Abstract
Resistance training to increase muscle mass and functional capacity is an integral part of diet and exercise programs for the management of obesity and type 2 diabetes. Low-intensity resistance training with slow movement and tonic force generation (LST) may be a practical and safe regimen for elderly obese individuals but the health benefits are uncertain. This study investigated the effects of LST on body composition and metabolic parameters in obese patients with type 2 diabetes. Twenty-six obese patients with type 2 diabetes engaged in LST training during hospitalization and were advised to maintain this regimen for 12 weeks after discharge. We compared lipid profile, arterial stiffness, and body composition before and after LST training. After 12 weeks of LST training, the ratio of lower extremity muscle mass to body weight increased significantly (0.176 ± 0.028 to 0.184 ± 0.023, mean ± SD), while body fat mass and body fat percentage decreased significantly (36.2 ± 10.9 kg to 34.3 ± 9.4 kg and 41.2 ± 8.6% to 40.1 ± 7.7%, respectively). Moreover, high-density lipoprotein cholesterol was significantly increased (42.2 ± 14 mg/dl to 46.3 ± 12.4 mg/dl) and both free fatty acids and lipoprotein(a) were decreased (665.2 ± 212.1 μEq/l to 525.4 ± 231.3 μEq/l and 15.4 ± 18 mg/dl to 13.8 ± 18 mg/dl, respectively). No significant change was observed in arterial stiffness. Although this study was a non-controlled investigation and some confounding factors including dietary intake, medication and compliance with training might affect the study result, a brief (12-week) LST training program may be a safe and effective strategy for the management of obesity and type 2 diabetes.
Introduction
Obesity is now a major health problem worldwide. Obese individuals generally have lower muscle strength, which increases the risk of disability [1]. The age-related decrease in muscle mass and accompanying increase in fat mass, termed sarcopenic obesity [2], may also increase the risk of disability and mortality [2,3]. Besides lower muscle strength, obesity is associated with fat infiltration into muscle, contribute to poor lower extremity physical performance [4]. A recent study also showed that lower limb muscle mass was associated with higher visceral fat mass in healthy men [5]. These studies suggest that obesity is perpetuated by poor lower extremity physical performance, and that resistance training (RT) for enhanced lower extremity strength is a good strategy for improving and preventing obesity. Hence resistance training in order to maintain or increase muscle mass and improve physical performance should be an integral part in the management of obesity and metabolic disorders.
Resistance training may also reduce cardiovascular disease (CVD) risk factors such as dyslipidemia and type 2 diabetes [6,7]. The American Diabetes Association recommends that patients with diabetes perform at least 150 min/week of moderate-intensity aerobic exercise as well as RT at least twice per week [8]. Furthermore, a recent meta-analysis concluded that RT improves glycemic control [9]. Thus, RT is recommended for both diabetes prevention and disease management. However, little evidence is available regarding the optimal intensity of RT for obese patients with type 2 diabetes.
A training regimen for gaining muscle size and strength that involved low-intensity resistance exercise with slow movement and tonic force generation (LST) was found to be effective in young men [10]. Previous studies showed that progressive resistance training safely and effectively improved glycemic control, insulin resistance and body composition in patients with type 2 diabetes [11–13]. However, obese patients with type 2 diabetes are recognized as having lower fitness levels than healthy individuals [14], and such patients cannot continue recommended exercise therapy in daily lives. On the other hand, LST training can also be performed without a considerable physical burden and should therefore be beneficial for rehabilitation from orthopedic injuries or as RT among the elderly [10].
To the best of our knowledge, however, no previous studies have examined the changes in body composition and metabolic parameters associated with LST training in obese patients with type 2 diabetes. In this study, we examine the effects of a brief LST training program on body composition and metabolic parameters in obese patients with type 2 diabetes.
Methods
Study participants
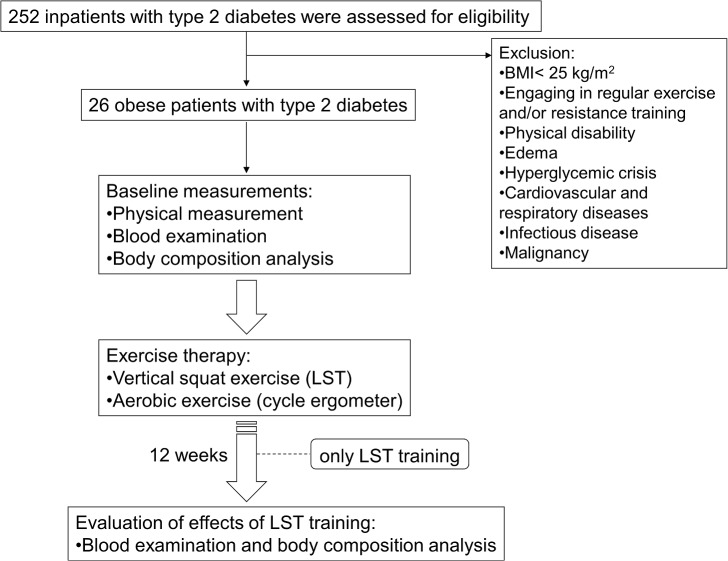
Of 252 patients with type 2 diabetes admitted to our hospital for glycemic control between September 2013 and August 2014, we recruited 26 eligible patients. We included patients with BMI > 25.0 kg/m2, which is defined as obesity by the Japan Society for the Study of Obesity [15], and who agreed to participate in our training program. We excluded patients with physical disability such as osteoarthritis, hyperglycemic crisis, stroke, cardiovascular and respiratory diseases, infectious disease, and malignancy. Patients with edema were excluded because we cannot accurately evaluate body composition using our bioelectrical impedance analysis method. Also excluded were individuals who engaged in regular exercise and/or RT. The Medical Ethics Committee of the National Center for Global Health and Medicine approved the study (Reference No. NCGM-G-001562-01), and all participants provided written informed consent to use their data in the study. The study was performed in accordance with the Declaration of Helsinki.
We performed baseline measurements during hospitalization. The participants received standard hospital diets (25−30 kcal/kg) during hospitalization and were instructed by certified nutritional educators to help continue the diet as outpatients. All participants also engaged in RT and aerobic exercise under the instruction of skilled physiotherapists. Approximately 12 weeks after discharge, we evaluated the changes in body composition, anthropometric parameters, and biochemical data. The study protocol is illustrated in Fig 1.
Fig 1. The protocol of this study.
Training protocol
The participants performed the following vertical half-squat exercise protocol: LST (without free weights) with 3–5 s for eccentric and concentric action, 1 s pause, and no relaxing phase [10]. They performed 8−10 repetitions × 3 sets or as many repetitions as possible. We also prescribed cycle ergometer at 50% of age-predicted maximum heart rate as calculated by the Karvonen method [16] [[(220 - age) - (resting heart rate)] × 50% + resting heart rate] as exercise therapy for diabetes. The average training time was 30–40 minutes per day. They did not engage in any other exercise. All participants performed LST training every other day during hospitalization and were advised to continue LST training only after discharge and were instructed not to take any other form of exercise except LST training. We checked compliance when patients visited our hospital every four weeks and after the 12-week study period.
Anthropometric measurements
Height and weight were measured with a rigid stadiometer and calibrated scale (seca 764, seca Co., Ltd, Birmingham, United Kingdom). BMI was calculated as body weight (kg) divided by the square of height (m). Waist circumference (WC) was measured in a standing posture at the umbilical level while breathing out.
Body composition analysis
We analyzed body composition using a bioelectrical impedance analysis device (InBody720, Biospace Co., Ltd, Tokyo, Japan) that measures the relative proportions of lean and fat tissues by differences in tissue impedance. The method is based on the principle that lean body tissue is almost all water and so is a better conductor of electrolytes than fat tissue. Segmental body composition was estimated using a patented 8-point tactile electrode system consisting of platform (feet) and gripping (hand) electrodes. The device uses six frequencies (1, 5, 50, 250, 500, and 1,000 kHz) and produces 30 impedance values for five body segments: right and left upper extremity, trunk, and right and left lower extremity [17].
Blood examination
Venous blood samples were taken after a 12-h overnight fast. Fasting plasma glucose was measured using an enzymatic method (GA-1170, Arkray, Inc., Kyoto, Japan). Glycated hemoglobin (HbA1c) was measured by high-performance liquid chromatography (HA-8180, Arkray, Inc., Kyoto, Japan). Total cholesterol, free fatty acid (FFA), triglyceride (TG), and high-density lipoprotein-cholesterol (HDL-C) were measured enzymatically using commercially available kits (T-CHO KL for total cholesterol, Sysmex Co., Hyogo, Japan; NEFA-SS for FFA, Eiken Chemical, Tochigi, Japan; Pureauto S TG-N and Cholestest N HDL, respectively, for TG and HDL-C, Sekisui Medical Co., Tokyo, Japan). Low-density lipoprotein-cholesterol (LDL-C) was obtained by the Friedwald formula [18]. Lipoprotein(a) (Lp(a)) was measured by a latex-enhanced turbidimetric immunoassay using Latex Daiichi (Sekisui Medical Co., Ltd, Tokyo, Japan).
Arterial stiffness and blood pressure
To estimate arterial stiffness, we measured brachial-ankle pulse wave velocity (baPWV) using a blood pressure pulse wave inspection apparatus (BP-203RPEIII, Omron Co., Ltd, Tokyo, Japan). To estimate arterial stiffness and peripheral vascular resistance, we also measured the heart rate-corrected augmentation index (AIx75) and central systolic blood pressure (cSBP) using a digital automatic sphygmomanometer (HEM-9000AI, Omron Co., Ltd, Tokyo, Japan).
Statistical analysis
All statistical analyses were performed using SPSS version 19 (IBM Co., Ltd, Chicago, IL). All values are expressed as mean ± standard deviation (SD). The Wilcoxon signed-rank test was applied to evaluate changes in body composition and other anthropometric and biochemical parameters following the LST regimen. Statistical significance was set at p < 0.05.
Results
Baseline characteristics
Demographic and baseline clinical characteristics are summarized in Table 1.
Table 1. Demographic and baseline clinical characteristics.
| n | 26 |
| Sex (men / Women) | 11 / 15 |
| Age (years) | 51.6 years) |
| Duration of disease (years) | 6.9 tion |
| Length of hospital stay (days) | 20.6 h of |
| Height (cm) | 161.9 (cm) |
| Weight (kg) | 87.6 t (kg) |
| Waist circumference (cm) | 106.5 circum |
| BMI (kg/m 2 ) | 33.4 kg/mc |
| Treatment | |
| Untreated | 5 |
| Oral hypoglycemic agents | 21 |
| Insulin | 5 |
| Anticholesteremic agents | 17 |
| Antihypertensive agents | 13 |
Data are expressed as mean ± SD. BMI: body mass index.
No patients dropped out of the training program. The 11 men and 15 women ranged in age from 27 to 75 years and all patients had BMI values > 25 kg/m2 (mean 33.4 ± 5.4 kg/m2). The majority (16/26, 62%) had HbA1c levels > 8.0%. Most patients (21/26) were currently receiving oral hypoglycemic agents and/or insulin injections, 17 were currently receiving lipid-lowering drugs, and 13 were currently receiving antihypertensive agents. The treatment for dyslipidemia was not changed during the study period. We changed hypoglycemic agents for glycemic control as needed. Daily metformin dosage was increased in 3 patients from 500 mg to 1000 mg, daily insulin glargine dosage was increased in 2 patients (from 15 units to 18 units and from 10 units to 16 units), daily 5 mg of linagliptin was added in one patient, daily 50 mg of miglitol was added in another patient. A patient started insulin therapy with 20 units of insulin glargine and taking daily 2 mg of glimepiride instead of decrease in daily metformin dosage from 1500 mg to 500 mg. In one patient, daily insulin glargine dosage was decreased from 36 units to 20 units. Antihypertensive drugs were changed in 2 patients. One patient started to take angiotensin II receptor blocker, daily 20 mg of olmesartan during the study period. The other patient changed to take an antihypertensive drug from daily 5 mg of amlodipine to daily 20 mg of olmesartan. After discharge, we instructed our patients to take diet directed by nutritional educators during hospitalization, however, some patients could not keep the diet therapy at home.
Changes in body composition, metabolic risk factors, and arterial stiffness
After 12 weeks of LST training, weight and BMI were significantly lower, although there was no significant change in WC. No significant change in skeletal muscle mass was found, but both body fat mass and body fat percentage decreased significantly, leading to a 4.6% increase in the ratio of lower extremity muscle mass to body weight (Table 2).
Table 2. Changes in body composition after LST training.
| Pre-training | Post-training | p | |
|---|---|---|---|
| Weight (kg) | 87.6 t (kg) | 85.4 t (kg) | 0.002 |
| Waist circumference (cm) | 106.5 circum | 103.1 circum | 0.184 |
| BMI (kg/m 2 ) | 33.4 kg/mc | 32.5 kg/mc | 0.002 |
| Body fat mass (kg) | 36.2 fat ma | 34.3 fat m | 0.021 |
| Body fat percentage (%) | 41.2 f 8.6 | 40.1 f 8.6 | 0.033 |
| Upper extremity muscle mass (kg) | 5.81 extre | 5.45 extre | 0.869 |
| Lower extremity muscle mass (kg) | 15.5 extr | 15.6 extr | 0.166 |
| Ratio of lower extremity muscle mass to body weight | 0.176 of lowe | 0.184 of lowe | 0.019 |
Data are expressed as mean ± SD. BMI: body mass index, LST: Low-intensity resistance training with slow movement and tonic force generation.
Plasma HbA1c levels, serum FFA, and Lp(a) were also significantly lower and serum HDL-C significantly was higher after LST training. However, there was no change in peripheral blood pressure, fasting plasma glucose, serum total cholesterol, TG, or LDL-C (Table 3).
Table 3. Changes in metabolic parameters.
| Pre-training | Post-training | p | |
|---|---|---|---|
| Plasma glucose (mg/dl) | 134.6 gluco | 151 6 glu | 0.407 |
| HbA1c (%) | 8.6 c (%) | 7.2 c (%) | 0.001 |
| Total cholesterol (mg/dl) | 181.3 choles | 182 3 chol | 0.378 |
| Triglycerides (mg/dl) | 153.9 ceride | 182.9 ceride | 0.284 |
| HDL cholesterol (mg/dl) | 42.2 hole | 46.3 holest | 0.002 |
| LDL cholesterol (mg/dl) | 108.9 oleste | 100.5 oleste | 0.667 |
| Free fatty acid (mEq/l) | 665.2 atty ac | 525.4 atty ac | 0.017 |
| Lipoprotein(a) (mg/dl) | 15.4 rote | 13.8 rote | 0.043 |
Data are expressed as mean ± SD. HDL: high-density lipoprotein, LDL: low-density lipoprotein.
We found no significant change in baPWV (1559 ± 280 cm/s to 1547 ± 292 cm/s, p = 0.484), AIx75 (80.1 ± 11.5% to 79.4 ± 14.7%, p = 0.682), or cSBP (135 ± 11 to 130 ± 22 mmHg, p = 0.266).
Discussion
In this study, a low-intensity resistance training program with slow movement and tonic force generation (LST) increased the ratio of lower extremity muscle mass to body weight, decreased the proportion of body fat, and improved lipid metabolism in obese patients with type 2 diabetes. Although skeletal muscle mass itself did not significantly increase, significant decreases were seen in weight (-2.2 kg), body fat mass (-2.1 kg), and body fat percentage (-1.1%). In sarcopenic obesity, muscle mass decreases while fat mass increases [19]. Thus, to detect sarcopenic obesity, it is necessary to measure not only weight, but also body composition. In addition, individuals with normal body weight but high body fat percentage are more likely to have metabolic syndrome [20] as well as higher CVD risk and all-cause mortality [21], indicating that both decreasing fat mass and increasing lean body mass are important aims in the treatment of metabolic syndrome. Our results also showed that LST training of the lower extremities was effective at maintaining muscle mass in obese patients with type 2 diabetes.
Obesity is associated with increased intramyocellular lipid accumulation in skeletal muscle [22–24] and usually with elevated serum FFA. Resistance training increases intramyocellular lipid oxidation during exercise [25,26], and lower extremity skeletal muscle is a major source of FFA uptake both at rest (15%−20%) and during exercise (30%−60%) [27]. Therefore, RT for enhancing lower extremity muscle mass is effective for improving FFA metabolism. In this study, even in the absence of increased muscle mass, serum FFA levels were significantly lower and HDL-C significantly higher after 12 weeks of LST training. Thus, even low-intensity RT without enhanced muscle mass can improve lipid metabolism in obese patients with type 2 diabetes.
A recent meta-analysis concluded that Lp(a) was an independent and modest risk factor for coronary heart disease and stroke [28]. Results of previous studies investigating the effect of exercise on Lp(a) concentration are controversial [29–34]. Rigla et al. reported a significant decrease in Lp(a) concentration in diabetic patients with higher baseline Lp(a) (> 9 mg/dl) after a 3-month physical exercise program [35], and Muls et al. reported that substantial weight loss in obese women resulted in lower Lp(a) concentration [36]. No previous studies have investigated the effect of low-intensity RT on Lp(a) concentration. To our knowledge, this study is the first to show that LST training also reduces Lp(a) concentrations in obese patients with type 2 diabetes. In the present study, Lp(a) concentrations significantly decreased with weight reduction and with an increased the L/W ratio. Further study is needed to elucidate the potential effects of LST training on Lp(a) concentrations.
High-intensity RT is known to be associated with increased arterial stiffness [37], although the influence of low-intensity RT on arterial stiffness remains unclear. We measured baPWV and AIx75 to evaluate arterial stiffness before and after 12 week-LST training. No significant changes were observed in these parameters, which suggests that LST training does not deteriorate arterial stiffness. Although the physiological mechanisms driving the increase in arterial stiffness by resistance training are not known, high-intensity RT stimulates sympathetic nervous system activity and increases arterial blood pressure [37]. LST training is different from high-intensity RT in that participants do not strain during training. It may be because LST training was performed without stimulation of the sympathetic nervous system and elevation of blood pressure that arterial stiffness showed no change. However, this lack of intensity has other benefits. For instance, four older participants (≥ 65 years) were able to perform the exercise program without injury, so this regimen may be suitable for sarcopenic obesity. LST training may have excellent cost-effectiveness because it does not require special instruments and the assistance of other people at home. In present study, the hospitalization for treatment of diabetes including the instruction of LST training cost 132,267 ± 55,554 yen ($1,102 ± 463). Coyle D et al. analyzed the data from the Diabetes Aerobic and Resistance Exercise (DARE) clinical trial and showed that resistance training program cost $38,300 [38]. Although we cannot simply compare the cost-effectiveness of our study with that of the DARE study because the DARE study is a 6 month-randomized controlled trial, the cost-effectiveness of LST training may be high.
This study has several limitations. First, the design of present study does not allow for any specific statement on the effect of LST training on lipid metabolism or body fat distribution because we did not investigate them in control group. Although we instructed our patients to continue the diet therapy after discharge, some patients could not keep the diet therapy, which may have confounded the results. The treatment for dyslipidemia was not changed during the study in all patients. The treatments for diabetes and hypertension were changed as needed, which could also confound our results. Second, we should have assessed insulin sensitivity to understand the associations of low-intensity resistance training with body composition and lipid profile. Third, we did not objectively evaluate patients' physical fitness or their muscle strength using a 1-repitition maximum test; we also did not standardize the training protocol. Forth, we did not supervise the participants engaging in LST training after discharge, so the degree of program compliance may have varied. The training duration was not standardized either. However, patients were instructed sufficiently during hospitalization (85% of the patients performed LST training six times or more) and none of them dropped out, suggesting that they found the routine feasible and that it could be used in the management of obese patients with type 2 diabetes in clinical practice. Since LST training was safe and feasible, we should investigate the effects of LST training in patients with physical disabilities in the future. To clarify the efficacy or non-inferiority to conventional recommended trainings, further studies, preferably randomized controlled trial should be needed in the future.
In conclusion, this study demonstrated that a 12-week LST training program can increase the ratio of lower extremity muscle mass to body weight, decrease body fat, and improve lipid metabolism in obese patients with type 2 diabetes. LST training may be a safe and feasible component of general management for obese patients with type 2 diabetes.
Supporting Information
(XLS)
Acknowledgments
This work was supported in part by a Grant-in-Aid for Research from the National Center for Global Health and Medicine (26A-201).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported in part by a Grant-in-Aid for Research from the National Center for Global Health and Medicine (26A-201). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Alley DE, Chang VW. The changing relationship of obesity and disability, 1988–2004. JAMA. 2007; 298(17): 2020–2027. [DOI] [PubMed] [Google Scholar]
- 2. Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin Nutr. 2012; 31(5): 583–601. 10.1016/j.clnu.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 3. Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008; 18(5): 388–395. 10.1016/j.numecd.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 4. Tuttle LJ, Sinacore DR, Mueller MJ. Intermuscular adipose tissue is muscle specific and associated with poor functional performance. J Ageing Res. 2012; 2012: 172957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yagi S, Kadota M, Aihara K, Nishikawa K, Hara T, Ise T, et al. Association of lower limb muscle mass and energy expenditure with visceral fat mass in healthy men. Diabetol Metab Syndr. 2014; 6(1): 27 10.1186/1758-5996-6-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hurley BF, Hanson ED, Sheaff AK. Strength training as a countermeasure to aging muscle and chronic disease. Sports Med. 2011; 41(4): 289–306. 10.2165/11585920-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 7. Braith RW, Stewart KJ. Resistance exercise training: its role in the prevention of cardiovascular disease. Circulation. 2006; 113(22): 2642–2650. [DOI] [PubMed] [Google Scholar]
- 8. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013; 36 Suppl 1: S11–S66. 10.2337/dc13-S011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strasser B, Siebert U, Schobersberger W. Resistance training in the treatment of the metabolic syndrome: a systematic review and meta-analysis of the effect of resistance training on metabolic clustering in patients with abnormal glucose metabolism. Sports Med. 2010; 40(5): 397–415. 10.2165/11531380-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 10. Tanimoto M, Ishii N. Effects of low-intensity resistance exercise with slow movement and tonic force generation on muscular function in young men. J Appl Physiol(1985). 2006; 100(4): 1150–1157. [DOI] [PubMed] [Google Scholar]
- 11. Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002; 25(12): 2335–2341. [DOI] [PubMed] [Google Scholar]
- 12. Misra A, Alappan NK, Vikram NK, Goel K, Gupta N, Mittal K, et al. Effect of supervised progressive resistance-exercise training protocol on insulin sensitivity, glycemia, lipids, and body composition in Asian Indians with type 2 diabetes. Diabetes Care. 2008; 31(7): 1282–1287. 10.2337/dc07-2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mavros Y, Kay S, Anderberg KA, Baker MK, Wang Y, Zhao R, et al. Changes in insulin resistance and HbA1c are related to exercise-mediated changes in body composition in older adults with type 2 diabetes: interim outcomes from the GREAT2DO trial. Diabetes Care. 2013; 36(8): 2372–2379. 10.2337/dc12-2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hills AP, Shultz SP, Soares MJ, Byrne NM, Hunter GR, King NA, et al. Resistance training for obese, type 2 diabetic adults: a review of the evidence. Obes Rev. 2010; 11(10): 740–749. 10.1111/j.1467-789X.2009.00692.x [DOI] [PubMed] [Google Scholar]
- 15. Examination Committee of Criteria for 'Obesity Disease' in Japan; Japan Society for the Study of Obesity. New criteria for 'obesity disease' in Japan. Circ J. 2002; 66(11): 987–992. [DOI] [PubMed] [Google Scholar]
- 16. Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957; 35(3): 307–315. [PubMed] [Google Scholar]
- 17. Anderson LJ, Erceg DN, Schroeder ET. Utility of multifrequency bioelectrical impedance compared with dual-energy x-ray absorptiometry for assessment of total and regional body composition varies between men and women. Nutr Res. 2012; 32(7): 479–485. 10.1016/j.nutres.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 18. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18: 499–502. [PubMed] [Google Scholar]
- 19. Kuh D, Bassey EJ, Butterworth S, Hardy R, Wadsworth ME; Musculoskeletal Study Team. Grip strength, postural control, and functional leg power in a representative cohort of British men and women: associations with physical activity, health status, and socioeconomic conditions. J Gerontol A Biol Sci Med Sci. 2005; 60(2): 224–231. [DOI] [PubMed] [Google Scholar]
- 20. Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab. 2004; 89(6): 2569–2575. [DOI] [PubMed] [Google Scholar]
- 21. Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010; 31(6): 737–746. 10.1093/eurheartj/ehp487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000; 49(4): 467–72. [DOI] [PubMed] [Google Scholar]
- 23. Hulver MW, Berggren JR, Cortright RN, Dudek RW, Thompson RP, Pories WJ, et al. Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Metab. 2003; 284(4): E741–E747. [DOI] [PubMed] [Google Scholar]
- 24. Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999; 277(6 Pt 1): E1130–E1141. [DOI] [PubMed] [Google Scholar]
- 25. Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GF, Hill RE, Grant SM. Effects of training duration on substrate turnover and oxidation during exercise. J Appl Physiol (1985). 1996; 81(5): 2182–2191. [DOI] [PubMed] [Google Scholar]
- 26. Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC, Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am J Physiol Regul Integr Comp Physiol. 2007; 292(3): R1271–R1278. [DOI] [PubMed] [Google Scholar]
- 27. Jensen MD. Fate of fatty acids at rest and during exercise: regulatory mechanisms. Acta Physiol Scand. 2003; 178(4): 385–390. [DOI] [PubMed] [Google Scholar]
- 28. Subramanian S, Chait A. Hypertriglyceridemia secondary to obesity and diabetes. Biochem Biophys Acta. 2012; 1821(5): 819–825. 10.1016/j.bbalip.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 29. Austin A1, Warty V, Janosky J, Arslanian S. The relationship of physical fitness to lipid and lipoprotein(a) levels in adolescents with IDDM. Diabetes Care. 1993; 16(2): 421–425. [DOI] [PubMed] [Google Scholar]
- 30. Hellsten G, Boman K, Hallmans G, Dahlén G. Lipids and endurance physical activity. Atherosclerosis. 1989; 75(1): 93–94. [DOI] [PubMed] [Google Scholar]
- 31. Hubinger L, Mackinnon LT. The effect of endurance training on lipoprotein(a) [Lp(a)] levels in middle-aged males. Med Sci Sports Exerc. 1996; 28(6): 757–764. [DOI] [PubMed] [Google Scholar]
- 32. Durstine JL, Ferguson MA, Szymanski LM, Davis PG, Alderson NL, Trost SG, et al. Effect of a single session of exercise on lipoprotein(a). Med Sci Sports Exerc. 1996; 28(10): 1277–1281. [DOI] [PubMed] [Google Scholar]
- 33. Hubinger L, Mackinnon LT, Barber L, McCosker J, Howard A, Lepre F. Acute effects of treadmill running on lipoprotein(a) levels in males and females. Med Sci Sports Exerc. 1997; 29(4): 436–442. [DOI] [PubMed] [Google Scholar]
- 34. Ponjee GA, Janssen EM, van Wersch JW. Long-term physical exercise and lipoprotein(a) levels in a previously sedentary male and female population. Ann Clin Biochem. 1995; 32(Pt 2): 181–185. [DOI] [PubMed] [Google Scholar]
- 35. Rigla M, Sánchez-Quesada JL, Ordóñez-Llanos J, Prat T, Caixàs A, Jorba O, et al. Effect of physical exercise on lipoprotein(a) and low-density lipoprotein modifications in type 1 and type 2 diabetic patients. Metabolism. 2000; 49(5): 640–647. [DOI] [PubMed] [Google Scholar]
- 36. Muls E, Kempen K, Vansant G, Cobbaert C, Saris W. The effects of weight loss and apolipoprotein E polymorphism on serum lipids, apolipoproteins A-I and B, and lipoprotein(a). Int J Obes Relat Metab Disord. 1993; 17(12): 711–716. [PubMed] [Google Scholar]
- 37. Miyachi M. Effects of resistance training on arterial stiffness: a meta-analysis. Br J Sports Med. 2013; 47(6): 393–396. 10.1136/bjsports-2012-090488 [DOI] [PubMed] [Google Scholar]
- 38. Coyle D, Coyle K, Kenny GP, Boulé NG, Wells GA, Fortier M, et al. Cost-effectiveness of exercise programs in type 2 diabetes. Int J Technol Assess Health Care. 2012; 28(3): 228–234. 10.1017/S0266462312000256 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.