Abstract
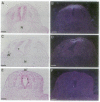
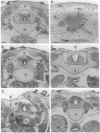
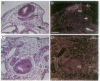
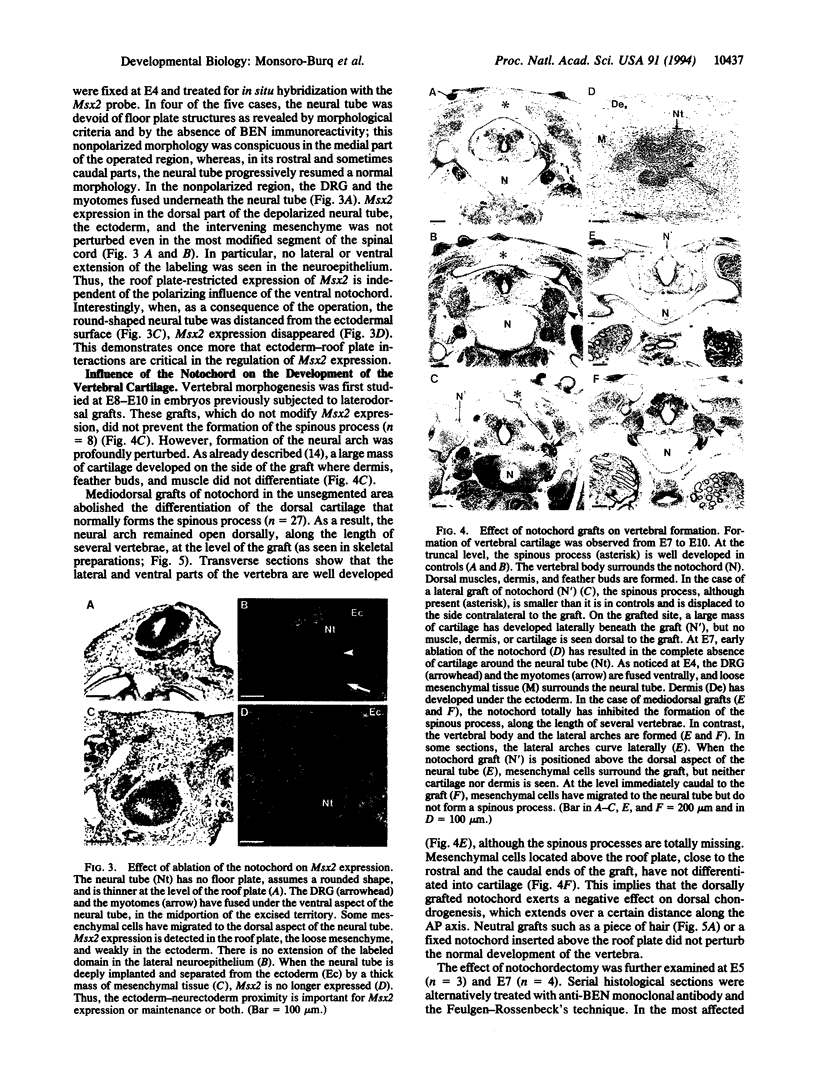
Vertebrae are derived from the sclerotomal moities of the somites. Sclerotomal cells migrate ventrally to surround the notochord, where they form the vertebral body, and dorsolaterally to form the neural arch, which is dorsally closed by the spinous process. Precursor cells of the spinous process as well as superficial ectoderm and roof plate express homeobox genes of the Msh family from embryonic day 2 (E2) to E6. The notochord has been shown to be responsible for the dorsoventral polarization of the somites and for the induction of sclerotomal cells into cartilage. Indeed, supernumerary notochord grafted laterally to the neural tube induces the conversion of the entire somite into cartilage. We report here that a mediodorsal graft of notochord prevents the sclerotomal cells migrating dorsally to the roof plate from differentiating into cartilage. Under these experimental conditions, expression of Msx genes is abolished. We thus demonstrate that cartilaginous, differentiation is differentially controlled in the dorsal part of the vertebra (spinous process) and in the neural arch and vertebral body.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bovolenta P., Dodd J. Perturbation of neuronal differentiation and axon guidance in the spinal cord of mouse embryos lacking a floor plate: analysis of Danforth's short-tail mutation. Development. 1991 Oct;113(2):625–639. doi: 10.1242/dev.113.2.625. [DOI] [PubMed] [Google Scholar]
- Brand-Saberi B., Ebensperger C., Wilting J., Balling R., Christ B. The ventralizing effect of the notochord on somite differentiation in chick embryos. Anat Embryol (Berl) 1993 Sep;188(3):239–245. doi: 10.1007/BF00188215. [DOI] [PubMed] [Google Scholar]
- Chalepakis G., Fritsch R., Fickenscher H., Deutsch U., Goulding M., Gruss P. The molecular basis of the undulated/Pax-1 mutation. Cell. 1991 Sep 6;66(5):873–884. doi: 10.1016/0092-8674(91)90434-z. [DOI] [PubMed] [Google Scholar]
- Coelho C. N., Krabbenhoft K. M., Upholt W. B., Fallon J. F., Kosher R. A. Altered expression of the chicken homeobox-containing genes GHox-7 and GHox-8 in the limb buds of limbless mutant chick embryos. Development. 1991 Dec;113(4):1487–1493. doi: 10.1242/dev.113.4.1487. [DOI] [PubMed] [Google Scholar]
- Couly G. F., Coltey P. M., Le Douarin N. M. The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development. 1992 Jan;114(1):1–15. doi: 10.1242/dev.114.1.1. [DOI] [PubMed] [Google Scholar]
- Couly G. F., Coltey P. M., Le Douarin N. M. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development. 1993 Feb;117(2):409–429. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- Deutsch U., Dressler G. R., Gruss P. Pax 1, a member of a paired box homologous murine gene family, is expressed in segmented structures during development. Cell. 1988 May 20;53(4):617–625. doi: 10.1016/0092-8674(88)90577-6. [DOI] [PubMed] [Google Scholar]
- Dupin E., Maus M., Fauquet M. Regulation of the quail tyrosine hydroxylase gene in neural crest cells by cAMP and beta-adrenergic ligands. Dev Biol. 1993 Sep;159(1):75–86. doi: 10.1006/dbio.1993.1222. [DOI] [PubMed] [Google Scholar]
- Hill R. E., Jones P. F., Rees A. R., Sime C. M., Justice M. J., Copeland N. G., Jenkins N. A., Graham E., Davidson D. R. A new family of mouse homeo box-containing genes: molecular structure, chromosomal location, and developmental expression of Hox-7.1. Genes Dev. 1989 Jan;3(1):26–37. doi: 10.1101/gad.3.1.26. [DOI] [PubMed] [Google Scholar]
- Hirano S., Fuse S., Sohal G. S. The effect of the floor plate on pattern and polarity in the developing central nervous system. Science. 1991 Jan 18;251(4991):310–313. doi: 10.1126/science.1987648. [DOI] [PubMed] [Google Scholar]
- Holland P. W. Cloning and evolutionary analysis of msh-like homeobox genes from mouse, zebrafish and ascidian. Gene. 1991 Feb 15;98(2):253–257. doi: 10.1016/0378-1119(91)90182-b. [DOI] [PubMed] [Google Scholar]
- Jabs E. W., Müller U., Li X., Ma L., Luo W., Haworth I. S., Klisak I., Sparkes R., Warman M. L., Mulliken J. B. A mutation in the homeodomain of the human MSX2 gene in a family affected with autosomal dominant craniosynostosis. Cell. 1993 Nov 5;75(3):443–450. doi: 10.1016/0092-8674(93)90379-5. [DOI] [PubMed] [Google Scholar]
- Jowett A. K., Vainio S., Ferguson M. W., Sharpe P. T., Thesleff I. Epithelial-mesenchymal interactions are required for msx 1 and msx 2 gene expression in the developing murine molar tooth. Development. 1993 Feb;117(2):461–470. doi: 10.1242/dev.117.2.461. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Monaghan A. P., Davidson D. R., Sime C., Graham E., Baldock R., Bhattacharya S. S., Hill R. E. The Msh-like homeobox genes define domains in the developing vertebrate eye. Development. 1991 Aug;112(4):1053–1061. doi: 10.1242/dev.112.4.1053. [DOI] [PubMed] [Google Scholar]
- Placzek M., Jessell T. M., Dodd J. Induction of floor plate differentiation by contact-dependent, homeogenetic signals. Development. 1993 Jan;117(1):205–218. doi: 10.1242/dev.117.1.205. [DOI] [PubMed] [Google Scholar]
- Pourquié O., Coltey M., Teillet M. A., Ordahl C., Le Douarin N. M. Control of dorsoventral patterning of somitic derivatives by notochord and floor plate. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5242–5246. doi: 10.1073/pnas.90.11.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert B., Lyons G., Simandl B. K., Kuroiwa A., Buckingham M. The apical ectodermal ridge regulates Hox-7 and Hox-8 gene expression in developing chick limb buds. Genes Dev. 1991 Dec;5(12B):2363–2374. doi: 10.1101/gad.5.12b.2363. [DOI] [PubMed] [Google Scholar]
- Robert B., Sassoon D., Jacq B., Gehring W., Buckingham M. Hox-7, a mouse homeobox gene with a novel pattern of expression during embryogenesis. EMBO J. 1989 Jan;8(1):91–100. doi: 10.1002/j.1460-2075.1989.tb03352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohkamm R. Degeneration and regeneration in neurons of the cerebellum. Adv Anat Embryol Cell Biol. 1977;53(6):1–118. doi: 10.1007/978-3-642-66818-0. [DOI] [PubMed] [Google Scholar]
- Satokata I., Maas R. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet. 1994 Apr;6(4):348–356. doi: 10.1038/ng0494-348. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Schoenwolf G. C. Notochordal induction of cell wedging in the chick neural plate and its role in neural tube formation. J Exp Zool. 1989 Apr;250(1):49–62. doi: 10.1002/jez.1402500107. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Bontoux M., Le Douarin N. M. Epithelio--mesenchymal interactions are critical for Quox 7 expression and membrane bone differentiation in the neural crest derived mandibular mesenchyme. EMBO J. 1991 Sep;10(9):2387–2393. doi: 10.1002/j.1460-2075.1991.tb07777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Le Douarin N. cDNA cloning of a quail homeobox gene and its expression in neural crest-derived mesenchyme and lateral plate mesoderm. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7482–7486. doi: 10.1073/pnas.87.19.7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Monsoro-Burq A. H., Bontoux M., Le Douarin N. M. A role for Quox-8 in the establishment of the dorsoventral pattern during vertebrate development. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10237–10241. doi: 10.1073/pnas.89.21.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio S., Karavanova I., Jowett A., Thesleff I. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell. 1993 Oct 8;75(1):45–58. [PubMed] [Google Scholar]
- Yamada T., Pfaff S. L., Edlund T., Jessell T. M. Control of cell pattern in the neural tube: motor neuron induction by diffusible factors from notochord and floor plate. Cell. 1993 May 21;73(4):673–686. doi: 10.1016/0092-8674(93)90248-o. [DOI] [PubMed] [Google Scholar]
- Yamada T., Placzek M., Tanaka H., Dodd J., Jessell T. M. Control of cell pattern in the developing nervous system: polarizing activity of the floor plate and notochord. Cell. 1991 Feb 8;64(3):635–647. doi: 10.1016/0092-8674(91)90247-v. [DOI] [PubMed] [Google Scholar]
- van Straaten H. W., Hekking J. W. Development of floor plate, neurons and axonal outgrowth pattern in the early spinal cord of the notochord-deficient chick embryo. Anat Embryol (Berl) 1991;184(1):55–63. doi: 10.1007/BF01744261. [DOI] [PubMed] [Google Scholar]
- van Straaten H. W., Thors F., Wiertz-Hoessels L., Hekking J., Drukker J. Effect of a notochordal implant on the early morphogenesis of the neural tube and neuroblasts: histometrical and histological results. Dev Biol. 1985 Jul;110(1):247–254. doi: 10.1016/0012-1606(85)90081-8. [DOI] [PubMed] [Google Scholar]