Abstract
Objective
Evidence suggests protective effects of vitamin D and anti-tumour immunity on colorectal cancer risk. Immune cells in tumour microenvironment can convert 25-hydroxyvitamin D [25(OH)D] to bioactive 1α,25-dihydroxyvitamin D3, which influences neoplastic and immune cells as an autocrine and paracrine factor. Thus, we hypothesised that the inverse association between vitamin D and colorectal cancer risk might be stronger for cancers with high-level immune response than those with low-level immune response.
Design
We designed a nested case-control study (318 rectal and colon carcinoma cases and 624 matched controls) within the Nurses’ Health Study and Health Professionals Follow-up Study, using molecular pathological epidemiology database. Multivariable conditional logistic regression was used to assess the association of plasma 25(OH)D with tumour subtypes according to the degree of lymphocytic reaction, tumour-infiltrating T-cells (CD3+, CD8+, CD45RO+ and FOXP3+ cells), microsatellite instability, or CpG island methylator phenotype.
Results
The association of plasma 25(OH)D with colorectal carcinoma differed by the degree of intratumoural periglandular reaction (Pheterogeneity=0.001); high 25(OH)D was associated with lower risk of tumour with high-level reaction [comparing the highest vs. lowest tertile: odds ratio, 0.10; 95% confidence interval, 0.03 to 0.35; Ptrend<0.001], but not risk of tumour with lower-level reaction (Ptrend>0.50). A statistically non-significant difference was observed for the associations of 25(OH)D with tumour subtypes according to CD3+ T-cell density (Pheterogeneity=0.03; adjusted statistical significance level of α=0.006).
Conclusion
High plasma 25(OH)D level is associated with lower risk of colorectal cancer with intense immune reaction, supporting a role of vitamin D in cancer immunoprevention through tumour-host interaction.
Keywords: 25-hydroxyvitamin D, anticancer immunity, colorectal cancer, epidemiology, exposure, immunology, immunotherapy, nutrition
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the fourth cause of cancer death worldwide. Studies have shown that high level of circulating vitamin D is associated with lower CRC risk,[1] supporting a preventive effect of vitamin D against CRC.[2] Obtained from food, supplements or photochemical synthesis in the skin, vitamin D is hydroxylated in the liver to the major circulating form, 25-hydroxyvitamin D [25(OH)D], and further hydroxylated to the biologically active form of vitamin D, 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3] by some specific cells in the body including immune cells.[3-5]
Accumulating evidence indicates an important role of vitamin D in regulation of immune function.[6, 7] Likewise, the multifaceted roles of host immunity and inflammation in regulating tumour evolution have long been recognised.[8-12] Local immunity status in tumour microenvironment may eliminate transformed cells or promote their tumourigenic potential, thus determining the fate of emerging tumour.[13] However, despite compelling evidence for the role of vitamin D in immunity and the role of immunity in tumour development, no study has yet examined whether the inverse association between vitamin D and CRC risk differs according to CRC subtypes classified by immunity status in the tumour microenvironment. When assessing cancer immunity, it is important to examine immune cells in the tumour microenvironment, which exhibit a substantial phenotypic difference from the same immune cell type in peripheral blood.[14] We speculated that immune cells in the tumour microenvironment might augment the anti-tumour effect of vitamin D, by means of their ability to enzymatically convert 25(OH)D to 1,25(OH)2D3. Therefore, we hypothesised that the lower CRC risk associated with high-level plasma vitamin D might be stronger for CRC subtype characterised by high-level immune cell infiltrates than for other CRC subtype with low-level immune cell infiltrates.
To test this hypothesis, we investigated the association of plasma 25(OH)D levels with risk of CRC subtypes according to the pattern and intensity of lymphocytic reaction to CRC, in a nested case-control study within two large prospective cohort studies, the Nurses’ Health Study and the Health Professionals Follow-up Study. We additionally examined densities of tumourinfiltrating T-cell subsets. Higher levels of lymphocytic reaction to CRC and tumour-infiltrating T-cells have been strongly associated with survival of CRC patients independent of tumour molecular features in these two cohorts.[15, 16] The two cohort studies offered us a unique opportunity to integrate data on prediagnostic plasma vitamin D level and immune cell evaluation in CRC tissue specimens in the longitudinal follow-up scheme. This integrative approach has enabled us to provide novel population-based evidence for possible interactive roles of vitamin D and host immunity in CRC prevention.
METHODS
Study population
The Nurses' Health Study (NHS) enrolled 121,701 registered female nurses in the U.S. who were aged 30-55 years at baseline in 1976, and the Health Professionals Follow-up Study (HPFS) included 51,529 U.S. male professionals who were aged 40-75 years at baseline in 1986.[17] In both cohorts, follow-up questionnaires were administered at baseline and biennially thereafter to collect and update medical, lifestyle, and other health-related information; validated food frequency questionnaires were completed every 4 years to update dietary information. More details about the two cohorts can be found in the Supplementary materials.
In both cohorts, when participants reported a diagnosis of colon or rectal carcinoma in biennial questionnaires, we asked for permission to acquire their medical records and pathologic reports. We identified deaths, including lethal unreported CRC cases, through the National Death Index and next-of-kin. For CRC deaths, we requested permission from next-of-kin to review medical records. A study physician, blinded to 25(OH)D information, reviewed records to confirm CRC diagnosis and extract relevant information on anatomic location, stage, and histological type of the cancer.
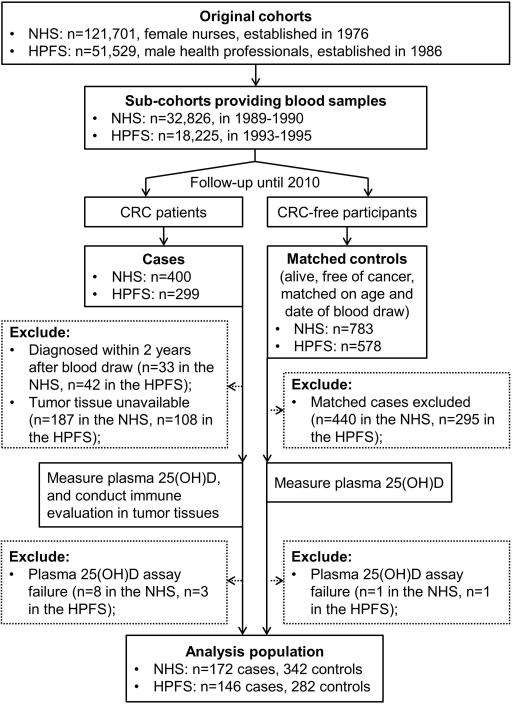
Blood specimens were collected from 32,826 women in the NHS between 1989 and 1990; and from 18,225 men in the HPFS between 1993 and 1995. The procedures for blood collection, handling and storage were similar for the two cohorts, as previously described.[18] Among participants who provided plasma samples, we documented 400 incident CRC cases in the NHS during follow-up through June 1, 2010, and 299 CRC cases in the HPFS through January 31, 2010. We collected paraffin-embedded archival tissue blocks from hospitals where participants with CRC had undergone tumour resection. For the current study, to minimize the influence of subclinical emerging tumour on plasma 25(OH)D level, we excluded CRC cases that were diagnosed within 2 years after blood draw. We also excluded cases if their plasma samples failed in 25(OH)D measurement or tumour lymphocytic reaction could not be determined. For each case, we used risk set sampling to randomly select up to 2 controls matched on sex (cohort), age (within 2 years) and year/month of blood draw (within 1 month in the same year) from eligible participants who were alive and free of cancer (except for non-melanoma skin cancer) at the time of diagnosis of the CRC case. As a result, 172 CRC cases and 342 controls from the NHS, and 146 cases and 282 controls from the HPFS were included in the analysis (Figure 1). The institutional review board at the Brigham and Women's Hospital and the Harvard School of Public Health approved this study. We obtained informed consent from all participants.
Figure 1.
Flow diagram of the nested case-control study design within the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS).
Plasma 25(OH)D assay
Plasma 25(OH)D was measured using a radioimmunosorbent assay at the laboratory of Dr. Bruce Hollis (Medical University of South Carolina, Charleston, SC) and Heartland Assays as described elsewhere.[18, 19] Samples from cases and their matched controls were handled together and analysed in the same batch. Quality control samples were randomly interspersed among the case-control samples. Personnel blinded to quality control and case-control status conducted all assays. The mean intra-assay coefficient of variation from quality control samples was <15% for all batches.
Plasma inflammatory marker assays
To account for the potential confounding effect by systemic inflammation on the plasma 25(OH)D-CRC association, we also measured three inflammatory markers in our study samples: C-reactive protein (CRP), interleukin 6 (IL6), and tumour necrosis factor receptor superfamily member 1B (TNFRSF1B, also known as soluble tumour necrosis factor receptor 2, sTNFR-2). We used a highly sensitive immunoturbidimetric assay (Denka Seiken Co, Tokyo, Japan) to measure CRP levels, an ultra-sensitive enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) to measure IL6, and an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) to measure TNFRSF1B levels. More details regarding the measurements can be found in previous publications.[20, 21]
Tumour immunity and molecular analyses
A pathologist (S.O.) evaluated tissue sections of CRC patients for the four components of lymphocytic reaction, including intratumoural periglandular reaction, tumour-infiltrating lymphocytes, Crohn's-like lymphoid reaction, and peritumoural lymphocytic reaction.[16] Each component was evaluated as absent, mild, moderate, or marked, and an agreement study was conducted as previously described.[16] In the current analyses, we combined moderate and marked lymphocytic infiltrate categories (as “high”) because of low case counts in these categories.
We also constructed tissue microarray,[22] to assess the density of tumour-infiltrating CD3+, CD8+, CD45RO+ (PTPRC), and FOXP3+ T-cells. We used immunohistochemistry techniques, an automated scanning microscope and Ariol image analysis system (Genetix, San Jose, CA, USA), to calculate the average density (cells/mm2) of each T-cell subset in tissue microarray cores, as previously described.[15] We dichotomised cases based on the density of each T-cell subset using the cutoff given in the footnote of Table 3.
Table 3.
Plasma 25-hydroxyvitamin D levels and colorectal cancer, overall and by tumour-infiltrating T-cell subset densitya
| Tertile 1 | Tertile 2 | Tertile 3 | P trend b | P heterogeneity c | ||
|---|---|---|---|---|---|---|
| Overalld | No. of cases/controls (151/301) | 64/106 | 50/99 | 37/96 | ||
| Crude OR (95% CI)e | 1 [Referent] | 0.83 (0.53-1.31) | 0.61 (0.36-1.02) | 0.06 | ||
| Multivariable OR (95% CI)f | 1 [Referent] | 0.84 (0.53-1.34) | 0.57 (0.33-0.99) | 0.05 | ||
| CD3+ cells | Low | |||||
| No. of cases/controls (65/129) | 22/50 | 24/41 | 19/38 | |||
| Crude OR (95% CI)e | 1 [Referent] | 1.33 (0.66-2.69) | 1.13 (0.50-2.57) | 0.69 | 0.05 | |
| Multivariable OR (95% CI)f | 1 [Referent] | 1.39 (0.67-2.85) | 1.07 (0.46-2.49) | 0.77 | 0.03 | |
| High | ||||||
| No. of cases/controls (86/172) | 42/56 | 26/58 | 18/58 | |||
| Crude OR (95% CI)e | 1 [Referent] | 0.59 (0.33-1.08) | 0.40 (0.20-0.79) | 0.008 | ||
| Multivariable OR (95% CI)f | 1 [Referent] | 0.57 (0.31-1.06) | 0.36 (0.17-0.75) | 0.006 | ||
| CD8+ cellsg | Low | |||||
| No. of cases/controls (73/145) | 28/51 | 23/52 | 22/42 | |||
| Crude OR (95% CI)e | 1 [Referent] | 0.83 (0.44-1.57) | 0.97 (0.46-2.02) | 0.86 | 0.19 | |
| Multivariable OR (95% CI)f | 1 [Referent] | 0.87 (0.45-1.69) | 0.97 (0.45-2.12) | 0.90 | 0.12 | |
| High | ||||||
| No. of cases/controls (76/152) | 34/56 | 27/45 | 15/51 | |||
| Crude OR (95% CI)e | 1 [Referent] | 0.92 (0.47-1.78) | 0.46 (0.21-0.98) | 0.05 | ||
| Multivariable OR (95% CI)f | 1 [Referent] | 0.86 (0.43-1.71) | 0.38 (0.16-0.88) | 0.03 | ||
| CD45RO+ cellsh | Low | |||||
| No. of cases/controls (77/153) | 31/60 | 25/46 | 21/47 | |||
| Crude OR (95% CI)e | 1 [Referent] | 1.04 (0.54-2.04) | 0.85 (0.41-1.75) | 0.66 | 0.20 | |
| Multivariable OR (95% CI)f | 1 [Referent] | 1.12 (0.56-2.24) | 0.86 (0.40-1.86) | 0.72 | 0.11 | |
| High | ||||||
| No. of cases/controls (73/146) | 33/47 | 25/51 | 15/48 | |||
| Crude OR (95% CI)e | 1 [Referent] | 0.72 (0.39-1.33) | 0.42 (0.20-0.91) | 0.03 | ||
| Multivariable OR (95% CI)f | 1 [Referent] | 0.66 (0.35-1.25) | 0.35 (0.16-0.80) | 0.01 | ||
| FOXP3+ cellsi | Low | |||||
| No. of cases/controls (69/138) | 31/52 | 20/46 | 18/40 | |||
| Crude OR (95% CI)e | 1 [Referent] | 0.71 (0.36-1.43) | 0.71 (0.33-1.55) | 0.37 | 0.74 | |
| Multivariable OR (95% CI)f | 1 [Referent] | 0.73 (0.35-1.52) | 0.64 (0.28-1.46) | 0.28 | 0.83 | |
| High | ||||||
| No. of cases/controls (77/153) | 31/52 | 28/49 | 18/52 | |||
| Crude OR (95% CI)e | 1 [Referent] | 0.97 (0.52-1.78) | 0.56 (0.27-1.14) | 0.13 | ||
| Multivariable OR (95% CI)f | 1 [Referent] | 0.96 (0.51-1.80) | 0.53 (0.25-1.13) | 0.12 | ||
Abbreviations: CI, confidence interval; OR, odds ratio.
Cutoff for low and high tumour-infiltrating T-cell subset density (cells/mm2): 244.97 for CD3+ cells, 236.65 for CD8+ cells, 376.97 for CD45RO+ cells, and 26.36 for FOXP3+ cells.
Test for trend was performed using the median value of each tertile of plasma 25-hydroxyvitamin D.
Comparing the risk of colorectal cancer associated with median value of each tertile of plasma 25-hydroxyvitamin D across subtypes of tumours.
Analysis was based on all cases having data on specific T-cells subsets and their matched controls.
Conditional logistic regression adjusted for the matching factors (sex, age and time of blood draw).
Adjusted for the same set of covariates as in Table 2.
Two cases with missing data on CD8+ cells and the matched four controls were excluded.
One case with missing data on CD45RO+ cells and the matched two controls were excluded.
Five cases with missing data on FOXP3+ cells and the matched 10 controls were excluded.
Because lymphocytic reaction to CRC has been associated with microsatellite instability (MSI) and CpG island methylator phenotype (CIMP) in CRC,[16] we also assessed MSI and CIMP status using the DNA extracted from tissue specimens as previously described.[23-25] More details are provided in the Supplementary materials.
Statistical analysis
Details of the statistical analysis are provided in the Supplementary materials. We used SAS 9.3 for all analyses (SAS Institute Inc., Cary, NC, USA). All statistical tests were two sided. Our primary hypothesis testing was the heterogeneity test between “the association of plasma 25(OH)D with lymphocyte-rich CRC subtype” and “that with lymphocyte-poor CRC subtype”. To account for multiple testing for the eight primary hypotheses associated with the eight immunity variables (degrees of intratumoural periglandular reaction, tumour-infiltrating lymphocytes, Crohn's-like reaction, and peritumoural reaction; and densities of CD3+, CD8+, CD45RO+, and FOXP3+ T-cells), we corrected the statistical significance level to α = 0.05/8 = 0.006 by the Bonferroni correction. All other assessments including evaluation of individual odds ratio (OR) estimates represented our secondary analyses. We recognised the use of multiple comparisons, and interpreted our data cautiously.
Plasma 25(OH)D levels were categorised into tertiles within each batch of measurement on the basis of the distribution among controls. We used multivariable conditional logistic regression to estimate ORs for CRC subtypes in relation to tertiles of plasma 25(OH)D. Test for trend was performed using the median value for each tertile as a continuous variable in the regression models. To examine the heterogeneity in the associations with various CRC subtypes, we used likelihood ratio test with one degree of freedom by comparing the model in which the association with plasma 25(OH)D was allowed to vary by tumour subtypes (ordinal or binary) to a model in which a common association was assumed across tumour subtypes.[22, 26]
We tested whether plasma 25(OH)D-CRC association varied by cohort using the Q statistic before pooling.[27] The association was similar in the two cohorts (comparing extreme tertiles: multivariable OR, 0.71; 95% CI, 0.50 to 1.01; Ptrend = 0.05 in the NHS; OR, 0.79; 95% CI, 0.52 to 1.21; Ptrend = 0.29 in the HPFS), and no statistically significant difference was detected (Pheterogeneity = 0.66 for Cochran's Q test). Therefore, for our main analyses, we pooled data from both cohorts.
RESULTS
Baseline characteristics of study participants
Table 1 shows basic characteristics of our study population. Compared to controls, CRC cases tended to be obese and smoke before age 30 in men (P = 0.02). In contrast, compared to cases, controls tended to take aspirin regularly and consume less alcohol, more folate and calcium in women (P < 0.05). The median of plasma 25(OH)D concentrations was higher among controls (27.8 ng/mL in women, 29.2 ng/mL in men) than cases (26.1 ng/mL in women, 27.8 ng/mL in men) (P = 0.02 in women, P = 0.10 in men).
Table 1.
Age-adjusted basic characteristics of case and control participants in women (1990) and men (1994)a
| Variable | Women |
Men |
||||
|---|---|---|---|---|---|---|
| Cases (n=172) | Controls (n=342) | P | Cases (n=146) | Controls (n=282) | P | |
| Age at blood draw, yearb | 58.6 (6.6) | 58.7 (6.5) | - | 66.0 (8.4) | 66.1 (8.4) | - |
| Body mass index, kg/m2 | 25.7 (4.9) | 25.4 (4.6) | 0.43 | 26.1 (3.3) | 25.5 (3.1) | 0.06 |
| Physical activity, MET-h/wk | 16.6 (20.9) | 16.5 (19.4) | 0.84 | 32.1 (26.8) | 30.5 (24.6) | 0.56 |
| Pack-year of smoking before age 30 | 3.6 (6.1) | 3.1 (4.5) | 0.18 | 6.1 (7.1) | 4.4 (6.4) | 0.02 |
| Current smoker, % | 13 | 14 | 0.61 | 4 | 5 | 0.67 |
| Family history of colorectal cancer, % | 20 | 21 | 0.85 | 22 | 16 | 0.15 |
| History of previous endoscopy, % | 38 | 38 | 0.92 | 54 | 62 | 0.11 |
| Current multivitamin use, % | 37 | 39 | 0.58 | 46 | 53 | 0.22 |
| Regular aspirin use, %c | 35 | 47 | 0.01 | 42 | 48 | 0.25 |
| Regular NSAID use, %d | 12 | 16 | 0.20 | 9 | 11 | 0.76 |
| Postmenopausal, % | 87 | 87 | 0.95 | - | - | - |
| Current hormone use, %e | 35 | 40 | 0.30 | - | - | - |
| Alcohol consumption, g/d | 4.0 (6.3) | 5.2 (9.5) | 0.01 | 13.3 (17.5) | 10.5 (13.0) | 0.31 |
| Folate intake, μg/d | 421 (207) | 447 (226) | 0.05 | 506 (273.1) | 541 (269) | 0.10 |
| Calcium intake, mg/d | 994 (592) | 1,060 (504) | 0.003 | 960 (422.2) | 943 (435) | 0.67 |
| Total fiber intake, g/d | 18.3 (5.8) | 18.8 (5.1) | 0.06 | 22.4 (7.2) | 23.6 (7.0) | 0.08 |
| DASH diet score | 23.5 (4.2) | 24.6 (4.2) | 0.06 | 24.2 (4.6) | 24.9 (4.6) | 0.13 |
| Plasma 25-hydroxyvitamin D, median (interquartile range), ng/mLf | 26.1 (19.5 to 31.7) | 27.8 (21.0 to 33.2) | 0.02 | 27.8 (22.2 to 33.3) | 29.2 (22.5 to 35.0) | 0.10 |
Abbreviations: DASH, Dietary Approaches to Stop Hypertension; MET, metabolic equivalent = (caloric need/kilogram body weight per hour activity)/(caloric need/kilogram body weight per hour at rest); NSAID, non-steroidal anti-inflammatory drug.
Among participants who had data on lymphocytic reaction. Numbers in parenthesis indicate standard deviation for means unless otherwise specified.
Age is one of the matching variables and is not age-standardised.
A standard tablet contains 325-mg aspirin, and regular users were defined as those who used at least 2 tablets per week.
Regular users were defined as those who used at least 2 tablets per week.
Percentage is among postmenopausal women.
Adjusted for measurement batch using the average batch correction method.
By comparing the baseline characteristics of cases with and without tumour immunity data, we did not find any substantial difference between the two groups except for lower alcohol consumption and higher proportion of stage II and III tumours among cases that had lymphocytic reaction data than those without lymphocyte data in women (Supplementary table s1).
We observed that plasma 25(OH)D level was not significantly associated with lower risk of overall CRC in our nested case-control set (Ptrend = 0.09; Table 2). Such association did not appreciably differ by the availability of tumour immunity data (Pheterogeneity > 0.60), when the CRC cases in the current study were compared to the CRC patients who were excluded due to the unavailable tumour immunity data (Supplementary table s2).
Table 2.
Plasma 25-hydroxyvitamin D levels and colorectal cancer, overall and by components of lymphocytic reactiona
| Tertile 1 | Tertile 2 | Tertile 3 | P trend b | P heterogeneity c | ||
|---|---|---|---|---|---|---|
| Overalld | Median of 25(OH)D, ng/mL | 19.0 | 27.9 | 37.4 | ||
| No. of cases/controls (318/624) | 122/212 | 109/208 | 87/204 | |||
| Crude OR (95% CI)e | 1 [Referent] | 0.90 (0.65-1.23) | 0.69 (0.48-1.00) | 0.05 | ||
| Multivariable OR (95% CI)f | 1 [Referent] | 0.90 (0.64-1.25) | 0.71 (0.48-1.05) | 0.09 | ||
| Intratumoural periglandular reaction | Absent | |||||
| No. of cases/controls (41/79) | 14/27 | 16/25 | 11/27 | |||
| Crude OR (95% CI)e | 1 [Referent] | 1.24 (0.52-2.96) | 0.72 (0.27-1.94) | 0.58 | <0.001 | |
| Multivariable OR (95% CI)f | 1 [Referent] | 1.15 (0.47-2.85) | 0.71 (0.26-1.95) | 0.55 | 0.001 | |
| Mild | ||||||
| No. of cases/controls (230/451) | 85/165 | 79/154 | 66/132 | |||
| Crude OR (95% CI)e | 1 [Referent] | 0.99 (0.68-1.45) | 0.95 (0.62-1.45) | 0.81 | ||
| Multivariable OR (95% CI)f | 1 [Referent] | 1.00 (0.67-1.47) | 0.98 (0.62-1.54) | 0.93 | ||
| High | ||||||
| No. of cases/controls (47/94) | 23/20 | 14/29 | 10/45 | |||
| Crude OR (95% CI)e | 1 [Referent] | 0.33 (0.12-0.88) | 0.10 (0.03-0.35) | <0.001 | ||
| Multivariable OR (95% CI)f | 1 [Referent] | 0.33 (0.12-0.90) | 0.10 (0.03-0.35) | <0.001 | ||
| Tumour-infiltrating lymphocytes | Absent | |||||
| No. of cases/controls (223/434) | 83/158 | 80/142 | 60/134 | |||
| Crude OR (95% CI)e | 1 [Referent] | 1.06 (0.73-1.55) | 0.80 (0.52-1.24) | 0.37 | 0.08 | |
| Multivariable OR (95% CI)f | 1 [Referent] | 1.02 (0.69-1.51) | 0.81 (0.51-1.30) | 0.42 | 0.13 | |
| Mild | ||||||
| No. of cases/controls (64/128) | 25/40 | 24/45 | 15/43 | |||
| Crude OR (95% CI)e | 1 [Referent] | 0.80 (0.39-1.65) | 0.50 (0.22-1.17) | 0.11 | ||
| Multivariable OR (95% CI)f | 1 [Referent] | 0.91 (0.44-1.89) | 0.50 (0.21-1.20) | 0.13 | ||
| High | ||||||
| No. of cases/controls (31/62) | 14/12 | 8/21 | 9/29 | |||
| Crude OR (95% CI)e | 1 [Referent] | 0.31 (0.10-0.98) | 0.20 (0.05-0.73) | 0.02 | ||
| Multivariable OR (95% CI)f | 1 [Referent] | 0.33 (0.10-1.05) | 0.22 (0.06-0.87) | 0.03 | ||
| Crohn's-like lymphoid reactiong | Absent | |||||
| No. of cases/controls (189/369) | 72/140 | 66/112 | 51/117 | |||
| Crude OR (95% CI)e | 1 [Referent] | 1.13 (0.75-1.70) | 0.81 (0.50-1.29) | 0.43 | 0.01 | |
| Multivariable OR (95% CI)f | 1 [Referent] | 1.08 (0.71-1.66) | 0.81 (0.49-1.33) | 0.45 | 0.09 | |
| Mild | ||||||
| No. of cases/controls (54/107) | 21/30 | 18/38 | 15/39 | |||
| Crude OR (95% CI)e | 1 [Referent] | 0.64 (0.29-1.41) | 0.47 (0.19-1.18) | 0.11 | ||
| Multivariable OR (95% CI)f | 1 [Referent] | 0.72 (0.31-1.65) | 0.55 (0.20-1.49) | 0.24 | ||
| High | ||||||
| No. of cases/controls (16/32) | 8/5 | 7/12 | 1/15 | |||
| Crude OR (95% CI)e | 1 [Referent] | 0.37 (0.09-1.50) | 0.05 (0.00-0.50) | 0.008 | ||
| Multivariable OR (95% CI)f | 1 [Referent] | 0.56 (0.13-2.36) | 0.08 (0.01-0.86) | 0.03 | ||
| Peritumoural lymphocytic reaction | Absent | |||||
| No. of cases/controls (49/94) | 19/27 | 15/31 | 15/36 | |||
| Crude OR (95% CI)e | 1 [Referent] | 0.71 (0.31-1.67) | 0.53 (0.22-1.31) | 0.16 | 0.05 | |
| Multivariable OR (95% CI)f | 1 [Referent] | 0.69 (0.28-1.68) | 0.55 (0.22-1.38) | 0.19 | 0.08 | |
| Mild | ||||||
| No. of cases/controls (201/396) | 74/149 | 74/139 | 53/108 | |||
| Crude OR (95% CI)e | 1 [Referent] | 1.06 (0.71-1.58) | 0.98 (0.62-1.55) | 0.95 | ||
| Multivariable OR (95% CI)f | 1 [Referent] | 1.06 (0.70-1.59) | 0.98 (0.60-1.59) | 0.95 | ||
| High | ||||||
| No. of cases/controls (68/134) | 29/36 | 20/38 | 19/60 | |||
| Crude OR (95% CI)e | 1 [Referent] | 0.66 (0.32-1.37) | 0.32 (0.14-0.73) | 0.006 | ||
| Multivariable OR (95% CI)f | 1 [Referent] | 0.64 (0.30-1.36) | 0.34 (0.14-0.81) | 0.02 | ||
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; CI, confidence interval; OR, odds ratio.
Component of lymphocytic reaction was categorised as: absent, mild, and high (moderate or marked).
Test for trend was performed using the median value of each tertile of plasma 25-hydroxyvitamin D.
Global test comparing the risk of colorectal cancer associated with median value of each tertile of plasma 25-hydroxyvitamin D across subtypes of tumours.
Analysis was based on all cases having data on specific components of lymphocytic reaction and their matched controls.
Conditional logistic regression adjusted for the matching factors (sex, age and time of blood draw).
In addition to the matching factors, multivariable conditional logistic regression adjusted for family history of colorectal cancer, endoscopic screening, regular use of aspirin or non-steroidal anti-inflammatory drug, pack-years of smoking before age 30 years old (continuous), body mass index (continuous, kg/m2), physical activity (continuous, metabolic equivalent hour per week), alcohol consumption (continuous, g/day), and Dietary Approaches to Stop Hypertension score (continuous).
Fifty nine cases with missing data on Crohn's-like lymphoid reaction and the matched 116 controls were excluded.
Plasma 25(OH)D level and CRC subtypes classified by degrees of lymphocytic reactions and densities of T-cell subsets
We examined degrees of lymphocytic reaction in tissue sections of CRC. Table 2 shows the association of plasma 25(OH)D level with risk of CRC subtypes classified by the degrees of lymphocytic reactions. Our primary hypothesis testing was on heterogeneity between “the association of plasma 25(OH)D with risk of lymphocyte-rich CRC” and “that with risk of lymphocyte-poor CRC” in the combined cohort, and the statistical significance level was adjusted to α = 0.006 to account for multiple testing. Notably, the association of plasma 25(OH)D with risk of CRC subtypes differed by the degree of intratumoural periglandular reaction (Pheterogeneity = 0.001).
High plasma 25(OH)D level was statistically significantly associated with lower risk of CRC subtype possessing high-level intratumoural periglandular reaction (comparing the highest vs. the lowest tertiles: multivariable OR, 0.10; 95% CI, 0.03 to 0.35; Ptrend < 0.001 across tertiles of 25(OH)D level), but not with CRC subtypes possessing absent or mild reaction (Ptrend = 0.93 for the mild-reaction subtype; and Ptrend = 0.55 for the absent-reaction subtype). A similar but attenuated difference was observed between risks of CRC subtypes classified by tumourinfiltrating lymphocytes, Crohn's-like reaction, or peritumoural reaction; and the heterogeneity test did not reach statistical significance (Pheterogeneity > 0.07).
We additionally subclassified CRC according to densities of each of the four T-cell subsets (CD3+, CD8+, CD45RO+, and FOXP3+ cells) within CRC tissues (Table 3). Similar to the results on intratumoural periglandular reaction, the association between plasma 25(OH)D level and CRC risk differed by CD3+ T-cell density (Pheterogeneity = 0.03), although this difference was not significant at the stringent statistical significance level (α = 0.006). High level of plasma 25(OH)D was associated with lower risk of colorectal tumours that were infiltrated by high density of CD3+ cells (P = 0.006), but not with tumours having low density of CD3+trend cells (Ptrend = 0.77). The association of plasma 25(OH)D with risk of CRC did not significantly differ by the density of CD8+, CD45RO+, or FOXP3+ cells (Pheterogeneity > 0.10).
Plasma 25(OH)D level and CRC subtypes classified by MSI or CIMP status
Because lymphocytic reaction to CRC has been associated with MSI and CIMP in CRC,[16] we also classified tumours by MSI and CIMP status as our secondary analyses. The association of plasma 25(OH)D with CRC subtypes did not significantly differ by MSI (Pheterogeneity = 0.02) or CIMP status (Pheterogeneity = 0.76; Supplementary table s3) at the stringent statistical significance level (α = 0.006).
Sensitivity analysis
To further control for potential confounding by lifestyle factors, instead of adjusting for the Dietary Approaches to Stop Hypertension (DASH) score, we adjusted for individual dietary factors that have been related to CRC risk, including multivitamins, calcium, red meat and processed meat, and total fiber, in our multivariable model. The results remained very similar, and the P value for heterogeneity was 0.002 for the associations between plasma 25(OH)D and risk of CRC subtypes classified by intratumoural periglandular reaction (Supplementary table s4). Given the potential influence of systemic inflammation on plasma 25(OH)D status and CRC development,[28, 29] we also adjusted for quartiles of each of the three inflammatory markers (i.e., CRP, IL6 and TNFRSF1B) in the multivariable model. As shown in Supplementary table s5, the results did not essentially change, and the plasma 25(OH)D-CRC associations remained statistically significantly different according to the degree of intratumoural periglandular reaction (Pheterogeneity < 0.001).
DISCUSSION
We conducted this study to test the hypothesis that the inverse association of plasma vitamin D level with risk of CRC might be stronger for CRC subtype with high-level lymphocytic reaction than for CRC subtype with low-level reaction. We found that the relationship between plasma 25(OH)D and risk of CRC differed by intratumoural periglandular reaction to CRC; high 25(OH)D was associated with lower risk of tumours possessing high-level lymphocytic reaction, but not with tumours having low-level or no lymphocytic reaction. Although statistical significance was not reached at the stringent level (α = 0.006), we also observed that the inverse association of plasma 25(OH)D with CRC risk appeared to be stronger for tumours infiltrated with high density of CD3+ T-cells, than for tumours with lower density of CD3+ T-cells. Our data provide evidence for a possible role of tumour stromal immune cells in generating bioactive 1,25(OH)2D3 to augment the influence of vitamin D on neoplastic and nonneoplastic cells in an autocrine and paracrine fashion.
As cancer immunotherapy has become an attractive strategy, integrated analyses of tumour molecular features and host factors including dietary and environmental exposures and immune response to tumour are increasingly important.[30-33] The degree of lymphocytic infiltrate in CRC tissue has been associated with MSI status and better patient survival.[34-38] However, there is a paucity of data on epidemiologic exposures (such as plasma 25(OH)D level) combined with tumour molecular features and immune response in the tumour microenvironment in population-based studies. Our current study aimed to address this challenge.
Although numerous epidemiologic studies have shown a lower CRC risk associated with high vitamin D level, it is still of considerable debate about whether this represents a causal relationship or arises from confounding. In a large randomised trial, daily supplementation with 400 IU of vitamin D combined with 1000 mg of elemental calcium for 7 years had no detectable benefit for CRC occurrence.[39] However, methodological limitations of this trial, including inadequate vitamin D dose, duration and compliance, might have contributed to the null findings. In this context, investigation of the influence of vitamin D on CRC subtypes characterised by immunity-related pathologic features may not only provide important insight into the causality of the vitamin D-CRC relationship but also reveal a complex interaction between exposures, host factors and tumour cells.[40] Our findings provide the first line of population-based evidence for the role of host immunity in vitamin D-mediated CRC prevention, therefore generating some mechanistic hypotheses for further investigation.
One possible mechanism through which immune response may modulate the effect of vitamin D on carcinogenesis is that immune cells can convert 25(OH)D to bioactive 1,25(OH)2D3, and thereby enhancing the effect of vitamin D on behaviors of both neoplastic and non-neoplastic cells. Macrophages, dendritic cells, T cells and B cells have all been shown to express enzymes critical for vitamin D metabolism and have an immune autocrine/paracrine activity.[3-5] Locally synthesised 1,25(OH)2D3 can then bind to the vitamin D receptor (VDR) and regulate transcription of genes that control cell proliferation, apoptosis and differentiation.[2] In contrast, colorectal neoplasia with low lymphocytic infiltrates may not have sufficient bioactive vitamin D in the tumour microenvironment to mediate the influence of plasma 25(OH)D level on neoplasia evolution.
As an alternative mechanism, modulation of immune function by vitamin D may help maintain immune homeostasis of the intestine, thus favoring tumour-suppressive effects over tumour-promoting effect of lymphocytic infiltrates.[7] It is plausible that the tumour-suppressive effect of vitamin D may be more pronounced in emerging tumours with abundant immune cells than in those with fewer immune cells. The role of inflammation caused by gut microbiota or other stimuli in colorectal carcinogenesis has been increasingly recognised.[41, 42] Vitamin D exerts an inhibitory action on the adaptive immune system through suppressing proinflammatory TH1 cell activity,[43] and enhancing anti-inflammatory TH2 cell activity.[44] There is evidence suggesting that adequate vitamin D and VDR expression are required for T-cell antigen receptor signaling and subsequent T-cell activation.[45] Recently, vitamin D has been found to prevent inflammation-associated colon cancer through suppression of inflammatory responses during initiation of carcinogenesis.[46] These experimental data may be consistent with our observation of the strong inverse association of high plasma 25(OH)D with risk of CRC subtype with high-level lymphocytic reactions.
Our current study has limitations. First, the sample size is limited due to the necessity of both prediagnostic plasma and CRC tissue specimens, and therefore our results should be interpreted cautiously. Given the uniqueness of the current study, our findings need to be replicated in independent datasets. Second, potential selection bias might arise from our exclusion of CRC cases without available tumour tissue data. However, the distribution of risk factors among included cases did not appreciably differ from excluded cases. Moreover, the association between plasma 25(OH)D and risk of overall CRC did not appreciably differ by the availability (vs. unavailability) of tumour tissue data. Third, the study was observational and subject to influence of confounding. However, adjustment for a wide range of risk factors for CRC had minimal impact on our results.
Our study has several strengths. First, this longitudinal study was conducted within two well-defined cohorts, and our nested case-control design enabled us to match each CRC case with controls from the same background population, which represents a substantial advantage over ordinary case-control design. Second, we measured 25(OH)D in plasma specimens obtained from cohort participants when they had not known if they would develop CRC or not in the future. These prediagnostic plasma specimens represent a precious resource to evaluate plasma biomarkers for a potential risk assessment tool in clinical settings. Third, we collected detailed information on potential confounders and had a high follow-up rate of the cohorts. Fourth, this study represents a unique integrative molecular pathological epidemiology [47-52] analysis of prediagnostic plasma vitamin D and immunity status in tumour tissue, which has enabled us to provide novel epidemiologic evidence on the potential role of vitamin D in cancer immunoprevention.
In conclusion, high-level plasma 25(OH)D is associated with a lower risk of the CRC subtype characterised by intense intratumoural periglandular lymphocytic reaction, but not with risk of CRC subtypes with less intense reaction. Our findings suggest a potential interplay of vitamin D and immune system that may operate to prevent CRC development. Further research is needed to confirm our findings and to examine potential mechanisms for CRC immunoprevention.
Supplementary Material
SUMMARY.
What is already known about this subject?
Vitamin D has been associated with lower risk of colorectal cancer (CRC).
Vitamin D plays an important role in regulation of immune function.
The multifaceted roles of host immunity and inflammation in regulating tumour evolution have long been recognised.
What are the new findings?
The association of high-level plasma vitamin D and lower risk of CRC differs by CRC subtypes classified by the degree of lymphocytic reaction to CRC.
Plasma vitamin D level is associated with lower risk of the CRC subtype characterised by high-degree intratumoural periglandular reaction, but not risk of the CRC subtype with low-degree reaction.
How might it impact on clinical practice in the foreseeable future?
Our findings provide the first line of population-based evidence for a role of vitamin D in cancer immunoprevention through tumour-host interaction.
In the future, host immunity status may serve as a potential biomarker to predict the benefit from vitamin D supplementation or other vitamin D-augmenting intervention for CRC prevention.
ACKNOWLEDGMENTS
We deeply thank hospitals and pathology departments throughout the U.S. for generously providing us with tissue specimens. We also would like to thank the participants and staff of the Nurses' Health Study and the Health Professionals Follow-up Study, for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
FUNDING
This work was supported by U.S. National Institutes of Health (NIH) grants [P01 CA87969 to S.E. Hankinson; R01 CA49449 to S.E. Hankinson; UM1 CA186107 to M.J. Stampfer; P01 CA55075 to W.C. Willett; UM1 CA167552 to W.C. Willett; R03 CA176717 to X.Z.; R01 CA137178 to A.T.C.; K24 DK098311 to A.T.C.; P50 CA127003 to C.S.F.; DF/HCC GI SPORE Developmental Project to S.O.; R01 CA151993 to S.O.; K07 CA190673 to R.N.; and K07 CA148894 to K.N.], and grants from The Paula and Russell Agrusa Fund for Colorectal Cancer Research (to C.S.F.), the Friends of the Dana-Farber Cancer Institute (S.O.), the Bennett Family Fund, and the Entertainment Industry Foundation. A.T.C. is a Damon Runyon Clinical Investigator. M.S. is a trainee of the Harvard Transdisciplinary Research Center on Energetics and Cancer (TREC). K.I. is supported by a Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad and by Takashi Tsuruo Memorial Fund. S.A.K. is supported by an early exchange postdoctoral fellowship grant from Asian medical center.
Abbreviations
- 1,25(OH)2D3
1α,25-dihydroxyvitamin D3
- 25(OH)D
25-hydroxyvitamin D
- CI
confidence interval
- CIMP
CpG island methylator phenotype
- CRC
colorectal cancer
- DASH
Dietary Approaches to Stop Hypertension
- HPFS
Health Professionals Follow-up Study
- MET
metabolic equivalent
- MSI
microsatellite instability
- NHS
Nurses' Health Study
- NSAID
non-steroidal anti-inflammatory drug
- OR
odds ratio
Footnotes
We use Human Genome Organisation (HUGO) Gene Nomenclature Committee (HGNC)-approved symbols for genes and gene products, including CD3, CD8, CRP, FOXP3, IL6, PTPRC, TNFRSF1B, and VDR; all of which are described at www.genenames.org.
AUTHOR CONTRIBUTIONS
Drs Song and Ogino have full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Song, Nishihara, Wang and Chan contributed equally. Drs Fuchs, Giovannucci, Wu and Ogino contributed equally.
Study concept and design: Drs Song, Giovannucci, Wu and Ogino.
Acquisition of data: Drs Song, Nishihara, Wang, Chan, Qian, Inamura, Zhang, Ng, Kim, Mima, Sukawa, Nosho, Fuchs, Giovannucci, Wu and Ogino.
Analysis and interpretation of data: Drs Song, Nishihara, Wang, Chan, Qian, Inamura, Zhang, Ng, Kim, Mima, Sukawa, Nosho, Fuchs, Giovannucci, Wu and Ogino.
Drafting of the manuscript: Drs Song and Ogino.
Critical revision of the manuscript for important intellectual content: Drs Song, Chan, Zhang, Giovannucci, Wu and Ogino.
Statistical analysis: Drs Song, Nishihara, Wang and Ogino.
Funding acquisition: Drs Chan, Fuchs, Giovannucci and Ogino.
Administrative, technical, or material support: Drs Chan, Fuchs, Giovannucci and Ogino.
Study supervision: Dr Ogino.
COMPETING INTERESTS
A.T.C. previously served as a consultant for Bayer Healthcare, Millennium Pharmaceuticals, and Pfizer Inc. This study was not funded by Bayer Healthcare, Millennium Pharmaceuticals, or Pfizer Inc. No other conflict of interest exists.
REFERENCES
- 1.Ma Y, Zhang P, Wang F, et al. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol. 2011;29:3775–82. doi: 10.1200/JCO.2011.35.7566. [DOI] [PubMed] [Google Scholar]
- 2.Feldman D, Krishnan AV, Swami S, et al. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–57. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 3.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 4.Edfeldt K, Liu PT, Chun R, et al. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci U S A. 2010;107:22593–8. doi: 10.1073/pnas.1011624108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sigmundsdottir H, Pan J, Debes GF, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–93. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 6.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veldhoen M, Brucklacher-Waldert V. Dietary influences on intestinal immunity. Nat Rev Immunol. 2012;12:696–708. doi: 10.1038/nri3299. [DOI] [PubMed] [Google Scholar]
- 8.Fridman WH, Pages F, Sautes-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 9.Xia D, Wang D, Kim SH, et al. Prostaglandin E2 promotes intestinal tumor growth via DNA methylation. Nat Med. 2012;18:224–6. doi: 10.1038/nm.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marzbani E, Inatsuka C, Lu H, et al. The invisible arm of immunity in common cancer chemoprevention agents. Cancer Prev Res (Phila) 2013;6:764–73. doi: 10.1158/1940-6207.CAPR-13-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Caro G, Marchesi F, Laghi L, et al. Immune cells: plastic players along colorectal cancer progression. J Cell Mol Med. 2013;17:1088–95. doi: 10.1111/jcmm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocca YS, Roberti MP, Arriaga JM, et al. Altered phenotype in peripheral blood and tumor-associated NK cells from colorectal cancer patients. Innate immunity. 2013;19:76–85. doi: 10.1177/1753425912453187. [DOI] [PubMed] [Google Scholar]
- 15.Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350–66. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogino S, Nosho K, Irahara N, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15:6412–20. doi: 10.1158/1078-0432.CCR-09-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao X, Lochhead P, Nishihara R, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596–606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu K, Feskanich D, Fuchs CS, et al. A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst. 2007;99:1120–9. doi: 10.1093/jnci/djm038. [DOI] [PubMed] [Google Scholar]
- 19.Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol. 1997;282:174–86. doi: 10.1016/s0076-6879(97)82106-4. [DOI] [PubMed] [Google Scholar]
- 20.Song M, Wu K, Ogino S, et al. A prospective study of plasma inflammatory markers and risk of colorectal cancer in men. Br J Cancer. 2013;108:1891–8. doi: 10.1038/bjc.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan AT, Ogino S, Giovannucci EL, et al. Inflammatory markers are associated with risk of colorectal cancer and chemopreventive response to anti-inflammatory drugs. Gastroenterology. 2011;140:799–808. doi: 10.1053/j.gastro.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–42. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 23.Nosho K, Irahara N, Shima K, et al. Comprehensive biostatistical analysis of CpG island methylator phenotype in colorectal cancer using a large population-based sample. PLoS One. 2008;3:e3698. doi: 10.1371/journal.pone.0003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogino S, Nosho K, Kirkner GJ, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–6. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogino S, Kawasaki T, Brahmandam M, et al. Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J Mol Diagn. 2006;8:209–17. doi: 10.2353/jmoldx.2006.050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–32. [PubMed] [Google Scholar]
- 27.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 28.Ghashut RA, Talwar D, Kinsella J, et al. The effect of the systemic inflammatory response on plasma vitamin 25 (OH) D concentrations adjusted for albumin. PLoS One. 2014;9:e92614. doi: 10.1371/journal.pone.0092614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou B, Shu B, Yang J, et al. C-reactive protein, interleukin-6 and the risk of colorectal cancer: a meta-analysis. Cancer Causes Control. 2014;25:1397–405. doi: 10.1007/s10552-014-0445-8. [DOI] [PubMed] [Google Scholar]
- 30.Bishehsari F, Mahdavinia M, Vacca M, et al. Epidemiological transition of colorectal cancer in developing countries: environmental factors, molecular pathways, and opportunities for prevention. World J Gastroenterol. 2014;20:6055–72. doi: 10.3748/wjg.v20.i20.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colussi D, Brandi G, Bazzoli F, et al. Molecular pathways involved in colorectal cancer: implications for disease behavior and prevention. Int J Mol Sci. 2013;14:16365–85. doi: 10.3390/ijms140816365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galon J, Pages F, Marincola FM, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li P, Wu H, Zhang H, et al. Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: a meta-analysis. Gut. 2014 doi: 10.1136/gutjnl-2014-308260. [DOI] [PubMed] [Google Scholar]
- 34.Alexander J, Watanabe T, Wu TT, et al. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158:527–35. doi: 10.1016/S0002-9440(10)63994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koshiol J, Lin SW. Can tissue-based immune markers be used for studying the natural history of cancer? Ann Epidemiol. 2012;22:520–30. doi: 10.1016/j.annepidem.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grizzi F, Bianchi P, Malesci A, et al. Prognostic value of innate and adaptive immunity in colorectal cancer. World J Gastroenterol. 2013;19:174–84. doi: 10.3748/wjg.v19.i2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmqvist R, Wikberg ML, Ling A, et al. The association of immune cell infiltration and prognosis in colorectal cancer. Curr Colorectal Cancer Rep. 2013;9:372–9. [Google Scholar]
- 38.Dahlin AM, Henriksson ML, Van Guelpen B, et al. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol. 2011;24:671–82. doi: 10.1038/modpathol.2010.234. [DOI] [PubMed] [Google Scholar]
- 39.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–96. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 40.Ogino S, Galon J, Fuchs CS, et al. Cancer immunology--analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011;8:711–9. doi: 10.1038/nrclinonc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahn J, Sinha R, Pei Z, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105:1907–11. doi: 10.1093/jnci/djt300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome research. 2012;22:292–8. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lemire JM, Archer DC, Beck L, et al. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr. 1995;125:1704S–8S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- 44.Boonstra A, Barrat FJ, Crain C, et al. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–80. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 45.von Essen MR, Kongsbak M, Schjerling P, et al. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010;11:344–9. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- 46.Meeker S, Seamons A, Paik J, et al. Increased dietary vitamin D suppresses MAPK signaling, colitis and colon cancer. Cancer Res. 2014 doi: 10.1158/0008-5472.CAN-13-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogino S, Stampfer M. Lifestyle factors and microsatellite instability in colorectal cancer: the evolving field of molecular pathological epidemiology. J Natl Cancer Inst. 2010;102:365–7. doi: 10.1093/jnci/djq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogino S, Chan AT, Fuchs CS, et al. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397–411. doi: 10.1136/gut.2010.217182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffmeister M, Blaker H, Kloor M, et al. Body mass index and microsatellite instability in colorectal cancer: a population-based study. Cancer Epidemiol Biomarkers Prev. 2013;22:2303–11. doi: 10.1158/1055-9965.EPI-13-0239. [DOI] [PubMed] [Google Scholar]
- 50.Hughes LA, Khalid-de Bakker CA, Smits KM, et al. The CpG island methylator phenotype in colorectal cancer: progress and problems. Biochimica et biophysica acta. 2012;1825:77–85. doi: 10.1016/j.bbcan.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Epplein M, Bostick RM, Mu L, et al. Challenges and Opportunities in International Molecular Cancer Prevention Research: An ASPO Molecular Epidemiology and the Environment and International Cancer Prevention Interest Groups Report. Cancer Epidemiol Biomarkers Prev. 2014;23:2613–7. doi: 10.1158/1055-9965.EPI-14-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campbell PT, Deka A, Briggs P, et al. Establishment of the Cancer Prevention Study II Nutrition Cohort Colorectal Tissue Repository. Cancer Epidemiol Biomarkers Prev. 2014;23:2694–702. doi: 10.1158/1055-9965.EPI-14-0541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.