Abstract
Background
Early diagnosis and treatment of complications after major abdominal surgery can decrease associated morbidity and mortality. Postoperative CRP levels have shown a strong correlation with complications. Aim of this systematic review and pooled-analysis was to assess postoperative values of CRP as a marker for major complications and construct a prediction model.
Study design
A systematic review was performed for CRP levels as a predictor for complications after major abdominal surgery (MAS). Raw data was obtained from seven studies, including 1427 patients. A logit regression model assessed the probability of major complications as a function of CRP levels on the third postoperative day. Two practical cut-offs are proposed: an optimal cut-off for safe discharge in a fast track protocol and another for early identification of patients with increased risk for major complications.
Results
A prediction model was calculated for major complications as a function of CRP levels on the third postoperative day. Based on the model several cut-offs for CRP are proposed. For instance, a two cut-off system may be applied, consisting of a safe discharge criterion with CRP levels below 75 mg/L, with a negative predictive value of 97.2%. A second cut-off is set at 215 mg/L (probability 20%) and serves as a predictor of complications, indicating additional CT-scan imaging.
Conclusions
The present study provides insight in the interpretation of CRP levels after major abdominal surgery, proposing a prediction model for major complications as a function of CRP on postoperative day 3. Cut-offs for CRP may be implemented for safe early-discharge in a fast-track protocol and, secondly as a threshold for additional examinations, such as CT-scan imaging, even in absence of clinical signs, to confirm or exclude major complications. The prediction model allows for setting a cut-off at the discretion of individual surgeons or surgical departments.
Introduction
Twenty percent of patients after major abdominal surgery (MAS) have a major postoperative complication, which requires invasive treatment and is associated with increased morbidity and mortality [1, 2].
Early diagnosis and treatment of major complications is associated with improved outcomes [3, 4]. However, early detection may be challenging and can be difficult to distinguish from the physiological postoperative systemic inflammatory response syndrome (SIRS) [5, 6]. Major complications, such as anastomotic leakage, may be clinically silent and not become evident until critical illness develops after a median of seven days [7].
A standardized postoperative quality-control algorithm for risk assessment of complications should aim at early identification of patients for a safe early discharge—as in a fast-track protocol—or help to identify patients at risk of developing major complications. This policy could decrease associated morbidity and mortality [8].
Several examinations contributed to the development of such a postoperative quality control algorithm. Postoperatively, clinical parameters, laboratory markers and imaging all contribute to identification of complications. Clinical parameters have shown to be non-specific, especially in the early postoperative phase, warranting additional examinations [1]. The acute phase protein CRP (C-reactive protein) has shown to have a strong correlation with postoperative complications [9, 10]. Postoperative CRP levels rise in response to the initial surgery in order to peak 48–72 hours postoperatively and decrease thereafter [11]. In patients with a complicated postoperative course, postoperative CRP levels remain elevated [9, 12].
Many studies aimed to establish a cut-off value for CRP as a predictor for postoperative complications, such as anastomotic leakage or septic complications [10, 13–17]. Results are however conflicting, postoperative complications are not stratified and most studies merely aimed to set a cut-off for CRP as a marker for anastomotic leakage after colorectal surgery [10, 13, 16, 17].
With such a variance in cut-offs and definitions, it would be of interest to assess the predictive value of CRP levels as a continuous value. This would enable assessment of separate CRP levels, allowing surgical wards to set a cut-off for additional examinations or safe discharge at their own discretion. Aim of this study was to conduct a systematic review and pooled-analysis of literature assessing the prediction of major complications as a function of continuous levels of CRP.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA statement) [18].
Definitions
All complications were stratified according to the Clavien-Dindo (CD) classification, which grades complications according to the required treatment [19, 20]. In line with recent literature, we further divided the CD classification into two groups, minor and major complications respectively [21]. Minor complications consist of grade I and grade II complications, which require no treatment or pharmacological treatment. Wound infections drained at the bedside are also considered minor complications. Major complications consist of CD grades III and up. These complications require surgical, endoscopic or radiological intervention and might lead to Intensive Care Unit admission or death. These complications include for instance reoperation for anastomotic leak or percutaneous drainage of abscesses.
Data sources and searches
Systematic electronic searches were conducted in the bibliographic database MEDLINE, Embase and the Cochrane Library (via Wiley) from inception to August 2014, in collaboration with a medical librarian. Search terms included controlled terms from MeSH as well as free text terms in PubMed, EMtree in Embase. We used free text terms only in The Cochrane library. An additional manual-check of article references was done in order to identify potential records of interest. The search strategy was altered for Embase and the Cochrane Library databases. We applied a language restriction; only English, German and Dutch articles were included. The search was further limited to “clinical studies” and articles with an abstract. The separate results from all searches were reconciled for duplicate articles. The abstracts obtained by the search in the three databases were combined and were used to select suitable articles by two reviewers independently (J.S. and A.H.), after which the full-text versions were retrieved and independently reviewed for inclusion by the two reviewers (J.S. and A.H.). Investigators with previous experience in reviews conducted the systematic review (A.H., E.J. and J.S.).
Inclusion and exclusion
Studies reporting on analyzing the diagnostic accuracy of the serum CRP as marker for major postoperative complications after MAS, which we defined as a surgical resection performed on upper-gastrointestinal, hepato-pancreatico-biliary and colorectal organs, as carried out by either primary anastomosis and/or creation of definitive stoma, were included. For instance, cholecystectomy is not included, since no anastomosis or ostomy is performed. Previous work has shown no statistically significant differences in postoperative CRP levels after upper-gastrointestinal, hepato-pancreatico-biliary and colorectal surgery [1].
The studies had to provide adequate information on the time of CRP measurement, the outcome and unit in which it was measured and also postoperative complications. Sensitivity, specificity, positive and negative predictive values, and the area under the curve had to be measured using logistic regression analysis (ROC curve). Exclusion criteria were: 1) duplicate studies of previously published data; 2) data pooled from several different postoperative days (POD); 3) no clear delineation provided of the time of CRP measurement; 4) postoperative complications that consisted only of minor surgical site infections (SSI); and 5) pediatric studies.
Quality Assessment
The quality of the studies was appraised using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) criteria [22]. The QUADAS-tool assesses the internal and external validity of diagnostic accuracy studies, by reviewing the quality of the studies, the risk of bias and the concerns regarding applicability. Two reviewers (J.S. and A.H.) independently applied the QUADAS-tool to all the studies included in the pooled-analysis for making an overall risk-of-bias judgment.
Data extraction and statistical analysis
Raw data was collected and pooled ROC-analysis was performed. True-/false positive, true-/false negative, sensitivity, specificity, area under the curve (AUC), positive-/ negative predictive and true cut-off values were calculated for each individual study. Data on true-/false positive, true-/false negative, sensitivity, specificity and the AUC were calculated. The cut-off of the pooled data was determined by maximizing the sum of the sensitivity and specificity (Youden index) [23]. Heterogeneity with respect to sensitivity and specificity was tested by a likelihood-ratio test for the variance component in a random-effects model. Positive predictive value (PPV) and negative predictive value (NPV) are study dependent and can therefore not be pooled. The risk of major complications was predicted from continuous CRP values by a logit regression model [24]. Reported ninety-five percent confidence intervals were obtained via Wald’s method [25].
Diagnostic accuracy for CRP as marker for major complications was calculated by ROC-analysis, maximizing the sum of the sensitivity and specificity (Youden-index). With the highest-measured accuracy for CRP on POD 3, a logit regression model of the probability of major complications as a function of continuous CRP levels was calculated. Based on this regression model, two practical cut-offs were calculated: a) the first as a marker with a high negative predictive value, which could be used for safe early discharge in a fast-track protocol; and b) a marker with a high positive predictive value, to identify patients in whom a CT-scan is strictly indicated for confirming or excluding a major postoperative complication.
Results
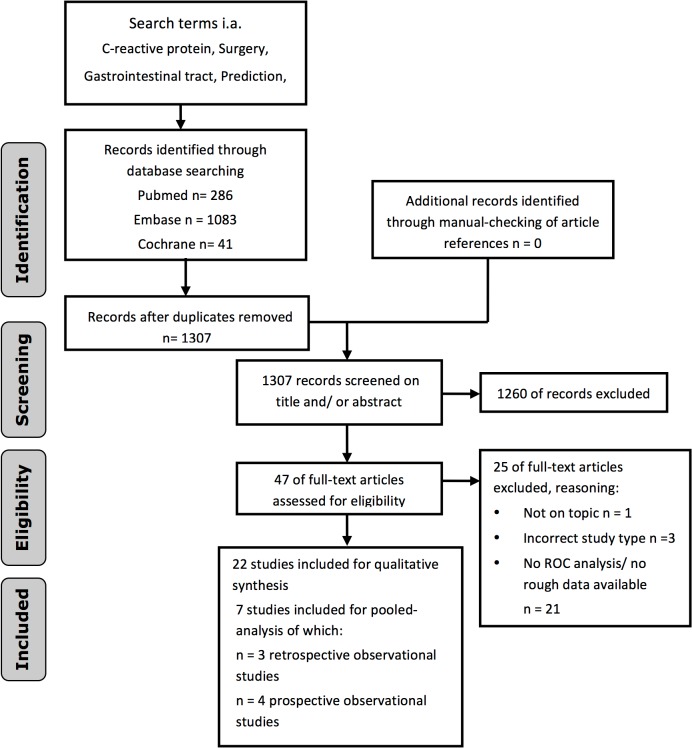
A total of 1307 articles were initially identified and screened. A flow-chart for the literature search is depicted in Fig 1. Based on abstract and title, 1260 articles were discarded. Forty-six articles were assessed in full-text format, whereas 25 articles were excluded because of different reasons as depicted in the flowchart of Fig 1. Finally a total of 22 studies were identified that stated an optimal cut-off value for CRP as marker for safe discharge, or as a marker for major postoperative complications or a certain type of complication, such as anastomotic leak. These articles are summarized in Table 1. Thirteen studies proposed a cut-off for CRP as a marker for postoperative complications [1, 10, 12–15, 17, 26–31]. Definitions varied widely, ranging from one study including all complications, to another study including only postoperative sepsis. Nine studies proposed a cut-off for CRP as a marker for anastomotic leakage [9, 16, 32–38]. Again, definitions of leakage varied widely, ranging from studies including all patients with clinical signs of leakage to studies including only patients with a leak confirmed by imaging or upon reoperation. Table 1 portrays an overview of definitions of complications used.
Fig 1. Flow chart for literature search.
Table 1. Overview of definitions used for complications and anastomotic leakage in the selected studies.
Abreviations; postoperative day (POD), anastomotic leak (AL), systemic inflammatory response syndrome (SIRS), computed tomography scan (CT), urinary tract infection (UTI).
| CRP as marker for postoperative complications | |||||
| Study | year | Organ | Cut-off | POD | Definition |
| Adamina [26] | 2014 | Colorectal | 56 mg/L | 4 | Infectious complications, graded according to Clavien-Dindo. Cut-off applies in absence of clinical signs. |
| Kørner [27] | 2009 | Colorectal | 190 mg/L | 3 | Intraabdominal infection; AL, abscess or diffuse peritonitis |
| Lane [28] | 2012 | Colorectal | 150 mg/L | 2 | Adverse events: including infective complications, postoperative organ dysfunction and prolonged length of stay |
| MacKay [15] | 2009 | Colorectal | 145 mg/L | 4 | All infective complications |
| Mokart [29] | 2005 | All abdominal | 93 mg/L | 1 | Postoperative sepsis (SIRS + infection) |
| Nason [30] | 2014 | Colorectal | 148 mg/L | 4 | Infective complications; AL confirmed by CT, wound infection with purulent drainage |
| Platt [14] | 2012 | Colorectal | 170 mg/L | 3 | Postoperative infective complications (surgical site and remote site infection) |
| Straatman [1] | 2014 | Major abdominal surgery | 145 mg/L | 3 | Postoperative complications defined by Clavien-Dindo, with a cut-off for major complications (grades 3 and up) |
| Warschkow [31] | 2012 | Pancreas | 94 mg/L | 7 | Postoperative inflammatory complications; pancreatic fistula, anastomotic leak, cholangitis, pancreatitis, wound infections, abscesses, pneumonia, UTI |
| Warschkow [13] | 2012 | Gastro-esophageal | 141 mg/L | 4 | Postoperative infections; AL, abscess, pneumonia, wound infection, UTI, colitis |
| Warschkow [17] | 2012 | Colorectal | 135 mg/L | 4 | Postoperative infectious complications: Any septic event, both intra- and extra- abdominal infections |
| Warschkow [10] | 2011 | Colorectal | 123 mg/L | 4 | Inflammatory complications; AL (confirmed by imaging or operation), UTI, wound infection, pneumonia, central line infections. |
| Welsch [12] | 2008 | Pancreas | 140 mg/L | 4 | Fistula, leak, abscess, wound infection, pneumonia, cholangitis, central line infection, UTI, necrotizing pancreatitis, infectious bilioma or pleural effusion |
| CRP as marker for anastomotic leakage (AL) | |||||
| Study | year | Organ | Cut-off | POD | Definition |
| Almeida [32] | 2012 | Colorectal | 140 mg/L | 3 | AL defined as free feacal fluid in the abdomen diagnosed by drain production or CT-scan imaging |
| Deitmar [33] | 2009 | Oesophagus | 135 mg/L | 2 | Leak of anastomosis or gastric staple line confirmed with endoscopy |
| Dutta [34] | 2011 | Esophago-gastric | 180 mg/L | 3 | AL confirmed with CT, contrast study or upor reoperation |
| Garcia-Granero [35] | 2013 | Colorectal | 147 mg/L | 3 | AL confirmed with imaging or upon reoperation |
| Oberhofer [36] | 2012 | Colorectal | 99 mg/L | 3 | AL (imaging), abscess, wound infection, pneumonia, central line infection, UTI |
| Ortega- Deballon [16] | 2010 | Colorectal | 125 mg/L | 4 | AL: feacal drain production, collection at anastomosis site with imaging, dehiscence during reoperation |
| Pedersen [37] | 2012 | Colorectal (MIS) | 200 mg/L | 3 | AL diagnosed in patients with acute abdomen, upon imaging or upon reoperation |
| Scepanovic [38] | 2013 | Stomach, small bowel, colon | 135 mg/L | 3 | AL defined as clinical presence of enteric contents within the drains |
| Welsch [9] | 2007 | Rectal | 140 mg/L | 3&4 | AL (imaging), abscess, wound infection, pneumonia, central line infection, UTI |
All authors of articles selected were contacted via e-mail or telephone and asked to provide raw data regarding collected CRP values and complications as graded according to the Clavien-Dindo classification in their studies in order to conduct a pooled analysis of the raw data. Authors of 11 studies never replied, of two studies no contact details were provided and the authors of two studies replied, stating they had no time or interest to participate. Seven authors provided complete raw data [1, 27, 32, 35, 37–39]. All authors reassessed their data regarding all complications using the Clavien-Dindo classification. These seven studies were included for pooled-analysis comprising a total of 1427 patients. The characteristics of the seven studies are provided in Table 2. Four studies had a prospective design. All seven studies included both malignant and benign indications for surgical intervention. CRP values were available in six studies on POD 3, in five studies on POD 4 and in six studies on POD 5.
Table 2. Characteristics of the studies included for pooled-analysis.
Abbreviations: months (mo), minimally invasive surgery (MIS).
| Study | Design | Study interval | Operation | Indication (acute/elective) | n | Major Complication |
|---|---|---|---|---|---|---|
| Almeida et at. (2012) [32] | Retrospective | 22 mo | Colorectal resection | Both | 173 | 21 of 173 (12.1%) |
| Garcia-Granero et al. (2013)[35] | Prospective | 17 mo | Colorectal resection | Both | 205 | 17 of 205 (8.3%) |
| Kørner et al. (2009) [27] | Retrospective | 12 mo | Colorectal resection | Both | 231 | 23 of 231 (10%) |
| Lagoutte et al. (2012) [39] | Retrospective | 14 mo | Colorectal resection | Both | 100 | 20 of 100 (20%) |
| Pedersenet al. (2012) [37] | Retrospective | 12 mo | MIS Colorectal resection | Both | 163 | 41 of 163 (25.2%) |
| Scepanovic et al. (2013) [38] | Prospective | 18 mo | Al digestive resections with anastomosis | Both | 156 | 15 of 156 (9.6%) |
| Straatman et al. (2014) [1] | Retrospective | 24 mo | All digestive resections with anastomosis/ostomy | Both | 399 | 82 of 399 (20.6%) |
Risk of bias
The methodological quality of the included studies varied. There were five studies on colorectal surgery and two including all digestive tract operations. There was considerable variation in the quality of the included studies as depicted in Table 3. Two items were scored as “no” for each of the included studies, namely the uninterpretable/intermediate test results were not reported or if withdrawals from the study were explained, as they were not relevant and applicable. Only five items were included in all studies. For the remaining eight items, it was of special concern that high numbers of the studies were not reported if patients received the same reference standard regardless of the index test result and if the reference standard was independent of the index test.
Table 3. The QUADAS tool for classification of accuracy of the studies selected from literature, according to number of studies in each response category.
| QUADAS criteria | Number of articles in each category | ||
|---|---|---|---|
| Yes No Unclear | |||
| Was the spectrum of patients representative of the patients who will receive the test in practice? | 7 | - | - |
| Were selection criteria clearly described? | 7 | - | - |
| Is the reference standard likely to correctly classify the target condition? RS: x, CT-scan of diag lap | 7 | - | - |
| Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | 7 | - | - |
| Did the whole sample or a random selection of the sample, receive verification using a reference standard of diagnosis? | - | 7 | - |
| Did patients receive the same reference standard regardless of the index test result? | 2 | 5 | - |
| Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | 1 | 6 | - |
| Was the execution of the index test described in sufficient detail to permit replication of the test? | 4 | 3 | - |
| Was the execution of the reference standard described in sufficient detail to permit its replication? | 6 | 1 | - |
| Were the index test results interpreted without knowledge of the results of the reference standard? | 5 | 1 | 1 |
| Were the reference standard results interpreted without knowledge of the results of the index test? | 7 | ||
| Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | 7 | ||
| Were uninterpretable/ intermediate test results reported? | 1 | 5 | 1 |
| Were withdrawals from the study explained? | 2 | 4 | 1 |
CRP prediction model
Raw data from the seven studies was pooled and analyzed. Major complications, as classified according to the Clavien-Dindo grades 3, 4 and 5, occurred in an average 15.4% of patients (range 8.3–25.2%). Average CRP levels in patients with major complications and patients with no or minor complications for postoperative days 3, 4 and 5 are depicted in Table 4.
Table 4. Median CRP levels and interquartile ranges (IQR) for each postoperative day in the included studies for patients with major complications versus patients with an uncomplicated course or minor complication.
NA = Not Available
| Study | CRP POD 3 | CRP POD 4 | CRP POD 5 | |||
|---|---|---|---|---|---|---|
| Uncomplicated or minor complication | Major complication | Uncomplicated or minor complication | Major complication | Uncomplicated or minor complication | Major complication | |
| Almeida [32] | 99 (78–122) | 167 (127–307) | 58 (37–93) | 179 (117–237) | 28 (22–35) | 124 (104–262) |
| Garcia-Granero [35] | 125 (71–186) | 255 (188–270) | 85 (50–138) | 260 (120–278) | 56 (31–100) | 248 (137–285) |
| Korner [27] | 113 (69–199) | 256 (180–317) | NA | NA | 54 (30–117) | 193 (95–313) |
| Lagoutte [39] | 112 (83–170) | 227 (140–270) | 80 (50–127) | 160 (100–284) | NA | NA |
| Pedersen [37] | NA | NA | NA | NA | 113 (69–223) | 194 (125–289) |
| Scepanovic [38] | 114 (87–140) | 168 (113–195) | 79 (62–113) | 145 (84–190) | 57 (39–98) | 127 (61–157) |
| Straatman [1] | 157 (108–229) | 245 (163–331) | 124 (82–171) | 189 (125–296) | 94 (54–172) | 159 (89–253) |
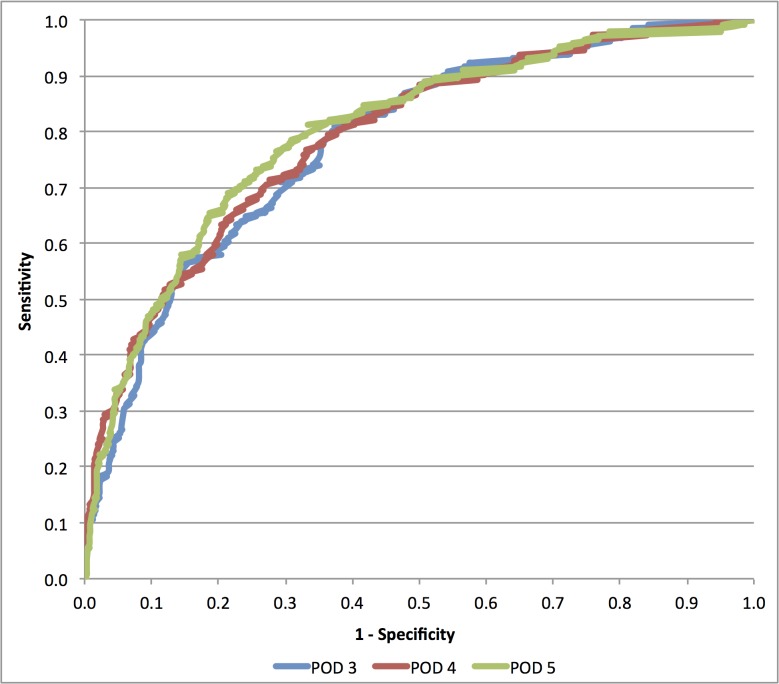
First pooled-analysis with receiver operator characteristic (ROC) curve analysis was used to assess the highest sensitivity and AUC for CRP as a marker of major complications, as depicted in Fig 2. According sensitivity and specificity are depicted in Table 5. With the highest measured sensitivity for CRP on POD 3, a logit regression model of the probability of major complications as a function of continuous CRP levels was calculated for postoperative day 3.
Fig 2. Receiver Operator Characteristics (ROC) curve for CRP levels on postoperative day (POD) 3,4 and 5.
Table 5. Receiver Operator Characteristics (ROC) curve analysis for pooled data with area under the curve (AUC), positive predictive values(PPV) and negative predictive values (NPV) for each postoperative day (POD).
Positive and negative likelihood ratios (LR+ and LR-) were calculated to assess the difference in odds of complications pre- and post CRP measurement.
| POD | cut-off | AUC | 95% CI | Sensitivity | Specificity | PPV | NPV | LR+ | LR- |
|---|---|---|---|---|---|---|---|---|---|
| POD 3 | 140 | 0.783 | 0.742–0.824 | 81.7% | 61.6% | 25.7% | 95.4% | 2.13 | 0.30 |
| POD 4 | 130 | 0.79 | 0.744–0.836 | 71.4% | 72.5% | 31.6% | 93.4% | 2.60 | 0.39 |
| POD 5 | 101 | 0.800 | 0.740–0.840 | 76.6% | 71.0% | 34.9% | 93.7% | 2.64 | 0.33 |
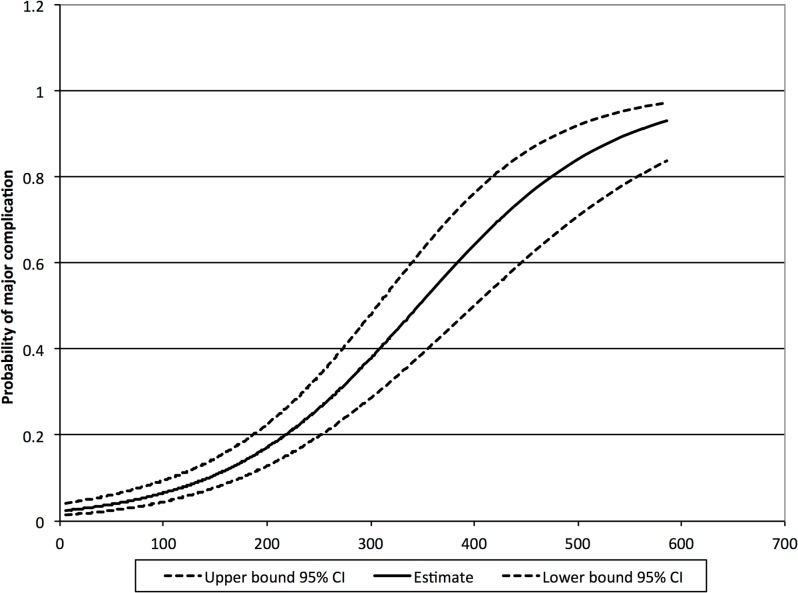
The prediction model is depicted in Fig 3 and Table 6 with according 95% confidence intervals. Following this risk assessment, cut-offs may be established at the discretion of the surgeon or surgical department. For instance an optimal cut-off was observed at 140 mg/L, with a sensitivity of 81.7% and specificity of 61.6%. One may also wish to establish two practical cut-offs. First, a cut-off for early safe discharge with a predictive value below 5% (95% CI: 3.3–7.5%) is calculated at 75 mg/L on POD 3 with a sensitivity of 96.2%, specificity of 21.7%, positive predictive value of 16.6% and a negative predictive value of 97.2%. Second, a cut-off that can be used as an identifier of those patients at high risk of developing major complications thereby indicating additional examinations; demonstrating a predictive value for CRP above 20% (95% CI: 14.7–25.6%). The latter cut-off was calculated at 215 mg/L for POD 3, with a sensitivity of 57.3%, specificity of 82.8%, positive predictive value of 35% and negative predictive value of 92.5%.
Fig 3. Prediction for the probability of major complications as a function of measured CRP levels on postoperative day three, with 95% confidence interval.
Depicts probability of complications for individual CRP measurements.
Table 6. Probability of major complications for different CRP levels, with the probability of the upper cut-off of 215 mg/L depicted.
| CRP level | Probability | 95% confidence interval | |
|---|---|---|---|
| mg/L | % | Lower | Upper |
| 50 | 3.94% | 2.51% | 6.13% |
| 75 | 5% | 3.33% | 7.55% |
| 100 | 6.51% | 4.46% | 9.39% |
| 150 | 10.77% | 7.83% | 14.64% |
| 200 | 17.16% | 12.85% | 22.53% |
| 215 | 20.00% | 14.70% | 25.60% |
| 250 | 26.42% | 19.96% | 34.10% |
| 300 | 37.88% | 28.63% | 48.10% |
| 350 | 52.48% | 40.07% | 64.59% |
| 400 | 62.98% | 48.98% | 75.09% |
Discussion
The presented systematic review and pooled-analysis, assesses the role of CRP as a marker for major complications.
Different studies have assessed CRP as a marker for complications and aimed to set a cut-off. Twenty-two studies were identified with cut-offs for CRP ranging from 140–190 mg/L on postoperative day three and 54–148 mg/L on postoperative day four. The wide range in cut-offs can be explained by the variety in definitions for complications depicted in the studies. Implementation of the Clavien-Dindo classification for complications allows for more reproducible assessment of complications [19].
The logistic regression model predicts major complications following major abdominal surgery as a function of continuous CRP levels on postoperative day three. The prediction models allows for assessment of separate CRP values, but also enables surgical wards to establish a cut-off for CRP at their own discretion. This cut-off can be established to differentiate between those patients at risk of developing major complications and those patients with an uncomplicated postoperative course or minor complications.
The positive predictive value of this practical cut-off may seem low, indicating that CRP levels also may be high in patients with a prolonged systemic inflammatory response syndrome based on severity of surgical trauma and not due to major complications. Yet it will most certainly help to identify major complications in an early phase in more than one-third of patients after MAS [15]. If clinical symptoms are not clear, determining whether CRP levels are decreasing or increasing in the following days can provide indications whether patients can be discharged safely or that a CT-scan has to be performed.
Positive predictive values remain low in all studies, further demonstrating that a high CRP-level alone is not sufficient for diagnosis of major complications but serves as an important indicative.
Given that CRP is non-specific for location or type of complication in combination with the low positive predictive value, it warrants additional examination. Computed Tomography (CT) is currently the most readily available imaging in the work-up of major complications [5, 40, 41]. Several studies have assessed the role of CT-scan imaging in diagnosis of major complications such as anastomotic leaks and found a sensitivity of 97%, therefore it is considered to be the imaging modality of choice [42, 43].
The prediction model allows for establishment of a cut-off at the discretion of the surgeon. A statistically optimal cut-off on POD 3 of 140 mg/L was calculated as an initial marker to identify major complications after MAS, with a sensitivity of 81.7% and specificity of 61.6%. Diagnostic accuracy for CRP was similar on postoperative day 3, 4 and 5 [3, 4, 44]. The results are in concordance with recent literature [14, 26, 44]. The relatively low sensitivity of 81.7% and a positive predictive value of 25.6% imply that a CT-scan would be performed whilst only one in four patients will be diagnosed with a major complication.
A more practical example would entail a double cut-off system. The first cut-off should correspond with a high negative predictive value in order to ensure early safe discharge in a fast track protocol. The second cut-off should aim at identifying patients with a high probability (a.s. >20%) of major complications, indicating whether additional examinations are necessary. Using the logit regression model for the probability of major complications calculated as a function of continuous CRP levels we were able to assess the two cut-offs. The first cut-off, with a value of CRP of 75 mg/L has a negative predictive value of 97.2%, indicating that patients with CRP levels below this cut-off may be discharged safely [26]. The second cut-off was set at 215 mg/L as predictor for complications.
Differences between the included studies in study design, methodology and patient population cause the pooled-analysis to be limited by its heterogeneity. Three of the seven included studies had a retrospective design [1, 27, 37]; this impacts the selection bias as well as the information bias. One study had data available for all patients on each day, suggesting selective measurement of CRP levels in the other studies [38]. Five out of the seven studies for pooled analysis included only colorectal surgery, compared to all digestive resections in the latter two studies. All were included since previous studies have shown no statistically significant differences in CRP levels between the different operated organ groups [1]. Only 7 out of 22 contacted authors provided data, possibly creating an inclusion bias. All types of surgery and organ groups are equally included in the 7 studies that provided data. Blinding was not performed in any of the studies. By requesting raw data for CRP levels and all complications being re-graded according to the Clavien-Dindo classification, the risk-of-bias by differences in criteria for complications is diminished [19, 20]. The Clavien-Dindo classification does not provide information on the type and localization of complications.
The present study provides insight in the interpretation of CRP levels measured after major abdominal surgery, proposing a logit regression model of a pooled analysis of seven studies, one for safe early-discharge in a fast-track protocol. The other concerning a reasonable high positive predictive value in which a CT-scan, independently of clinical symptoms, will confirm or exclude a major complication. The prediction model provides further insight and allows for setting a cut-off at the discretion of individual surgeons.
Definitive results should be obtained in a prospective manner. A prospective randomized trial is currently in progress, in order to assess the role of standardized measurements of CRP in a step-up quality-control algorithm using as primary endpoint a decrease in morbidity and mortality after major abdominal surgery.
Supporting Information
(PDF)
The document displays our search strategy for the Medline database. The search was adapted accordingly for the Embase and Cochrane databases
(DOCX)
Acknowledgments
We would like to thank the research groups of A.B. Almeida, M. Frasson, A. Garcia-Granero, H. Kørner, N. Lagoutte, T. Pedersen and M.S. Pedersen for supplying us with their raw data, thereby facilitating this study.
Data Availability
All relevant data are within the paper and/or the Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1. Straatman J, Cuesta MA, Gisbertz SS, van der Peet DL Value of a step-up diagnosis plan: CRP and CT-scan to diagnose and manage postoperative complications after major abdominal surgery. Rev Esp Enferm Dig. 2014; 106(8):515–21. [PubMed] [Google Scholar]
- 2. Velasco E, Thuler LC, Martins CA, Dias LM, Conalves VM Risk factors for infectious complications after abdominal surgery for malignant disease. Am J Infect Control. 1996; 24(1):1–6. [DOI] [PubMed] [Google Scholar]
- 3. Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001; 345(19):1368–77. [DOI] [PubMed] [Google Scholar]
- 4. Rivers EP, Coba V, Whitmill M Early goal-directed therapy in severe sepsis and septic shock: a contemporary review of the literature. Curr Opin Anaesthesiol. 2008; 21(2):128–40. 10.1097/ACO.0b013e3282f4db7a [DOI] [PubMed] [Google Scholar]
- 5. DuBrow RA, David CL, Curley SA Anastomotic leaks after low anterior resection for rectal carcinoma: evaluation with CT and barium enema. American Journal of Roentgenology. 1995; 165(3):567–71. [DOI] [PubMed] [Google Scholar]
- 6. Castelli GP, Pognani C, Cita M, Paladini R Procalcitonin as a prognostic and diagnostic tool for septic complications after major trauma. Crit Care Med. 2009; 37(6):1845–49. 10.1097/CCM.0b013e31819ffd5b [DOI] [PubMed] [Google Scholar]
- 7. Nesbakken A, Nygaard K, Lunde OC, Blücher J, Gjertsen Ø, Dullerud R Anastomotic leak following mesorectal excision for rectal cancer: true incidence and diagnostic challenges. Colorectal Disease. 2005; 7:576–81. [DOI] [PubMed] [Google Scholar]
- 8. den Dulk M, Noter SL, Hendriks ER, Brouwers MAM, van der Vlies CH, Oostenbroek RJ, et al. Improved diagnosis and treatment of anastomotic leakage after colorectal surgery. EJSO. 2009; 35(4):420–6. 10.1016/j.ejso.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 9. Welsch T, Müller SA, Ulrich A, Kischlat A, Hinz U, Kienle P, et al. C-reactice protein as early predictor for infectious complications in rectal surgery. Int J Colorectal Dis. 2007; 22:1499–507. [DOI] [PubMed] [Google Scholar]
- 10. Warschkow R, Tarantino I, Torzewski M, Näf F, Lange J, Steffen T Diagnostic accuracy of C-reactive protein and white blood cell counts in the early detection of inflammatory complications after open resection of colorectal cancer: a retrospective study of 1,187 patients. Int J Colorectal Dis. 2011; 26(11):1405–13. 10.1007/s00384-011-1262-0 [DOI] [PubMed] [Google Scholar]
- 11. Kragsbjerg P, Holmberg H, Vikerfors T Serum concentrations of interleukin-6, tumour necrosis factor alpha and C-reactive protein in patients undergoing major operations. Eur J Surg. 1995; 161(1):17–22. [PubMed] [Google Scholar]
- 12. Welsch T, Frommhold K, Hinz U, Weigand MA, Kleeff J, Friess H, et al. Persisting elevation of C-reactive protein after pancreatic resections can indicate developing inflammatory complications. Surgery. 2008; 143(1):20–8. [DOI] [PubMed] [Google Scholar]
- 13. Warschkow R, Tarantino I, Ukegjini K, Beutner U, Müller SA, Schmied BM, et al. Diagnostic study and meta-analysis of C-reactive protein as a predictor of postoperative inflammatory complications after gastroesophageal cancer surgery. Langenbecks Arch Surg. 2012; 397(5):727–36. 10.1007/s00423-012-0944-6 [DOI] [PubMed] [Google Scholar]
- 14. Platt JJ, Ramanathan ML, Crosbie RA, Anderson JH, McKee RF, Horgan PG, et al. C-reactive protein as a predictor of postoperative infective complications after curative resection in patients with colorectal cancer. Ann Surg Oncol. 2012; 19(13):4168–77. 10.1245/s10434-012-2498-9 [DOI] [PubMed] [Google Scholar]
- 15. MacKay GJ, Molloy RG, O'Dwyer PJ C-reactive protein as a predictor of postoperative infective complications following elective colorectal resection. Colorectal Disease. 2011; 13(5):583–7. 10.1111/j.1463-1318.2010.02236.x [DOI] [PubMed] [Google Scholar]
- 16. Ortega-Deballon P, Radais F, Facy O, d'Athis P, Masson D, Charles PE, et al. C-reactive protein is an early predictor of septic complications after elective colorectal surgery. World J Surg. 2010; 34(4):808–14. 10.1007/s00268-009-0367-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Warschkow R, Beutner U, Steffen T, Müller SA, Schmied BM, Güller U, et al. Safe and early discharge after colorectal surgery due to C-reactive protein; A diagnostic meta-analysis of 1832 patients. Ann Surg. 2012; 256(2):245–50. 10.1097/SLA.0b013e31825b60f0 [DOI] [PubMed] [Google Scholar]
- 18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009; 339:b2700 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications; five year experience. Ann Surg. 2009; 250(2):187–96. 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 20. Dindo D, Demartines N, Clavien PA Classification of surgical complications; a new proposal with evaluation in a cohort of 6336 patient and results of a survey. Ann Surg. 2004; 240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strong VE, Devaud N, Allen PJ, Gonen M, Brennan MF, Coit D Laparoscopic versus open subtotal gastrectomy for adenocarcinoma: a case-control study. Ann Surg Oncol. 2009; 16:1507–13. 10.1245/s10434-009-0386-8 [DOI] [PubMed] [Google Scholar]
- 22. Whiting P, Rutjes A, Westwood M, Mallett S, Deeks J, Reitsma H, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155(8):529–36. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 23. Youden WJ Index for rating diagnostic tests. Cancer. 1950; 3(1):32–5. [DOI] [PubMed] [Google Scholar]
- 24. Nelder J, Wedderburn R Generalized Linear Models. Journal of the Royal Statistical Society. 1972; 135(3):370–84. [Google Scholar]
- 25. Wald A Tests of statistical hypotheses concerning several parameters when the number of observation is large. Transactions of the American Mathematical Society. 1943; 54(3):426–82. [Google Scholar]
- 26. Adamina M, Warschkow R, Naf F, Hummel B, Rduch T, Lange J, et al. Monitoring c-reactive protein after laparoscopic colorectal surgery excludes infectious complications and allows for safe and early discharge. Surg Endosc. 2014; 28:2939–48. 10.1007/s00464-014-3556-0 [DOI] [PubMed] [Google Scholar]
- 27. Kørner H, Nielsen HJ, Søreide JA, Nedrebø BS, Søreide K, Knapp JC Diagnostic accuracy of C-reactive protein for intraabdominal infections after colorectal resections. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2009; 13:1599–606. [DOI] [PubMed] [Google Scholar]
- 28. Lane JC, Wright S, Burch J, Kennedy H, Jenkins JT Early prediction of adverse events in enhanced recovery based upon the host systemic inflammatory response. Colorectal Disease. 2012; 15:224–30. [DOI] [PubMed] [Google Scholar]
- 29. Mokart D, Merlin M, Sannini A, Brun JP, Delpero JR, Houvenaegel G, et al. Procalcitonin, interleukin 6 and systemic inflammatory response syndrome (SIRS): early markers of postoperative sepsis after major surgery. British Journal of Anaesthesia. 2005; 94(6):767–73. [DOI] [PubMed] [Google Scholar]
- 30. Nason GJ, Barry BD, Obinwa O, McMacken E, Rajaretnam NS, Neary PC Early rise in C-reactive protein is a marker for infective complications in laparoscopic colorectal surgery. Surgical Laparoscopy, Endoscopy and Percutaneous Techniques. 2014; 24(1):57–61. 10.1097/SLE.0b013e31828fa03e [DOI] [PubMed] [Google Scholar]
- 31. Warschkow R, Ukegjini K., Tarantino I., Steffen T., Müler S.A., Schmied B.M., Marti L. Diagnostic study and meta-analysis of C-reactive protein as a predictor of postoperative inflammatory complications after pancreatic surgery. J Hepatobiliary Pancreat Sci. 2012; 19(4):492–500. 10.1007/s00534-011-0462-x [DOI] [PubMed] [Google Scholar]
- 32. Almeida AB, Faria G, Moreira H, Pinto-de Sousa J, Correia-da-Silva P, Costa Maia J Elevated serum C-reactive protein as a predictive factor for anastomotic leakage in colorectal surgery. Int J Surg. 2012; 10(2):87–91. 10.1016/j.ijsu.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 33. Deitmar S, Anthoni C, Palmes D, Haier J, Senninger N, Brüwer M Sind Leukozyten und C-reactives Protein geeignete Parameter als Frühindikatoren de Anastomoseninsuffizienz nach Ösophagusresektion? Zentralbl Chir. 2009; 134(1):83–9. [DOI] [PubMed] [Google Scholar]
- 34. Dutta S, Fullarton GM, Forshaw MJ, Horgan PG, McMillan DC Persistent elevation of C-reactive protein following esophagogastric cancer resection as a predictor of postoperative surgical site infectious complications. World J Surg. 2011; 35(5):1017–25. 10.1007/s00268-011-1002-1 [DOI] [PubMed] [Google Scholar]
- 35. Garcia-Granero A, Frasson M, Flor-Lorente B, Blanco F, Puga R, Carratala A, et al. Procalcitonin and C-reactive protein as early predictors of anastomotic leak in colorectal surgery: a prospective observational study. Dis Colon Rectum. 2013; 56(4):475–83. 10.1097/DCR.0b013e31826ce825 [DOI] [PubMed] [Google Scholar]
- 36. Oberhofer D, Juras J, Pavičic AM, Žuric IR, Rumenjak V Comparison of C-reactive protein and procalcitonin as predictors of postoperative infectious complications after elective colorectal surgery. Croat Med J. 2012; 53(6):612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pedersen T, Roikjær O, Jess P Increased levels of C-reactive protein and leukocyte count are poor predictors of anastomotic leakage following laparoscopic colorectal resection. Dan Med J. 2012; 59(12):e1–4. [PubMed] [Google Scholar]
- 38. Scepanovic MS, Kovacevic B, Cijan V, Antic A, Petrovic Z, Asceric R, et al. C-reactive protein as an early predictor for anastomotic leakage in elective abdominal surgery. Tech Coloproctol. 2013; 17(5):541–7. 10.1007/s10151-013-1013-z [DOI] [PubMed] [Google Scholar]
- 39. Lagoutte N, Facy O, Ravoire A, Chalumeau C, Jonval L, Rat P, et al. C-reactive protein and procalcitonin for the early detection of anastomotic leakage after elective colorectal surgery: pilot study in 100 patients. J Visc Surg. 2012; 149(5):e345–9. 10.1016/j.jviscsurg.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 40. Power N, Atri M, Ryan S, Haddad R, Smith A CT assessment of anastomotic bowel leak. Clinical Radiology. 2007; 62(1):37–42. [DOI] [PubMed] [Google Scholar]
- 41. Khoury W, Ben-Yehuda A, Ben-Haim M, Klausner JM, Szold O Abdominal computed tomography for diagnosing postoperative lower gastrointestinal tract leaks. J Gastrointest Surg. 2009; 13(8):1454–8. 10.1007/s11605-009-0925-4 [DOI] [PubMed] [Google Scholar]
- 42. Eckmann C, Kujath P, Schiedeck THK, Shekarriz H, Bruch HP Anastomotic leakage following low anterior resection: results of a standardized diagnostic and therapeutic approach. Int J Colorectal Dis. 2004; 19(2):128–33. [DOI] [PubMed] [Google Scholar]
- 43. Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA Anastomotic leaks after intestinal anastomosis: it’s later than you think. Ann Surg. 2007; 245(2):254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Singh PP, Zeng IS, Srinivasa S, Lemanu DP, Connolly AB, Hill AG Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg. 2014; 101(4):339–46. 10.1002/bjs.9354 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
The document displays our search strategy for the Medline database. The search was adapted accordingly for the Embase and Cochrane databases
(DOCX)
Data Availability Statement
All relevant data are within the paper and/or the Supporting Information files.