Abstract
Anti-Thy1 glomerulonephritis is a rat nephritis model closely simulating human mesangial proliferative glomerulonephritis. It affects primarily the mesangium, yet displays substantial proteinuria during the course. This study investigated the molecular signals underlying proteinuria in this disease and the modulation of which by the known antiproteinuric agent, pentoxifylline. Male Wistar rats were randomly divided into a control group and nephritic groups with or without treatment with IMD-0354 (an IκB kinase inhibitor), SB431542 (an activin receptor–like kinase inhibitor) or pentoxifylline. Kidney sections were prepared for histological examinations. Glomeruli were isolated for mRNA and protein analysis. Urine samples were collected for protein and nephrin quantitation. One day after nephritis induction, proteinuria developed together with ultrastructural changes of the podocyte and downregulation of podocyte mRNA and protein expression. These were associated with upregulation of tumor necrosis factor (TNF)-α and transforming growth factor (TGF)-β/activins mRNAs and activation of nuclear factor (NF)-κB p65 and Smad2/3. IMD-0354 attenuated proteinuria on d 1, whereas SB431542 decreased proteinuria on d 3 and 5, in association with partial restoration of downregulated podocyte mRNA and protein expression. Pentoxifylline attenuated proteinuria and nephrinuria through the course, plus inhibition of p-NF-κB p65 (d 1) and p-Smad2/3 (d 5) and partial reversal of downregulated podocyte mRNA and protein. Our data show that the pathogenesis of proteinuria in anti-Thy1 glomerulonephritis involves TNF-α and TGF-β/activin pathways, and the evolution of this process can be attenuated by pentoxifylline via downregulation of NF-κB and Smad signals and restoration of the podocyte component of the glomerular filtration barrier.
INTRODUCTION
Proteinuria is a hallmark of glomerular injury and a predictor for end-stage renal disease (1,2). Current data indicate that disruptions of the glomerular filtration barrier play a central role in the pathogenesis of glomerular proteinuria (3–5). Within the glomerular filtration barrier, the podocyte slit diaphragm has been most extensively studied and is considered to be the key component in determining glomerular permselectivity (6). In line with this notion, proteinuric animal models can be induced by genetic knockdown of podocyte-related genes or selective injury to the podocyte slit diaphragm with agents such as puromycin aminonucleoside or adriamycin (7). By contrast, the anti-Thy1 glomerulonephritis is characterized by complement-dependent mesangiolysis at the outset and manifests substantial proteinuria during the course (8). The mechanisms underlying the development of proteinuria in this disease have not been fully elucidated.
Inflammatory cytokines and fibrogenic mediators have been shown to modulate podocyte motility and albumin permeability in vitro (9–13). And mesangial- derived cytokines such as tumor necrosis factor (TNF)-α or transforming growth factor (TGF)-β1 can inhibit the expression of nephrin and ezrin by podocytes and induce podocyte death and detachment (14,15). These observations imply the existence of a potential mesangial cell-podocyte crosstalk (16–18), and raise the possibility that certain cytokines produced by activated mesangial cells in anti-Thy1 glomerulonephritis may act in a paracrine manner on adjacent podocytes, which then cause proteinuria via alterations of glomerular permselectivity. The objective of this study was to investigate the potential mechanisms whereby proteinuria developed in anti-Thy1 disease and the modulation of which by selective signal transduction inhibitors, including the known antiproteinuric agent pentoxifylline (PTX) (19–24).
MATERIALS AND METHODS
Induction of Anti-Thy1 Glomerulonephritis and Experimental Design
The study was carried out under a protocol approved by Institutional Animal Care and Use Committee of National Taiwan University and complied with standards delineated in the ARRIVE guidelines for animal research (25).
Male Wistar rats weighing 190–220 g were obtained from and housed at the animal center of our institute (temperature: 22 ± 2°C; humidity: 50 ± 20%) and fed with LabDiet® 5001 (LabDiet, St. Louis, MO, USA) with free access to drinking water. The animals were randomly divided into a control group of 15 rats and 4 nephritic groups with or without treatment of 18 rats each. The control rats (group A) received 0.2 mL of 1× phosphate-buffered saline (pH 7.4) on d 0. The nephritic rats (group B) received an intravenous injection of 250 μg of a mouse anti-rat Thy1 monoclonal antibody (Cedarlane, Burlington, ON, Canada) diluted in 0.9% saline (via tail veins) on d 0 and were treated with vehicle from d 1 to d 5. Groups C–E were treated with the same dose of anti-Thy1 antibody as group B, plus an IκB kinase inhibitor, IMD-0354 (10 mg/kg, Tocris Bioscience, Bristol, UK) (group C), or an activin receptor–like kinase (ALK) inhibitor, SB431542 (20 mg/kg, Tocris Bioscience) (group D), administered intraperitoneally daily from d 1 to 5, or PTX (20 mg/kg, Sanofi-aventis, Laval, Quebec, Canada) (group E), administered intravenously daily from day −2 to d 5. The doses of IMD-0354 and SB431542 administered to animals were determined and prepared according to references provided by the manufacturer, while the dose of PTX was determined according to our previous study, which also showed a greater antiproteinuric efficacy of the drug by administration before, as opposed to after, nephritis induction (8). All animals in groups A–E were anesthetized with a single intraperitoneal injection of 8 mg/kg xylazine and 80 mg/kg ketamine, and groups of three to six rats were sacrificed at the end of d 0, 1, 3, 5 or 7 (group A only).
Measurement of Proteinuria and Urinary Nephrin Excretion
Twenty-four–hour urine collections were taken at different time points, and protein concentrations were quantitated by the Bradford method (Bio-Rad, Hercules, CA, USA). Separate sets of urine samples were collected, and nephrin concentrations were measured using a commercially available enzyme-linked immunosorbent assay kit (Exocell, Philadelphia, PA, USA), adjusted for creatinine levels determined by a standard colorimetric method (Jaffe rate reaction).
Renal Histopathology and Electron Microscopy
Kidney sections were fixed with 4% buffered formaldehyde and embedded in paraffin. Three-micrometer-thick sections were stained with hematoxylin and eosin, or periodic acid–Schiff reagents and examined by light microscopy (Nikon Instruments, Nikon Corporation, Tokyo, Japan). For immunohistological staining, kidney samples were fixed with 4% paraformaldehyde and embedded in paraffin. Three-micrometer sections were deparaffinized and rehydrated. Sections were treated with 0.3% H2O2 in methanol for 15 min to quench endogenous peroxide activity and boiled for 10 min in 0.1 mol/L citrate buffer to retrieve antigens. To detect activation of TNF-α and TGF-β signaling pathways, sections were incubated with anti–phospho-NF-κB p65 (Ser276) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and anti-phospho-Smad2/3 (Ser465/467) (Cell Signaling Technology, Danvers, MA, USA) overnight at 4°C, washed in 1× phosphate-buffered saline and incubated with ImmPRESS™ polymer reagent (Vector Laboratories Inc., Burlingame, CA, USA) for 30 min at room temperature. After washing, sections were incubated with the VECTOR NovaRED kit (Vector Laboratories) for 2–15 min to produce a brown red product. To detect podocyte-related proteins, sections were incubated with the following primary antibodies: anti-nephrin, anti-synaptopodin, anti-Wilms tumor (WT)-1 (Santa Cruz Biotechnology) or anti-podocin (Sigma-Aldrich) at 4°C overnight. After washing, sections were incubated with the fluorophore-conjugated secondary antibody and then mounted and subjected to fluorescence microscopy (Leica DMRA, Leica Microsystems, Wetzlar, Germany).
The number of podocytes per glomerulus was assayed by counting WT-1–positive cells. The ratio of the immunofluorescent staining area to the total area of the glomerulus were calculated as the area positively stained for podocyte-related proteins (synaptopodin, nephrin, podocin) per glomerular area by automated computer analysis with Image-Pro plus 6.0 (Media Cybernetics, Rockville, MD, USA), based on the method reported elsewhere (26).
For electron microscopic examinations, small blocks of kidneys were fixed in 2.5% buffered glutaraldehyde, postfixed in 2% osmium tetroxide, dehydrated in graded ethanol and embedded in epoxy resin. Ultrathin sections (0.1-μm-thick) were stained with uranyl acetate and lead citrate and examined by an electron microscope (Hitachi H-7100, Tokyo, Japan).
Quantitative Polymerase Chain Reaction (PCR)
The gene expression of isolated glomeruli was analyzed as follows. Complementary DNA was generated from glomerular total RNA using an iScript™ cDNA Synthesis Kit (Bio-Rad). The abundance of mRNAs for TNF-α, interleukin (IL)-1β, monocyte chemoattractant protein (MCP)-1, TGF-β1, TGF-β2, TGF-β3, activin-βA and activin-βB, as well as various filtration barrier proteins, were quantified by using the Bio-Rad MyiQ™ single color real-time PCR detection system. The specific primer pairs used are listed in Supplementary Table S1. Briefly, 1 μL of the cDNA was mixed with 200 nmol/L of primers and 1× Bio-Rad iQ™ SYBR Green Supermix. The gene expression levels of all cDNA samples were normalized by using 18S rRNA. The mean levels of the target gene/18S rRNA gene in normal glomeruli were arbitrarily defined as 1.0, and quantitative data were shown as the relative increase/decrease from control glomeruli.
Western Blot Analysis
Isolated glomeruli, before and after treatment with IMD-0354, SB431542 or PTX to the experimental animals, were lysed on ice in radioimmunoprecipitation assay buffer that contained 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L sodium orthovanadate and 1 μg/mL each of aprotinin, leupeptin and pepstatin. Lysates were centrifuged at 17,608g, and supernatants (50 μg protein/lane) were separated by 9% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto an Immobilon filter (Millipore, Bedford, MA, USA), as described previously (27). Temporal changes of α-smooth muscle actin (SMA) (Sigma-Aldrich), nuclear factor (NF)-κB p65, phospho-Smad2/3, podocyte-related proteins (nephrin, podocin, synaptopodin, WT-1 and zonula occludens [ZO]-1) (Santa Cruz Biotechnology), phospho-NF-κB p65 and Smad2/3 (Cell Signaling Technology) were then detected according to the manufacturers’ instructions.
Statistical Analysis
Data were expressed as mean ± standard error of the mean (SEM). One-way analysis of variance was performed by using PASW Statistics for Windows, version 18.0.0 (SPSS [IBM, Armonk, NY, USA]) to detect any differences among different groups of experimental animals. A p value of <0.05 was considered statistically significant.
All supplementary materials are available online at www.molmed.org.
RESULTS
Temporal Relationships between Cytokine Induction, Alteration of Filtration Barrier and Evolution of Proteinuria in Anti-Thy1 Glomerulonephritis
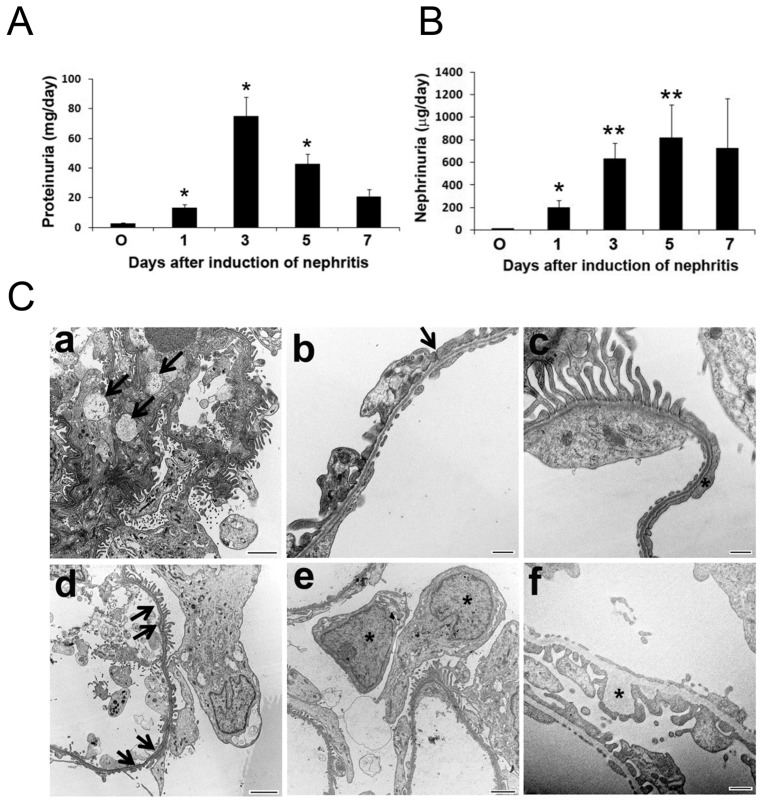
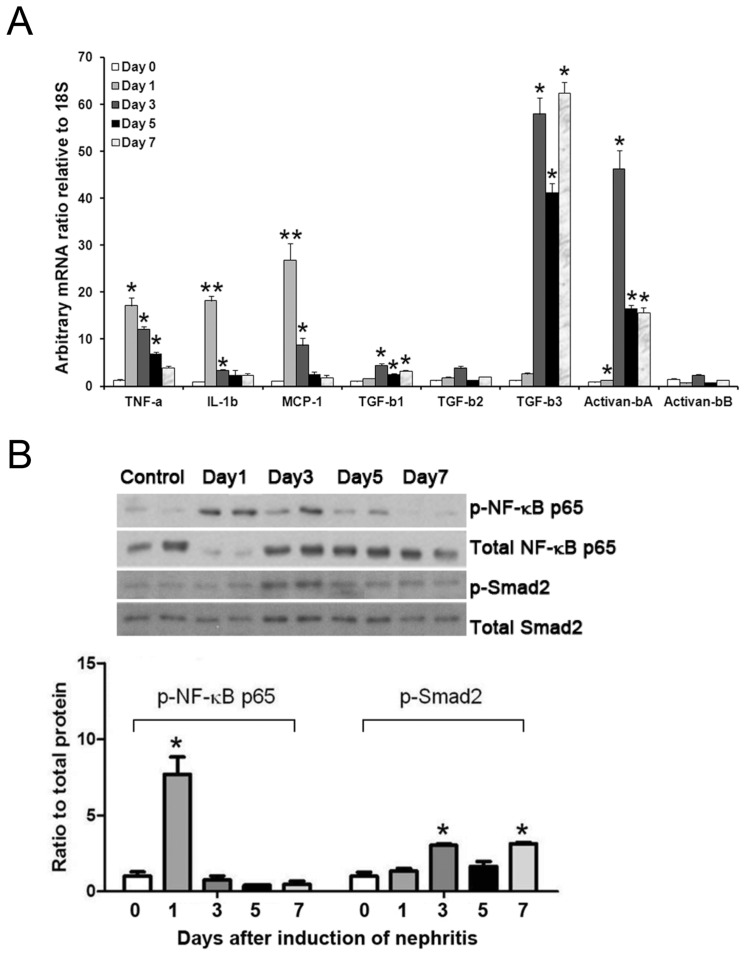
After induction of the nephritis, proteinuria started to develop from d 1 and sustained to d 5 (Figure 1A). Appreciable amounts of nephrin were excreted in the urine during the same period (Figure 1B). On electron microscopy, mesangiolytic changes within the mesangium were seen on d 1. Ultrastructural changes of the podocytes and endothelial cells were noted at different time points of the disease (Figure 1C). In parallel to these findings, quantitative PCR showed upregulation of glomerular mRNAs for inflammatory cytokines (TNF-α, IL-1β, and MCP-1) and fibrogenic molecules (TGF-β1, -2, and -3 and activin-βA), with TNF-α and IL-1β prevailing in the early phase, and TGF-β and activin-βA dominating in the later stage (Figure 2A). Immunoblotting results demonstrated induction of p-NF-κB p65 earlier than that of p-Smad2/3 within the glomeruli (Figure 2B).
Figure 1.
Induction of anti-Thy1 nephritis resulted in increased urinary protein excretion (A) and urinary nephrin excretion (B). Each bar represents the mean ± SEM for a group of three to six rats. *p < 0.05; **p < 0.01 versus control at d 0. (C) Representative transmission electron micrographs showing kidney sections of rats after nephritis induction at different time points. (a) changes of the mesangial matrix (arrows) on d 1; (b) deformation of foot processes and loss of slit diaphragms (arrow) on d 1; (c) effacement of foot processes (asterisk) and (d) disruptions of endothelial layer of the filtration barrier (arrows) on d 3; (e) podocytes (asterisks) detached from glomerular basement membrane in the urinary space on d 3; (f) irregular thickening of glomerular basement membrane (asterisk) on d 5. Bar size, 2 μm (a, d, e); 500 nm (b, c, f).
Figure 2.
(A) Quantitative PCR results showing upregulation of glomerular mRNAs for inflammatory cytokines (TNF-α, IL-1β, MCP-1) and fibrogenic molecules (TGF-β1, TGF-β3, activin-βA) after induction of the nephritis. (B) Representative immunoblotting results showing activation of p-NF-κB p65 and p-Smad2 after induction of the nephritis. Each bar represents the mean ± SEM of three experiments. *p < 0.05; **p < 0.01 versus control at d 0.
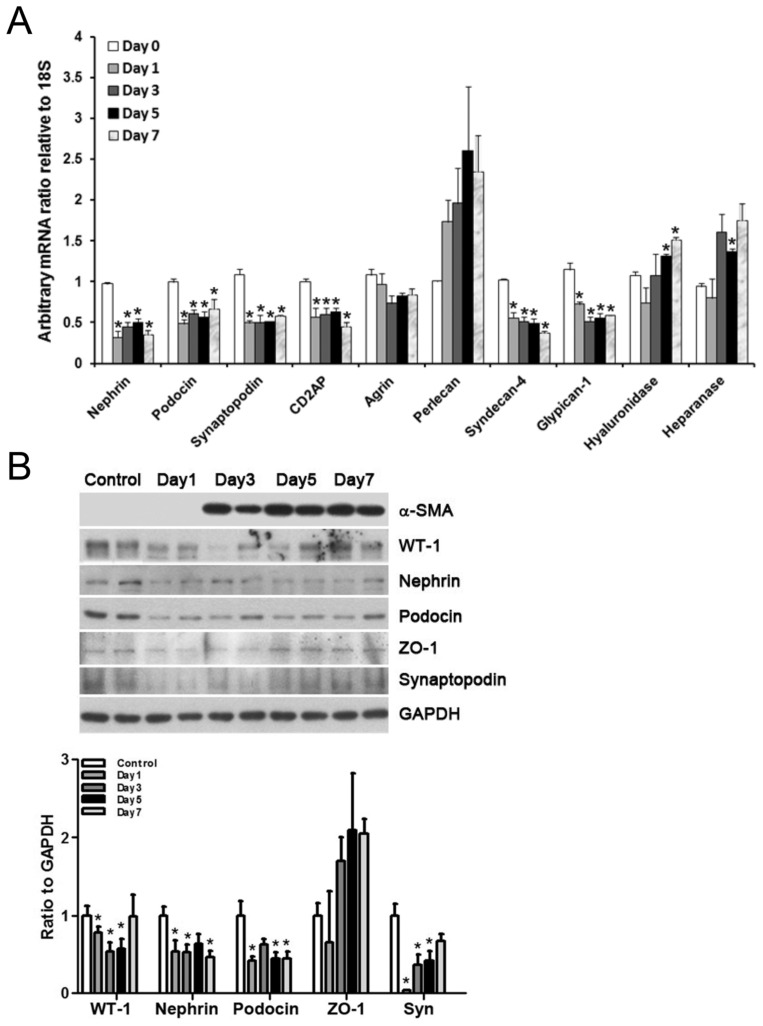
Quantitative PCR showed significant downregulation of glomerular mRNAs for podocyte-related nephrin, podocin, synaptopodin, CD2-associated protein (CD2AP) and endothelium-derived syndecan-4 and glipican-1 after induction of the nephritis. We also observed upregulation of mRNAs for hyaluronidase and heparanase, but not agrin or perlecan, at some points of the disease (Figure 3A). Immunoblotting results showed downregulation of glomerular WT-1, nephrin, podocin and synaptopodin from the outset, and induction of glomerular α-SMA from d 3, which coincided temporally with rebound proliferation of mesangial cells (Figure 3B).
Figure 3.
(A) Quantitative PCR results showing downregulation of glomerular mRNAs for filtration barrier proteins (nephrin, podocin, synaptopodin, CD2AP, syndecan-4, glipican-1) after induction of the nephritis. (B) Representative immunoblotting results showing temporal changes of α-SMA and podocyte-related proteins (WT-1, nephrin, podocin, ZO-1, synaptopodin) during the nephritis. Syn, synaptopodin. Each bar represents the mean ± SEM of three experiments. *p < 0.05 versus control at d 0.
Effects of IκB Kinase Inhibition on Proteinuria in Anti-Thy1 Glomerulonephritis
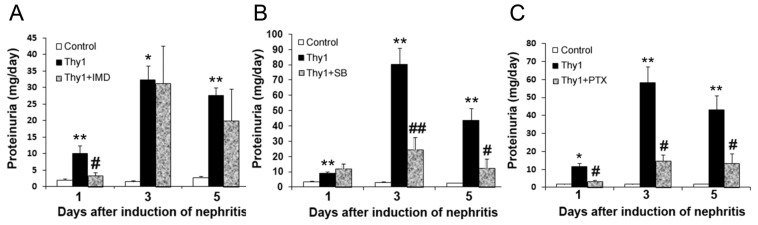
Treatment with an IκB kinase inhibitor, IMD-0354, resulted in attenuation of proteinuria on d 1, but not d 3 or 5, of the nephritis (Figure 4A). IMD-0354 attenuated the activation of NF-κB p65 within the glomeruli on d 1 (Supplementary Figure S1A) and partially restored the downregulated glomerular nephrin, synaptopodin and CD2AP mRNAs (Supplementary Figure S1B) as well as the downregulated glomerular WT-1, nephrin and synaptopodin proteins (Supplementary Figure S1C).
Figure 4.
(A) IMD-0354 (IMD) attenuated proteinuria on d 1 of the nephritis. (B) SB431542 (SB) attenuated proteinuria on d 3 and 5 of the nephritis. (C) PTX attenuated proteinuria through the course of the nephritis. Each bar represents the mean ± SEM for a group of six rats. *p < 0.05, **p < 0.01 versus control rats; #p < 0.05, ##p < 0.01, versus vehicle-treated nephritic rats. Thy1, vehicle-treated nephritic rats; Thy1 + IMD, nephritic rats treated with IMD-0354; Thy1 + SB, nephritic rats treated with SB431542; Thy1 + PTX, nephritic rats treated with PTX.
Effects of Activin Receptor–Like Kinase Inhibition on Proteinuria in Anti-Thy1 Glomerulonephritis
Treatment with an ALK inhibitor, SB431542, led to attenuation of proteinuria on d 3 and 5, but not d 1, of the nephritis (Figure 4B). SB431542 attenuated the activation of Smad2/3 within the glomeruli on d 5 (Supplementary Figure S2A) and partially restored the downregulated glomerular nephrin, synaptopodin, CD2AP and podocin mRNAs (Supplementary Figure S2B) as well as the downregulated glomerular WT-1, nephrin, podocin and synaptopodin proteins (Supplementary Figure S2C).
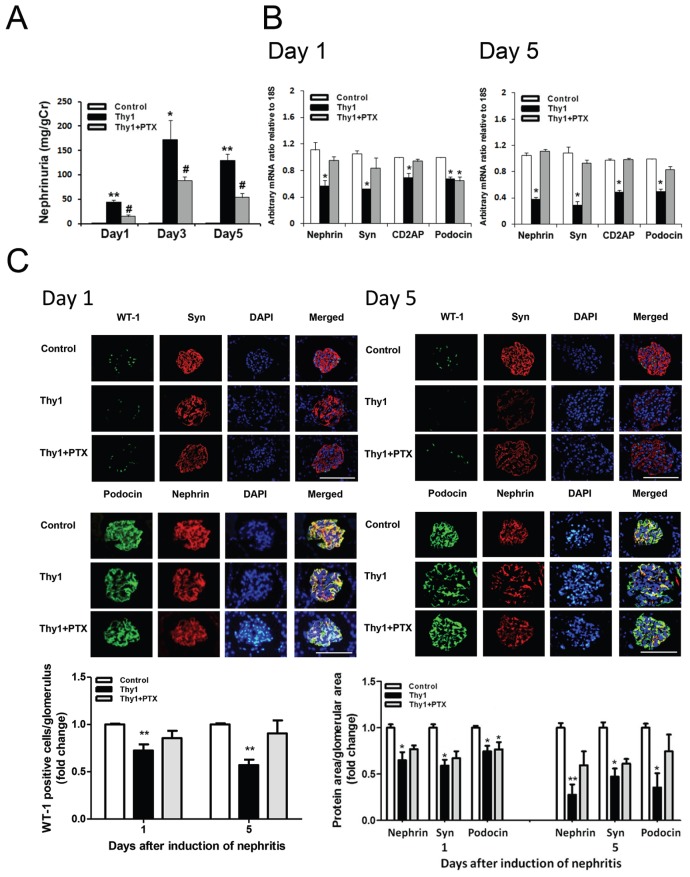
Mechanisms Whereby PTX Reduces Proteinuria in Anti-Thy1 Glomerulonephritis
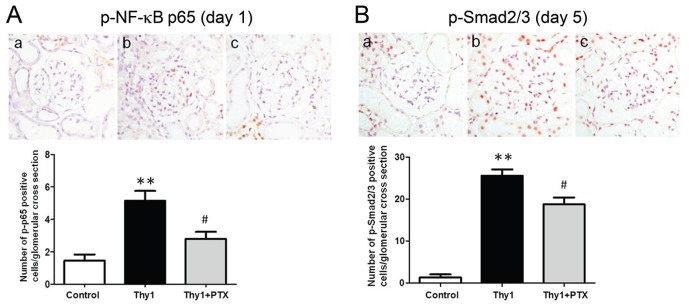
The above results revealed that blocking the inflammatory or fibrotic pathway could attenuate glomerular injury at different time points. We then examined whether PTX, an agent capable of inhibiting both pathways (20,27,28), might exert a more complete control of proteinuria. Our results showed that treatment with PTX attenuated proteinuria (Figure 4C) and nephrinuria (Figure 5A) through the course of the disease. The treatment partially reversed the downregulated expression of podocyte-related mRNAs and proteins on d 1 (except podocin) and d 5 of the disease (Figures 5B, C). More importantly, the activation of glomerular NF-κB p65 and Smad2/3 was attenuated by PTX treatment on d 1 and 5, respectively (Figure 6).
Figure 5.
(A) PTX attenuated urinary nephrin excretion through the course of the nephritis. Each bar represents the mean ± SEM for a group of six rats. *p < 0.05, **p < 0.01, versus control rats; #p < 0.05 versus vehicle-treated nephritic rats. (B) PTX partially reversed down-regulated mRNAs for nephrin, synaptopodin and CD2AP on d 1 (left) and nephrin, synaptopodin, CD2AP and podocin on d 5 (right). Each bar represents the mean ± SEM of three experiments. *p < 0.05 versus control rats. (C) Representative immunofluorescent stainings of WT-1 and synaptopodin (upper panels), as well as podocin and nephrin (lower panels) within the glomeruli on d 1 (left) and d 5 (right). Bar size, 100 μm. Lower graphs show that, semiquantitatively, PTX partially reversed the reduction of WT-1–positive cells per glomerulus and the area positively stained for podocyte-related proteins per glomerular area. Values are the mean ± SEM of 25–30 glomeruli. *p < 0.05, **p < 0.01, versus control rats. Thy1, vehicle-treated nephritic rats; Thy1 + PTX, PTX-treated nephritic rats.
Figure 6.
Representative immunohistochemical staining showing p-NF-κB p65 (A) and p-Smad2/3 signals (B) on d 1 and 5, respectively (a, b and c denote control rats, vehicle-treated nephritic rats and PTX-treated nephritic rats, respectively; original magnification 400×). Bar graphs show semiquantitative results of p-p65–positive and p-Smad2/3–positive cells per glomerular cross-section. Values are the mean ± SEM of 25–30 glomeruli. **p < 0.01 versus control rats; #p < 0.05 versus vehicle-treated nephritic rats. Thy1, vehicle-treated nephritic rats; Thy1 + PTX, PTX-treated nephritic rats.
DISCUSSION
Anti-Thy1 glomerulonephritis is a well-established nephritis model that simulates human mesangial proliferative glomerulonephritis. It is characterized by glomerular injury affecting primarily the mesangium yet displaying substantial proteinuria during the course. The precise mechanism of proteinuria in this disease has not been definitively established. One possibility is secondary podocyte damage due to physical stretching resulted from mesangial cell destruction, destabilization of capillary structure and formation of intraglomerular microaneurysms (29). This idea can be exemplified by an early study that showed occurrence of transient proteinuria in parallel with morphological changes of the podocyte and foot processes during the initial stage of the disease (30). Schaefer et al. (31) reported alterations in the expression of glomerular nephrin, podocin and CD2AP and suggested dysregulation of slit-diaphragm as a cause for proteinuria. Besides, we demonstrated ultrastructural changes of the filtration barrier structure, including alterations of the podocyte foot processes and podocytes as well as disrupted endothelium, irregularly thickened glomerular basement membrane and detached podocytes at different time points of the nephritis. In addition to physical forces, an alternative mechanism for proteinuria could be attributable to humoral factors that are induced during the disease. For example, inflammatory cytokines or fibrogenic mediators had been shown to modulate podocyte motility and albumin permeability, mRNA expression of podocyte markers such as nephrin and ezrin as well as podocyte survival and adhesion (9–15). Presumably, through mesangial cell-podocyte communication (16–18), these effects might lead to breakdown of the glomerular permeability with resultant formation of proteinuria.
In this study, we observed downregulation of glomerular nephrin, podocin, CD2AP and synaptopodin mRNAs in parallel with induction of TNF-α and IL-1β mRNAs on d 1. These data suggest a role of proinflammatory cytokines in the development of proteinuria during the early phase of the nephritis. In support of this hypothesis, the administration of an IκB kinase inhibitor, IMD-0354, to nephritic rats resulted in reduction of proteinuria and attenuation of glomerular p-NF-κB signals on d 1. Furthermore, the downregulated nephrin, synaptopodin and CD2AP mRNAs and WT-1, nephrin and synaptopodin proteins were restored partially by IMD-0354, indicating a possible pathogenetic role of TNF-α/IL-1β/NF-κB signaling in proteinuria formation. IMD-0354 exerted its antiproteinuric effect only in the early phase but not at a later time. The reason for this result was not clear, but could be due in part to the rapid up-and-down expression of TNF-α and IL-1β mRNAs, and activation of NF-κB p65. We surmise that mechanisms other than the TNF-α/IL-1β/NF-κB cascade might be involved more substantially in proteinuria after d 3, which could not be modulated by treatment with IMD-0354.
The TGF-β/activin family for which expression prevailed in the mid to late phase of the nephritis may be a plausible candidate underlying proteinuria during that period. Apart from its profibrotic action, TGF-β1 induces podocyte dedifferentiation and causes albumin influx across the podocyte monolayer (11,12). In TGF-β1 transgenic mice, podocytes undergo apoptosis in association with depletion of podocytes early during progressive glomerulosclerosis (32). Furthermore, TGF-β1 overexpression in glomeruli has been shown to induce proteinuria along with effacement of the podocyte foot process and downregulation of nephrin and synaptopodin (33). Similarly, activin A is an activator of renal glomerular and interstitial fibrosis (34,35). Nevertheless, compared with TGF-β1, relatively little information is available concerning the link between activin and proteinuria formation. In this study, we observed downregulation of glomerular nephrin, podocin and synaptopodin mRNAs and proteins that coincided temporally with induction of TGF-β1, TGF-β3 and activin-βA mRNAs during the mid and late phase of the nephritis. These temporal associations implicate a role for TGF-β/activin pathways in the pathogenesis of proteinuria in anti-Thy1 disease.
An early study evaluating the pathogenic role of Smad3 signaling in proteinuria formation demonstrated attenuation of streptozotocin-induced albuminuria in Smad3 knockout mice (36). In a subsequent report, TGF-β1 transgenic mice deficient for Smad3 exhibited less podocyte apoptosis, whereas mice heterozygous for CD2AP displayed reduced PI3K/Akt signaling as well as more severe podocyte apoptosis and proteinuria (37). These observations suggest that loss of CD2AP expression and/or function in podocytes may sensitize these cells to TGF-β/Smad-dependent apoptosis, thereby contributing to proteinuria formation. In that context, the suppressed glomerular CD2AP mRNA and concomitant increased glomerular TGF-β1 and TGF-β3 mRNAs might act cooperatively to potentiate the pathogenic role of TGF-β-Smad3 signaling in proteinuria during the later phase of anti-Thy1 glomerulonephritis.
By using pharmacological inhibitors, Grygielko et al. (38) showed that SB525334, a selective ALK5 inhibitor, reduced proteinuria in adriamycin-induced nephrosis in rats. ALK inhibitors act by suppression of TGF-β/Smad signals and inhibition of autoinduction of TGF-β and possibly activin βA (39). In line with these findings, we found that administration of SB431542, a prototypic ALK inhibitor, to rats with anti-Thy1 disease reduced proteinuria in association with attenuation of glomerular p-Smad2/3 signals and preservation of podocyte filtration barrier proteins (WT-1, nephrin, synaptopodin, podocin) during mid- to late-stage nephritis. These findings coincide with earlier reports showing similar inhibitory effects of proteinuria with distinct anti–TGF-β strategies in the same model (40–42). Treatment with SB431542 to nephritic rats, however, did not attenuate proteinuria in the early phase. This result could be due to a lack of significant induction of TGF-β at the outset of the disease, implying an involvement of other pathways in proteinuria formation during the early phase. In support of this notion, a recent study showed that inhibiting TGF-β with soluble TGF-β-receptor antibody prevented fibrotic responses, but not proteinuria in murine adriamycin nephropathy (43). Given the differential induction patterns of cytokines, and the insufficiency of either SB431542 or IMD-0354 to attenuate proteinuria through the entire course, it is tempting to speculate that agents capable of blocking multiple pathogenic pathways (for example, TNF-α, IL-1β and TGF-β/activins) may be required to achieve more complete control of proteinuria.
PTX is a clinically available phospho-diesterase inhibitor that has been shown to reduce proteinuria in various human kidney diseases, including mesangial proliferative glomerulonephritis and IgA nephropathy (19–24). The antiproteinuric effect of this drug is largely ascribed to its inhibitory action on TNF-α and/or TGF-β pathways (20,27,28). In agreement with these reports, the present study shows that PTX can reduce proteinuria in association with attenuation of nephrinuria, downregulation of glomerular NF-κB and Smad signals and restoration of podocyte-related filtration barrier proteins. The possibility of nonspecific interference with proteinuria by administration of PTX before the induction of nephritis was excluded in our previous study (8). However, it should be pointed out that the reversal of downregulated nephrin and podocin mRNA/protein by PTX was not complete, which likely accounted for residual proteinuria and nephrinuria despite treatment. In addition, alterations in other elements of the glomerular filtration barrier, such as the endothelial syndecan-4 and glypican-1, and the glycosaminoglycan degrading hyaluronidase and heparanase, have been shown to participate in the development of proteinuria (5,44–46). Because transcriptional regulation of these genes was not likely TNF-α– or TGF-β–dependent (47,48), PTX might not be able to modulate the dysregulated mRNA expression as seen in the present model.
CONCLUSION
Our data indicate that traditional pro-inflammatory and fibrogenic cytokines, such as TNF-α and TGF-β/activin, participate in the pathogenesis of proteinuria in anti-Thy1 glomerulonephritis. And the evolution of this process can be attenuated by PTX via downregulation of NF-κB and Smad signals and restoration of the podocyte component of the glomerular filtration barrier. These data may form the basis for the development of novel therapeutic agents aiming to treat proteinuric patients with mesangial proliferative types of glomerulonephritis.
Supplemental Data
ACKNOWLEDGMENTS
We are grateful to professor Kuo-Shyan Lu and his members for technical support in the use of electron microscopy at the Department of Anatomy and Cell Biology, College of Medicine, National Taiwan University. This project was supported by grants from the National Science Council, Executive Yuan (grant nos. 96-2314-B-002-059-MY3, 99-2628-B-002-011-MY2), National Taiwan University Hospital (grant no. 100-S1568), the Ta-Tung Kidney Foundation and the Mrs. Hsiu-Chin Lee Kidney Research Fund, Taipei, Taiwan.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Cite this article as: Chen YM, et al. (2015) Pentoxifylline attenuates proteinuria in anti-Thy1 glomerulonephritis via downregulation of nuclear factor-κB and Smad2/3 signaling. Mol. Med. 21:276–84.
REFERENCES
- 1.Keane W. Proteinuria: its clinical importance and role in progressive renal disease. Am J Kidney Dis. 2000;35:S97–105. doi: 10.1016/s0272-6386(00)70237-x. [DOI] [PubMed] [Google Scholar]
- 2.Iseki K, Ikemiya Y, Iseki C, Takishita S. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 2003;63:1468–74. doi: 10.1046/j.1523-1755.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- 3.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 4.Holthofer H. Molecular architecture of the glomerular slit diaphragm: lessons learnt for a better understanding of disease pathogenesis. Nephrol Dial Transplant. 2007;22:2124–8. doi: 10.1093/ndt/gfm344. [DOI] [PubMed] [Google Scholar]
- 5.Haraldsson B, Nyström J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88:451–87. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 6.Shankland SL. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–47. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 7.Simic I, Tabatabaeifar M, Schaefer F. Animal models of nephrotic syndrome. Pediatr Nephrol. 2013;28:2079–88. doi: 10.1007/s00467-012-2376-5. [DOI] [PubMed] [Google Scholar]
- 8.Chen YM, et al. Pentoxifylline attenuates experimental mesangial proliferative glomerulonephritis. Kidney Int. 1999;56:932–43. doi: 10.1046/j.1523-1755.1999.00636.x. [DOI] [PubMed] [Google Scholar]
- 9.Reiser J, et al. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem. 2004;279:34827–32. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- 10.Burt D, et al. The monocyte chemoattractant protein-1/cognate CC chemokine receptor 2 system affects cell motility in cultured human podocytes. Am J Pathol. 2007;171:1789–99. doi: 10.2353/ajpath.2007.070398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, et al. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol. 2008;172:299–308. doi: 10.2353/ajpath.2008.070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen M, et al. Oral administration of GW788388, an inhibitor of TGF-beta type I and II receptor kinases, decreases renal fibrosis. Kidney Int. 2008;73:705–15. doi: 10.1038/sj.ki.5002717. [DOI] [PubMed] [Google Scholar]
- 13.Lee EY, et al. The monocyte chemoattractant protein-1/CCR2 loop, inducible by TGF-beta, increases podocyte motility and albumin permeability. Am J Physiol Renal Physiol. 2009;297:F85–94. doi: 10.1152/ajprenal.90642.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai KN, et al. Podocyte injury induced by mesangial-derived cytokines in IgA nephropathy. Nephrol Dial Transplant. 2009;24:62–72. doi: 10.1093/ndt/gfn441. [DOI] [PubMed] [Google Scholar]
- 15.Zhu L, et al. Synergistic effect of mesangial cell-induced CXCL1 and TGF-β1 in promoting podocyte loss in IgA nephropathy. PLoS One. 2013;8:e73425. doi: 10.1371/journal.pone.0073425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlöndorff D, Banas B. The mesangial cell revisited: no cell is an island. J Am Soc Nephrol. 2009;20:1179–87. doi: 10.1681/ASN.2008050549. [DOI] [PubMed] [Google Scholar]
- 17.Menon MC, Chuang PY, He CJ. The glomerular filtration barrier: components and crosstalk. Int J Nephrol. 2012;2012;749010 doi: 10.1155/2012/749010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haraldsson BS. The endothelium as part of the integrative glomerular barrier complex. Kidney Int. 2014;85:8–11. doi: 10.1038/ki.2013.317. [DOI] [PubMed] [Google Scholar]
- 19.Ducloux D, Bresson-Vautrin C, Chalopin JM. Use of pentoxifylline in membranous nephropathy. Lancet. 2001;357:1672–3. doi: 10.1016/s0140-6736(00)04830-3. [DOI] [PubMed] [Google Scholar]
- 20.Navarro JF, Mora C, Muros M, Garca J. Additive antiproteinuric effect of pentoxifylline in patients with type 2 diabetes under angiotensin II receptor blockade: a short-term, randomized, controlled trial. J Am Soc Nephrol. 2005;16:2119–26. doi: 10.1681/ASN.2005010001. [DOI] [PubMed] [Google Scholar]
- 21.Chen YM, Lin SL, Chiang WC, Wu KD, Tsai TJ. Pentoxifylline ameliorates proteinuria via suppression of renal monocyte chemoattractant protein-1 in subnephrotic primary glomerular diseases. Kidney Int. 2006;69:1410–5. doi: 10.1038/sj.ki.5000302. [DOI] [PubMed] [Google Scholar]
- 22.Lin SL, Chen YM, Chiang WC, Wu KD, Tsai TJ. Effect of pentoxifylline in addition to losartan on proteinuria and GFR in CKD: a 12-month randomized trial. Am J Kidney Dis. 2008;52:464–74. doi: 10.1053/j.ajkd.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Renke M, et al. Effect of pentoxifylline on proteinuria, markers of tubular injury and oxidative stress in non-diabetic patients with chronic kidney disease: placebo controlled, randomized, cross-over study. Acta Biochim Pol. 2010;57:119–23. [PubMed] [Google Scholar]
- 24.Navarro-González JF, et al. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: the PREDIAN trial. J Am Soc Nephrol. 2015;26:220–9. doi: 10.1681/ASN.2014010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: ARRIVE guidelines. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyauchi M, et al. Hypertrophy and loss of podocytes in diabetic nephropathy. Intern Med. 2009;48:1615–20. doi: 10.2169/internalmedicine.48.2137. [DOI] [PubMed] [Google Scholar]
- 27.Chen YM, et al. Dual regulation of tumor necrosis factor-α-induced CCL2/monocyte chemoattractant protein-1 expression in vascular smooth muscle cells by nuclear factor-κB and activator protein-1: modulation by type III phosphodiesterase inhibition. J Pharmacol Exp Ther. 2004;309:978–86. doi: 10.1124/jpet.103.062620. [DOI] [PubMed] [Google Scholar]
- 28.Lin SL, et al. Pentoxifylline attenuates tubulointerstitial fibrosis by blocking smad3/4-activated transcription and profibrogenic effects of connective tissue growth factor. J Am Soc Nephrol. 2005;16:2702–13. doi: 10.1681/ASN.2005040435. [DOI] [PubMed] [Google Scholar]
- 29.Yu D, et al. Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol. 2005;16:1733–41. doi: 10.1681/ASN.2005020159. [DOI] [PubMed] [Google Scholar]
- 30.Bagchus WM, Hoedemaeker PJ, Rozing J, Bakker WW. Glomerulonephritis induced by monoclonal anti-Thy 1.1 antibodies: a sequential histological and ultrastructural study in the rat. Lab Invest. 1986;55:680–7. [PubMed] [Google Scholar]
- 31.Schaefer L, et al. Nephrin expression is increased in anti-Thy1.1-induced glomerulonephritis in rats. Biochem Biophys Res Commun. 2004;324:247–54. doi: 10.1016/j.bbrc.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 32.Schiffer M, et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108:807–16. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghayur A, et al. Adenovirus-mediated gene transfer of TGF-β1 to the renal glomeruli leads to proteinuria. Am J Pathol. 2012;180:940–51. doi: 10.1016/j.ajpath.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita S, Maeshima A, Kojima I, Nojima Y. Activin A is a potent activator of renal interstitial fibroblasts. J Am Soc Nephrol. 2004;15:91–101. doi: 10.1097/01.asn.0000103225.68136.e6. [DOI] [PubMed] [Google Scholar]
- 35.Gaedeke J, Boehler T, Budde K, Neumayer HH, Peters H. Glomerular activin A overexpression is linked to fibrosis in anti-Thy1 glomerulonephritis. Nephrol Dial Transplant. 2005;20:319–28. doi: 10.1093/ndt/gfh653. [DOI] [PubMed] [Google Scholar]
- 36.Fujimoto M, et al. Mice lacking Smad3 are protected against streptozotocin-induced diabetic glomerulopathy. Biochem Biophys Res Commun. 2009;305:1002–7. doi: 10.1016/s0006-291x(03)00885-4. [DOI] [PubMed] [Google Scholar]
- 37.Xavier S, et al. TbetaRI independently activates Smad- and CD2AP-dependent pathways in podocytes. J Am Soc Nephrol. 2009;20:2127–37. doi: 10.1681/ASN.2008070806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grygielko ET, et al. Inhibition of gene markers of fibrosis with a novel inhibitor of transforming growth factor-beta type I receptor kinase in puromycin-induced nephritis. J Pharmacol Exp Ther. 2005;313:943–51. doi: 10.1124/jpet.104.082099. [DOI] [PubMed] [Google Scholar]
- 39.Sharma K, Jin Y, Guo J, Ziyadeh FN. Neutralization of TGF-beta by anti-TGF-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes. 1996;45:522–30. doi: 10.2337/diab.45.4.522. [DOI] [PubMed] [Google Scholar]
- 40.Border WA, Okuda S, Languino L, Sporn MB, Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor β1. Nature. 1990;346:371–4. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- 41.Isaka Y, et al. Gene therapy by skeletal muscle expression of decorin prevent fibrotic disease in rat kidney. Nat Med. 1996;2:418–23. doi: 10.1038/nm0496-418. [DOI] [PubMed] [Google Scholar]
- 42.Kasuga H, et al. Effects of anti-TGF-β type II receptor antibody on experimental glomerulonephritis. Kidney Int. 2001;60:1745–55. doi: 10.1046/j.1523-1755.2001.00990.x. [DOI] [PubMed] [Google Scholar]
- 43.Finer G, et al. Divergent roles of Smad3 and PI3-kinase in murine adriamycin nephropathy indicate distinct mechanisms of proteinuria and fibrogenesis. Kidney Int. 2012;82:525–36. doi: 10.1038/ki.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pyke C, Kristensen P, Ostergaard PB, Oturai PS, Rømer J. Proteoglycan expression in the normal rat kidney. Nephron. 1997;77:461–70. doi: 10.1159/000190325. [DOI] [PubMed] [Google Scholar]
- 45.Meuwese MC, et al. Endothelial surface layer degradation by chronic hyaluronidase infusion induces proteinuria in apolipoprotein E-deficient mice. PLoS One. 2010;5:e14262. doi: 10.1371/journal.pone.0014262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szymczak M, Kuźniar J, Klinger M. The role of heparanase in diseases of the glomeruli.ź. Arch Immunol Ther Exp (Warsz) 2010;58:45–56. doi: 10.1007/s00005-009-0061-6. [DOI] [PubMed] [Google Scholar]
- 47.Gil N, et al. Heparanase is essential for the development of diabetic nephropathy in mice. Diabetes. 2012;61:208–16. doi: 10.2337/db11-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sachs N, Sonnenberg A. Cell-matrix adhesion of podocytes in physiology and disease. Nat Rev Nephrol. 2013;9:200–10. doi: 10.1038/nrneph.2012.291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.