant treatment
Introduction
Vulvar cancer is a gynecologic malignancy that will be diagnosed in an estimated 4,850 women in the United States in 2014.1, 2 Squamous cell carcinoma (vSCC) comprises the majority (90%) of vulvar cancers, and primarily affects elderly women between ages 75-84.2, 3 Surgical excision is the only curative treatment option for vSCC, ranging from wide local excision to complete radical vulvectomy, and depending on the extent of surgical resection, vSCC can be a disfiguring malignancy with long term physical and psychological morbidities.4-7 Although distant metastases are rare, nodal involvement and local recurrence are important clinical outcomes of vSCC. Adjuvant chemotherapy or radiation treatments have proven to be effective at preventing recurrence and improving outcomes for vSCC patients, yet despite the presence of these adjuvant therapies, recurrence rates for vSCC remain as high as 40%, indicating ineffective utilization of adjuvant treatment options.8-11 Current criteria for use of post-surgical radiation and chemotherapy vary widely between surgeons and institutions. These criteria often include nodal involvement, depth of invasion, and positive or close surgical margins, but do not take into account histopathologic features of vSCC that may be associated with increased tumor aggressiveness and recurrence. Identifying pathologic features relevant to outcome and prognosis in these patients may be key in successful adjuvant treatment of vSCC.
Perineural Invasion
Perineural invasion (PNI) is a pathologic feature that is inconsistently included in pathology reports and is not currently required for TNM staging of vSCC.12 PNI occurs when tumor cells surround or invade the layers of the nerve sheath, and has been associated with worse outcomes in prostate cancer, head and neck squamous cell carcinoma, colorectal carcinoma, as well as in other cancers.13-19 Additionally, several studies suggest that PNI may play a role in local tumor recurrence in cancers such as prostate and pancreatic carcinoma.20, 21 Despite evidence that PNI is an important pathologic feature, its relevance to clinical outcomes in vSCC is not well defined. Understanding the relevance of PNI to local tumor aggressiveness and recurrence in vSCC could alter the current approach to adjuvant treatment options for vSCC patients by allowing more efficient identification of aggressive cases that could benefit from additional therapeutic options.
The purpose of this study was to investigate the association of PNI with clinicopathologic features of vSCC to determine if PNI could be a useful marker of tumor aggressiveness during treatment planning.
Materials and Methods
Case Acquisition
Cases of vSCC were identified using SoftPath IV software. Search criteria included all University of Arkansas for Medical Sciences (UAMS) archived surgical report cases from 1997-2013 with the terms “vulva” and “squamous cell carcinoma” in the final diagnosis. Biopsy specimens and cases with carcinoma in situ only were not included. Cases identified as vSCC were collected, and for each case all H&E slides were reviewed by 2 board certified gynecologic pathologists (CMQ and SKJ) to confirm diagnosis and determine which sections contained tumor. One hundred and three cases were identified, and corresponding archived formalin-fixed paraffin-embedded (FFPE) blocks containing tumor were collected from the UAMS pathology archives. Clinicopathologic data for each confirmed case of vSCC was obtained from corresponding pathology reports and clinical records and included clinical stage at diagnosis, depth of invasion (DOI), recurrence, nodal involvement, and lymph-vascular invasion (LVI), summarized in Table 1. Presence or absence of PNI was noted if included on the pathology report.
Table 1.
Clinicopathologic parameters of 103 cases of vulvar squamous cell carcinoma
| Parameters | No. of Cases | % of Cases |
|---|---|---|
| Recurrence in Primary Tumors | ||
| Present | 31 | 30.1 |
| Absent | 63 | 61.2 |
| Recurrent tumor | 9 | 8.7 |
| Nodal Involvement | ||
| Present | 25 | 24.3 |
| Absent | 50 | 48.5 |
| Unknown* | 28 | 27.2 |
| Lymph-vascular Invasion | ||
| Present | 22 | 21.4 |
| Absent | 78 | 75.7 |
| Unknown | 3 | 2.9 |
| Tumor Stage | ||
| Stage I | 43 | 41.7 |
| Stage II | 20 | 19.4 |
| Stage III | 19 | 18.5 |
| Stage IV | 7 | 6.8 |
| Unknown | 14 | 13.6 |
| Depth of Invasion** | ||
| <2.0 cm | 91 | 88.3 |
| ≥2.0 cm | 12 | 11.7 |
| Patient Age*** | ||
| <60 | 35 | 34.0 |
| ≥60 | 67 | 65.1 |
| Unknown | 1 | 0.9 |
Nodal excision not performed
Depth of invasion ranged from 0.1-6.4 cm
Age ranged from 21-93
Assessment of Perineural Invasion
For each case of vSCC, a dual-chromogen immunohistochemistry (IHC) approach was used to determine the presence of PNI in all FFPE blocks containing tumor. The number of blocks stained per case ranged from 1-25 with an average of 6 blocks per case. FFPE tissue blocks were sectioned at 4 μm and heat fixed on glass slides. Slides were deparaffinized with xylene and hydrated with graded alcohol solutions, according to standard protocol. Antigen retrieval was performed using Dako Proteinase K for 15 minutes at room temperature. Dual- staining was performed using the Dako EnVision G/2 Doublestain System, Rabbit/Mouse (DAB+/Permanent red) per manufacturer's instructions. Antibody against cytokeratin AE1/3 (Dako M3515) for epithelial cells was applied to slides at 0.844 μg/mL for 30 minutes at room temperature and visualized with DAB+; S100 antibody (Dako Z0311) for nerve tissue was applied to slides at 10 μg/mL for 30 minutes at room temperature and visualized with permanent red chromogen. Slides were counterstained with hematoxylin for 2 minutes, rinsed, then manually coverslipped using Dako aqueous mounting media.
For every case of vSCC, each dual-stained slide was reviewed for presence of PNI, which was defined as tumor cells invading into nerve tissue or encompassing at least 33% of the nerve circumference (Figure 1) as previously described. 14 Slides were visualized at low power (4x-10x) to determine location of tumor and nerve regions. Invasion of tumor cells into nerve tissue was confirmed at high power (20x-40x). The presence or absence of PNI and the number of nerves invaded by tumor per slide was recorded for each case.
Figure 1.
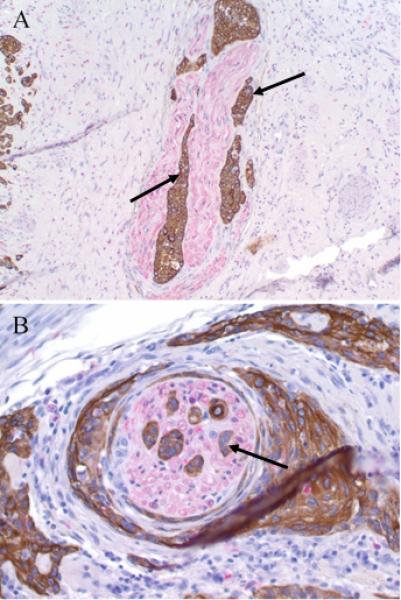
Perineural invasion in vSCC – Tumor cells (arrow) surround and invade into nearby nerves (red chromogen) in vSCC. Slides were stained using antibodies to cytokeratin AE1/3 and S100. (A) 20x magnification; (B) 40x magnification.
Statistical Analysis
Fisher's exact test was used to analyze categorical data, specifically when comparing PNI status with tumor recurrence, nodal involvement, stage, and LVI, while the Wilcoxon rank sum test with continuity correction was used to test the association between depth of invasion (DOI) and PNI status, and between DOI and tumor recurrence. A multivariable logistic regression model was run to test the combined association of PNI status and DOI to tumor recurrence; DOI was log-transformed because of right-skewness. Associations were tested at significance level 0.05.
Results
One hundred and three cases of vSCC representing 94 patients with primary vSCC and 9 recurrent tumors were identified and analyzed. Patient age ranged from 21 to 93 (mean 64 ± SD 16). Eighty-seven (92.5%) of the women were Caucasian and 7 (7.5%) were African American. Forty-five of the 94 patients were lost to follow up since their primary diagnosis, and their current health status is unknown. The remaining 49 patients had an average follow-up time of 28 months, and of these, 2 are alive with disease, 26 are alive without evidence of disease, and 21 patients died (16 died from vSCC). Of 103 total cases, 75 received lymph node dissection, with 25 showing lymph node involvement in at least one lymph node. Depth of tumor invasion ranged from 0.1 cm to 6.4 cm with an average of 0.99 cm. Additional pathologic features are highlighted in Table 1. Importantly, the presence or absence of PNI was noted in the pathology report for only 67 cases, revealing that PNI status was previously undocumented in at least 35% of cases. Furthermore, 18 of the 67 cases (27%) were defined as “PNI negative” on the pathology report, but were determined to contain PNI using the dual-stain method.
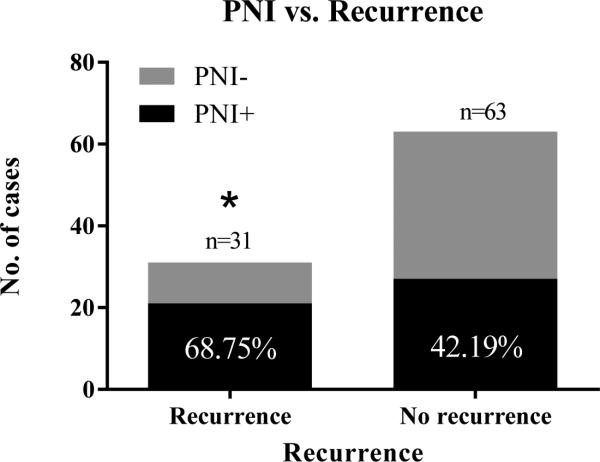
Perineural invasion was identified in 54 of the 103 tumors (52%). Of the 94 primary tumors, PNI was present in 21 of 31 tumors that recurred (69%), versus only 27 of 63 (42%) non-recurrent cases (p = 0.0290; OR = 2.8) (Figure 2). In addition, vSCCs with PNI had greater depth of tumor invasion than those without PNI (n = 103; p = 0.0047) (Figure 3). DOI was not, however, a statistically significant predictor of recurrence (p = 0.2220). Because DOI was correlated with PNI, a multivariable logistic regression model was fitted to estimate the independent contributions of PNI and DOI to risk of recurrence (Table 2). PNI was an independent, statistically significant predictor of recurrence (adjOR = 2.613, p = 0.045) while depth of invasion (DOI) was not (adjOR = 1.104 per doubling of DOI, p = 0.599). There was no significant association between PNI and LVI (n = 100) or nodal involvement (n = 75). Although there was no statistically significant association of PNI with tumor stage (n = 89), 6 of 7 (85%) Stage IV cases contained PNI suggesting that PNI may be associated with advanced tumor stage.
Figure 2.
PNI and recurrence – The majority of recurrent vSCC contained PNI (68.75%) compared with less than half (42.19%) of non-recurrent tumors containing PNI (p = 0.0290, OR = 2.8).
Figure 3.
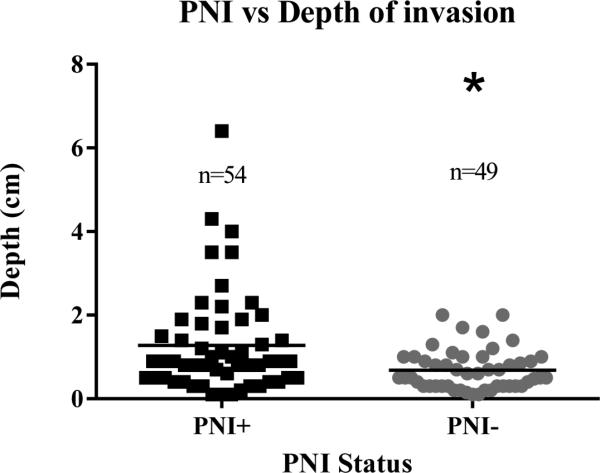
PNI and depth of invasion – vSCC displaying PNI had greater depth of tumor invasion than tumors that did not contain PNI (p = 0.0015).
Table 2.
Multivariable logistic regression model of recurrence
| Parameter Estimate | Standard Error | z-statistic | Adjusted Odds Ratio | p-value | |
|---|---|---|---|---|---|
| PNI | 0.961 | 0.479 | 2.006 | 2.613 | 0.045 |
| log2(DOI) | 0.099 | 0.189 | 0.526 | 1.104 | 0.599 |
Parameter estimates from a model with perineural invasion (PNI) and depth of invasion (DOI) as predictors of tumor recurrence. PNI is an independent, statistically significant predictor of recurrence (adjOR = 2.613, p = 0.045) while DOI is not (adjOR = 1.104 per doubling of DOI, p = 0.599)
Discussion
Although no standardized protocol for adjuvant treatment of vSCC has been enforced, adjuvant treatment planning for these patients following surgery relies heavily upon depth of invasion, nodal involvement, and status of resection margins. There is little focus on histopathologic features of the tumor, likely due to the fact that few major pathologic features have been associated with outcomes or tumor aggressiveness in vulvar carcinoma. Perineural invasion has been linked to increased mortality, increased recurrence, and overall worse outcomes in a number of cancers. Specifically, evidence showing the association of PNI with poor outcomes in head and neck SCC and improvement of these outcomes with adjuvant therapies has led to the required inclusion of PNI status on pathological assessment for carcinomas of the larynx, lip and oral cavity, salivary glands, and pharynx.12, 19, 22, 23 However, PNI still remains understudied in gynecological cancers such as vulvar squamous cell carcinoma.
To investigate the importance of PNI in determining tumor aggressiveness and potential for recurrence in vSCC, we used a dual-stain method that allowed reliable identification of PNI within FFPE tumor samples. Once we identified the presence or absence of PNI in each case, we investigated the association of PNI status with clinicopathologic parameters. Our results show that perineural invasion is associated with recurrence of vSCC, independent of nodal status or depth of invasion. Importantly, association of PNI with recurrence suggests that PNI might be a mechanism for recurrence in both deeply and minimally invasive tumors, and that the presence of PNI in any tumor, regardless of depth of invasion, indicates increased risk for recurrence and should therefore be considered in treatment planning. However, the current lack of required reporting of PNI status in these tumors may hinder the usefulness of this tumor feature. Our results showed that at least 35% of pathology reports revealed no information concerning PNI status, and 27% of those that were reported inaccurately referred to PNI as absent when it was actually present within the tumor. This inconsistency of PNI reporting may reflect the lack of understanding of importance of PNI in vSCC or the absence of required reporting, but may also be in part due to the time consuming nature of analyzing nerves in each patient sample. Successful use of the dual-stain method in this study indicates that when used in conjunction with traditional H&E, a more specific histologic assessment of PNI may be helpful in identifying PNI and reducing PNI reporting error.
The association of PNI with recurrence but not nodal involvement suggests that neural involvement in vulvar carcinoma may be a significant part of the mechanism for local recurrence in this cancer, but does not play a role in regional metastases of vSCC. Specifically, neural invasion may serve as a reservoir for tumor cells, protecting these cells from attack by the immune system, and allowing recurrent growth or spread to local structures even after primary tumor resection. Adjuvant chemo- or radiation-therapies could overcome this local failure, making PNI an important tool for determining post-surgical treatment options. Further studies investigating interactions between nerves and tumor or stromal cells in vulvar carcinoma will be important in understanding the mechanism of increased risk for recurrence in these patients. As with any retrospective study, there are some limitations within our dataset. For example, many patients received their primary diagnosis and surgical treatment at our facility, and their follow up care elsewhere. In these cases, recurrence data may be lacking, resulting in an underestimation of the actual recurrence rate. In addition, we were unable to determine the association of PNI with overall survival because almost half of the patients were lost to follow-up. Inclusion of more patients might be able to overcome these limitations.
In conclusion, the results of this study show that perineural invasion is a significant independent indicator of risk for recurrence in vSCC. Taken further, our results suggest that PNI may be an additional pathologic indicator for adjuvant therapy and should be routinely evaluated in vSCC patients.
Acknowledgements
The authors would like to acknowledge Jennifer James and the UAMS Experimental Pathology Core Facility for technical assistance.
Source of Funding
This study was supported by the Translational Research Institute, grants UL1TR000039 and KL2TR000063 through the NIH National Center for Research Resources and National Center for Advancing Translational Sciences, institutional training grant T32GM106999, and funds from the University of Arkansas for Medical Sciences Department of Pathology and the Winthrop P. Rockefeller Cancer Institute.
Footnotes
Conflicts of Interest
The authors of this manuscript have no conflicts of interest to disclose.
References
- 1. [September 2, 2014];Cancer Facts & Figures. 2014 Available at: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/index.
- 2. [July 30, 2014];SEER Cancer Statistics Fact Sheets: Vulvar Cancer. 2014 Available at: http://seer.cancer.gov/statfacts/html/vulva.html.
- 3.Eifel PJBJ, Markman MA. Cancer: Principles and Practice of Oncology. Lippincott Williams & Wilkins; Philadelphia, PA: 2011. Cancer of the cervix, vagina, and vulva. pp. 1311–1344. [Google Scholar]
- 4.Benedet JL, Bender H, Jones H, 3rd, et al. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2000;70:209–262. [PubMed] [Google Scholar]
- 5.Canavan TP, Cohen D. Vulvar cancer. American family physician. 2002;66:1269–1274. [PubMed] [Google Scholar]
- 6.Andersen BL, van Der Does J. Surviving gynecologic cancer and coping with sexual morbidity: an international problem. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 1994;4:225–240. doi: 10.1046/j.1525-1438.1994.04040225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton DP. The prevention and management of treatment related morbidity in vulval cancer. Best practice & research Clinical obstetrics & gynaecology. 2003;17:683–701. doi: 10.1016/s1521-6934(03)00045-2. [DOI] [PubMed] [Google Scholar]
- 8.Faul CM, Mirmow D, Huang Q, et al. Adjuvant radiation for vulvar carcinoma: improved local control. International journal of radiation oncology, biology, physics. 1997;38:381–389. doi: 10.1016/s0360-3016(97)82500-x. [DOI] [PubMed] [Google Scholar]
- 9.Han SN, Vergote I, Amant F. Weekly paclitaxel/carboplatin in the treatment of locally advanced, recurrent, or metastatic vulvar cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2012;22:865–868. doi: 10.1097/IGC.0b013e31824b4058. [DOI] [PubMed] [Google Scholar]
- 10.Parthasarathy A, Cheung MK, Osann K, et al. The benefit of adjuvant radiation therapy in single-node-positive squamous cell vulvar carcinoma. Gynecologic oncology. 2006;103:1095–1099. doi: 10.1016/j.ygyno.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Maggino T, Landoni F, Sartori E, et al. Patterns of recurrence in patients with squamous cell carcinoma of the vulva. A multicenter CTF Study. Cancer. 2000;89:116–122. doi: 10.1002/1097-0142(20000701)89:1<116::aid-cncr16>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Sobin LH, Gospodarowicz MK, Wittekind C, et al. TNM classification of malignant tumours. Wiley-Blackwell; Chichester, West Sussex, UK ; Hoboken, NJ: 2010. [Google Scholar]
- 13.Harnden P, Shelley MD, Clements H, et al. The prognostic significance of perineural invasion in prostatic cancer biopsies: a systematic review. Cancer. 2007;109:13–24. doi: 10.1002/cncr.22388. [DOI] [PubMed] [Google Scholar]
- 14.Liebig C, Ayala G, Wilks JA, et al. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 15.Jiang N, Deng JY, Liu Y, et al. Incorporation of perineural invasion of gastric carcinoma into the 7th edition tumor-node-metastasis staging system. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014 doi: 10.1007/s13277-014-2258-5. [DOI] [PubMed] [Google Scholar]
- 16.Loeb S, Epstein JI, Humphreys EB, et al. Does perineural invasion on prostate biopsy predict adverse prostatectomy outcomes? BJU international. 2010;105:1510–1513. doi: 10.1111/j.1464-410X.2009.08845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beard CJ, Chen MH, Cote K, et al. Perineural invasion is associated with increased relapse after external beam radiotherapy for men with low-risk prostate cancer and may be a marker for occult, high-grade cancer. International journal of radiation oncology, biology, physics. 2004;58:19–24. doi: 10.1016/s0360-3016(03)01433-0. [DOI] [PubMed] [Google Scholar]
- 18.Duraker N, Sisman S, Can G. The significance of perineural invasion as a prognostic factor in patients with gastric carcinoma. Surgery today. 2003;33:95–100. doi: 10.1007/s005950300020. [DOI] [PubMed] [Google Scholar]
- 19.Harrison LB, Sessions RB, Hong WK. Head and neck cancer : a multidisciplinary approach. Wolters Kluwer Health/Lipppincott Williams & Wilkins; Philadelphia: 2009. Prognostic factors in patients with head and neck cancer. p. xxii.p. 960.p. 916. of plates. [Google Scholar]
- 20.Feng FY, Qian Y, Stenmark MH, et al. Perineural invasion predicts increased recurrence, metastasis, and death from prostate cancer following treatment with dose-escalated radiation therapy. International journal of radiation oncology, biology, physics. 2011;81:e361–367. doi: 10.1016/j.ijrobp.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 21.Fouquet T, Germain A, Brunaud L, et al. Is perineural invasion more accurate than other factors to predict early recurrence after pancreatoduodenectomy for pancreatic head adenocarcinoma? World journal of surgery. 2014;38:2132–2137. doi: 10.1007/s00268-014-2465-7. [DOI] [PubMed] [Google Scholar]
- 22.Fagan JJ, Collins B, Barnes L, et al. Perineural invasion in squamous cell carcinoma of the head and neck. Archives of otolaryngology--head & neck surgery. 1998;124:637–640. doi: 10.1001/archotol.124.6.637. [DOI] [PubMed] [Google Scholar]
- 23.Miller ME, Palla B, Chen Q, et al. A novel classification system for perineural invasion in noncutaneous head and neck squamous cell carcinoma: histologic subcategories and patient outcomes. American journal of otolaryngology. 2012;33:212–215. doi: 10.1016/j.amjoto.2011.06.003. [DOI] [PubMed] [Google Scholar]