Abstract
Increased incidence of erectile dysfunction (ED) has been reported among patients with sleep apnea (SA). However, this association has not been confirmed in a large-scale study. We therefore performed a population-based cohort study using Taiwan National Health Insurance (NHI) database to investigate the association of SA and ED. From the database of one million representative subjects randomly sampled from individuals enrolled in the NHI system in 2010, we identified adult patients having SA and excluded those having a diagnosis of ED prior to SA. From these suspected SA patients, those having SA diagnosis after polysomnography were defined as probable SA patients. The dates of their first SA diagnosis were defined as their index dates. Each SA patient was matched to 30 randomly-selected, age-matched control subjects without any SA diagnosis. The control subjects were assigned index dates as their corresponding SA patients, and were ensured having no ED diagnosis prior to their index dates. Totally, 4,835 male patients with suspected SA (including 1,946 probable SA patients) were matched to 145,050 control subjects (including 58,380 subjects matched to probable SA patients). The incidence rate of ED was significantly higher in probable SA patients as compared with the corresponding control subjects (5.7 vs. 2.3 per 1000 patient-year; adjusted incidence rate ratio = 2.0 [95% CI: 1.8-2.2], p<0.0001). The cumulative incidence was also significantly higher in the probable SA patients (p<0.0001). In multivariable Cox regression analysis, probable SA remained a significant risk factor for the development of ED after adjusting for age, residency, income level and comorbidities (hazard ratio = 2.0 [95%CI: 1.5-2.7], p<0.0001). In line with previous studies, this population-based large-scale study confirmed an increased ED incidence in SA patients in Chinese population. Physicians need to pay attention to the possible underlying SA while treating ED patients.
Introduction
Sleep apnea (SA) is the most common form of sleep disordered breathing characterized by repetitive cessation of breathing during sleep, usually associated with intermittent hypoxia and sleep fragmentation [1–3]. Results from Wisconsin Sleep Cohort study estimate the prevalence of SA was 9% for women and 24% for men [4]. The diagnosis of SA is usually made by frequent apnea/hypopnea events during sleep, i.e. increased apnea-hypopnea index (AHI), on nocturnal polysomnography (PSG) [5]. More than 90% of patients have obstructive sleep apnea (OSA), characterized by recurrent upper airway collapse, while less than 10% of patients have central sleep apnea (CSA), which is related to losing neurological drives of respiratory effort [1,2].
Erectile dysfunction (ED), defined as the inability to obtain and maintain an erection sufficient for satisfactory sexual intercourse, is both highly prevalent and inadequately treated [6]. Over 152 million men were estimated to have ED in 1995, and this prevalence was projected to an estimation of 322 million by 2025 [7]. Because sexual health is important for overall wellbeing, ED is associated with significantly lower quality of life [8]. More importantly, ED has been recognized as an important sentinel event for peripheral artery disease, coronary artery disease (CAD), stroke and even all cause-mortality [9–11].
SA is associated with various diseases such as hypertension, ischemic heart disease, diabetes mellitus, stroke, and hormonal dysfunction [12,13]. Many previous studies have demonstrated that patients with SA have significant risk for erectile dysfunction (ED), and some studies have also shown high prevalence of SA in men with ED [6,14–20]. Although the association between SA and ED has been widely discussed, however, this association has not been confirmed in a large-scale cohort study. We therefore conducted this large population-based cohort study to further strengthen the association between SA and ED.
Methods
Data Sources
The Taiwan National Health Insurance (NHI) has covered ambulatory care, hospital inpatient care, dental services, and prescription drugs since March, 1995. The NHI coverage rate was 96.16% of the whole population of 23 million in 2000 and rose to 99% by the end of 2004 [21]. The NHI medical reimbursement claims database is managed by the National Health Research Institutes in Taiwan. The dataset used for this study is Longitudinal Health Insurance Database 2010 (LHID2010), a cohort of 1 million randomly sampled subjects in the NHI system in 2010 and included their reimbursement information until the end of 2010. Patient identification numbers were encrypted for confidentiality. The study protocol was approved by the Kaohsiung Medical University Hospital Institutional Review Board (KMUH-IRB-EXEMPT-20130034).
Sleep Apnea Cohorts
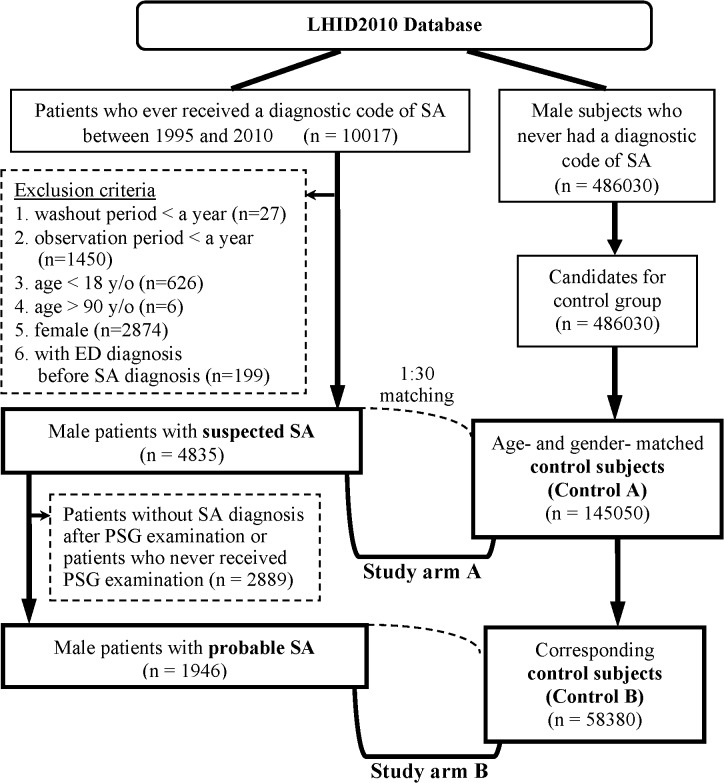
A total of 4,835 male patients were identified as the “suspected SA cohort” by the algorithm (Fig 1). In brief, patients with SA diagnosis between March 1, 1995 and December 31, 2010 were identified initially. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes of 327.20, 327.21, 327.23, 327.27, 327.29, 780.51, 780.53, and 780.57 (Table A in S1 File) were used for diagnosis of SA [1–3]. The ICD-9-CM codes of 607.84 and 302.72 were used for diagnosis of ED [22,23]. Each patient was followed from the date of his initial SA diagnosis, defined as the index date, to either their first diagnosis of ED, end of the study period, or termination of the record because of death or withdrawal from the insurance program, whichever came first. In order to increase the likelihood of including only newly diagnosed SA cases, patients with washout periods (from NHI enrollment to the index date) less than a year were excluded. Patients with diagnosis of ED before the index date were excluded. Patients with follow-up periods less than a year were excluded to ensure enough follow-up periods. Besides, patients younger than 18 years old or elder than 90 years old on the index date were also excluded.
Fig 1. Algorithm for identifying the study population.
(Abbreviations: SA = sleep apnea; ED = erectile dysfunction; PSG = polysomnography).
From the 4,835 patients with suspected SA, those who had ever received PSG examination and remained having SA diagnosis after PSG were further extracted; these 1,946 patients were defined as the “probable SA cohort.”
Control Cohorts
For each patient with suspected SA, thirty age-matched control subjects were randomly selected. The control subjects were given the same index date as their corresponding SA patients. During the matching process, the same exclusion criteria for the SA patients were also applied while selecting control subjects to ensure enough washout periods and follow-up periods and the absence of any ED diagnosis before the index date. Finally, 145,050 control subjects (control A cohort) were matched to the patients with suspected SA (suspected SA cohort), and those who matched to the patients with probable SA (probable SA cohort) were also extracted as control B cohort (n = 58,380). As the SA patients, the control subjects were followed from the index date to either the development of ED, end of the study period, or termination of the record because of death or withdrawal from the insurance program, whichever came first.
Criteria and definitions of variables
The endpoint of this study was the development of ED, defined by the first appearance of ED diagnosis. To increase the reliability of the diagnosis, only those with ED diagnosis for at least three times in the ambulatory claim database or at least once in the inpatient claim database were considered having ED.
The presence of comorbidity is identified by the presence of any corresponding diagnostic codes before the index date in the claim databases and confirmed by the presence of the codes for at least three times in the ambulatory claim database or at least once in the inpatient claim database. Based on the comorbidities, Charlson Comorbidity Index (CCI) score was calculated [24].
Statistical analysis
This study composed of two study arms with identical statistical analyses. Study arm A compared the data between the suspected SA cohort and control A cohort; study arm B compared the data between the probable SA cohort and control B cohort.
First, the demographic data, comorbidities and CCI score were compared between the SA cohorts and the corresponding control cohorts using the Pearson χ2 test for categorical variables or Student’s t test for continuous variables, as appropriate. The ED incidence rate was calculated as the number of ED developed during the follow-up period divided by the total person-year. The ED incidence rates of SA cohorts and control cohorts were further compared by calculating the incidence rate ratio (IRR), defined as the ratio of the ED incidence rates of SA cohort and the corresponding control cohort. The 95% confidence intervals (95% CIs) for the IRRs were estimated under the assumption that the observed number of ED followed a Poisson probability distribution. Stratified analyses were also performed, by classifying the subjects with age group, residency, income level or the presence of any comorbidity. The adjusted IRRs were calculated by multivariable analyses adjusting for age, residency, income and the presence of various comorbidities (except for the variable used for stratification). Cumulative incidence of ED was calculated and compared with Kaplan-Meier method and log-rank test. To further assess the effect of SA, multivariable Cox proportional hazards regression analyses were performed with adjustment of age, residency, income level and comorbidities. After excluding the subjects having events in the initial 1–5 years of the follow-up period, sensitivity analyses were performed on the remaining SA patients and ten randomly-selected corresponding control subjects with the new follow-up periods starting from 1–5 years after the index dates (Table D in S1 File).
Extraction and computation of data, data linkage, processing and sampling and all statistical analyses were performed using SAS system (version 9.3 for Windows, SAS Institute Inc., Cary, NC, USA). The statistical significance level was set at a two-sided p value of < 0.05.
Results
Totally, 4,835 male patients with suspected SA, including 1,946 patients with probable SA, were identified and matched to age-matched control subjects by the algorithm (Fig 1). The baseline characteristics of study cohorts are presented in Table 1. The mean (± standard deviation (SD)) age was 45.6±14.2 years in the suspected SA cohort and the control A cohort; the mean (±SD) age was 46.4±13.1 years in the probable SA cohort and the control B cohort. As compared with the subjects in corresponding control cohorts, patients in the suspected SA cohort and probable SA cohort had more comorbidities, in terms of heart diseases, major neurological disorders, chronic pulmonary diseases, connective tissue diseases, peptic ulcer disease, liver disease, diabetes mellitus, renal disease and cancer (Table 1).
Table 1. Baseline characteristics of the study population.
| Study arm A | Study arm B | |||||
|---|---|---|---|---|---|---|
| Control A | Suspected SA | P value | Control B | Probable SA | P value | |
| N | 145050 (100%) | 4835 (100%) | 58380 (100%) | 1946 (100%) | ||
| Age (year), mean ± SD | 45.6 ± 14.2 | 45.6 ± 14.2 | 46.4 ± 13.1 | 46.4 ± 13.1 | ||
| Age (year), n (%) | ||||||
| ≤ 40 | 56940 (39%) | 1898 (39%) | 20400 (35%) | 680 (35%) | ||
| 40 < age ≤ 50 | 38760 (27%) | 1292 (27%) | 16920 (29%) | 564 (29%) | ||
| > 50 | 49350 (34%) | 1645 (34%) | 21060 (36%) | 702 (36%) | ||
| Residency | ||||||
| Northern Taiwan | 48356 (33%) | 1809 (37%) | <0.0001 | 19133 (33%) | 800 (41%) | <0.0001 |
| Other areas | 96694 (67%) | 3026 (63%) | 39247 (67%) | 1146 (59%) | ||
| Monthly income (NT$), median(IQR) | 21000 (1249–40100) | 25200 (1249–45800) | <0.0001 | 21000 (1249–42000) | 30300 (17280–50600) | <0.0001 |
| Monthly income (NT$), n (%) | ||||||
| ≤ 24000 | 86823 (60%) | 2401 (50%) | <0.0001 | 33925 (58%) | 850 (44%) | <0.0001 |
| > 24000 | 58227 (40%) | 2434 (50%) | 24455 (42%) | 1096 (56%) | ||
| Wash-out period (day), median (IQR) | 11.2 (8.6–13.1) | 11.3 (8.7–13.1) | 0.0269 | 11.4 (8.9–13.1) | 11.4 (9.1–13.1) | 0.0561 |
| Follow-up period (day), median (IQR) | 4.3 (2.5–6.7) | 4.3 (2.5–6.7) | 0.6427 | 4.2 (2.5–6.4) | 4.1 (2.5–6.4) | 0.5845 |
| CCI score, mean ± SD | 0.7 ± 1.3 | 1.2 ± 1.7 | <0.0001 | 0.7 ± 1.3 | 1.3 ± 1.6 | <0.0001 |
| CCI score, n (%) | ||||||
| = 0 | 93086 (64%) | 2113 (44%) | <0.0001 | 36910 (63%) | 766 (39%) | <0.0001 |
| = 1 | 28002 (19%) | 1240 (26%) | 11579 (20%) | 535 (27%) | ||
| ≥ 2 | 23962 (17%) | 1482 (31%) | 9891 (17%) | 645 (33%) | ||
| Underlying diseases, n (%) | ||||||
| Heart disease | 2740 (2%) | 200 (4%) | <0.0001 | 1051 (2%) | 87 (4%) | <0.0001 |
| Myocardial infarction | 1075 (1%) | 63 (1%) | <0.0001 | 431 (1%) | 25 (1%) | 0.0062 |
| Congestive heart failure | 1934 (1%) | 156 (3%) | <0.0001 | 735 (1%) | 68 (3%) | <0.0001 |
| Peripheral vascular disease | 1024 (1%) | 42 (1%) | 0.1854 | 425 (1%) | 18 (1%) | 0.3167 |
| Major neurological disorder | 6955 (5%) | 432 (9%) | <0.0001 | 2769 (5%) | 185 (10%) | <0.0001 |
| Cerebral Vascular disease | 6598 (5%) | 409 (8%) | <0.0001 | 2608 (4%) | 176 (9%) | <0.0001 |
| Dementia | 624 (0%) | 48 (1%) | <0.0001 | 217 (0%) | 16 (1%) | 0.0016 |
| Hemiplegia | 913 (1%) | 40 (1%) | 0.0886 | 397 (1%) | 16 (1%) | 0.4543 |
| Chronic pulmonary disease | 18230 (13%) | 1266 (26%) | <0.0001 | 7363 (13%) | 570 (29%) | <0.0001 |
| Connective tissue disease | 1034 (1%) | 54 (1%) | 0.0011 | 437 (1%) | 22 (1%) | 0.0564 |
| Peptic ulcer disease | 22658 (16%) | 1219 (25%) | <0.0001 | 9345 (16%) | 502 (26%) | <0.0001 |
| Liver disease | 16850 (12%) | 1081 (22%) | <0.0001 | 7076 (12%) | 483 (25%) | <0.0001 |
| Diabetes mellitus | 10496 (7%) | 487 (10%) | <0.0001 | 4390 (8%) | 212 (11%) | <0.0001 |
| Renal disease | 3020 (2%) | 184 (4%) | <0.0001 | 1239 (2%) | 71 (4%) | <0.0001 |
| Cancer | 3344 (2%) | 199 (4%) | <0.0001 | 1330 (2%) | 77 (4%) | <0.0001 |
| Others (non-CCI items) | ||||||
| Hypertension | 26545 (18%) | 1629 (34%) | <0.0001 | 11011 (19%) | 763 (39%) | <0.0001 |
Abbreviation: SA = sleep apnea; CCI = Charlson Comorbidity Index.
Patients with suspected SA had significantly higher ED incidence rate than subjects in control A cohort did (4.4 vs. 2.3 per 1000 patient-year; adjusted IRR = 1.7 [95% CI: 1.6–1.8], p<0.0001); significantly higher ED incidence rate was also noted in patients with probable SA as compared with subjects in control B cohort (5.7 vs. 2.3 per 1000 patient-year; adjusted IRR = 2.0 [95% CI: 1.8–2.2], p<0.0001) (Table 2). On stratified analyses, patients with SA had significantly higher ED incidence rate as compared with subjects in the corresponding control cohort in all strata (Table 2).
Table 2. Incidence rate of erectile dysfunction after the index date in each group.
| Study arm A | Study arm B | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control A | Suspected SA | Crude IRR [95% CI] | Adjusted IRR [95% CI] | Control B | Probable SA | Crude IRR [95% CI] | Adjusted IRR [95% CI] | |||||||||||||
| N | ED | PY | IR | N | ED | PY | IR | N | ED | PY | IR | N | ED | PY | IR | |||||
| Whole study population | 145050 | 1614 | 705703.1 | 2.3 | 4835 | 103 | 23430.0 | 4.4 | 1.9 [1.8–2.1] *** | 1.7 [1.6–1.8] *** | 58380 | 643 | 277701.5 | 2.3 | 1946 | 52 | 9188.7 | 5.7 | 2.4 [2.2–2.7] *** | 2.0 [1.8–2.2] *** |
| Stratified analyses | ||||||||||||||||||||
| Age | ||||||||||||||||||||
| ≤ 50 | 95700 | 670 | 476028.0 | 1.4 | 3190 | 55 | 15787.5 | 3.5 | 2.5 [2.3–2.7] *** | 1.9 [1.8–2.1] *** | 37320 | 260 | 181850.4 | 1.4 | 1244 | 29 | 6003.0 | 4.8 | 3.4 [3.0–3.8] *** | 2.5 [2.2–2.8] *** |
| > 50 | 49350 | 944 | 229675.1 | 4.1 | 1645 | 48 | 7642.4 | 6.3 | 1.5 [1.3–1.7] *** | 1.4 [1.2–1.6] *** | 21060 | 383 | 95851.1 | 4.0 | 702 | 23 | 3185.7 | 7.2 | 1.8 [1.5–2.2] *** | 1.6 [1.3–1.9] *** |
| Residents in | ||||||||||||||||||||
| Northern Taiwan | 48356 | 675 | 251968 | 2.7 | 1809 | 49 | 9624.7 | 5.1 | 1.9 [1.7–2.1] *** | 1.7 [1.5–1.9] *** | 19133 | 263 | 97702.3 | 2.7 | 800 | 27 | 4304.1 | 6.3 | 2.3 [2.0–2.7] *** | 2.0 [1.7–2.4] *** |
| Other areas | 96694 | 939 | 453735.1 | 2.1 | 3026 | 54 | 13805.3 | 3.9 | 1.9 [1.7–2.1] *** | 1.6 [1.5–1.8] *** | 39247 | 380 | 179999.2 | 2.1 | 1146 | 25 | 4884.5 | 5.1 | 2.4 [2.1–2.8] *** | 2.0 [1.7–2.3] *** |
| Monthly income | ||||||||||||||||||||
| ≤ NT$24000 | 86823 | 882 | 420118.2 | 2.1 | 2401 | 42 | 11524.7 | 3.6 | 1.7 [1.6–1.9] *** | 1.5 [1.4–1.7] *** | 33925 | 351 | 160024.6 | 2.2 | 850 | 18 | 3921.4 | 4.6 | 2.1 [1.8–2.5] *** | 1.7 [1.5–2.0] *** |
| > NT$24000 | 58227 | 732 | 285584.9 | 2.6 | 2434 | 61 | 11905.2 | 5.1 | 2.0 [1.8–2.2] *** | 1.7 [1.6–1.9] *** | 24455 | 292 | 117676.9 | 2.5 | 1096 | 34 | 5267.2 | 6.5 | 2.6 [2.3–3.0] *** | 2.2 [1.9–2.5] *** |
| Comorbidity | ||||||||||||||||||||
| No (CCI score = 0) | 93086 | 795 | 477442.7 | 1.7 | 2113 | 51 | 11079.9 | 4.6 | 2.8 [2.5–3.0] *** | 2.8 [2.6–3.1] *** v | 36910 | 281 | 185073.5 | 1.5 | 766 | 22 | 3955.5 | 5.6 | 3.7 [3.2–4.2] *** | 3.6 [3.1–4.1] *** |
| Yes (CCI score ≥1) | 51964 | 819 | 228260.4 | 3.6 | 2722 | 52 | 12350.0 | 4.2 | 1.2 [1.0–1.3] ** | 1.1 [1.0–1.3] * | 21470 | 362 | 92628.0 | 3.9 | 1180 | 30 | 5233.2 | 5.7 | 1.5 [1.3–1.7] *** | 1.4 [1.2–1.7] *** |
The adjusted IRRs were calculated by multivariable analyses adjusting for age, residency, income and the presence of various comorbidities (except for the variable used for stratification).
*p<0.05
**p<0.01
***p<0.0001
Abbreviation: SA = sleep apnea; CCI = Charlson Comorbidity Index
N = number of patients; ED = number of patients with erectile dysfunction; PY = total patient-years
IR = incident rate, as expressed as ED incidence per 1000 patient-years; IRR = incidence rate ratio; CI = confidence interval.
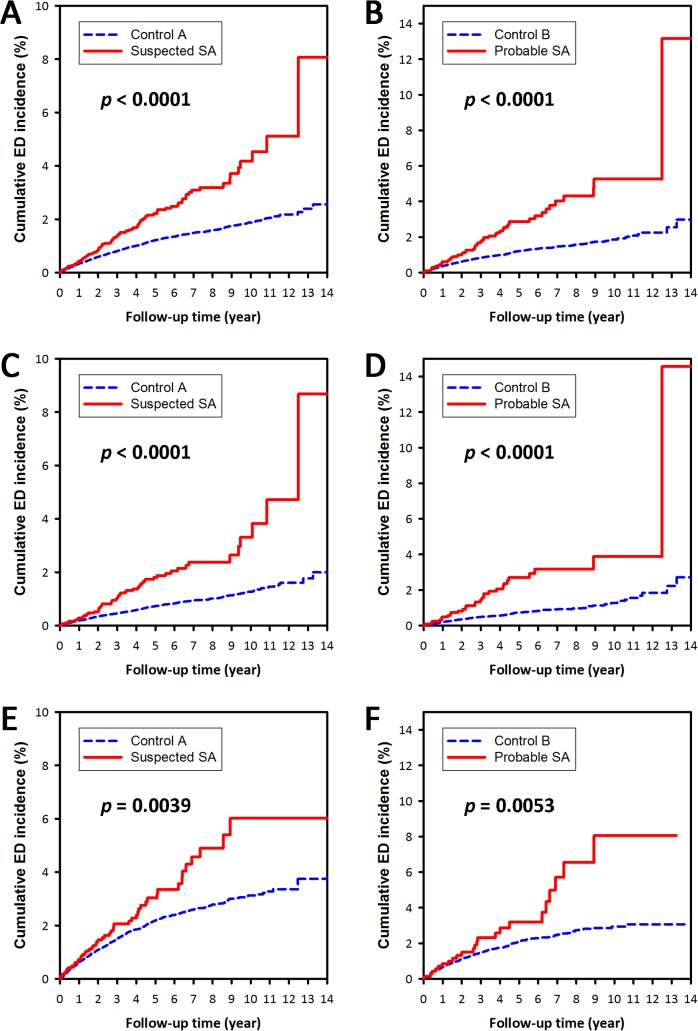
The cumulative ED incidence was significantly higher for both suspected SA cohort (as compared with control A cohort, p<0.0001) and probable SA cohort (as compared with control B cohort, p<0.0001) (Fig 2). On stratified analyses, the SA cohorts had a significantly higher cumulative ED incidence as compared with the corresponding control cohorts in strata of either subjects with age up to 50 years old, or subjects older than 50 years old (all p<0.01) (Fig 2).
Fig 2. The cumulative incidences of erectile dysfunction (ED).
The blue dashed lines and red continuous lines show the cumulative incidence of ED for the control cohort and the sleep apnea (SA) cohort, respectively. (A, C, E) study arm A (suspected SA vs. control A); (B, D, F) study arm B (probable SA vs. control B); (A, B) whole study population; (C, D) subjects ≤ 50 years old; (E, F) subjects >50 years old.
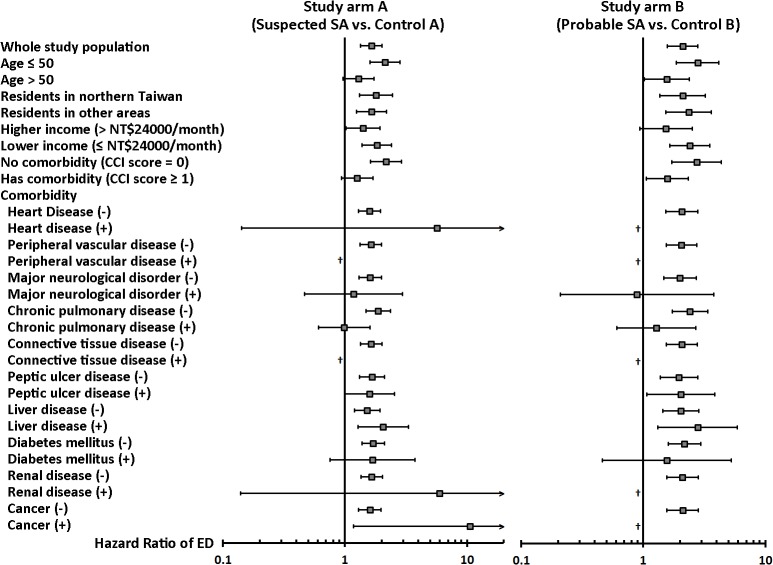
On multivariable Cox proportional hazards regression analyses comparing the ED incidences in probable SA cohort and control B cohort, SA was an independent risk factor for developing ED (hazard ratio [HR]: 2.0, 95% CI: 1.5–2.7, p<0.0001) (Table 3 and Table C in S1 File). The analysis comparing suspected SA cohort and control A cohort (study arm A) showed a consistent result (Table 3 and Table B in S1 File). On stratified analyses, the presence of either suspected SA or probable SA was associated with a higher risk for developing ED both in patients ≤ 50 years old and those > 50 years old (Fig 3). The increased ED risk related to SA was more significant in patients without comorbidities, whereas the effect of SA was modest in those with comorbidities.
Table 3. Multivariable Cox regression analysis of the factors contributing to erectile dysfunction – maximal models.
| Study arm A (Suspected SA vs. Control A) | Study arm B (Probable SA vs. Control B) | |||
|---|---|---|---|---|
| HR [95% CI] | P value | HR [95% CI] | P value | |
| SA patients vs. Control subjects | 1.7 [1.4–2.1] | <0.0001 | 2.0 [1.5–2.7] | <0.0001 |
| Age > 50 vs. Age ≤ 50 | 2.7 [2.5–3.0] | <0.0001 | 2.3 [2.0–2.8] | <0.0001 |
| Residency (Northern Taiwan vs. Other areas) | 1.4 [1.3–1.6] | <0.0001 | 1.4 [1.2–1.6] | <0.0001 |
| Higher income (> NT$24000) vs. lower income (≤ NT$24000) | 1.4 [1.3–1.6] | <0.0001 | 1.3 [1.1–1.5] | 0.0004 |
| Presence of underlying diseases: | ||||
| Heart disease | 0.7 [0.5–1.0] | 0.0625 | 0.9 [0.6–1.4] | 0.7094 |
| Peripheral vascular disease | 0.5 [0.3–1.1] | 0.0700 | 0.4 [0.1–1.3] | 0.1164 |
| Major neurological disorder | 1.1 [0.9–1.3] | 0.5071 | 1.3 [1.0–1.7] | 0.0779 |
| Chronic pulmonary disease | 1.1 [1.0–1.3] | 0.0895 | 1.1 [0.9–1.3] | 0.4791 |
| Connective tissue disease | 0.8 [0.4–1.3] | 0.3522 | 0.7 [0.3–1.8] | 0.4883 |
| Peptic ulcer disease | 1.0 [0.9–1.2] | 0.8412 | 1.2 [1.0–1.4] | 0.1063 |
| Liver disease | 1.5 [1.3–1.7] | <0.0001 | 1.4 [1.1–1.7] | 0.0015 |
| Diabetes mellitus | 1.6 [1.4–1.8] | <0.0001 | 1.9 [1.6–2.4] | <0.0001 |
| Renal disease | 1.0 [0.7–1.3] | 0.8166 | 1.0 [0.7–1.6] | 0.8486 |
| Cancer | 1.1 [0.8–1.4] | 0.5013 | 1.5 [1.1–2.2] | 0.0210 |
*Abbreviations: SA = sleep apnea; CCI = Charlson Comorbidity Index; HR = hazard ratio; CI = confidence interval.
Sensitivity analyses showed consistent results (Table D in S1 File).
Fig 3. Stratified analyses of the multivariable Cox proportional hazards regression analyses.
The results are presented with adjusted HRs (95% CI) of sleep apnea, which are adjusted for age, residency, income and the presence of various comorbidities (except for the variable used for stratification). *Abbreviations: SA = sleep apnea; CCI = Charlson Comorbidity Index; HR = hazard ratio; CI = confidence interval. †: Due to small sample size, hazard ratio cannot be estimated.
Discussion
This large population-based cohort study revealed that patients with SA had significantly higher incidence of ED than subjects without ED. The analyses using SA patients identified with merely the diagnostic codes (i.e., suspected SA cohort in study arm A) and the analyses using SA patients identified with the presence of diagnosis after PSG (i.e., probable SA cohort in study arm B) showed consistent results. Multivariable Cox regression analyses showed that SA remained an independent risk factor for developing ED after adjusting for age and comorbidities.
To the best of our knowledge, this is the first long-term nationwide population-based cohort study to investigate ED incidence in SA patients. Many studies to date have demonstrated the association between SA and ED since 1990, when Hirshkowitz et al. reported 43.8% of ED patients had apnea index ≥5 [6,14–20]. A study using questionnaires in a health-screening program showed that both SA and ED were prevalent and might be related to each other in adult men [17]. Other studies using questionnaires also found that the ED risk was significantly higher in patients with severe obstructive SA and the correlation between the severity of SA and the severity of ED was strong [14,16]. A case-control study further showed that obstructive SA patients had significantly more ED and sexual dissatisfaction as compared with age-matched control [25]. However, it had remained uncertain whether the association between SA and ED was maintained in the presence of other risk factors for ED until a prospective cross-sectional analysis by Budweiser et al. confirmed that SA was an independent risk factor for ED after adjusting for many known risk factors, such as age and hypertension [19]. A population-based cross-sectional study in Sao Paolo, Brazil also showed obstructive SA was significantly associated with a higher risk of ED [26]. Although many studies have been done in discussing the relationship between SA and ED, these studies are cross-sectional studies and are relatively small in size. Our population-based study not only showed results consistent with the previous studies, but also demonstrated a temporal relationship using a cohort study design. The sensitivity analyses using “incubation time” design further strengthened the evidence showing the association between SA and ED. In line with previous studies, we found old age, diabetes mellitus, hypertension, and liver diseases were also independent risk factors for ED from the multivariable Cox regression analysis [26–28]. Interestingly, the effect of SA was less significant in the elder patients and patients without comorbidities than in younger patients and patients with comorbidities, respectively. The findings might be explained by possible less awareness of sexual dysfunction in the elder population and patients with comorbidities in Taiwan. However, as a more reasonable explanation in our opinion, the ED risk had already been increased by aging or comorbidities, so the effect of SA was somewhat obscured. Further prospective studies are needed to clarify this point.
Intermittent hypoxia, a hallmark of SA, plays an important role in the health effect of SA, and ED is no exception. A previous study showed that the degree of hypoxemia correlated with the score on a questionnaire-based scale evaluating erectile function [29]. The role of hypoxia was also well demonstrated by an animal study showing that chronic intermittent hypoxia during sleep was associated with emergence of ED and decreased libido in mice [30]. Indeed, treating with continuous positive airway pressure (CPAP), the gold standard in treating SA, may also improve the sexual performance in patients having both SA and ED [18,25,31–34]. Other treatment strategies for SA, such as oral appliances (mandibular advancement devices) and surgical intervention (uvulopalatopharyngoplasty), might also improve ED [25,35]. Some studies suggested that sildenafil was superior to CPAP in treating ED in SA patients [31,34,36,37]. However, due to the detrimental effect of sildenafil on respiratory and desaturation events during sleep [38], this drug should be used carefully. Combination of CPAP and oral sildenafil might be a more effective and safer treatment modality for ED in SA patients [36,37].
The pathogenic mechanisms contributing to the development of ED in SA patients, including abnormalities in neural, hormonal and vascular regulation, have been discussed [20]. The development of peripheral nerve dysfunction, as shown by altered bulbocavernosus reflex, was associated with ED in SA patients [39]. Although low testosterone level was associated with ED, studies showed that testosterone level was not significantly changed in patients receiving treatment for SA or in mice treated with intermittent hypoxia [25,26,30,40]. In the recent decade, much attention has been paid to the role of endothelial dysfunction, which is also one of the main mechanisms for cardio-metabolic consequences of SA [6]. A study of serum inflammatory markers and cytokines showed elevated serum concentration of high-sensitivity C-reactive protein, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-8 (IL-8) in SA patients with ED, as compared with those without ED; this study supported the possible involvement of endothelial dysfunction in the development of ED in SA patients [41]. The study using murine model also found decreased expression of nitric oxide synthase (NOS) in erectile tissue after chronic intermittent hypoxia [30]. In addition, a rat study showed that long-term intermittent hypoxia increased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation, which decreased the expression and activity of constitutive NOS, including endothelial NOS and neuronal NOS, and thereby contributed to the development of ED [42]. Taken together, endothelial dysfunction related to intermittent hypoxia might be the most important mechanism underlying ED related to SA. Since CPAP has been demonstrated to be able to reverse endothelial dysfunction in SA patients [43], it is not surprising that CPAP treatment improves sexual function in patients having both SA and ED [18,25,31–34].
Our study has two major strengths. First, our study is the largest study to date discussing about the association between SA and ED. As compared with lab-based or hospital-based setting, the nationwide, population-based setting minimizes selection bias and provides a broad view of the real world. Second, this is a cohort study with a long follow-up period. As compared with the previous studies using cross-sectional design, our study provides better evidence about the association between SA and ED by demonstrating a temporal trajectory.
Nevertheless, this study had several limitations. First, the diagnosis based on diagnostic codes might be less accurate than those made according to standardized criteria in clinical trials. Therefore, in addition to the analyses using SA patients identified with merely the diagnostic codes (i.e., suspected SA cohort), we performed another set of analyses using SA patients having SA diagnosis after PSG (i.e., probable SA cohort) and found consistent results. Besides, the method using diagnostic codes to identify ED in the NHI database has been used in many previous studies [22,23]. Second, it is not possible to adequately distinguish OSA and CSA based on the diagnostic codes in our database. It is therefore not possible to assess whether OSA and CSA affect the risk of ED in the same way. However, because the vast majority of SA is OSA, it is OSA that might substantially increase the risk of ED [1,2]. Third, some well-known potentially important clinical risk factors for ED, such as smoking history, obesity, and alcoholism, were not available in the NHI database, so interpretation of our results must be careful to account for possible impact of these risk factors. Nevertheless, SA remained an independent risk factor even in the multivariable Cox regression analyses adjusting for various comorbidities, which minimized the effect of these confounders. Fourth, because SA patients seemed having higher income level, this could potentially introduced a bias toward more awareness to sexual dysfunction in the higher socio-economic group and thus leaded to higher incidence of ED in the SA cohorts. We therefore incorporated the residency and income level into the multivariable models to control these possible confounders. Finally, information about the severity of SA and major treatment modalities, such as CPAP and oral appliances, were not available in the NHI database. However, the inclusion of milder SA patients and SA patients receiving treatment might abate the extent of increased ED risk from SA, resulting in underestimation of the hazard ratios in our study.
In conclusion, the present large nationwide population-based cohort study confirms SA as an independent risk factor for ED. Clinicians should consider ED while seeing patients with SA, and men with ED might also benefit from sleep evaluation.
Supporting Information
Table B. Multivariable Cox regression analysis of the factors contributing to erectile dysfunction (reduced models of study arm A). Table C. Multivariable Cox regression analysis of the factors contributing to erectile dysfunction (reduced models of study arm B). Table D. Sensitivity analyses.
(PDF)
Acknowledgments
The authors thank the help from the Statistical Analysis Laboratory, Department of Internal Medicine and the Statistical Analysis Laboratory, Department of Medical Research, Kaohsiung Medical University Hospital and Center of Teaching and Research, Kaohsiung Municipal Ta-Tung Hospital.
This study is based on data from the National Health Insurance Research Database provided by the National Health Insurance Administration, Ministry of Health and Welfare and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of National Health Insurance Administration, Ministry of Health and Welfare or National Health Research Institutes (NHRI). Due to legal and ethical restrictions, researchers should contact NHRI (http://nhird.nhri.org.tw/index.htm) for access of the data after approved by institutional review board.
Data Availability
This study is based on data from the National Health Insurance Research Database provided by the National Health Insurance Administration, Ministry of Health and Welfare and managed by National Health Research Institutes (NHRI). Due to legal and ethical restrictions, researchers should contact NHRI (http://nhird.nhri.org.tw/) for access of the data after approved by institutional review board.
Funding Statement
The authors have no support or funding to report.
References
- 1. Chou PS, Chang WC, Chou WP, Liu ME, Lai CL, Liu CK, et al. Increased risk of benign prostate hyperplasia in sleep apnea patients: a nationwide population-based study. PLoS One. 2014;9(3):e93081 10.1371/journal.pone.0093081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chou KT, Huang CC, Chen YM, Su KC, Shiao GM, Lee YC, et al. Sleep apnea and risk of deep vein thrombosis: a non-randomized, pair-matched cohort study. Am J Med. 2012;125(4):374–80. 10.1016/j.amjmed.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 3. Kang JH, Keller JJ, Chen YK, Lin HC. Association between obstructive sleep apnea and urinary calculi: a population-based case-control study. Urology. 2012;79(2):340–5. 10.1016/j.urology.2011.08.040 [DOI] [PubMed] [Google Scholar]
- 4. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–5. 10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- 5. Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea?: The Rational Clinical Examination systematic review. JAMA. 2013;310(7):731–41. 10.1001/jama.2013.276185 [DOI] [PubMed] [Google Scholar]
- 6. Hoyos CM, Melehan KL, Phillips CL, Grunstein RR, Liu PY. To ED or not to ED—Is erectile dysfunction in obstructive sleep apnea related to endothelial dysfunction? Sleep Med Rev. 2015;20:5–14. 10.1016/j.smrv.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 7. Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84(1):50–6. [DOI] [PubMed] [Google Scholar]
- 8. Jonler M, Moon T, Brannan W, Stone NN, Heisey D, Bruskewitz RC. The effect of age, ethnicity and geographical location on impotence and quality of life. Br J Urol. 1995;75(5):651–5. [DOI] [PubMed] [Google Scholar]
- 9. Meller SM, Stilp E, Walker CN, Mena-Hurtado C. The link between vasculogenic erectile dysfunction, coronary artery disease, and peripheral artery disease: role of metabolic factors and endovascular therapy. J Invasive Cardiol. 2013;25(6):313–9. [PubMed] [Google Scholar]
- 10. Gandaglia G, Briganti A, Jackson G, Kloner RA, Montorsi F, Montorsi P, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol. 2014;65(5):968–78. 10.1016/j.eururo.2013.08.023 [DOI] [PubMed] [Google Scholar]
- 11. Vlachopoulos CV, Terentes-Printzios DG, Ioakeimidis NK, Aznaouridis KA, Stefanadis CI. Prediction of cardiovascular events and all-cause mortality with erectile dysfunction: a systematic review and meta-analysis of cohort studies. Circ Cardiovasc Qual Outcomes. 2013;6(1):99–109. 10.1161/CIRCOUTCOMES.112.966903 [DOI] [PubMed] [Google Scholar]
- 12. Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172(11):1447–51. 10.1164/rccm.200505-702OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172(12):1590–5. 10.1164/rccm.200504-637OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Teloken PE, Smith EB, Lodowsky C, Freedom T, Mulhall JP. Defining association between sleep apnea syndrome and erectile dysfunction. Urology. 2006;67(5):1033–7. 10.1016/j.urology.2005.11.040 [DOI] [PubMed] [Google Scholar]
- 15. Hirshkowitz M, Karacan I, Arcasoy MO, Acik G, Narter EM, Williams RL. Prevalence of sleep apnea in men with erectile dysfunction. Urology. 1990;36(3):232–4. [DOI] [PubMed] [Google Scholar]
- 16. Margel D, Cohen M, Livne PM, Pillar G. Severe, but not mild, obstructive sleep apnea syndrome is associated with erectile dysfunction. Urology. 2004;63(3):545–9. 10.1016/j.urology.2003.10.016 [DOI] [PubMed] [Google Scholar]
- 17. Heruti R, Shochat T, Tekes-Manova D, Ashkenazi I, Justo D. Association between erectile dysfunction and sleep disorders measured by self-assessment questionnaires in adult men. J Sex Med. 2005;2(4):543–50. 10.1111/j.1743-6109.2005.00072.x [DOI] [PubMed] [Google Scholar]
- 18. Goncalves MA, Guilleminault C, Ramos E, Palha A, Paiva T. Erectile dysfunction, obstructive sleep apnea syndrome and nasal CPAP treatment. Sleep Med. 2005;6(4):333–9. 10.1016/j.sleep.2005.03.001 [DOI] [PubMed] [Google Scholar]
- 19. Budweiser S, Enderlein S, Jorres RA, Hitzl AP, Wieland WF, Pfeifer M, et al. Sleep apnea is an independent correlate of erectile and sexual dysfunction. J Sex Med. 2009;6(11):3147–57. 10.1111/j.1743-6109.2009.01372.x [DOI] [PubMed] [Google Scholar]
- 20. Zias N, Bezwada V, Gilman S, Chroneou A. Obstructive sleep apnea and erectile dysfunction: still a neglected risk factor? Sleep Breath. 2009;13(1):3–10. 10.1007/s11325-008-0212-8 [DOI] [PubMed] [Google Scholar]
- 21. Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308(18):1906–14. [DOI] [PubMed] [Google Scholar]
- 22. Huang CC, Chan WL, Chen YC, Chen TJ, Chung CM, Huang PH, et al. Herpes simplex virus infection and erectile dysfunction: a nationwide population-based study. Andrology. 2013;1(2):240–4. 10.1111/j.2047-2927.2012.00037.x [DOI] [PubMed] [Google Scholar]
- 23. Su VY, Liu CJ, Lan MY, Chen YM, Su KC, Lee YC, et al. Allergic rhinitis and risk of erectile dysfunction—a nationwide population-based study. Allergy. 2013;68(4):440–5. 10.1111/all.12100 [DOI] [PubMed] [Google Scholar]
- 24. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. [DOI] [PubMed] [Google Scholar]
- 25. Hoekema A, Stel AL, Stegenga B, van der Hoeven JH, Wijkstra PJ, van Driel MF, et al. Sexual function and obstructive sleep apnea-hypopnea: a randomized clinical trial evaluating the effects of oral-appliance and continuous positive airway pressure therapy. J Sex Med. 2007;4(4 Pt 2):1153–62. 10.1111/j.1743-6109.2006.00341.x [DOI] [PubMed] [Google Scholar]
- 26. Andersen ML, Santos-Silva R, Bittencourt LR, Tufik S. Prevalence of erectile dysfunction complaints associated with sleep disturbances in Sao Paulo, Brazil: a population-based survey. Sleep Med. 2010;11(10):1019–24. 10.1016/j.sleep.2009.08.016 [DOI] [PubMed] [Google Scholar]
- 27. Maiorino MI, Bellastella G, Esposito K. Diabetes and sexual dysfunction: current perspectives. Diabetes Metab Syndr Obes. 2014;7:95–105. 10.2147/DMSO.S36455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huyghe E, Kamar N, Wagner F, Capietto AH, El-Kahwaji L, Muscari F, et al. Erectile dysfunction in end-stage liver disease men. J Sex Med. 2009;6(5):1395–401. 10.1111/j.1743-6109.2008.01169.x [DOI] [PubMed] [Google Scholar]
- 29. Shin HW, Rha YC, Han DH, Chung S, Yoon IY, Rhee CS, et al. Erectile dysfunction and disease-specific quality of life in patients with obstructive sleep apnea. Int J Impot Res. 2008;20(6):549–53. 10.1038/ijir.2008.39 [DOI] [PubMed] [Google Scholar]
- 30. Soukhova-O'Hare GK, Shah ZA, Lei Z, Nozdrachev AD, Rao CV, Gozal D. Erectile dysfunction in a murine model of sleep apnea. Am J Respir Crit Care Med. 2008;178(6):644–50. 10.1164/rccm.200801-190OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perimenis P, Karkoulias K, Konstantinopoulos A, Perimeni PP, Katsenis G, Athanasopoulos A, et al. Sildenafil versus continuous positive airway pressure for erectile dysfunction in men with obstructive sleep apnea: a comparative study of their efficacy and safety and the patient's satisfaction with treatment. Asian J Androl. 2007;9(2):259–64. 10.1111/j.1745-7262.2007.00085.x [DOI] [PubMed] [Google Scholar]
- 32. Khafagy AH, Khafagy AH. Treatment of obstructive sleep apnoea as a therapeutic modality for associated erectile dysfunction. Int J Clin Pract. 2012;66(12):1204–8. 10.1111/j.1742-1241.2012.02990.x [DOI] [PubMed] [Google Scholar]
- 33. Budweiser S, Luigart R, Jorres RA, Kollert F, Kleemann Y, Wieland WF, et al. Long-term changes of sexual function in men with obstructive sleep apnea after initiation of continuous positive airway pressure. J Sex Med. 2013;10(2):524–31. 10.1111/j.1743-6109.2012.02968.x [DOI] [PubMed] [Google Scholar]
- 34. Pastore AL, Palleschi G, Ripoli A, Silvestri L, Maggioni C, Pagliuca G, et al. Severe obstructive sleep apnoea syndrome and erectile dysfunction: a prospective randomised study to compare sildenafil vs. nasal continuous positive airway pressure. Int J Clin Pract. 2014;68(8):995–1000. 10.1111/ijcp.12463 [DOI] [PubMed] [Google Scholar]
- 35. Shin HW, Park JH, Park JW, Rhee CS, Lee CH, Min YG, et al. Effects of surgical vs. nonsurgical therapy on erectile dysfunction and quality of life in obstructive sleep apnea syndrome: a pilot study. J Sex Med. 2013;10(8):2053–9. 10.1111/jsm.12128 [DOI] [PubMed] [Google Scholar]
- 36. Perimenis P, Konstantinopoulos A, Karkoulias K, Markou S, Perimeni P, Spyropoulos K. Sildenafil combined with continuous positive airway pressure for treatment of erectile dysfunction in men with obstructive sleep apnea. Int Urol Nephrol. 2007;39(2):547–52. 10.1007/s11255-006-9079-4 [DOI] [PubMed] [Google Scholar]
- 37. Li X, Dong Z, Wan Y, Wang Z. Sildenafil versus continuous positive airway pressure for erectile dysfunction in men with obstructive sleep apnea: a meta-analysis. Aging Male. 2010;13(2):82–6. 10.3109/13685530903406789 [DOI] [PubMed] [Google Scholar]
- 38. Roizenblatt S, Guilleminault C, Poyares D, Cintra F, Kauati A, Tufik S. A double-blind, placebo-controlled, crossover study of sildenafil in obstructive sleep apnea. Arch Intern Med. 2006;166(16):1763–7. 10.1001/archinte.166.16.1763 [DOI] [PubMed] [Google Scholar]
- 39. Fanfulla F, Malaguti S, Montagna T, Salvini S, Bruschi C, Crotti P, et al. Erectile dysfunction in men with obstructive sleep apnea: an early sign of nerve involvement. Sleep. 2000;23(6):775–81. [PubMed] [Google Scholar]
- 40. Knapp A, Myhill PC, Davis WA, Peters KE, Hillman D, Hamilton EJ, et al. Effect of continuous positive airway pressure therapy on sexual function and serum testosterone in males with type 2 diabetes and obstructive sleep apnoea. Clin Endocrinol (Oxf). 2014;81(2):254–8. 10.1111/cen.12401 [DOI] [PubMed] [Google Scholar]
- 41. Bouloukaki I, Papadimitriou V, Sofras F, Mermigkis C, Moniaki V, Siafakas NM, et al. Abnormal cytokine profile in patients with obstructive sleep apnea-hypopnea syndrome and erectile dysfunction. Mediators Inflamm. 2014;2014:568951 10.1155/2014/568951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu K, Liu XS, Xiao L, Shang J, Li MC, Xu YJ, et al. NADPH oxidase activation: a mechanism of erectile dysfunction in a rat model of sleep apnea. J Androl. 2012;33(6):1186–98. 10.2164/jandrol.112.016642 [DOI] [PubMed] [Google Scholar]
- 43. Kohler M, Stoewhas AC, Ayers L, Senn O, Bloch KE, Russi EW, et al. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2011;184(10):1192–9. 10.1164/rccm.201106-0964OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table B. Multivariable Cox regression analysis of the factors contributing to erectile dysfunction (reduced models of study arm A). Table C. Multivariable Cox regression analysis of the factors contributing to erectile dysfunction (reduced models of study arm B). Table D. Sensitivity analyses.
(PDF)
Data Availability Statement
This study is based on data from the National Health Insurance Research Database provided by the National Health Insurance Administration, Ministry of Health and Welfare and managed by National Health Research Institutes (NHRI). Due to legal and ethical restrictions, researchers should contact NHRI (http://nhird.nhri.org.tw/) for access of the data after approved by institutional review board.