Abstract
Polychlorinated biphenyls (PCBs) are persistent environmental pollutants that contribute to the initiation of cardiovascular disease. Exercise has been shown to reduce the risk of cardiovascular disease; however, whether exercise can modulate PCB-induced vascular endothelial dysfunction and associated cardiovascular risk factors is unknown. We examined the effects of exercise on coplanar PCB- induced cardiovascular risk factors including oxidative stress, inflammation, impaired glucose tolerance, hypercholesteremia, and endothelium-dependent relaxation. Male ApoE−/− mice were divided into sedentary and exercise groups (voluntary wheel running) over a 12 week period. Half of each group was exposed to vehicle or PCB 77 at weeks 1, 2, 9, and 10. For ex vivo studies, male C57BL/6 mice exercised via voluntary wheel training for 5 weeks and then were administered with vehicle or PCB 77 24 hours before vascular reactivity studies were performed. Exposure to coplanar PCB increased risk factors associated with cardiovascular disease, including oxidative stress and systemic inflammation, glucose intolerance, and hypercholesteremia. The 12 week exercise intervention significantly reduced these pro-atherogenic parameters. Exercise also upregulated antioxidant enzymes including phase II detoxification enzymes. Sedentary animals exposed to PCB 77 exhibited endothelial dysfunction as demonstrated by significant impairment of endothelium-dependent relaxation, which was prevented by exercise. Lifestyle modifications such as aerobic exercise could be utilized as a therapeutic approach for the prevention of adverse cardiovascular health effects induced by environmental pollutants such as PCBs. Keywords: exercise, polychlorinated biphenyl, endothelial function, antioxidant response, cardiovascular disease, inflammation, oxidative stress
Keywords: exercise, polychlorinated biphenyl, endothelial function, antioxidant response, cardiovascular disease, inflammation, oxidative stress
Introduction
Cardiovascular disease remains the leading cause of death in developed nations. A number of different factors including environmental and chemical exposures are contributors to cardiovascular diseases. An accumulating body of evidence from epidemiological, in vitro, and in vivo studies link cardiovascular disease to environmental pollution, including exposure to persistent organic pollutants such as dioxins and polychlorinated biphenyls (PCBs) (L. Lind & Lind, 2012). Dioxin exposure appears to be associated with mortality from cardiovascular disease (Humblet, Birnbaum, Rimm, Mittleman, & Hauser, 2008). Furthermore, residing near sites contaminated with PCBs is associated with increased rates of hospitalization for coronary heart disease and acute myocardial infarction (Sergeev & Carpenter, 2005), and circulating levels of PCBs were associated with atherosclerotic plaques in humans (P. M. Lind, van Bavel, Salihovic, & Lind, 2012).
Because the endothelium is in immediate contact with blood, endothelial cells are particularly vulnerable to environmental contaminants present in the circulation and which can induce inflammation and endothelial dysfunction (Eske et al., 2013; Hennig et al., 2002). The majority of pro-inflammatory effects from coplanar PCBs are mediated through the aryl hydrocarbon receptor (AhR) (reviewed in (Petriello, Newsome, & Hennig, 2013)). Activation of the AhR leads to transcription of the detoxifying enzyme cytochrome p450 1A1 (CYP1A1). CYP1A1 induction aides in the detoxification of PCB 77 to multiple metabolites including hydroxyl and sulfate versions which are ultimately excreted (Robertson &Hansen 2001). Importantly, when in the presence of PCB 77, CYP1A1 can become uncoupled and increase the levels of cellular reactive oxygen species (ROS), leading to induction of pro-inflammatory genes and subsequent vascular dysfunction (Kopf & Walker, 2010). Our laboratory has demonstrated previously that exposure to PCB 77 increases the expression of vascular cell adhesion molecule-1 (VCAM-1) (S G Han, Eum, Toborek, Smart, & Hennig, 2010), endothelial-derived monocyte chemoattractant protein-1 (MCP-1) (Majkova, Smart, Toborek, & Hennig, 2009), and interleukin-6 (IL-6) (Hennig et al., 2002). Other groups have shown that PCBs impair endothelium-dependent dilation (Helyar et al., 2009) and promote obesity-associated atherosclerosis (Arsenescu, Arsenescu, King, Swanson, & Cassis, 2008).
As pollutant emissions continue to increase (i.e., manufacturing and agriculture), human exposure to these pollutants will rise, thus leading to the need for physiological buffers to protect against pollutant-induced adverse health effects such as cardiovascular disease. Data from our laboratory and other groups have provided strong evidence that nutrition can modulate cardiovascular toxicity of environmental pollutants (Du et al., 2012; Sung Gu Han, Han, Toborek, & Hennig, 2012; Majkova et al., 2011; Ramadass, Meerarani, Toborek, Robertson, & Hennig, 2003; L. Wang, Reiterer, Toborek, & Hennig, 2008); however, the effect of other lifestyle modifications such as exercise on health risks associated with exposure to persistent organic pollutants remains relatively unexplored. One such PCB study examined the effects of exercise on the gut microbiome and found that 5 weeks of voluntary exercise attenuated PCB-induced alterations in proteobacteria (Choi et al., 2013). Exercise has been well-established as an effective primary and secondary intervention for atherosclerotic cardiovascular disease. Studies in both human and animal models have provided evidence that exercise exerts beneficial effects on atherogenesis and coronary artery disease (reviewed in (Pinto et al., 2012; Szostak & Laurant, 2011; Whayne & Maulik, 2012)). Exercise improves traditional cardiovascular disease risk factors including, hyperlipidemia, obesity, insulin sensitivity and hypertension, as well as vascular function affected by changes in redox status and inflammation (Whyte & Laughlin, 2010). It has been shown that aerobic exercise decreases atherosclerotic plaque formation (Gielen, Schuler, & Hambrecht, 2001; Ulrich Laufs et al., 2004; Napoli et al., 2006) and reduces neointima formation after carotid artery injury (Pynn, Schafer, Konstantinides, & Halle, 2004). Exercise has also been shown to reduce proinflammatory cytokines in humans with coronary artery disease (Y.-J. Kim et al., 2008) and in animal models of cardiovascular disease (Yang & Chen, 2003). In ApoE−/− mice, exercise improves endothelium-dependent vasodilation or relaxation in isolated aortas and decreases vascular oxidative stress (Ajijola et al., 2009; U Laufs et al., 2005).
By using ApoE−/− mice, a well-documented model of atherosclerosis (Alan Daugherty, 2002), we studied the relationship between exercise and PCB 77 exposure. Our results indicate that exposure to a coplanar PCB increased cardiovascular disease-related risk factors including oxidative stress, vascular inflammation, hyperlipidemia, and glucose intolerance, which were attenuated in exercised animals.
Materials & Methods
Chemicals
PCB 77 was purchased from Accustandard Inc. (New Haven, CT). Phenylephrine, Acetylcholine, Tempol, L-NG-Nitroarginine Methyl Ester (L-NAME), Sodium Nitroprusside (SNP), and acetonitrile were obtained from Sigma Aldrich (St. Louis, MO).
Animal treatment & sample collection
Male ApoE−/− mice were obtained from the Jackson Laboratories (Bar Harbor, ME). Each mouse was individually caged, handled, and used in compliance with the Animal Care and Use Committee of the University of Kentucky. Mice were given ad libitum access to food (rodent standard chow) and water and housed in a pathogen-free environment for 12 weeks. Mice were administered vehicle (0.2 mL tocopherol-stripped safflower oil, Dyets, Inc. Bethlehem, PA), or PCB 77 (170 µM/kg) by oral gavage as separate doses during weeks 1, 2, 9, and 10, and the dosage was based on earlier studies demonstrating glucose intolerance (Baker et al., 2013). Our model of exercise was the widely used voluntary running-wheel model, previously described (Esser et al., 2007). Each mouse randomized to exercise was placed in a modified cage with the wheel attached to a magnetic sensing mechanism from which the corresponding distance, speed, and amount of time spent running were obtained via ClockLab software (Actimetrics, Wilmette, IL). Mice were placed in a metabolic cage system (Techniplast, Inc., Philadelphia, PA) during week 12 to collect urine and feces. Fat mass and lean body mass were measured by an echo magnetic resonance imaging system (Echo-MRI; Echo Medical Systems, Houston, TX). At the study end point, mice were euthanized with CO2 for exsanguination and tissue harvest (liver, soleus muscle, visceral adipose tissue, lungs, and kidney).
Quantification of plasma cholesterol, lipoproteins and cytokines/chemokines
Plasma cholesterol concentrations and lipoprotein distributions were determined as described previously (A Daugherty, Manning, & Cassis, 2000; Manning, Cassis, & Daugherty, 2003). Cytokines and chemokines were measured simultaneously by using an 18-plex kit (Millipore, St. Charles, MO) (Majkova et al., 2009).
Glucose tolerance test
Mice were fasted for 6 hours prior to the glucose tolerance test performed during week 6 of the study. Blood was collected from the tail vein and tested for glucose concentration with a glucometer (Freedom Freestyle Lite; Abbott Laboratories, Abbott Park, IL). Mice were administered D-glucose (20% in saline, oral gavage) and blood glucose was quantified at 0, 15, 30, 60, 90 and 120 min. Total area under the curve (AUC; arbitrary units) calculates the area below the observed concentrations without the presence of a baseline value (Baker et al., 2013).
Quantification of PCBs and F2-isoprostanes
To determine systemic PCB and metabolite concentrations, PCB 77 and its hydroxyl metabolites were isolated from plasma and tissue samples. Briefly, homogenates were extracted in acetonitrile with the appropriate internal standards, dried under N2 and reconstituted (Newsome et al., 2014). Measurement of urinary F2-Isoprostanes (F2-IsoPs) is considered the gold standard for assessment of in vivo oxidative stress (Morrow, 2005), and the assay was performed as described by us elsewhere (Newsome et al., 2014). After extractions were performed, plasma, tissue, and urinary levels of PCB 77, hydroxyl metabolites, and F2-IsoPs were analyzed using a Shimadzu ultra-fast liquid chromatography system coupled with an AB Sciex 4000-Qtrap hybrid linear ion trap triple quadrupole mass spectrometer in multiple reaction monitoring (MRM) mode (Newsome et al., 2014).
Gene expression of CYP1A1 and antioxidant enzymes
Total mRNA was extracted from liver using the TRIZOL reagent (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer’s protocol and as reported elsewhere (Newsome et al., 2014). Primer sequences [see Online Resource, Table S1) for SYBR Green chemistry were designed using the Primer Express Software 3.0 for RT-PCR (Applied Biosystems) and procured from Integrated DNA Technologies, Inc. (Coralville, IA).
Ex vivo vascular reactivity studies
Forty Male C57BL/6 mice were randomized to exercise or sedentary groups for five weeks. Individually caged sedentary controls were handled for the same procedures and amount of time as the exercised mice. Twenty-four hours before euthanasia, mice were intraperitoneally injected with 170 µM/kg of PCB 77 or vehicle (an average of 0.2 mL tocopherol-stripped safflower oil per animal (Dyets, Inc. Bethlehem, PA). This dose was chosen based on a pilot study from our laboratory that demonstrated impaired endothelial-dependent relaxation in these mice (unpublished data). Measurement of contractile activity was performed using aortic rings as described previously (Rateri et al., 2011).
Statistical analysis
Data are represented as mean ± SEM. Two-way ANOVA was used, followed by a post-hoc Tukey’s test to measure differences using SigmaStat software (Systat Software, Version 12, Point Richmond, CA). Differences with a value of p<0.05 were considered statistically significant. For vascular reactivity studies, Two-way Repeated Measures ANOVA was performed.
Results
Exercise reduces cardiovascular disease risk factors in PCB 77-treated mice
All treatment groups gained weight, and specifically lean body mass [see Online Resource, Figure S1A], suggesting that the dosing regimen of PCB did not produce signs of PCB-induced wasting syndrome.
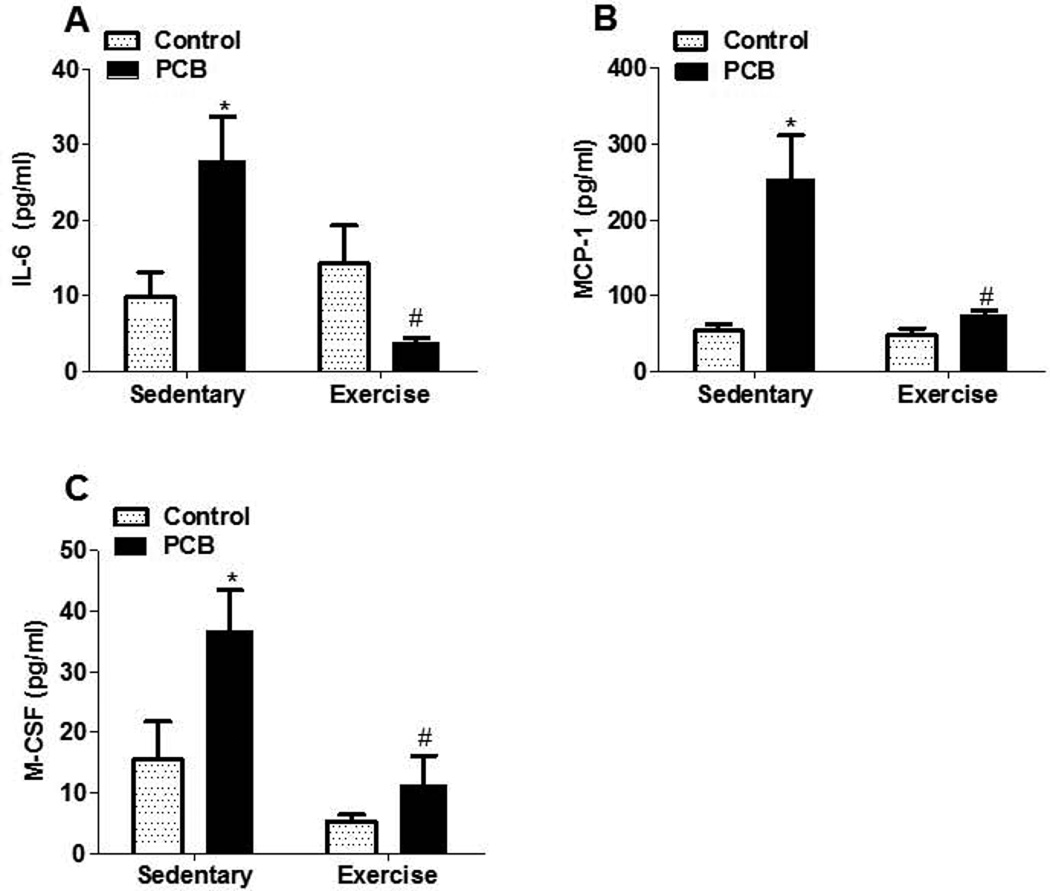
Because cardiovascular disease is recognized as an inflammatory disease (Libby, 2002), selected inflammatory parameters were investigated. For example, interleukin 6 (IL-6), monocyte chemoattractant protein 1 (MCP-1), and macrophage colony stimulating factor (M-CSF) levels all markedly increased in sedentary animals as a result of PCB exposure (Figure 1). In contrast, exercising the PCB-exposed mice resulted in a significant decrease of all these inflammatorily parameters, such as IL-6, MCP-1 and M-CSF, compared to their sedentary counterparts (Figure 1).
Fig. 1.
Exercise prevents upregulation of proinflammatory cytokines induced by PCB 77 exposure. Plasma samples were analyzed for IL-6, MCP-1, and M-CSF levels. Data represent the mean± SEM (n=5). * Significantly different compared to vehicle control, sedentary (p<0.05). #Significantly different compared to PCB77-treated sedentary mice (p<0.05)
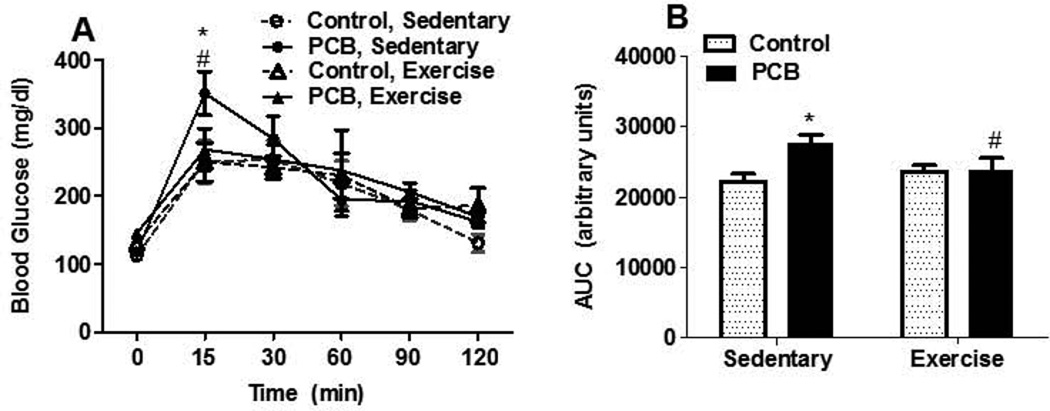
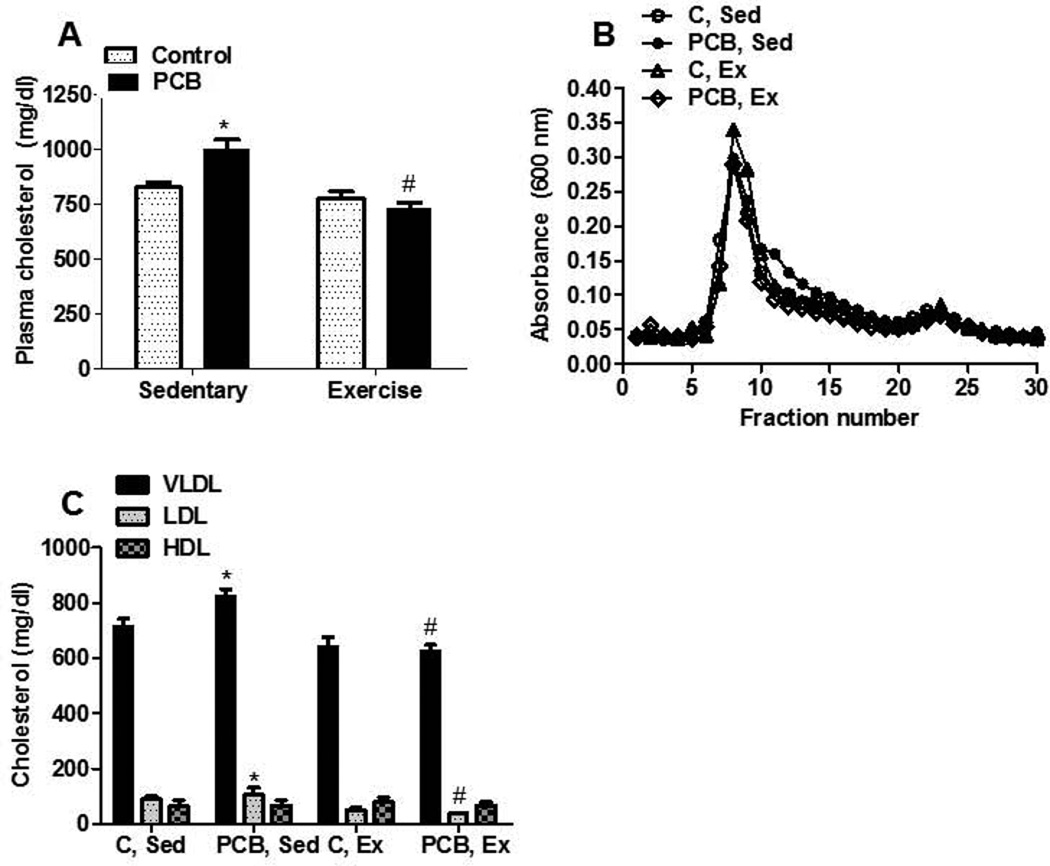
We also examined the effects of exercise on other risk factors associated with cardiovascular disease including impaired glucose tolerance and hypercholesterolemia. Sedentary animals treated with PCB 77 showed a significant increase in blood glucose concentrations, compared with vehicle control and exercise groups, 15 min after a response to a bolus of administered glucose (Figure 2A). The total AUC for blood glucose was significantly increased in sedentary mice treated with PCB 77 (Figure 2B) and exercise attenuated this response. Because hypercholesterolemia (Arsenescu et al. 2008; Lee et al. 2011) is associated with PCB exposure and exercise has been shown by several groups to lower blood cholesterol and cardiovascular disease (Laughlin, 1999; Leung et al., 2008; Thompson et al., 2003), total plasma cholesterol and lipoprotein fractions were measured. PCB treatment increased plasma cholesterol, which was significantly reduced in exercised animals (Figure 3A). Resolution of lipoproteins through size exclusion chromatography followed by nonlinear curve fitting analysis determined that exercise significantly decreased both the VLDL (Figure 3B,C) and LDL cholesterol concentrations (Figure 3B,C) in PCB 77-treated mice compared to sedentary counterparts. No differences in HDL concentrations were found among groups.
Fig. 2.
Exercise attenuates PCB 77-impaired glucose intolerance. A) Blood glucose concentrations. B) Total area under the curve (AUC) calculates the area below the observed concentrations. Data present mean ± SEM (n=8). *Significantly different compared to vehicle control, sedentary (p<0.05) #Significantly different compared to PCB 77-treated, sedentary mice (p<0.05)
Fig. 3.
Exercise reduces total plasma cholesterol and VLDL and LDL cholesterol concentrations. (A) Plasma cholesterol concentrations. (B) Lipoproteins were resolved by size exclusion chromatography. (C) Plasma cholesterol concentrations of lipoprotein fractions. Data represent the mean ± SEM of 6 animals. * Significantly different compared to vehicle control, sedentary (p<0.05). # Significantly different compared to PCB77-treated sedentary mice (p<0.01)
Exercise reduces systemic oxidative stress an d upregulates antioxidant enzymes
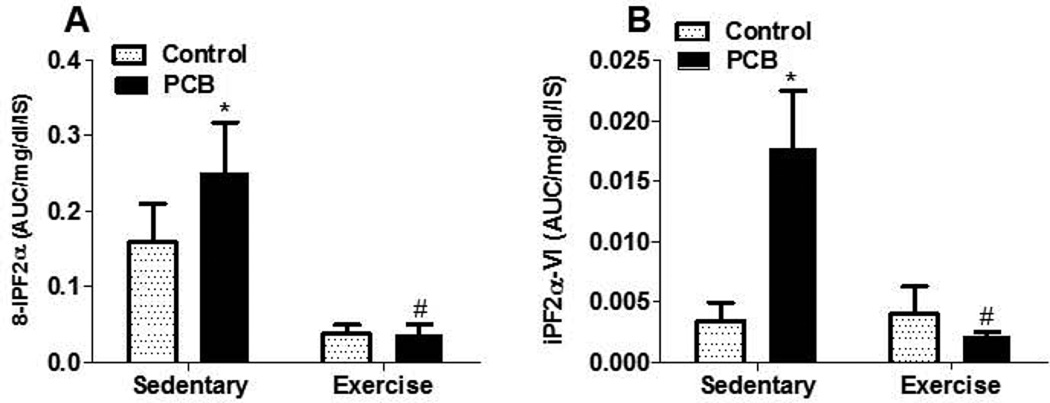
Because inflammatory diseases, such as atherosclerosis, are redox sensitive, we also assessed in vivo systemic oxidative stress. F2-isoprostanes (F2-IsoPs), prostaglandin-like eicosanoids formed during fatty acid peroxidation, were measured in urinary samples from all treatment groups. In PCB-treated animals, exercised mice had significantly lower levels of 8- iPF2 α and iPF2 α-VI compared to sedentary mice (Figure 4). Because of this 8-fold reduction of oxidative stress in exercised animals, we next examined expression of antioxidant enzymes. Overall, exercise significantly upregulated the expression of catalase, glutathione peroxidase (Gpx), glutathione S-reductase (GSR) and glutathione S-transferase (GST) [See Online Resource, Figure S2]. Additionally, exercised groups exposed to PCB 77 had a significant downregulation of CYP1A1 compared to sedentary counterparts, which could contribute to the lower levels of oxidative stress [See Online Resource, Figure S3]. To assess whether exercise had an effect on body burden, PCB 77 and its hydroxyl metabolite OH-PCB 77 were quantified in the plasma, liver, lungs, soleus, kidney, retroperitoneal white adipose tissue, epididymal white adipose tissue, and subcutaneous white adipose tissue. PCB 77 and OH-PCB 77 levels were undetectable in tissues from vehicle-treated mice or PCB 77 treated mice at the conclusion of the study. OH-PCB 77 levels in feces from sedentary mice were approximately 4 fold higher than those from exercised mice [See Online Resource, Figure S3B].
Fig. 4.
Exercise modulates PCB 77-induced oxidative stress. Urine F2-isoprostane levels were measured by HPLC/MS MS. All values were normalized to urine creatinine levels and for IS recovery. Data are represented as mean ± SEM (n=5). * Significantly different compared to vehicle control, sedentary (p<0.05). # Significantly different compared to PCB77-treated sedentary mice (p<0.05)
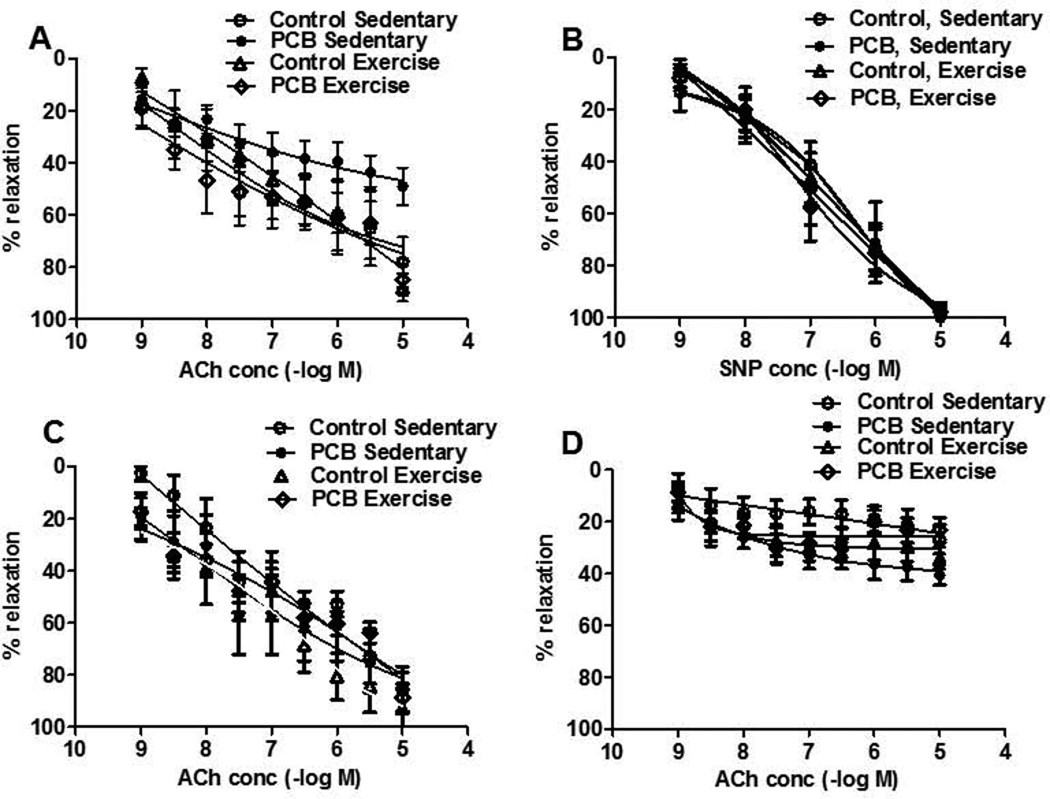
Exercise restores endothelium-dependent relaxation in PCB 77-treated mice
To substantiate the PCB-induced dysfunction of the vascular endothelium, vascular functional studies with isolated vessels were performed. PCB 77 impaired ACh-dependent relaxation, an endothelial-dependent event (Figure 5A). Two way ANOVA RM revealed a statistically significant interaction between treatment groups and concentration (p=0.002). This reduced relaxation was rescued in vessels derived from exercised but not from sedentary animals. There were no differences in groups treated with the NO donor sodium nitroprusside (SNP), indicating that impairment occurred within the vascular endothelium (Figure 5B). Pre incubation with Tempol, a superoxide dismutase mimetic, was necessary to improve the PCB-induced relaxation impairment observed in sedentary animals (Figure 5C). Pre-incubation with L-NAME, a NO inhibitor, significantly reduced endothelium dependent relaxation in all groups (Figure 5D). As previously reported (Kopf, Huwe, & Walker, 2008), there were no differences in endothelium independent relaxation among all treatment groups implicating the importance of the vascular endothelium and not smooth muscle cells.
Fig. 5.
Exercise restores endothelium-dependent relaxation in PCB77 impaired vessels. A) ACh-induced vasodilation was significantly attenuated in PCB, Sedentary mice. p value of the curve (p=0.002). B) No differences were observed in vasorelaxation response to sodium nitroprusside (SNP). C) Pre-incubation with Tempol (10−3 M) restored vasodilation in PCB, Sedentary animals (n=7–9). D) Incubation of L-NAME (10−5 M) significantly reduced ACh-dilation in Control, Sedentary; Control, Exercise; and PCB, Exercise groups but not in PCB, sedentary mice (n=7–9).
Discussion
There is substantial evidence that exposure to persistent organic pollutants including dioxin and PCBs are linked to the incidence of cardiovascular disease and heart failure which remain the leading cause of death in developed nations (Humblet et al., 2008; Sjöberg Lind, Lind, Salihovic, van Bavel, & Lind, 2013). We have shown previously that coplanar PCBs, including PCB 77 and PCB 126 are proinflammatory and atherogenic in vascular endothelial cells (Hennig et al., 2002; Toborek et al., 1995). Coplanar PCBs have also been shown to promote obesity (Arsenescu et al., 2008), atherosclerosis (Arsenescu et al., 2011), and diabetes (Baker et al., 2013; Fierens et al., 2003.).
In most people, body burdens of environmental pollutants are prevalent, and prevention against environmental chemical-induced disease pathologies remains a challenge. Positive lifestyle changes such as healthful nutrition and an increase in physical activity tend to protect against the development of inflammatory diseases such as atherosclerosis, obesity and diabetes (Gil, Ortega, & Maldonado, 2011; Hamer & Stamatakis, 2012; C.-Z. Wang, Mehendale, Calway, & Yuan, 2011). Evidence from our laboratory suggests that antioxidant nutrients and related bioactive compounds found in fruits and vegetables protect against environmental toxic insult to the vascular endothelium by increasing antioxidant defense and by down-regulation of proinflammatory signaling (Hennig et al., 2007; Newsome et al., 2014); however, the role of exercise remains largely unknown.
Data from this study provide evidence for the protective pr operties of physical activity against cardiovascular disease initiated by exposure to a persistent organic pollutant. Exposure to PCB 77 in sedentary animals elevated several risk factors associated with cardiovascular disease including oxidative stress, systemic inflammation, glucose intolerance, and hypercholesteremia. Baker et al. have previously reported that coplanar PCBs induce rapid and sustained glucose intolerance in an AhR-dependent manner (Baker et al., 2013). We demonstrate glucose intolerance in sedentary, PCB-treated animals that is prevented in exercised animals. In the current study, sedentary, PCB-treated animals had significantly higher levels of plasma cholesterol, predominately in the VLDL and LDL fractions. Our results extend previous findings which have shown that dietary exposure to PCB 77 significantly increases hypercholesteremia specifically within the VLDL fraction that is associated with increased atherosclerosis (Arsenescu et al., 2008). Furthermore, studying residents of Anniston, Alabama revealed an association between increased concentrations of PCBs and elevated levels of total serum lipids, total cholesterol and triglycerides (Aminov, Haase, Pavuk, & Carpenter, 2013). Voluntary exercise attenuated the hypercholesteremia observed in PCB-treated mice. These findings suggest that exercise can prevent and/or delay risk factors associated with cardiovascular disease.
Low-grade inflammation is a hallmark of endothelial dysfunction and atherosclerosis (Libby, 2002). We have demonstrated previously that coplanar PCBs can cause endothelial dysfunction as evidenced by an upregulation of inflammatory mediators including the cytokine IL-6 and chemokine MCP-1 (Eske et al. 2013; Han et al. 2012; Majkova et al. 2009). In fact, PCB 77-treated animals that were exposed to exercise had levels of inflammation that were not different from control sedentary animals. Exercise attenuated the PCB 77-mediated induction of inflammatory cytokines and chemokines including MCP-1, IL-6, and M-CSF. Because inflammation is sensitive to redox changes or an increase in oxidative stress, we also assessed in vivo systemic oxidative stress. Results from our study indicate that exposure to PCB 77 leads to a dramatic increase in F2-isoprostanes in sedentary mice, with much lower levels (8-fold) found in exercised animals, suggesting that exercise protects against systemic oxidative stress associated with PCB 77 exposure.
Mechanisms of protective properties of physical exercise such as voluntary exercise are not simple and may involve induction of phase II antioxidant enzymes (Dieter & Vella, 2013; Galassetti & Riddell, 2013; Kelly & Pomp, 2013; Schmidt, Endres, Dimeo, & Jungehulsing, 2013). Our findings demonstrate an upregulation of several phase II antioxidant enzymes, such as Gpx1, GST, and GSR. Additionally, our results show a downregulation of the phase I enzyme, CYP1A1, which has been implicated in contributing to oxidative stress in the presence of coplanar PCBs (Schlezinger, Struntz, Goldstone, & Stegeman, 2006). Furthermore, superoxide can uncouple eNOS (Michel & Vanhoutte, 2010), the enzyme responsible for the production of the potent dilator nitric oxide, thus producing peroxynitrite and reducing endothelial-dependent relaxation. We have previously shown in cultured endothelial cells that exposure to PCB 77 can lead to an increase in peroxynitrite (Lim, Smart, Toborek, & Hennig, 2007).
Endothelial dysfunction is a critical step in the development of cardiovascular disease including atherosclerosis (Libby, Aikawa, & Jain, 2006; Sena, Pereira, & Seiça, 2013). To further investigate the role of the vascular endothelium in PCB exposure, we performed ex vivo vascular reactivity studies. Exposure to PCB 77 led to severe impairment of endothelium dependent dilation or relaxation in sedentary animals. Exercise was able to prevent the PCB-induced impairment. Reduced NO bioavailability is thought to be a primary cause of endothelial dysfunction (Vanhoutte, Shimokawa, Tang, & Feletou, 2009). Our data show that ACh-induced relaxation is blocked in all groups but PCB 77-treated sedentary animals. Because the relaxation response in exercised and control groups is significantly blunted when treated with the NO inhibitor L-NAME, one could argue that increased bioavailability of NO is one of the protective mechanisms of exercise within the vascular endothelium. In fact, pre-incubation with L-NAME significantly reduced endothelium dependent relaxation in all groups, except in PCB-treated sedentary animals. The superoxide mimetic Tempol rescued the reduced vasodilation in PCB-treated sedentary animals, suggesting a relationship between PCB exposure, increased oxidative stress and dysfunction of the vascular endothelium. Previous publications have reported rescue of dioxin-induced endothelial dysfunction by TEMPOL in sedentary mice (Kopf & Walker, 2010). This implies that exercise might normalize the redox status within the vascular endothelium, thus improving relaxation. Because pre-incubation with L-NAME, a NO inhibitor, significantly reduced endothelium dependent relaxation in all groups, except in PCB-treated sedentary animals, NO-mediated mechanism may also contribute to the protective effects of exercise against PCB-induced dysfunction. Our work in cultured endothelial cells suggests that PCB can cause dysfunctional NO signaling (Lim et al., 2007).
In addition to well-established protective mechanisms of exercise, including down-regulation of inflammation through upregulation of antioxidant genes and improved endothelial-dependent relaxation, our data with PCBs also implicate increased metabolism of lipophilic compounds such as PCBs. Compared to sedentary animals, we found that exercised animals exposed to PCB 77 had significantly less OH-PCB 77 metabolites within their feces. This suggests indirectly that exercise can increase drug metabolism and that most of the PCB 77 may have been metabolized and/or excreted prior to the time of measurements (at the end of study). Although extensive human data are lacking, biotransformation and metabolism of PCB 77 can lead to multiple PCB metabolites including hydroxylated and sulfated versions in mammalian species (Robertson &Hansen 2001). Within this study, the hydroxylated PCB metabolite OH-PCB 77 was measured; however the metabolism of PCB 77 can produce additional metabolites such as 4’-OH-PCB 79, which have been shown to induce AhR-mediated activity (Machala et al., 2004).
Also, the sustained CYP1A1 induction in the absence of measurable PCB 77 may be due to epigenetic changes induced by the exposure to the environmental pollutant. PCBs have been shown to produce epigenetic changes including changes in DNA methylation global patterns, modifying the histone pattern of the CYP1A1 promoter, and causing histone post-translational modifications in the liver (Casati, Sendra, Poletti, Negri-Cesi, & Celotti, 2013). Interestingly, PCB 77 causes a low level of AhR recruitment to the gene enhancer that lasts for a longer period of time than activation by other AhR ligands including TCDD, PCB 126, or PCB 169 (Ovesen, Schnekenburger, & Puga, 2011). This increased period of transcriptional activation could account for the continued gene expression of CYP1A1 thus leading to increases in oxidative stress which contribute to PCB-induced cardiovascular risk factors.
A better understanding of the distribution, metabolism, and excretion of PCB 77 and related coplanar PCBs may allow for more effective biomedically-based therapeutic strategies. Nutritional intervention, such as with olestra, can interfere with the absorption of dietary PCBs and has been shown to result in their reduced systemic accumulation (Jandacek, Rider, Keller, & Tso, 2010). Similar to such nutritional intervention that suggests modification of enterohepatic circulation or reduced re-absorption of organochlorine compounds (Jandacek &Genuis 2013), PCBs and their metabolites may undergo enhanced enterohepatic circulation during exercise. Moreover, it has been reported that voluntary wheel running increases whole-body cholesterol turnover in mice (Meissner et al. 2010) as well as increased bile acid and cholesterol excretion (Meissner et al. 2011). Voluntary wheel running could modify enterohepatic circulation and thus lead to increased excretion of lipophilic compounds including PCBs. Exercise also can alter pharmacokinetics by affecting drug absorption and hepatic and renal clearance of drugs (Persky, Eddington, & Derendorf, 2003). Four weeks of voluntary exercise in C57BL/6 mice led to an upregulation of CYP7A1 and CYP27, two CYP enzymes that play a role in cholesterol metabolism (Wilund, Feeney, Tomayko, Chung, & Kim, 2008). Interestingly, in a recent human study it was shown that drastic weight loss in humans can lead to a temporary increase in serum POPs and, within 6–12 months, to a significant 15% decrease in total PCB body burden (M. J. Kim et al., 2011). Because exercise is a successful weight loss strategy, future studies should assess the impact of exercise on PCB body burden chan ges over an extended period of time following PCB exposure.
In summary, our study provides experimental evidence that exercise is beneficial for protecting the vasculature against PCB-induced oxidative stress and inflammation. Coplanar PCBs are persistent and a significant risk factor for endothelial injury and associated cardiovascular disease. More studies are needed to determine the amount of exercise required to prevent or mitigate adverse health effects associated with PCB exposure. This work suggests that lifestyle modifications including aerobic exercise can be utilized as a therapeutic approach for the prevention of adverse health effects induced by environmental pollutants such as PCBs.
Supplementary Material
Acknowledgements
We thank the University of Kentucky Center of Research in Obesity and Cardiovascular Disease COBRE P20 GM103527-06 for its use of its core facilities, specifically the EchoMRI machine to measure body composition. We thank Alan Daugherty and Jess Moorleghen for their assistance in vascular reactivity studies.
Funding: This work was supported by the National Institute of Environmental Health Sciences at the National Institutes of Health [P42ES007380], the University of Kentucky Agricultural Experiment Station, and the UK Center for Muscle Biology.
References
- Ajijola OA, Dong C, Herderick EE, Ma Q, Goldschmidt-Clermont PJ, Yan Z. Voluntary running suppresses proinflammatory cytokines and bone marrow endothelial progenitor cell levels in apolipoprotein-E-deficient mice. Antioxid Redox Signal. 2009;11(1):15–23. doi: 10.1089/ars.2008.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminov Z, Haase RF, Pavuk M, Carpenter DO. Analysis of the effects of exposure to polychlorinated biphenyls and chlorinated pesticides on serum lipid levels in residents of Anniston, Alabama. Environmental Health : A Global Access Science Source. 2013;12:108. doi: 10.1186/1476-069X-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect. 2008;116(6):761–768. doi: 10.1289/ehp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenescu V, Arsenescu R, Parulkar M, Karounos M, Zhang X, Baker N, Cassis LA. Polychlorinated biphenyl 77 augments angiotensin II-induced atherosclerosis and abdominal aortic aneurysms in male apolipoprotein E deficient mice. Toxicol Appl Pharmacol. 2011;257(1):148–154. doi: 10.1016/j.taap.2011.08.028. doi:S0041-008X(11)00342-5 [pii] 10.1016/j.taap.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NA, Karounos M, English V, Fang J, Wei Y, Stromberg A, Cassis LA. Coplanar polychlorinated biphenyls impair glucose homeostasis in lean C57BL/6 mice and mitigate beneficial effects of weight loss on glucose homeostasis in obese mice. Environmental Health Perspectives. 2013;121(1):105–110. doi: 10.1289/ehp.1205421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casati L, Sendra R, Poletti A, Negri-Cesi P, Celotti F. Androgen receptor activation by polychlorinated biphenyls: epigenetic effects mediated by the histone demethylase Jarid1b. Epigenetics : Official Journal of the DNA Methylation Society. 2013;8(10):1061–1068. doi: 10.4161/epi.25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JJ, Eum SY, Rampersaud E, Daunert S, Abreu MT, Toborek M. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environmental Health Perspectives. 2013;121(6):725–730. doi: 10.1289/ehp.1306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A. Mouse models of atherosclerosis. The American Journal of the Medical Sciences. 2002;323(1):3–10. doi: 10.1097/00000441-200201000-00002. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11814139. [DOI] [PubMed] [Google Scholar]
- Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105(11):1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieter BP, Vella CA. A proposed mechanism for exercise attenuated methylglyoxal accumulation: Activation of the ARE-Nrf pathway and increased glutathione biosynthesis. Medical Hypotheses. 2013;81(5):813–815. doi: 10.1016/j.mehy.2013.08.034. [DOI] [PubMed] [Google Scholar]
- Du Z-Y, Zhang J, Wang C, Li L, Man Q, Lundebye A-K, Frøyland L. Risk-benefit evaluation of fish from Chinese markets: nutrients and contaminants in 24 fish species from five big cities and related assessment for human health. The Science of the Total Environment. 2012;416:187–199. doi: 10.1016/j.scitotenv.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Eske K, Newsome B, Han SG, Murphy M, Bhattacharyya D, Hennig B. PCB 77 dechlorination products modulate pro-inflammatory events in vascular endothelial cells. Environmental Science and Pollution Research International. 2013 doi: 10.1007/s11356-013-1591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser KA, Su W, Matveev S, Wong V, Zeng L, McCarthy JJ, Gong MC. Voluntary wheel running ameliorates vascular smooth muscle hyper-contractility in type 2 diabetic db/db mice. Applied Physiology, Nutrition, and Metabolism = Physiologie Appliquée, Nutrition et Métabolisme. 2007;32(4):711–720. doi: 10.1139/H07-058. [DOI] [PubMed] [Google Scholar]
- Fierens S, Mairesse H, Heilier J-F, De Burbure C, Focant J-F, Eppe G, Bernard A. Dioxin/polychlorinated biphenyl body burden, diabetes and endometriosis: findings in a population-based study in Belgium. Biomarkers : Biochemical Indicators of Exposure, Response, and Susceptibility to Chemicals. 2003;8(6):529–534. doi: 10.1080/1354750032000158420. [DOI] [PubMed] [Google Scholar]
- Galassetti P, Riddell MC. Exercise and type 1 diabetes (T1DM) Comprehensive Physiology. 2013;3(3):1309–1336. doi: 10.1002/cphy.c110040. [DOI] [PubMed] [Google Scholar]
- Gielen S, Schuler G, Hambrecht R. Exercise training in coronary artery disease and coronary vasomotion. Circulation. 2001;103(1):E1–E6. doi: 10.1161/01.cir.103.1.e1. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11136704. [DOI] [PubMed] [Google Scholar]
- Gil A, Ortega RM, Maldonado J. Wholegrain cereals and bread: a duet of the Mediterranean diet for the prevention of chronic diseases. Public Health Nutrition. 2011;14(12A):2316–2322. doi: 10.1017/S1368980011002576. [DOI] [PubMed] [Google Scholar]
- Goncharov A, Pavuk M, Foushee HR, Carpenter DO. Blood pressure in relation to concentrations of PCB congeners and chlorinated pesticides. Environmental Health Perspectives. 2011;119(3):319–325. doi: 10.1289/ehp.1002830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M, Stamatakis E. Low-dose physical activity attenuates cardiovascular disease mortality in men and women with clustered metabolic risk factors. Circulation. Cardiovascular Quality and Outcomes. 2012;5(4):494–499. doi: 10.1161/CIRCOUTCOMES.112.965434. [DOI] [PubMed] [Google Scholar]
- Han SG, Eum SY, Toborek M, Smart E, Hennig B. Polychlorinated biphenyl-induced VCAM-1 expression is attenuated in aortic endothelial cells isolated from caveolin-1 deficient mice. Toxicol Appl Pharmacol. 2010;246(1–2):74–82. doi: 10.1016/j.taap.2010.04.009. doi:S0041-008X(10)00136-5 [pii] 10.1016/j.taap.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SG, Han S-S, Toborek M, Hennig B. EGCG protects endothelial cells against PCB 126-induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes. Toxicology and Applied Pharmacology. 2012;261(2):181–188. doi: 10.1016/j.taap.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helyar SG, Patel B, Headington K, El Assal M, Chatterjee PK, Pacher P, Mabley JG. PCB-induced endothelial cell dysfunction: role of poly(ADP-ribose) polymerase. Biochem Pharmacol. 2009;78(8):959–965. doi: 10.1016/j.bcp.2009.06.019. doi:S0006-2952(09)00488-2 [pii] 10.1016/j.bcp.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Ettinger AS, Jandacek RJ, Koo S, McClain C, Seifried H, Suk WA. Using nutrition for intervention and prevention against environmental chemical toxicity and associated diseases. Environ Health Perspect. 2007;115(4):493–495. doi: 10.1289/ehp.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Meerarani P, Slim R, Toborek M, Daugherty A, Silverstone AE, Robertson LW. Proinflammatory properties of coplanar PCBs: in vitro and in vivo evidence. Toxicol Appl Pharmacol. 2002;181(3):174–183. doi: 10.1006/taap.2002.9408. doi:S0041008X02994081 [pii] [DOI] [PubMed] [Google Scholar]
- Humblet O, Birnbaum L, Rimm E, Mittleman MA, Hauser R. Dioxins and cardiovascular disease mortality. Environmental Health Perspectives. 2008;116(11):1443–1448. doi: 10.1289/ehp.11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandacek RJ, Genuis SJ. An assessment of the intestinal lumen as a site for intervention in reducing body burdens of organochlorine compounds. The Scientific World Journal. 2013;2013:205–621. doi: 10.1155/2013/205621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandacek RJ, Rider T, Keller ER, Tso P. The effect of olestra on the absorption, excretion and storage of 2,2’,5,5' tetrachlorobiphenyl; 3,3',4,4' tetrachlorobiphenyl; and perfluorooctanoic acid. Environment International. 2010;36(8):880–883. doi: 10.1016/j.envint.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SA, Pomp D. Genetic determinants of voluntary exercise. Trends in Genetics : TIG. 2013;29(6):348–357. doi: 10.1016/j.tig.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Marchand P, Henegar C, Antignac JP, Alili R, Poitou C, Clement K. Fate and complex pathogenic effects of dioxins and polychlorinated biphenyls in obese subjects before and after drastic weight loss. Environ Health Perspect. 2011;119(3):377–383. doi: 10.1289/ehp.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-J, Shin Y-O, Bae J-S, Lee J-B, Ham J-H, Son Y-J, Yang H-M. Beneficial effects of cardiac rehabilitation and exercise after percutaneous coronary intervention on hsCRP and inflammatory cytokines in CAD patients. Pflügers Archiv : European Journal of Physiology. 2008;455(6):1081–1088. doi: 10.1007/s00424-007-0356-6. [DOI] [PubMed] [Google Scholar]
- Kopf PG, Huwe JK, Walker MK. Hypertension, cardiac hypertrophy, and impaired vascular relaxation induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin are associated with increased superoxide. Cardiovasc Toxicol. 2008;8(4):181–193. doi: 10.1007/s12012-008-9027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf PG, Walker MK. 2,3,7,8-tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1. Toxicol Appl Pharmacol. 2010;245(1):91–99. doi: 10.1016/j.taap.2010.02.007. doi:S0041-008X(10)00058-X [pii] 10.1016/j.taap.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs U, Wassmann S, Czech T, Munzel T, Eisenhauer M, Bohm M, Nickenig G. Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25(4):809–814. doi: 10.1161/01.ATV.0000158311.24443.af. doi:01.ATV.0000158311.24443.af [pii] 10.1161/01.ATV.0000158311.24443.af. [DOI] [PubMed] [Google Scholar]
- Laufs U, Werner N, Link A, Endres M, Wassmann S, Jürgens K, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109(2):220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- Laughlin MH. Cardiovascular response to exercise. The American Journal of Physiology. 1999;277(6 Pt 2):S244–S259. doi: 10.1152/advances.1999.277.6.S244. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10644251. [DOI] [PubMed] [Google Scholar]
- Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, Jacobs DR., Jr Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS One. 2011;6(1):e15977. doi: 10.1371/journal.pone.0015977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung FP, Yung LM, Laher I, Yao X, Chen ZY, Huang Y. Exercise, vascular wall and cardiovascular diseases: an update (Part 1) Sports Med. 2008;38(12):1009–1024. doi: 10.2165/00007256-200838120-00005. doi:5 [pii] [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. doi:10.1038/nature01323 nature01323 [pii] [DOI] [PubMed] [Google Scholar]
- Libby P, Aikawa M, Jain MK. Vascular endothelium and atherosclerosis. Handbook of Experimental Pharmacology. 2006;(176 Pt 2):285–306. doi: 10.1007/3-540-36028-x_9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16999230. [DOI] [PubMed]
- Lim EJ, Smart EJ, Toborek M, Hennig B. The role of caveolin-1 in PCB77-induced eNOS phosphorylation in human-derived endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293(6):H3340–H3347. doi: 10.1152/ajpheart.00921.2007. doi:00921.2007 [pii] 10.1152/ajpheart.00921.2007. [DOI] [PubMed] [Google Scholar]
- Lind L, Lind PM. Can persistent organic pollutants and plastic-associated chemicals cause cardiovascular disease? Journal of Internal Medicine. 2012;271(6):537–553. doi: 10.1111/j.1365-2796.2012.02536.x. [DOI] [PubMed] [Google Scholar]
- Lind PM, van Bavel B, Salihovic S, Lind L. Circulating levels of persistent organic pollutants (POPs) and carotid atherosclerosis in the elderly. Environmental Health Perspectives. 2012;120(1):38–43. doi: 10.1289/ehp.1103563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machala M, Bláha L, Lehmler H-J, Plísková M, Májková Z, Kapplová P, Robertson LW. Toxicity of hydroxylated and quinoid PCB metabolites: inhibition of gap junctional intercellular communication and activation of aryl hydrocarbon and estrogen receptors in hepatic and mammary cells. Chemical Research in Toxicology. 2004;17(3):340–347. doi: 10.1021/tx030034v. [DOI] [PubMed] [Google Scholar]
- Majkova Z, Layne J, Sunkara M, Morris AJ, Toborek M, Hennig B. Omega-3 fatty acid oxidation products prevent vascular endothelial cell activation by coplanar polychlorinated biphenyls. Toxicol Appl Pharmacol. 2011;251(1):41–49. doi: 10.1016/j.taap.2010.11.013. doi:S0041-008X(10)00445-X [pii] 10.1016/j.taap.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkova Z, Smart E, Toborek M, Hennig B. Up-regulation of endothelial monocyte chemo attractant protein-1 by coplanar PCB77 is caveolin-1-dependent. Toxicol Appl Pharmacol. 2009;237(1):1–7. doi: 10.1016/j.taap.2009.02.016. doi:S0041-008X(09)00084-2 [pii] 10.1016/j.taap.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning MW, Cassis LA, Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2003;23(3):483–488. doi: 10.1161/01.ATV.0000058404.92759.32. doi:10.1161/01.ATV.0000058404.92759.32 01.ATV.0000058404.92759.32 [pii] [DOI] [PubMed] [Google Scholar]
- Meissner M, Lombardo E, Havinga R, Tietge UJ, Kuipers F, Groen AK. Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis. 2011;218:323–329. doi: 10.1016/j.atherosclerosis.2011.06.040. [DOI] [PubMed] [Google Scholar]
- Meissner M, Havinga R, Boverhof R, Kema I, Groen AK, Kuipers F. Exercise enhances whole-body cholesterol turnover in mice. Medicine and Science in Sports and Exercise. 2010;42(8):1460–1468. doi: 10.1249/MSS.0b013e3181cfcb02. [DOI] [PubMed] [Google Scholar]
- Michel T, Vanhoutte PM. Cellular signaling and NO production. Pflugers Arch. 2010;459(6):807–816. doi: 10.1007/s00424-009-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005;25(2):279–286. doi: 10.1161/01.ATV.0000152605.64964.c0. doi:01.ATV.0000152605.64964.c0 [pii] 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- Napoli C, Williams-Ignarro S, de Nigris F, Lerman LO, D’Armiento FP, Crimi E, Ignarro LJ. Physical training and metabolic supplementation reduce spontaneous atherosclerotic plaque rupture and prolong survival in hypercholesterolemic mice. Proc Natl Acad Sci U S A. 2006;103(27):10479–10484. doi: 10.1073/pnas.0602774103. doi:0602774103 [pii] 10.1073/pnas.0602774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome BJ, Petriello MC, Han SG, Murphy MO, Eske KE, Sunkara M, Hennig B. Green tea diet decreases PCB 126-induced oxidative stress in mice by up-regulating antioxidant enzymes. The Journal of Nutritional Biochemistry. 2014;25(2):126–135. doi: 10.1016/j.jnutbio.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovesen JL, Schnekenburger M, Puga A. Aryl hydrocarbon receptor ligands of widely different toxic equivalency factors induce similar histone marks in target gene chromatin. Toxicological Sciences : An Official Journal of the Society of Toxicology. 2011;121(1):123–131. doi: 10.1093/toxsci/kfr032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persky AM, Eddington ND, Derendorf H. A review of the effects of chronic exercise and physical fitness level on resting pharmacokinetics. International Journal of Clinical Pharmacology and Therapeutics. 2003;41(11):504–516. doi: 10.5414/cpp41504. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14620948. [DOI] [PubMed] [Google Scholar]
- Petriello MC, Newsome B, Hennig B. Influence of nutrition in PCB-induced vascular inflammation. Environmental Science and Pollution Research International. 2013 doi: 10.1007/s11356-013-1549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Di Raimondo D, Tuttolomondo A, Buttà C, Milio G, Licata G. Effects of physical exercise on inflammatory markers of atherosclerosis. Current Pharmaceutical Design. 2012;18(28):4326–4349. doi: 10.2174/138161212802481192. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22390642. [DOI] [PubMed] [Google Scholar]
- Pynn M, Schafer K, Konstantinides S, Halle M. Exercise training reduces neointimal growth and stabilizes vascular lesions developing after injury in apolipoprotein e-deficient mice. Circulation. 2004;109(3):386–392. doi: 10.1161/01.CIR.0000109500.03050.7C. doi:10.1161/01.CIR.0000109500.03050.7C 01.CIR.0000109500.03050.7C [pii] [DOI] [PubMed] [Google Scholar]
- Ramadass P, Meerarani P, Toborek M, Robertson LW, Hennig B. Dietary flavonoids modulate PCB-induced oxidative stress, CYP1A1 induction, and AhR-DNA binding activity in vascular endothelial cells. Toxicol Sci. 2003;76(1):212–219. doi: 10.1093/toxsci/kfg227. doi:10.1093/toxsci/kfg227 kfg227 [pii] [DOI] [PubMed] [Google Scholar]
- Rateri DL, Moorleghen JJ, Balakrishnan A, Owens aP, Howatt Da, Subramanian V, Daugherty A. Endothelial cell-specific deficiency of Ang II type 1a receptors attenuates Ang II-induced ascending aortic aneurysms in LDL receptor−/− mice. Circulation Research. 2011;108(5):574–581. doi: 10.1161/CIRCRESAHA.110.222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson L, Hansen L. PCB: recent advances in environmental toxicology and health effects. Lexington, KY: The University press of Kentucky; 2001. p. 461. [Google Scholar]
- Schlezinger JJ, Struntz WDJ, Goldstone JV, Stegeman JJ. Uncoupling of cytochrome P450 1A and stimulation of reactive oxygen species production by co-planar polychlorinated biphenyl congeners. Aquatic Toxicology (Amsterdam, Netherlands) 2006;77(4):422–432. doi: 10.1016/j.aquatox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Schmidt W, Endres M, Dimeo F, Jungehulsing GJ. Train the vessel, gain the brain: physical activity and vessel function and the impact on stroke prevention and outcome in cerebrovascular disease. Cerebrovascular Diseases (Basel, Switzerland) 2013;35(4):303–312. doi: 10.1159/000347061. [DOI] [PubMed] [Google Scholar]
- Sena CM, Pereira AM, Seiça R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochimica et Biophysica Acta. 2013;1832(12):2216–2231. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Sergeev AV, Carpenter DO. Hospitalization rates for coronary heart disease in relation to residence near areas contaminated with persistent organic pollutants and other pollutants. Environmental Health Perspectives. 2005;113(6):756–761. doi: 10.1289/ehp.7595. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1257602&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg Lind Y, Lind PM, Salihovic S, van Bavel B, Lind L. Circulating levels of persistent organic pollutants (POPs) are associated with left ventricular systolic and diastolic dysfunction in the elderly. Environmental Research. 2013;123:39–45. doi: 10.1016/j.envres.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Szostak J, Laurant P. The forgotten face of regular physical exercise: a “natural” anti-atherogenic activity. Clinical Science (London, England : 1979) 2011;121(3):91–106. doi: 10.1042/CS20100520. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Wenger NK. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical. Circulation. 2003;107(24):3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. doi:10.1161/01.CIR.0000075572.40158.77 107/24/3109 [pii] [DOI] [PubMed] [Google Scholar]
- Toborek M, Barger SW, Mattson MP, Espandiari P, Robertson LW, Hennig B. Exposure to polychlorinated biphenyls causes endothelial cell dysfunction. J Biochem Toxicol. 1995;10(4):219–226. doi: 10.1002/jbt.2570100406. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8568836. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 2009;196(2):193–222. doi: 10.1111/j.1748-1716.2009.01964.x. doi:APS1964 [pii] 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- Wang C-Z, Mehendale SR, Calway T, Yuan C-S. Botanical flavonoids on coronary heart disease. The American Journal of Chinese Medicine. 2011;39(4):661–671. doi: 10.1142/S0192415X1100910X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Reiterer G, Toborek M, Hennig B. Changing ratios of omega-6 to omega-3 fatty acids can differentially modulate polychlorinated biphenyl toxicity in endothelial cells. Chem Biol Interact. 2008;172(1):27–38. doi: 10.1016/j.cbi.2007.11.003. doi:S0009-2797(07)00317-1 [pii] 10.1016/j.cbi.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whayne TF, Maulik N. Nutrition and the healthy heart with an exercise boost. Canadian Journal of Physiology and Pharmacology. 2012;90(8):967–976. doi: 10.1139/y2012-074. [DOI] [PubMed] [Google Scholar]
- Whyte JJ, Laughlin MH. The effects of acute and chronic exercise on the vasculature. Acta Physiologica (Oxford, England) 2010;199(4):441–450. doi: 10.1111/j.1748-1716.2010.02127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilund KR, Feeney LA, Tomayko EJ, Chung HR, Kim K. Endurance exercise training reduces gallstone development in mice. Journal of Applied Physiology (Bethesda, Md. : 1985) 2008;104(3):761–765. doi: 10.1152/japplphysiol.01292.2007. [DOI] [PubMed] [Google Scholar]
- Yang A-L, Chen H-I. Chronic exercise reduces adhesion molecules/iNOS expression and partially reverses vascular responsiveness in hypercholesterolemic rabbit aortae. Atherosclerosis. 2003;169(1):11–17. doi: 10.1016/s0021-9150(03)00013-3. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12860246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.