Abstract
Introduction
ColoPulse tablets are an innovative development in the field of oral dosage forms characterized by a distal ileum and colon-specific release. Previous studies in humans showed release in the ileo-colonic region, but the relationship between gastrointestinal pH and release was not experimentally proven in vivo. This information will complete the in vivo release-profile of ColoPulse tablets.
Materials and Methods
Release from ColoPulse tablets was studied in 16 healthy volunteers using the dual label isotope strategy. To determine gastrointestinal pH profiles and transit times the IntelliCap system was used. A ColoPulse tablet containing 13C-urea and an uncoated, immediate release tablet containing 15N2-urea were taken simultaneously followed by a standardized breakfast after three hours. Five minutes after intake of the tablets the IntelliCap capsule was swallowed and pH was measured until excretion in the feces. Breath and urine samples were collected for isotope analysis.
Results
Full analysis could be performed in 12 subjects. Median bioavailability of 13C -urea was 82% (95% CI 74–94%, range 61–114%). The median lag time (5% release of 13C) was 5:42 h (95% CI 5:18–6:18 h, range 2:36–6:36 h,) There was no statistically significant difference between lag time based on isotope signal and colon arrival time (CAT) based on pH (median 5:42 vs 5:31 h p = 0.903). In all subjects an intestinal pH value of 7.0 was reached before release of 13C from the ColoPulse tablet occurred.
Discussion and Conclusions
From the combined data from the IntelliCap system and the 13C -isotope signal it can be concluded that release from a ColoPulse tablet in vivo is not related to transit times but occurs in the ileo-colonic region after pH 7.0 is reached. This supports our earlier findings and confirms that the ColoPulse system is a promising delivery system for targeting the distal ileum and colon.
Trial Registration
ISRCTN Registry 18301880
Introduction
Distal ileum and Colon-specific delivery of medicines is clinically relevant because their efficacy can be improved, side effects can be reduced and the bioavailability of drugs that are metabolized or poorly absorbed in the higher parts of the small intestine can be enhanced. This offers interesting perspectives for the treatment of for instance inflammatory bowel diseases with peptides [1].
In the literature different strategies for colon-specific delivery have been described. They include pH-responsive systems, time-based systems and systems triggered by the colon flora, as well as combinations of such systems [1,2]. The recently developed ColoPulse technology is a promising pH-responsive system being able to specifically deliver the active substance to the ileo-colonic region. Because of the non-percolating incorporation of a super-disintegrant in the coating, by which a more reliable and pulsatile release is achieved, it differs from other pH-responsive systems. The dissolution of this coating is triggered by the physiologically occurring increase in pH from 5.5 in the upper small intestine to 7.5 in the ileo-colonic region [3].
Until now bioavailability from ColoPulse dosage forms has been studied in healthy volunteers and in patients with Crohn’s disease using stable isotopes of urea [4,5,6]. All studies showed a mean total bioavailability of 13C-urea of > 76%, but the relationship between gastrointestinal pH and release from a ColoPulse dosage form has so far not been proven experimentally in vivo. To obtain more insight in the behaviour and functioning of ColoPulse tablets and capsules in vivo, studies on the gastrointestinal pH with the concomitant administration of a ColoPulse tablet are justified.
In the literature different devices for gastrointestinal pH measurement have been described [7]. They can be divided in two subgroups: the “static” devices and the “freely moving” devices. With representatives of the first group only the oesophageal, intra-gastric or peri-mucosal colonic pH can be measured. Their main disadvantage is the inability to measure the pH along the entire length of the gastrointestinal tract. The second group mainly comprises pH sensitive wireless radiotelemetry capsules (RTC). After administration of a RTC the subject is able to perform normal daily activities. Emitted radio signals are detected by a recorder mostly worn around the waist. If the device functions properly, measuring ends when the RTC is excreted with the feces. Disadvantages of the RTCs used so far are the frequent loss of signals, batteries running out of power before excretion, large pH-drift (up to approximately 1 unit) and difficulties in determining the exact location of the capsule in the gastrointestinal tract. In Table 1 a summary of the available literature on freely moving devices studied in inflammatory bowel diseases and / or healthy volunteers is presented [8–21].
Table 1. Summary of available literature on gastrointestinal pH measurement with freely moving devices in healthy volunteers and / or inflammatory bowel diseases.
| Study | Device | Subjects | Battery life | pH Sampling interval | Position detection | Food intake during study | Data loss | pH drift device during study | Total transit time | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|
| Watson et al, 1972 [8] | Radiotelemetry Capsule | 2 healthy subjects 7 patients with miscellaneous gastrointestinal disorders | 10 days | 60 min | Abdominal x-ray | Device intake after breakfast. No restrictions in food and beverages | - a | 0.1 unit | - | |
| Evans et al, 1988 [9] | Radiotelemetry capsule (Remote control systems Ltd, UK) | 72 healthy subjects | - | 12 seconds | “locator” to detect highest signal intensity | Device intake after overnight fasting. breakfast after leaving the stomach. No restrictions in food and beverages | In 14 subjects > 75% loss in the small intestine | pH 4: < 0.6 unit pH 9.2 < 1.0 unit | Mean: 23.3 h | Measurement up to 48 h. Median signal loss 20.4%. 2 subjects > 1.0 unit pH drift |
| Fallingborg et al, 1989 [10] | Radiotelemetry Capsule (Remote control systems Ltd, UK) | 39 healthy subjects | - | 15–120 min (not between 11 pm and 8 am) | Fluoroscopy | Device intake after overnight fasting; breakfast after leaving the stomach. Food and beverages according to the protocol | - | < 0.9 unit | 9–129 h | |
| Raimundo A et al 1992 [11] | Radiotelemetry Capsule | 7 patients with acute colitis 6 patients with ulcerative colitis in remission | - | - | Based on pH | - | - | - | - | |
| Fallingborg et al, 1993 [12] | Radiotelemetry Capsule | 7 patients with ulcerative colitis | - | 30 min (not between 11 pm and 8 am) | Fluoroscopy | Device intake after overnight fasting; breakfast after leaving the stomach. No restrictions in food and beverages | - | < 0.4 unit | 8 - > 123 h | Measurement max 39h. |
| Sasaki et al, 1997 [13] | Radiotelemetry Capsule (Remote control systems Ltd, UK) | 4 healthy subjects 4 patients with Crohn’s disease | 1 | 1 second | Based on pH, x-ray, contrast colonogram and a radio-directional probe | Device intake after overnight fasting; breakfast after leaving the stomach. Food according to the protocol | - | < 0.5unit | - | |
| Press et al, 1998 [14] | Radiotelemetry Capsule (7036, Oakfield instruments, UK) | 12 healthy subjects 11 patients with ulcerative colitis 15 patients with Crohn’s disease | - | - | “locator” to detect highest signal intensity | Device intake after overnight fasting; breakfast after leaving the stomach. No restrictions in food and beverages | In 4 subjects > 75% loss in 24h | < 0.5 unit | - | Measurement in the colon was marked as unpredictable. 4 subjects had to repeat the study |
| Fallingborg et al, 1998 [15] | Radiotelemetry Capsule (remote control systems Ltd, UK) | 13 healthy subjects 9 patients with Crohn’s disease | - | 10–15 min | Fluoroscopy | Device intake after > 8h fasting; breakfast after leaving the stomach | - | <0.4 unit | - | Difference in small intestine transit time between resected patients and healthy volunteers |
| Ewe et al, 1999 [16] | Radiotelemetry Capsule (7036, Oakfield instruments, UK) | 15 healthy subjects 15 patients with Crohn’s Disease 5 patients with ulcerative colitis | 24 h | 6 seconds | Metal detector | Device intake after > 8h fasting; breakfast after leaving the stomach | 6 subjects excluded, several reasons | - | Median 24–31 h | In 1 subject > 2 weeks retention of RTC |
| Maqbool et al, 2009 [17] | SmartPill, (Buffalo, NY, USA) | 10 healthy subjects | 5 days | 5 seconds, after 24 h 20 seconds | Based on pH | 2000 kcal diet, 30% fat. Device intake after breakfast | - | - | - | |
| Rubin et al, 2009 [18] | Smartpill, (Buffalo, NY, USA) | 10 patients with active ulcerative colitis | - | - | Based on pH, motility and temperature | - | - | - | Median 24.6 h | No complications with the device |
| Lalezari et al, 2012 [19] | SmartPill, (Buffalo, NY, USA) | 10 healthy subjects 9 patients with IBS | 5 days | 5 seconds | Based on pH | Device intake after > 8h fasting; breakfast after leaving the stomach | - | - | - | |
| Schaar et al, 2013 [20] / Koziolek et al, 2014 [21] | (IntelliCap MedimetricsEindhoven, NL) | 2x 10 healthy volunteers | > 48 h | 10 seconds | Study 1: based on pH and temperature. Study 2: also based on 99MTc | Device intake with water after overnight fasting. Food 4 h after device intake | Mean 3.5% (one subject 13%) | - | Average 30:34 h | Two publications, same studies |
a- = no information in publication.
Recently a medical device for the in vivo measurement of pH and temperature in the gastrointestinal tract was developed by Medimetrics (Eindhoven, The Netherlands): the IntelliCap system [20,21]. Furthermore the system can be used for electronically controlled drug delivery in defined sections of the gastrointestinal tract to quantify regional drug absorption [22]. It differs from the so far available freely moving RTCs by more accurate and more frequent measurements, minimal signal loss, a built-in drug reservoir and improved battery power (> 72 h) to ensure reliable and complete data acquisition. By combining diagnostic functionalities and the capability to generate adjustable controlled drug release profiles, the IntelliCap system can play a promising role in pharmaceutical drug profiling and formulation development.
In this paper we describe a study performed in healthy volunteers in which we studied the relationship between gastrointestinal pH obtained with the IntelliCap system as well as the release from a ColoPulse tablet to prove that release from a ColoPulse tablet indeed does occur in the ileo-colonic region and after a pH value of ≥ 7.0 is reached.
Materials and Methods
Subjects
Sixteen healthy volunteers (10 male, 6 female, age 18–65) were initially included in this study (Table 2). Participant recruitment started January 2011 and ended April 2011. Written informed consent was obtained from all participants. They had no history of gastrointestinal diseases or gastrointestinal surgery. None of the subjects used antibiotics or drugs influencing the gastrointestinal transit time for at least three months prior to the start of the study. A possible Helicobacter pylori infection was excluded with a 13C-urea breath test (INFAI, Köln, Germany).
Table 2. Demographics of included subjects (healthy volunteers).
| Median (range) | |
|---|---|
| Sex (male / female) | 10 / 6 |
| Age (year) | 27.5 (19–63) |
| Weight (kg) | 77.0 (54.5–121.4) |
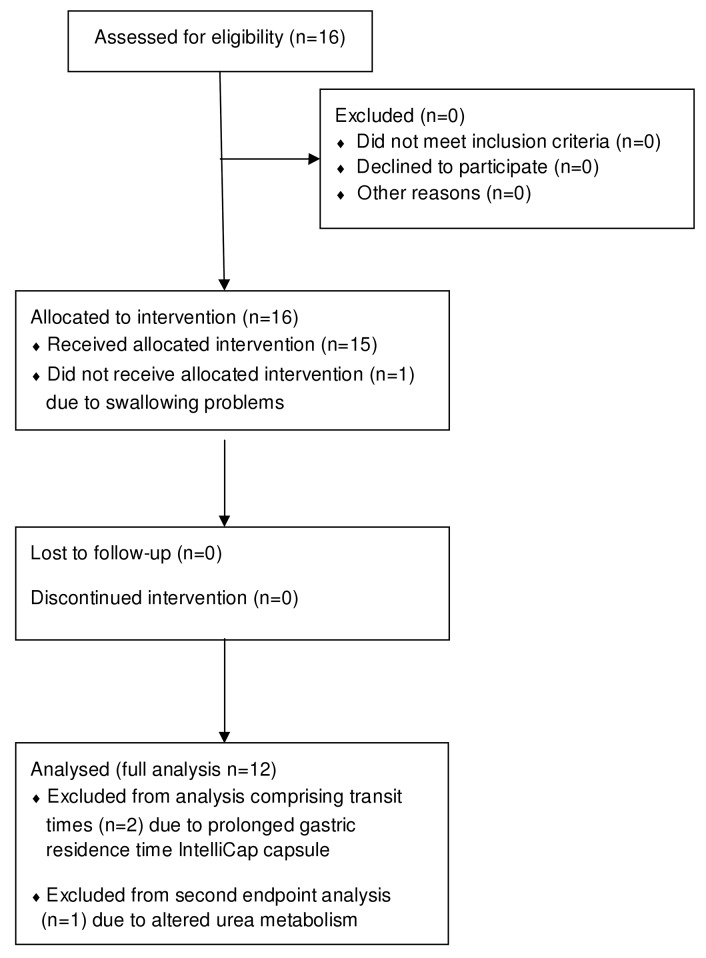
A flowchart summarizing recruitment and analysis is shown in Fig 1.
Fig 1. Flowchart.
Study-design
This bioavailability study was performed as an open-label, non-randomized, single arm clinical trial and was part of a study described by Maurer et al [6]. The design was based on a previous feasibility study in which two stable isotopes of urea are administered simultaneously [23]. Subjects were fasted from 8 p.m. the day before the test day. Only water, apple juice (until 11 p.m.) and unsweetened tea without sugar were allowed. On the test day an uncoated tablet containing 50 mg 15N2-urea and a ColoPulse tablet containing 50 mg 13C-urea were taken simultaneously at around 8 a.m. with 100 mL apple juice. Five minutes thereafter the IntelliCap capsule was swallowed with another 100 mL apple juice. A standardized breakfast was taken three hours after the intake of the tablets. The meal consisted of a standardized double sandwich and 200 mL unsweetened tea. Approximately 6 and 10 hours after tablet intake, respectively lunch and dinner were taken. There were no food-restrictions for lunch and dinner except foods rich in 13C, like corn products, cane sugar and pineapple. During the test day, (that ended at 8 a.m. the next morning) water, apple juice and tea were the only beverages allowed.
Sampling, administration of the tablets and IntelliCap capsule took place in a controlled facility until 5 p.m. Thereafter subjects went home were they continued sampling of breath and urine according to the study protocol. All necessary information was recorded in a diary. A summary of the study design is shown in Table 3.
Table 3. Study schedule, activities are marked with an X (T0 is 8 a.m.).
| Time (h) | -12 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 24 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arrival, start fasting | X | |||||||||||||||||
| Intake tablets | X | |||||||||||||||||
| Intake IntelliCap | X | |||||||||||||||||
| Meal | X | X | X | |||||||||||||||
| Urine Sample | X | X | X | X | X | X | X | |||||||||||
| Breath sample | X | X | X | X | XX | XX | X | XX | XX | XX | XX | XX | X | X | X | X | ||
| Return home | X |
Ethics statement
The study was approved by the ethical committee of the University Medical Center Groningen (ref 2009.188 / EudraCT 2009–01347121) and was performed according to the principles of the Declaration of Helsinki. The study has been registered in the ISRCTN register (ISRCTN18301880). This was not done before the start of recruitment because at that time this was not required by internal procedures and the ethical committee that approved this study.
IntelliCap system
The IntelliCap system was supplied by Medimetrics (Eindhoven, The Netherlands) and consisted of a capsule and a portable unit. The size of the IntelliCap capsule was 27 x 11 mm. A complete description including illustrations of the IntelliCap system can be found in the literature [20]. The drug reservoir was filled with normal saline solution which had no function in this study and was not expelled from the IntelliCap capsule during the experiments.
Data were measured until excretion of the IntelliCap capsule or until the battery ran out of power. The excretion of the IntelliCap capsule from the body had to be confirmed by collecting the device from the stool. If the IntelliCap capsule was not retrieved within 96 hours it was probably missed and its absence from the body was confirmed with an abdominal X-ray. No follow up was required after retrieving the IntelliCap capsule or confirmation of its absence by X-ray.
Analysis of pH profiles
The pH profiles were analysed for gastrointestinal landmarks (ingestion, pylorus, ileocecal valve and excretion) using the following criteria:
-
▪
Ingestion: rapid and sustained rise in temperature from room to body temperature and a rapid drop of > 3 pH units
-
▪
Pylorus: rapid and sustained rise of at least 3 pH units
-
▪
Ileocecal valve (cecum): first and rapid drop of > 0.8 pH units at least 1 h after the pylorus to pH ≤ 6.5
-
▪
Excretion: rapid and sustained drop in temperature from body to room temperature
Gastrointestinal residence and transit times were derived from the identification of gastrointestinal landmarks and are defined as follows:
-
▪
Gastric residence time (GRT): elapsed time between ingestion and pylorus
-
▪
Small intestine transit time (SBTT): elapsed time between pylorus and ileocecal valve
-
▪
Colonic arrival time (CAT): elapsed time between ingestion and ileocecal valve
-
▪
Colonic transit time (CTT): elapsed time between ileocecal valve and excretion
-
▪
Whole gut transit time (WGTT): elapsed time between ingestion and excretion
Chemicals, isotopes and coated tablets
All substances were of pharmacopoeial grade (Ph. Eur. or USP) and were obtained via a certified wholesaler as described before [6]. The stable isotopes 13C-urea and 15N2-urea (AP 99%) were obtained from an FDA-controlled facility (Isotec, USA). Tablet cores containing 50 mg 13C- or 50 mg 15N2-urea and 25 mg caffeine were compounded in the Department of Hospital and Clinical Pharmacy of the University Medical Center Groningen and analysed according to the European Pharmacopoeia 7th edition
A ColoPulse coating of 13–17 mg/cm2 was applied on the tablets containing 13C-urea.The coating was composed of a mixture of Eudragit S-100:PEG 6000:Ac-di-sol:talc in a ratio of 7:1:3:2 (w/w). The solvent was an acetone-water 97:3 mixture (w/w). Coating thickness was determined and expressed as the amount of Eudragit S100 applied per cm2. Caffeine was added to the 13C-urea tablet cores for quality control purposes and was used as a marker substance for the in vitro determination of the release characteristics lag- and pulse time in the in vitro dissolution test. Caffeine was also added to the 15N2-urea tablet cores to obtain comparable tablet cores, with no particular function in this tablet. All tablets, coated and uncoated, met established pharmaceutical quality control criteria [6].
Urea-kinetics
To study the bioavailability from a ColoPulse tablet in the ileo-colonic region the difference in kinetics and fate between 13C-urea and 15N2-urea was used. An overview of the relevant kinetic steps can be found in Maurer et al [23,24]. Release of 13C-urea in the ileo-colonic region (urease-rich) from a ColoPulse tablet leads to in situ fermentation of 13C-urea into 13CO2 which is subsequently exhaled in breath. The delivery of the isotope in the colon can therefore be established by measuring the 13CO2 response in breath. Unfermented urea (i.e. release in the small intestine, urease-poor) can be measured as the amount of 13C-urea in urine. The second stable isotope of urea, 15N2-urea, in an uncoated tablet functions as a reference and reflects 100% release in a urease-poor region. Release of 15N2-urea in the small intestine from an uncoated capsule leads to recovery of 15N2-urea in urine. Bioavailability can be described by the difference between kinetics of 13C- and 15N2-urea [23,24].
Sample collections and analysis
Breath samples were collected every 0.5–1 h up to 15 h after intake of the tablets (Table 2) and were analysed as described before [23]. Briefly, 13C/12C isotope ratios in the CO2 of breath samples were analysed by using a validated breath 13C-analyser (Thermo Fisher Scientific, Bremen, Germany) based on isotope ratio mass spectrometry (IRMS).
Urine samples were collected during 24 h after intake of the tablets at prescribed intervals (Table 2) in 500 or 1000 mL containers containing an aliquot of 6M HCl. Urine volumes were recorded and 20 mL samples were stored at -80°C until analysis. Concentrations of total 15N and 13C were determined as described before using an elemental analyzer interfaced with IRMS [23].
Calculations
The Percentage of the administered Dose Recovered (PDR) of 13C and 15N in each urine sample, the ratio of the PDRs 13C versus 15N (the 13C/15N-ratio), the fermented (Ffermented) and not-fermented (Fnot-fermented) fraction of 13C urea were calculated as described before [23,24]. In short:
-
▪
Ffermented was calculated as the cumulative (c)PDR of 13C in breath over a 15 h time period
-
▪
Fnot-fermented = cPDR 13C / cPDR 15N in a 24 h urine collection
-
▪
Bioavailability = Ffermented + Fnot-fermented
-
▪
The lag time was derived from the cPDR of 13C in breath and was defined as de time between administration of the tablets and the time the cPDR reached the value of 5% of cPDR at t = 15 h
All data were corrected for baseline-concentrations of 13C and 15N in breath and /or urine. Furthermore, breath data were corrected for CO2-retention as described before [4].
Statistical procedures
This study was performed as a bioavailability study. Based on previous data on transit times of a ColoPulse tablet a sample size of 10 patients is needed to detect a clinically relevant difference of 15% between lag-time based on isotope-signal and colon arrival time based on pH with 80% power and a significance level of α = 0.05 (two sided). Because this study was part of another study [6] requiring a higher sample size and anticipating some drop-out 16 subjects were included.
The results were evaluated by descriptive statistics with SPSS version 22. Normal distribution of the data was investigated with the Shapiro-Wilk test. The center was characterized by the mean and standard deviation (pH-data) or the median and corresponding bootstrap based 95% confidence intervals (95% CI). The dispersion was characterized by the coefficient of variation (CV) and range because not all data were normally distributed. A (parametric) paired-samples t-test (two tailed, α = 0.05) was used to compare the results within groups when data were normally distributed for both variables. A (non-parametric) Wilcoxon signed rank test was performed to compare the results when at least one of the variables was not normally distributed. Differences were considered significant when p < 0.05.
Endpoints
The endpoint was to investigate the relationship between the gastrointestinal pH-profile obtained with the IntelliCap system and release of 13C-urea from a ColoPulse tablet and to confirm that release occurs in the ileo-colonic region after pH 7.0 has been reached.
Results
The results of 15 out of 16 healthy volunteers initially included in the study were evaluated (Fig 1). One volunteer could not swallow the IntelliCap capsule and was therefore excluded without replacement. Two other subjects (6 and 15) appeared to have a prolonged gastric residence time and the IntelliCap capsule was still in the stomach when breakfast and the following meals were taken. Because their gastric residence time was respectively 17 and 22 h data of these subjects were excluded from any analysis comprising transit times. Their lag time based on the isotope signal was within the normal range. Subject 5 was also excluded from analysis comprising lag time and bioavailability, because of a probably altered urea metabolism. This was concluded from the fact that the cPDR 13C in breath was <6.5% after 15h combined with a cDPR of unfermented 13C in urine of 70% at t = 24 h. The pH profile of this subject was normal with a GRT of 0:15 h and a SBTT of 3.15 h. The coating functioned well, because the appearance of 13C in the urine sample could be seen in the sample collected between t = 4 and 7 h and not earlier. This means that 13C-urea in stead of being fermented was absorbed into the bloodstream when it was released at the ileo-colonic region. The data from the remaining 12 subjects were available for all analyses.
IntelliCap capsules could be recovered from the feces within 72 hours after intake in 13 out of 15 subjects. For two subjects the temperature data indicated that the IntelliCap capsule had left the body, but the subjects failed to retrieve it from the feces. Absence from the body was confirmed with an abdominal X-ray. No adverse events potentially related to the IntelliCap system were observed during the study.
In three subjects the portable unit ran out of power after circa 60 hours. This did not influence the data collection necessary for the endpoint analysis because in all subjects the IntelliCap capsule already passed the cecum. However, for these subjects time of excretion and whole gut transit time (WGTT) could not be determined.
In three other subjects the communication between the capsule and portable unit was interrupted varying from 4 to 12 h because the subjects did not wear the portable unit close enough to the body or did not wear it. This also did not influence the data collection for endpoint analysis because all interruptions occurred more than 24 h after intake, when the IntelliCap capsule already had passed the cecum. For one of these subjects excretion, CTT and WGTT could not be determined because excretion occurred during the period of interrupted communication. No other loss of data was encountered in the study.
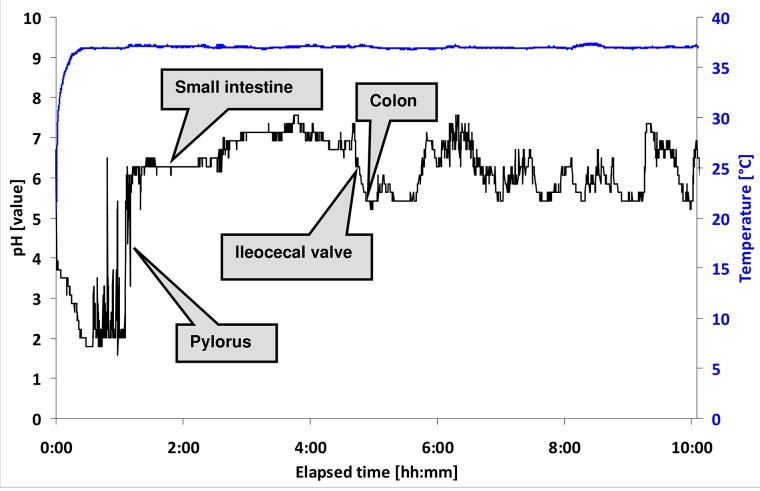
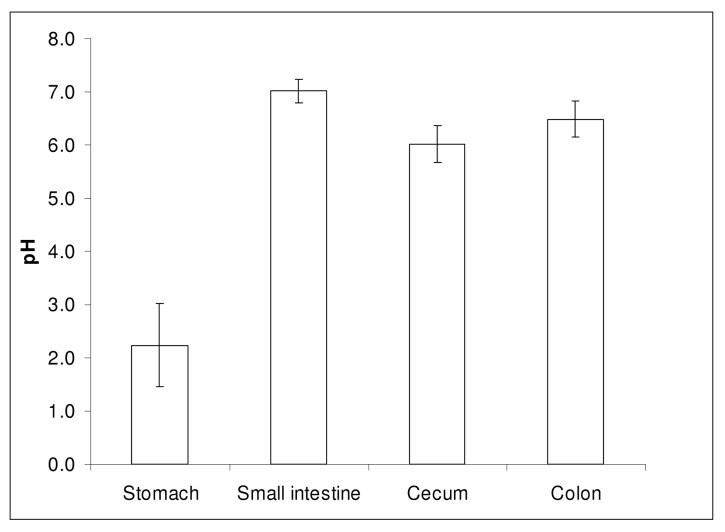
All gastrointestinal pH profiles recorded with the IntelliCap system were analysed according to the mentioned methods. A summary of the gastrointestinal transit times is shown in Fig 2 and a representative example of a pH and temperature profile is shown in Fig 3. The residence in the stomach, passage of the pylorus, course of pH in the small intestine and the ileocecal valve (cecum) are all clearly visible in this figure. From Fig 2 it is obvious that there are large inter-individual differences in transit times. For example, the colon arrival time (CAT) differs from 3:25–8:20 h (median 5:31 h, 95% CI 4:51–5:48 h, CV 26%) and the whole gut transit time for the IntelliCap capsule was 10:01–59:39 h (median 27:08 h, 95% CI 22:49–59:11 h, CV 52%). Gastric residence time varied between 0:15 and 3:14 h (median 1:30 h, 95% CI 1:05–2:08 h, CV 59%). The median difference between the time when pH 7.0 was reached and the CAT was 2.26 h and in most subjects pH remained > 7.0 until the cecum was reached. A summary of measured pH values in the stomach, small intestine and colon is shown in Fig 4.
Fig 2. Mean (gastrointestinal residence and transit times determined with the IntelliCap system.
Data are presented mean and standard deviation of 13 evaluable subjects (for CTT and WGTT n = 9).
Fig 3. Example of pH-profile of the first 10 hours after intake of the IntelliCap capsule (subject 14).
Fig 4. Summary of pH in the stomach, small intestine and colon as measured with the IntelliCap system.
Data are presented as mean and standard deviation of 15 evaluable subjects.
Lag time and bioavailability of 13C-urea from a ColoPulse tablet were calculated as described. The median lag time was 5:42 h (95% CI 5:18–6:18 h, range 2:36–6:36 h, CV 18%) and median bioavailability was 82% (95% CI 74–94%) range 61–114%, CV 10%). More detailed results can be found in S1 summary and in Maurer et al [6].
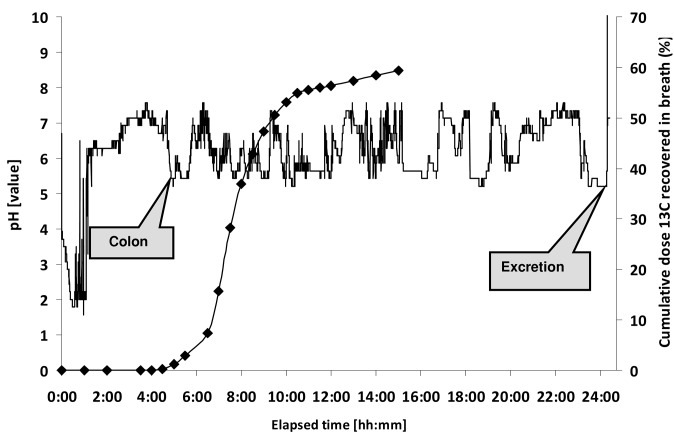
There was no statistically significant difference between CAT based on pH-data (IntelliCap) and the lag time of the ColoPulse tablet based on the stable isotope signal of 13C-urea in breath (median 5:31 vs 5:42 h, p = 0.903, parametric test). A representative example is shown in Fig 5. Information about all subjects can be found in Fig 6.
Fig 5. Colon arrival time based on pH (-) corresponds with release of 13C (♦) (subject 14).
See also Fig 4 for the first 10 hours.
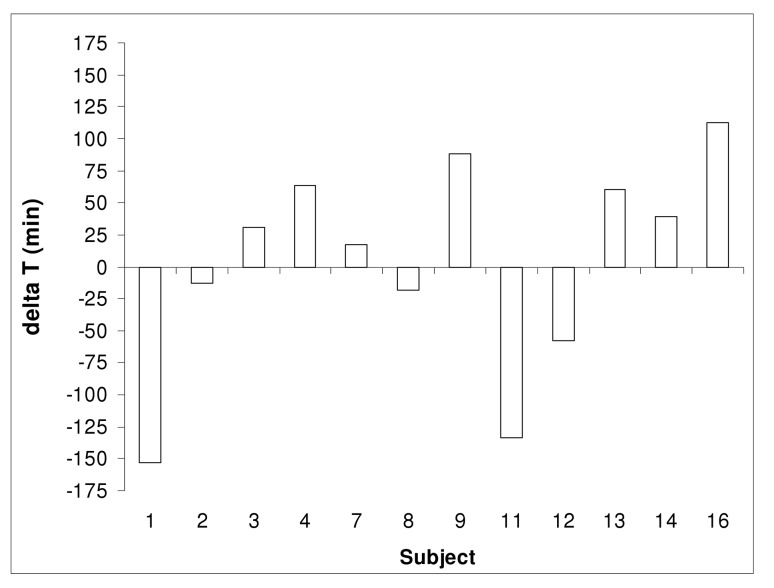
Fig 6. Difference (delta T) between lag-time (based on isotope signal) and CAT (based on pH) for each subject (n = 12).
In all subjects a pH value of 7.0 was reached before release of 13C from the ColoPulse tablet occurred, as measured in exhaled breath. There was a statistically significant difference between the time when pH 7.0 was reached and the lag time (185 vs 342 minutes, p = 0.002, non-parametric test).
Discussion
This is the first study in humans combining release from ColoPulse tablets and in vivo gastrointestinal pH measurements using the IntelliCap system. There was no difference between CAT (pH-data) and lag time (isotope data) found in this study which delivers proof that release of ColoPulse tablets occurs in the ileo-colonic region. Furthermore the results show that release from the ColoPulse tablet does not occur before an intestinal pH value of 7.0 is reached.
Several studies have been published with radiotelemetry capsules (RTC). Through the years the functioning of RTCs improved considerably. The first RTCs were used in the 1970’s and had logging intervals from 5 to 120 minutes to save battery capacity [8,10]. Furthermore high data loss (75%) has been described [9]. Thereafter RTCs with shorter logging intervals up to 5 seconds became available and battery life improved considerably [16,17,19]. However in some studies data loss was still described mostly attributed to the angle between the RTC and the antenna [16]. In the current study with the IntelliCap system data loss was observed in three subjects out of 15. This didn’t occur in the first 8 hours after intake of the capsule, when data were also sent to the control center, but only when the subjects were at home and did not keep their receiver close enough to the body. Therefore this event did not influence the outcome of our study, because the IntelliCap capsule already passed the cecum at the time data loss occurred. On the other hand, this shows that an overnight stay in a controlled facility is preferable when longer pH profiling is needed.
The IntelliCap system was able to record complete gastrointestinal pH and temperature profiles as well as derived transit-times from intake to excretion. The observed gastrointestinal transit times for the small intestine and the colon are within the range of earlier published data of healthy volunteers, only the median GRT appeared to be increased [10,25]. Published data collected with RTC and dosage forms labeled with gamma emitting radionuclides mention an average GRT of about ≤ 1 h in fasted, healthy volunteers while we observed an average gastric residence time of 1:30 h in the evaluable subjects. A likely explanation is the intake of the tablets with apple juice, since it is known that food increases the GRT of pharmaceutical dosage forms [25]. Due to its caloric content apple juice apparently also has a delaying effect. However in this study, the apple juice was given to get the same study design as previous studies with ColoPulse formulations. This was done to be able to compare the functioning of ColoPulse tablets used in this study with capsules which were used in previous studies. In the future ColoPulse formulations can also be administered with water.
All subjects showed an elevated gastric pH (pH 3–4) immediately after administration probably due to administration with apple juice, which decreased to a more acidic level of around pH 1.6 in about 30 minutes. Because in our study the determination of the location of the IntelliCap capsule was only based on pH, pH values of the different segments of the small intestine and colon could not be determined. The median pH values of the stomach, small intestines and colon as observed in the majority of subjects are consistent with published data from fasted, healthy volunteers [7].
No difference was found between the colon arrival time based on the 13C-isotope signal (lag time of ColoPulse tablets) and pH-measurements (from IntelliCap system). This proves the site-specific release of the active substance from the ColoPulse tablets in the ileo-colonic region. Simultaneous migration of the ColoPulse tablet and the IntelliCap capsule after leaving the stomach is supported by the literature. According to Davis et al [25] no difference in intestinal transit times was seen between solid dosage forms with the same size of ColoPulse tablets and the IntelliCap capsule. Gastric emptying of large single unit systems however, was highly influenced by the presence of food in the stomach. Even a light breakfast delayed emptying in some subjects. This may explain the increased stomach residence time of the IntelliCap capsule in two subjects as seen in the current study. In these subjects colon arrival time and bioavailability based on isotope signal were within the normal range. However the relatively large IntelliCap capsule was retained in the stomach for respectively 17 and 21 h, probably because they returned to their “normal” meal intakes when the IntelliCap capsule was still in the stomach. After pylorus passage of these two IntelliCap capsules intestinal transit times were comparable to those of the other subjects.
In this study we observed no relation between the lag-time of a ColoPulse tablet and the time when pH 7.0 was reached or the CAT. However, in none of the subjects release from the ColoPulse tablet occurred before pH 7.0 was reached.
The difference between the time when pH 7.0 was reached and the CAT was approximately 2.5 hours, supporting the fact that release occurs in the distal ileum and colon. Remarkably, this difference in time is relatively large and differs from parameters used in vitro dissolution tests that were performed with the ColoPulse tablets in the gastrointestinal simulation system (GISS) [26]. We use this in vitro test for quality control of ColoPulse tablets and normally dissolution of the coating and subsequent release occurs within 30 minutes after raising pH from 6.8 to 7.5. However, the volumes of intestinal fluid in vivo differ from the volumes used in the GISS. According to Schiller et al [27] the fluid volume of the small intestine has a maximum of 319 mL while the volume in this stage of the GISS is as high as 940 mL. Furthermore the fluid is not distributed homogenously along the small intestine in vivo with water pockets and “dry” segments randomly scattered. This contributes to the relatively slow dissolution of the Eudragit-S coating and is probably the cause of the relatively large difference between the time point at which pH 7 was reached and the CAT. The clinical relevance of this phenomenon seems to be limited because median bioavailability was 82%.
Conclusion
Based on the combined data from the IntelliCap system and the urea-isotope signal from a ColoPulse tablet as obtained in this study in healthy volunteers it can be concluded that release from ColoPulse tablets indeed occurs in the distal ileum and colon and after pH 7.0 is reached. This supports our earlier observations and confirms that the ColoPulse system is a promising delivery system for site-specific delivery and local therapy in inflammatory bowel diseases present in the distal ileum and colon.
Supporting Information
(DOC)
(DOC)
(DOC)
(DOC)
Acknowledgments
The authors thank Theo de Boer for analyzing the breath samples.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Medimetrics Personalized Drug Delivery, Eindhoven, The Netherlands, provided support in the form of salaries for authors CW and VI and IntelliCap systems, but did not have any role in study design and decision to publish. The specific roles of these authors are articulated in the author contributions section.
References
- 1. McConnell EL, Liu F, Basit AW. Colonic treatments and targets: issues and opportunities. J Drug Target 2009;17: 335–363. 10.1080/10611860902839502 [DOI] [PubMed] [Google Scholar]
- 2. Singh BN. Modified-release solid formulations for colonic delivery. Recent Pat Drug Deliv Formul 2007;1: 53–63. [DOI] [PubMed] [Google Scholar]
- 3. Schellekens RCA, Baltink JH, Woesthuis EM, Stellaard F, Kosterink JG, Woerdenbag HJ et al. Film coated tablets (ColoPulse technology) for targeted delivery in the lower intestinal tract: Influence of the core composition on release characteristics. Pharm Dev Technol 2012;17: 40–47. 10.3109/10837450.2010.513986 [DOI] [PubMed] [Google Scholar]
- 4. Schellekens RCA, Olsder GG, Langenberg SMCH, Boer T, Woerdenbag HJ, Frijlink HW et al. Proof-of-concept study on the suitability of 13C-urea as a marker substance for assessment of in vivo behaviour of oral colon-targeted dosage forms. Br J Pharmacol 2009;158: 532–540. 10.1111/j.1476-5381.2009.00302.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schellekens RCA, Stellaard F, Olsder GG, Woerdenbag HJ, Frijlink HW, Kosterink JGW. Oral ileocolonic drug delivery by the ColoPulse-system: A bioavailability study in healthy volunteers. J Control Release 2010;146: 334–340. 10.1016/j.jconrel.2010.05.028 [DOI] [PubMed] [Google Scholar]
- 6. Maurer JM, Schellekens RC, Rieke HM van, Stellaard F, Wutzke KD, Buurman DJ et al. ColoPulse tablets perform comparably in healthy volunteers and Crohn’s patients and show no influence of food and time of food intake on bioavailability. J Control Release 2013;172: 618–624 10.1016/j.jconrel.2013.09.021 [DOI] [PubMed] [Google Scholar]
- 7. Nugent SG, Kumar D, Rampton DS, Evans DF. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 2001;48: 571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watson BW, Meldrum SJ, Riddle MC, Brown RL, Sladen GE. pH profile of gut as measured by radiotelemetry capsule. Br Med J 1972;8: 104–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 1988;29: 1035–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fallingborg J, Christensen LA, Ingeman-Nielsen M, Jacobsen BA, Abildgaard K, Rasmussen HH. pH-profile and regional transit times of the normal gut measured by a radiotelemetry device. Aliment Pharmacol Therap 1989;3: 605–613 [DOI] [PubMed] [Google Scholar]
- 11. Raimundo AH, Evans DF, Rogers J, Silk DBA. Gastrointestinal pH profiles in ulcerative colitis. Gastroenterology 1992; 102: A681 [Google Scholar]
- 12. Fallingborg J, Christensen LA, Jacobsen BA, Rasmussen SN. Very low intraluminal colonic pH in patients with active ulcerative colitis. Dig Dis Sci 1993;38: 1989–1993 [DOI] [PubMed] [Google Scholar]
- 13. Sasaki Y, Hada R, Nakajima H, Fukuda S, Munakata A. Improved localizing method of radiopill in measurement of entire gastrointestinal pH profiles: colonic luminal pH in normal subjects and patients with Crohn’s disease. Am J Gastroenterol 1997;92: 114–118 [PubMed] [Google Scholar]
- 14. Press AG, Hauptmann IA, Hauptmann L, Fuchs B, Fuchs M, Ewe K et al. Gastrointestinal pH profiles in patients with inflammatory bowel disease. Aliment Pharmacol Ther 1998;12: 673–678 [DOI] [PubMed] [Google Scholar]
- 15. Fallingborg J, Pedersen P, Jacobsen BA. Small intestinal transit time and intraluminal pH in ileocecal resected patients with Crohn’s Disease. Dig Dis Sci 1998; 43: 702–705 [DOI] [PubMed] [Google Scholar]
- 16. Ewe K, Schwartz S, Petersen S, Press AG. Inflammation does not decrease intraluminal pH in chronic inflammatory bowel disease. Dig Dis Sci 1999;44: 1434–1439 [DOI] [PubMed] [Google Scholar]
- 17. Maqbool S, Parkman HP, Friedenberg FK. Wireless capsule motility: comparison of the SmartPill GI monitoring system with scintigraphy for measuring whole gut transit. Dig Dis Sci 54 2009: 2167–2174 10.1007/s10620-009-0899-9 [DOI] [PubMed] [Google Scholar]
- 18. Rubin DT, Bunnag AP, Surma BL, Mikolajczyk A. Measurement of luminal pH in patients with mildly to moderately active UC: a pilot study using SmartPill. Gastroenterology 2009;136: M1097 [Google Scholar]
- 19. Lalezari D. Gastrointestinal pH profile in subjects with irritable bowel syndrome. Ann Gastroenterol 2012; 25: 333–337 [PMC free article] [PubMed] [Google Scholar]
- 20. van der Schaar PJ, Dijksman JF, Broekhuizen-de Gast H, Shimizu J, van Lelyveld N, Zou H et al. A novel ingestible electronic drug delivery and monitoring device. Gastrointest Endosc 2013;78: 520–528 10.1016/j.gie.2013.03.170 [DOI] [PubMed] [Google Scholar]
- 21. Koziolek M, Grimm M, Becker D, Iordanov V, Zou H, Shimizu J et al. Investigation of PH and temperature profiles in the GI tract of fasted human subjects using the IntelliCap system. J Pharm Sci 2014. ( 10.1002/jps.24274) [DOI] [PubMed] [Google Scholar]
- 22. Becker D, Zhang J, Heimbach T, Penland RC, Wanke C, Shimizu J et al. Novel orally swallowable Intellicap device to quantify regional drug absorption in human GI tract using diltiazem as model drug. AAPS PharmSciTech 2014:15: 1490–1497 10.1208/s12249-014-0172-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maurer JM, Schellekens RCA, Wutzke KD, Dijkstra G, Woerdenbag HJ, Frijlink HW et al. A non-invasive, low-cost study design to determine the release profile of colon drug delivery systems: a feasibility study. Pharm Res 2012;29: 2070–2078 10.1007/s11095-012-0735-3 [DOI] [PubMed] [Google Scholar]
- 24. Maurer JM, Schellekens RC, Wutzke KD, Stellaard F. Isotope-labelled urea to test colon drug delivery devices in vivo: principles, calculations and interpretations. Isotopes Environ Health Stud 2013;49: 473–491 10.1080/10256016.2013.803099 [DOI] [PubMed] [Google Scholar]
- 25. Davis SS, Hardy JG and Fara JW. Transit of pharmaceutical dosage forms through the small intestine. Gut 1986;27: 886–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schellekens RC, Stuurman FE, van der Weert FH, Kosterink JG, Frijlink HW. A novel dissolution method relevant to intestinal release behaviour and its application in the evaluation of modified release mesalazine products. Eur J Pharm Sci 2007;30: 15–20 [DOI] [PubMed] [Google Scholar]
- 27. Schiller C, Fröhlich CP, Giessmann T, Siegmund W, Mönnikes H, Hosten N et al. Intestinal fluid volumes and transit of dosage forms as assessed by magnetic resonance imaging. Aliment Pharmacol Ther 2005;22: 971–979 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.