Abstract
Background and Aims
For HIV-positive individuals who use illicit opioids, engagement in methadone maintenance therapy (MMT) can contribute to improved HIV treatment outcomes. However, to our knowledge, the role of methadone dosing in adherence to antiretroviral therapy (ART) has not yet been investigated. We sought to examine the relationship between methadone dose and ART adherence among a cohort of persons who use illicit opioids.
Design and Setting
We used data from the ACCESS study, an ongoing prospective observational cohort of HIV-positive persons who use illicit drugs in Vancouver, Canada, confidentially linked to comprehensive HIV treatment data in a setting of universal no-cost medical care including medications. We evaluated the longitudinal relationship between methadone dose and the likelihood of ≥ 95% adherence to ART among ART-exposed participants during periods of engagement in MMT.
Participants
297 ART-exposed individuals on MMT were recruited between December 2005 and May 2013 and followed for a median of 42.1 months.
Measurements
We measured methadone dose at ≥ 100 vs < 100 mg/day and the likelihood of ≥ 95% adherence to ART.
Findings
In adjusted generalized estimating equation (GEE) analyses, MMT dose ≥ 100 mg/day was independently associated with optimal adherence to ART (adjusted odds ratio [AOR] = 1.38; 95% confidence interval [CI]: 1.08 – 1.77, p = 0.010). In a sub-analysis, we observed a dose-response relationship between increasing MMT dose and ART adherence (AOR = 1.06 per 20 mg/day increase, 95% CI: 1.00 – 1.12, p = 0.041).
Conclusion
Among HIV-positive individuals in methadone maintenance therapy, those receiving higher doses of methadone (≥ 100 mg/day) are more likely to achieve ≥ 95% adherence to antiretroviral therapy than those receiving lower doses.
Keywords: HIV, adherence, illicit drug use, methadone, antiretroviral therapy
INTRODUCTION
The introduction of antiretroviral therapy (ART) has resulted in substantial reductions in HIV/AIDS-associated morbidity and mortality among many seropositive groups worldwide (1–4). The success of ART however, relies upon sustained adherence to the regimen. Previous studies have shown that optimal ART adherence (≥ 95% adherence) is associated with sustained HIV viral suppression (5, 6), increased CD4+ cell count (7), increased survival (8) and decreased progression to AIDS (9).
Unfortunately, the improvements in survival seen in HIV-infected populations overall have been less pronounced among HIV-positive persons who use illicit drugs (10, 11). This disparity is largely attributable to lower rates of ART initiation and optimal ART adherence (12), and treatment initiation at more advanced disease stages (13). Ongoing alcohol and illicit drug use is consistently associated with poorer adherence to ART (14–16), and persons who use illicit drugs often experience worse virologic and immunologic outcomes (17, 18). Nevertheless, persons who use illicit drugs who achieve optimal adherence to ART can experience the same benefits from therapy as other HIV-infected groups (19).
Encouragingly, persons who use illicit drugs who are engaged in substance abuse treatment, particularly opioid-addicted persons engaged on opioid agonist therapy, such as methadone maintenance therapy (MMT), have improved access to ART (20, 21), achieved higher levels of ART adherence (22, 23), and lower ART discontinuation rates (24). In one study among ART-exposed persons who inject drugs, enrolment in MMT was associated with reduced heroin use, improved adherence to ART, HIV-RNA viral suppression and CD4 cell count rise (25).
Higher methadone doses, specifically doses of at least 60mg/day, have been associated with decreased illicit opioid use (26, 27), improved retention in treatment programs (26, 28), and lower mortality rates (29) when compared to lower methadone doses. Although the optimal dose of methadone needs to be individualized, minimum doses of 60 mg/day are consistently recommended and most MMT programs advise providing average doses in the range of 80–100 mg/day (26, 30, 31). Additionally, there is increasing evidence demonstrating improved treatment outcomes with high-dose methadone (i.e., ≥ 100 mg/day) (32–35). Despite these recommendations, D’Aunno et al. (2014) demonstrated that many individuals on MMT remain under-dosed. Using 23 years of longitudinal data from MMT programs across the United States, they observed that while the proportion of patients receiving ≥ 60 mg/day of methadone has increased, as of 2011, 23% of patients remained on sub-therapeutic doses (< 60 mg/day), and only 59% of patients on methadone received doses of at least 80 mg/day (30).
While there is a growing body of evidence evaluating the effect of high-dose methadone (≥ 100 mg/day) on drug use outcomes, we are unaware of any study that has investigated the effect of methadone dose within the context of HIV treatment. Therefore, this study sought to test whether individuals on high-dose methadone (i.e., ≥ 100 mg/day) exhibited higher levels of optimal ART adherence (i.e., ≥ 95%) in multivariable models adjusted for relevant confounders using longitudinal data from a long-running observational cohort of HIV-positive illicit drug users.
METHODS
Study Participants
Data for this study was drawn from the AIDS Care Cohort to evaluate Exposure to Survival Services (ACCESS), an ongoing prospective cohort of HIV-positive persons who use illicit drugs in Vancouver, Canada which has previously been described in detail (25, 36). The participants are recruited through snowball sampling and extensive street outreach, beginning in 2005, in the city’s Downtown Eastside neighborhood, an area with an open drug market, high levels of injection drug use, poverty, and HIV infection (37–39). Individuals are eligible for enrolment in ACCESS if they are HIV-positive, aged ≥ 18 years, have a history of illicit drug use other than cannabis in the previous month, and provide written informed consent. At baseline and at every six-month follow-up interview, participants answer a standardized interviewer-administered questionnaire and provide blood samples for serologic analysis. The questionnaire elicits detailed demographic data, as well as information pertaining to drug use patterns and related exposures. All participants are remunerated $30 (CAD) for each study visit and, when appropriate, are referred to additional healthcare services, including addiction treatment. Ethical approval is provided annually by the University of British Columbia/Providence Health Care Research Ethics Board.
In this study, we included participants who were ART-exposed at recruitment and individuals who initiated ART during the study period, and included all interview periods from study entry or ART initiation forward, respectively. In addition, we restricted our analytic sample to individuals with at least one CD4+ and plasma HIV-1 RNA viral load (VL) measurement within ± 180 days of their baseline interview. To focus on the effect of MMT dose on ART adherence, we only included 180-day periods during which individuals reported any dispensation of methadone.
HIV/AIDS Drug Treatment Program
The information obtained from the semi-annual questionnaire is augmented with data on HIV treatment and clinical outcomes from the British Columbia Centre for Excellence in HIV/AIDS (BC-CfE) Drug Treatment Programme, as described previously (36). In brief, a province-wide centralized ART pharmacy provides complete information on all antiretroviral medications dispensed to each participant during the study period. Additionally, a complete clinical profile for each participant, including CD4+ cell counts and plasma HIV-1 RNA levels (as measured by Amplicor Monitor Assay, Roche Molecular Systems) is also provided from the BC-CfE’s treatment registry. All HIV/AIDS treatment and care including all medications and HIV clinical monitoring is available free of cost to all HIV-infected residents of British Columbia through the province’s universal no-cost healthcare system.
ART adherence
The outcome of interest was optimal adherence to ART based on pharmacy refill data. Information on exposure to ART was obtained through a confidential linkage to treatment records from the comprehensive provincial ART dispensary described previously (36). We measured adherence to therapy in each six-month period as the number of days for which ART was dispensed over the number of days since that individual had first been prescribed ART, capped at 180 days. We have previously demonstrated the clinical validity of ART pharmacy refill data and shown that it reliably predicts virologic suppression (5, 40–42) and survival (8, 36). Optimal ART adherence was considered to be adherence at ≥ 95%, therefore in our analysis we dichotomized adherence at ≥ 95% vs < 95%.
Methadone Program
In British Columbia, standard pharmacotherapy for opioid dependence involves oral solution methadone, a long-acting synthetic opioid agonist that is prescribed by physicians. Methadone is provided free-of-charge through the provincial healthcare system and prescribed primarily through doctor offices and clinics. In the majority of cases, pharmacists supervise the ingestion of methadone in the community on a daily basis (43). Given that sustained adherence to methadone has been shown to decrease risk of relapse to opioid use and decrease overall mortality, a treatment philosophy emphasizing indefinite maintenance of MMT has become widely accepted in Canadian settings (44). The primary explanatory variable of interest for this study was current receipt of high-dose methadone (≥ 100 vs. < 100 mg/day), gathered through self-report. We dichotomized the dose at ≥ 100mg/day as there is growing evidence demonstrating improved drug treatment outcomes associated with doses ≥ 100mg/day (32–35). Previous research has shown that persons who use illicit drugs who are on methadone are able to give valid self-reports of their methadone dosage (weighted kappa = 0.97) (45).
Secondary Explanatory Variables
To best estimate the relationship between ART adherence and methadone dose, we also considered secondary explanatory variables that we hypothesized might confound this relationship. These included the following socio-demographic variables: age (per 10 years older), gender (male vs. non-male), ethnicity (Caucasian vs. non-Caucasian), and level of education (< high school diploma vs. ≥ high school diploma). Gender was dichotomized as male vs. non-male for the purpose of the statistical analysis in order to include a small proportion of our study population who identifies as transgender, including Indigenous individuals who self-reported being Two Spirited. Ethnicity was dichotomized as Caucasian vs. non-Caucasian, as Caucasian participants are the most numerous ethnic group in the cohort.
Other variables included: employment (yes vs. no), homelessness (yes vs. no), incarceration (yes vs. no), crack smoking (≥ daily vs. < daily), cocaine injection (≥ daily vs. < daily), methamphetamine use (≥ daily vs. < daily), and heavy alcohol use (yes vs. no). As we hypothesize that changing levels of heroin use are on the causal pathway between methadone dose and optimal adherence, we did not include it as a possible confounding variable. Employment referred to having salaried or temporary work in the licit labor market at any time during the previous six months. Employment has been associated with better ART adherence in previous studies (46). Homelessness referred to living on the street or having no fixed address in the previous six months. Studies have shown poor housing status is a risk factor for ART non-adherence (47). Heavy alcohol use was defined as ≥ 4 drinks/day on average in the previous six months.
We included a number of clinical characteristics of antiretroviral treatment or methadone treatment that might confound the relationship between methadone dose and ART adherence including: time since ART initiation (per year later), time since methadone initiation (per year later), ART side effects hinder adherence (yes vs. no), Hepatitis C Virus (HCV) serostatus (negative vs. positive), HIV physician experience (<6 patients vs. ≥ 6 patients) and CD4+ cell count (per 100 cells/mm3). Time since ART initiation and time since methadone initiation were treated as time-updated continuous variables which measured the interval between the interview date and the participant’s earliest dispensation of ART and methadone, as recorded by pharmacy dispensation records and self-report, respectively. The ART side effects hinder adherence variable is a time-updated dichotomous variable which reflects if an individual reported finding ART difficult to take due to side-effects in the 180 day period prior to the study interview. Inclusion of this variable is warranted as side-effects with ART are a commonly cited reason for non-adherence (48). For CD4+ cell count (per 100 cells/mm3), we used the median of all available observations in the previous six months; if none were available, we used the most recent observation. In line with previous analysis in this setting, an HIV experienced physician was defined as one who had previously enrolled six or more individuals into the HIV/AIDS treatment registry at the time the participant initiated treatment (49). Inclusion of this variable is warranted given that physician experience with HIV-infected patients is associated with improved survival (50). Since participants were continuously enrolled in the treatment program during the study period, a physician could become experienced over time, however, the experience level assigned was based on the physician’s HIV-related experience at the time of the participant’s first interview following ART initiation.
All variables except gender, ethnicity (Caucasian vs. non-Caucasian) and HIV physician experience were time-updated. All behavioral variables referred to the six-month period before the interview.
Statistical Analysis
First, we compared characteristics of participants who were on methadone ≥ 100 mg/day and < 100 mg/day at baseline, using the Chi-square test for binary measures and Wilcoxon rank sum test for continuous measures. We then estimated the bivariate relationships between ≥ 95% adherence and all explanatory variables over the study period using generalized estimating equations (GEE). This form of regression modeling was used to account for the correlation among observations gathered from the same individual as well as between individuals observed at the same time point to estimate the independent effect of methadone dose on ART adherence. Interpretation of parameter estimates from GEE models differ from standard logistic regression models, as GEE are used to make inferences about population means between groups over time. Specifically, we compared mean levels of optimal adherence between high-dose and low-dose methadone patients over time, accounting for the correlation within individual-level observations.
To account for possible confounding and calculate the best effect estimate, we constructed a multivariate model using a priori-defined modeling strategy (51, 52). Several studies have successfully used this technique to estimate the relationship between an outcome of interest and a selected explanatory variable (52–54). First, we fit a full model, including the primary explanatory variable and all secondary explanatory variables that were associated with ≥ 95% adherence in bivariate analyses at p < 0.10. To determine the final set of variables, we used a manual stepwise approach based on changes in the value of the coefficient for the primary explanatory variable when individual secondary variables were removed from the full model, as in previous analyses (55, 56).
To test the robustness of our models, we also conducted three sensitivity analyses. First, to further explore the effect of methadone dose on ART adherence, we conducted a sub-analysis using a Cochran-Armitage test to investigate the likelihood of ≥ 95% adherence with increasing methadone dose (per 50 mg/day increase). Using GEE we also conducted a sub-analysis to examine the longitudinal relationship between the likelihood of optimal adherence and methadone dose expressed per 20 mg/day increase.
Second, to further investigate the effect of the completeness of CD4 data on our results, we removed all 180 day observation periods lacking ≥ 1 CD4 cell count observations and repeated the bivariable and multivariable modeling.
Third, to further investigate the possible interaction of methadone dose and the presence of specific antiretroviral agents shown to effect the bioavailability of methadone (57), we repeated our bivariable and multivariable modeling, including a time-updated explanatory variable indicating whether an individual had been dispensed at least one dose of delaviridine (DEL), efavirenz (DMP) or nevaripine (NEV) in the previous 180 days. To avoid biased estimates in this sensitivity analysis, the analyses were restricted to periods in which an individual had been dispensed ≥ 1 day of ART.
RESULTS
Between December 2005 and May 2013, 297 ART-exposed individuals reported MMT use and were included in our analyses. Among them 133 (44.8%) reported a methadone dose ≥ 100 mg/day (median dose: 145 mg/day; interquartile range [IQR]: 115 – 180), and 142 (47.8%) reported a dose of < 100 mg/day (median dose: 60 mg/day; IQR: 40 – 80) at baseline. The median methadone dose for all patients in the study at baseline was 90 mg/day (IQR: 60 – 140). The median duration of follow-up was 42.1 months (IQR: 19.0 – 68.7), contributing to 1056.3 person-years of follow-up.
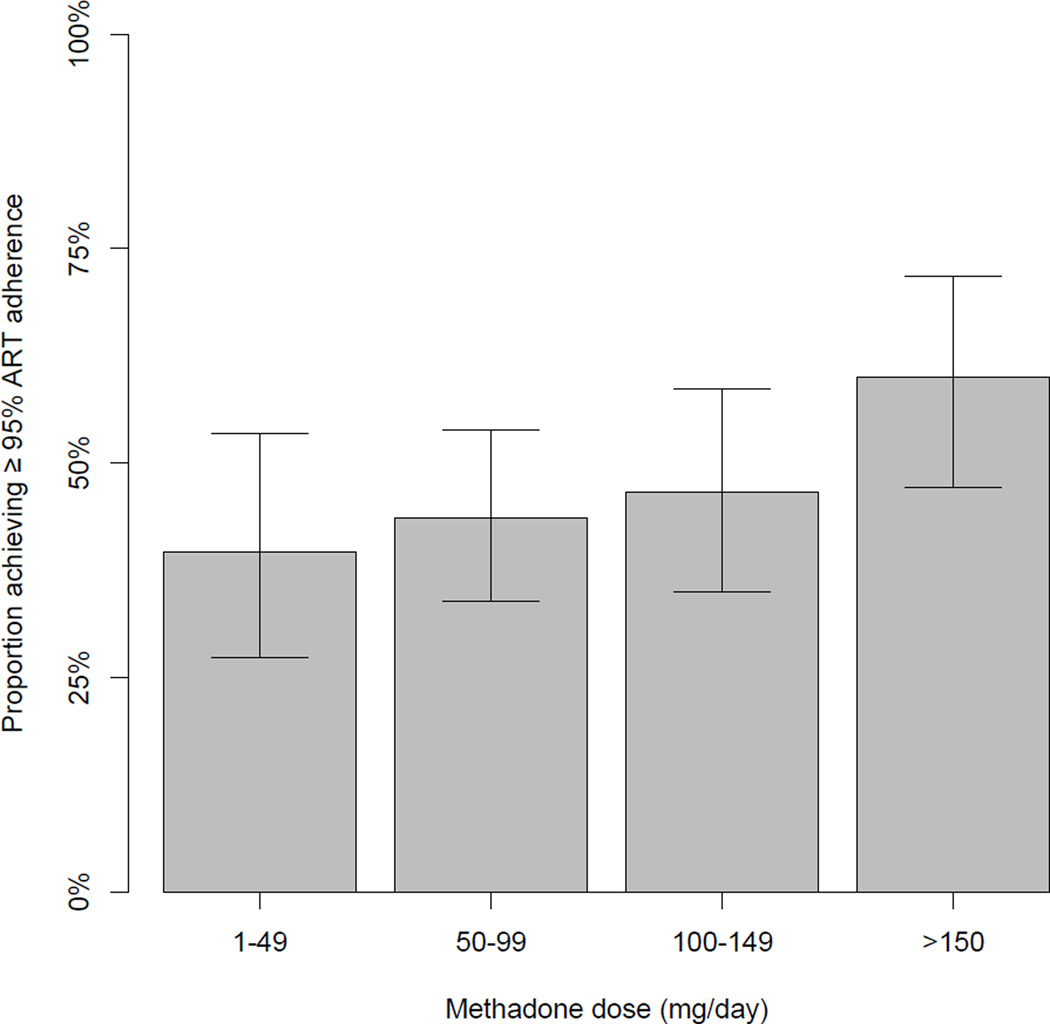
The proportion of participants achieving ≥ 95% adherence to ART at baseline stratified by methadone dose (i.e., 1 – 49 mg/day; 50 – 99; 100 – 149; > 150) is depicted in Figure 1. A Cochran-Armitage test indicates a significant trend between increasing dose and the proportion of optimally adherent participants (p = 0.021.) The characteristics of the participants at their baseline interview stratified by methadone dose (≥ 100 vs. < 100 mg/day) are presented in Table 1.
Figure 1.
Proportion with 95% confidence intervals of individuals achieving at least 95% adherence to antiretroviral therapy in the previous six months at baseline stratified by methadone dose (n = 297). Cochran-Armitage test for trend: p-value = 0.021
Table 1.
Baseline characteristics of 297 antiretroviral therapy-exposed persons who use illicit drugs engaged in methadone maintenance therapy stratified by methadone dose
| Characteristic | Total n (%) |
Methadone dose | Odds Ratio (95% CI) |
p - value | |
|---|---|---|---|---|---|
| ≥ 100 mg n (%) |
< 100 mg n (%) |
||||
| Age | |||||
| Age in years | 42.8 | 43.6 | 42.7 | 0.167 | |
| (IQR) | (37.7 – 48.3) | (38.7 – 49.0) | (37.3 – 47.6) | ||
| Gender | |||||
| Male | 175 (58.9) | 72 (54.1) | 91 (64.1) | ||
| Female | 116 (39.1) | 58 (43.6) | 48 (33.8) | 1.53 (0.93 – 2.50) | 0.091 |
| Transgender | 6 (2.0) | 3 (2.3) | 3 (2.1) | 1.26 (0.25 – 6.45) | 0.778 |
| Ethnicity | |||||
| Caucasian | 175 (58.9) | 76 (57.1) | 90 (63.4) | 0.77 (0.47 – 1.25) | 0.291 |
| Non-Caucasian | 122 (41.1) | 57 (42.9) | 52 (36.6) | ||
| Level of Education | |||||
| < High school | 157 (52.9) | 70 (52.6) | 79 (55.6) | 0.93 (0.58 – 1.51) | 0.775 |
| ≥ High school | 132 (44.4) | 58 (43.6) | 61 (43.0) | ||
| Employment € † | |||||
| Yes | 48 (16.2) | 21 (15.8) | 21 (14.8) | 1.08 (0.56 – 2.08) | 0.818 |
| No | 249 (83.8) | 112 (84.2) | 121 (85.2) | ||
| Homelessness € | |||||
| Yes | 70 (23.6) | 27 (20.3) | 37 (26.1) | 0.72 (0.41 – 1.26) | 0.245 |
| No | 226 (76.1) | 106 (79.7) | 104 (73.2) | ||
| Crack Smoking € | |||||
| ≥ Daily | 122 (41.1) | 64 (48.1) | 46 (32.4) | 1.94 (1.19 – 3.16) | 0.008 |
| < Daily | 175 (58.9) | 69 (51.9) | 96 (67.6) | ||
| Cocaine injection € | |||||
| ≥ Daily | 32 (10.8) | 13 (9.8) | 16 (11.3) | 0.85 (0.39 – 1.83) | 0.672 |
| < Daily | 263 (88.6) | 120 (90.2) | 125 (88.0) | ||
| Methamphetamine Use € | |||||
| ≥ Daily | 7 (2.4) | 2 (1.5) | 4 (2.8) | 0.52 (0.09 – 2.90) | 0.685£ |
| < Daily | 288 (97.0) | 131 (98.5) | 137 (96.5) | ||
| Heavy Alcohol Use € ‡ | |||||
| Yes | 4 (1.4) | 1 (0.8) | 2 (1.4) | 0.53 (0.05 – 5.92) | 1.000£ |
| No | 293 (98.7) | 132 (99.3) | 140 (98.6) | ||
| Incarceration € | |||||
| Yes | 38 (12.8) | 20 (15.0) | 16 (11.3) | 1.39 (0.69 – 2.82) | 0.354 |
| No | 259 (87.2) | 113 (85.0) | 126 (88.7) | ||
| Time since ART Initiation | |||||
| Per year | 6.3 | 6.0 | 6.9 | 0.251 | |
| (IQR) | (2.4 – 8.8) | (2.2 – 8.3) | (3.0 – 8.9) | ||
| Time since MMT Initiation | |||||
| Per year | 6.0 | 7.7 | 4.5 | <0.001 | |
| (IQR) | (1.9 – 11.1) | (3.7 – 12.9) | (1.1 – 9.5) | ||
| ART SE Hinder Adherence € α | |||||
| Yes | 70 (23.6) | 37 (27.8) | 23 (16.2) | 1.99 (1.11 – 3.58) | 0.028 |
| No | 227 (76.4) | 96 (72.2) | 119 (83.8) | ||
| HCV serostatus | |||||
| Negative | 4 (1.3) | 3 (2.3) | 1 (0.7) | 0.31 (0.03 – 2.99) | 0.283 |
| Positive | 293 (98.7) | 130 (97.7) | 141 (99.3) | ||
| HIV physician experience | |||||
| < 6 patients | 45 (15.2) | 17 (12.8) | 26 (18.3) | 0.62 (0.32 – 1.21) | 0.156 |
| ≥ 6 patients | 235 (79.1) | 111 (83.5) | 105 (73.9) | ||
| CD4+ count € | |||||
| Per 100 cells/mm3 | 2.95 | 3.10 | 2.65 | 0.027 | |
| (IQR) | (1.85 – 4.30) | (2.10 – 4.40) | (1.45 – 4.05) | ||
| Dispensed DEL, DMP or NEV € β | |||||
| No | 220 (74.1) | 91 (68.4) | 111 (78.2) | 1.65 (0.96 – 2.84) | 0.067 |
| ≥ 1 dose | 77 (25.9) | 42 (31.6) | 31 (21.8) | ||
Refers to the six month period prior to the baseline interview
Defined as having a regular job, temporary job or self-employment
Heavy alcohol use defined as ≥ 4 drinks/day on average
Fisher’s exact test
SE refers to side effects
Refers to being dispensed delaviridine, efavirenz or nevaripine
Of the 1976 interview periods, 982 (49.7%) were characterized by less than 95% adherence to ART. Table 2 presents bivariable associations of ≥ 95% ART adherence with the primary and secondary explanatory variables over the study period. Compared to individuals on < 100mg/day of methadone, those on high-dose methadone had significantly increased rates of optimal ART adherence (OR = 1.40; 95% CI: 1.09 – 1.80). In the final multivariable model, methadone dose of ≥ 100 mg/day remained positively and independently associated with optimal ART adherence (adjusted odds ratio [AOR] = 1.38; 95% CI: 1.08–1.77) after adjustment for confounders including crack smoking and CD4+ cell count.
Table 2.
Bivariate, multivariate and final multivariate GEE analysis of factors associated with ART adherence (≥ 95%)
| Characteristic | Odds Ratio (95% Confidence Interval) | ||
|---|---|---|---|
| Bivariate | Multivariate | Final Confounding Model |
|
|
Methadone dose
€ (≥100mg vs. <100mg) |
1.40 (1.09 – 1.80) | 1.35 (1.04 – 1.74) | 1.38 (1.08 – 1.77) |
|
Age (per 10 years older) |
1.47 (1.16 – 1.86) | 1.21 (0.90 – 1.63) | |
|
Gender (Male vs. non-male) |
0.92 (0.65 – 1.31) | 1.00 (0.68 – 1.49) | |
|
Ethnicity (Caucasian vs. non-Caucasian) |
0.95 (0.67 – 1.33) | 0.77 (0.52 – 1.14) | |
|
Level of Education (< high school vs. ≥ high school) |
0.90 (0.64 – 1.28) | 0.80 (0.55 – 1.18) | |
|
Employment
€
† (Yes vs. no) |
0.93 (0.72 – 1.20) | 0.99 (0.76 – 1.30) | |
|
Homelessness
€ (Yes vs. no) |
0.79 (0.61 – 1.02) | 0.89 (0.67 – 1.19) | |
|
Crack smoking
€ (≥ Daily vs. < daily) |
0.70 (0.57 – 0.86) | 0.72 (0.58 – 0.91) | 0.74 (0.60 – 0.92) |
|
Cocaine injection
€ (≥ Daily vs. < daily) |
1.12 (0.75 – 1.67) | 1.27 (0.83 – 1.94) | |
|
Methamphetamine Use
€ (≥ Daily vs. < daily) |
1.29 (0.61 – 2.75) | 1.56 (0.67 – 3.65) | |
|
Heavy Alcohol Use
€ (Yes vs. no) |
0.72 (0.38 – 1.34) | 0.59 (0.31 – 1.1) | |
|
Incarceration
€ (Yes vs. no) |
0.91 (0.67 – 1.25) | 1.07 (0.75 – 1.50) | |
|
Time since ART Initiation (per year older) |
1.03 (0.99 – 1.06) | 0.97 (0.93 – 1.01) | |
|
Time since MMT Initiation (per year older) |
1.04 (1.02 – 1.06) | 1.03 (1.00 – 1.05) | |
|
ART SE Hinder Adherence
€
α (Yes vs. no) |
0.76 (0.60 – 0.97) | 0.88 (0.68 – 1.13) | |
|
HCV Serostatus (Negative vs. positive) |
0.48 (0.09 – 2.50) | ||
|
HIV physician experience (< 6 patients vs. ≥ 6 patients) |
0.84 (0.54 – 1.30) | 0.96 (0.58 – 1.58) | |
|
CD4+ count
€ (per 100 cells/ mm3) |
1.18 (1.08 – 1.28) | 1.17 (1.06 – 1.28) | 1.16 (1.07 – 1.26) |
Refers to the six month period prior to the interview
Defined as having a regular or temporary job
Heavy alcohol use defined as ≥ 4 drinks/day on average
SE refers to side effects
As models including HCV serostatus failed to converge, we excluded it from multivariable models. This failure was likely a result of the extremely small number of observation periods characterized by negative HCV antibody status (14 [0.7%] of 1976 periods among 4 [1.3%] of 297 participants.) HCV serostatus was not significantly associated with either ART adherence or MMT dose in bivariable analyses (p-values > 0.25, results not shown.)
The results of the three sensitivity analyses are reported below. To further explore the effect of methadone dose on ART adherence, we conducted a sub-analysis to examine the longitudinal relationship between the likelihood of ≥ 95% ART adherence and methadone dose expressed as continuous variable. In a GEE model, increasing methadone dose (per 20mg/day increase) was significantly associated with optimal ART adherence (OR = 1.06, 95% CI: 1.00 – 1.22).
In the second sensitivity analysis, we determined that almost all (1910, 96.7%) of the 1976 six-month observation periods in the study contained at least one CD4 cell count observation. The median number of CD4 counts in each 180 day observation period was 3 (IQR: 2 – 4). When we removed the 66 observation periods lacking a CD4 count result and repeated the modeling procedure, the crude and adjusted odds ratios for methadone dose were largely unchanged and the set of terms in the final multivariable model was identical (results not shown).
The third sensitivity analysis was conducted to investigate the possible interaction of methadone dose and the presence of specific antiretroviral agents shown to affect the metabolization of methadone (delavirdine, efavirenz or nevaripine). In models fit to observation periods with ≥ 1 day of ART dispensed in the last 180 days, the adjusted odds ratio between methadone dose and ≥ 95% adherence in both the full and final models did not substantially differ when compared to the original analysis (original analysis AOR = 1.38, 95% CI: 1.08 – 1.77; sensitivity analysis AOR = 1.36, 95% CI: 1.04 – 1.76). Additionally, we fit a GEE model of optimal adherence with an interaction term for methadone dose (≥ 100 mg/day vs. < 100 mg/day) and the presence of DEL, DMP or NEV, and the interaction term was not statistically significant (p = 0.431).
DISCUSSION
This study demonstrates an independent and positive association between high-dose methadone (≥ 100 mg/day) and a greater likelihood of optimal ART adherence (≥ 95%). Additionally, a significant positive dose-response relationship was observed between methadone dose (per 20 mg/day increase) and likelihood of optimal ART adherence.
Several biological, clinical and structural lines of evidence support our observations. First, high-dose methadone may sufficiently saturate opioid receptors and thus effectively block the positively reinforcing euphoric effects of illicit opioids (e.g., heroin) (33), leading to attenuated illicit opioid use. With decreased illicit opioid use, patients have increased overall stability and are better able to adhere to their prescribed medications, including ART. Secondly, previous literature has demonstrated that methadone doses equal to or above 100 mg/day are associated with improved long-term retention in methadone treatment programs (58, 59). Engagement in a structured methadone maintenance program, particularly over extended periods of time, allows for regular contact with health care professionals, facilitating optimal engagement in medical care, including treatment for HIV/AIDS (25). A five-year longitudinal study by Roux et al. (2009) showed that longer durations of retention in opioid substitution therapy were associated with higher likelihood of long-term virological success (60). Third, persons who use illicit opioids on MMT have lower rates of criminal activity and incarceration than those who are not on methadone (61). Bellin et al. (1999) demonstrated that individuals discharged from custody on high-dose methadone (defined as ≥ 60 mg/day in their study) had lower criminal recidivism rates compared to individuals discharged on low-dose methadone (62). This is notable as both incarceration and release from custody pose substantial barriers to continued ART adherence (55).
Given that improved health outcomes among people living with HIV are contingent on optimal ART adherence, our findings have important public health implications. These results are particularly relevant as persons who inject drugs account for more than 40% of new HIV infections in some countries (63). Unfortunately, in many countries with uncontrolled outbreaks of HIV and other blood-borne pathogens among people who use drugs, there are systemic barriers to implementing the delivery of high-dose methadone to improve ART adherence. For example, in Thailand, methadone is used primarily for detoxification through methadone tapers, and there are few long-term maintenance programs established (64, 65). This occurs despite evidence from systematic reviews and meta-analyses showing that methadone used for detoxification alone is associated with an increased risk of HIV transmission (66). In Russia, federal laws prohibit the use opioid substitution therapy in the treatment of opioid dependence, and ongoing illicit drug use excludes individuals from being eligible for HIV treatment (67, 68). Additionally, in many countries where opioid substitution therapy is available, individuals may be reluctant to up-titrate methadone to therapeutic doses for a variety of reasons, including fears of being constrained to long-term treatment (69). The current study provides more evidence in support of the immediate scaling-up of methadone programs to improve access and ensure adequate doses are prescribed in order to curb HIV/AIDS-associated morbidity and mortality among opioid-dependent individuals worldwide.
As ART regimens continue to be simplified to once daily dosing, the co-administration of both methadone and ART as daily witnessed doses at an individual’s pharmacy is a promising intervention to improve adherence. ART directly observed treatment (DOT) programs have been successfully implemented in community settings, including methadone clinics, and have shown improved rates of viral suppression (70, 71). A systematic review of interventions to improve ART adherence and virologic outcomes among HIV infected persons who use drugs found that directly administered ART and medication assisted therapy, including MMT, improved short-term adherence and virologic outcomes, however, these outcomes were not sustained after intervention cessation (72).
There are several limitations in our study. While previous research has demonstrated the clinical validity of ART pharmacy refill data and has shown that it reliably predicts virologic suppression (5, 40–42) and survival (8, 36), adherence rates were calculated based on the number of days where ART was dispensed. This may not necessarily correspond to the number of days that the medication was taken, and therefore may overestimate adherence rates. Second, information regarding participation in MMT and methadone dose for this study was based on self-report. However, our validated outcome of interest, ART adherence, was acquired from an administrative database and we do not believe individuals differentially reported methadone dose based on their recent ART adherence. Additionally, Langendendam et al. (1999) has demonstrated that persons who use illicit opioids are able to give valid self-reports of their methadone dosages (45). Third, our study focused on overall ART adherence, however we did not look specifically at which ART regimens patients were prescribed as a secondary explanatory variable. Several commonly prescribed antiretroviral medications, particularly the non-nucleoside reverse transcriptase inhibitors (NNRTIs) delavirdine, efavirenz and nevaripine, may accelerate the metabolism of methadone (13, 73). The combination of these NNRTIs with methadone can result in lower serum concentrations of methadone, and methadone dose increases between 20–50% are sometimes necessary to maintain therapeutic effects (73). If methadone doses are not adequately adjusted when patients are started on certain antiretrovirals, they may remain under-dosed and as result may not benefit from the stabilizing benefits of methadone to the same degree as those whose doses were increased. In order to account for this we did a sensitivity analysis to account for patients prescribed delavirdine, efavirenz or nevaripine. The results were not affected substantially and we did not find a significant interaction. Fourth, our ART data is limited in that we do not have information on pill burden or the frequency of dosing. Both have previously been identified as being important factors in ART adherence (74). Fifth, the number of reports of some secondary explanatory variables (e.g., heavy alcohol use) were low, which might have hindered their consideration as confounding variables. Finally, while the ACCESS cohort was recruited using extensive street outreach and snowball sampling, it is not a random sample. Therefore, our results might not be generalizable of all HIV-positive persons who use illicit opioids in this setting or in others.
In conclusion, the present study demonstrated improved ART adherence among participants who reported a methadone dose of ≥ 100mg/day. Additionally, a significant dose-response relationship between increasing methadone dose and ART adherence was also observed. These findings underscore the need to improve access to and delivery of effective methadone doses for individuals who use illicit opioids in an effort to engage individuals in structured programs that may facilitate and maximize ART adherence and ultimately improve HIV outcomes.
Acknowledgments
The authors thank the study participants for their contribution to the research, as well as current and past researchers and staff. The study was supported by the US National Institutes of Health (R01DA021525). This research was undertaken, in part, thanks to funding from the Canada Research Chairs program through a Tier 1 Canada Research Chair in Inner City Medicine which supports Dr. Evan Wood. Dr. Milloy is supported in part by the US National Institutes of Health. Dr. Lappalainen, Dr. Ahamad and Dr. Nolan are supported in part by the Canada Addiction Medicine Research Fellowship, a US National Institute on Drug Abuse (NIDA)-funded research training fellowship. Dr. Montaner is supported with grants paid to his institution by the British Columbia Ministry of Health and by the US National Institutes of Health (R01DA036307). He has also received limited unrestricted funding, paid to his institution, from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare.
REFERENCES
- 1.Palella FJ, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. Journal of acquired immune deficiency syndromes. 2006;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. The New England journal of medicine. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Hogg RS, Yip B, Kully C, Craib KJ, O'Shaughnessy MV, Schechter MT, et al. Improved survival among HIV-infected patients after initiation of triple-drug antiretroviral regimens. CMAJ: Canadian Medical Association Journal. 1999;160(5):659–665. [PMC free article] [PubMed] [Google Scholar]
- 4.Ray M, Logan R, Sterne JA, Hernandez-Diaz S, Robins JM, Sabin C, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. Aids. 2010;24(1):123–137. doi: 10.1097/QAD.0b013e3283324283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Low-Beer S, Yip B, O'Shaughnessy MV, Hogg RS, Montaner JS. Adherence to triple therapy and viral load response. Journal of acquired immune deficiency syndromes. 2000;23(4):360–361. doi: 10.1097/00126334-200004010-00016. [DOI] [PubMed] [Google Scholar]
- 6.Nolan S, Milloy MJ, Zhang R, Kerr T, Hogg RS, Montaner JS, et al. Adherence and plasma HIV RNA response to antiretroviral therapy among HIV-seropositive injection drug users in a Canadian setting. AIDS care. 2011;23(8):980–987. doi: 10.1080/09540121.2010.543882. [DOI] [PubMed] [Google Scholar]
- 7.Wood E, Hogg RS, Yip B, Harrigan PR, O'Shaughnessy MV, Montaner JS. The impact of adherence on CD4 cell count responses among HIV-infected patients. Journal of acquired immune deficiency syndromes. 2004;35(3):261–268. doi: 10.1097/00126334-200403010-00006. [DOI] [PubMed] [Google Scholar]
- 8.Wood E, Hogg RS, Yip B, Harrigan PR, O'Shaughnessy MV, Montaner JS. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4+ cell count is 0.200 to 0.350 × 10(9) cells/L. Annals of internal medicine. 2003;139(10):810–816. doi: 10.7326/0003-4819-139-10-200311180-00008. [DOI] [PubMed] [Google Scholar]
- 9.Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. Aids. 2001;15(9):1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 10.Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bulletin of the World Health Organization. 2013;91(2):102–123. doi: 10.2471/BLT.12.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lert F, Kazatchkine MD. Antiretroviral HIV treatment and care for injecting drug users: an evidence-based overview. The International journal on drug policy. 2007;18(4):255–261. doi: 10.1016/j.drugpo.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. Journal of general internal medicine. 2002;17(5):377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376(9738):367–387. doi: 10.1016/S0140-6736(10)60829-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez A, Barinas J, O'Cleirigh C. Substance use: impact on adherence and HIV medical treatment. Current HIV/AIDS reports. 2011;8(4):223–234. doi: 10.1007/s11904-011-0093-5. [DOI] [PubMed] [Google Scholar]
- 15.Lucas GM. Substance abuse, adherence with antiretroviral therapy, and clinical outcomes among HIV-infected individuals. Life sciences. 2011;88(21–22):948–952. doi: 10.1016/j.lfs.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein MD, Rich JD, Maksad J, Chen MH, Hu P, Sobota M, et al. Adherence to antiretroviral therapy among HIV-infected methadone patients: effect of ongoing illicit drug use. The American journal of drug and alcohol abuse. 2000;26(2):195–205. doi: 10.1081/ada-100100600. [DOI] [PubMed] [Google Scholar]
- 17.Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. Journal of acquired immune deficiency syndromes. 2001;27(3):251–259. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- 18.Palepu A, Tyndall M, Yip B, O'Shaughnessy MV, Hogg RS, Montaner JS. Impaired virologic response to highly active antiretroviral therapy associated with ongoing injection drug use. Journal of acquired immune deficiency syndromes. 2003;32(5):522–526. doi: 10.1097/00126334-200304150-00009. [DOI] [PubMed] [Google Scholar]
- 19.Wood E, Montaner JS, Yip B, Tyndall MW, Schechter MT, O'Shaughnessy MV, et al. Adherence to antiretroviral therapy and CD4 T-cell count responses among HIV-infected injection drug users. Antiviral therapy. 2004;9(2):229–235. [PubMed] [Google Scholar]
- 20.Sambamoorthi U, Warner LA, Crystal S, Walkup J. Drug abuse, methadone treatment, and health services use among injection drug users with AIDS. Drug and alcohol dependence. 2000;60(1):77–89. doi: 10.1016/s0376-8716(99)00142-8. [DOI] [PubMed] [Google Scholar]
- 21.Palepu A, Horton NJ, Tibbetts N, Meli S, Samet JH. Uptake and adherence to highly active antiretroviral therapy among HIV-infected people with alcohol and other substance use problems: the impact of substance abuse treatment. Addiction. 2004;99(3):361–368. doi: 10.1111/j.1360-0443.2003.00670.x. [DOI] [PubMed] [Google Scholar]
- 22.Turner BJ, Zhang D, Laine C, Pomerantz RJ, Cosler L, Hauck WW. Association of provider and patient characteristics with HIV-infected women's antiretroviral therapy regimen. Journal of acquired immune deficiency syndromes. 2001;27(1):20–29. doi: 10.1097/00126334-200105010-00004. [DOI] [PubMed] [Google Scholar]
- 23.Malta M, Strathdee SA, Magnanini MM, Bastos FI. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: a systematic review. Addiction. 2008;103(8):1242–1257. doi: 10.1111/j.1360-0443.2008.02269.x. [DOI] [PubMed] [Google Scholar]
- 24.Reddon H, Milloy MJ, Simo A, Montaner J, Wood E, Kerr T. Methadone maintenance therapy decreases the rate of antiretroviral therapy discontinuation among HIV-positive illicit drug users. AIDS and behavior. 2014;18(4):740–746. doi: 10.1007/s10461-013-0584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palepu A, Tyndall MW, Joy R, Kerr T, Wood E, Press N, et al. Antiretroviral adherence and HIV treatment outcomes among HIV/HCV co-infected injection drug users: the role of methadone maintenance therapy. Drug and alcohol dependence. 2006;84(2):188–194. doi: 10.1016/j.drugalcdep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Faggiano F, Vigna-Taglianti F, Versino E, Lemma P. Methadone maintenance at different dosages for opioid dependence. The Cochrane database of systematic reviews. 2003;(3) doi: 10.1002/14651858.CD002208. CD002208. [DOI] [PubMed] [Google Scholar]
- 27.Strain EC, Bigelow GE, Liebson IA, Stitzer ML. Moderate- vs high-dose methadone in the treatment of opioid dependence: a randomized trial. JAMA : the journal of the American Medical Association. 1999;281(11):1000–1005. doi: 10.1001/jama.281.11.1000. [DOI] [PubMed] [Google Scholar]
- 28.Hser YI, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109(1):79–87. doi: 10.1111/add.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao DL, Chen PC, Chen CH, Hsieh CJ, Huang YF, Shih WY, et al. Higher methadone doses are associated with lower mortality in patients of opioid dependence in Taiwan. Journal of psychiatric research. 2013;47(10):1530–1534. doi: 10.1016/j.jpsychires.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 30.D'Aunno T, Pollack HA, Frimpong JA, Wuchiett D. Evidence-based treatment for opioid disorders: A 23-year national study of methadone dose levels. Journal of substance abuse treatment. 2014;47(4):245–250. doi: 10.1016/j.jsat.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selby P, Kahan M. Methadone Maintenance: A Physician's Guide to Treatment. Second Edition. Toronto, ON CAN: Centre for Addiction and Mental Health; 2011. p. 270. 2011. [Google Scholar]
- 32.Fareed A, Casarella J, Roberts M, Sleboda M, Amar R, Vayalapalli S, et al. High dose versus moderate dose methadone maintenance: is there a better outcome? Journal of addictive diseases. 2009;28(4):399–405. doi: 10.1080/10550880903183042. [DOI] [PubMed] [Google Scholar]
- 33.Donny EC, Brasser SM, Bigelow GE, Stitzer ML, Walsh SL. Methadone doses of 100 mg or greater are more effective than lower doses at suppressing heroin self-administration in opioid-dependent volunteers. Addiction. 2005;100(10):1496–1509. doi: 10.1111/j.1360-0443.2005.01232.x. [DOI] [PubMed] [Google Scholar]
- 34.Maxwell S, Shinderman M. Optimizing response to methadone maintenance treatment: use of higher-dose methadone. Journal of psychoactive drugs. 1999;31(2):95–102. doi: 10.1080/02791072.1999.10471730. [DOI] [PubMed] [Google Scholar]
- 35.Maxwell S, Shinderman MS. Optimizing long-term response to methadone maintenance treatment: a 152-week follow-up using higher-dose methadone. Journal of addictive diseases. 2002;21(3):1–12. doi: 10.1300/J069v21n03_01. [DOI] [PubMed] [Google Scholar]
- 36.Wood E, Hogg RS, Lima VD, Kerr T, Yip B, Marshall BD, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA : the journal of the American Medical Association. 2008;300(5):550–554. doi: 10.1001/jama.300.5.550. [DOI] [PubMed] [Google Scholar]
- 37.Strathdee SA, Patrick DM, Currie SL, Cornelisse PG, Rekart ML, Montaner JS, et al. Needle exchange is not enough: lessons from the Vancouver injecting drug use study. Aids. 1997;11(8):F59–F65. doi: 10.1097/00002030-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Strathdee SA, van Ameijden EJ, Mesquita F, Wodak A, Rana S, Vlahov D. Can HIV epidemics among injection drug users be prevented? Aids. 1998;12(Suppl A):S71–S79. [PubMed] [Google Scholar]
- 39.Tyndall MW, Currie S, Spittal P, Li K, Wood E, O'Shaughnessy MV, et al. Intensive injection cocaine use as the primary risk factor in the Vancouver HIV-1 epidemic. Aids. 2003;17(6):887–893. doi: 10.1097/00002030-200304110-00014. [DOI] [PubMed] [Google Scholar]
- 40.Palepu A, Yip B, Miller C, Strathdee SA, O'Shaughnessy MV, Montaner JS, et al. Factors associated with the response to antiretroviral therapy among HIV-infected patients with and without a history of injection drug use. Aids. 2001;15(3):423–424. doi: 10.1097/00002030-200102160-00021. [DOI] [PubMed] [Google Scholar]
- 41.Wood E, Montaner JS, Yip B, Tyndall MW, Schechter MT, O'Shaughnessy MV, et al. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2003;169(7):656–661. [PMC free article] [PubMed] [Google Scholar]
- 42.Grossberg R, Zhang Y, Gross R. A time-to-prescription-refill measure of antiretroviral adherence predicted changes in viral load in HIV. Journal of clinical epidemiology. 2004;57(10):1107–1110. doi: 10.1016/j.jclinepi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Nosyk B, Marsh DC, Sun H, Schechter MT, Anis AH. Trends in methadone maintenance treatment participation, retention, and compliance to dosing guidelines in British Columbia, Canada: 1996–2006. Journal of substance abuse treatment. 2010;39(1):22–31. doi: 10.1016/j.jsat.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Caplehorn JR, Dalton MS, Cluff MC, Petrenas AM. Retention in methadone maintenance and heroin addicts' risk of death. Addiction. 1994;89(2):203–209. doi: 10.1111/j.1360-0443.1994.tb00879.x. [DOI] [PubMed] [Google Scholar]
- 45.Langendam MW, van Haastrecht HJ, van Ameijden EJ. The validity of drug users' self-reports in a non-treatment setting: prevalence and predictors of incorrect reporting methadone treatment modalities. International journal of epidemiology. 1999;28(3):514–520. doi: 10.1093/ije/28.3.514. [DOI] [PubMed] [Google Scholar]
- 46.Nachega JB, Uthman OA, Peltzer K, Richardson LA, Mills EJ, Amekudzi K, et al. Association between antiretroviral therapy adherence and employment status: systematic review and meta-analysis. Bulletin of the World Health Organization. 2015;93(1):29–41. doi: 10.2471/BLT.14.138149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palepu A, Milloy MJ, Kerr T, Zhang R, Wood E. Homelessness and adherence to antiretroviral therapy among a cohort of HIV-infected injection drug users. Journal of urban health : bulletin of the New York Academy of Medicine. 2011;88(3):545–555. doi: 10.1007/s11524-011-9562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mills EJ, Nachega JB, Bangsberg DR, Singh S, Rachlis B, Wu P, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS medicine. 2006;3(11):e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood E, Hogg RS, Yip B, Harrigan PR, O'Shaughnessy MV, Montaner JS. Is there a baseline CD4 cell count that precludes a survival response to modern antiretroviral therapy? Aids. 2003;17(5):711–720. doi: 10.1097/00002030-200303280-00009. [DOI] [PubMed] [Google Scholar]
- 50.Kitahata MM, Koepsell TD, Deyo RA, Maxwell CL, Dodge WT, Wagner EH. Physicians' experience with the acquired immunodeficiency syndrome as a factor in patients' survival. The New England journal of medicine. 1996;334(11):701–706. doi: 10.1056/NEJM199603143341106. [DOI] [PubMed] [Google Scholar]
- 51.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Third ed. New York, New York: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 52.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. American journal of epidemiology. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 53.Lima V, Fernandes K, Rachlis B, Druyts E, Montaner J, Hogg R. Migration adversely affects antiretroviral adherence in a population-based cohort of HIV/AIDS patients. Social science & medicine. 2009;68(6):1044–1049. doi: 10.1016/j.socscimed.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 54.Marshall BD, Kerr T, Shoveller JA, Patterson TL, Buxton JA, Wood E. Homelessness and unstable housing associated with an increased risk of HIV and STI transmission among street-involved youth. Health & place. 2009;15(3):753–760. doi: 10.1016/j.healthplace.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milloy MJ, Kerr T, Buxton J, Rhodes T, Guillemi S, Hogg R, et al. Dose-response effect of incarceration events on nonadherence to HIV antiretroviral therapy among injection drug users. The Journal of infectious diseases. 2011;203(9):1215–1221. doi: 10.1093/infdis/jir032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lima VD, Geller J, Bangsberg DR, Patterson TL, Daniel M, Kerr T, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. Aids. 2007;21(9):1175–1183. doi: 10.1097/QAD.0b013e32811ebf57. [DOI] [PubMed] [Google Scholar]
- 57.Bruce RD, Moody DE, Altice FL, Gourevitch MN, Friedland GH. A review of pharmacological interactions between HIV or hepatitis C virus medications and opioid agonist therapy: implications and management for clinical practice. Expert review of clinical pharmacology. 2013;6(3):249–269. doi: 10.1586/ecp.13.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peles E, Schreiber S, Adelson M. Factors predicting retention in treatment: 10-year experience of a methadone maintenance treatment (MMT) clinic in Israel. Drug and alcohol dependence. 2006;82(3):211–217. doi: 10.1016/j.drugalcdep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 59.Nosyk B, MacNab YC, Sun H, Fischer B, Marsh DC, Schechter MT, et al. Proportional hazards frailty models for recurrent methadone maintenance treatment. American journal of epidemiology. 2009;170(6):783–792. doi: 10.1093/aje/kwp186. [DOI] [PubMed] [Google Scholar]
- 60.Roux P, Carrieri MP, Cohen J, Ravaux I, Poizot-Martin I, Dellamonica P, et al. Retention in opioid substitution treatment: a major predictor of long-term virological success for HIV-infected injection drug users receiving antiretroviral treatment. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;49(9):1433–1440. doi: 10.1086/630209. [DOI] [PubMed] [Google Scholar]
- 61.Werb D, Kerr T, Marsh D, Li K, Montaner J, Wood E. Effect of methadone treatment on incarceration rates among injection drug users. European addiction research. 2008;14(3):143–149. doi: 10.1159/000130418. [DOI] [PubMed] [Google Scholar]
- 62.Bellin E, Wesson J, Tomasino V, Nolan J, Glick AJ, Oquendo S. High Dose Methadone Reduces Criminal Recidivism in Opiate Addicts. Addiction Research & Theory. 1999;7(1):19–29. [Google Scholar]
- 63.UNAIDS: Joint United Nations Programme on HIV/AIDS. Global Report: UNAIDS report on the global AIDS epidemic 2013. UNAIDS; 2013. pp. 1–198. [Google Scholar]
- 64.Barrett M, Thomson N, Aramrattana A. Rapid assessment and response: Preparation for the scale-up of comprehensive harm reduction services in Thailand. Chiangmai, Thailand: Chiangmai University; 2010. Jun, 2010. Report No.: 1. [Google Scholar]
- 65.Fairbairn N, Hayashi K, Kaplan K, Suwannawong P, Qi J, Wood E, et al. Factors associated with methadone treatment among injection drug users in Bangkok, Thailand. Journal of substance abuse treatment. 2012;43(1):108–113. doi: 10.1016/j.jsat.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 66.MacArthur GJ, Minozzi S, Martin N, Vickerman P, Deren S, Bruneau J, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. Bmj. 2012;345:e5945. doi: 10.1136/bmj.e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Russia's punitive drug laws. Lancet. 2011;377(9783):2056. doi: 10.1016/S0140-6736(11)60903-3. [DOI] [PubMed] [Google Scholar]
- 68.Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet. 2010;376(9738):355–366. doi: 10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]
- 69.Sanders JJ, Roose RJ, Lubrano MC, Lucan SC. Meaning and methadone: patient perceptions of methadone dose and a model to promote adherence to maintenance treatment. Journal of addiction medicine. 2013;7(5):307–313. doi: 10.1097/ADM.0b013e318297021e. [DOI] [PubMed] [Google Scholar]
- 70.Conway B, Prasad J, Reynolds R, Farley J, Jones M, Jutha S, et al. Directly observed therapy for the management of HIV-infected patients in a methadone program. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;38(Suppl 5):S402–S408. doi: 10.1086/421404. [DOI] [PubMed] [Google Scholar]
- 71.Berg KM, Litwin A, Li X, Heo M, Arnsten JH. Directly observed antiretroviral therapy improves adherence and viral load in drug users attending methadone maintenance clinics: a randomized controlled trial. Drug and alcohol dependence. 2011;113(2–3):192–199. doi: 10.1016/j.drugalcdep.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Binford MC, Kahana SY, Altice FL. A systematic review of antiretroviral adherence interventions for HIV-infected people who use drugs. Current HIV/AIDS reports. 2012;9(4):287–312. doi: 10.1007/s11904-012-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maas B, Kerr T, Fairbairn N, Montaner J, Wood E. Pharmacokinetic interactions between HIV antiretroviral therapy and drugs used to treat opioid dependence. Expert opinion on drug metabolism & toxicology. 2006;2(4):533–543. doi: 10.1517/17425255.2.4.533. [DOI] [PubMed] [Google Scholar]
- 74.Atkinson MJ, Petrozzino JJ. An evidence-based review of treatment-related determinants of patients' nonadherence to HIV medications. AIDS patient care and STDs. 2009;23(11):903–914. doi: 10.1089/apc.2009.0024. [DOI] [PubMed] [Google Scholar]