Abstract
The follitropin or follicle-stimulating hormone receptor (FSHR) belongs to a highly conserved subfamily of the G protein-coupled receptor (GPCR) superfamily and is mainly expressed in specific cells in the gonads. As any other GPCR, the newly synthesized FSHR has to be correctly folded and processed in order to traffic to the cell surface plasma membrane and interact with its cognate ligand. In this chapter, we describe in detail the conditions and procedures used to study outward trafficking of the FSHR from the endoplasmic reticulum to the plasma membrane. We also describe some methods to analyze phosphorylation, β-arrestin recruitment, internalization, and recycling of this particular receptor, which have proved useful in our hands for dissecting its downward trafficking and fate following agonist stimulation.
1. INTRODUCTION
The pituitary gonadotropic hormones (GPH), follitropin (FSH) and lutropin (LH), as well as placental choriongonadotropin (hCG), are glycoprotein hormones that play an essential role in gonadal function. Their cognate receptors (FSHR and LHCGR) belong, together with the thyroid-stimulating hormone receptor, to a highly conserved subfamily of the G protein-coupled receptor (GPCR) superfamily. FSHR and LHCGR are expressed by specific cells in the gonads (Vassart, Pardo, & Costagliola, 2004). The FSHR is expressed in ovarian granulosa cells where its action is required for growth and maturation of ovarian follicles and for granulosa cell estrogen production. In the testis, FSH supports the metabolism of Sertoli cells, thereby indirectly maintaining spermatogenesis. GPH receptors are characterized by a large NH2-terminal extracellular domain (ECD), where recognition and binding of their cognate ligands occur. This ectodomain comprises of a central structural motif of nine imperfect leucine-rich repeats (LRRs), a motif that is shared with a number of other membrane receptors that are involved in ligand selectivity and specific protein–protein interactions (Bogerd, 2007). The carboxyl-terminal end of the large ECD displays the so-called hinge region, which has recently been structurally characterized (Jiang et al., 2012). The hinge region structurally links the leucine-rich ECD with the serpentine transmembrane domain of GPH receptors and depending on the GPH receptor, this region may be involved in high-affinity hormone binding, receptor activation, intramolecular signal transduction, and/or silencing the basal activity of the receptor in the absence of ligand (Mueller, Jaeschke, Gunther, & Paschke, 2009). It is interesting to note that in contrast to the LHCGR, which appears insensitive to effects on hormone binding when trafficking is impaired by mutagenesis, the FSHR is particularly sensitive to mutations of the primary sequence. For example, when mutations impair trafficking of this receptor from the endoplasmic reticulum (ER) to the cell surface plasma membrane (PM) [at the ECD, exoloop 3 (Rozell, Wang, Liu, & Segaloff, 1995), and the carboxyl-terminal domain (C-tail) (Song, Ji, Beauchamp, Isaacs, & Ji, 2001)], it is not possible to recover hormone-binding activity (Nechamen & Dias, 2000; Rozell et al., 1995; Song et al., 2001). This may be due to an inherent flexibility of the human (h) FSHR that results in metastability and, conversely, a greater stability of the LHCGR. Along these lines, it is worth noting that, with one exception (Peltoketo et al., 2010; Tao, Mizrachi, & Segaloff, 2002), activating mutations in the LHCGR do not appear to translate into activating mutations when analogous mutations are made in the FSHR. In humans, the FSHR gene is located on chromosome 2p21–p16 (Gromoll, Ried, Holtgreve-Grez, Nieschlag, & Gudermann, 1994). The coding region of this gene consists of 10 exons, ranging in size between 69 and 1234 bp, and nine introns, ranging in size between 108 and 15 kb (Gromoll, Pekel, & Nieschlag, 1996). The receptor protein consists of 695 amino acid residues; the first 17 amino acids encode a signal sequence, resulting in a mature FSHR of 678 amino acid residues long, with a molecular weight of ~75 kDa as predicted from its cDNA (Dias et al., 2002). However, glycosylation of its three or four possible glycosylation sites may give rise to receptor forms with approximate weights of 80–87 kDa for the mature receptor (see below).
Upon agonist binding, the activated FSHR stimulates a number of intracellular signaling pathways. The canonical Gαs/cAMP/PKA signaling pathway has been recognized as a key effector mechanism of LH and FSH biological action for more than 20 years. However, gonadotropin receptors have also been reported to couple to other G protein subtypes and activate a number of distinct effector enzymes (reviewed in Ulloa-Aguirre, Crepieux, Poupon, Maurel, & Reiter, 2011) depending on the particular developmental stage of the host cells (Musnier et al., 2009).
One interesting signaling cascade involved in the so-called functional selectivity of the FSHR is that mediated by β-arrestins (Ulloa-Aguirre et al., 2011). β-Arrestins are versatile adapter proteins that form complexes with GPCRs following receptor activation. These particular scaffold molecules play a major role in desensitization and downward trafficking (internalization and recycling) of many GPCRs (see below). In addition, they play an instrumental role as regulators of intracellular signaling events via activation of several pathways, including the MAPK pathway (Reiter & Lefkowitz, 2006). In the case of the FSHR, biased extracellular signal-regulated kinase signaling has been shown to occur at the FSHR bound to a modified agonist (Wehbi, Decourtye, et al., 2010; Wehbi, Tranchant, et al., 2010) or when its PM expression is severely reduced (Tranchant et al., 2011). In this case, β-arrestins recruited to the agonist-bound receptor assemble a MAPK module, whereas G protein-dependent signaling is impaired.
Similar to other proteins, GPCRs have to be correctly folded in order to pass through the ER quality control system (Ulloa-Aguirre & Conn, 2009). In fact, mutations that affect the folding process of these receptors result in intracellular retention or increased degradation of the folding intermediates. Several GPCR-interacting proteins that support trafficking to the PM have been identified. Among these are calnexin and calreticulin, which bind a broad range of glycoproteins facilitating proper folding of intermediate molecules (Helenius, Trombetta, Hebert, & Simons, 1997). In the case of the FSHR, coimmunoprecipitation experiments have shown that the folding process of the precursor involves interactions with these two chaperones (Mizrachi & Segaloff, 2004; Rozell, Davis, Chai, & Segaloff, 1998) as well as with the protein disulfide isomerase (PDI; Mizrachi & Segaloff, 2004). PDI is an ER-resident enzyme involved in disulfide bond formation of folding intermediates and that probably acts as a cochaperone with calnexin and calreticulin during their association with the glycoprotein hormone receptors (Mizrachi & Segaloff, 2004).
Properly folded and assembled secretory proteins are segregated from ER-resident proteins into COPII-coated vesicles for exporting to the Golgi to be further processed before being sent to their final destination. Several recently identified motifs have been shown to be involved in GPCR exit from the ER and the Golgi. Among these motifs is the F(x)6LL sequence identified in the C-tail of several GPCRs, including the glycoprotein hormone receptors (Duvernay, Zhou, & Wu, 2004). In the hFSHR, this export motif is located between amino acid residues 616 (residues are numbered according to the mature receptor, minus the 17 amino acid residues leader sequence) and 624 (Ulloa-Aguirre & Conn, 2009). The C-tail of the hFSHR contains the minimal BBXXB motif reversed in its juxtamembrane region (in the rat this motif is rather BBXB), which in other GPCRs is involved in G protein coupling (Timossi et al., 2004). The last two residues of the BXXBB motif (R617 and R618) and the preceding F616 constitute the NH2 end of the highly conserved F(x)6LL motif. Consequently, mutations in these residues impair receptor trafficking and cell membrane localization of the receptor (Timossi et al., 2004; Zarinan et al., 2010). The third intracellular loop (iL) of the hFSHR also contains this motif and replacement of all its basic residues with alanine impairs trafficking of the receptor to the PM (Timossi et al., 2004). Receptor mutants with this sequence (e.g., R556A) are retained intracellularly and, when present in the heterozygous state, exert dominant negative effects on their wild-type (WT) counterpart, probably through forming misfolded mutant:WT receptor complexes (Zarinan et al., 2010) (see below).
Posttranslational modifications are important for GPCR export to the cell surface (Ulloa-Aguirre & Conn, 2009). Three modifications are particularly important: glycosylation, palmitoylation, and phosphorylation. Glycosylation facilitates folding of protein precursors by increasing their solubility and stabilizing protein conformation (Helenius & Aebi, 2004). The hFSHR bears four potential glycosylation sites (as defined by the consensus sequence N-X-Ser/Thr, where X is any amino acid except proline) at positions 174, 182, 276, and 301 of the extracellular NH2-terminal domain (Dias et al., 2002). The only direct biochemical evidence that exists as to which sites are glycosylated in the hFSHR is derived from the crystal structure of the ECD residues 25–250 (Fan et al., 2005). The structure shows that carbohydrate is attached at residue N174 which protrudes into solvent, whereas no carbohydrate is attached at residue N182, which protrudes from the flat beta sheet into the hormone–receptor binding interface. No structural information is available for residues 276 and 301 at this time. The naturally occurring mutations Ala172Val and Asn174Ile cause a profound defect in targeting the receptor protein to the cell membrane (Huhtaniemi & Themmen, 2005), confirming the importance of glycosylation of FSHR at position 174 and that glycosylation at this particular location is essential for trafficking of the receptor to the PM. In contrast, it has been shown that the rat FSHR is glycosylated at two of three glycosylation consensus sequences (174, 182, and 276) and that the presence of carbohydrates at either one of these residues (N174 or N276) is sufficient for receptor folding and trafficking to the PM (Davis, Liu, & Segaloff, 1995).
Another posttranslational modification important for GPCR trafficking to the PM is palmitoylation. Cysteine residues in the COOH-terminus of several GPCR, belonging mainly to family A, have been shown to be the target for S-acylation with palmitic acid, and this posttranslational modification is often required for efficient delivery of the protein to the cell membrane. In this vein, the FSHR is not an exception. In fact, the hFSHR exhibits in its COOH-terminus (C-tail) two conserved cysteine residues (at positions 629 and 655) and one nonconserved Cys residue at position 627 (Uribe et al., 2008). Employing site-directed mutagenesis, it has been recently shown that the hFSHR is palmitoylated at all cysteine residues, regardless of their location in the C-tail of the receptor (Uribe et al., 2008). Apparently, S-acylation at C627 and C655 is not essential for efficient FSHR cell surface membrane expression, whereas S-acylation at C629 is, as replacement of this residue with glycine or alanine reduced detection of the mature form of the receptor by ~40–70%. Further, when all palmitoylation sites are removed from the FSHR, cell surface PM expression is reduced to ~10–30%.
Agonist-induced phosphorylation is a general regulatory mechanism affecting downward trafficking of most GPCRs (Reiter & Lefkowitz, 2006). The FSHR has been reported to be phosphorylated by second messenger-dependent kinases PKA and PKC but also by G protein-coupled receptor kinases (GRK) 2, 3, 5, and 6 in various models (Kara et al., 2006; Krishnamurthy, Galet, & Ascoli, 2003; Lazari, Liu, Nakamura, Benovic, & Ascoli, 1999; Marion et al., 2002; Nakamura, Hipkin, & Ascoli, 1998; Troispoux et al., 1999). PKA and PKC contribute to both agonist-dependent (homologous) and agonist-independent (heterologous) desensitization of the receptor. GRK-mediated phoshorylation leads to more complex effects as they are centrally involved in homologous desensitization while simultaneously regulating β-arrestin recruitment and subsequent β-arrestin effects on receptor internalization through clathrin-coated pits and G protein-independent signaling. A cluster of five serines and threonines located in the C-tail of the FSHR has been shown to account for the bulk of FSH-induced phosphorylation as a result of GRK2 action (Kara et al., 2006). It is also well documented that β-arrestins are recruited to the GRK-phosphorylated and agonist-occupied FSHR (Kara et al., 2006; Krishnamurthy, Galet, et al., 2003; Lazari et al., 1999; Marion et al., 2002; Nakamura et al., 1998; Troispoux et al., 1999). In addition to GRK2, GRK5 and 6 have also been found to contribute to the same processes in HEK293 cells, though to a lesser extent (Kara et al., 2006). Interestingly, β-arrestins recruited to GRK2- or GRK5/6-phosphorylated FSHR have been suggested to exert distinct intracellular functions (Kara et al., 2006; Reiter & Lefkowitz, 2006). It is well established that β-arrestin 1 and 2 binding to GRK-phosphorylated FSHR leads to the internalization and recycling of the receptor (Kara et al., 2006; Kishi, Krishnamurthy, Galet, Bhaskaran, & Ascoli, 2002; Lazari et al., 1999; Nakamura et al., 1998; Piketty, Kara, Guillou, Reiter, & Crepieux, 2006). As also reported for other GPCR, GRK2-phosphorylated FSHR has been reported to predominate in the β-arrestin-mediated desensitization process whereas GRK5 and 6-induced phosphorylation of the activated FSHR is required for β-arrestin-dependent signaling pathway in HEK293 cells (Kara et al., 2006; Reiter & Lefkowitz, 2006).
Given the importance of cellular trafficking and correct placement of the receptor in the PM for normal function, in the following sections, we will briefly describe the basic methods used to analyze: (1) Human FSHR protein expression by radioligand binding assays and immunoblotting, (2) cell surface PM and intracellular association of FSHRs by acceptor photobleaching fluorescence resonance energy transfer (FRET) and coimmunoprecipitation, (3) phosphorylation and β-arrestins recruitment to the FSHR, and (4) internalization and recycling of the FSHR. Sections start with brief background information related to the application of the method(s) described.
2. OUTWARD TRAFFICKING DEFECTIVE FSHR MUTANTS. STUDYING PLASMA MEMBRANE EXPRESSION OF THE FSHR
As mentioned above, the hFSHR is unusually sensitive to mutations. In fact, all naturally occurring mutations in the ECD of this receptor (I143T, A172V, N174I, D207V, and P329R) drastically impair targeting of the receptor to the PM and thereby limit hormone binding and intracellular signaling. Mutations at or near the transmembrane region have minimal effects on FSH binding but impair to variable extent signal transduction. Therefore, it seems that the location, rather than nature of the amino acid alteration, is a stronger determinant of the functional response. As described above, A172 is the first amino acid in the perfectly conserved stretch of five amino acids in gonadotropin receptors (AFNQT), which is also the locus of loss-of-function mutations in the LHCGR (Gromoll et al., 2002). Valine in this position as well as isoleucine in position 174 may interfere with structural integrity of the LRR which hosts the glycosylation site, and perturbation of this structure likely impairs proper receptor LRR formation, especially the alpha helical portion of the LRR. The putative loss of glycosylation may affect trafficking to the PM. In fact, it has been shown that when the A172V mutant is overexpressed in vitro, only a very small proportion of the mutated receptor is present on the PM but is functional, though it has yet to be determined whether this form of the receptor is glycosylated or not at the N174 site. Instead, most of the mutated receptor is sequestered inside the cell, explaining the inactivation mechanism (Huhtaniemi & Alevizaki, 2007; Rannikko et al., 2002). Mutations at the NH2-terminal end of the hFSHR ectodomain also affect expression of the receptor. Alanine scanning mutagenesis of this region has identified two regions encompassing amino acids 12–14 and 22–30 whose primary sequence is important for receptor trafficking (Nechamen & Dias, 2000, 2003). In particular, mutations at F13, I23, D26, L27, R29, and N30 considerably reduced cell surface expression due to impaired intracellular trafficking (Nechamen & Dias, 2003). Remarkably, mutations at these sites impair proper glycosylation of the receptor but this is likely due to inappropriate amino terminal folding and trapping of these intermediates by surveillance proteins which then block appropriate glycosylation processing of endoglycosidase H (an enzyme that cleaves asparagine-linked mannose-rich oligosaccharides, but not highly processed complex oligosaccharides from glycoproteins)-sensitive molecules in the ER–Golgi (Nechamen & Dias, 2003).
Radioligand receptor-binding assays, which detect only functional (i.e., ligand bindable) receptor molecules and Western immunoblotting, which detects both immature and mature forms of receptor have been used to detect PM expression of the FSHR. For the latter, the anti-human FSHR antibody mAb 106.105 has been employed (Lindau-Shepard, Brumberg, Peterson, & Dias, 2001). This antibody was generated by immunization of mice with the ECD of the hFSHR (residues 1–350) expressed in insect cells and purified. The monoclonal antibody maps to the peptide epitope comprised by amino acid residues 300–315 (317–332 when leader sequence is included), without showing reactivity for the rat FSHR. For mature, binding-competent FSHRs both methods appear to correlate well with each other (Nechamen & Dias, 2003; Zarinan et al., 2010). Additional FSHR antibodies prepared against bacterial-expressed FSHR-ECD are available from the American Tissue Type Collection, which appear suitable for this method (Vannier, Loosfelt, Meduri, Pichon, & Milgrom, 1996) (see also Section 4.1 for NH2-terminally tagged FSHR constructs).
2.1. Methods to study FSH binding in FSHR-expressing human embryonic kidney-293 cells
Cultured human embryonic kidney-293 (HEK-293) cells stably or transiently expressing the WT hFSHR or mutants are used. In the case of transiently transfected cells, binding assays may be performed 48 h after transfection.
2.1.1 Radioreceptor assay to determine affinity constant and number of binding sites
This assay is an equilibrium displacement binding isotherm assay necessary to calculate the KD and the number of cell surface receptors, and is performed under conditions where no internalization can occur. The radioreceptor assay (RRA) method we have used is the following:
Prepare low specific activity (25–30 μCi/μg protein) 125I-FSH by diluting 125I-FSH in RRA buffer (50 mM Tris, pH 7.5; 25 mM MgCl2; 0.3% bovine serum albumin) so that final concentration of tracer is 20 ng/ml (~5×105cpm/50 μl). In our experience, iodination of 20–50 μg highly purified pituitary FSH using lactoperoxidase yields a FSH tracer with the desired specific activity (Weiner & Dias, 1992). Highly purified natural or recombinant hFSH is available from Prospec (Ness-Ziona, Israel). Prepare unlabeled pituitary FSH samples in 100 μl RRA buffer. Make eight dilutions and assay each in triplicate (1000, 300, 100, 30, 10, 3, 1, and 0 ng); the 1000 ng dose will be employed to calculate the nonspecific binding (NSB). Alternatively, untransfected HEK-293 may be used to determine NSB. Thereafter, wash transfected HEK-293 cells (cultured in 100×20 mm dishes) twice with unsupplemented medium and detach cells with 1 ml trypsin solution. This brief treatment of cells with trypsin does not decrease radiolabeled FSH binding, but this condition should be verified in each laboratory because the quality of trypsin preparations may differ. Add 9 ml supplemented 10% bovine serum albumin-containing medium and count cells. Remove medium by centrifugation and resuspend cells in RRA buffer at a density of 150,000 cells/150 μl. Based on the results of total (specific) 125I-FSH binding (see above), adjust the number of cells transfected with expression-deficient FSHR plasmids to yield a comparable cpm of 125I-FSH binding in the RRA in order to accurately compare KD values between WT receptor and mutants. Add 150 μl of the cell suspension to culture tubes, then each dose of unlabeled FSH (in 100 μl), and finally 50 μl of the 125I-FSH solution. Allow equilibrium by incubating the mixture for 18 h at room temperature (RT) with constant shaking. At the end of the incubation period, add 3-ml ice-cold RRA buffer and centrifuge at 1500×g for 30 min at 4 °C. Transfer the tubes to sponge racks for holding and decanting assay tubes into a proper container, and dry the pellet by inverting the tubes over a paper towel, allowing the sample to air dry for 2–3 min. Count pellets in a gamma counter, plot data (Uribe et al., 2008), and analyze using any available computer software (Cohen, Bariteau, Magenis, & Dias, 2003).
2.2. Methods to detect total FSHR expression by gel electrophoresis (SDS-PAGE) and Western immunoblotting
The anti-human FSHR antibody mAb 106.105 detects both mature (m.w. ~80 kDa), membrane-anchored as well as immature, underglycosylated, high mannose ER-localized forms (m.w. ~75 kDa) of the receptor (Lindau-Shepard et al., 2001; Nechamen & Dias, 2003; Fig. 2.1A). Expression-defective hFSHR mutants are retained intracellularly and endoglycosidase H treatment causes a gel shift of the 75 kDa band representing immature, incompletely processed FSHR down to the deglycosylated 55 kDa band, while the mature form band is unaffected (Nechamen & Dias, 2003). HEK-293 cells stably or transiently expressing the FSHR and cultured in 60×20 mm culture plates may be used to detect the FSHR by SDS-PAGE and Western immunoblotting. If transiently transfected cells are used, this procedure may be performed 48 h after transfection.
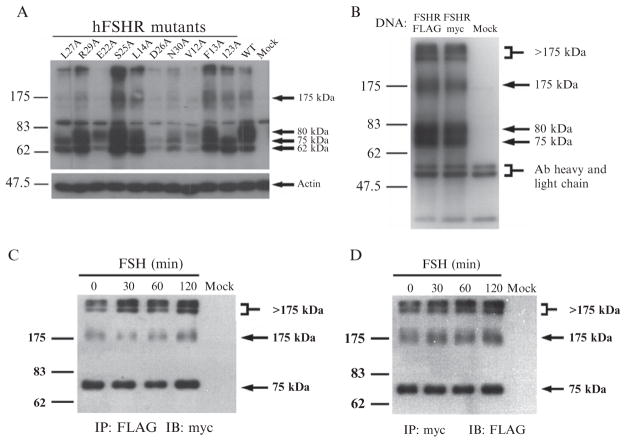
Figure 2.1.
(A) and (B) Visualization of the hFSHR by immunoblotting. HEK 293 cells were transiently transfected with the hFSHR constructs indicated using Lipofectamine reagent according to the manufacturer’s recommendations. Forty-eight hours later, cells were solubilized in Igepal/DOC lysis buffer, protein content of lysates was quantified by BCA protein assay, equal amounts of protein were analyzed by SDS-PAGE and Western blot (A) or immunoprecipitated with 5 μg of mAb 106.105, and then analyzed by SDS-PAGE and Western blot (B). Both blots were probed with 5 μg of mAb 106.105 followed by goat anti-mouse HRP conjugate secondary antibody at 1:10,000. In (A), the blot was reprobed with anti-actin Ab (1:5000) followed by goat anti-rabbit HRP conjugate secondary Ab (1:10,000). For reasons which are unclear at present, the 62 kDa form of receptor is not coimmunoprecipitated. The 75 and 175 kDa bands represent immature forms of the receptor which have not yet had the C-tail clipped. The 175 kDa form is either irreversibly aggregated or irreversibly bound to, for example, a chaperone protein. (C) and (D) Detection of intracellular oligomerization by coimmunoprecipitation and immunoblotting. HEK 293 cells were cotransfected with FLAG- and myc-tagged FSHR or mock transfected and were then treated with 1.2 nM FSH for the indicated times. Cell lysates were immunoprecipitated with anti-FLAG (C) or anti-myc (D) mAbs and then analyzed by SDS and Western blots. Immunoblots were probed with biotinylated myc mAb and HRP-conjugated streptavidin to detect myc-tagged FSHR in FLAG immunoprecipitates (C) or with HRP-conjugated FLAG mAb to detect FLAG-tagged FSHR in myc immunoprecipitates (D). Figures B–D are reproduced from Thomas et al. (2007) with permission from the authors and The Endocrine Society. Copyright 2007, The Endocrine Society.
2.2.1 SDS-PAGE
Prepare resolving and stacking gel solutions for 7.5% discontinuous buffer system SDS-PAGE (Laemmli, 1970) and polymerize in an assembled casting frame and stand for a 8.3×7.3 cm gel electrophoresis apparatus (e.g., Mini-PROTEAN II for 1-D vertical gel electrophoresis; Bio-Rad, Hercules, CA); also prepare the sample buffer (1 × SDS sample buffer: 0.0625 M Tris, pH 6.8; 2% SDS; 10% glycerol; 0.1 M DTT; 0.01% bromophenol blue) and running electrophoresis buffer (1 × electrophoresis buffer: 0.025 M Tris, 0.192 M glycine, 0.1% SDS). To prepare protein extracts, remove medium from FSHR-expressing cultured cells, wash twice with 1 ml ice-cold 1 × PBS, and harvest cells in Igepal–DOC lysis buffer (10 mM Tris, pH 7.5; 1% Igepal; 0.4% deoxycholate; 140 mM NaCl; 5 mM EDTA) supplemented with protease inhibitor cocktail tablets (Roche Diagnostics, Indianapolis, IN). Using a Dounce homogenizer, extracts are prepared with 10 strokes using a tight fitting pestle. Clean extracts by centrifugation at 13,000 rpm for 10 min at 4 °C and transfer supernatants to 1.5 ml microfuge vials. Determine protein concentration to be loaded onto gels as well as the volume to load per well, depending on different comb configurations (e.g., 10–15 μg protein/gel lane in a volume of 15–20 μl for 0.75 mm combs). If samples are too concentrated, dilute with 1 × PBS before adding sample buffer (2 μl sample+18 μl 1 × PBS+3.3 μl 6 × sample buffer). Warm samples for 5 min at 60 °C; it is critically important to not boil the samples as is usually done with SDS gels because this will cause aggregation of the receptor/sample. Spin tubes at 13,000 rpm for ~1 min at RT in microfuge and load samples using appropriate gel-loading tips. Place electrode assembly with loaded samples into the electrophoresis chamber, fill the electrode assembly carefully and then the electrophoresis chamber both with 1 × electrophoresis buffer. Run gels at 85 V for stacking gel and 100–150 V for resolving gel (can increase to 200 V for resolving gel if running on ice).
2.2.2 Western immunoblotting
After SDS-PAGE, transfer mini-gels to PVDF (polyvinylidene difluoride) membranes (0.45-μm pore size) by electroblotting (wet transfer or semidry transfer); make sure that prestained molecular weight markers transfer to the membrane matrix. After protein electrotransfer, wash membrane with Ponceau-S red staining solution (0.1% (w/v) Ponceau-S in 1% (v/v) acetic acid) to rapidly determine efficiency and evenness of transfer of electrophoresed proteins, then destain with 1 × TBST wash buffer (0.01 M Tris–HCl, 0.15 M NaCl, 0.05% Tween 20). Incubate membranes in 10-ml blocking buffer (5% (w/v) nonfat milk/1 × TBST) for 1 h at RT or overnight at 4 °C on platform rocker. After blocking, wash blots with 50 ml 1 × TBST three times, 5–10 min each on an orbital shaker. Incubate blots with blocking buffer containing 5 μg purified anti-human FSHR antibody mAb 106.105 overnight at 4 °C or 1 h at RT on platform rocker. Wash blots again 3 × with 50 ml 1 × TBST on orbital shaker, and then incubate blots with goat anti-mouse antibody (secondary antibody) conjugated to horseradish peroxidase (HRP) (at a 1:10,000 dilution in blocking buffer) for 1 h at RT or overnight at 4 °C on rocker. After incubation with the secondary antibody, wash blots with 50 ml 1×TBST on orbital shaker three times, 5–10 min each, and then add 5 ml enhanced chemiluminescent substrate reagent and incubate for 5 min. Place blot in between transparency film, gently remove air bubbles and expose to autoradiography film (e.g., Kodak Biomax light autoradiography film, 13×18 cm; Perkin Elmer Life Sciences, Santa Clara, CA) in a dark-room. It is recommended to expose for 1 min to check signal intensity and then continue with longer or shorter exposures as needed (maximal. exposure length=60 min, minimal exposure length=1 s). For high backgrounds, wash blot in 1 × TBST then redevelop. Figure 2.1A shows a typical autoradiogram from an immunoblot of cells expressing WT hFSHR and traffic-defective hFSHR mutants. In the above described SDS-PAGE system, the 80-kDa band represents the mature receptor with complex oligosaccharides; this form is the only species of FSHR biotinylated by cell-impermeant biotin (Thomas et al., 2007). The 62-kDa band corresponds to the high mannose precursor glycoprotein localized in the ER and the 75-kDa band represents immature form of FSHR not yet expressed on the cell surface.
2.2.3 Immunoprecipitation of the FSHR
In order to detect the interaction of FSHR with other proteins or to study the posttranslational modifications of FSHR, it is necessary to isolate the receptor from cellular constituents. Since the mAb 106.105 antibody recognizes both mature and immature forms of the hFSHR, SDS-PAGE and Western blotting also can be applied to visualize this receptor and its interacting proteins from cell extracts immunoprecipitated with this antibody (Nechamen & Dias, 2003). To this end, confluent hFSHR-expressing cells cultured in two 60 mm dishes (which provide enough extract for at least two immunoprecipitations) are lysed as described above with Igepal/DOC lysis buffer. Five hundred micrograms of protein from each lysate are precleared by incubating with 100 μl protein A-agarose beads (Millipore, Billerica, MA) for 30 min at 4 °C with end-over-end rotation. Agarose beads are pelleted in a microfuge (5 min) and the supernatants are carefully poured off into a clean tube. Immunoprecipitations are performed with 5 μg mAb 106.105 (or mouse IgG2b isotype control) overnight at 4 °C (rocking is not needed). Antibody–protein complexes are then pulled-down by adding 100 μl protein A-agarose beads followed by incubation at 4 °C for 2 h with end-over-end rotation. After incubation, extracts are underlayered with a 0.5 ml cushion of 0.5 × lysis buffer/ 30% sucrose and spun in a microfuge at 13,000 rpm for 20 s. Beads are washed twice more with lysis buffer (0.5×) and finally resuspended in 60 μl 2 × Laemmli buffer and stored at −20 °C until used for SDS-PAGE and Western immunoblotting. Before SDS-PAGE, the suspension is heated to 60 °C for 5 min, spun for 1 min, and the supernatants are carefully poured off into clean tubes. Usually 40–60 μl/tube are recovered.
When immunoblotting is performed using monoclonal anti-hFSHR antibody, both the mature and immature forms of the FSHR are detected. Importantly, this procedure also allows a better identification of high-molecular-weight FSHR bands with molecular weights ≥175 kDa, which may represent immature FSHRs complexed with a chaperone or a cargo protein that are not dissociable by SDS (Thomas et al., 2007; Fig. 2.1B).
3. STUDYING OLIGOMERIZATION OF INTRACELLULAR AND CELL SURFACE-EXPRESSED FSHRs
As with many other GPCRs, the FSHR forms dimers or oligomers early during receptor biosynthesis (Thomas et al., 2007), which may be important to allow correct intracellular trafficking of the complex to the PM as well as for signal diversification through coupling to multiple G proteins (Nechamen, Thomas, & Dias, 2007). The mechanism and extent of FSHR self-association is not known. The ligand-bound ectodomain of the FSHR has been crystallized in two forms. (Fan et al., 2005; Jiang et al., 2012). The structures show how follicle-stimulating hormone binds to the curved inner surface of the receptor ectodomaininahand-claspfashionandbothα-andβ-subunitsinteractwiththe β-strandresiduesofthereceptor.Inonecrystalstructureoftheectodomain,two monomers were weakly associated, suggesting that the FSHR could form a dimer of two occupied receptors, if the interacting surface was via the alpha helical portion of the LRR ectodomain (Fan & Hendrickson, 2005, 2007). However, the FSHR-ECD/FSHR-ECD-interacting interface is weak and is not likely to be the major stabilizing force for dimerization. Further, the area of contact is small and may not represent a physiologically relevant protein interaction site for dimerization. The more recent crystal structure, which included the entire 350 amino acid ectodomain demonstrated an additional mode of association of hormone with the extracellular domain that included the hinge regions of the receptor, and a trimeric receptor structure (Jiang et al, 2012). Also chimeric, mutational, and interfering peptide fragment studies have suggested that the association between hFSHR monomers might occur through the TM domains (Guan et al., 2010; Osuga et al., 1997; Zarinan et al., 2010) or the C-tail (Zarinan et al.,2010), as it has been demonstrated for the β2-adrenergic receptor (Hebert et al., 1996) and the dopamine D2-receptor homodimerization (Lee, O’Dowd, Rajaram, Nguyen, & George, 2003; Ng et al., 1996) and μ- and δ-opiod receptors hetero-oligomerization (Fan et al., 2005).
3.1. Detection of FSHR self-association at the plasma membrane by cell surface fluorescence resonance energy transfer
Membrane receptor oligomerization has to date been primarily studied using FRET which occurs over intermolecular distances between 1.0 and 10.0 nm and is thus a way to assess protein–protein interaction even under conditions of very small clusters of proteins in the PM (Kenworthy, 2001; Kenworthy & Edidin, 1998; Siegel et al., 2000). The FSHR poses a special problem because, unlike other GPCRs or even the LHCGR which has been studied extensively with FRET assays (Lei et al., 2007), the FSHR is clipped at the C-tail and, therefore, it is not possible to employ fluorescent protein fusions at the carboxyl-terminus of the receptor, as is the standard of practice with other GPCRs. In an alternative approach, it has been possible to employ the mAb 106.105 conjugated to Alexa 568 and to Alexa 647 to successfully detect the presence of FSHR oligomers at the PM (Thomas et al., 2007). The use of differentially labeled mAb 106.105 or mAb 106.105-Fab′ fragments for hFSHR FRET obviates the need for C-tail fusion proteins, which may be useful to detect FSHR–FSHR interactions that occur within the cell but not at the PM level as the C-tail-terminal end of the FSHR (but not that of the LHCGR) is subjected to proteolytic processing during biosynthesis (Thomas et al., 2007). In fact, C-tail epitope tags in mature, cell surface-expressed FSHR usually are undetectable.
The Alexa 568 and Alexa 647 chromofluor pair meets the requisite characteristic to produce FRET, in which the emission spectrum of the donor (Alexa 568) overlaps the absorption spectrum of the acceptor (Alexa 647). The efficiency of energy transfer (E) from donor to acceptor is highly dependent on the distance between the chromofluors, and is often reported as E%. The easiest way to quantify the absolute efficiency of transfer is to measure donor emission before and after photobleaching of the acceptor. The increase in emission (dequenching) of the donor is a direct measure of the FRET efficiency (E), and is calculated from: E %=[1–(donor emission prior to acceptor photobleach)/(donor emission after acceptor photobleach)] × 100. Analyses are performed directly on captured confocal images, using a cell or its subregion as its own internal standard after photobleaching (Siegel et al., 2000). This procedure is called acceptor photobleaching FRET. The acceptor photobleaching cell surface fluorescence resonance energy transfer (CSFRET) method employed to detect dimerization of the hFSHR is the following:
Human embryonic kidney-293 cells stably expressing the hFSHR and plated in 35-mm Petri dishes with glass coverslip bottoms (MatTek Corp, Ashland, MA) are placed on ice following exchange of tissue culture medium with ice-cold PBS. Cells are then incubated with a 1:1 (μg/μg) mixture of mAb 106.105-Alexa 568 or mAb 106.105-Alexa 647 (directly labeled according to the manufacturer’s instructions with Alexa 568 or Alexa 647; Molecular Probes, Inc, Eugene, OR), for 30 min at 4 °C. The cells are washed twice with ice-cold PBS for 5 min, and then fixed with 4% formaldehyde in PBS freshly prepared from a 16% formaldehyde solution. Following fixation, the cells are washed in PBS at RT two times for 5 min. The cells are then covered with PBS and imaged on a Zeiss LSM 510 META confocal microscope system on an Axiomat 200 M inverted microscope equipped with a 63 × 1.4 NA, oil immersion differential interference contrast lens. Alexa 568 images are collected using the 540-nm laser line from a HeNe laser and 545 dichroic mirror and a band pass filter of 565–615 nm. Alexa 647 images are collected using the 633-nm laser line from a HeNe laser and 545 dichroic mirror and a band pass filter of 650–710 nm. The pinhole is set at 1.32 Airy units and a Z resolution of ~2.0 μm. Images may be collected at 12-bit intensity resolution over 512×512 pixels at a pixel dwell time of 6.4 μs. FRET is recorded by examining the dequenching of the Alexa 568 in a region of interest (ROI) following photobleaching of the Alexa 647 by the 633 nm HeNe laser line for 30–90 s at maximum power. This irradiation results in greater than 95% photodestruction of the acceptor fluor.
Images may be analyzed using, for example, a LSM FRET tool that is integrated with the LSM 510 collection software (Zeiss, Inc. Thornwood, NY). FRET analyses are performed on ROI drawn directly on captured confocal images. The analysis uses a cell or its subregion as its own internal standard after photobleaching (Siegel et al., 2000).
3.2. Coimmunoprecipitation of c-myc-tagged and FLAG-tagged FSHR to detect intracellular association of FSHRs
Dimerization of intracellular FSHRs may be detected by coimmunoprecipitating FSHR–FSHR complexes in cell extracts from HEK-293 cells (grown to 90–100% confluency on 60×20-mm dishes) previously cotransfected with 1 μg each of plasmids (pRK5 vector) encoding either FLAG (DYKDDDDK) or c-myc (EQKLISEEDL) carboxyl-terminally tagged FSHR, and incubated for an additional 24–48 h. Epitope tags, which are inserted at the carboxyl-terminal end of the FSHR C-tail, are subject to proteolytic processing before trafficking to the PM (Thomas et al., 2007). Coimmunoprecipitation with either anti-FLAG or anti-c-myc antibodies should only detect associated, immature forms of the FSHR (Fig. 2.1C and D). The immunoprecipitation procedure is performed as described above employing mAb 106.105, anti-FLAG M2 mAb (Sigma Chemical Co., St. Louis, MO), or anti-c-myc clone 9E10 mAb (American Type Culture Collection, Manassas, VA) at a dilution of 5 μg Ab/500 μg protein extract. Immunoprecipitates are then analyzed by 7.5% SDS-PAGE and Western immunoblotting.
Blots from FLAG immunoprecipitates are probed with 10 μg c-myc mAb labeled with biotin [following the manufacturer’s recommendations for EZ-Link Sulfo-NHS-LC-biotin reagent (Pierce, Rockford, IL)]. Blots are then washed and incubated with 10 μg HRP-conjugated-streptavidin (Pierce) to reveal c-myc-tagged FSHR in FLAG immunoprecipitates. Meanwhile, extracts immunoprecipitated with anti-c-myc mAb are probed with HRP-conjugated FLAG M2 mAb (Sigma) (1:5000) to detect FLAG-tagged FSHR in c-myc immunoprecipitates. Signal is developed using enhanced chemiluminescence substrate reagent as described above. In both cases (as well as when mAb 106.105 immunoprecipitates are probed with either biotinylated anti-c-myc or with HRP-conjugated FLAG M2 mAb), blots will mainly show immature and high molecular weight forms of the FSHR. Note that the membrane expressed form of FSHR represented as the 80-kDa band in Fig. 2.1B is absent in Fig. 2.1C and D when staining with the tag-specific antibodies, indicating that the tag and some portion of the C-tail is clipped before insertion into the PM.
4. PHOSPHORYLATION, INTERNALIZATION, AND RECYCLING OF THE FSHR (DOWNWARD TRAFFICKING)
As with other GPCRs, the FSHR undergoes homologous desensitization. The receptor becomes rapidly phosphorylated on serine and threonine residues located at iLs-1 and -3 and the C-tail in response to agonist stimulation, and this phosphorylation facilitates agonist-induced functional uncoupling and internalization through β-arrestins-mediated mechanisms (Kara et al., 2006). The FSHR bears a conserved Ser/Thr cluster in its C-tail and, accordingly, may be recognized as a “class B” seven-transmembrane receptor, in which high-affinity recruitment of both β-arrestin-1 and -2 equally occurs (Reiter & Lefkowitz, 2006). In addition to promoting receptor/G protein uncoupling, β-arrestins target such desensitized receptors to clathrin-coated pits for endocytosis by functioning as adaptor proteins that link the receptors to components (e.g., clathrin molecules) of the endocytic machinery. The majority of the internalized FSH/FSHR complexes then accumulates in endosomes and subsequently recycles back to the cell surface PM, where bound hormone dissociates, allowing preservation of potential responsiveness of the receptor to further hormonal stimulation (Krishnamurthy, Kishi, et al., 2003). Nevertheless, a fraction of FSH/FSHR complexes may be routed to the degradation pathway, which takes place in the lysosomes (Dias, 1986; Krishnamurthy, Kishi, et al., 2003). Cell surface residence of the FSHR at the cell membrane is additionally governed by ubiquitination of the receptor at residues located in the iL-3. Residues that regulate downward trafficking (internalization and postendocytic fate) of the FSHR from the PM are mainly located in the intracellular domains of the receptor, and include T371, S373, T378 (iL-1), S546–S549 (iL-3), and T639–T644 (C-tail) (phosphorylation and β-arrestins interaction); D550 (iL-3), P671, L672, H674, Q677, and N678 (C-tail) (postendocytic fate); and K555 (plus other still unidentified residues at the iL-3) (ubiquitination) (Cohen et al., 2003; Krishnamurthy, Galet, et al., 2003; Krishnamurthy, Kishi, et al., 2003; Ulloa-Aguirre, Zarinan, Pasapera, Casas-Gonzalez, & Dias, 2007).
In this section, we will briefly describe the methods to study FSHR phosphorylation and coupling to β-arrestins as well as some methods to analyze FSHR internalization and recycling.
4.1. Phosphorylation of the FSHR and β-arrestin recruitment
Detection of either phosphorylation or β-arrestin recruitment necessitates immunoprecipitation of the FSHR. In the two following sections, we describe an immunoprecipitation procedure that relies on a FLAG-tagged version of the hFSHR which is different from the one described in Section 3.2. In this construct, FLAG epitope was inserted in the NH2-terminal of the FSHR and the native signal sequence was replaced by the one from a hemagglutinin of influenza virus (Guan, Kobilka, & Kobilka, 1992; Reiter et al., 2001). Alternatively, FSHR could be immunoprecipitated with mAb 106.105 antibody as described in Section 2.2.3. For both protocols, HEK-293 cells are grown in DMEM supplemented with 10% heat-inactivated FBS, 10 U/ml penicillin, and 10 μg/ml streptomycin. When 70–80% confluent, cells are transiently transfected with 66 ng/cm2 of the plasmid encoding the FLAG-tagged FSHR.
4.1.1 Method to detect phosphorylation of the FSHR
All experiments should be performed with cells expressing equivalent amounts of FSHR at the PM. One 10-cm Petri dish is prepared for each experimental condition. Forty to 72 h after transfection, cells are incubated for 1 h at 37 °C in phosphate-free DMEM (Invitrogen, Life Technologies, Inc.) containing 0.1 mCi 32P/ml (Kara et al., 2006). Cells are then stimulated for 5 min with 100 ng/ml of FSH. After stimulation, cells are placed on ice, washed thrice with ice-cold PBS and harvested in 1 ml glycerol buffer (50 mM HEPES; 0.5% Nonidet P-40; 250 mM NaCl; 2 mM EDTA; and 10% glycerol, pH 8.0) containing protease and phosphatase inhibitors (0.2 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, and 10 μg/ml aprotinin). The lysates are tumbled for 3 h at 4 °C and then spun at 15,000 rpm for 20 min at 4 °C. At this stage, aliquots of total lysates are saved for each condition and stored at −20 °C until further analysis. The remaining supernatants are then immunoprecipitated by tumbling overnight at 4 °C with M2-conjugated agarose beads (Sigma). After immunoprecipitation, the beads are washed five times with Tris-buffered saline and eluted with 50 μl of Laemmli buffer. Eluates are then incubated at 42 °C for 30 min and loaded on a 10% SDS-PAGE. After migration, the gel is dried on a paper and autoradiographed. The phosphorylation signal can be revealed and analyzed using either a PhosphorImager (Molecular Dynamics, Amersham Biosciences, Pittsburgh, PA) or X-ray film. Autoradiographic analysis should reveal an agonist-induced band corresponding to the FSHR molecular weight. Little to no signal should be observed with the WT FSHR in the absence of FSH stimulation.
4.1.2 Method to analyze β-arrestin recruitment to the FSHR
Endogenous β-arrestins are coimmunoprecipitated with the activated receptor after sulfo-dithio-bis[succinimidyl propionate] (DSP, Pierce, Rockford, IL) cross-linking using a method adapted from Luttrell et al. (2001). All experiments should be performed with cells expressing equivalent amounts of FSHR at the PM. Prepare one 10-cm culture dish per condition. Forty to 72 h after transfection, cells are serum-starved for 4 h in DMEM supplemented with antibiotics, 10 mM HEPES, and 0.1% BSA. Cells are then stimulated with 100 ng/ml FSH in 4 ml of PBS/10 mM HEPES per culture dish. Approximately 10–15 min before the end of stimulation, prepare fresh DSP reagent in a 50-ml conical tube. Dilute 8 mg of DSP in 4 ml DMSO and then add 4 ml PBS, 10 mM HEPES drop-wise. Incubate the DSP mixture for 5–10 min at RT. Slowly add 1 ml of the DSP mixture to the cells placed on an orbital shaker (minimum speed). Incubate cell dishes at RT for 30 min with gentle agitation. The cross-linking reaction is quenched with Tris–HCl (pH 7.3) to a 20 mM final concentration. Culture dishes are then placed on ice and washed three times with ice-cold PBS/10 mM HEPES. After carefully removing the final wash, ~600 μl of RIPA buffer (150 mM NaCl; 50 mM Tris, pH 8.0; 5 mM EDTA; 1% Nonidet P-40) with freshly added proteases inhibitor cocktail (4 μg/ml aprotinin, 20 μg/ml leupeptin, 2 mM phenylmethylsulfonyl fluoride, 1 μg/ml pepstatin A, 2 mM benzamidin, 2 mM NaVO3, 10 μg/ml trypsin inhibitor) are added to each dish and cells are scraped. The lysates are tumbled for at least 4 h at 4 °C. Thereafter, the pellets are centrifuged at 15,000 rpm for 20 min at 4 °C. Aliquots of total lysates are collected and stored at −20 °C until further analysis.
The remaining lysates are incubated with M2-agarose beads (25 μl of bead suspension) overnight at 4 °C. The beads are washed three times with RIPA buffer and eluted with 50 μl of Laemmli buffer. The samples are incubated at RT for 1 h before loading on 10% SDS-PAGE. Proteins are transferred to nitrocellulose membrane (Whatman, Inc., Piscataway, NJ) and incubated overnight with an anti-β-arrestin antibody. The polyclonal A1CT antibody (from Dr. R. J. Lefkowitz, Duke University Medical Center, Durham, NC) cross-reacting with β-arrestins 1 and 2, can be used at a 1:3000 dilution in Tris-buffered saline, 0.1% Tween 20, and 5% nonfat dry milk. Alternatively, the commercially available anti-pan arrestin antibody (ab2914) from Abcam (Cambridge, MA) can also be used (at a 1:1000 dilution). FLAG M2 monoclonal antibody (1:20,000) in Tris-buffered saline, 0.1% Tween 20, and 5% nonfat dry milk is used to monitor the levels of immunoprecipitated FSHR across the different conditions. The blots are revealed using an enhanced chemiluminescence reaction ECL (Amersham Pharmacia Biotech, Pittsburgh, PA). Two bands corresponding to β-arrestin 1 (~51 kDa) and β-arrestin 2 (~49 kDa) are detected in FSH-stimulated cells.
4.2. Internalization in equilibrium and nonequilibrium conditions
It is not clear if internalization of FSHR occurs in the absence of ligand occupancy, in a constitutive manner. Measurement of FSHR internalization is done indirectly using radiolabeled FSH. Upon occupancy, FSHR internalization proceeds concomitant with activation of adenylate cyclase, association with arrestins, endocytosis, degradation, and recycling (Fig. 2.2). Consideration of whether to use equilibrium or nonequilibrium assay conditions is based on the outcome measurements desired (Cohen et al., 2003; Wiley & Cunningham, 1982). In the case on nonequilibrium conditions, initial rates of internalization are calculated, uninfluenced by recycling, and degradation of the radiolabeled tracer. It is important to remember that the tracer is the beacon for internalization, and that it is degraded and recycled under equilibrium conditions. Internalization rate of the receptor has never been measured directly by measuring the receptor itself. In addition, the reversibility of FSH binding decreases with increasing incubation time (Andersen, Curatolo, & Reichert, 1983). Measuring internalization under equilibrium conditions provides an estimate of internalization rate that is undoubtedly affected by recycling and degradation, and could in essence be considered a beta phase internalization rate. Concentration of receptor is also an issue to consider, as well as the genetic background of the cells used to study internalization. Thus, internalization rates of FSH studied in native primary cultures of Sertoli cells (Dias, 1986) are different from internalization rates determined in HEK293 cells overexpressing FSHR (Cohen et al., 2003).
Figure 2.2.
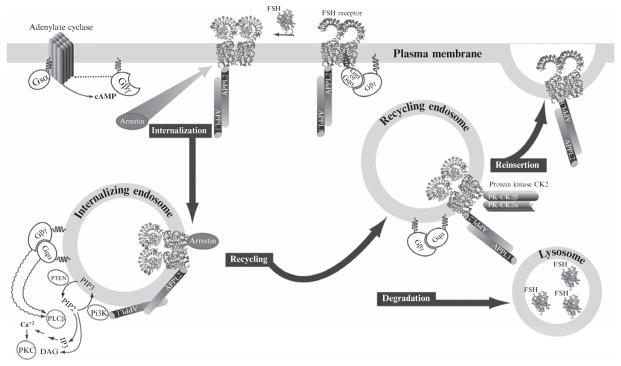
Downward traffic of the FSHR is linked to intracellular signaling pathways. FSH binding to FSHR induces the exchange of GDP from the stimulatory subunit (Gαs) of the G protein heterotrimer. Upon binding of GTP, the G protein heterotrimer dissociates and Gαs activates adenylate cyclase, while the βγ dimer activates phospholipase Cβ. These are the canonical pathways for FSH activation. The receptor iL1 and iL3 are subject to phosphorylation by GRKs and subsequent binding of arrestins. Internalization via arrestins likely follows the same early endosome pathway as that of APPL proteins; the latter interacts constitutively with the iL1 of the FSHR. Mutation of K376A in FSHR prevents association of APPL1 with FSHR and loss of FSH-induced intracellular calcium release and inositol triphosphate (IP3) production, with no effect on internalization. Knockdown of arrestin results in a loss of FSH-stimulated ERK phosphorylation. FSHR is ubiquitinated, likely enabling its sorting into recycling vesicles with bound FSH or fused with lysosomes where unliganded FSH is degraded. Recycled unoccupied receptor in theory can engage FSH for another round of FSH stimulation.
In internalization assays, cells remain attached to plates so it is possible to distinguish between surface binding and internalized radiolabeled ligand.
For studying internalization in equilibrium conditions, seed cells into 24-well plate(s) at a density of 1×105 cells/well in 1 ml DMEM supplemented with antibiotics and 10% FBS (seed extra wells if possible to normalize binding data to cell number or protein concentration). Incubate at 37 °C, 5% CO2 in a humidified incubator until ~70–80% confluent. Transfect cells with the FSHR or control cDNA and incubate cells at 37 °C, 5% CO2 for 48 h. The day of the assay, remove medium and replace with 250 μl serum-free medium only or serum-free medium containing 1 μg purified unlabeled FSH (to NSB wells) to account for nonspecific binding. Preincubate for 30 min at 37 °C, 5% CO2. At the end of the preincubation time, add 50 μl of a 125I-FSH solution (~1×106cpm/50 μl) to all wells to achieve a final concentration of ~40 ng/ml; final volume should be 300 μl/ well. Incubate at 37 °C, 5% CO2 for one additional hour and thereafter as-pirate media gently, put plates on ice and wash twice with 1 ml ice-cold 1 × PBS. Add 300 μl ice-cold elution buffer (50 mM glycine; 100 mM NaCl, pH 3.0). Incubate on ice for 20 min and then remove and transfer elution buffer to glass tubes. Count each tube’s radioactivity content in a gamma counter to measure “cell surface”-bound 125I-FSH. Add 500 μl of 2 N NaOH to wells at RT and place on orbital shaker for 1 h (at RT) to solubilize cells. Remove and transfer NaOH samples to glass tubes and count in a gamma counter to measure “cell-associated” 125I-FSH (Kluetzman, Thomas, Nechamen, & Dias, 2011). A protein or DNA assay or cell count on extra wells can be done to normalize binding data with respect to protein concentration or cell number.
For nonequilibrium conditions, it is essential to use a concentration of radiolabeled FSH that will achieve rapid saturation of all receptors. However, this is counterbalanced by the high level of radioactivity that must be handled. Considering these two issues, typically 125I-FSH is diluted in serum-free medium so that final concentration is 40 ng/ml (~1×106cpm/50 μl), which is high enough to assure almost immediate binding (Cohen et al., 2003; Kluetzman et al., 2011). In the nonequilibrium internalization assay, 125I-FSH tracer is allowed to bind for short periods of time (0–50 min) and cells are harvested in order to study initial phases of internalization. Transfected cells are seeded into 24-well plates at 1.5×105 cells/ml/well and incubated until subconfluency. On the day of the experiment, aspirate a row of four wells (time point=50 min), add 250-μl serum-free medium with (one well) or without (three wells) 1 μg purified FSH and then add 50 μl of 125I-FSH to all wells; stagger time points (t=0, 5, 10, 20, and 30) for adding tracer so that all wells are harvested at same time. Incubate at 37 °C, 5% CO2 and continue until time point zero. Immediately after adding trace to time zero wells, aspirate all incubation wells, put plate on ice, wash two times with ice-cold PBS, and then add 300 μl ice-cold elution buffer to recover cell surface-bound 125I-FSH. Thereafter, wash cells once with cold PBS, and add 500 μl 2 N NaOH to measure cell-associated 125I-FSH after transferring the NaOH samples to glass tubes. Do cell count on a separate 24-well plate to normalize counts with respect to cell number.
4.3. Recycling and degradation of internalized FSHRs
Note that in Section 4.2, the procedures result in a readout that represents a “snapshot” of radioligand binding, which does not account for radiolabeled tracer that was bound and internalized prior to acid elution of surface-bound radioligand. In addition, the acid-soluble fraction likely includes recycled radioligand depending on when the sample is taken (Kluetzman et al., 2011). The base solubilized fraction of radiolabeled ligand represents internalized hormone but also includes hormone, which is in the process of recycling to the cell membrane. This fraction of recycled hormone can be assessed by incubating cells with excess unlabeled ligand following removal of radioligand from the cell surface and washing. Subsequent acid elution will yield recycled radioligand (Kluetzman et al., 2011).
4.3.1 Assessment of recycled FSHR
Following internalization, the radiolabeled FSH/FSHR complex can be targeted to the lysosomes or it may recycle to the cell surface. Upon recycling to the cell surface, the radiolabeled FSH can be quantified by acid elution, provided that sufficient unlabeled FSH is present to chase the labeled FSH. The following procedure was described recently and reflects a way to assess this fraction of internalized FSHR (Kluetzman et al., 2011).
Forty-eight hours following transfection (see above), transfected cells are incubated in the presence of excess (40 ng/ml) 125I-FSH for 1 h at 37 °C, 5% CO2 in SFM (to allow for internalization). After the incubation, 125I-FSH is removed, monolayers are washed twice with cold PBS, and surface-bound hormone is eluted by incubating in cold isotonic pH 3.0 buffer on ice for 2 min. Cells are then washed twice again with cold PBS and placed back into the incubator in warm assay medium containing 1 μg/ml unlabeled FSH (to prevent the reassociation of 125I-FSH released from the cells back into the medium. If this is not done, it will not be possible to determine recycled FSH because the capacity of FSH binding is such that radiolabeled FSH does not get chased to the cell surface), and a second incubation at 37 °C for different times (0–120 min) is conducted to allow the cells to process the hormone that had been internalized during the first incubation. At each time point, medium is removed for trichloroacetic acid (TCA) precipitation and then cell surface-bound 125I-hFSH is eluted as above, followed by lysis with 2 N NaOH to determine internalized radiolabeled hormone. At time point zero, unlabeled hormone is immediately removed, surface-bound 125I-hFSH is eluted, and cell-associated 125I-hFSH is released by 2 N NaOH lysis. Cell-associated radioactivity at this time point represents “initial 125I-hFSH internalized” that is used for relative comparisons at later time points. The total recycled 125I-hFSH represents the summation of surface-eluted 125I-hFSH and TCA-precipitable material at each time point analyzed (see below).
4.3.2 Assessment of degraded FSH
Radiolabeled FSH, which is internalized and then trafficked to lysosomes, can be released into the acid compartment of the lysosome and then degraded. Degraded radiolabeled FSH is an indication of the pool of FSHR which has trafficked to the lysosome (Kluetzman et al., 2011).
Following binding and internalization, it is possible to assess the TCA-soluble radioactivity in the media, which represents degraded 125I-FSH. In contrast, TCA-insoluble radioactivity released from the cells subsequent to acid elution of surface-bound radiolabeled FSH, represents 125I-FSH that has been internalized and then recycled to the cell surface. These two parameters are determined by addition of an equal volume of 20% TCA to medium collected during the second incubation (during which an excess of unlabeled FSH was present). Insoluble material is removed by centrifugation at 3000×g for 30 min. The pellets are washed with an additional aliquot of 10% TCA, and then tubes are spun again, supernatant is removed, and the radioactivity in pellets, supernatants, and washes is determined in a gamma counter. The radioactivity determined in the wash supernatant is added to that determined in the supernatant (TCA-soluble) fractions.
In some experiments, it may be desired to inhibit internalization of radiolabeled FSH as a control to be used in assessing degradation. Two approaches can be used. In one approach, cells are incubated with 0.45 M sucrose in DMEM, 10% FBS for 1 h prior to and during 125I-hFSH incubation in serum-free medium. An additional approach uses dominant negative dynamin expression to inhibit internalization (Damke, Baba, Warnock, & Schmid, 1994). Both approaches prevent the internalization of receptor and radiolabeled FSH. However, the sucrose method may affect binding, whereas the dynamin method does not have this effect (Kluetzman et al., 2011).
Acknowledgments
The authors wish to thank supports from CONACyT (Mexico) (grant 86881 to A. U.-A.), The National Institutes of Health (Bethesda, MD) (grants HD18407 to J. A. D., and AG029531 to G. R. B.), The Wellcome Trust and Academy of Finland (to I. H.), and the Région Centre, Institut National de la Recherche Agronomique and Agence Nationale de la Recherche (E. R.). We also acknowledge LE STUDIUM (Orleans, France) for supporting sCORTS. R. Thomas and T. Zariñán assisted in the preparation of this chapter.
References
- Andersen TT, Curatolo LM, Reichert LE., Jr Follitropin binding to receptors in testis: Studies on the reversibility and thermodynamics of the reaction. Molecular and Cellular Endocrinology. 1983;33:37–52. doi: 10.1016/0303-7207(83)90055-2. [DOI] [PubMed] [Google Scholar]
- Bogerd J. Ligand-selective determinants in gonadotropin receptors. Molecular and Cellular Endocrinology. 2007;260–262:144–152. doi: 10.1016/j.mce.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Cohen BD, Bariteau JT, Magenis LM, Dias JA. Regulation of follitropin receptor cell surface residency by the ubiquitin-proteasome pathway. Endocrinology. 2003;144:4393–4402. doi: 10.1210/en.2002-0063. [DOI] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. The Journal of Cell Biology. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D, Liu X, Segaloff DL. Identification of the sites of N-linked glycosylation on the follicle-stimulating hormone (FSH) receptor and assessment of their role in FSH receptor function. Molecular Endocrinology. 1995;9:159–170. doi: 10.1210/mend.9.2.7776966. [DOI] [PubMed] [Google Scholar]
- Dias JA. Effect of transglutaminase substrates and polyamines on the cellular sequestration and processing of follicle-stimulating hormone by rat Sertoli cells. Biology of Reproduction. 1986;35:49–58. doi: 10.1095/biolreprod35.1.49. [DOI] [PubMed] [Google Scholar]
- Dias JA, Cohen BD, Lindau-Shepard B, Nechamen CA, Peterson AJ, Schmidt A. Molecular, structural, and cellular biology of follitropin and follitropin receptor. Vitamins and Hormones. 2002;64:249–322. doi: 10.1016/s0083-6729(02)64008-7. [DOI] [PubMed] [Google Scholar]
- Duvernay MT, Zhou F, Wu G. A conserved motif for the transport of G protein-coupled receptors from the endoplasmic reticulum to the cell surface. The Journal of Biological Chemistry. 2004;279:30741–30750. doi: 10.1074/jbc.M313881200. [DOI] [PubMed] [Google Scholar]
- Fan QR, Hendrickson WA. Structure of human follicle-stimulating hormone in complex with its receptor. Nature. 2005;433:269–277. doi: 10.1038/nature03206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan QR, Hendrickson WA. Assembly and structural characterization of an authentic complex between human follicle stimulating hormone and a hormone-binding ectodomain of its receptor. Molecular and Cellular Endocrinology. 2007;260–262:73–82. doi: 10.1016/j.mce.2005.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T, Varghese G, Nguyen T, Tse R, O’Dowd BF, George SR. A role for the distal carboxyl tails in generating the novel pharmacology and G protein activation profile of mu and delta opioid receptor hetero-oligomers. The Journal of Biological Chemistry. 2005;280:38478–38488. doi: 10.1074/jbc.M505644200. [DOI] [PubMed] [Google Scholar]
- Gromoll J, Pekel E, Nieschlag E. The structure and organization of the human follicle-stimulating hormone receptor (FSHR) gene. Genomics. 1996;35:308–311. doi: 10.1006/geno.1996.0361. [DOI] [PubMed] [Google Scholar]
- Gromoll J, Ried T, Holtgreve-Grez H, Nieschlag E, Gudermann T. Localization of the human FSH receptor to chromosome 2 p21 using a genomic probe comprising exon 10. Journal of Molecular Endocrinology. 1994;12:265–271. doi: 10.1677/jme.0.0120265. [DOI] [PubMed] [Google Scholar]
- Gromoll J, Schulz A, Borta H, Gudermann T, Teerds KJ, Greschniok A, et al. Homozygous mutation within the conserved Ala-Phe-Asn-Glu-Thr motif of exon 7 of the LH receptor causes male pseudohermaphroditism. European Journal of Endocrinology. 2002;147:597–608. doi: 10.1530/eje.0.1470597. [DOI] [PubMed] [Google Scholar]
- Guan XM, Kobilka TS, Kobilka BK. Enhancement of membrane insertion and function in a type IIIb membrane protein following introduction of a cleavable signal peptide. The Journal of Biological Chemistry. 1992;267:21995–21998. [PubMed] [Google Scholar]
- Guan R, Wu X, Feng X, Zhang M, Hebert TE, Segaloff DL. Structural determinants underlying constitutive dimerization of unoccupied human follitropin receptors. Cellular Signalling. 2010;22:247–256. doi: 10.1016/j.cellsig.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert TE, Moffett S, Morello JP, Loisel TP, Bichet DG, Barret C, et al. A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. The Journal of Biological Chemistry. 1996;271:16384–16392. doi: 10.1074/jbc.271.27.16384. [DOI] [PubMed] [Google Scholar]
- Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annual Review of Biochemistry. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- Helenius A, Trombetta E, Hebert D, Simons JF. Calnexin, calreticulin and the folding glycoproteins. Trends in Biochemical Sciences. 1997;7:193–200. [Google Scholar]
- Huhtaniemi I, Alevizaki M. Mutations along the hypothalamic-pituitary-gonadal axis affecting male reproduction. Reproductive Biomedicine Online. 2007;15:622–632. doi: 10.1016/s1472-6483(10)60529-9. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi IT, Themmen AP. Mutations in human gonadotropin and gonadotropin-receptor genes. Endocrine. 2005;26:207–217. doi: 10.1385/ENDO:26:3:207. [DOI] [PubMed] [Google Scholar]
- Jiang X, Liu H, Chen X, Chen PH, Fischer D, Sriraman V, et al. Structure of follicle-stimulating hormone in comlex with the entire ectodoman of its receptor. Proceedngs of the National Academy of Sciences of the United States of America. 2012;109:12491–12496. doi: 10.1073/pnas.1206643109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara E, Crepieux P, Gauthier C, Martinat N, Piketty V, Guillou F, et al. A phosphorylation cluster of five serine and threonine residues in the C-terminus of the follicle-stimulating hormone receptor is important for desensitization but not for beta-arrestin-mediated ERK activation. Molecular Endocrinology. 2006;20:3014–3026. doi: 10.1210/me.2006-0098. [DOI] [PubMed] [Google Scholar]
- Kenworthy AK. Imaging protein-protein interactions using fluorescence resonance energy transfer microscopy. Methods. 2001;24:289–296. doi: 10.1006/meth.2001.1189. [DOI] [PubMed] [Google Scholar]
- Kenworthy AK, Edidin M. Distribution of a glycosylphosphatidylinositol-anchored protein at the apical surface of MDCK cells examined at a resolution of <100 A using imaging fluorescence resonance energy transfer. The Journal of Cell Biology. 1998;142:69–84. doi: 10.1083/jcb.142.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi H, Krishnamurthy H, Galet C, Bhaskaran RS, Ascoli M. Identification of a short linear sequence present in the C-terminal tail of the rat follitropin receptor that modulates arrestin-3 binding in a phosphorylation-independent fashion. The Journal of Biological Chemistry. 2002;277:21939–21946. doi: 10.1074/jbc.M110894200. [DOI] [PubMed] [Google Scholar]
- Kluetzman KS, Thomas RM, Nechamen CA, Dias JA. Decreased degradation of internalized follicle-stimulating hormone caused by mutation of aspartic acid 6.30(550) in a protein kinase-CK2 consensus sequence in the third intracellular loop of human follicle-stimulating hormone receptor. Biology of Reproduction. 2011;84:1154–1163. doi: 10.1095/biolreprod.110.087965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy H, Galet C, Ascoli M. The association of arrestin-3 with the follitropin receptor depends on receptor activation and phosphorylation. Molecular and Cellular Endocrinology. 2003;204:127–140. doi: 10.1016/s0303-7207(03)00088-1. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy H, Kishi H, Shi M, Galet C, Bhaskaran RS, Hirakawa T, et al. Postendocytotic trafficking of the follicle-stimulating hormone (FSH)-FSH receptor complex. Molecular Endocrinology. 2003;17:2162–2176. doi: 10.1210/me.2003-0118. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazari MF, Liu X, Nakamura K, Benovic JL, Ascoli M. Role of G protein-coupled receptor kinases on the agonist-induced phosphorylation and internalization of the follitropin receptor. Molecular Endocrinology. 1999;13:866–878. doi: 10.1210/mend.13.6.0289. [DOI] [PubMed] [Google Scholar]
- Lee SP, O’Dowd BF, Rajaram RD, Nguyen T, George SR. D2 dopamine receptor homodimerization is mediated by multiple sites of interaction, including an intermolecular interaction involving transmembrane domain 4. Biochemistry. 2003;42:11023–11031. doi: 10.1021/bi0345539. [DOI] [PubMed] [Google Scholar]
- Lei Y, Hagen GM, Smith SM, Liu J, Barisas G, Roess DA. Constitutively-active human LH receptors are self-associated and located in rafts. Molecular and Cellular Endocrinology. 2007;260–262:65–72. doi: 10.1016/j.mce.2005.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau-Shepard B, Brumberg HA, Peterson AJ, Dias JA. Reversible immunoneutralization of human follitropin receptor. Journal of Reproductive Immunology. 2001;49:1–19. doi: 10.1016/s0165-0378(00)00079-6. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, et al. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion S, Robert F, Crepieux P, Martinat N, Troispoux C, Guillou F, et al. G protein-coupled receptor kinases and beta arrestins are relocalized and attenuate cyclic 3′,5′-adenosine monophosphate response to follicle-stimulating hormone in rat primary Sertoli cells. Biology of Reproduction. 2002;66:70–76. doi: 10.1095/biolreprod66.1.70. [DOI] [PubMed] [Google Scholar]
- Mizrachi D, Segaloff DL. Intracellularly located misfolded glycoprotein hormone receptors associate with different chaperone proteins than their cognate wild-type receptors. Molecular Endocrinology. 2004;18:1768–1777. doi: 10.1210/me.2003-0406. [DOI] [PubMed] [Google Scholar]
- Mueller S, Jaeschke H, Gunther R, Paschke R. The hinge region: An important receptor component for GPHR function. Trends in Endocrinology and Metabolism. 2009;21:111–122. doi: 10.1016/j.tem.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Musnier A, Heitzler D, Boulo T, Tesseraud S, Durand G, Lecureuil C, et al. Developmental regulation of p70 S6 kinase by a G protein-coupled receptor dynamically modelized in primary cells. Cellular and Molecular Life Sciences. 2009;66:3487–3503. doi: 10.1007/s00018-009-0134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Hipkin RW, Ascoli M. The agonist-induced phosphorylation of the rat follitropin receptor maps to the first and third intracellular loops. Molecular Endocrinology. 1998;12:580–591. doi: 10.1210/mend.12.4.0087. [DOI] [PubMed] [Google Scholar]
- Nechamen CA, Dias JA. Human follicle stimulating hormone receptor trafficking and hormone binding sites in the amino terminus. Molecular and Cellular Endocrinology. 2000;166:101–110. doi: 10.1016/s0303-7207(00)00281-1. [DOI] [PubMed] [Google Scholar]
- Nechamen CA, Dias JA. Point mutations in follitropin receptor result in ER retention. Molecular and Cellular Endocrinology. 2003;201:123–131. doi: 10.1016/s0303-7207(02)00424-0. [DOI] [PubMed] [Google Scholar]
- Nechamen CA, Thomas RM, Dias JA. APPL1, APPL2, Akt2 and FOXO1a interact with FSHR in a potential signaling complex. Molecular and Cellular Endocrinology. 2007;260–262:93–99. doi: 10.1016/j.mce.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng GY, O’Dowd BF, Lee SP, Chung HT, Brann MR, Seeman P, et al. Dopamine D2 receptor dimers and receptor-blocking peptides. Biochemical and Biophysical Research Communications. 1996;227:200–204. doi: 10.1006/bbrc.1996.1489. [DOI] [PubMed] [Google Scholar]
- Osuga Y, Hayashi M, Kudo M, Conti M, Kobilka B, Hsueh AJ. Co-expression of defective luteinizing hormone receptor fragments partially reconstitutes ligand-induced signal generation. The Journal of Biological Chemistry. 1997;272:25006–25012. doi: 10.1074/jbc.272.40.25006. [DOI] [PubMed] [Google Scholar]
- Peltoketo H, Strauss L, Karjalainen R, Zhang M, Stamp GW, Segaloff DL, et al. Female mice expressing constitutively active mutants of FSH receptor present with a phenotype of premature follicle depletion and estrogen excess. Endocrinology. 2010;151:1872–1883. doi: 10.1210/en.2009-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piketty V, Kara E, Guillou F, Reiter E, Crepieux P. Follicle-stimulating hormone (FSH) activates extracellular signal-regulated kinase phosphorylation independently of beta-arrestin- and dynamin-mediated FSH receptor internalization. Reproductive Biology and Endocrinology. 2006;4:33. doi: 10.1186/1477-7827-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannikko A, Pakarinen P, Manna PR, Beau I, Misrahi M, Aittomaki K, et al. Functional characterization of the human FSH receptor with an inactivating Ala189Val mutation. Molecular Human Reproduction. 2002;8:311–317. doi: 10.1093/molehr/8.4.311. [DOI] [PubMed] [Google Scholar]
- Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: Roles in receptor silencing, trafficking and signaling. Trends in Endocrinology and Metabolism. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Reiter E, Marion S, Robert F, Troispoux C, Boulay F, Guillou F, et al. Kinase-inactive G-protein-coupled receptor kinases are able to attenuate follicle-stimulating hormone-induced signaling. Biochemical and Biophysical Research Communications. 2001;282:71–78. doi: 10.1006/bbrc.2001.4534. [DOI] [PubMed] [Google Scholar]
- Rozell TG, Davis DP, Chai Y, Segaloff DL. Association of gonadotropin receptor precursors with the protein folding chaperone calnexin. Endocrinology. 1998;139:1588–1593. doi: 10.1210/endo.139.4.5881. [DOI] [PubMed] [Google Scholar]
- Rozell TG, Wang H, Liu X, Segaloff DL. Intracellular retention of mutant gonadotropin receptors results in loss of hormone binding activity of the follitropin receptor but not of the lutropin/choriogonadotropin receptor. Molecular Endocrinology. 1995;9:1727–1736. doi: 10.1210/mend.9.12.8614409. [DOI] [PubMed] [Google Scholar]
- Siegel RM, Chan FK, Zacharias DA, Swofford R, Holmes KL, Tsien RY, et al. Measurement of molecular interactions in living cells by fluorescence resonance energy transfer between variants of the green fluorescent protein. Science’s STKE. 2000;2000:l1. doi: 10.1126/stke.2000.38.pl1. [DOI] [PubMed] [Google Scholar]
- Song YS, Ji I, Beauchamp J, Isaacs NW, Ji TH. Hormone interactions to Leu-rich repeats in the gonadotropin receptors. I. Analysis of Leu-rich repeats of human luteinizing hormone/chorionic gonadotropin receptor and follicle-stimulating hormone receptor. The Journal of Biological Chemistry. 2001;276:3426–3435. doi: 10.1074/jbc.M003772200. [DOI] [PubMed] [Google Scholar]
- Tao YX, Mizrachi D, Segaloff DL. Chimeras of the rat and human FSH receptors (FSHRs) identify residues that permit or suppress transmembrane 6 mutation-induced constitutive activation of the FSHR via rearrangements of hydrophobic interactions between helices 6 and 7. Molecular Endocrinology. 2002;16:1881–1892. doi: 10.1210/me.2001-0199. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Nechamen CA, Mazurkiewicz JE, Muda M, Palmer S, Dias JA. Follice-stimulating hormone receptor forms oligomers and shows evidence of carboxyl-terminal proteolytic processing. Endocrinology. 2007;148:1987–1995. doi: 10.1210/en.2006-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timossi C, Ortiz-Elizondo C, Pineda DB, Dias JA, Conn PM, Ulloa-Aguirre A. Functional significance of the BBXXB motif reversed present in the cytoplasmic domains of the human follicle-stimulating hormone receptor. Molecular and Cellular Endocrinology. 2004;223:17–26. doi: 10.1016/j.mce.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Tranchant T, Durand G, Gauthier C, Crepieux P, Ulloa-Aguirre A, Royere D, et al. Preferential beta-arrestin signalling at low receptor density revealed by functional characterization of the human FSH receptor A189 V mutation. Molecular and Cellular Endocrinology. 2011;331:109–118. doi: 10.1016/j.mce.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Troispoux C, Guillou F, Elalouf JM, Firsov D, Iacovelli L, De Blasi A, et al. Involvement of G protein-coupled receptor kinases and arrestins in desensitization to follicle-stimulating hormone action. Molecular Endocrinology. 1999;13:1599–1614. doi: 10.1210/mend.13.9.0342. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Conn PM. Targeting of G protein-coupled receptors to the plasma membrane in health and disease. Frontiers in Bioscience. 2009;14:973–994. doi: 10.2741/3290. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Crepieux P, Poupon A, Maurel MC, Reiter E. Novel pathways in gonadotropin receptor signaling and biased agonism. Reviews in Endocrine and Metabolic Disorders. 2011;12:259–274. doi: 10.1007/s11154-011-9176-2. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Zarinan T, Pasapera AM, Casas-Gonzalez P, Dias JA. Multiple facets of follicle-stimulating hormone receptor function. Endocrine. 2007;32:251–263. doi: 10.1007/s12020-008-9041-6. [DOI] [PubMed] [Google Scholar]
- Uribe A, Zarinan T, Perez-Solis MA, Gutierrez-Sagal R, Jardon-Valadez E, Pineiro A, et al. Functional and structural roles of conserved cysteine residues in the carboxyl-terminal domain of the follicle-stimulating hormone receptor in human embryonic kidney 293 cells. Biology of Reproduction. 2008;78:869–882. doi: 10.1095/biolreprod.107.063925. [DOI] [PubMed] [Google Scholar]
- Vannier B, Loosfelt H, Meduri G, Pichon C, Milgrom E. Anti-human FSH receptor monoclonal antibodies: Immunochemical and immunocytochemical characterization of the receptor. Biochemistry. 1996;35:1358–1366. doi: 10.1021/bi952290f. [DOI] [PubMed] [Google Scholar]
- Vassart G, Pardo L, Costagliola S. A molecular dissection of the glycoprotein hormone receptors. Trends in Biochemical Sciences. 2004;29:119–126. doi: 10.1016/j.tibs.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Wehbi V, Decourtye J, Piketty V, Durand G, Reiter E, Maurel MC. Selective modulation of follicle-stimulating hormone signaling pathways with enhancing equine chorionic gonadotropin/antibody immune complexes. Endocrinology. 2010;151:2788–2799. doi: 10.1210/en.2009-0892. [DOI] [PubMed] [Google Scholar]
- Wehbi V, Tranchant T, Durand G, Musnier A, Decourtye J, Piketty V, et al. Partially deglycosylated equine LH preferentially activates beta-arrestin-dependent signaling at the follicle-stimulating hormone receptor. Molecular Endocrinology. 2010;24:561–573. doi: 10.1210/me.2009-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner RS, Dias JA. Biochemical analyses of proteolytic nicking of the human glycoprotein hormone alpha-subunit and its effect on conformational epitopes. Endocrinology. 1992;131:1026–1036. doi: 10.1210/endo.131.3.1380433. [DOI] [PubMed] [Google Scholar]
- Wiley HS, Cunningham DD. The endocytotic rate constant. A cellular parameter for quantitating receptor-mediated endocytosis. The Journal of Biological Chemistry. 1982;257:4222–4229. [PubMed] [Google Scholar]
- Zarinan T, Perez-Solis MA, Maya-Nunez G, Casas-Gonzalez P, Conn PM, Dias JA, et al. Dominant negative effects of human follicle-stimulating hormone receptor expression-deficient mutants on wild-type receptor cell surface expression. Rescue of oligomerization-dependent defective receptor expression by using cognate decoys. Molecular and Cellular Endocrinology. 2010;321:112–122. doi: 10.1016/j.mce.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]