Abstract
Background
Organ transplantation has seen an increased utilization of organs from older donors over the past decades in an attempt to meet the globally growing shortage of donor organs. However, inferior transplant outcomes when utilizing older donor organs represent a growing challenge.
Methods and Results
Here, we characterize the impact of donor age on solid organ transplantation using a murine cardiac transplantation model. We found a compromised graft survival when utilizing older hearts. Shorter graft survival of older hearts was independent of organ age per se, as chimeric young or old organs repopulated with young passenger leukocytes showed comparable survival times. Transplantation of older organs triggered more potent alloimmune responses via intragraft CD11c+ dendritic cells (DCs) augmenting CD4+ and CD8+ T cell proliferation and pro-inflammatory cytokine production, particularly that of IL-17A. Of note, depletion of donor CD11c+ DCs prior to engraftment, neutralization of IL-17A or transplantation of older hearts into IL-17A-/- mice delayed rejection and reduced alloimmune responses to levels observed when transplanting young hearts.
Conclusions
These results demonstrate a critical role of old donor CD11c+ DCs in mounting age-dependent alloimmune responses with an augmented IL-17A response in recipient animals. Targeting IL-17A may serve as a novel therapeutic approach when transplanting older organs.
Keywords: transplantation, immunology, rejection, aging
Introduction
With the success of organ transplantation, age limits have essentially been lifted. Older organs have been increasingly utilized to compensate the increasing organ shortage 1. Organ age on the other hand has been linked to intrinsic chronic interstitial inflammation in general referred to as inflamm-aging, with reduced clearance of self- as well as exogenous antigens as hallmark findings 2. Moreover, older donor organs have not only shown greater fragility and increased susceptibility to injury, but have also elicited more potent systemic recipient immune responses through augmented immunogenicity. At the same time, mechanistic links between organ age and alloimmune responses remain unclear.
Consistent with previous reports, we have previously shown in a large-scale clinical analysis that rates of acute rejection increase in parallel to donor age regardless of recipient age 3. It is well established that aging is associated with significant and broad changes of the immune system 4,5. The impact of these changes on transplant outcome, however, remains poorly understood.
Increasing evidence indicates that there is an age-associated dysregulation in Th1 and Th2 cytokine synthesis 6. While the IL-2 signaling machinery has been shown to be critical for Th1 differentiation and proliferation, IL-2 has been established as an inhibitor of Th17 development 7. Of significance for studies on immunosenescence and alloimmune responses, CD4+ T cells isolated from splenocytes of old mice produced higher amounts of IL-17 8.
Using a fully allogenic heart transplant mouse model, we demonstrate that old cardiac allografts were rejected more rapidly, consistent with our clinical observations. Our data indicate that older CD11c+ dendritic cells (DCs) were the primary instigators of augmented alloimmune responses via IL-17A. Collectively, our data demonstrate that older organs skew the recipient immune response towards CD4+IL-17A+ T cells via resident cardiac allograft CD11c+ cells in an age-dependent manner. At the same time, blocking IL-17A prolonged survival of older organs, thus revealing novel therapeutic opportunities.
Methods
Study approval
Animal use and care were in accordance with National Institutes of Health and Institutional Animal Care and Use Committee guidelines.
Animals
8-12 week old wild type (WT) male C57BL/6 or DBA/2J WT male mice were purchased from Charles River Laboratories (Wilmington, MA). 18 month old WT male C57BL/6 mice were purchased from the National Institute of Aging (NIA, Bethesda, MD). 8-12 week old BALB/c IL-17-/- mice were bred at the Harvard School of Public Health Animal Facility; breeder pairs were kindly provided by Yoichiro Iwakura, Center for Experimental Medicine, Institute of Medical Science, University of Tokyo, Tokyo, Japan.
Heterotopic heart transplantation
Using a modified cuff technique, fully vascularized cardiac grafts from either old or young B6 donor mice were heterotopically transplanted into young DBA/2J, young BALB/c WT or BALB/c IL-17-/- recipients, respectively. Hearts were anastomosed to recipient's common carotid artery and internal jugular vein. Transplantation into the recipient's cervical region facilitated reliable functional assessment through palpation. Ischemic times were kept consistently at 40 min with an anastomosis time of 12 min. Graft function was measured daily by palpation, and allograft rejection was defined as the complete cessation of palpable contractility. Graft survival is shown as the median survival time (MST) in days.
In vivo treatment protocols
For the generation of chimeric donors, young and old prospective cardiac donor C57BL/6 mice were lethally irradiated (11 Gy), eliminating all passenger leukocytes within the heart. Bone marrow was procured from young syngeneic C57BL/6 mice and transfused intravenously 24 hours after irradiation (10×106 cells/animal). Mice were then used as cardiac allograft donors after six weeks, when cardiac tissue had been repopulated with leukocytes derived from the transplanted bone marrow. Successful repopulation was validated by immunohistochemistry.
For the depletion of DCs, young and old cardiac donor C57BL/6 mice were treated intravenously with 0.5mg liposomal clodronate (Encapsula NanoSciences, Nashville, TN) at day 8, 5 and 1 prior to graft procurement. This regimen insured depletion of intragraft CD11c+ dendritic cells as documented by flow cytometry analysis.
For blockage of IL-17A, 100μg of anti-IL-17A (R&D systems, Minneapolis, USA) were administered intravenously to the recipient animals every other day following cardiac transplantation starting at day 0 until the day of complete rejection; the control group for this experiment was injected with isotype control antibodies using the same protocol.
ELISpot Assay, ELISA
ELISpot assays were performed using mouse IFNγ or mouse IL-6 ELISpot Kits (BD Biosciences, San Diego, CA), respectively. Briefly, 0.5×106 unselected splenocytes from DBA/2J recipients were used as responder cells and restimulated with 0.5×106 irradiated splenocytes from naïve donor-type B6 animals within 96 well ELISpot plates for 24 hours under standard cell culture conditions (using RPMI 1640 medium supplemented with 10% FCS, 1% penicillin/streptomycin and 1% L-glutamine; 37°C; 5% CO2). Alternatively, 1×106 splenocytes from naïve DBA/2J mice used as responder cells to 104 flow-sorted splenic CD11c+ DCs (untreated or stimulated for 24 hours with 100 ng/ml lipopolysaccharide, LPS) isolated from either young or old naïve B6 mice. The ELISpot assay was adapted to measure the frequency of alloreactive T cells producing IFNγ or IL-6, respectively. The resulting spots were counted on a computer-assisted enzyme-linked immunospot image analyzer (Cellular Technology), and frequencies were expressed as numbers of cytokine-producing spots per 0.5×106 or 1×106 responder cells.
For detection of IL-17A in vitro, ELISA was performed. Mouse IL-17A was measured using commercial kits (eBioscience). Briefly, ELISA plates were coated with 100μl of anti-cytokine capture antibody at 4°C overnight. Plates were then washed 5x with 0.05% PBS-Tween (PBST) and coated for 1 hour with the blocking buffer provided by the manufacturer. Samples or standards were added in duplicates (100μl/well) and incubated at 4°C overnight. Wells were washed 5x with PBST and incubated with 100μl of anti-cytokine detection antibody at 4°C overnight. Wells were then washed 5x with PBST and incubated with 100μl of avidin-HRP at room temperature for 30 minutes. Thereafter, wells were washed 7x with PBST and incubated with 100μl/well of a substrate. The reaction was stopped after 15min with 1M H2SO4 and absorbance was measured using a multiplate microplate fluorescence reader (Synergy HT, Biotek, Winooski, VT) at 405nm.
Cell isolation
Single-cell leukocyte suspensions were obtained from spleens of young (8-12 weeks) DBA/2J WT mice by negative selection using anti-mouse antibodies against CD8, CD11b, CD11c, CD19, CD24, CD25, CD44, CD45R, CD49b, TCRγ/δ and TER119 using the EasySep™ Mouse Naïve CD4+ T Cell Isolation Kit (Stemcell Technologies, Vancouver, British Columbia, Canada) according to manufacturer's protocol. For isolation of CD11c+ DCs, single cell suspensions were obtained from hearts of young (8-12 weeks) and old (18 months) C57BL/6 WT mice. Briefly, hearts were procured and washed x3 with Ca2+- and Mg2+-free PBS. Tissue was then cut into 5 mm pieces and placed in tissue extraction buffer (5mM EDTA, 2mM 2-ME in PBS), and incubated with continuous, brisk stirring at 37°C for 30 minutes; suspension was then filtrated through a 70μm filter. CD11c+ DCs were then isolated using EasySep™ Mouse CD11c Positive Selection Kit (Stemcell Technologies) according to the manufacturer's protocol.
Statistical analysis
Kaplan-Meier survival graphs were constructed and log-rank comparisons of groups were used to calculate P values. Student's t-test was used for comparison of means between two groups. No multiple testing correction was performed. A P value <0.05 was considered as statistically significant. Data were expressed as mean ± standard error of the mean (SEM). GraphPad Prism version 6, GraphPad Software, La Jolla, CA, USA, was used for statistical analysis.
Additional methods are detailed in the online Supplemental Material.
Results
Cardiac allografts from old donors are rejected more rapidly
We have previously shown in a large-scale clinical study that episodes of acute rejection were more frequent when utilizing older organs for transplantation 3. To shed light on this clinical observation and to understand underlying mechanisms, we transplanted fully MHC-mismatched cardiac allografts from either young (10-12 weeks) or old (18 months) C57BL/6 donor mice into young DBA/2J recipients. In this heterotopic and fully vascularized cardiac transplant model, old hearts showed a significantly shorter graft survival (Fig. 1a). Furthermore, old cardiac allografts displayed more advanced ISHLT rejection scores compared to allografts procured from young donors (Fig. 1b). Moreover, intragraft immunohistological analysis demonstrated increased CD4+ and CD8+ T cell infiltrates (Fig. 1c). Collectively, these findings documented age-dependent differences in cardiac allograft survival correlating with increased lymphocellular infiltration and advanced structural changes.
Figure 1.
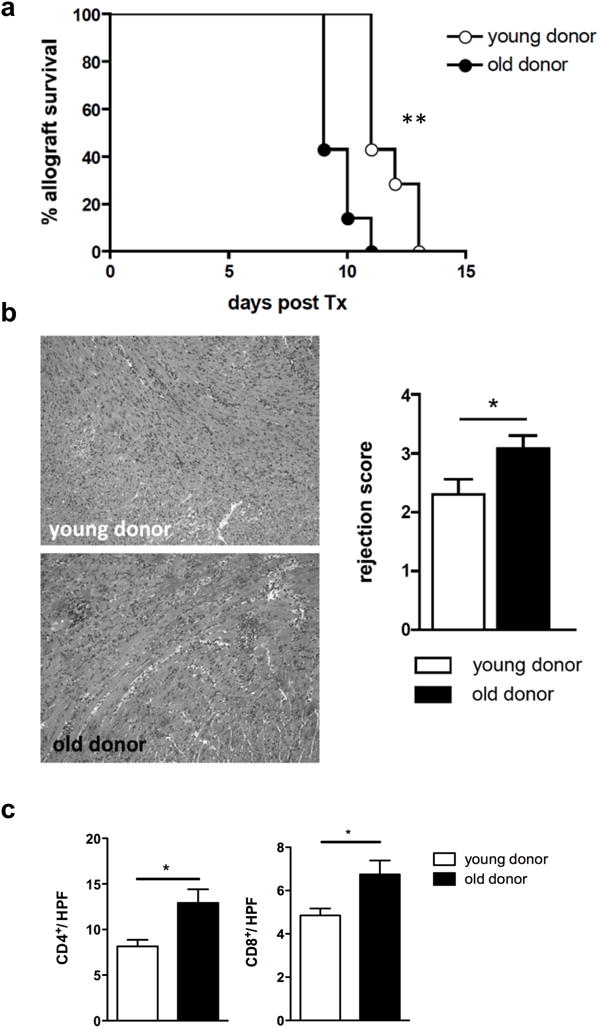
Old cardiac allografts are rejected more rapidly. a, Hearts from old and young C57BL/6 mice were transplanted into young DBA/2J mice and mean survival times (MST) were analyzed. (n=7/group; **P<0.01; data are representative of two independent sets of experiments). b, 7 days after transplantation, mice were euthanized and transplanted hearts were procured; H&E staining was performed to asses lymphocytic infiltration and structural changes; samples were scored based on the International Society for Heart and Lung Transplantation (ISHLT) rejection score (n=8/group, magnification, 10x; *P<0.05). c, Paraffin sections were stained for CD4/CD8 to assess lymphocytic infiltration; data is shown as cells per high-power field (HPF) (n=8/group; *P<0.05; data are representative of two independent sets of experiments).
Accelerated rejection of cardiac allografts from old donors is associated with an enhanced systemic alloimmune response
To determine donor age-related effects on systemic alloimmune responses within recipients, frequencies of splenic CD4+/CD8+ effector T cells and CD4+ regulatory T cells (Tregs) were assessed by day 7 after transplantation. While frequencies of CD4+CD25+FoxP3+ Tregs were age-independent, recipients of cardiac allografts from old donors showed significantly increased frequencies of CD8+CD44highCD62low and higher rates of CD4+CD44highCD62low effector T cells (Supplemental Fig. 1a). Importantly, transplantation of old cardiac allografts also led to elevated frequencies of CD8+IFNγ+ T cells among recipient splenocytes as assessed by intracellular cytokine staining (Supplemental Fig. 1b).
Properties of recipient splenocytes were further characterized in vitro by restimulation with donor-type antigen and subsequent measurements of cytokine production and proliferative responses. ELISpot data indicated increased frequencies of responder T cells that produced IFNγ and IL-6 upon donor- antigen stimulation (Supplemental Fig. 1c). Moreover, an increased proliferation of alloreactive splenocytes responding to donor-type antigen was noted (Supplemental Fig. 1d). Taken together, these results indicated that older cardiac allografts enhanced systemic alloimmune responses of recipient mice.
Passenger leucocytes mediate donor age-dependent transplant survival and alloimmune responses
Passenger leukocytes have been shown to play an important role in allograft rejection 9-11. The age of passenger leukocytes has previously been shown to impact allograft rejection 12-14. However, age-related changes in parenchymal tissue may also be of relevance in allograft rejection 15-17. Thus, to delineate whether the observed effects of donor age on allograft survival and systemic alloimmune responses were linked to age-dependent changes in parenchymal tissue or mediated by age-dependent modifications of passenger leukocytes residing within the graft, we generated chimeric C57BL/6 donor animals through lethal total body irradiation and subsequent hematopoietic reconstitution with bone marrow procured from young naïve syngeneic C57BL/6 mice. By 6 weeks, young or old hearts from chimeric animals where transplanted into young WT DBA/2J animals. To validate this chimeric model, CD11c+ staining was performed 6 weeks after repopulation (Fig. 2a).
Figure 2.
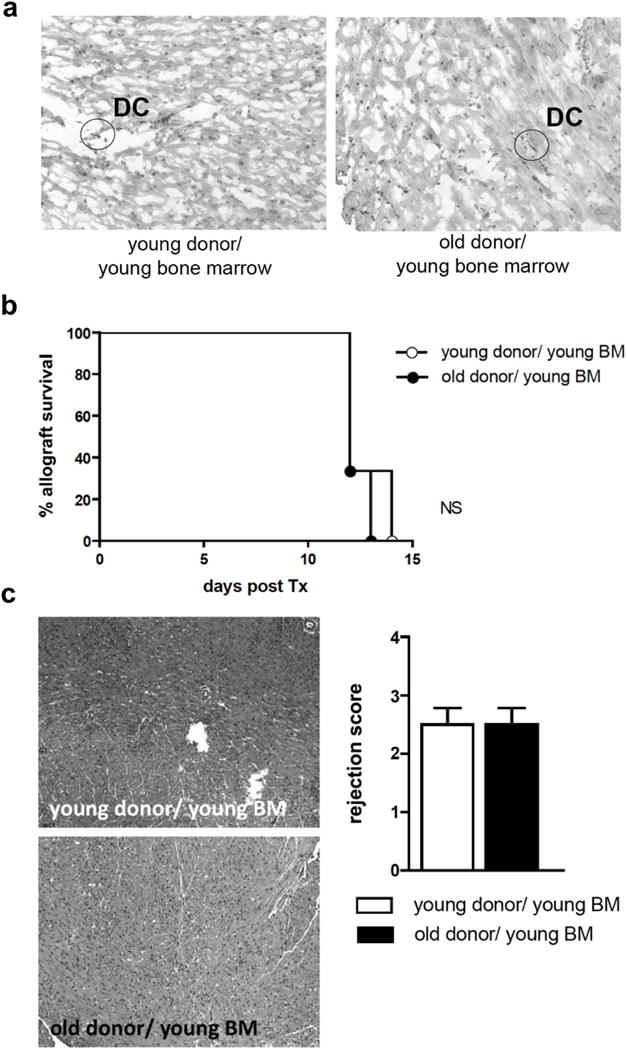
Old cardiac allografts from chimeric animals showed survival and rejection scores comparable to young cardiac allografts. Young and old C57BL/6 mice were lethally irradiated (11 Gy), thus eliminating passenger leukocytes. 24 hours after irradiation, bone marrow from young syngeneic C57BL/6 mice was injected intravenously to reconstitute passenger leukocytes within donor hearts. a, immunostaining for CD11c was performed 6 weeks after bone marrow transplantation to confirm successful repopulation of prospective donor hearts with DCs (representative slides; 10x). b-c, Hearts from young and old chimeric animals were transplanted into young WT DBA/2J mice; (b) mean survival time and (c) rejection scores by H&E staining were analyzed. (n=4/group; NS, non-significant; data are representative of two independent sets of experiments).
When chimeric old or young hearts repopulated with young passenger leukocytes had been transplanted into young DBA/2J mice, graft survival was no longer donor age-dependent (Fig. 2b). Similarly, histomorphological changes, as assessed by ISHLT rejection scores at day 7, were no longer age-dependent (Fig. 2c). Moreover, systemic immune responses following the engraftment of chimeric grafts demonstrated comparable frequencies of CD4+ and CD8+ effector T cells (Supplemental Fig. 2a). No significant difference was observed in frequencies of CD4+CD25+FoxP3+ cells (Supplemental Fig. 2a). In addition, following re-stimulation of recipient responder splenocytes with donor-type antigen, frequencies of IFNγ-producing alloreactive splenocytes did not differ in recipients of either old or young chimeric hearts (Supplemental Fig. 2b). Of note, there was a significant decrease in frequencies of IL-6 producing alloreactive splenocytes in recipients of old chimeric hearts (Supplemental Fig. 2b). Moreover, proliferation rates of recipient-derived splenocytes following transplantation of chimeric hearts were independent of organ age (Supplemental Fig. 2c).
Collectively, these results suggest that the impact of donor age on alloimmune responses is not related to the age of cardiac parenchyma but rather to the age of passenger leukocytes.
CD11c+dendritic cells mediate donor age-related effects of augmented alloimmune responses
Due to their ability to migrate to secondary lymphoid tissues and to present alloantigens, donor DCs are considered instigators of adaptive alloimmune responses 10,11,18. Thus, to determine whether CD11c+ DCs play a role in mediating the observed augmented alloimmune response when transplanting older organs, donor DCs were depleted by liposomal clodronate prior to transplantation. Consistent with previous studies 19,20, liposomal clodronate pretreatment resulted in a dramatic depletion (>98%) of CD11c+ DCs in donor hearts (Fig. 3a). Of note, pretreatment with liposomal clodronate had only minor effects on macrophage populations residing within cardiac tissue (Fig. 3a). Next, CD11c+ DC-depleted donor cardiac allografts were transplanted into young recipients and survival was assessed. Depletion of CD11c+ DCs prolonged survival of old cardiac allografts to levels comparable to that observed when young allografts had been transplanted (Fig. 3b). Moreover, ISHLT rejection scores were comparable in both, young and old cardiac allografts subsequent to clodronate treatment (Fig. 3c). In addition, systemic alloimmune responses were age-independent following donor treatment with clodronate as assessed by splenic T cell populations (Supplemental Fig. 3a), intracellular cytokine staining (Supplemental Fig. 3b), frequencies of IFNγ and IL-6 producing alloreactive splenocytes (Supplemental Fig. 3c), and proliferation of alloreactive splenocytes (Supplemental Fig. 3d). Taken together, these findings suggest that intragraft CD11c+ DCs are the primary mediators of age-dependent immune responses and accelerated allograft rejection observed when transplanting old cardiac allografts.
Figure 3.
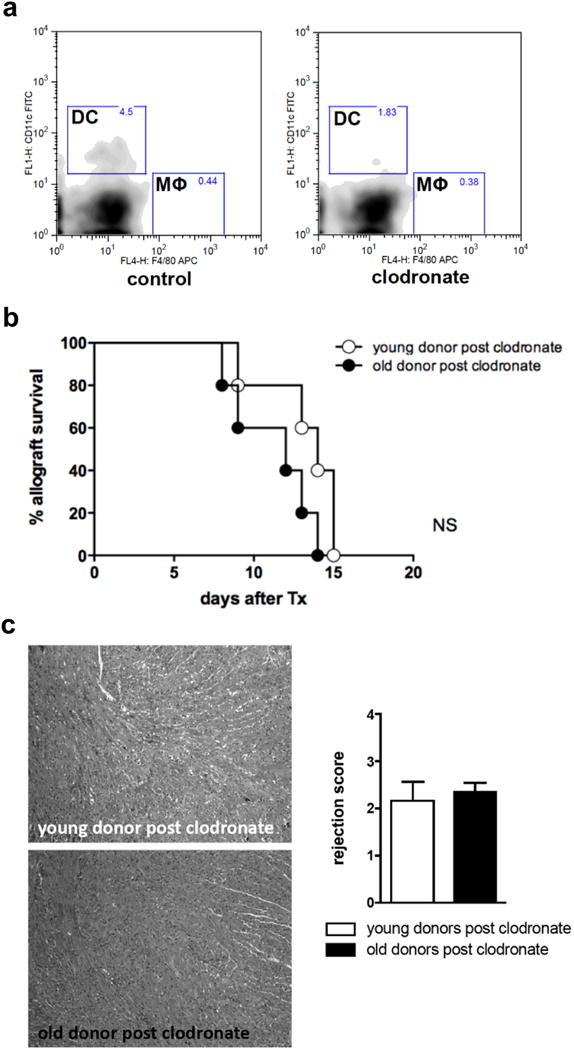
Clodronate depletion of DCs in older donors abolished previously observed accelerated rejection in younger recipients. Young and old C57BL/6 donors were treated intravenously with liposomal clodronate at -8, -5 and -1 days prior to graft procurement. a, This regimen insured depletion of intragraft CD11c+ DCs as documented by flow cytometry;, empty liposomes without clodronate were used as controls (n=3). b, DC-depleted hearts from young and old donors were transplanted into young DBA/2J mice and (b) mean survival time as well as (c) rejection scores as determined by H&E staining were analyzed. (n=5/group; NS, non significant; data are representative of two independent sets of experiments).
Aged CD11c+dendritic cells demonstrate increased allostimulatory capacities in vitro
To investigate mechanisms of donor DC-mediated effects on alloimmunity in detail and to delineate the impact of immunosenescence on DCs, flow-sorted naïve splenic CD11c+ DCs were characterized. Both naïve and LPS-stimulated CD11c+ DCs isolated from old C57BL/6 mice used as allogeneic stimulators to splenocytes from young naïve DBA/2J mice evoked significantly stronger alloimmune responses with elevated frequencies of IFNγ-producing responder cells (Fig. 4a) in addition to increased proliferation rates (Fig. 4b). Pre-activation with LPS increased the overall allostimulatory level of both young and old DCs in a dose-dependent manner. Moreover, flow cytometry revealed a significant increase in surface expression of activation and maturation markers including I-Ab (MHC-II), CD40, CD80, and CD86 on old DCs (Fig. 4c), supporting the concept of intrinsic functional modifications in older DCs.
Figure 4.
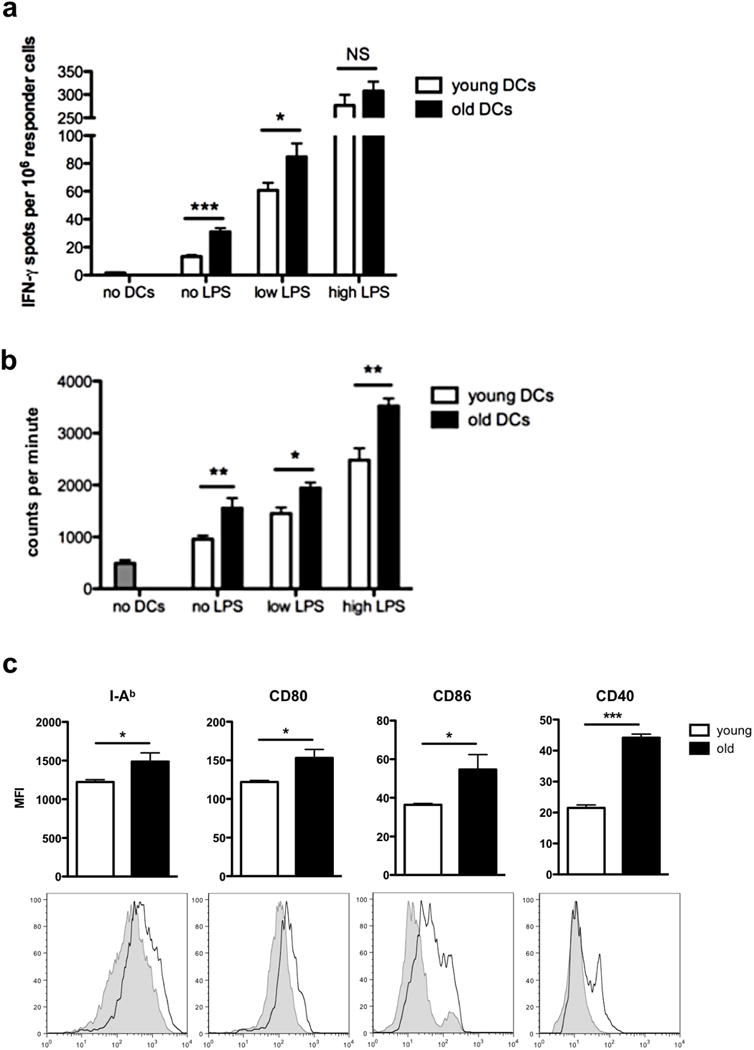
Old DCs induce enhanced alloimmune responses. a-b, flow-sorted splenic CD11c+ DCs from either young or old naïve C57BL/6 mice were used as allogeneic stimulators of young DBA/2 splenocytes in ELISpot and proliferation assays either without activation or lipopolysaccharide (LPS) activation at low (10ng/ml) and high (100ng/ml) concentrations; (a) frequencies of IFNγ-producing responder splenocytes (n=6/group; *P<0.05; ***P<0.001; experiments were performed in triplicates; data are representative of three independent sets of experiments) and (b) proliferation rates of responder splenocytes by 3H thymidine incorporation (n=6/group; *P<0.05; **P<0.001; experiments performed in triplicates; data are representative of three independent sets of experiments) were analyzed. c, Characterization of activation and maturation markers (I-Ab/MHC-II, CD40, CD80, and CD86) was performed using mean fluorescence intensity (MFI) (n=3/group; *P<0.05; ***P<0.001; grey tinted: young DCs, black line: old DCs; representative histogram plots of three independent sets of experiments are shown).
IL-17A is critical in enhancing the rejection of older allografts
Elevated frequencies of Th17 cells have been observed in old mice and humans 21,22. More importantly, Th17 cells have been linked to critical pathways in allograft rejection 23. To dissect the role of Th17 cells in this process in more detail, we explored whether elevated levels of intragraft IL-17A were immanent to organ age per se or linked to age-dependent alloimmune responses elicited by old passenger leukocytes residing within the graft.
Cardiac allografts were collected prior to rejection (day 7) and IL-17A mRNA levels were quantified by real-time PCR. Results indicated a dramatic increase in IL-17A mRNA levels in old cardiac allografts (> 30-fold increase, Fig. 5a). Of note, old and young cardiac allografts showed equally low levels of IL-17A mRNA following the engraftment of organs that were pre-treated with liposomal clodronate (Fig. 5a). Next, isolated DCs of hearts from old (18 months) or young (8-12 weeks) C57BL/6 mice were co-cultured with isolated naïve CD4+ cells (ratio 1:5) for 7 days in media alone, under Th17 polarizing conditions alone, in LPS alone or under Th17 polarizing conditions in the presence of LPS. LPS-activated DCs from older cardiac allografts in Th17-polarizing conditions increased frequencies of CD4+IL-17A+ T cells dramatically (49.3% vs. 24.2% in DCs from younger hearts; P<0.0001, Fig. 5b). Of note, neither old or young DCs in media alone, Th17 polarizing conditions alone or in the presence of LPS alone demonstrated significant differences in the proliferation of CD4+IL-17A+ T cells, suggesting that both, activation of donor DCs and Th17 polarizing conditions are required during the rejection of older allografts. These results were confirmed by ELISA (Fig. 5c). Taken together, these findings suggest a prominent role for IL-17A in mounting an augmented immune response linked to the engraftment of older donor organs.
Figure 5.
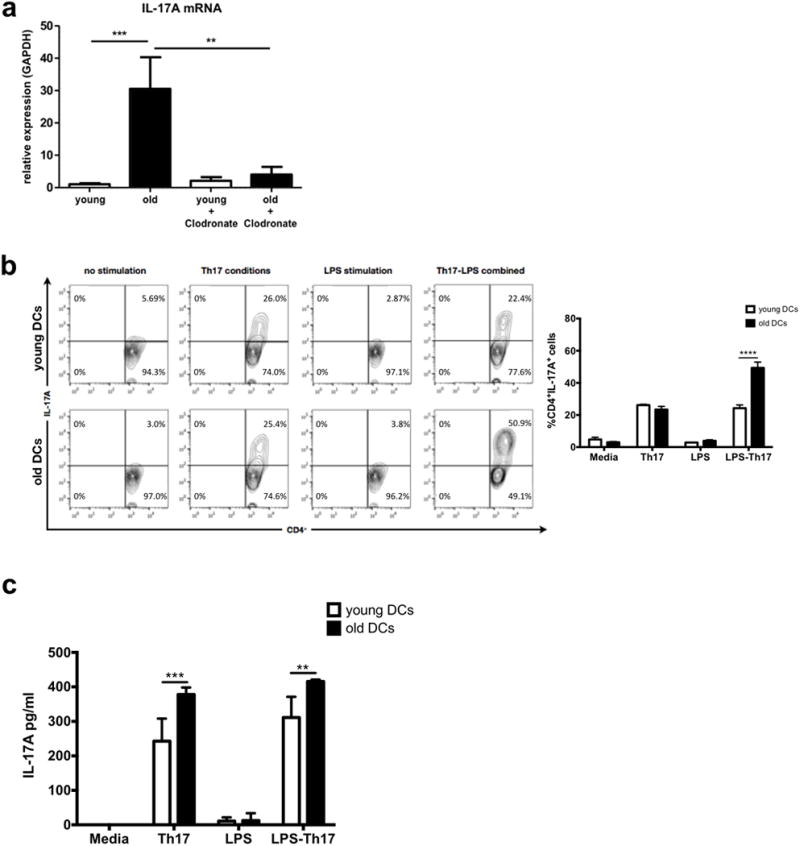
IL-17A has a critical role in age-dependent immune responses. a, 7 days after transplantation hearts were procured and intragraft levels of IL-17A were analyzed by real-time PCR (n=4/group; **, p<0.01; ***, p<0.001). b-c, DCs were isolated from old and young hearts (C57BL/6) and co-cultured with naïve CD4+ cells isolated from spleens (DBA/2J) in media alone, in Th17-polarizing conditions alone, in presence of lipopolysaccharide (LPS) alone or under Th17 polarizing conditions in presence of LPS. After 7 days, (b) frequencies of CD4+IL-17A+ cells were assessed by flow cytometry gating on CD4+ cells and (c) cytokine secretion was assessed by ELISA (n=3/group; **P<0.01, ***P<0.001 ****P<0.0001).
Blocking IL-17 in recipient animals prolongs the survival of old but not young cardiac allografts
Our results thus far suggested a critical role of IL-17A in alloimmune responses subsequent to the transplantation of older grafts. To evaluate the role of IL-17A in vivo, we first performed a functional blockade of IL-17A by treating recipients of young or old C57BL/6 allografts with anti-IL-17A starting at the day of transplantation. Survival of older allografts was significantly prolonged subsequent to the application of anti-IL-17A. Of note, anti-IL-17A treatment did not significantly prolong the survival of young allografts (Fig. 6a).
Figure 6.
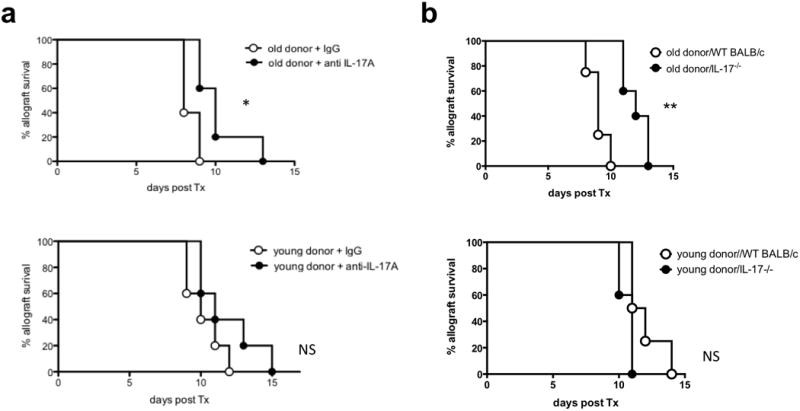
Targeting IL-17A in solid organ transplantation abolished donor age-related alloimmune responses. a, hearts from old and young C57BL/6 mice were transplanted into young DBA/2J recipient mice; recipient mice were treated with 100μg of anti-IL-17A or with isotype control antibodies (IgG) every other day starting at day 0 and mean survival times (MST) were analyzed (n=5/group; *P<0.05; NS=non-significant). b, hearts from old and young C57BL/6 mice were transplanted into young BALB/c IL-17A-/-mice and MST was analyzed (n=4-5/group; *P<0.05).
To explore the critical role of IL-17A in vivo further, hearts of old and young donors were transplanted into IL-17-/- BALB/c mice. Indeed, the absence of IL-17 prolonged graft survival of old hearts (MST 12 vs. 9 days in IL-17-/- mice compared to WT recipients; P<0.05, Fig. 6b), while survival of young cardiac allografts remained unaltered (WT vs. IL-17-/- mice recipients; MST 11.5 vs. 11 days; NS; Fig. 6c).
These in vivo results emphasize the prominent role for IL-17A in donor age-dependent alloimmune responses.
Discussion
The growing discrepancy between demand and supply in organ transplantation has resulted in an increased utilization of organs from old donors 24,25. Over the last decade, more than half of all transplanted kidneys were procured from donors older than 50 years 26. At the same time, transplant outcomes of extended criteria donor organs were found to be inferior to those observed in organs from younger donors 12. In cardiac transplantation, donations have dropped dramatically since 2000. While significantly more older recipients are receiving cardiac transplants, the age of heart donors is in general lower compared to the age of other solid organ transplant donors 27. Nevertheless, understanding age-specific aspects of immunogenicity and repair may provide desperately needed novel resources for cardiac transplantation. In a large retrospective analysis, we have recently shown that the transplantation of older organs is linked to more frequent rates of acute rejection 3. Despite vast clinical implications, experimental studies dissecting the effects of donor age on allograft immunogenicity and recipient immune responses remain scarce 28. Thus, dissecting mechanistic aspects of donor age-related immune responses appears of major clinical relevance.
Our experimental data reflect clinical observations showing that older organs are rejected earlier. Albeit small, the difference in graft survival of 2 days had been highly significant. We wish to point out that we used a cardiac transplant model with a fully MHC-mismatched donor-/recipient combination leading to graft dysfunction subsequent to acute rejection in less than 2 weeks. Moreover, aspects of age-dependent injury/repair, functional reserve or mismatching in donor/recipient size are likely to be superseded by the vigorous alloimmune response in this model.
Furthermore, using a chimeric mouse model, we demonstrated that age-dependent alloimmune responses were not linked to the age of cardiac parenchyma. This model of complete depletion of intragraft leukocytes through lethal total-body irradiation and subsequent reconstitution with leukocytes derived from transplanted bone marrow has been established and used extensively by other investigators in the analysis of repopulation and residual APCs using flow cytometry and RT-PCR 29,30. For this work, repopulation of prospective allografts was confirmed by immunohistochemical staining (Fig. 2a).
Impaired graft function correlated with organ age is most likely related to both, limited functional reserve and augmented immunogenicity. Physiological changes related to aging have been put forward as non-immunological factors influencing graft survival. In theory, increased susceptibility to tissue damage during the transplantation procedure subsequent to ischemia in addition to impaired repair mechanisms may be linked to a pro-inflammatory milieu including increased DAMP signaling, eliciting and perpetuating secondary allospecific immune responses. While physiological aspects of aging may, at least in theory, impact organ function following transplantation, our results suggest that the age of cardiac parenchyma per se did not mediate donor age-dependent differences of alloimmunity, as old donor hearts containing young passenger leukocytes did not augment alloimmune responses.
Previous publications have illustrated the central role of IFNγ as well as CD4+ and CD8+ cells in allograft rejection 23,31,32. In these studies, a feedback loop leading to the production of IL-2 by Th1 cells after allogenic stimulation has been characterized. Those events promote alloreactive cytotoxic CD8+ cells, which in turn produce IFNγ, enhancing Th1 responses even further. Our data confirm these findings by showing increased frequencies of CD4+IFNγ+ and CD8+IFNγ+ effector cells subsequent to the engraftment of both, young and old cardiac allografts. Of note, we observed higher frequencies of CD8+IFNγ+ T cells when old allografts were transplanted.
Recent clinical and experimental studies have shown the relevance of a novel CD4+ T helper population coined Th17 as instigators of allograft rejections. Th17 cells have been characterized by their signature cytokine IL-17A, a potent pro-inflammatory cytokine 33-35. Of note, studies outside of transplantation have shown that aging is associated with a general increase in levels of Th17 cells 36-38. However, the role of Th17 responses with respect to organ age remains unclear. Our results show a significant increase of intragraft IL-17A mRNA levels in old transplanted allografts. These results suggest a strong relationship between IFNγ-producing CD4+ and CD8+ as well as IL-17A-producing CD4+ cells in accelerating the rejection of older organs. To characterize the functional relevance and the origin of the highly elevated IL-17A levels in older allografts in more detail, we co-cultured intragraft DCs of old and young C57BL/6 mice with splenic naïve CD4+ cells. Those experiments allowed us to distinguish whether elevated IL-17 levels were intrinsically related to aging or linked to the encounter with old passenger DCs. Our data demonstrated a significant increase of CD4+IL-17A+ cells after DC-activation in Th17-polarizing conditions, thus confirming that old passenger DCs trigger a more potent IL-17A response. The role of IL-17A in allograft rejection was subsequently tested in recipient animals deficient in IL-17A or those that were pretreated with anti-IL-17A. Both recipients showed not only an improved allograft survival compared to untreated controls but also a prolonged survival of older compared to younger allografts, thus demonstrating the critical role of IL-17A in the rejection of older grafts. Taken together, these results underscore a novel and clinical relevant role of IL-17A in the rejection of older allografts.
It is recognized that donor-derived APCs residing in the interstitium of allografts, in particular macrophages and DCs, are instigators of primary allospecific immune responses via direct alloantigen presentation to recipient responder cells within secondary lymphatic tissue 11,14,39. However, due to their potent ability to instigate an adaptive immune response by activating naïve T cells, DCs are generally regarded as primary initiators of allograft rejection 39,40. Both, clinical and experimental studies have shown that old APCs elicit enhanced alloimmune responses. Moreover, APCs collected from healthy elderly individuals co-cultured with purified T cells have elicited enhanced T cell proliferation in both, syngeneic and allogeneic settings 41. However, these studies failed to characterize phenotypic and functional aspects driving this process. In our studies, we focused on the role of DCs and characterized phenotypic and functional aspects. Our functional assays demonstrated that old DCs increased the proliferation and production of IFNγ in allogeneic responder cells (CD4+ and CD8+ cells). Of note, augmented IFNγ production was also observed subsequent to a stimulation with LPS, suggesting age-dependent allostimulatory capacities of old DCs even following unspecific preactivation. Moreover, depletion of old CD11c+ DCs by liposomal clodronate treatment blocked the recipients' Th17 response and extended allograft survival, suggesting that DCs play a central role in this process. Furthermore, old CD11c+ DCs presented advanced activation and maturity as shown by increased expression of MHC-II, CD80, CD86, and CD40. These data are in accordance with results by others, showing an increased immunogenicity of old DCs in experimental graft-versus-host-disease models 42.
Administered in a liposomal formulation, clodronate is selectively taken up by phagocytic leukocytes, particularly dendritic cells and macrophages 43. Following intracellular disruption of the liposomes through lysosomal phospholipases, accumulating clodronate leads to selective apoptosis of target cells 44. The observed depletion of DCs in our model (Fig. 3a) may be related to decreased permeability of cardiac capillaries to liposomal clodronate 45,46. Thus, effects in our model targeted predominantly circulating phagocytic leukocytes. With the turnover of resident macrophage being much slower than that of DCs 47, we do not assume a sizable effect of clodronate on cardiac macrophage populations subsequent to 1 week of treatment. Although our detailed in vitro and in vivo analysis supported the critical role of old DCs as instigators of organ age-dependent alloimmune responses, we cannot entirely rule out that other APCs including macrophages have been playing a role in organ age-dependent immune responses.
Although data originating from experimental models have inherent shortcomings and might not always imply biological or even clinical significance, this work explains mechanistic findings of a previous clinical observation. Our experimental data show that donor age impacts not only organ quality but also recipient alloimmune responses through passenger leukocytes independent of unspecific or procedure-related injuries. This observation is intriguing as therapeutically interventions may influence graft survival and overall transplant outcome. Furthermore, we were able to identify the critical role of the age of DCs rather than organ age per se as a driving force of organ age-dependent alloimmune responses. These findings may not only advance our overall understanding of allograft rejection, but can potentially also lead to new specific translational strategies in recipients of older cardiac transplants.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by grants from the NIH (RO1AG039449) and the Carlos Slim Foundation de la Salud. R.A. was supported by a grant from the NIH (5R01AI091930-04).
Footnotes
Disclosures: None.
References
- 1.Kocot A, Giessing M. Increasing the donor and recipient pool-expanded criteria in living kidney donors. Transplant Proc. 2013;45:1245–1247. doi: 10.1016/j.transproceed.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 2.Cevenini E, Monti D, Franceschi C. Inflamm-ageing. Curr Opin Clin Nutr Metab Care. 2013;16:14–20. doi: 10.1097/MCO.0b013e32835ada13. [DOI] [PubMed] [Google Scholar]
- 3.Tullius SG, Milford E. Kidney Allocation and the Aging Immune Response. N Engl J Med. 2011;364:1369–1370. doi: 10.1056/NEJMc1103007. [DOI] [PubMed] [Google Scholar]
- 4.Romanyukha AA, Yashin AI. Age related changes in population of peripheral T cells: towards a model of immunosenescence. Mech Ageing Dev. 2003;124:433–443. doi: 10.1016/s0047-6374(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 5.Rink L, Cakman I, Kirchner H. Altered cytokine production in the elderly. Mech Ageing Dev. 1998;102:199–209. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Rodríguez L, López-Hoyos M, Muñoz-Cacho P, Martínez-Taboada VM. Aging is associated with circulating cytokine dysregulation. Cell Immunol. 2012;273:124–132. doi: 10.1016/j.cellimm.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Liao W, Lin JX, Wang L, Li P, Leonard WJ. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat Immunol. 2011;12:551–559. doi: 10.1038/ni.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang MC, Liao JJ, Bonasera S, Longo DL, Goetzl EJ. Nuclear factor-kappaB-dependent reversal of aging-induced alterations in T cell cytokines. FASEB J. 2008;22:2142–2150. doi: 10.1096/fj.07-103721. [DOI] [PubMed] [Google Scholar]
- 9.Steinmuller D. Passenger leukocytes and the immunogenicity of skin allografts. J Invest Dermatol. 1980;75:107–115. doi: 10.1111/1523-1747.ep12521331. [DOI] [PubMed] [Google Scholar]
- 10.Sekine Y, Bowen LK, Heidler KM, Van Rooijen N, Brown JW, Cummings OW, Wilkes DS. Role of passenger leukocytes in allograft rejection: effect of depletion of donor alveolar macrophages on the local production of TNF-alpha, T helper 1/T helper 2 cytokines, IgG subclasses, and pathology in a rat model of lung transplantation. J Immunol. 1997;159:4084–4093. [PubMed] [Google Scholar]
- 11.Kreisel D, Petrowsky H, Krasinskas AM, Krupnick AS, Szeto WY, McLean AD, Popma SH, Gelman AE, Traum MK, Furth EE, Moore JS, Rosengard BR. The role of passenger leukocyte genotype in rejection and acceptance of rat liver allografts. Transplantation. 2002;73:1501–1507. doi: 10.1097/00007890-200205150-00022. [DOI] [PubMed] [Google Scholar]
- 12.Reutzel-Selke A, Jurisch A, Denecke C, Pascher A, Martins PNA, Kessler H, Tamura A, Utku N, Pratschke J, Neuhaus P, Tullius SG. Donor age intensifies the early immune response after transplantation. Kidney Int. 2007;71:629–636. doi: 10.1038/sj.ki.5002098. [DOI] [PubMed] [Google Scholar]
- 13.Reutzel-Selke A, Filatenkov A, Jurisch A, Denecke C, Martins PNA, Pascher A, JONAS S, Pratschke J, Neuhaus P, Tullius SG. Grafts from elderly donors elicit a stronger immune response in the early period posttransplantation: a study in a rat model. Transplant Proc. 2005;37:382–383. doi: 10.1016/j.transproceed.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Denecke C, Ge X, Jurisch A, Kleffel S, Kim IK, Padera RF, Weiland A, Fiorina P, Pratschke J, Tullius SG. Modified CD4(+) T-cell response in recipients of old cardiac allografts. Transpl Int. 2012;25:328–336. doi: 10.1111/j.1432-2277.2011.01417.x. [DOI] [PubMed] [Google Scholar]
- 15.Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev. 2007;128:36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 17.Slegtenhorst BR, Dor FJMF, Elkhal A, Rodriguez H, Yang X, Edtinger K, Quante M, Chong AS, Tullius SG. Mechanisms and Consequences of Injury and Repair in Older Organ Transplants. Transplantation. 2014;97:1091–1099. doi: 10.1097/TP.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lechler R, Ng WF, Steinman RM. Dendritic cells in transplantation--friend or foe? Immunity. 2001;14:357–368. doi: 10.1016/s1074-7613(01)00116-9. [DOI] [PubMed] [Google Scholar]
- 19.Ward NL, Loyd CM, Wolfram JA, Diaconu D, Michaels CM, McCormick TS. Depletion of antigen-presenting cells by clodronate liposomes reverses the psoriatic skin phenotype in KC-Tie2 mice. Br J Dermatol. 2011;164:750–758. doi: 10.1111/j.1365-2133.2010.10129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferenbach DA, Sheldrake TA, Dhaliwal K, Kipari TMJ, Marson LP, Kluth DC, Hughes J. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int. 2012;82:928–933. doi: 10.1038/ki.2012.207. [DOI] [PubMed] [Google Scholar]
- 21.Lee JS, Lee WW, Kim SH, Kang Y, Lee N, Shin MS, Kang SW, Kang I. Age-associated alteration in naive and memory Th17 cell response in humans. Clin Immunol. 2011;140:84–91. doi: 10.1016/j.clim.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim MA, Lee J, Park JS, Jhun JY, Moon YM, Cho ML, Kim HY. Increased Th17 differentiation in aged mice is significantly associated with high IL-1β level and low IL-2 expression. Exp Gerontol. 2014;49:55–62. doi: 10.1016/j.exger.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Fan H, Jiang S. CD4(+) T-cell subsets in transplantation. Immunol Rev. 2013;252:183–191. doi: 10.1111/imr.12038. [DOI] [PubMed] [Google Scholar]
- 24.Wittwer T, Wahlers T. Marginal donor grafts in heart transplantation: lessons learned from 25 years of experience. Transpl Int. 2008;21:113–125. doi: 10.1111/j.1432-2277.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- 25.Livi U, Bortolotti U, Luciani GB, Boffa GM, Milano A, Thiene G, Casarotto D. Donor shortage in heart transplantation. Is extension of donor age limits justified? J Thorac Card Surg. 1994;107:1346–54. discussion 1354–5. [PubMed] [Google Scholar]
- 26.Fritsche L, Hörstrup J, Budde K, Reinke P, Giessing M, Tullius S, Loening S, Neuhaus P, Neumayer HH, Frei U. Old-for-old kidney allocation allows successful expansion of the donor and recipient pool. Am J Transplant. 2003;3:1434–1439. doi: 10.1046/j.1600-6135.2003.00251.x. [DOI] [PubMed] [Google Scholar]
- 27.Colvin Adams M, Smithy JM, Heubner BM, Skeans MA, Edwards LB, Waller C, Schnitzler MA, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2012 Annual Data Report: heart. Am J Transplant. 2013;14(Suppl 1):113–138. doi: 10.1111/ajt.12583. [DOI] [PubMed] [Google Scholar]
- 28.Holinski S, Modersohn D, Proch C, Meyer R, Konertz W. Age-dependent chronic rejection after experimental heart transplantation. J Heart Lung Transplant. 2006;25:1099–1102. doi: 10.1016/j.healun.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Eiref, Zhang, Popma, Shah, Moore, Rosengard Creation of Chimeric Hearts: A Tool for Testing the “Passenger Leukocyte” Hypothesis. Ann Thorac Surg. 1997;64:6–6. doi: 10.1016/s0003-4975(97)00617-6. [DOI] [PubMed] [Google Scholar]
- 30.Krasinskas AM, Eiref SD, McLean AD, Kreisel D, Gelman AE, Popma SH, Moore JS, Rosengard BR. Replacement of graft-resident donor-type antigen presenting cells alters the tempo and pathogenesis of murine cardiac allograft rejection. Transplantation. 2000;70:514–521. doi: 10.1097/00007890-200008150-00020. [DOI] [PubMed] [Google Scholar]
- 31.He C, Heeger PS. CD8 T Cells Can Reject Major Histocompatibility Complex Class I-Deficient Skin Allografts. Am J Transplant. 2004;4:698–704. doi: 10.1111/j.1600-6143.2004.00416.x. [DOI] [PubMed] [Google Scholar]
- 32.Jiang S, Herrera O, Lechler RI. New spectrum of allorecognition pathways: implications for graft rejection and transplantation tolerance. Curr Opin Immunol. 2004;16:550–557. doi: 10.1016/j.coi.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Syrjälä SO, Keränen MAI, Tuuminen R, Nykänen AI, Tammi M, Krebs R, Lemström KB. Increased Th17 rather than Th1 alloimmune response is associated with cardiac allograft vasculopathy after hypothermic preservation in the rat. J Heart Lung Transplant. 2010;29:1047–1057. doi: 10.1016/j.healun.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Deteix C, Attuil-Audenis V, Duthey A, Patey N, McGregor B, Dubois V, Caligiuri G, Graff-Dubois S, Morelon E, Thaunat O. Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J Immunol. 2010;184:5344–5351. doi: 10.4049/jimmunol.0902999. [DOI] [PubMed] [Google Scholar]
- 35.Tsaur I, Gasser M, Aviles B, Lutz J, Lutz L, Grimm M, Lange V, Lopau K, Heemann U, Germer CT, Chandraker A, Waaga-Gasser AM. Donor antigen-specific regulatory T-cell function affects outcome in kidney transplant recipients. Kidney Int. 2011;79:1005–1012. doi: 10.1038/ki.2010.533. [DOI] [PubMed] [Google Scholar]
- 36.André S, Tough DF, Lacroix-Desmazes S, Kaveri SV, Bayry J. Surveillance of antigen-presenting cells by CD4+ CD25+ regulatory T cells in autoimmunity: immunopathogenesis and therapeutic implications. Am J Pathol. 2009;174:1575–1587. doi: 10.2353/ajpath.2009.080987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 38.Schmitt V, Rink L, Uciechowski P. The Th17/Treg balance is disturbed during aging. Exp Gerontol. 2013;48:1379–1386. doi: 10.1016/j.exger.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 39.O'Connell PJ, Burlingham WJ. Donor dendritic cell persistence in organ allograft recipients in the absence of immunosuppression. J Leukoc Biol. 1999;66:301–305. doi: 10.1002/jlb.66.2.301. [DOI] [PubMed] [Google Scholar]
- 40.Wyburn KR, Jose MD, Wu H, Atkins RC, Chadban SJ. The role of macrophages in allograft rejection. Transplantation. 2005;80:1641–1647. doi: 10.1097/01.tp.0000173903.26886.20. [DOI] [PubMed] [Google Scholar]
- 41.Castle SC, Uyemura K, Crawford W, Wong W, Makinodan T. Antigen presenting cell function is enhanced in healthy elderly. Mech Ageing Dev. 1999;107:137–145. doi: 10.1016/s0047-6374(98)00141-9. [DOI] [PubMed] [Google Scholar]
- 42.Ordemann R, Hutchinson R, Friedman J, Burakoff SJ, Reddy P, Duffner U, Braun TM, Liu C, Teshima T, Ferrara JLM. Enhanced allostimulatory activity of host antigen-presenting cells in old mice intensifies acute graft-versus-host disease. J Clin Invest. 2002;109:1249–1256. doi: 10.1172/JCI14793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naito M, Nagai H, Kawano S, Umezu H, Zhu H, Moriyama H, Yamamoto T, Takatsuka H, Takei Y. Liposome-encapsulated dichloromethylene diphosphonate induces macrophage apoptosis in vivo and in vitro. J Leukoc Biol. 1996;60:337–344. doi: 10.1002/jlb.60.3.337. [DOI] [PubMed] [Google Scholar]
- 44.Rabinovich GA, Riera CM, Iribarren P. Granulocyte-macrophage colony-stimulating factor protects dendritic cells from liposome-encapsulated dichloromethylene diphosphonate-induced apoptosis through a Bcl-2-mediated pathway. Eur J Immunol. 1999;29:563–570. doi: 10.1002/(SICI)1521-4141(199902)29:02<563::AID-IMMU563>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 45.Danenberg HD, Fishbein I, Gao J, Mönkkönen J, Reich R, Gati I, Moerman E, Golomb G. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation. 2002;106:599–605. doi: 10.1161/01.cir.0000023532.98469.48. [DOI] [PubMed] [Google Scholar]
- 46.van Rooijen N, Hendrikx E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol. 2010;605:189–203. doi: 10.1007/978-1-60327-360-2_13. [DOI] [PubMed] [Google Scholar]
- 47.Spencer SC, Fabre JW. Characterization of the tissue macrophage and the interstitial dendritic cell as distinct leukocytes normally resident in the connective tissue of rat heart. J Exp Med. 1990;171:1841–1851. doi: 10.1084/jem.171.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


