Abstract
Both activities of daily living (ADL) and some blood biomarkers (such as albumin) have been associated with mortality in very elderly people, but scarce data is available on the predictive performance of them in isolation or in combination, which is important for clinicians in decision making. Here, based on prospective mortality data over a 6-year follow-up period from 433 long-lived individuals (LLIs) aged 95+ years in the Rugao longevity cohort, we aimed to evaluate Cox proportional hazard ratios (HRs) and discriminative power (ROC curve) of 14 biomarkers and ADL for all-cause mortality. We found that six biomarkers (total triglyceride, albumin, low-density lipoprotein cholesterol, platelet count, lymphocyte count, and neutrophil count) were associated with mortality with a p < .10 in the univariate model. Significant associations of albumin and neutrophil count with mortality were observed when they were simultaneously included in a multivariate model, with HRs of 0.97 (95 % CI 0.94, 0.99; p = .005) and 1.09 (95 % CI 1.00, 1.18; p = .043). With respect to ADL, the corresponding HR was 1.10 (95 % CI 1.07, 1.14; p < .001). Low albumin (<40 g/L) combined with ADL dependent had a significantly increased mortality risk (HR = 2.19; 95 % CI 1.63, 2.95). Albumin and ADL separately showed good discriminative accuracies (area under the curve [AUC] = 0.68 and 0.66, respectively), and their combination had an increased predictive utility (AUC = 0.73). In conclusion, both albumin and ADL are efficient predictors of all-cause mortality in long-lived populations and their combination further increases discriminative power. The preliminary findings, if validated and translated, would help clinicians to identify the elderly people at varying mortality risk.
Keywords: Activities of daily living, Serum albumin, Long-lived individuals, Mortality
Introduction
With health improvement in recent decades, more and more older adults have survived to advanced age, resulting a rapidly growing segment of the population, e.g., the very elderly people. As a frail age group experiencing physical disability and comorbidity, their health trajectories in the later years of a long life have received increasing attention (Collerton et al. 2007; Poon et al. 2007). In the context of costly long-term care services, identifying persons at high risk for mortality might be a priority in clinical decision making. But it challenges researchers and clinicians for this age group with extended life span are very heterogeneous.
To date, several risk factors have been associated with mortality in very elderly people in some studies (Broeska et al. 2013; Cesari et al. 2008; de Ruijter et al. 2009; den Elzen et al. 2009; Gondo et al. 2006; Gueresi, 2010; Mossakowska et al. 2014; Nakazawa et al. 2012; Nybo et al. 2003; Shimizu et al., 2001; van Houwelingen et al. 2013), including functional status, cognitive performance, nutritional status, and blood biomarkers (e.g., albumin). Of these factors, functional status, usually measured by activities of daily living (ADL), has been widely used as a predictor of mortality for its simplification and generalization across various populations and different clinical settings (Cesari et al. 2008; Mossakowska et al. 2014; Nakazawa et al. 2012; Nybo et al. 2003; Shimizu et al., 2001). We have paid much attention to ADL in long-lived individuals (LLIs) over 95 years of age in a previous work (Liu et al. 2014b) and also explored the effects of blood biomarkers. We noted that some blood biomarkers (such as serum albumin) had been linked to mortality in the very elderly (Boland et al. 2014; Gueresi, 2010; Izaks et al. 2003; Shimizu et al., 2001; van Houwelingen et al. 2013). It is well known that blood biomarkers are routinely examined in clinical laboratory diagnosis, which is objective, fast, and relatively easy-to-use. ADL is also an easily measured factor, although subjective. However, to our best knowledge, no specific study have evaluated the predictive performance of them in isolation or in combination in a long-lived population over 95 years of age, a heterogeneous age group, which is very important for clinicians in decision making.
Based on the data from the Rugao longevity cohort, this study aimed to evaluate the potential associations of 14 biomarkers and ADL assessed at baseline with all-cause mortality in a Chinese long-lived population. Additionally, the discrimination power of them in isolation or in combination was also evaluated. This study intends to shed light on the predictive value of routine clinical laboratory testing on mortality, which may provide preliminary evidence for identifying very elderly people at varying risk.
Methods
Study population
We used data from 463 LLIs enrolled in the Rugao longevity cohort, a population-based prospective study conducted between December 24, 2007, and February 29, 2008, in Rugao, Jiangsu Province, China. Details regarding the cohort are provided elsewhere (Cai et al. 2009; Liu et al. 2014a). Briefly, after strict four-step age verifications, 705 persons aged 95+ years (149 men and 556 women) including 102 centenarians were identified in Rugao. Of them, 463 (103 men and 360 women) participants were recruited, representing 65.7 % of the LLIs in Rugao, a typical medium-sized city of China (from the perspective of population and economy). No significant difference in age and gender ratio was found between non-responders and responders (all p > .05). Nineteen participants were excluded due to acute infections, known malignant tumor, severely physical disorder (e.g., being ill in bed for a long time), or taking anti-inflammatory drug (e.g., nonsteroidal anti-inflammatory drugs) in the previous 12 months at the baseline, and two participants died during the baseline investigation. Nine participants had not been identified as alive or dead until the date of censoring according to the local government (the Bureau of Civil Affairs), leaving a study sample of 433 LLIs with relatively good health status in this study.
As described previously (Cai et al. 2009; Liu et al. 2014a), a structured questionnaire was administered by trained field staff that delved into areas including demographic characteristics, histories of chronic disease (e.g., chronic obstructive pulmonary disease, coronary heart disease, malignant tumor), functional status (assessed by the Katz Index of ADL), and medications (e.g., anti-inflammatory drug). Physical examinations were accomplished, and fasting blood specimens were drawn for laboratory examination. All interviews were tape recorded, and 5 % of the recorded interviews were evaluated for interviewing quality. Written informed consent was obtained from each participant or a member of his/her immediate family. The Human Ethnics Committee of Fudan University School of Life Sciences approved the research.
Blood biomarker
Blood biomarkers including total triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, serum albumin, serum uric acid, total bilirubin, direct bilirubin, alanine transaminase, glutamic oxaloacetic transaminase, red blood cell count, white blood cell, platelet count, lymphocyte count, and neutrophil count which were routinely examined in clinical diagnosis were considered in this study. The biomarkers were measured using Olympus AU5811 clinical chemistry analyzer (Tokyo, Japan) with standard laboratory techniques (performed by a technician in the biochemistry laboratory of Jiangsu Rugao Hospital of Traditional Chinese Medicine).
ADL
As described previously (Liu et al. 2014b), the Katz Index was used to assess ADL in this study. The Katz Index is on the basis of six daily tasks: eating, dressing, bathing, indoor transferring, going to the toilet, and cleaning oneself afterwards (Katz et al. 1963). Each task has the following three response alternatives: strongly independent, somewhat dependent, and strongly dependent, with a score of 1, 2, and 3 points, respectively. Lower scores indicated better functional status. Based on the total summed scores, a binary variable including ADL independent (total score = 6) and ADL dependent (total score >6) was constructed.
Mortality
Dates of death were obtained through the Bureau of Civil Affairs of Rugao. Survival time (in months) was calculated from February 2008 to the date of death or of censoring on April 2014. The Bureau of Civil Affairs in Rugao is responsible for providing pensions for persons aged 80 years or over every month. The penalty for any family trying to conceal the death information of the elderly is high.
Statistical analyses
Baseline characteristics were expressed as means ± standard deviation (SD) for normally distributed continuous variables, median (interquartile range) for non-normal continuous variables, or by the percentage for categorical variables. To calculate hazard ratios (HRs) and 95 % confidence intervals (CIs) of the biomarkers and ADL for all-cause mortality, we firstly used a univariate model. Biomarkers with a p < .10 in the univariate model were then simultaneously included in a multivariate model adjusted for age and sex. Similarly, the corresponding HR and 95%CI of ADL for all-cause mortality were also calculated. Since the aim of the study was to evaluate the performance of predictors, and not to explore the causes of disease, no adjustments were made for potential confounders except for age and sex as other studies did (van Houwelingen et al. 2013).
We assessed the discriminative power of the identified biomarkers and ADL on all-cause mortality by means of receiver operation characteristic (ROC) curves and obtained the area under the curve (AUC) with 95 % CI for each potential predictor. To observe the combined effects of efficient predictors (i.e., serum albumin and ADL in this study) on mortality, we categorized them into binary variables and then included them in the Cox proportional hazard model. In addition, a Kaplan-Meier plot was also used for presentation of survival curves that were compared with the log-rank test. All analyses in this study were carried out in the total sample, since non-significant difference in survival curve between males and females was found. SAS software (version 9.3; SAS Institute, Cary, NC) was used for all analyses except for ROC curves, which was performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). A p value of <.05 (two-tailed) was considered statistically significant.
Results
Baseline characteristics of the study participants are shown in Table 1. The median age was 97.0 years (range of 95–107 years). About 77.8 % (337) were female. Approximately 50 % were classified as ADL independent (ADL = 6, data not shown). The means ± SD or median (interquartile range) of blood biomarkers were presented in Table 1. Of the 433 participants, 394 (91.0 %) died over a 6-year follow-up period.
Table 1.
Baseline characteristics of the study subjects from the Rugao longevity cohort
| Characteristicsa | No. | |
|---|---|---|
| Age (years) | 433 | 97.0 (96.0–98.0) |
| Female, n (%) | 433 | 337 (77.8) |
| ADL | 420 | 8.4 ± 3.4 |
| TG (mmol/L) | 422 | 0.98 (0.75–1.32) |
| HDL-C (mmol/L) | 422 | 1.35 (1.16–1.58) |
| LDL-C (mmol/L) | 422 | 2.46 (2.02–2.97) |
| Serum albumin (g/L) | 421 | 42.4 ± 4.6 |
| SUA (μmol/L) | 416 | 269.0 (216.0–338.0) |
| TBIL (μmol/L) | 423 | 14.0 (10.6–17.1) |
| DBIL (μmol/L) | 423 | 3.2 (2.4–4.8) |
| ALT (U/T) | 422 | 11.0 (9.0–14.0) |
| Got (U/T) | 422 | 24.0 (21.0–28.0) |
| RBC count (×1012/L) | 419 | 3.9 (3.5–4.3) |
| WBC count (×109/L) | 419 | 5.2 (4.3–6.1) |
| Platelet count (×109/L) | 419 | 159.0 (121.0–204.0) |
| Lymphocyte count (×109/L) | 417 | 1.6 (1.3–2.1) |
| Neutrophil count (×109/L) | 419 | 2.9 (2.3–3.8) |
ADL activities of daily living, TG total triglyceride, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, SUA serum uric acid, TBIL total bilirubin, DBIL direct bilirubin, ALT alanine transaminase, GOT glutamic oxaloacetic transaminase, RBC red blood cell, WBC white blood cell
aNormally distributed continuous variables were expressed as mean ± standard deviation (SD); non-normal continuous variables were expressed as median (interquartile range); categorical variables were presented by the percentage
Table 2 shows all-cause mortality risks associated with the biomarkers. Six (total triglyceride, albumin, low-density lipoprotein cholesterol, platelet count, lymphocyte count, and neutrophil count) of them were found associated with all-cause mortality with a p < .10 in the univariate model, and they were simultaneously included in a multivariate model adjusted for age and sex. Significant associations of albumin and neutrophil count with mortality were observed, with HRs of 0.97 (95 % CI 0.94, 0.99; p = .005) and 1.09 (95 % CI 1.00, 1.18; p = .043).With respect to ADL, as expected, the corresponding HR for mortality was 1.10 (95 % CI 1.07, 1.14; p < .001, data not shown) in a univariate model.
Table 2.
Cox proportional hazard ratios (HRs) of the biomarkers for all-cause mortality: the Rugao longevity cohort
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| HRa (95 % CI) | p value | HRa (95 % CI) | p value | |
| TG (mmol/L) | 0.84 (0.69, 1.01) | .069 | 0.88 (0.72, 1.09) | .244 |
| HDL-C (mmol/L) | 1.00 (0.99, 1.01) | .982 | – | – |
| LDL-C (mmol/L) | 0.86 (0.74, 1.00) | .043 | 0.91 (0.78, 1.06) | .229 |
| Serum albumin (g/L) | 0.95 (0.93, 0.97) | <.001 | 0.97 (0.94, 0.99) | .005 |
| SUA (μmol/L) | 1.00 (1.00, 1.00) | .286 | – | – |
| TBIL (μmol/L) | 1.00 (0.98, 1.02) | .743 | – | – |
| DBIL (μmol/L) | 1.02 (0.98, 1.06) | .391 | – | – |
| ALT (U/T) | 1.00 (0.98, 1.01) | .804 | – | – |
| Got (U/T) | 1.00 (0.99, 1.01) | .912 | – | – |
| RBC count (×1012/L) | 0.90 (0.76, 1.06) | .204 | – | – |
| WBC count (×109/L) | 1.03 (0.96, 1.10) | .378 | – | – |
| Platelet count (×109/L) | 1.00 (1.00, 1.00) | .093 | 1.00 (1.00, 1.00) | .064 |
| Lymphocyte count (×109/L) | 0.84 (0.71, 0.99) | .035 | 0.86 (0.73, 1.02) | .087 |
| Neutrophil count (×109/L) | 1.10 (1.01, 1.19) | .023 | 1.09 (1.00, 1.18) | .043 |
Model 1: unadjusted (a univariate model); model 2: included the biomarkers with a p < .10 in the univariate model, age, and sex
TG total triglyceride, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, SUA serum uric acid, TBIL total bilirubin, DBIL direct bilirubin, ALT alanine transaminase, GOT glutamic oxaloacetic transaminase, RBC red blood cell, WBC white blood cell, CI confidence interval
We evaluated the predictive performances of albumin and neutrophil count and ADL separately. The AUC for neutrophil count were 0.60 (95 % CI 0.51, 0.68; p = .050), with marginal significance. Albumin and ADL showed good discriminative powers, with AUCs of 0.68 (95 % CI 0.61, 0.76; p < .001) and 0.66 (95 % CI 0.58, 0.73; p = .001, Fig. 1), respectively. Thus, albumin and ADL were selected as potential efficient predictors of mortality. As expected, combination of these two predictors had an increased predictive utility (AUC = 0.73; 95 % CI 0.65, 0.80; p < .001, Fig. 1).
Fig. 1.
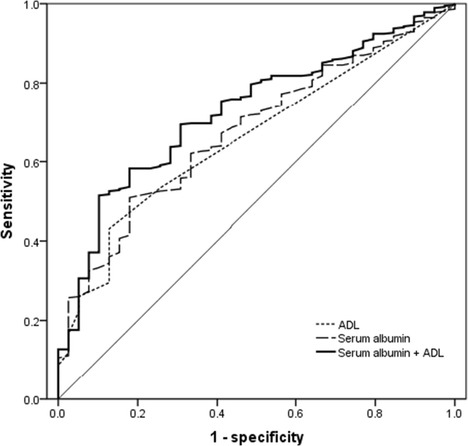
Receiver operating characteristic (ROC) curves showing predictive performance of albumin and ADL, in isolation and in combination, for all-cause mortality in long-lived individuals from the Rugao longevity cohort. The area under the curve (AUC) with 95 % confidence intervals (CIs) for albumin (0.68 [0.61, 0.76], p < .001), for ADL (0.66 [0.58, 0.73], p = .001), and for albumin and ADL in combination (0.73 [0.65, 0.80], p < .001) were documented
To observe the combined effects of serum albumin and ADL on mortality, we categorized them into binary variables and then included them in the Cox proportional hazard model. Albumin was classified as two categories (low and high albumin) according to clinic cutoffs of 40 g/L used in previous studies (Neal et al. 2009). We found that participants with low albumin (<40 g/L) or being ADL dependent (ADL>6) had increased all-cause mortality risks (all log-rank tests p < .001, Fig. 2). The corresponding HRs were 1.61 (95 % CI 1.30, 2.01) and 1.56 (95 % CI 1.27, 1.91), respectively (Table 3). As expected, in participants who were ADL independent, mortality was significantly higher in those with low albumin when compared with those with high albumin (HR = 1.66; 95 % CI 1.17, 2.36; p = .005, Table 3). Low albumin (<40 g/L) combined with ADL dependent had a significantly increased mortality risk (HR = 2.19; 95 % CI 1.63, 2.95; p < .001, Table 3).
Fig. 2.
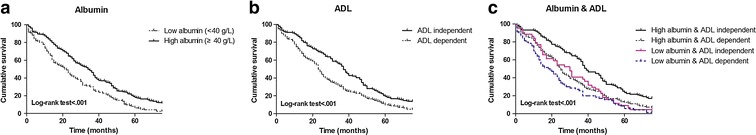
Kaplan-Meier cumulative survival curve according to a albumin groups (low albumin [<40 g/L] vs. high albumin [≥40 g/L]); b ADL groups (ADL independent [ADL = 6] vs. ADL dependent [ADL > 6]); c albumin and ADL groups (four categories: high albumin and independent; high albumin and dependent; low albumin and independent; low albumin and dependent) in long-lived individuals from the Rugao longevity cohort
Table 3.
The combined effects of serum albumin and ADL on all-cause mortality in long-lived individuals from the Rugao longevity cohort: Cox proportional hazard model
| Case (%) | Age- and sex-adjusted model | ||
|---|---|---|---|
| HRa (95 % CI) | p value | ||
| Albumin | |||
| High albumin (≥40 g/L) | 263 (88.0) | 1.00 (reference) | – |
| Low albumin (<40 g/L) | 119 (97.5) | 1.61 (1.30, 2.01) | <.001 |
| ADL | |||
| Independent (ADL = 6) | 180 (86.1) | 1.00 (reference) | – |
| Dependent (ADL > 6) | 198 (95.2) | 1.56 (1.27, 1.91) | <.001 |
| Combination of albumin and ADL | |||
| High albumin (≥40 g/L) and independent (ADL = 6) | 129 (82.7) | 1.00 (reference) | – |
| High albumin (≥40 g/L) and dependent (ADL>6) | 126 (93.3) | 1.62 (1.26, 2.08) | <.001 |
| Low albumin (<40 g/L) and independent (ADL = 6) | 42 (95.5) | 1.66 (1.17, 2.36) | .005 |
| Low albumin (<40 g/L) and dependent (ADL>6) | 69 (98.6) | 2.19 (1.63, 2.95) | <.001 |
ADL activities of daily living, HR hazard ratio, CI confidence interval
Discussion
The main finding of this study was that both serum albumin and ADL are efficient predictors of all-cause mortality in LLIs from the Rugao longevity cohort, and their combinations increases discriminative power (AUC = 0.73). Notably, when the conventional predictor ADL shows independence, albumin alone could discriminate people with an increased mortality risk. The preliminary findings, if validated and translated, would help clinicians to identify the elderly people at varying risk, which provide important evidence for healthy aging research.
Our findings support observations of other studies that low serum albumin is associated with mortality in very elderly people (Gondo et al. 2006; Gueresi, 2010; Sahyoun et al. 1996; Shimizu et al., 2001; Takata et al. 2010). More importantly, we observed the acceptable predictive utility of albumin in Chinese long-lived population. Serum albumin plays certain vital physiologic roles in health maintenance for many organs. For instance, it acts as a transport protein for fatty acids and maintains the intravascular oncotic pressure. Generally, albumin is considered as an indicator of nutritional risk in older adults, though the perspective remains controversial (Kuzuya et al. 2007). Recently, Ji and colleagues observed that lower albumin was associated with poor nutritional status in community-living Chinese aged 90 and over (Ji et al. 2012). It is well known that nutrition has a strong effect on immunity. To some degree, maintained or improved nutritional status could reduce morbidity and increase survival (Sanchez Garcia et al. 2012). Whether the efficient predictive value of albumin on all-cause mortality in this study is attributed to the particularly important role that nutrition serves in healthy aging needs further scrutiny.
Impressively, we observed stronger discriminative power of serum albumin on mortality in comparison to ADL in LLIs. As far as we know, there is no previous study comparing their performance in such individuals. ADL, one important factor in characterizing one’s health status, has been regarded as a conventional predictor of mortality in large numbers of epidemiologic studies (Cesari et al. 2008; Mossakowska et al. 2014; Nakazawa et al. 2012; Nybo et al. 2003; Shimizu et al., 2001). We have confirmed the statement in our heterogeneous sample. This association might be explained by the effects of morbidity, physical inactivity, or the process of aging itself (Mossakowska et al. 2014). Compared with ADL, albumin shows a similar predictive utility (AUC = 0.68 for albumin, and =0.66 for ADL) in this study. More importantly, albumin did work when participants were categorized as independent in ADL (Table 3), with a HR of 1.66 (95 % CI 1.17, 2.36) for mortality. This may help clinicians identify more patients at high mortality risk and provide primary preventions accordingly. Furthermore, combinations of albumin and ADL are recommended for an increase discriminative power (AUC = 0.73). These inexpensive, easy-to-use screening methods could have potential application value in the context of costly long-term care services in such a frail age group.
Neutrophil count showed a positive association with all-cause mortality in LLIs, albeit it is not a powerful predictor. To date, the predictive utility of neutrophil count has not been evaluated separately from white blood cell (WBC) count. As one important type of WBC, the effect of neutrophils on mortality has not been well addressed. It has been postulated that neutrophils could produce cytokines, oxidation metabolites, and free radicals that might cause oxidative damages to various tissues (e.g., muscles) and organ systems (Wu et al. 2009), and evidently, this process could lead to an increased mortality risk (Babior 1978). It could be a potential limitation for many studies that did not differentiate WBC to scrutinize the effect of different WBC subtypes (Willems et al. 2010). It is particularly intriguing to examine whether the identified associations of WBC with mortality in current studies are mostly accounted for by neutrophil or not (Ruggiero et al. 2007). In our sample, we had examined the association between WBC count and all-cause mortality, but we failed to observe a positive relation, partially because of the characteristics of study participants. This needs future validations across various elderly people.
Our study has at least two strengths. First, the population-based approach and the reasonably large sample size increase the generalizability of our findings to long-lived populations, as the study participants were recruited from the whole city. In a city with a population of 1,362,500, the subjects of this study represent 65.7 % of the Rugao LLIs (n = 705). Rugao is a typical medium-sized city (from the pespective of population and economy) of China. The findings from such a representative sample may have the potentials to be generalized to most elderly people in China. Second, the simple predictors found in this study could be used as a prognostic tool. Albumin was routinely examined in clinical laboratory measurements, which is fast, inexpensive, and objective, and could be examined in community health centers (CHCs) in China. ADL is a subjective tool to determine the levels of care that one should receive, but it can also be easily used by general practitioners in CHCs of China.
Some weak points of this study should be also noted. First, the biomarkers were not measured repeatedly, which would have nonetheless allow us to observe the changing trend of biomarkers over time and their associations with mortality. Then, no validation cohort was available to confirm the preliminary findings of this study, and this is necessary and crucial for guideline development. Finally, nine participants (2.0 %) had not been identified as alive or dead until the date of censoring according to the local Bureau of Civil Affairs. This phenomenon also existed in other studies in China (Flaherty et al. 2011) and might be attributed to the unique traditional Chinese culture despite the high penalty for concealing death information.
In conclusion, for the first time, this study suggested that both serum albumin and ADL are efficient predictors of all-cause mortality in long-lived population and their combinations further increases discriminative power. The predictive value of the inexpensive, easy-to-use screening methods needs future validations in other cohorts.
Acknowledgments
We acknowledge all the people who participated in the Rugao longevity cohort and, especially, the long-lived individuals for participating in the study. This work was supported by a grant from the National Natural Science Foundation (31171216), a grant from the National Basic Research Program (2012CB944600), and a grant from the National Science and Technology Support Program (2011BAI09B00). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Zuyun Liu and Guangzhen Zhong contributed equally to this work.
References
- Babior BM. Oxygen-dependent microbial killing by phagocytes (first of two parts) N Engl J Med. 1978;298:659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Boland BS, Dong MH, Bettencourt R, Barrett-Connor E, Loomba R. Association of serum bilirubin with aging and mortality. J Clin Exp Hepatol. 2014;4:1–7. doi: 10.1016/j.jceh.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeska VE, Lengyel CO, Tate RB. Nutritional risk and 5-year mortality of older community-dwelling Canadian men: the Manitoba follow-up study. J Nutr Gerontol Geriatr. 2013;32:317–329. doi: 10.1080/21551197.2013.840256. [DOI] [PubMed] [Google Scholar]
- Cai XY, et al. Association of mitochondrial DNA haplogroups with exceptional longevity in a Chinese population. PLoS One. 2009;4:e6423. doi: 10.1371/journal.pone.0006423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, et al. Physical function and self-rated health status as predictors of mortality: results from longitudinal analysis in the ilSIRENTE study. BMC Geriatr. 2008;8:34. doi: 10.1186/1471-2318-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collerton J, et al. The Newcastle 85+ study: biological, clinical and psychosocial factors associated with healthy ageing: study protocol. BMC Geriatr. 2007;7:14. doi: 10.1186/1471-2318-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruijter W, Westendorp RG, Assendelft WJ, den Elzen WP, de Craen AJ, le Cessie S, Gussekloo J. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ. 2009;338:a3083. doi: 10.1136/bmj.a3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Elzen WP, Willems JM, Westendorp RG, de Craen AJ, Assendelft WJ, Gussekloo J. Effect of anemia and comorbidity on functional status and mortality in old age: results from the Leiden 85-plus study. Can Med Assoc J. 2009;181:151–157. doi: 10.1503/cmaj.090040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty JH, Dong B, Wu H, Zhang Y, Guralnik JM, Malmstrom TK, Morley JE. Observational study of 1-year mortality rates before and after a major earthquake among Chinese nonagenarians. J Gerontol Ser A Biol Sci Med Sci. 2011;66:355–361. doi: 10.1093/gerona/glq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondo Y, et al. Functional status of centenarians in Tokyo, Japan: developing better phenotypes of exceptional longevity. J Gerontol Ser A Biol Sci Med Sci. 2006;61:305–310. doi: 10.1093/gerona/61.3.305. [DOI] [PubMed] [Google Scholar]
- Gueresi PMR. Determinants of further survival in centenarians from the province of Mantova. Statistica. 2010;70:16. [Google Scholar]
- Izaks GJ, Remarque EJ, Becker SV, Westendorp RG. Lymphocyte count and mortality risk in older persons. The Leiden 85-plus study. J Am Geriatr Soc. 2003;51:1461–1465. doi: 10.1046/j.1532-5415.2003.51467.x. [DOI] [PubMed] [Google Scholar]
- Ji L, Meng H, Dong B. Factors associated with poor nutritional status among the oldest-old. Clin Nutr. 2012;31:922–926. doi: 10.1016/j.clnu.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- Kuzuya M, Izawa S, Enoki H, Okada K, Iguchi A. Is serum albumin a good marker for malnutrition in the physically impaired elderly? Clin Nutr. 2007;26:84–90. doi: 10.1016/j.clnu.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Liu Z, et al. Association between subjective well-being and exceptional longevity in a longevity town in China: a population-based study. Age. 2014;36:9632. doi: 10.1007/s11357-014-9632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, et al. Blood biomarkers and functional disability among extremely longevous individuals: a population-based study. J Gerontol Ser A Biol Sci Med Sci. 2014 doi: 10.1093/gerona/glu229. [DOI] [PubMed] [Google Scholar]
- Mossakowska M, et al. Cognitive performance and functional status are the major factors predicting survival of centenarians in Poland. J Gerontol Ser A Biol Sci Med Sci. 2014;69:1269–1275. doi: 10.1093/gerona/glu003. [DOI] [PubMed] [Google Scholar]
- Nakazawa A, Nakamura K, Kitamura K, Yoshizawa Y. Association between activities of daily living and mortality among institutionalized elderly adults in Japan. J Epidemiol Jpn Epidemiol Assoc. 2012;22:501–507. doi: 10.2188/jea.JE20110153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal CP, et al. Evaluation of the prognostic value of systemic inflammation and socioeconomic deprivation in patients with resectable colorectal liver metastases. Eur J Cancer. 2009;45:56–64. doi: 10.1016/j.ejca.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Nybo H, et al. Predictors of mortality in 2,249 nonagenarians—the Danish 1905-Cohort Survey. J Am Geriatr Soc. 2003;51:1365–1373. doi: 10.1046/j.1532-5415.2003.51453.x. [DOI] [PubMed] [Google Scholar]
- Poon LW, et al. Methodological considerations in studying centenarians: lessons learned from the Georgia centenarian studies. Annu Rev Gerontol Geriatr. 2007;27:231–264. [PMC free article] [PubMed] [Google Scholar]
- Ruggiero C, et al. White blood cell count and mortality in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2007;49:1841–1850. doi: 10.1016/j.jacc.2007.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun NR, Jacques PF, Dallal G, Russell RM. Use of albumin as a predictor of mortality in community dwelling and institutionalized elderly populations. J Clin Epidemiol. 1996;49:981–988. doi: 10.1016/0895-4356(96)00135-7. [DOI] [PubMed] [Google Scholar]
- Sanchez Garcia E, Montero Errasquin B, Sanchez Castellano C, Cruz-Jentoft AJ. Importance of nutritional support in older people. Nestle Nutr Inst Workshop Ser. 2012;72:101–108. doi: 10.1159/000339998. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Arai Y, Gondo Y, Wakida Y. Determinants of further survival in centenarians. Geriatr Gerontol Int. 2001;1:4. doi: 10.1046/j.1444-1586.2001.00005.x. [DOI] [Google Scholar]
- Takata Y, et al. Serum albumin levels as an independent predictor of 4-year mortality in a community-dwelling 80-year-old population. Aging Clin Exp Res. 2010;22:31–35. doi: 10.1007/BF03324812. [DOI] [PubMed] [Google Scholar]
- van Houwelingen AH, den Elzen WP, Mooijaart SP, Heijmans M, Blom JW, de Craen AJ, Gussekloo J. Predictive value of a profile of routine blood measurements on mortality in older persons in the general population: the Leiden 85-plus Study. PLoS One. 2013;8:e58050. doi: 10.1371/journal.pone.0058050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems JM, Trompet S, Blauw GJ, Westendorp RG, de Craen AJ. White blood cell count and C-reactive protein are independent predictors of mortality in the oldest old. J Gerontol Ser A Biol Sci Med Sci. 2010;65:764–768. doi: 10.1093/gerona/glq004. [DOI] [PubMed] [Google Scholar]
- Wu IC, Shiesh SC, Kuo PH, Lin XZ. High oxidative stress is correlated with frailty in elderly Chinese. J Am Geriatr Soc. 2009;57:1666–1671. doi: 10.1111/j.1532-5415.2009.02392.x. [DOI] [PubMed] [Google Scholar]


