SUMMARY
Salmonella enterica infections are common causes of bloodstream infection in low-resource areas, where they may be difficult to distinguish from other febrile illnesses and may be associated with a high case fatality ratio. Microbiologic culture of blood or bone marrow remains the mainstay of laboratory diagnosis. Antimicrobial resistance has emerged in Salmonella enterica, initially to the traditional first-line drugs chloramphenicol, ampicillin, and trimethoprim-sulfamethoxazole. Decreased fluoroquinolone susceptibility and then fluoroquinolone resistance have developed in association with chromosomal mutations in the quinolone resistance-determining region of genes encoding DNA gyrase and topoisomerase IV and also by plasmid-mediated resistance mechanisms. Resistance to extended-spectrum cephalosporins has occurred more often in nontyphoidal than in typhoidal Salmonella strains. Azithromycin is effective for the management of uncomplicated typhoid fever and may serve as an alternative oral drug in areas where fluoroquinolone resistance is common. In 2013, CLSI lowered the ciprofloxacin susceptibility breakpoints to account for accumulating clinical, microbiologic, and pharmacokinetic-pharmacodynamic data suggesting that revision was needed for contemporary invasive Salmonella infections. Newly established CLSI guidelines for azithromycin and Salmonella enterica serovar Typhi were published in CLSI document M100 in 2015.
INTRODUCTION
Salmonella enterica is a leading cause of community-acquired bloodstream infections in many low- and middle-income countries (1, 2). Salmonella enterica serovars Typhi, Paratyphi A, Paratyphi B, and Paratyphi C may be referred to collectively as typhoidal Salmonella, whereas other serovars are grouped as nontyphoidal Salmonella (NTS). Typhoidal Salmonella strains are human host-restricted organisms that cause typhoid fever and paratyphoid fever, together referred to as enteric fever. NTS strains may be host generalists, infecting or colonizing a broad range of vertebrate animals, or may be adapted or restricted to particular nonhuman animal species (3).
We review invasive Salmonella infections with respect to epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management. In particular, we focus on the development of antimicrobial resistance and recent changes to the interpretation of antimicrobial susceptibility tests for fluoroquinolones and to establishment of methods and interpretive criteria for azithromycin.
EPIDEMIOLOGY AND CLINICAL ASPECTS
Typhoidal Salmonella
Burden of disease.
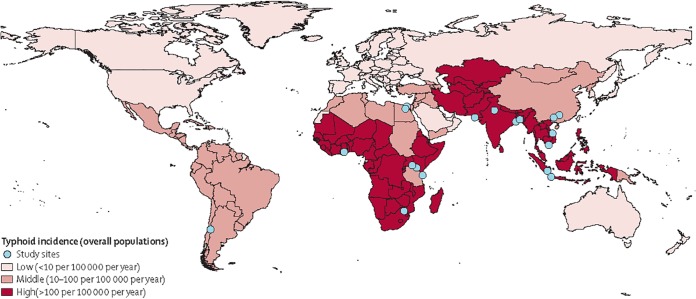
In 2000, typhoid fever was estimated to cause approximately 21.7 million illnesses and 216,000 deaths and paratyphoid fever 5.4 million illnesses (4). Typhoid and paratyphoid fevers were included in the Global Burden of Disease 2010 (GBD 2010) project, when they were together estimated to account for 12.2 million disability-adjusted life years (5) and 190,200 deaths (6). Children in south-central and southeast Asia are at particular risk (4). The International Vaccine Institute estimated that there were 11.9 million typhoid fever illnesses and 129,000 deaths in low- and middle-income countries in 2010 (Fig. 1) (7). Typhoid fever appears to have become more common in sub-Saharan African countries (8) or to have been underappreciated there in the past (9). In some Asian countries, Salmonella serovar Paratyphi A has accounted for a growing proportion of enteric fever (10, 11).
FIG 1.
Typhoid incidence in low-income and middle-income countries (risk adjusted and corrected for blood culture sensitivity). (Reprinted from reference 7 with permission from Elsevier.)
Sources and modes of transmission.
Typhoidal Salmonella is transmitted predominantly through water or food contaminated with human feces. The risk for infection is high in low- and middle-income countries where typhoidal Salmonella is endemic and that have poor sanitation and lack of access to safe food and water (4). Enteric fever in high-income countries is usually acquired abroad and is associated with travel to areas of endemicity (12), although clusters may be associated with food preparers who are chronic carriers of Salmonella serovar Typhi (13).
Host risk and protective factors.
A range of host risk and protective factors have been identified for typhoidal Salmonella infection. Salmonella enterica is acid susceptible and must survive the gastric acid barrier to successfully establish infection in the terminal ileum. Gastric acid secretion has been shown to be suppressed during acute enteric fever, subsequently returning to normal and with the degree of acid suppression relating to the infection severity (14, 15). The acid tolerance of the organism may be an important determinant of transition to the small intestine and can vary with the infecting serovar (16).
Past infection with Helicobacter pylori has been suggested to be associated with typhoid fever, perhaps because both diseases are associated with reduced gastric acidity. In a case-control study in India, the presence of serum anti-H. pylori immunoglobulin G antibodies was associated with typhoid fever with an adjusted odds ratio (OR) of 2.03 (95% confidence interval [CI], 1.02 to 4.01) (17). In this study, illiteracy, being part of a nuclear family, nonuse of soap, and consumption of ice cream were also associated with an increased risk of typhoid. H. pylori IgG antibodies develop 1 to 3 months after acute infection and so could indicate either active or previous infection. In a similar case-control study done in Jakarta, Indonesia, with an age-stratified analysis, the level of H. pylori IgG but not IgA antibody was higher in typhoid fever patients than in community controls (18). Furthermore, plasma gastrin levels, indicative of hypochlorhydria, were not significantly elevated in typhoid fever cases compared to controls. In a multivariable analysis, there was an association of H. pylori IgG seropositivity with typhoid fever with an odds ratio of 1.93 (95% CI, 1.10 to 3.40). However, the authors suggested that the association may result from common environmental exposure to poor hygiene rather than implying a causal relationship through reduced gastric acid secretion.
A limited number of studies have demonstrated host genetic factors that influence susceptibility to enteric fever. The cystic fibrosis transmembrane conductance regulator (CFTR) is a protein expressed on the gastric mucosa. In vitro experiments have shown that the wild-type protein facilitates adherence and entry of Salmonella serovar Typhi, but not Salmonella serovar Typhimurium, into intestinal epithelial cells (19). This binding and entry are mediated by an interaction between Salmonella serovar Typhi lipopolysaccharide (LPS) and type IVb pilus and CFTR protein residues (20, 21). Expression of CFTR on the intestinal epithelium is stimulated by the presence of Salmonella serovar Typhi and commensal bacteria in the intestine (22, 23). Mutations in CFTR, such as F508del, are associated with cystic fibrosis. In the presence of this mutation there is no uptake of Salmonella serovar Typhi into intestinal epithelial cells, and in heterozygotes uptake into cells is reduced (19). Thus, the F508del mutant may provide protection against infection following exposure to Salmonella serovar Typhi. A case-control study in Jakarta, Indonesia, of mutations in the CFTR allele and enteric fever found no participants with the F508del mutation. It is possible that variations in CFTR other than F508del may provide protection against enteric fever. A microsatellite polymorphism in intron 8, IVS8CA, of the CFTR gene was associated with protection from enteric fever (P = 0.003) (24). In a further analysis of additional regions, the presence of one or more protein-expressing variations, including the IVS8 TG11TG12 genotype, provided a modest protection from enteric fever (OR, 0.57; P < 0.01) (25).
Immune defense against enteric fever is likely to depend in part at least on cellular immunity. A study in Vietnam showed associations between major histocompatibility complex (MHC) class II and class III genes and enteric fever (26). Alleles of HLA-DR, HLA-DQ, and the proinflammatory gene TNFA were associated with either resistance or susceptibility to enteric fever among hospitalized patients. Circulating levels of both tumor necrosis factor alpha (TNF-α) and soluble TNF receptor have been reported to be elevated in enteric fever and higher in severe disease (27, 28). The capacity to secrete TNF-α following ex vivo stimulation has been shown to be reduced in the acute phase of typhoid fever, with the degree correlating with severity of disease and delayed recovery (28, 29). The association with susceptibility to enteric fever and the polymorphism in the tumor necrosis factor alpha gene (TNFA-308) could not be replicated in an Indonesian population with community-diagnosed enteric fever, and there were no associations with polymorphisms in a number of other proinflammatory genes (30). A possible explanation for the discrepancy in the results is that the association of TNFA polymorphisms is with disease severity rather than susceptibility to infection. Of interest, HLA-DR haplotypes were associated with protection from severe enteric fever among Indonesian patients, although this could not be linked with TNF-α production capacity (31). Further analysis of the Vietnamese population again suggested that a haplotype in the TNF region gives protection against enteric fever, but the causative disease locus remains to be determined (32). In a recent genome-wide association study of patients in Vietnam and Nepal with blood culture-confirmed enteric fever, a strong association was found for HLA-DRB1 as a major contributor to resistance against enteric fever, presumably through antigen presentation (33).
Toll-like receptors (TLRs) mediate the innate immune responses to bacterial pathogens. TLR5 binds to bacterial flagellin and TLR4 to LPS. Among Vietnamese patients with enteric fever there were no significant associations with the TLR5392STOP polymorphisms (34). There were also no associations with TLR4 polymorphism. However, because of low gene frequencies, the sample may have been inadequate to give a definitive answer (35). In the mouse model of typhoid fever, control of Salmonella serovar Typhimurium infection is dependent on the natural resistance-associated macrophage protein 1 (Nramp1), but in Vietnamese patients with enteric fever, there was no allelic association between the NRAMP1 alleles and typhoid fever susceptibility (36). Polymorphisms in the PARK2/PACRG gene cluster, linked to ubiquitination, and proteasome-mediated protein degradation previously found to be associated with susceptibility to infection with Mycobacterium leprae were weakly associated with susceptibility to enteric fever in an Indonesian population (37).
The relationship between HIV infection and enteric fever has not been studied in detail. In the early 1990s, Peruvian patients with HIV infection were reported to be at increased risk of Salmonella serovar Typhi disease (38). Subsequent reports in Africa and Asia highlighted the association of nontyphoidal Salmonella serovars, rather than Salmonella serovar Typhi, with HIV (1, 3). In a study of unselected hospitalized adults with fever in Tanzania, HIV appeared to be protective against Salmonella serovar Typhi infection (odds ratio, 0.12; 95% CI, 0.03 to 0.49; P = 0.001) (39). The 26 positive Salmonella serovar Typhi cultures were taken from 24 (9.8%) of 244 HIV-uninfected patients and two (1.2%) of 161 HIV-infected patients. A similar inverse association between typhoid and HIV infection was noted in a meta-analysis of bloodstream infection studies from Africa (1). The use of hospitalized patients may be a source of bias, and the relationship requires further study.
Presenting symptoms and signs.
In areas of endemicity, patients admitted to hospital are usually school-aged children or young adults between 5 and 25 years of age, and both sexes are affected equally (40–42). Many patients do not require admission to hospital, due to either mild disease, self-medication, or being treated in health stations, clinics, or as hospital outpatients (43–45). These community-managed cases may be of nonspecific illness that is not recognized clinically as enteric fever, especially among children under 5 years of age (46–48).
After ingestion of Salmonella serovar Typhi or Paratyphi A, an asymptomatic period follows that usually lasts 7 to 14 days (range, 3 to 60 days). Human challenge models, both in the 1950s to 1970s (49) and more recently (50), contributed to the understanding of incubation and very early symptoms in typhoid fever. These studies have shown that a higher infecting dose is associated with a higher attack rate and a shorter interval to bacteremia but has no influence on the time to symptom development or disease severity. Recent human challenge studies have also demonstrated that a proportion of patients develop a subclinical or asymptomatic bacteremia and that fecal shedding can occur in the period before symptom development, during primary infection (51). As symptomatic disease develops, the predominant symptom is the fever (40–42, 52). The temperature rises gradually during the first week of the illness and reaches a high plateau of 39 to 40°C the following week. There is little diurnal variation, although the pattern may be modified by anti-pyretic medications. Patients can have influenza-like symptoms, a dull frontal headache, malaise, anorexia, a dry cough, sore throat, and occasionally epistaxis. Constipation is a frequent early symptom although many patients will experience diarrhea at some point. Enteric fever can present as a diarrheal illness and occasionally with bloody diarrhea. Most patients have abdominal pain that is diffuse and poorly localized. Nausea is common, and vomiting occurs in more severe cases. It is unusual for a patient hospitalized with typhoid to have no abdominal symptoms and normal bowel movements. Rigors are uncommon and this can be a useful feature to distinguish the illness from malaria (53).
Besides fever, physical examination findings may be few. A slightly distended abdomen with a “doughy” consistency and diffuse tenderness is common. Occasionally the pain and tenderness is intense in the right iliac fossa, mimicking appendicitis, or may be more generalized, raising the possibility of peritonitis. Moderate soft and tender hepatomegaly and splenomegaly eventually develop in most patients. A relative bradycardia is described as being common in enteric fever, although some reports suggest that a tachycardia is more common and that a relative bradycardia, when present, is not specific for enteric fever (54–57). Rose spots, a blanching erythematous maculopapular rash with lesions approximately 2 to 4 mm in diameter, have been reported in 1 to 30% of cases (Fig. 2) (41, 58). They usually occur on the abdomen and chest and more rarely on the back, arms, and legs. Rose spots are easily missed in dark-skinned patients. Abnormal lung sounds, especially scattered wheezes, are common and can suggest pneumonia, but if the chest radiograph is normal and fever high, enteric fever should be considered. There may be a history of intermittent confusion, and many patients have a characteristic apathetic affect. Important differences in children, compared to adults, are a greater frequency of diarrhea and vomiting, jaundice, febrile convulsions, nephritis, or typhoid meningitis (59–62). Enteric fever may also complicate pregnancy and rarely cause neonatal infection (63, 64).
FIG 2.
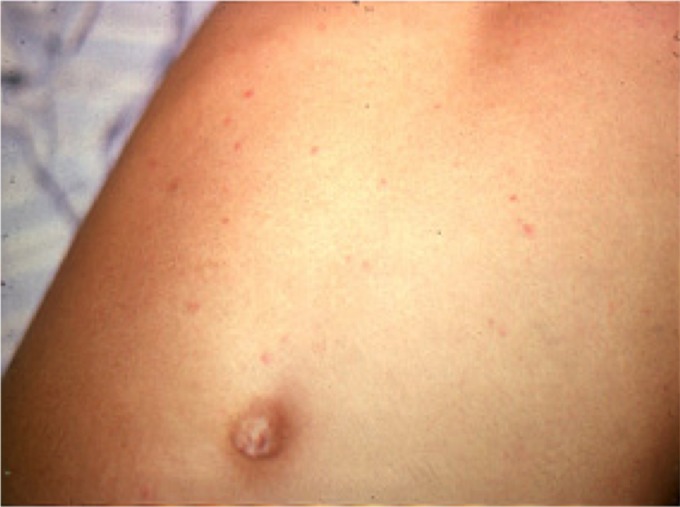
Rose spots on the abdomen of a patient with typhoid fever. (Reprinted from reference 603.)
If the disease is untreated, by the second to fourth week patients may become increasingly sick with weight loss, weakness, and an altered mental state, and complications develop (40–42). Complications occur in 10 to 15% of hospitalized patients and are particularly likely in patients who have been ill for more than 2 weeks. Many complications have been described (Table 1), of which gastrointestinal bleeding, intestinal perforation, and typhoid encephalopathy are most closely associated with risk for death (65–67). Other complications of enteric fever include psychiatric disturbance and pneumonia. Paratyphoid fever has been described as a less severe infection than typhoid fever. However, recent studies suggest that Salmonella serovar Paratyphi A, an increasing problem in many areas in Asia (10, 11), can cause a disease with severity equal to that of Salmonella serovar Typhi (68).
TABLE 1.
Complications of enteric fever
| Complication |
|---|
| Gastrointestinal bleeding |
| Intestinal perforation (usually of terminal ileum, occasionally of colon) |
| Encephalopathy accompanied by hemodynamic shock |
| Hepatitis |
| Cholecystitis |
| Pneumonia (may be due to secondary infection with other organisms such as Streptococcus pneumoniae) |
| Myocarditis |
| Acute kidney injury, nephritis |
| Deep-seated abscess (e.g., spleen, large joint, bone) |
| Anemia |
| Meningitis (in infants) |
| Neurological disturbance (cerebellar ataxia) |
| Miscarriage |
| Psychiatric disturbance |
| Disseminated intravascular coagulation |
| Relapse |
| Chronic carriage (fecal or urinary carriage for >1 yr) |
| Carcinoma of gallbladder |
Patient outcomes.
(i) Complications.
Gastrointestinal bleeding resulting from the erosion of a necrotic ileal Peyer's patch through the wall of an enteric vessel may develop in up to 10% of hospitalized patients (41, 69, 70). Bleeding is usually slight and self-limited, resolving without the need for blood transfusion. However, in some cases bleeding is substantial, and it can be rapidly fatal when a large vessel is involved. Evidence for silent gastrointestinal bleeding may be sudden collapse of the patient or a steadily falling hematocrit.
Intestinal perforation, usually involving the ileum but occasionally involving the colon, is a serious complication of enteric fever (41, 69–74). This may occur in 1 to 3% of hospitalized patients (41, 70, 75) and be manifest by an acute abdomen or more insidiously by worsening abdominal pain, a rising pulse, and falling blood pressure in an already-sick patient. Severely ill patients often display restlessness, hypotension, and tachycardia. A chest radiograph may show free gas under the diaphragm. Abdominal ultrasound is useful for demonstrating and aspirating feculent fluid in the peritoneal cavity.
Studies from Indonesia and Papua New Guinea have revealed an important subgroup of patients with a high case fatality ratio associated with mental confusion or shock (systolic blood pressure of <90 mm Hg in adults or <80 mm Hg in children) and with evidence of decreased skin, cerebral, or renal perfusion (76–78). The mental state of the patient may range from apathetic although rousable to severely agitated, delirious, or obtunded. Complete stupor or coma is infrequent. Patients with advanced illness may display the “typhoid facies,” described as a thin, flushed face with a staring, apathetic expression. Mental apathy may progress to an agitated delirium, frequently accompanied by hand tremor, tremulous speech, and gait ataxia. If the patient's condition deteriorates further, the features described in the writings of Louis and Osler may occur, including muttering delirium, twitching of the fingers and wrists, agitated plucking at the bedclothes, and a staring and unrousable stupor, also known as coma vigil (40).
A wide range of other complications has been described. The most common include cholecystitis, hepatitis, pneumonia, acute kidney injury, and myocarditis (69, 70). Typhoid fever during pregnancy may be complicated by miscarriage but appears to be mitigated by antimicrobial treatment (63). Mother-to-child transmission may lead to neonatal typhoid, a rare but severe and life-threatening illness (64, 79). Meningitis occurs among children below 1 year of age (62).
In the preantimicrobial era, enteric fever carried a case fatality ratio of approximately 10 to 30% (40, 41, 80). With effective antimicrobials, the case fatality ratio is usually less than 1%. Reported case fatality ratios have varied from less than 2% in Pakistan (59) and Vietnam (66, 70) to 30 to 50% in some areas of Indonesia (76, 77) and Papua New Guinea (78). Severe and fatal disease has been associated with both male and female sex, extremes of age, and antimicrobial drug resistance (59, 65, 67, 70, 73, 81). The most important contributor to a poor outcome is probably a delay in instituting effective antimicrobial treatment.
(ii) Relapse and reinfection.
Typhoid fever relapse is manifest by a second episode of fever, often but not always milder than the first, occurring 1 week to 3 weeks after the recovery from the first episode (52). Relapse may occur in as many as 5 to 10% of untreated or chloramphenicol-treated typhoid patients. Typically relapsing patients have an isolate with an antimicrobial susceptibility pattern identical to that during the first episode and can be managed with the same drug (82, 83). Reinfection has also been described and is distinguished by differences in the susceptibility pattern, Vi phage type, or molecular type of isolates (82–84).
(iii) Fecal shedding and chronic carriage.
Most patients with acute enteric fever continue to excrete Salmonella serovar Typhi or Paratyphi A in their stool or urine for some days after starting antimicrobial treatment, and up to 10% may do so for up to 3 months. Approximately 1 to 4% of patients still excrete the organism at 3 months and are unlikely to cease shedding. Those excreting at 1 year meet the formal definition of “chronic carrier.” Among carriers detected by screening, one-quarter give no history of acute typhoid fever. Fecal carriage is more frequent among individuals with gallbladder disease and is most common among women over 40 years of age. In the Far East there is an association between fecal carriage and opisthorchiasis, and urinary carriage is associated with schistosomiasis and nephrolithiasis. Most chronic carriers have no symptoms, although acute typhoid fever has been reported among carriers on occasions. Chronic carriage can occur with both Salmonella serovar Typhi and Paratyphi A (85). Chronic Salmonella serovar Typhi carriers have an increased risk of carcinoma of the gallbladder (86, 87). In one of the few attempts to measure the prevalence of Salmonella serovar Typhi carriage in a population, in Santiago, Chile, in 1980, there were estimated to be 694 chronic carriers per 100,000 population (88).
The detection of chronic carriers depends on the labor-intensive isolation of the bacteria from stool or urine. Excretion can be intermittent, and at least three negative stool cultures are required for patients to be considered free of infection (89). Identification of carriers of Salmonella serovar Typhi can be accomplished by serial stool culture. The detection of IgG to the Vi antigen has been proposed as a method to detect chronic carriers, and the test has proved valuable in the context of outbreak investigations (90–93). Its role in detecting carriers in the general population of areas of endemicity where background levels of IgG to Vi may be high, or where the Vi vaccine is widely used, is less clear (94). Nucleic acid amplification tests of gallbladder contents may be a diagnostic addition in the future (95).
Prevention and control.
Enteric fever in Western Europe and North America declined in parallel with the introduction of treatment of municipal water, pasteurization of dairy products, and exclusion of human feces from food production (96). More recently declines have occurred in Latin America (4) and in some Asian countries (97) in parallel with economic transition and water and sanitation improvements. Strategies for enteric fever prevention include improving sanitation, ensuring the safety of food and water supplies, identification and treatment of chronic carriers of Salmonella serovar Typhi, and the use of typhoid vaccines to reduce the susceptibility of hosts to infection or disease. Reducing the proportion of people without access to safe drinking water is a component of Millenium Development Goal 7 (98).
Two typhoid vaccines are currently available in the United States. The Ty21a vaccine is a live, attenuated, oral vaccine containing the chemically attenuated Salmonella serovar Typhi strain Ty21a, and the parenteral Vi vaccine is based on the Salmonella serovar Typhi Vi capsular polysaccharide antigen. Ty21a is available as orally administered enteric capsules and is licensed in the United States for use in children ≥6 years of age and elsewhere for children as young as 2 years of age. The Vi-based vaccine is licensed in the United States for children aged ≥2 years. The cumulative protective efficacy over 3 years has been estimated to be 48% for Ty21a and 55% for Vi polysaccharide vaccine in a Cochrane systematic review (99). The effectiveness of the parenteral Vi vaccine has recently been confirmed in young children, and the protection of unvaccinated neighbors of Vi vaccinees has been demonstrated (100). The earliest Vi conjugate vaccine to reach clinical trials, Vi-rEPA, has been shown to be safe and immunogenic in Vietnamese children aged 2 to 5 years, providing protective efficacy of 91.5% (101) and an estimated 89% cumulative efficacy after 3.8 years (99). Phase 2 studies of other Vi conjugate vaccines have been completed (102). A number of Vi conjugate constructs are now in development. However, Vi-based monovalent vaccines do not offer protection against most paratyphoid fever, because only Salmonella serovars Typhi, Paratyphi C, and Dublin carry the Vi antigen. Salmonella serovar Typhi Ty21a does not express the Vi antigen, and clinical field trials suggest that while Ty21a may provide limited protection against Salmonella serovar Paratyphi B, it does not offer cross-protection against Salmonella serovar Paratyphi A (103, 104). The development of effective vaccines for paratyphoid fever is an important priority.
Nontyphoidal Salmonella
Burden of disease.
In industrialized countries, nontyphoidal Salmonella is transmitted predominantly by commercially produced food contaminated by animal feces, and it usually causes a self-limited enterocolitis with diarrhea in humans. Bloodstream infection occurs in approximately 6% of patients with diarrheal enterocolitis; infants, young children, the elderly, and the immunocompromised are at particular risk for bacteremia (105–108). Nontyphoidal Salmonella serovars are diverse in their host range and epidemiology and vary in their propensity to cause bloodstream infection and severe human disease. Salmonella serovar Typhimurium is considered to be a typical host generalist with a broad host range and modest likelihood of causing invasive disease, while other nontyphoidal Salmonella serovars such as Salmonella serovars Heidelberg, Dublin, and Choleraesuis are markedly more likely than Salmonella serovar Typhimurium to cause hospitalization, invasive disease, or death. Some serovars, such as Salmonella serovar Newport, are associated with a lower case fatality ratio (0.3%) than Salmonella serovar Typhimurium (108, 109). The reasons for differences in host specificity among Salmonella serovars are complex and incompletely understood. Genome degradation appears to be associated with host specificity in Salmonella Typhi and Salmonella Paratyphi A, which have absent and deactivated genes compared with Salmonella Typhimurium. In addition, typhoidal Salmonella serovars elicit a dampened immune response compared with nontyphoidal Salmonella serovars associated with diarrhea and also produce a unique exotoxin, which may explain differences in the clinical phenotype (110, 111).
Modes of nontyphoidal Salmonella transmission in low- and middle-income countries are less well understood than in industrialized countries. Malaria and malnutrition predispose to invasive disease among children, and HIV does so among both children and young adults (3). Worldwide in 2006, enteric NTS was estimated to cause 93.8 million diarrheal illnesses and 155,000 deaths (112). In the Institute for Health Metrics and Evaluation Global Burden of Disease 2010 project, enteric nontyphoidal Salmonella was estimated to account for 4.8 million disability-adjusted life years (5) and 81,300 deaths (6). In 2010, nontyphoidal Salmonella was estimated to cause approximately 3.4 million invasive infections and 681,000 deaths; 57% of these illnesses and deaths occurred in Africa (113).
The most widely reported serovars associated with invasive disease across Africa are Salmonella serovar Typhimurium and Salmonella serovar Enteritidis (114–123). Some serovars are prominent in localized areas, such as Salmonella serovar Concord in Ethiopia (124), Salmonella serovar Bovismorbificans in Malawi (125), Salmonella serovar Stanleyville and Salmonella serovar Dublin in Mali (126), and Salmonella serovar Isangi in South Africa (127), the last being associated with a nosocomial outbreak.
A novel Salmonella serovar Typhimurium multilocus sequence type, ST313, has been described and currently accounts for much invasive disease in sub-Saharan Africa. This sequence type has a unique prophage repertoire and a degraded genome that shows some convergence with that of Salmonella serovar Typhi, raising the possibility of increased host specialization or invasiveness (128). One putative virulence gene, ST313td, has been described in Salmonella serovar Typhimurium ST313 and also found in other human invasive and enteric pathovars, notably Salmonella serovar Dublin (129). However, to date no other candidate virulence genes have been associated with invasive nontyphoidal Salmonella strains from HIV-infected patients (130). Ongoing genomic and phenotypic investigations of Salmonella serovar Typhimurium ST313 from Africa may be an area promising further new insights.
Sources and modes of transmission.
While sources and modes of transmission of enteric nontyphoidal Salmonella have been studied extensively in industrialized countries (131), epidemiologic studies of nontyphoidal Salmonella infections in areas of endemicity in sub-Saharan Africa are very limited (132). In industrialized countries, animal products and, increasingly, produce contaminated with animal feces are important sources of nontyphoidal Salmonella (131). Contact with animals, such as reptiles, and with animal environments are important sources of nontyphoidal Salmonella infection not associated with food (133). Although transmission by food contaminated with animal feces must be considered, greater roles than in industrialized countries for waterborne transmission, transmission directly from animals and their environments, and transmission between people, independent of a nonhuman animal reservoir, have been hypothesized. Partial genome molecular studies of nontyphoidal Salmonella strains from invasive human infections and those carried by animals among the households of children with invasive disease in Africa have so far failed to find similarities between strains, while family members of index cases have been found to have more closely related isolates (134, 135). Although it was hypothesized that the degraded genome of Salmonella serovar Typhimurium ST313 might reflect a reduced host range and human restriction of the sequence type, recent studies have indicated that ST313 also displays a severe invasive phenotype in chickens but reduced potential for cecal colonization (136). The emergence of two distinct clades of Salmonella serovar Typhimurium ST313 across Africa shows temporal relationships to acquired antimicrobial resistance determinants, particularly to the first-line antimicrobial chloramphenicol, and to the emergence of HIV on the continent (137). This suggests that transmission among humans may have exerted genomic selection pressure. Little is known about the epidemiology or phenotype of prevalent enteric nontyphoidal Salmonella strains in Africa. Nontyphoidal Salmonella was not a common cause of moderate to severe diarrheal disease in African sites participating in the Global Enterics Multicenter Study (138). However, asymptomatic carriage of nontyphoidal Salmonella appears to be relatively common (134, 138). The contribution to asymptomatic carriage or diarrheal disease in Africa of Salmonella serovar Typhimurium ST313 is still unclear, although the diversity of nontyphoidal Salmonella strains from enteric samples is likely to be wide (139).
Host risk and protective factors.
Previous gastric surgery, pernicious anemia, and medications that reduce the acid barrier, such as antacids, H2 antagonists, and proton pump inhibitors, increase susceptibility to nontyphoidal Salmonella enteric infection (140–144). Individuals at the extremes of age are at increased risk of invasive nontyphoidal Salmonella. In the case of older people, this may be because of multiple comorbidities, including diabetes, renal disease, or medications. The recent use of antimicrobials is associated with increased risk of multidrug-resistant Salmonella infection (145).
In Africa, there is a clear bimodal age distribution that contrasts with the continuous age distribution through childhood to adulthood of typhoid fever. Children aged from 6 to 18 months and adults aged 25 to 40 years, in whom HIV prevalence is also highest, show the highest incidences of invasive nontyphoidal Salmonella in Africa (146). Among children, there is a relatively low incidence of invasive nontyphoidal Salmonella below the age of 6 months. This may be attributable to protection afforded by breastfeeding, by lack of exposure to contaminated water or food during exclusive breastfeeding, or by transplacental transfer of protective IgG (147). However, neonatal invasive nontyphoidal Salmonella does occur, particularly among children born outside a health care facility (148, 149).
Unlike enteric fever, for which where there are no clear clinical associations with classic immunocompromising conditions, invasive nontyphoidal Salmonella is associated with many forms of immunocompromise (150). These include disorders of oxidative cellular killing, such as chronic granulomatous disease, in which nontyphoidal Salmonella is described as the most common cause of bloodstream infection and the third leading cause of all infections (151). Children who are homozygous for sickle cell disease are susceptible to invasive nontyphoidal Salmonella infections (152). In addition, inherited deficiencies of cytokines that are known to be critical for intracellular killing, particularly interleukin-12 (IL-12) and IL-23, are associated with invasive nontyphoidal Salmonella (153).
There is an overwhelming association of invasive nontyphoidal Salmonella with advanced HIV disease among African adults, with >95% of cases being HIV infected (1, 122, 150, 154–157). Among children, HIV is a risk factor (OR, 3.2) for infection with NTS (158). In cohorts of African children with invasive nontyphoidal Salmonella, around 20% are typically HIV infected (116). Several immune defects have been described that could contribute to the apparent exquisite susceptibility of adults with advanced HIV to recurrent invasive nontyphoidal Salmonella. These include the loss of IL-17-producing CD4 cells in the gut mucosa, permitting rapid invasion (159), and dysregulated excess production of anti-LPS IgG that inhibits serum killing of extracellular Salmonella in a concentration-dependent fashion (160, 161). Nontyphoidal Salmonella establishes an intracellular niche during invasive disease in HIV infection (162). It is likely that this is facilitated by the ability of the bacteria to be rapidly internalized before serum killing can occur (163). Once in the intracellular niche, reduction and dysregulation of proinflammatory cytokine responses in HIV-infected individuals, observed in vivo (162, 164) and ex vivo (165), allow intracellular survival and persistence, leading to frequent recrudescence and relapses of bacteremia with identical strains of nontyphoidal Salmonella (154, 166). In contrast with HIV-infected adults, a lack of protective antibody appears to be implicated in the susceptibility of African children <18 months of age to invasive nontyphoidal Salmonella disease, and antibody is likely to be important both for cellular and cell-free control of nontyphoidal Salmonella disease in children (147, 167, 168).
An association of invasive nontyphoidal Salmonella with malaria among African children was first noted in 1987 in West Africa (117). Since then, recent malaria (116), acute severe malaria (169), and severe malarial anemia, but not cerebral malaria (170), have all been specifically described as risk factors for invasive nontyphoidal Salmonella. The co-relationship of malaria with invasive nontyphoidal Salmonella among children has also been described both spatially, contrasting high- and low-incidence areas for malaria (171, 172), and temporally, with both rising (172) and falling (173, 174) background incidences of malaria being associated with corresponding changes in the incidence of invasive nontyphoidal Salmonella. However, this association is not necessarily specific for invasive nontyphoidal Salmonella, and a strong association of malaria with all bacteremias has been noted in Kenyan children, where the bacteremia incidence rate ratio associated with malaria parasitemia is 6.69 (175). A causal relationship with malaria was inferred by a reduced odds ratio for sickle cell trait, which is protective against malaria, among children with bacteremia, particularly due to Gram-negative organisms (175).
Malnutrition is also associated with invasive nontyphoidal Salmonella in African children (116, 176). In rural Kenya, nontyphoidal Salmonella bacteremia was associated with child malnutrition, with an odds ratio (95% confidence interval) of 1.68 (1.15 to 2.44) (158). Invasive nontyphoidal Salmonella disease is strongly seasonal among both adults and children, coinciding with the rainy season (114). It is not clear whether this reflects waterborne transmission, seasonal malaria transmission during periods of increased rainfall, associated food scarcity and malnutrition, or a combination of these factors.
Presenting symptoms and signs.
The clinical presentation of invasive nontyphoidal Salmonella infection is nonspecific among both children and adults. Therefore, recognition and management are challenging, particularly in settings without facilities for the laboratory diagnosis of bloodstream infection. Invasive nontyphoidal Salmonella presents as a febrile illness. Respiratory symptoms are frequently present, and diarrhea is often not a prominent feature. However, often the clinician is faced with a febrile patient without an obvious clinical focus of infection. Features on physical examination include abnormal respiratory findings, such as rapid respiratory rate or chest crepitations suggestive of pneumonia, and hepatosplenomegaly in 30 to 45% of cases (116, 154, 177, 178). Splenomegaly has been shown to be a useful clinical feature to predict nontyphoidal Salmonella bacteremia among adults in areas where HIV seroprevalence is high (179). The lack of current or recent diarrhea in invasive nontyphoidal Salmonella infection among immunosuppressed patients has been well described in many settings (180, 181). Patients with invasive nontyphoidal Salmonella also often display the features of underlying conditions such as anemia, malnutrition, and advanced HIV disease. It has been widely recognized that the nonspecific presentation among children often fulfills empirical algorithms for lower respiratory tract infection. In such circumstances, health care workers may commence antimicrobial treatment that is inappropriate for invasive nontyphoidal Salmonella, especially when antimicrobial resistance among Salmonella enterica is common (182, 183). In addition, febrile presentations are often not identified as bloodstream infection by pediatric guidelines (184, 185), resulting in delayed or missed antimicrobial treatment.
Patient outcomes.
Nontyphoidal Salmonella meningitis may occur among children. It has poor outcomes and a high case fatality ratio (186). Although lower respiratory tract infection-related symptoms and signs are common in invasive nontyphoidal Salmonella infection, these are frequently caused by other pathogens (154). Lobar pneumonia caused by nontyphoidal Salmonella has been described in a child (187).
Recurrent invasive nontyphoidal Salmonella infection was quickly recognized as a defining feature of AIDS in the 1980s. Recurrence typically occurs in 20 to 30% of treated adults with HIV, usually within 4 to 6 months (154, 162). Partial- and whole-genome sequencing has been used to establish that 80% of cases of recurrence are due to recrudescence with an identical strain, likely arising from intracellular persistence and latency. Only 20% of recurrences represent reinfection with a new strain (154, 166). Patients with HIV infection, once established on effective antiretroviral treatment after an episode of invasive nontyphoidal Salmonella, appear to be less likely to experience recurrence (188). In areas where HIV seroprevalence is not high, persistent nontyphoidal Salmonella bacteremia may suggest mycotic aneurysm. Nontyphoidal Salmonella intravascular, bone, and joint infections are frequently reported in industrialized countries (189).
Mortality from invasive nontyphoidal Salmonella infection is high in all subgroups, even if appropriate antimicrobial therapy is given. Case fatality ratios among HIV-infected adults from African case series were ≥50% early in the HIV epidemic (122, 154). A study from Thailand reported a case fatality ratio of 36% among all cases of invasive nontyphoidal Salmonella but 59% among cases who were HIV infected (190). The reported case fatality ratio among adults appears to have fallen gradually in Africa, after initial reports in the 1980s and 1990s, to around 20 to 25% more recently. This has been attributed largely to improved recognition and more prompt and effective management (191). It is possible but not certain that more-effective antimicrobials, such as fluoroquinolones replacing chloramphenicol, may also have contributed to these improving outcomes (114). More recently, with the wide availability of HIV care and treatment services, including antiretroviral therapy, across Africa, there are reports of a reduced incidence of and mortality from invasive nontyphoidal Salmonella and other bloodstream infections among adults (192). The case fatality ratio among cohorts of children with invasive nontyphoidal Salmonella disease across Africa has been reported to be 20 to 28% and highest among children <2 years of age. This is comparable to the case fatality ratio associated with other bloodstream infections (116, 158, 177).
Prevention and control.
Food safety from farm to fork is fundamental to the control of nontyphoidal Salmonella in industrialized countries. The very limited evidence base on sources and modes of transmission of nontyphoidal Salmonella in low- and middle-income countries hampers the development of evidence-based prevention advice. However, some host risk factors for invasive nontyphoidal Salmonella disease are modifiable, and interventions to prevent these mitigate disease risk. Efforts to prevent and effectively manage malaria are likely to be important in this regard. Indeed, the prevalence of invasive nontyphoidal Salmonella has declined in parallel with that of malaria in a number of areas (173–175). HIV care and treatment services, including the use of trimethoprim-sulfamethoxazole prophylaxis to prevent opportunistic infections (193, 194) and antiretroviral therapy to reverse immunosuppression, are likely to reduce the risk for invasion by nontyphoidal Salmonella and in turn disease incidence (188, 195).
Despite invasive nontyphoidal Salmonella being overlooked in evaluations of disease burden to date (5, 6, 196), some vaccine development efforts are under way (197). However, despite their availability for livestock and poultry, there are currently no nontyphoidal Salmonella vaccines available for humans. The occurrence of invasive nontyphoidal Salmonella disease predominantly among immunocompromised persons challenges vaccine development (160, 198).
Challenge of Distinguishing Invasive Salmonella Infections from Other Febrile Conditions in Areas of Endemicity
In settings where enteric fever and invasive salmonellosis are endemic, most patients with fever self-treat by visiting a local pharmacy or shop selling antimicrobials. Those who do not respond to self-treatment may then present to a health center, outpatient clinic, or hospital. Many other viral, bacterial, and protozoal infections resemble enteric fever and invasive salmonellosis (9). Enteric fever and invasive nontyphoidal salmonellosis should always be considered when suspected malaria has not been confirmed or the illness has not responded to antimalarial therapy. In areas of endemicity, rickettsial infections, leptospirosis, brucellosis, and dengue should be considered in the differential diagnosis. Noninfectious conditions characterized by fever, including lymphoproliferative disorders and vasculitides, should not be overlooked. Clinical judgment can be unreliable, and broad-spectrum antimicrobials may be needed in the initial management of severely ill febrile patients. A few clinical algorithms have been developed for febrile disease diagnosis (199–202), but these are limited by a lack of accuracy.
LABORATORY DIAGNOSIS
Diagnostic tests are needed for the diagnosis of invasive Salmonella infections, for the detection of convalescent and chronic fecal carriage of typhoidal Salmonella, and to estimate the burden of disease for public health assessment. Different tests and biological samples may be required for each situation (203). It may be important to be able to detect both Salmonella serovar Typhi and Salmonella serovar Paratyphi A infections, as they cannot be distinguished from each other clinically. Microbial culture is the mainstay of diagnosis. Antibody and antigen detection and nucleic acid amplification tests have limitations, as described below.
Bacterial Culture
The definitive diagnosis of enteric fever relies on the isolation of Salmonella enterica from normally sterile clinical samples, usually blood and bone marrow. Culture confirms the diagnosis and provides an isolate for antimicrobial susceptibility testing, epidemiologic typing, and molecular characterization. In untreated patients with enteric fever, the blood culture is positive in 80% of patients or more (41, 204, 205). In areas of endemicity where antimicrobials are frequently taken before evaluation, the yield from blood culture can be as low as 40%, and in this setting, bone marrow aspirate culture is usually considered the reference standard method, with a sensitivity of >80%. The importance of the volume of blood taken for blood culture relates to the number of bacteria in the blood (211). Invariably, the number of viable bacteria in each milliliter of blood is less than 10, and frequently it is only one or less (162, 206–210). The optimum period for detecting organisms circulating in the bloodstream is considered to be in the first or second week of the illness, although cultures can still remain positive in the third week in the absence of antimicrobial exposure (41, 204, 207). Quantitative bacteriology studies have shown declining counts with an increasing duration of disease (210). Various methods are used to improve the yield of blood culture (summarized in Table 2), but all remain limited by the low numbers of viable bacteria in blood.
TABLE 2.
Modifications of blood culture methods to improve detection of Salmonella Typhi and Salmonella Paratyphi A
| Methodological issue | Methoda | Advantages | References |
|---|---|---|---|
| Formulation of broth blood culture medium | Bile salt broth/Oxgall | Bile salts and Oxgall inhibit bactericidal activity of blood, improve detection of Salmonella Typhi and Salmonella Paratyphi A, unsuitable for isolation of non-enteric fever pathogens | 212–215, 219 |
| Supplementation of BHI broth or TSB with 0.05% SPS | SPS inhibits bactericidal activity of blood | 213, 214, 220 | |
| Soybean casein digest broth (Bactec system) | Automated detection, quality-assured medium, expense, complete system includes automated culture instrument | 216–218 | |
| Peptone-enriched TSB supplemented with BHI solids (BactAlert) | |||
| Inclusion of charcoal or resins in the medium to adsorb anticobials | Charcoal or resins in the medium should adsorb antimicrobials | 216, 218 | |
| Blood-to-broth dilution ratio: at least 1:10 is important, although higher dilutions may be beneficial | Reduce bactericidal activity of blood and dilute antimicrobials present | 221 | |
| Sample used | Clot culture: blood clot, after separation from serum, added to preprepared streptokinase broth | Removal of serum thought to reduce bactericidal activity of blood, improved yield even when on chloramphenicol treatment | 213, 221–225 |
| Lysis of cells in blood before start of culture | May release intracellular bacteria | 226, 227 | |
| Centrifugation of blood followed by direct culture on solid medium | May concentrate low numbers of bacteria and produce the bacteria growing on the plate more quickly | 226, 227 | |
| Duration of incubation | Prolonging duration of blood culture incubation | Incubation duration of longer than 7 days increased yield in older blood cultures systems; modern continuously monitored automated systems usually positive within 48 h | 204, 220 |
BHI, brain heart infusion; TSB, tryptic soy broth; SPS, sodium polyethanol sulfonate.
A number of studies have demonstrated a higher sensitivity from bone marrow culture aspirate than from blood culture, even after antimicrobials have been taken for several days and regardless of the duration of disease prior to sampling (162, 215, 228–233) (Table 3). The increased sensitivity of bone marrow culture compared with blood culture relates to the higher bacterial concentration in bone marrow (234). Bone marrow culture is more frequently positive in patients with severe and complicated disease (226). A bone marrow aspiration is an uncomfortable and specialized procedure, uncommonly performed outside research studies, although some authors have suggested that a fine-needle technique can be well tolerated (235). It is notable that most studies demonstrating a higher yield from bone marrow were performed before modern blood culture media and continuously monitored blood culture instruments were available and generally used low volumes of blood for culture (232, 233). It is possible that if a sufficiently large volume of blood is taken for culture using modern media and systems (211), blood culture may be as sensitive as bone marrow culture. Rose spot culture has been reported to be positive in 70% of patients, although in practice rose spots are rarely present (228). Cerebrospinal fluid culture is usually positive only in very young children (62).
TABLE 3.
Sensitivity of blood culture versus bone marrow culture for the diagnosis of enteric fever
| Author(s) (reference) | Yr published | No. of samples tested (blood and bone marrow culture) | Blood culture |
Bone marrow culture |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vol (ml) | Culture medium | Culture system | No. (%) positive | Vol (ml) | Culture medium | Culture system | No. (%) positive | |||
| Hirsowitz and Cassel (599) | 1951 | 28 | NAa | Ox bile broth | Manual | 13 (46.4) | NA | Ox bile broth | Manual | 18 (64.3) |
| Gilman et al. (228) | 1975 | 62 | 2 | Supplemented peptone broth | Manual | 25 (40.3) | NA | Supplemented peptone broth, Ruiz-Castaneda | Manual | 56 (90.3) |
| Guerra-Caceres et al. (229) | 1979 | 60 | 15 | Trypticase soy broth, Ruiz-Castaneda | Manual | 26 (43.3) | 0.5–1.0 | Trypticase soy broth, Ruiz-Castaneda | Manual | 57 (95.0) |
| Benavente et al. (236) | 1984 | 36 | 3 | Ox bile broth | Manual | 15 (41.7) | 0.5–1.0 | Ox bile broth | Manual | 27 (75.0) |
| Hoffman et al. (215) | 1984 | 118 | 3 | Ox bile broth | Manual | 64 (54.2) | 0.5–0.8 | Ox bile broth | Manual | 101 (85.6) |
| Vallenas et al. (230) | 1985 | 43 | 3 | Ox bile broth | Manual | 19 (44.2) | 0.5 | Ox bile broth | Manual | 36 (83.7) |
| Hoffman et al. (231) | 1986 | 61 | 3 | Ox bile broth | Manual | 27/56 (48.2) | NA | Ox bile broth | Manual | 56/61 (91.8) |
| Hoffman et al. (231) | 1986 | 61 | 8 | Ox bile broth | Manual | 38/56 (67.9) | NA | Ox bile broth | Manual | 56/61 (91.8) |
| Rubin et al. (209) | 1990 | 29 | 8 | Ox bile broth | Manual | 14 (48.3) | 0.5–0.8 | Ox bile broth | Manual | 25 (86.2) |
| Akoh (233) | 1991 | 31 | 2 | Thioglycolate broth | Manual | 11 (35.4) | 1.0–2.0 | Thioglycolate broth | Manual | 19 (61.3) |
| Dance et al. (600) | 1991 | 17 | 15 | Brain heart infusion broth with sodium polyanethol sulfonate | Manual | 14 (82.4) | 0.5–1.0 | Brain heart infusion broth with sodium polyanethol sulfonate | Manual | 15 (88.2) |
| Farooqui et al. (232) | 1991 | 88 | 5 | Brain heart infusion broth, thioglycolate broth | Manual | 58 (65.9) | 0.5–1.0 | Brain heart infusion broth, thioglycolate broth | Manual | 88 (100) |
| Chaicumpa et al. (292) | 1992 | 52 | 3 | Ox bile broth | Manual | 22 (42.3) | 0.5–0.8 | Ox bile broth | Manual | 32 (61.5) |
| Gasem et al. (601) | 1995 | 86 | 3 | Ox bile broth | Manual | 57 (66.3) | 1.0 | Ox bile broth | Manual | 70 (81.4) |
| Gasem et al. (601) | 1995 | 86 | 10 | Ox bile broth | Manual | 58 (67.4) | 1.0 | Ox bile broth | Manual | 70 (81.4) |
| Gasem et al. (602) | 2002 | 53 | 8–10 | Becton Dickinson Bactec/F medium | Continuously monitored | 43 (81.1) | 1.0–2.0 | Becton Dickinson Bactec/F medium | Continuously monitored | 53 (86.9) |
| Wain et al. (214) | 2008 | 73 | 5 | Brain heart infusion broth, brain heart infusion broth with sodium polyanethol sulfonate, ox bile broth | Manual | 57 (78.1) | 1.0 | NA | Manual | 69 (94.5) |
| Wain et al. (214) | 2008 | 68 | 15 | Brain heart infusion, brain heart infusion with sodium polyanethol sulfonate, ox bile broth | Manual | 59 (86.7) | 1.0 | NA | Manual | 61 (89.7) |
NA, not available.
Salmonella enterica may be isolated from feces in up to 30% of patients with typhoid fever and in <1% of urine samples, with the number of organisms recoverable from feces increasing throughout an untreated illness (41). The sensitivity of fecal culture increases from about 10% in a single sample to about 30% by testing multiple samples. Sensitivity is also improved by using whole feces rather than rectal swabs and by using a Selenite F enrichment step and selective media. Culture of bile obtained from an overnight duodenal string capsule provides a sensitivity similar to that for blood culture and offers an additional means to isolate typhoidal Salmonella from patients or carriers. Young children and those with severe disease may be unable to tolerate the procedure (215, 230, 236). A positive culture from feces, duodenal contents, or urine requires cautious interpretation. Although it may indicate acute enteric fever infection, it could also represent chronic carriage, with the acute infection syndrome caused by a different organism.
Isolates of Salmonella serovar Typhi and Salmonella serovar Paratyphi A should be handled with care in the laboratory, as they have been a common cause of laboratory-acquired infection. Many jurisdictions recommend handling these pathogens under biosafety level 3 conditions. Adequate disposal of specimens and cultures by autoclaving is essential.
Serologic Assays
Antibody detection.
The Widal test measures agglutinating antibodies against LPS (O) and flagellar (H) antigens of Salmonella serovar Typhi in the sera of individuals with suspected enteric fever (237). Although usually discouraged due to inaccuracy, it is simple and inexpensive to perform and is still widely used in some countries (238). The performance of the method has been hampered by a lack of standardization of reagents and inappropriate result interpretation (239, 240). The Widal test ideally requires both acute- and convalescent-phase serum samples taken approximately 10 days apart; a positive result is determined by a 4-fold increase in antibody titer. However, antibody titers in infected patients often rise before the clinical onset, making it difficult to demonstrate the required 4-fold rise between initial and subsequent samples (237, 241). In practice, the result from a single, acute-phase serum sample is often used, but false-negative and false-positive results are common. Knowledge of the background levels of antibodies in the local population may aid interpretation of the Widal test, and performance is best among patients with a high prior probability of enteric fever (242, 243). For example, in a study from Vietnam using Widal test data from patients with typhoid fever and both febrile and healthy control participants, it was found that a cutoff titer of ≥200 for O agglutination or ≥100 for H agglutination would correctly diagnose 74% of blood culture-positive patients. However, 14% of positive results were classified as false positive and 10% of negative results as false negative (243).
Enzyme-linked immunosorbent assays (ELISAs) have been used to study the normal antibody response during enteric fever to LPS, flagella, Vi capsular polysaccharide, or outer membrane protein antigens (244–251). Although ELISAs measuring anti-LPS antibodies and antiflagellum antigens are more sensitive than the Widal “O” and “H” antigen-based test, the results are still limited by lack of specificity. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) immunoblotting has also been used to detect serum antibodies against LPS and flagellar antigens of Salmonella serovar Typhi and Salmonella serovars Paratyphi A, Paratyphi B, and Paratyphi C (252).
Rapid serologic tests.
There are a number of commercially available point-of-care rapid serologic tests for enteric fever. Their characteristics are summarized in Table 4 and in a recent review (253). The tests currently available have different methods and formats. Most have been developed for use with blood, including venous whole-blood, serum, or capillary samples, and detect antibody directed against Salmonella antigens. The antibody class detected is usually IgM, which is suggestive of a current or recent infection. Some rapid tests detect IgG, which may indicate a current infection or previous exposure.
TABLE 4.
Characteristics of selected serologic tests used for the diagnosis of infection with Salmonella Typhi and Salmonella Paratyphi A
| Test | Test methodology | Reported characteristicsb | Reference(s) |
|---|---|---|---|
| Enterocheck-WB | Dipstick detecting anti-LPS IgM antibodies | Sensitivity, 89%; specificity, 97% | 268 |
| LifeAssay Test-it | Detects IgM antibodies against Salmonella Typhi LPS in an ICT LFA cassette format | Sensitivity, 59%; specificity, 98% | 265, 266 |
| Mega Salmonella | ELISA detecting IgG and IgM antibodies against an undefined Salmonella Typhi antigen | Sensitivity, 91%; specificity, 49% | 257 |
| Multi-Test-Dip-S-Ticks | Dipstick detecting anti-LPS IgG and IgM | Sensitivity, 89%; specificity, 53% | 259 |
| PanBio | ELISA detecting anti-LPS IgG and IgM | Sensitivity, 78%; specificity, 80% | 267 |
| SD Bioline | ICT LFA cassette detecting IgG and IgM antibodies against an undefined Salmonella Typhi antigen | Sensitivity, 69%; specificity, 79% | 257 |
| Tubex TF | Detects antibody against Salmonella Typhi LPS with an inhibition assay format and a visual result readout | Sensitivity, 56–100%; specificity, 58–100% | 249, 255–260, 263, 270 |
| Typhidot | Measures IgM and IgG antibodies against a 50-kDa outer membrane protein of Salmonella Typhi in an immunodot test format | Sensitivity, 67–98%; specificity, 58–100% | 254, 255, 257, 259, 260, 269 |
| Typhidot M | Measures IgM antibodies, after removal of IgG antibodies, against a 50-kDa outer membrane protein of Salmonella Typhi in a dot blot format | Sensitivity, 47–98%; specificity, 65–93% | 254, 255, 258 |
| TyphiRapid IgM and IgG IgM (Combo) | Measures IgM antibodies, after removal of IgG antibodies, against a 50-kDa outer membrane protein of Salmonella Typhi in an ICT LFAa cassette format | Sensitivity, 89–100%; specificity, 85–89% | 261, 262 |
| Widal test | Measures agglutinating antibodies against O and H antigens of Salmonella Typhi and Salmonella Paratyphi A; uses a tube or slide format | Very variable sensitivity and specificity; lack of standardized reagents | 237–240, 242, 243 |
ICT, immunochromatographic test; LFA, lateral-flow assay.
This column shows the range of sensitivity and specificity values from different studies with a range of sample sizes and methodologies. Where there is only one study, that value is given. For a systematic review of the studies examining Tubex TF and Typhidot, see reference 270.
Typhidot (Malaysian Biodiagnostic Research Sdn Bhd, Kuala Lumpur, Malaysia) detects specific IgM and IgG antibodies against a 50-kDa Salmonella serovar Typhi outer membrane protein (OMP) in an immunodot test format. Typhidot-M is a modified version of Typhidot but detects IgM to the same OMP and as a more specific marker of current acute infection (254–260). Reported sensitivities vary between 67 and 98% for Typhidot and between 47 and 98% for Typhidot-M, with specificities of 73 to 100% for Typhidot and 65 to 93% for Typhidot-M. The TyphiRapid IgM and TyphiRapid IgG IgM (Combo) are similar versions of the test but in an ICT cassette format (261, 262). Tubex TF (IDL Biotech, Sollentuna, Sweden) detects antibody against Salmonella serovar Typhi LPS with an inhibition assay format and visual result readout (249, 256–260, 263). Reported sensitivities for Tubex TF vary between 56 to 100%, with specificities of 58 to 100%. A modification for paratyphoid diagnosis has recently been developed (264). The Royal Tropical Institute (KIT), Netherlands, has developed a test detecting IgM against LPS using a dipstick, latex agglutination, and, most recently, a lateral-flow format (LifeAssay, Cape Town, South Africa) (249, 265, 266). Studies using the lateral-flow test report sensitivities of 60% and specificities of 98%. Other rapid test kits available include the following: an ELISA and dipstick (Multi-Test Dip-S-Ticks) from PanBio (PanBio Indx, Inc., Baltimore, MD) detecting anti-LPS IgG and IgM; Mega-Salmonella (Mega Diagnostics, Los Angeles, CA), which is an ELISA detecting IgG and IgM antibodies against an undefined Salmonella serovar Typhi antigen; the SD Bioline Typhoid rapid test (Standard Diagnostics, Kyonggi-do, South Korea), which uses an immunochromatographic method to detect IgG and IgM antibodies against another undefined Salmonella serovar Typhi antigen; and a dipstick test named Enterocheck-WB (Zephyr Biomedicals, Goa, India) that detects anti-LPS IgM antibodies (257, 259, 267, 268). A number of other tests are commercially available but have not been evaluated in published studies.
Although rapid serologic tests may represent some improvement over the Widal test, currently available results suggest that they still lack sensitivity and also specificity because of the background antibody levels in the general population (269) and the cross-reactive nature of the selected antigens. Accurate evaluation of the tests is also hampered by the absence of a satisfactory reference standard. A recent systematic review of studies of two widely used tests, Tubex TF and Typhidot, concluded that the performance characteristics did not justify their use (270).
Molecular Assays
Nucleic acid amplification tests, including conventional PCR and real-time PCR, have been developed for the detection of both Salmonella serovars Typhi and Paratyphi A, mainly in blood (271–288). Nucleic acid amplification methods have the potential to amplify small numbers of organisms and nonculturable bacteria, as well as dead organisms. Targets for Salmonella serovar Typhi PCR-based assays have included the Hd flagellin gene fliC-d (271), the Vi capsular gene viaB (272), the tyvelose epimerase gene (tyv) (previously rfbE), the paratose synthase gene (prt) (previously rfbS), groEL (273), the 16sRNA gene (274), hilA (a regulatory gene in Salmonella pathogenicity island 1 [SPI-1]) (275), the gene encoding the 50-kDa outer membrane protein ST50 (276), and the hypothetical protein gene ratA (277). Nested primers have been used in some studies to improve sensitivity, although this may lead to unspecific amplification and contamination.
The sensitivity of PCR without enrichment in blood-culture positive cases has exceeded 90% in some studies (271, 273, 278–286), although other studies have reported much lower sensitivities more consistent with the number of bacteria in the blood (266, 272, 287). Bone marrow aspirates were examined in one study, with 100% sensitivity (287), and positive results have also been found in urine samples (288). The specificity among patients with other conditions has usually been 100%. Several studies have reported patients with clinically suspected typhoid who are PCR positive and blood culture negative (266, 272). The absence of a perfect reference standard makes interpretation of these results challenging.
Other Diagnostic Tests
Assays to detect bacterial antigens such as Vi, O9, and Hd in urine have been used as diagnostic methods for enteric fever but with variable results (235, 289–293). The greatest level of sensitivity was found with the Vi antigen, whereas the O9 and Hd antigens were less sensitive. The sensitivity for Vi increased when multiple samples were examined, but the specificity was less satisfactory, particularly in patients with brucellosis. In studies of patients from Indonesia on detection of O9 antigen in urine using an ELISA and dot blot format, the sensitivity increased from 65% to 95% when between one and three urine samples were examined (292). In a further study in Vietnam, the sensitivity was 92% and specificity 72% when three serial urine samples were compared with blood culture (293). These data indicate the intermittent nature of antigen excretion in the urine during an infection. Studies detecting Vi and other antigens in serum have also been performed, with variable results (294–299).
New Diagnostic Approaches
There is a considerable research effort aimed at developing new diagnostics for enteric fever (203). Increasing knowledge of the Salmonella serovar Typhi and Salmonella serovar Paratyphi A genomes should lead to better targets for nucleic acid amplification tests (300–303). Methods to remove potential inhibitory human DNA from the sample may improve sensitivity (304), as may combining short periods of blood culture broth incubation with PCR amplification (305). Proteomic and immunoscreening approaches are being used to seek antibodies and antigens that have a higher level of specificity than those used currently used (306–311). Analysis of bacterial gene expression in typhoid and paratyphoid may also lead to new diagnostic tests (312–314).
Other Laboratory Findings In Enteric Fever
Most patients with enteric fever have a total white cell count within the normal range. Leukocytosis may suggest an intestinal perforation or another diagnosis. Eosinopenia is a common although nonspecific finding (51). A normochromic anemia, mild thrombocytopenia, and an increased erythrocyte sedimentation rate are common. There may be laboratory markers of a mild disseminated intravascular coagulation, although this rarely appears to be of clinical relevance (208). Elevation of alanine transaminases and aspartate transaminase to two to three times the normal level is a frequent accompaniment (52).
ANTIMICROBIAL RESISTANCE
Development of Antimicrobial Resistance among Typhoidal Salmonella Strains
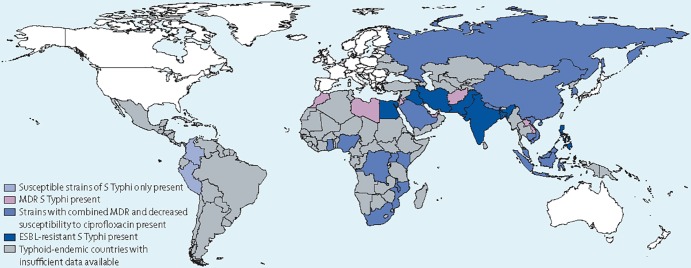
Figure 3 summarizes the current global distribution of antimicrobial drug resistance in Salmonella Typhi. Prior to the mid-1970s, chloramphenicol was the mainstay of treatment of enteric fever (315–317). However, reports on chloramphenicol-resistant isolates began to appear before 1970 (318, 319). In 1972, the first epidemic caused by a chloramphenicol-resistant strain was reported from Mexico (320). Soon thereafter, outbreaks involving chloramphenicol-resistant Salmonella serovar Typhi were reported from several different areas, including India (321), Vietnam (322, 323), South Korea (324), and Bangladesh (325). A common feature of the outbreak strains was that the chloramphenicol resistance determinant was located on a self-transmissible plasmid of the HI1 incompatibility type (IncHI) (325, 326). In addition to chloramphenicol resistance, these plasmids often carried genes conferring resistance to other drugs, such as streptomycin, sulfonamides, and tetracyclines (325, 326).
FIG 3.
Worldwide distribution of antimicrobial drug resistance in Salmonella enterica serovar Typhi. (Reprinted from reference 604 with permission from Elsevier.)
The development of chloramphenicol resistance led to increased use of two other first-line drugs, ampicillin and trimethoprim-sulfamethoxazole. Trimethoprim-sulfamethoxazole remained an effective drug until 1975, when resistance was reported from France (327). By the late 1980s, multiple-drug resistance (MDR), defined as resistance to ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazole, was reported from multiple countries (328–330). Cases of enteric fever caused by MDR Salmonella serovars Typhi and Paratyphi A continued to emerge in the 1990s and early 2000s (331–336). Among Salmonella Typhi isolates collected in Vietnam in 1993 to 1996, 80% were reported to be MDR (82). A similar prevalence was reported from Africa among isolates collected in Kenya and Nigeria in 2004 to 2006 (70 and 61% MDR, respectively) (338, 339). In Europe, a large retrospective study looking at 692 Salmonella serovar Typhi isolates collected in 2002 to 2003 documented a 22% prevalence of the MDR phenotype (337). A study in Delhi, India, found that MDR Salmonella serovar Typhi increased from 34% in 1999 to 66% in 2005 (340). Today, MDR Salmonella Typhi is considered endemic in many developing countries, especially in areas of South and Southeast Asia.
In response to the development and spread of MDR Salmonella serovar Typhi, the use of fluoroquinolone antimicrobials such as ciprofloxacin was recommended as an alternative for the treatment of typhoid fever (325, 342). However, resistance among Salmonella serovar Typhi isolates developed within a short period, with the first case being reported in 1992 from the United Kingdom (343). This was soon followed by similar reports from other countries, such as South Korea, India, and Vietnam (344, 345). The first epidemic involving a fluoroquinolone-resistant Salmonella serovar Typhi isolate occurred in Tajikistan in 1997 (346). The same year, 60% of Salmonella serovar Typhi isolates in India displayed a MIC of ≥2 μg/ml to ciprofloxacin (345). Similarly, an increase in Salmonella serovar Paratyphi A isolates with ciprofloxacin MICs of ≥2 μg/ml was reported from New Delhi in 2000 (347), and a ciprofloxacin-resistant isolate with a MIC of 128 μg/ml was isolated in Japan in 2002 (348). By 2008, there were reports of ciprofloxacin-resistant Salmonella serovar Typhi from multiple centers in India (349–352). In 2010, a tertiary hospital in Chandigarh, India, reported that 13.6% of isolates were ciprofloxacin resistant (353). In 2013, a tertiary care center in New Delhi reported that 37.9% of 344 typhoidal Salmonella isolates were ciprofloxacin resistant (354). In association with increased use of ciprofloxacin, several areas have observed a decline in chloramphenicol resistance and MDR prevalence among both Salmonella serovar Typhi and Paratyphi A isolates (355). Although this is an encouraging trend, the reintroduction of chloramphenicol as first-line treatment would likely result in reemergence of MDR isolates.
Decreased susceptibility to fluoroquinolones, often associated with nalidixic acid resistance, has also been observed in countries where these infections are not endemic, where the majority of cases are associated with international travel. In 1999, decreased susceptibility to fluoroquinolones was detected in 23% of all Salmonella serovar Typhi isolates in the United Kingdom (356). In the United States, the proportion of nalidixic acid-resistant isolates increased from 19% in 1999 to 42% in 2004 and in Canada, nalidixic acid resistant isolates rose from 40% to 80% between 2000 and 2006 (357, 358). A similar trend was observed in the United Kingdom, where the proportion of Salmonella serovar Typhi isolates with decreased susceptibility to ciprofloxacin increased from 35% in 2001 to 70% in 2006 (359).
In areas with a high prevalence of both MDR and fluoroquinolone resistance, azithromycin, an azalide antimicrobial that has demonstrated good efficacy against uncomplicated enteric fever in multiple clinical trials (360), and extended-spectrum cephalosporins (e.g., ceftriaxone) tend to be used for the treatment of enteric fever. However, Salmonella serovar Typhi isolates displaying resistance to extended-spectrum cephalosporins have been described. For example, extended-spectrum β-lactamase (ESBL) enzymes of the SHV-12 and CTX-M types and an AmpC β-lactamase of the ACC-1 type have been reported among Salmonella serovar Typhi isolates from Germany, the Philippines, Bangladesh, and India (361–364). In 2013, an MDR isolate of Salmonella serovar Paratyphi A harboring a CTX-M-15 β-lactamase was detected in a Japanese traveler returning from India (605). Similarly, there have been sporadic reports of azithromycin resistance. A recent study from India reported increasing prevalence of azithromycin resistance among typhoidal Salmonella isolates; among 344 isolates collected in 2010 to 2012, 7.3% were resistant to azithromycin using disk diffusion interpretive criteria suggested by the British Society for Antimicrobial Chemotherapy (BSAC) (354). Importantly, an increasing prevalence of azithromycin resistance was observed for both Salmonella serovars Typhi and Paratyphi A over the 2-year study period (354).
Development of Antimicrobial Resistance among Nontyphoidal Salmonella Strains
As for typhoidal Salmonella, an increasing prevalence of antimicrobial resistance has been observed in nontyphoidal Salmonella over recent decades. An important trend has been the development of MDR among isolates of Salmonella serovar Typhimurium but also other serovars. The MDR phenotype in Salmonella serovar Typhimurium began to appear in the early 1980s in the United Kingdom, where it was closely associated with a specific phage type called definitive type 104 (DT104) (365). These isolates displayed resistance to five antimicrobial agents—ampicillin, chloramphenicol, streptomycin, sulfonamides, and tetracycline—a resistance phenotype commonly referred to as R-type ACSSuT. By the 1990s, this phenotype had been reported from several other countries in Europe as well as the United States, Canada, Israel, Turkey, and Japan (366, 367). However, Salmonella serovar Typhimurium with R-type ACSSuT has been declining in many countries since that time. In the United States, this phenotype accounted for 35% of all Salmonella serovar Typhimurium isolates submitted to the U.S. National Antimicrobial Monitoring System (NARMS) in 1997. However, between 1998 and 2005 there was a steady decline from 32% to 22%, and in 2011 20% of all Salmonella serovar Typhimurium isolates collected from humans displayed this phenotype (368, 369).
Another important resistance trend among nontyphoidal Salmonella isolates has been the development of resistance to quinolones (i.e., nalidixic acid) and fluoroquinolones such as ciprofloxacin. Although nalidixic acid is not used for treatment, development of resistance to this drug is of clinical importance since it may be associated with reduced clinical effectiveness of fluoroquinolone treatments (370, 371). Reports of quinolone resistance among nontyphoidal Salmonella strains followed soon after the introduction of fluoroquinolones. In Denmark, quinolone resistance in Salmonella serovar Enteritidis increased from 0.8% in 1995 to 8.5% in 2000. In the early 2000s, a large European surveillance study including 27,000 isolates reported low-level fluoroquinolone resistance in 13% of Salmonella serovar Typhimurium, 8% of Salmonella serovar Enteritidis, 53% of Salmonella serovar Virchow, and 57% of Salmonella serovar Hadar (372) isolates. In Southeast Asia, several studies have documented the emergence of quinolone- and fluoroquinolone-resistant Salmonella strains. In 2009, a high prevalence of reduced susceptibility to ciprofloxacin was reported among Salmonella strains from the Philippines (15%), Singapore (25%), and Thailand (46.2%) (373). In 2011, 31% of isolates were fully resistant to ciprofloxacin in a study from Thailand (374). The prevalence of fluoroquinolone-resistant isolates in high-income countries has been found to be lower (107). Among 2,344 nontyphoidal Salmonella isolates collected in the United States in 2011, 0.2% were ciprofloxacin resistant (369).
The development of nontyphoidal Salmonella isolates resistant to extended-spectrum cephalosporins, such as ceftriaxone, represents another substantial public health concern. These drugs are important for treating invasive Salmonella infections, especially among children, among whom fluoroquinolones may be avoided. Resistance to extended-spectrum cephalosporins has been recognized in nontyphoidal Salmonella since the mid-1980s and is commonly mediated through β-lactamases of the ESBL or AmpC type. Some of the earliest reports on cephalosporin-resistant isolates of nontyphoidal Salmonella originated in North Africa. During 1984 and 1990, cephalosporin-resistant strains were frequently isolated in pediatric units of Tunisian hospitals (375, 376). In Southeast Asia, cephalosporin resistance has been reported from Singapore, the Philippines, and Thailand (373, 377). In Thailand, isolates harboring enzymes of the CMY and CTX-M types have been described (378, 379). Importantly, some of the cephalosporin-resistant nontyphoidal Salmonella isolates in Thailand also show resistance to fluoroquinolones (379–381). Such infections might require the use of more expensive drugs such as carbapenems or tigecycline.
In Europe, cephalosporin-resistant nontyphoidal Salmonella was first detected in France and Italy in 1989 and 1990, respectively (382, 383). A CTX-M-producing clone of Salmonella serovar Typhimurium spread in Russia, Hungary, and Greece between 1996 and 1999, and sporadic cases and outbreaks involving CMY-producing Salmonella serovar Newport were reported from France between 2000 and 2005 (375, 384).
In North America, reports on β-lactamase-producing isolates of nontyphoidal Salmonella started to appear in the mid-1990s. In the United States, a national survey of 4,003 Salmonella isolates collected in 1995 found 3 (<0.1%) ceftriaxone-resistant isolates, each of which had been acquired outside the United States (385). Early reports of ceftriaxone-resistant nontyphoidal Salmonella appeared in the mid- to late 1990s (386). The first ESBL-producing strain detected in Canada was an SHV-producing Salmonella serovar Typhimurium strain identified in 2000 (387). Two years later, the first CMY-producing Salmonella strain was reported during a small outbreak of Salmonella serovar Newport in Alberta, Canada (388). Since then, multiple studies have reported on the occurrence of CMY and ESBL enzymes among Salmonella isolates collected from human, animal, and retail meat sources in Canada and the United States (389–399). Perhaps the most notable trend in the United States has been the emergence of MDR Salmonella serovar Newport with an AmpC phenotype (400). These strains emerged in the early 2000s and harbored plasmids encoding cephalosporin resistance (blaCMY) and the R-type ACSSuT phenotype (400). According to NARMS, the proportion of Salmonella serovar Newport isolates with this phenotype peaked at 25% in 2001 but had declined to 13% by 2005. More recently ceftriaxone-resistant Salmonella serovar Heidelberg has emerged in the United States (390, 401).
Over recent years, several reports have documented the emergence of extensively drug-resistant isolates of nontyphoidal Salmonella. Salmonella serovar Typhimurium isolates resistant to 12 to 15 antimicrobial agents, comprising 6 or 7 CLSI drug classes, including cephems, have been reported from Malaysia and Vietnam (402–404). Similarly, Salmonella isolates resistant to 6 or 7 antimicrobial agents, comprising 3 to 5 CLSI drug classes, have been detected in Thailand (374). A recent trend is the development of extensively drug-resistant isolates of Salmonella serovar Kentucky. A ciprofloxacin-resistant strain of Salmonella serovar Kentucky (ST198-X1) that originally emerged in Egypt and spread throughout Africa and the Middle East from 2002 to 2008 has now acquired additional resistance (405). Since 2009, variants displaying resistance to extended-spectrum cephalosporins and carbapenems have been detected. Nontyphoidal Salmonella isolates displaying carbapenem resistance have been reported from multiple countries, including China, Columbia, Pakistan, and the United States. Among these isolates, Klebsiella pneumoniae carbapenemase and New Delhi metallo-beta-lactamase-1 enzymes were most prevalent (406–410). Of concern is that some of these isolates are also resistant to most aminoglycosides, trimethoprim-sulfamethoxazole, and azithromycin (405).
Molecular Mechanisms of Resistance
Multiple-drug resistance.
Among typhoidal Salmonella isolates, resistance to the traditional first-line antimicrobials ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazole is commonly caused by resistance determinants located on plasmids. Resistance to ampicillin is often mediated through β-lactamases (e.g., blaPSE and blaTEM), whereas several mechanisms may be associated with chloramphenicol resistance. Three types of chloramphenicol acetyltransferases (CATs) (types I to III) have been described in Gram-negative bacteria, but resistance due to the production of CAT of type I has been encountered most frequently in Enterobacteriaceae (411). Genes involving nonenzymatic resistance mechanisms, such as cmlA and floR, have also been found in Salmonella enterica (411, 412). Both trimethoprim and sulfamethoxazole are folate pathway inhibitors that prevent the synthesis of DNA. Resistance to both drugs occurs by acquisition of genes encoding folate pathway enzymes that do not bind these compounds. In Salmonella enterica, resistance to trimethoprim is mediated through various dihydrofolate reductase (dfr) genes, whereas sulfamethoxazole resistance is due to the presence of sul genes such as sul1 or sul2 (412).
The genes mediating R-type ACSSuT in nontyphoidal Salmonella are commonly clustered together in a chromosomal genetic element called Salmonella genomic island 1 (SGI-1). This element was first identified in Salmonella serovar Typhimurium DT104 but has since then been detected in many other serovars, including Salmonella serovars Agona, Albany, Cerro, Derby, Dusseldorf, Emek, Haifa, Infantis, Kentucky, Kiambu, Kingston, Meleagridis, Newport, Paratyphi B, and Tallahassee (413–415). In the classic SGI-1, a 14-kb region bracketed by two integron structures within this chromosomal element contains the antimicrobial resistance genes contributing to its ACSSuT properties: blaPSE-1 conferring resistance to ampicillin, floR to chloramphenicol and florfenicol, aadA2 to streptomycin, sul1 to sulfonamides, and tetG to tetracycline (416).
Fluoroquinolone resistance.
Fluoroquinolones target the two enzymes DNA gyrase and topoisomerase IV, whose subunits are encoded by the gyrA and gyrB and the parC and parE genes (417). Mutations in the quinolone resistance-determining regions (QRDRs) of these genes may confer reduced susceptibility or resistance to fluoroquinolones, depending on the number of mutations acquired (417, 418). A single point mutation in the gyrA gene, typically Ser-83 to Phe, Ser-83 to Tyr, Asp-87 to Asn, or Asp-87 to Gly, will confer resistance to the quinolone nalidixic acid and decreased susceptibility to ciprofloxacin. Two or more mutations in the gyrA gene or other topoisomerase genes are required to confer full clinical resistance to ciprofloxacin (MIC, ≥1 μg/ml) (418–421).
In addition to the chromosomally encoded mechanisms, plasmid-mediated resistance mechanisms have been described. The first plasmid-mediated quinolone resistance (PMQR) mechanism, qnrA, was described in the late 1990s, and since then, a variety of other plasmid-mediated mechanisms have been discovered in Enterobacteriaceae, including different qnr variants, aac(6′)-Ib-cr, qepA, and oqxAB (422, 423). Among nontyphoidal Salmonella strains, a number of qnr variants and the aac(6′)-Ib-cr mechanism have been detected (424). Reports on PMQR mechanisms among typhoidal Salmonella isolates are rare but include a qnrB2-producing isolate detected in Germany and three qnrS1-producing isolates detected in the United States (364, 425). The plasmid-mediated mechanisms typically confer decreased susceptibility to ciprofloxacin in the MIC range of 0.125 to 1.0 μg/ml and a modest increase in susceptibility to nalidixic acid in the MIC range of 8 to 32 μg/ml (422, 426, 427).
Cephalosporin resistance.
Cephalosporins belong to the group of β-lactam antimicrobials and disrupt the bacterial cell wall synthesis by targeting penicillin binding proteins (PBPs) and the cross-linking of the peptidoglycan (428). Resistance to cephalosporins, including extended-spectrum cephalosporins such as ceftriaxone, is commonly mediated through β-lactamases that inactivate the drug by cleaving the β-lactam ring. The β-lactamases mediating resistance to extended-spectrum cephalosporins can be divided into three groups: extended-spectrum β-lactamases (ESBLs), carbapenemases, and AmpC-type β-lactamases (429, 430). Among the AmpC plasmid-mediated β-lactamases, cephamycinases (CMY), encoded by blaCMY genes, are the predominant cause of cephalosporin resistance in nontyphoidal Salmonella. The genes encoding the β-lactamase enzymes are commonly located on mobile genetic elements such as plasmids, transposons, and integrons. Consequently, resistance may spread horizontally between isolates, clones, and serovars.
Macrolide resistance.
Macrolides inhibit protein synthesis by binding to the 50S subunit of the bacterial ribosome. Resistance mechanisms have been detected in Salmonella enterica isolates with elevated azithromycin MICs, here defined as MICs of >16 μg/ml. Among nontyphoidal Salmonella isolates, a macrolide-2′-phosphotransferase encoded by the mphA gene has been described (405, 431). The first case of azithromycin treatment failure in a patient with invasive Salmonella infection was reported in 2010 (432). This infection was due to a Salmonella serovar Paratyphi A isolate displaying an azithromycin MIC of 256 μg/ml (432).
ANTIMICROBIAL MANAGEMENT
The aims of management of invasive Salmonella infections are to resolve clinical symptoms by eliminating the infection with antimicrobials, to provide supportive treatment with fluids and nutrition, and to monitor for the development of complications. Effective antimicrobial therapy reduces mortality and complications and shortens the illness. In enteric fever, antimicrobial treatment may eradicate fecal carriage and reduce onward transmission. In areas of endemicity, antimicrobial treatment for typhoid fever is often started empirically based on the syndrome of fever for 3 to 4 days or more, constitutional symptoms (e.g., malaise and fatigue), symptoms or signs in the gastrointestinal system, a negative malaria smear, and no other clear source of infection. Antimicrobial agents are not recommended for treatment of nonsevere, nontyphoidal Salmonella diarrhea in healthy adults or children, but they are recommended for people with evidence of sepsis or extraintestinal infection or for specific populations at risk for bacteremia and disseminated disease.
Enteric fever is an intracellular infection involving the reticuloendothelial system, particularly bone marrow, liver, and spleen (52). Invasive nontyphoidal Salmonella has also been shown to establish an intracellular niche in the blood and bone marrow (162). Therefore, effective treatment depends on the ability of antimicrobials to penetrate intracellular sites of infection in the reticuloendothelial system and gallbladder. Some antimicrobials, including gentamicin and first- and second-generation cephalosporins such as cefuroxime, appear to be effective in vitro but are ineffective in vivo and should not be used in enteric fever (433).
Traditional First-Line Antimicrobials
Chloramphenicol was the first antimicrobial found to be effective in enteric fever and for many years was the standard treatment (315). Treatment with chloramphenicol led to symptom resolution within 4 to 6 days and transformed a prolonged, debilitating, and potentially fatal disease into a treatable condition with a low case fatality ratio. A physician using chloramphenicol to treat typhoid fever for the first time commented: “…the clinical improvement and complete transformation in a few days can only be appreciated by clinicians who have had previous experience of typhoid fever and have known their own helplessness in the past to affect its protracted course… its great value in saving life and curtailing morbidity in this disease is incontestable” (434). Oral chloramphenicol treatment results in serum concentrations between 5 and 20 μg/ml above the usual wild-type MIC (435). The succinate ester prodrug is used for intravenous or intramuscular administration and gives lower serum levels than the oral form (436, 437). Chloramphenicol has been shown to be clinically effective in a number of studies (341, 438–447). Disadvantages of chloramphenicol are its four-times-daily administration and the need to give it for at least 2 weeks to reduce the 10 to 15% risk of relapse. Dose-related and reversible bone marrow depression and irreversible bone marrow aplasia that is rare, unpredictable, and often fatal may occur (448). Amoxicillin and trimethoprim-sulfamethoxazole were shown to have efficacy generally comparable to that of chloramphenicol with less risk of toxicity, although they also needed to be given for at least 2 weeks to avoid relapse (439, 449–456). The experience with mecillinam was less convincing (456–458). Chloramphenicol, amoxicillin, and trimethoprim-sulfamethoxazole were widely available and affordable in areas of endemicity and remained the standard of care for many years. The emergence and spread of MDR strains of Salmonella serovars Typhi and Paratyphi A led to the evaluation of new antimicrobials. The extended-spectrum cephalosporins, fluoroquinolones, and azithromycin were established as effective alternatives.
Fluoroquinolones
The fluoroquinolones, in particular ciprofloxacin and ofloxacin but also including fleroxacin and pefloxacin, were evaluated for enteric fever treatment as the MDR strains emerged. Peak plasma levels of ciprofloxacin and ofloxacin after oral administration were initially well above the prevailing MICs of infecting strains (481–483). The drugs were concentrated intracellularly at the site of infection (484, 485) and were rapidly bactericidal in vitro (481, 486). When given for duration of 7 to 14 days, fluoroquinolones were often 100% effective with very low levels of relapse (329, 342, 443, 470, 487–505). The clinical response was rapid, with fever resolution within 3 to 5 days. A number of studies were conducted, predominantly in Vietnam, with a shorter course of treatment of 5 days (506, 507) or 2 or 3 days (507–510) in uncomplicated disease with broadly similar results. Fleroxacin and pefloxacin were equally effective, although norfloxacin is not recommended because of low tissue concentrations. As these fluoroquinolones could conveniently be given orally and local generically produced drugs were relatively affordable, they became widely used for treating suspected and confirmed cases of enteric fever.
For invasive nontyphoidal Salmonella there is only very limited historical experience of the use of chloramphenicol compared to ciprofloxacin. Ciprofloxacin was introduced following the emergence of resistance to chloramphenicol (114) in treatment of invasive nontyphoidal Salmonella in Africa. The reported recurrence ratio after treatment with chloramphenicol was 43% (154), compared to 30% following treatment with ciprofloxacin (162). This difference might be attributable to improved intracellular penetration of the fluoroquinolone. The case fatality ratio also fell gradually over the reported period, but this could be attributable to multiple effects other than the change in antimicrobial use (114).
Over time, physicians in areas of endemicity and those treating returning travelers began to report cases of fluoroquinolone failure when treating enteric fever despite the laboratory reporting a susceptible isolate (343, 419, 511–519). It became clear that a subset of strains of Salmonella serovars Typhi and Paratyphi A had emerged that were less susceptible to the fluoroquinolones. They showed decreased susceptibility to ciprofloxacin (MICs of 0.125 to 0.5 μg/ml, compared with the wild-type MIC of ≤0.03 μg/ml), and the decreased susceptibility is most commonly mediated by point mutations in genes encoding DNA gyrase, the target enzyme for the drug (486, 513, 514, 520–522). Isolates that have this decreased-susceptibility phenotype were not defined as resistant by the existing ciprofloxacin interpretive criteria for disk diffusion but were usually found to be nalidixic acid resistant. Nalidixic acid resistance has proved to be a useful, although not a completely accurate, laboratory marker of decreased fluoroquinolone susceptibility (337). The accumulating evidence that these strains are associated with an impaired response to ciprofloxacin and ofloxacin has led to a recent revision of the Clinical and Laboratory Standards Institute (CLSI) breakpoint guidelines such that they are now classified as intermediate (523, 524). Ciprofloxacin-intermediate strains have become common in Asia, sometime causing large outbreaks, with reports also in sub-Saharan Africa, and South America (486, 525–529). Studies with whole-genome sequencing of global Salmonella serovar Typhi collections have shown that a particular haplotype, H58, is more likely to be MDR and to have intermediate susceptibility to ciprofloxacin (530, 531). This haplotype has become dominant in many regions and may have a competitive advantage compared with other haplotypes (532–536).
Infection with Salmonella enterica isolates with intermediate susceptibility to ciprofloxacin may respond to higher doses or longer durations of treatment with ciprofloxacin or ofloxacin; failure does not occur in all cases (537). However, even if fluoroquinolone treatment of such infections is successful, higher levels of fecal shedding posttreatment may occur, driving further transmission of strains (520). Where possible, fluoroquinolones should be avoided in patients infected with strains with intermediate susceptibility to fluoroquinolones. Alternatives include extended-spectrum cephalosporins (e.g., ceftriaxone) and, in nonsevere cases, azithromycin or the traditional first-line antimicrobials, if susceptible. If fluoroquinolones are the only available option, for example, in outpatient management, they should be used at the maximum dose, such as 20 mg/kg/day of ciprofloxacin, for at least 7 days.
The later-generation fluoroquinolone gatifloxacin has been found to be an effective alternative for Salmonella infections with isolates with intermediate ciprofloxacin susceptibility. A single daily dose of 10 mg/kg for 7 days has achieved cure in more than 90% of patients and short fever clearance times (341, 480, 537, 538). Gatifloxacin targets both DNA gyrase and topoisomerase IV. Therefore, gatifloxacin is less inhibited by the common mutations of the gyrA gene of Salmonella serovar Typhi than are ciprofloxacin and ofloxacin (486). Fully fluoroquinolone-resistant Salmonella isolates with a ciprofloxacin MIC of ≥1 μg/ml are unlikely to respond to any of the fluoroquinolones. Such isolates are increasing in South Asia and are sporadically reported from other countries (350–352, 539–543).
There are some patient safety concerns with the fluoroquinolones. A caution with children is the potential for damage to growing weight-bearing joints and cartilage, based on studies in immature animals. The available evidence in humans suggests that if used in short courses the risk of an effect on growth and joint development is low and that musculo-skeletal side effects are reversible (544–551). In most countries the use of fluoroquinolones in children is relatively contraindicated except for use in multidrug resistant infections where there are no suitable alternative. The fluoroquinolones do carry a risk of tendon damage in patients over 60 years of age, those on concomitant corticosteroids, or those with a history of tendon disorders. Dysglycemia among the elderly and those with diabetes has been reported with gatifloxacin, leading to the withdrawal of the drug from the American and other markets (552). Careful monitoring of glucose levels in patients recruited to typhoid studies in Nepal have not shown adverse effects, and most typhoid fever cases in areas of endemicity occur in children and young adults rather than the elderly (341). While it is uncommon for patients with typhoid fever to have diabetes in areas of endemicity, diabetes is an emerging problem among adults in South Asia. Gatifloxacin should be avoided in patients over 50 years of age and in those with diabetes or renal failure.
Extended-Spectrum Cephalosporins
Ceftriaxone has been the principal cephalosporin evaluated in clinical trials of typhoid fever, although cefotaxime and cefoperazone have also proved effective in a few studies (459, 460). Peak drug levels of ceftriaxone of >140 μg/ml and trough levels of >22 μg/ml provide unbound concentrations well above a typical MIC of 0.03 to 0.06 μg/ml (461, 462). Ceftriaxone is generally safe to use, including in children, is slowly bactericidal against Salmonella serovar Typhi in vitro, and is able to penetrate and kill intracellular bacteria (463, 464). Ceftriaxone has been used to treat typhoid in durations of 14 days (465–467), 7 days (342, 468–470), 5 days (440, 471–473), and 3 days (442, 474, 475). The proportion of patients cured has varied from 70% to more than 90%. The resolution of symptoms is invariably slow, often with the fever clearance times of 6 to 8 days. Relapse may occur in more than 10% of patients in some studies, particularly when the duration of treatment is 7 days or less. The optimum duration of treatment is unclear. While the cost and inconvenience of parenteral administration of ceftriaxone are disadvantages, once-daily administration makes this easier. Oral extended-spectrum cephalosporins such as cefixime have also been studied. Although early results were promising (465, 472, 476–478), in two later studies the treatment response was slow and clinical failures and relapses were seen (479, 480).
Azithromycin
Azithromycin is increasingly being used for empiric treatment of uncomplicated enteric fever, especially in areas where MDR and fluoroquinolone-resistant infections are prevalent (444, 469, 501, 511, 516). The MICs of strains are usually in the range of 0.25 to 16 μg/ml (553–556). Serum levels after oral administration are 0.04 to 0.4 μg/ml (557). Azithromycin shows excellent penetration into most tissues and achieves intracellular concentrations inside macrophages and neutrophils that are 10 to 100 times greater than serum concentrations, with slow release from this intracellular site (558, 559). The drug has a long half-life of 2 to 3 days, allowing once-daily administration, and is safe to use in children. The immunomodulatory effects of azithromycin may also be important by reversing the immunoparalysis seen in typhoid (28, 29, 560). Although some initial clinical studies were disappointing (561), subsequent randomized controlled trials have confirmed azithromycin to be equivalent or superior to chloramphenicol, fluoroquinolones, and extended-spectrum cephalosporins for the management of uncomplicated typhoid fever and associated with a prompt resolution of clinical symptoms and low prevalence of relapse and convalescent fecal carriage (444, 469, 473, 501, 511, 516, 538). Doses have varied between 10 and 20 mg/kg/day for between 5 and 7 days, and the optimum dose and duration are yet to be determined. Occasional patients in these studies have demonstrated a slow clearance of bacteremia. Several studies have reported azithromycin MIC distributions for typhoidal and nontyphoidal Salmonella strains (501, 511, 562–565). These studies have led to a suggested epidemiologic MIC cutoff value of ≤16 μg/ml for wild-type strains (566, 567). Clinical interpretive criteria for disk diffusion and MIC testing have recently been adopted by CLSI for Salmonella serovar Typhi but not yet for Salmonella serovar Paratyphi A (570). There are sporadic reports of strains with an azithromycin MIC of ≥32 μg/ml but limited published data on the clinical response to azithromycin in such infections (432, 562, 563, 568, 569). The newly established CLSI guidelines for azithromycin disk diffusion and MIC interpretive criteria for Salmonella serovar Typhi were published in CLSI document M100 in 2015 (570).
Carbapenems and Tigecycline
If combined resistance to all first- and second-line drugs develops, the carbapenems (e.g., imipenem, meropenem, and ertapenem), and tigecycline could be potential alternatives (571). There are few reports describing the use of these potentially expensive drugs (572). In many areas of South Asia, increasing proportions of Salmonella isolates have regained susceptibility to the traditional first-line drugs chloramphenicol, ampicillin, and trimethoprim-sulfamethoxazole. These older drugs could be used again, although the prevalence of MDR Salmonella may rise again.
Antimicrobial Combinations
Combinations of fluoroquinolones, cephalosporins, and azithromycin are probably used quite frequently, particularly in patients who fail to respond promptly (517, 573). The potential advantages of antimicrobial combinations include a broadening of the spectrum of antimicrobial activity, particularly in the face of potential drug-resistant strains, utilization of any potential synergy between the drugs, and the potential to reduce the emergence of resistant strains during the course of treatment. However, there are few studies of the in vitro interactions between antimicrobials for Salmonella isolates (574, 575).
Pregnancy
Ampicillin, amoxicillin, and ceftriaxone are considered safe in pregnant women with enteric fever (63, 576). Fluoroquinolones have been occasionally used in pregnant patients infected with MDR Salmonella isolates (577, 578). Animal studies of azithromycin have not revealed evidence of fetotoxicity, although there are only limited controlled data in human pregnancy (579). The U.S. Food and Drug Administration has assigned azithromycin to pregnancy category B, indicating that it should be given during pregnancy only when benefit outweighs risk. Azithromycin is excreted into human milk, and the manufacturer recommends that caution be used when administering azithromycin to nursing women.
Treatment of Severe Disease
Gastrointestinal bleeding is usually self-limiting, and few patients require a blood transfusion. In exceptional circumstances, surgery, intra-arterial vasopressin, or colonoscopic interventions have been used to halt hemorrhage (580–582). The management of intestinal perforation includes nasogastric suction, administration of fluids to correct hypotension, and prompt surgery. Surgery has been demonstrated to lead to improved survival compared with a conservative approach (73). The survival of patients undergoing surgery for perforation is generally 70 to 75% but reaches 97% in the best series (72–74). In contrast, approximately 30% of conservatively managed patients survive. Simple closure of perforations is usually adequate, although procedures to bypass severely affected sections of the ileum are sometimes used. Closure of perforations should be accompanied by vigorous peritoneal toilet. Metronidazole or clindamycin should be added to the therapy of ceftriaxone- or fluoroquinolone-treated patients. Metronidazole and aminoglycosides are recommended for patients receiving chloramphenicol, ampicillin, or trimethoprim-sulfamethoxazole.
Enteric fever patients with altered consciousness and hemodynamic shock have high case fatality ratios. A study in Jakarta showed that high doses of dexamethasone substantially reduced the mortality of such severe cases. The criterion for entry to the study was marked mental confusion or shock. Among adults treated with chloramphenicol, dexamethasone at 3 mg/kg infused intravenously over half an hour, followed by eight doses of 1 mg/kg every 6 h, resulted in a 10% case fatality ratio, compared to 55.6% among controls (76). A study using historical controls demonstrated a comparable benefit in children (77). Whether lower doses of glucocorticosteroids would have a similar effect is unclear. A nonrandomized study in Papua New Guinea using lower equivalent doses of hydrocortisone failed to replicate this result (78). It is also unclear whether enteric fever caused by susceptible typhoidal Salmonella treated with a second-line antimicrobial agent such as ceftriaxone or a fluoroquinolone would achieve a similar result. It has proved difficult to replicate this study because the number of severe typhoid patients has decreased, possibly because of the ready availability of over-the-counter antimicrobials.
Treatment of Chronic Carriage
Eradication of chronic carriage has been achieved with longer durations of antimicrobial therapy than are required for management of acute infection. The choice of antimicrobial depends on the susceptibility of the strain. Ampicillin or amoxicillin, sometimes combined with probenecid, trimethoprim-sulfamethoxazole, and fluoroquinolones have been used with some success (583–591). Cholecystectomy can be considered if antimicrobials fail, but the surgery can carry risks, and there should be additional indications for operation. The success of surgery for the elimination of chronic carriage is increased by giving antimicrobials at the same time (592).
Evidence Needs for Antimicrobial Management
Most physicians base their therapeutic choices on international or country guidelines, when available, or expert opinion (593–596). Experience based on routine practice may be unreliable. In low-resource areas, the diagnosis of invasive Salmonella infection is usually unconfirmed, and patients treated as outpatients who do not improve or subsequently relapse may return to a different health care facility. Antimicrobials are easily available without prescription in pharmacies and shops in most developing countries, and counterfeit or substandard antimicrobials are likely to be common (597). The detection and management of convalescent and chronic carriage are of crucial public health importance, particularly in the light of widespread drug resistance, but have received little attention.
The evidence base of randomized controlled trials to guide enteric fever treatment is limited and particularly so for invasive nontyphoidal salmonellosis. Many randomized trials have been small and underpowered to demonstrate significant differences between antimicrobial choices. Systematic reviews of the evidence from existing studies are useful (505, 598). Further well-conducted randomized clinical trials, with detailed microbiology and accompanying pharmacokinetic and pharmacodynamic analysis, are essential to define safe and effective regimens for patients. Such trials are likely to require multicenter designs to be able to recruit sufficient patients over a reasonable period of time.
The appropriate timing of and early and late outcomes following antiretroviral therapy in patients presenting with invasive NTS are almost entirely unknown. This is an area where further study and practical recommendations would be useful.
CONCLUSIONS
Salmonella enterica infections are common causes of bloodstream infection in low-resource areas, where they may be difficult to distinguish from other febrile illnesses and may be associated with a high case fatality ratio. Microbiologic culture of blood or bone marrow remains the mainstay of laboratory diagnosis. Antimicrobial resistance has emerged in Salmonella enterica, initially to the traditional first-line drugs chloramphenicol, ampicillin, and trimethoprim-sulfamethoxazole. Decreased fluoroquinolone susceptibility and then fluoroquinolone resistance have developed in association with chromosomal mutations in the quinolone resistance-determining regions of genes encoding DNA gyrase and topoisomerase IV and also by plasmid-mediated resistance mechanisms. In 2013, CLSI lowered the ciprofloxacin susceptibility breakpoints to account for accumulating clinical, microbiologic, and pharmacokinetic-pharmacodynamic data suggesting that revision was needed for contemporary invasive Salmonella infections (523). Resistance to extended-spectrum cephalosporins has occurred more often in nontyphoidal than in typhoidal Salmonella strains. Azithromycin is effective for the management of uncomplicated typhoid fever and may serve as an alternative oral drug in areas where fluoroquinolone resistance is common. Newly established CLSI guidelines for azithromycin disk diffusion and MIC interpretive criteria for Salmonella serovar Typhi were published in CLSI document M100 in 2015 (570).
ACKNOWLEDGMENTS
We thank France Mentré for helpful discussions and Lorna Saint Ange for editing.
J.A.C. is supported by the joint U.S. National Institutes of Health-National Science Foundation Ecology and Evolution of Infectious Disease program (R01 TW009237), by the United Kingdom Biotechnology and Biological Sciences Research Council (BBSRC) (BB/J010367/1), and by United Kingdom BBSRC Zoonoses in Emerging Livestock Systems awards BB/L017679, BB/L018926, and BB/L018845. This work was supported in part by EU-FP7 R-Gnosis and EvoTAR programs and by OSEO-Nosobio.
The authors have no conflicts of interest.
The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the views, decisions, or policies of the U.S. Centers for Disease Control and Prevention.
Biographies

John A. Crump is McKinlay Professor of Global Health and Co-Director, Centre for International Health, University of Otago, New Zealand, Adjunct Professor of Medicine, Pathology, and Global Health, Duke University, and Guest Researcher, U.S. Centers for Disease Control and Prevention. He qualified in medicine from the University of Otago and trained in internal medicine, infectious diseases, medical microbiology, and medical epidemiology in New Zealand, Australia, the United Kingdom, the United States, and Tanzania. He led a clinical research program in Tanzania for a decade, and his ongoing collaborative research is based there and in Myanmar. His current research interests are in the prevention, diagnosis, and treatment of infectious diseases in developing countries, with particular focus on febrile illness, invasive bacterial diseases (especially the salmonelloses), bacterial zoonoses, and enteric infections.

Maria Sjölund-Karlsson is a Senior Service Fellow with the Division of Healthcare Quality Promotion at the U.S. Centers for Disease Control and Prevention (CDC). She received her Ph.D. in clinical bacteriology from the Faculty of Medicine at Uppsala University, Sweden. She subsequently worked at the Swedish Reference Laboratory for Antimicrobial Susceptibility Testing, which also serves as a reference laboratory for the European Committee on Antimicrobial Susceptibility Testing (EUCAST). In 2007, she joined the CDC, working with antimicrobial resistance surveillance of enteric bacteria through the U.S. National Antimicrobial Resistance Monitoring System (NARMS). With NARMS, she performed research related to cephalosporin and fluoroquinolone resistance development in Salmonella enterica and collaborated with the Clinical and Laboratory Standards Institute (CLSI) to enhance susceptibility testing methods for Salmonella enterica. In her current role with the CDC Division of Healthcare Quality Promotion, she oversees molecular characterization and typing studies of emerging health care-associated surveillance isolates, including Clostridium difficile and carbapenem-resistant Enterobacteriaceae.

Melita A. Gordon is a Reader in Gastroenterology at the University of Liverpool. She is a clinical scientist and gastroenterologist who has worked on invasive Salmonella disease in Africa since 1997. Since being a Wellcome Trust Fellow in Malawi, she has investigated the clinical epidemiology, the presentation and adverse clinical outcomes, and the host response and molecular and cellular pathogenesis of invasive nontyphoidal Salmonella disease in HIV-infected adults, describing in particular the dysregulation of the host response and the intracellular nature of infection. She has also described the emergence of sequential epidemics of typhoidal and nontyphoidal invasive Salmonella disease associated with the emergence of multidrug resistance and novel nontyphoidal Salmonella strains. She was awarded the Sir Francis Avery Jones research medal of the British Society of Gastroenterology and the SAGE award for Excellence in Gastroenterology.

Christopher M. Parry is a Professor at the School of Tropical Medicine and Global Health, Nagasaki University, Nagasaki, Japan, and Senior Lecturer at the London School of Hygiene and Tropical Medicine. He qualified in natural sciences and medicine from Cambridge University and trained in internal medicine, infectious diseases, clinical microbiology, and tropical medicine in the United Kingdom and Malawi. Over the past 20 years he has worked in the Oxford South East Asia Tropical Network in Vietnam, Cambodia, and Bangladesh and at the Liverpool School of Tropical Medicine and the University of Liverpool. His current research interests concern the epidemiology, diagnosis, and management of severe bacterial infections, including typhoid fever, leptospirosis, and tuberculosis, with ongoing studies in the Philippines and Cambodia. He teaches in the Masters in Tropical Medicine programs in London and Nagasaki.
REFERENCES
- 1.Reddy EA, Shaw AV, Crump JA. 2010. Community acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis 10:417–432. doi: 10.1016/S1473-3099(10)70072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deen J, von Seidlein L, Andersen F, Elle N, White NJ, Lubell Y. 2012. Community-acquired bacterial bloodstream infections in developing countries in south and southeast Asia: a systematic review. Lancet Infect Dis 12:480–487. doi: 10.1016/S1473-3099(12)70028-2. [DOI] [PubMed] [Google Scholar]
- 3.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. 2012. Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379:2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crump JA, Luby SP, Mintz ED. 2004. The global burden of typhoid fever. Bull World Health Organ 82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 5.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, et al. 2012. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 6.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mogasale V, Maskery B, Ochiai RL, Lee JS, Mogasale VV, Ramani E, Kim YE, Park JK, Wierzba TF. 2014. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob Health 2:e570–580. doi: 10.1016/S2214-109X(14)70301-8. [DOI] [PubMed] [Google Scholar]
- 8.Breiman RF, Cosmas L, Njuguna H, Audi A, Olack B, Ochieng JB, Wamola N, Bigogo GM, Awiti G, Tabu CW, Burke H, Williamson J, Oundo JO, Mintz ED, Feikin DR. 2012. Population-based incidence of typhoid fever in an urban informal settlement, Nairobi, Kenya: implications for typhoid vaccine use in Africa. PLoS One 7:e29119. doi: 10.1371/journal.pone.0029119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crump JA. 2012. Typhoid fever and the challenge of nonmalaria febrile illness in sub-Saharan Africa. Clin Infect Dis 54:1107–1109. doi: 10.1093/cid/cis024. [DOI] [PubMed] [Google Scholar]
- 10.Woods CW, Murdoch DR, Zimmerman MD, Glover WA, Basnyat B, Wolf L, Belbase RH, Reller LB. 2006. Emergence of Salmonella enterica serotype Paratyphi A as a major cause of enteric fever in Kathmandu, Nepal. Trans R Soc Trop Med Hyg 100:1063–1067. doi: 10.1016/j.trstmh.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Ochiai RL, Wang X, von Seidlein L, Yang J, Bhutta ZA, Bhattacharya SK, Agtini M, Deen JL, Wain J, Kim DR, Ali M, Acosta CJ, Jodar L, Clemens JD. 2005. Salmonella Paratyphi A rates, Asia. Emerg Infect Dis 11:1764–1766. doi: 10.3201/eid1111.050168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynch MF, Blanton EM, Bulens S, Polyak C, Vojdani J, Stevenson J, Medalla F, Barzilay E, Joyce K, Barrett T, Mintz ED. 2009. Typhoid fever in the United States, 1999-2006. JAMA 302:859–865. doi: 10.1001/jama.2009.1229. [DOI] [PubMed] [Google Scholar]
- 13.Olsen SJ, Bleasdale SC, Magnano AR, Landrigan C, Holland BH, Tauxe RV, Mintz ED, Luby SP. 2003. Outbreaks of typhoid fever in the United States, 1960-1999. Epidemiol Infect 130:13–21. doi: 10.1017/S0950268802007598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khosla SN, Jain N, Khosla A. 1993. Gastric acid secretion in typhoid fever. Postgrad Med J 69:121–123. doi: 10.1136/pgmj.69.808.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhalla S, Vij JC, Anand BS, Varghese A, Chuttani HK. 1985. Gastric acid secretion in patients with typhoid fever. Gut 26:491–494. doi: 10.1136/gut.26.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiwari RP, Sachdeva N, Hoondal GS, Grewal JS. 2004. Adaptive acid tolerance response in Salmonella enterica serovar Typhimurium and Salmonella enterica serovar Typhi. J Basic Microbiol 44:137–146. doi: 10.1002/jobm.200310333. [DOI] [PubMed] [Google Scholar]
- 17.Bhan MK, Bahl R, Sazawal S, Sinha A, Kumar R, Mahalanabis D, Clemens JD. 2002. Association between Helicobacter pylori infection and increased risk of typhoid fever. J Infect Dis 186:1857–1860. doi: 10.1086/345762. [DOI] [PubMed] [Google Scholar]
- 18.Vollaard AM, Verspaget HW, Ali S, Visser LG, Veenendaal RA, Van Asten HA, Widjaja S, Surjadi CH, Van Dissel JT. 2006. Helicobacter pylori infection and typhoid fever in Jakarta, Indonesia. Epidemiol Infect 134:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pier GB, Grout M, Zaidi T, Meluleni G, Mueschenborn SS, Banting G, Ratcliff R, Evans MJ, Colledge WH. 1998. Salmonella Typhi uses CFTR to enter intestinal epithelial cells. Nature 393:79–82. doi: 10.1038/30006. [DOI] [PubMed] [Google Scholar]
- 20.Lyczak JB, Zaidi TS, Grout M, Bittner M, Contreras I, Pier GB. 2001. Epithelial cell contact-induced alterations in Salmonella enterica serovar Typhi lipopolysaccharide are critical for bacterial internalization. Cell Microbiol 3:763–772. doi: 10.1046/j.1462-5822.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- 21.Balakrishna AM, Saxena AM, Mok HY, Swaminathan K. 2009. Structural basis of typhoid: Salmonella Typhi type IVb pilin (PilS) and cystic fibrosis transmembrane conductance regulator interaction. Proteins 77:253–261. doi: 10.1002/prot.22500. [DOI] [PubMed] [Google Scholar]
- 22.Lyczak JB, Pier GB. 2002. Salmonella enterica serovar Typhi modulates cell surface expression of its receptor, the cystic fibrosis transmembrane conductance regulator, on the intestinal epithelium. Infect Immun 70:6416–6423. doi: 10.1128/IAI.70.11.6416-6423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyczak JB. 2003. Commensal bacteria increase invasion of intestinal epithelium by Salmonella enterica serovar Typhi. Infect Immun 71:6610–6614. doi: 10.1128/IAI.71.11.6610-6614.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van de Vosse E, Ali S, de Visser AW, Surjadi C, Widjaja S, Vollaard AM, van Dissel JT. 2005. Susceptibility to typhoid fever is associated with a polymorphism in the cystic fibrosis transmembrane conductance regulator (CFTR). Hum Genet 118:138–140. doi: 10.1007/s00439-005-0005-0. [DOI] [PubMed] [Google Scholar]
- 25.van de Vosse E, de Visser AW, Al-Attar S, Vossen R, Ali S, van Dissel JT. 2010. Distribution of CFTR variations in an Indonesian enteric fever cohort. Clin Infect Dis 50:1231–1237. doi: 10.1086/651598. [DOI] [PubMed] [Google Scholar]
- 26.Dunstan SJ, Stephens HA, Blackwell JM, Duc CM, Lanh MN, Dudbridge F, Phuong CX, Luxemburger C, Wain J, Ho VA, Hien TT, Farrar J, Dougan G. 2001. Genes of the class II and class III major histocompatibility complex are associated with typhoid fever in Vietnam. J Infect Dis 183:261–268. doi: 10.1086/317940. [DOI] [PubMed] [Google Scholar]
- 27.Bhutta ZA, Mansoorali N, Hussain R. 1997. Plasma cytokines in paediatric typhoidal salmonellosis: correlation with clinical course and outcome. J Infect 35:253–256. doi: 10.1016/S0163-4453(97)93004-8. [DOI] [PubMed] [Google Scholar]
- 28.Keuter M, Dharmana E, Gasem MH, van der Ven-Jongekrijg J, Djokomoeljanto R, Dolmans WM, Demacker P, Sauerwein R, Gallati H, van der Meer JW. 1994. Patterns of proinflammatory cytokines and inhibitors during typhoid fever. J Infect Dis 169:1306–1311. doi: 10.1093/infdis/169.6.1306. [DOI] [PubMed] [Google Scholar]
- 29.House D, Chinh NT, Hien TT, Parry CM, Ly NT, Diep TS, Wain J, Dunstan S, White NJ, Dougan G, Farrar JJ. 2002. Cytokine release by lipopolysaccharide-stimulated whole blood from patients with typhoid fever. J Infect Dis 186:240–245. doi: 10.1086/341298. [DOI] [PubMed] [Google Scholar]
- 30.Ali S, Vollaard AM, Kremer D, de Visser AW, Martina CA, Widjaja S, Surjadi C, Slagboom E, van de Vosse E, van Dissel JT. 2007. Polymorphisms in proinflammatory genes and susceptibility to typhoid fever and paratyphoid fever. J Interferon Cytokine Res 27:271–279. doi: 10.1089/jir.2006.0129. [DOI] [PubMed] [Google Scholar]
- 31.Dharmana E, Joosten I, Tijssen HJ, Gasem MH, Indarwidayati R, Keuter M, Dolmans WM, Van Der Meer JW. 2002. HLA-DRB1*12 is associated with protection against complicated typhoid fever, independent of tumour necrosis factor alpha. Eur J Immunogenet 29:271–280. [DOI] [PubMed] [Google Scholar]
- 32.Dunstan SJ, Nguyen TH, Rockett K, Forton J, Morris AP, Diakite M, Mai NL, Le TP, House D, Parry CM, Ha V, Nguyen TH, Dougan G, Tran TH, Kwiatowski D, Farrar JJ. 2007. A TNF region haplotype offers protection from typhoid fever in Vietnamese patients. Hum Genet 122:51–61. doi: 10.1007/s00439-007-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunstan SJ, Hue NT, Han B, Li Z, Tram TT, Sim KS, Parry CM, Chinh NT, Vinh H, Lan NP, Thieu NT, Vinh PV, Koirala S, Dongol S, Arjyal A, Karkey A, Shilpakar O, Dolecek C, Foo JN, Phuong LT, Lanh MN, Do T, Aung T, Hon DN, Teo YY, Hibberd ML, Anders KL, Okada Y, Raychaudhuri S, Simmons CP, Baker S, de Bakker PI, Basnyat B, Hien TT, Farrar JJ, Khor CC. 2014. Variation at HLA-DRB1 is associated with resistance to enteric fever. Nat Genet 46:1333–1336. doi: 10.1038/ng.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunstan SJ, Hawn TR, Hue NT, Parry CP, Ho VA, Vinh H, Diep TS, House D, Wain J, Aderem A, Hien TT, Farrar JJ. 2005. Host susceptibility and clinical outcomes in Toll-like receptor 5-deficient patients with typhoid fever in Vietnam. J Infect Dis 191:1068–1071. doi: 10.1086/428593. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen TH, Mai NL, Le TP, Ha V, Nguyen TC, Tran TH, Nguyen TH, Farrar JJ, Dunstan SJ. 2009. Toll-like receptor 4 (TLR4) and typhoid fever in Vietnam. PLoS One 4:e4800. doi: 10.1371/journal.pone.0004800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunstan SJ, Ho VA, Duc CM, Lanh MN, Phuong CX, Luxemburger C, Wain J, Dudbridge F, Peacock CS, House D, Parry C, Hien TT, Dougan G, Farrar J, Blackwell JM. 2001. Typhoid fever and genetic polymorphisms at the natural resistance-associated macrophage protein 1. J Infect Dis 183:1156–1160. doi: 10.1086/319289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali S, Vollaard AM, Widjaja S, Surjadi C, van de Vosse E, van Dissel JT. 2006. PARK2/PACRG polymorphisms and susceptibility to typhoid and paratyphoid fever. Clin Exp Immunol 144:425–431. doi: 10.1111/j.1365-2249.2006.03087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gotuzzo E, Frisancho O, Sanchez J, Liendo G, Carrillo C, Black RE, Morris JG Jr. 1991. Association between the acquired immunodeficiency syndrome and infection with Salmonella Typhi or Salmonella Paratyphi in an endemic typhoid area. Arch Intern Med 151:381–382. [PubMed] [Google Scholar]
- 39.Crump JA, Ramadhani HO, Morrissey AB, Saganda W, Mwako MS, Yang L-Y, Chow S-C, Morpeth SC, Reyburn H, Njau BN, Shaw AV, Diefenthal HC, Shao JF, Bartlett JA, Maro VP. 2011. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin Infect Dis 52:341–348. doi: 10.1093/cid/ciq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osler W. 1912. The principles and practice of medicine: designed for the use of practitioners and students of medicine, 8th ed D Appleton and Company, New York, NY. [Google Scholar]
- 41.Stuart BM, Pullen RL. 1946. Typhoid: clinical analysis of 360 cases. Arch Intern Med 78:629–661. doi: 10.1001/archinte.1946.00220060002001. [DOI] [PubMed] [Google Scholar]
- 42.Huckstep RL. 1962. Typhoid fever and other Salmonella infections. E & S Livingstone, Edinburgh, Scotland. [Google Scholar]
- 43.Lin F-YC, Vo AH, Phan VB, Nguyen TT, Bryla D, Tran CT, Ha BK, Dang DT, Robbins JB. 2000. The epidemiology of typhoid fever in the Dong Thap Province, Mekong Delta region of Vietnam. Am J Trop Med and Hyg 62:644–648. [DOI] [PubMed] [Google Scholar]
- 44.Sinha A, Sazawal S, Kumar R, Sood S, Reddaiah VP, Singh B, Rao M, Naficy A, Clemens JD, Bhan MK. 1999. Typhoid fever in children aged less than 5 years. Lancet 354:734–737. doi: 10.1016/S0140-6736(98)09001-1. [DOI] [PubMed] [Google Scholar]
- 45.Kasper MR, Sokhal B, Blair PJ, Wierzba TF, Putnam SD. 2010. Emergence of multidrug-resistant Salmonella enterica serovar Typhi with reduced susceptibility to fluoroquinolones in Cambodia. Diagn Microbiol Infect Dis 66:207–209. doi: 10.1016/j.diagmicrobio.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Ferreccio C, Levine MM, Manterola A, Rodriguez G, Rivara I, Prenzel I, Black RE, Mancuso T, Bulas D. 1984. Benign bacteremia caused by Salmonella Typhi and Paratypi in children aged younger than 2 years. J Pediatr 104:899–900. doi: 10.1016/S0022-3476(84)80492-8. [DOI] [PubMed] [Google Scholar]
- 47.Topley JM. 1986. Mild typhoid fever. Arch Dis Child 61:164–167. doi: 10.1136/adc.61.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahle WT, Levine MM. 1993. Salmonella Typhi infection in children younger than five years of age. Pediatr Infect Dis J 12:627–631. doi: 10.1097/00006454-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 49.Waddington CS, Darton TC, Pollard AJ. 2014. The challenge of enteric fever. J Infect 68:S38–S50. doi: 10.1016/j.jinf.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Waddington CS, Darton TC, Woodward WE, Angus B, Levine MM, Pollard AJ. 2014. Advancing the management and control of typhoid fever: a review of the historical role of human challenge studies. J Infect 68:405–418. doi: 10.1016/j.jinf.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Waddington CS, Darton TC, Jones C, Haworth K, Peters A, John T, Thompson BA, Kerridge SA, Kingsley RA, Zhou L, Holt KE, Yu LM, Lockhart S, Farrar JJ, Sztein MB, Dougan G, Angus B, Levine MM, Pollard AJ. 2014. An outpatient, ambulant-design, controlled human infection model using escalating doses of Salmonella Typhi challenge delivered in sodium bicarbonate solution. Clin Infect Dis 58:1230–1240. doi: 10.1093/cid/ciu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. 2002. Typhoid fever. N Engl J Med 347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 53.Cunha BA, Gran A, Munoz-Gomez S. 2013. Typhoid fever vs. malaria in a febrile returning traveler: typhomalaria revisited: an Oslerian perspective. Travel Med Infect Dis 11:66–69. doi: 10.1016/j.tmaid.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Ellis ME, Moosa A, Hillier V. 1990. A review of typhoid fever in South African black children. Postgrad Med J 66:1032–1036. doi: 10.1136/pgmj.66.782.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ostergaard L, Huniche B, Andersen PL. 1996. Relative bradycardia in infectious diseases. J Infect 33:185–191. doi: 10.1016/S0163-4453(96)92225-2. [DOI] [PubMed] [Google Scholar]
- 56.Davis TM, Makepeace AE, Dallimore EA, Choo KE. 1999. Relative bradycardia is not a feature of enteric fever in children. Clin Infect Dis 28:582–586. doi: 10.1086/515143. [DOI] [PubMed] [Google Scholar]
- 57.Cunha BA. 2000. The diagnostic significance of relative bradycardia in infectious disease. Clin Microbiol Infect 6:633–634. doi: 10.1046/j.1469-0691.2000.0194f.x. [DOI] [PubMed] [Google Scholar]
- 58.Maskey AP, Basnyat B, Thwaites GE, Campbell JI, Farrar JJ, Zimmerman MD. 2008. Emerging trends in enteric fever in Nepal: 9124 cases confirmed by blood culture 1993-2003. Trans R Soc Trop Med Hyg 102:91–95. doi: 10.1016/j.trstmh.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Bhutta ZA. 1996. Impact of age and drug resistance on mortality in typhoid fever. Arch Dis Child 75:214–217. doi: 10.1136/adc.75.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scragg J, Rubidge C, Wallace HL. 1969. Typhoid fever in African and Indian children in Durban. Arch Dis Child 44:18–28. doi: 10.1136/adc.44.233.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duggan MB, Beyer L. 1975. Enteric fever in young Yoruba children. Arch Dis Child 50:67–71. doi: 10.1136/adc.50.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abuekteish F, Daoud AS, Massadeh H, Rawashdeh M. 1996. Salmonella Typhi meningitis in infants. Indian Pediatr 33:1037–1040. [PubMed] [Google Scholar]
- 63.Seoud M, Saade G, Uwaydah M, Azoury R. 1988. Typhoid fever in pregnancy. Obstet Gynecol 71:711–714. [PubMed] [Google Scholar]
- 64.Reed RP, Klugman KP. 1994. Neonatal typhoid fever. Pediatr Infect Dis J 13:774–777. doi: 10.1097/00006454-199409000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Butler T, Islam A, Kabir I, Jones PK. 1991. Patterns of morbidity and mortality in typhoid fever dependent on age and gender: review of 552 hospitalized patients with diarrhea. Rev Infect Dis 13:85–90. doi: 10.1093/clinids/13.1.85. [DOI] [PubMed] [Google Scholar]
- 66.Hoa NTT, Diep TS, Wain J, Parry CM, Hien TT, Smith MD, Walsh AL, White NJ. 1998. Community-acquired septicaemia in southern Viet Nam: the importance of multidrug-resistant Salmonella Typhi. Trans R Soc Trop Med Hyg 92:503–508. doi: 10.1016/S0035-9203(98)90891-4. [DOI] [PubMed] [Google Scholar]
- 67.Walia M, Gaind R, Mehta R, Paul P, Aggarwal P, Kalaivani M. 2005. Current perspectives of enteric fever: a hospital-based study from India. Ann Trop Paediatr 25:161–174. doi: 10.1179/146532805X58085. [DOI] [PubMed] [Google Scholar]
- 68.Maskey AP, Day JN, Phung QT, Thwaites GE, Campbell JI, Zimmerman M, Farrar JJ, Basnyat B. 2006. Salmonella enterica serovar Paratyphi A and S. enterica serovar Typhi cause indistinguishable clinical syndromes in Kathmandu, Nepal. Clin Infect Dis 42:1247–1253. doi: 10.1086/503033. [DOI] [PubMed] [Google Scholar]
- 69.Bhan MK, Bahl R, Bhatnagar S. 2005. Typhoid and paratyphoid fever. Lancet 366:749–762. doi: 10.1016/S0140-6736(05)67181-4. [DOI] [PubMed] [Google Scholar]
- 70.Parry CM, Thompson C, Vinh H, Chinh NT, Phuong LT, Ho VA, Hien TT, Wain J, Farrar JJ, Baker S. 2014. Risk factors for the development of severe typhoid fever in Vietnam. BMC Infect Dis 14:73. doi: 10.1186/1471-2334-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang DB, DuPont HL. 2005. Problem pathogens: extra-intestinal complications of Salmonella enterica serotype Typhi infection. Lancet Infect Dis 5:341–348. doi: 10.1016/S1473-3099(05)70138-9. [DOI] [PubMed] [Google Scholar]
- 72.Bitar R, Tarpley J. 1985. Intestinal perforation in typhoid fever: a historical and state-of-the-art review. Rev Infect Dis 7:257–271. doi: 10.1093/clinids/7.2.257. [DOI] [PubMed] [Google Scholar]
- 73.Butler T, Knight J, Nath SK, Speelman P, Roy SK, Azad MAK. 1985. Typhoid fever complicated by intestinal perforation: a persisting fatal disease requiring surgical management. Rev Infect Dis 7:244–256. doi: 10.1093/clinids/7.2.244. [DOI] [PubMed] [Google Scholar]
- 74.van Basten JP, Stockenbrugger R. 1994. Typhoid perforation: a review of the literature since 1960. Trop Geogr Med 46:336–339. [PubMed] [Google Scholar]
- 75.Walia M, Gaind R, Paul P, Mehta R, Aggarwal P, Kalaivani M. 2006. Age-related clinical and microbiological characteristics of enteric fever in India. Trans R Soc Trop Med Hyg 100:942–948. doi: 10.1016/j.trstmh.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 76.Hoffman SL, Punjabi NH, Kumala S, Moechtar MA, Pulungsih SP, Rivai AR, Rockhill RC, Woodward TE, Loedin AA. 1984. Reduction in mortality in chloramphenicol-treated severe typhoid fever by high-dose dexamethasone. N Engl J Med 310:82–88. doi: 10.1056/NEJM198401123100203. [DOI] [PubMed] [Google Scholar]
- 77.Punjabi NH, Hoffman SL, Edman DC, Sukri N, Laughlin LW, Pulungsih SP, Rivai AR, Sututo Moechtar A, Woodward TE. 1988. Treatment of severe typhoid fever in children with high dose dexamethasone. Pediatr Infect Dis J 7:598–600. doi: 10.1097/00006454-198808000-00002. [DOI] [PubMed] [Google Scholar]
- 78.Rogerson SJ, Spooner VJ, Smith TA, Richens J. 1991. Hydrocortisone in chloramphenicol-treated severe typhoid fever in Papua New Guinea. Trans R Soc Trop Med Hyg 85:113–116. doi: 10.1016/0035-9203(91)90180-7. [DOI] [PubMed] [Google Scholar]
- 79.Mohanty S, Gaind R, Sehgal R, Chellani H, Deb M. 2009. Neonatal sepsis due to Salmonella Typhi and Paratyphi A. J Infect Dev Ctries 3:633–638. [DOI] [PubMed] [Google Scholar]
- 80.van den Bergh ETAM, Hussein GM, Keuter M, Dolmans MV. 1999. Outcome in three groups of patients with typhoid fever in Indonesia between 1948 and 1990. Trop Med Int Health 4:211–215. doi: 10.1046/j.1365-3156.1999.43374.x. [DOI] [PubMed] [Google Scholar]
- 81.Khan M, Coovadia YM, Connolly C, Sturm AW. 1999. Influence of sex on clinical features, laboratory findings, and complications of typhoid fever. Am J Trop Med Hyg 61:41–46. [DOI] [PubMed] [Google Scholar]
- 82.Wain J, Hien TT, Connerton P, Ali T, Parry CM, Chinh NT, Vinh H, Phuong CX, Ho VA, Diep TS, Farrar JJ, White NJ, Dougan G. 1999. Molecular typing of multiple-antibiotic-resistant Salmonella enterica serovar Typhi from Vietnam: application to acute and relapse cases of typhoid fever. J Clin Microbiol 37:2466–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hermans PW, Saha SK, van Leeuwen WJ, Verbrugh HA, van Belkum A, Goessens WH. 1996. Molecular typing of Salmonella Typhi strains from Dhaka (Bangladesh) and development of DNA probes identifying plasmid-encoded multidrug-resistant isolates. J Clin Microbiol 34:1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marmion DE, Naylor GR, Stewart IO. 1953. Second attacks of typhoid fever. J Hyg (Lond) 51:260–267. doi: 10.1017/S0022172400015680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Caygill CP, Braddick M, Hill MJ, Knowles RL, Sharp JC. 1995. The association between typhoid carriage, typhoid infection and subsequent cancer at a number of sites. Eur J Cancer Prev 4:187–193. doi: 10.1097/00008469-199504000-00010. [DOI] [PubMed] [Google Scholar]
- 86.Welton JC, Marr JS, Friedman SM. 1979. Association between hepatobiliary cancer and typhoid carrier state. Lancet 313:791–794. doi: 10.1016/S0140-6736(79)91315-1. [DOI] [PubMed] [Google Scholar]
- 87.Shukla VK, Singh H, Pandey M, Upadhyay SK, Nath G. 2000. Carcinoma of the gallbladder: is it a sequel of typhoid? Dig Dis Sci 45:900–903. doi: 10.1023/A:1005564822630. [DOI] [PubMed] [Google Scholar]
- 88.Levine MM, Black RE, Lanata C, Chilean Typhoid Committee . 1982. Precise estimation of the numbers of chronic carriers of Salmonella Typhi in Santiago, Chile, an endemic area. J Infect Dis 146:724–726. doi: 10.1093/infdis/146.6.724. [DOI] [PubMed] [Google Scholar]
- 89.Braddick MR, Crump BJ, Yee ML. 1991. How long should patients with Salmonella Typhi or Salmonella Paratyphi be followed-up? A comparison of published guidelines. J Public Health Med 13:101–107. [PubMed] [Google Scholar]
- 90.Losonsky GA, Ferreccio C, Kotloff KL, Kaintuck S, Robbins JB, Levine MM. 1987. Development and evaluation of an enzyme-linked immunosorbent assay for serum Vi antibodies for detection of chronic Salmonella Typhi carriers. J Clin Microbiol 25:2266–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin F-YC, Becke JM, Groves C, Lim BP, Israel E, Becker EF, Helfrich RM, Swetter DS, Cramton T, Robbins JB. 1988. Restaurant-associated outbreak of typhoid fever in Maryland: identification of carrier facilitated by measurement of serum Vi antibodies. J Clin Microbiol 26:1194–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nolan CM, Feeley JC, White PC, Hambie EA, Brown SL, Wong KH. 1980. Evaluation of a new assay for Vi antibody in chronic carriers of Salmonella Typhi. J Clin Microbiol 12:22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Engleberg NC, Barrett TJ, Fisher H, Porter B, Hurtado E, Hughes JM. 1983. Identification of a carrier by using Vi enzyme-linked immunosorbent assay serology in an outbreak of typhoid fever in an Indian reservation. J Clin Microbiiol 18:1320–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gupta A, My Thanh NT, Olsen SJ, Sivapalasingam S, My Trinh TT, Phuong Lan NT, Hoekstra RM, Bibb W, Minh NT, Danh TP, Cam PD, Mintz ED. 2006. Evaluation of community-based serologic screening for identification of chronic Salmonella Typhi carriers in Vietnam. Int J Infect Dis 10:309–314. doi: 10.1016/j.ijid.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 95.Nath G, Mauryal P, Gulati AK, Singh TB, Srivastava R, Kumar K, Tripathi SK. 2010. Comparison of Vi serology and nested PCR in diagnosis of chronic typhoid carriers in two different study populations in typhoid endemic area of India. Southeast Asian J Trop Med Pub Health 41:636–640. [PubMed] [Google Scholar]
- 96.Anonymous. 1920. Typhoid in the large cities of the United States in 1919: eighth annual report. JAMA 74:672–675. [Google Scholar]
- 97.Nga TV, Parry CM, Le T, Lan NP, Diep TS, Campbell JI, Hoang NV, Dung LT, Wain J, Dolecek C, Farrar JJ, Chau NV, Hien TT, Day JN, Baker S. 2012. The decline of typhoid and the rise of non-typhoid salmonellae and fungal infections in a changing HIV landscape: bloodstream infection trends over 15 years in southern Vietnam. Trans R Soc Trop Med Hyg 106:26–34. doi: 10.1016/j.trstmh.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 98.United Nations. 2014. Millenium development goals. http://www.un.org/millenniumgoals/ Accessed 1 October 2014.
- 99.Fraser A, Goldberg E, Acosta CJ, Paul M, Leibovici L. 2007. Vaccines for preventing typhoid fever. Cochrane Database Syst Rev 3:CD001261. [DOI] [PubMed] [Google Scholar]
- 100.Sur D, Ochiai RL, Bhattacharya SK, Ganguly NK, Ali M, Manna B, Dutta S, Donner A, Kanungo S, Park JK, Puri MK, Kim DR, Dutta D, Bhaduri B, Acosta CJ, Clemens JD. 2009. A cluster-randomized effectiveness trial of Vi typhoid vaccine in India. N Engl J Med 361:335–344. doi: 10.1056/NEJMoa0807521. [DOI] [PubMed] [Google Scholar]
- 101.Lin FY, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC, Kossaczka Z, Bryla DA, Shiloach J, Robbins JB, Schneerson R, Szu SC. 2001. The efficacy of a Salmonella Typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med 344:1263–1269. doi: 10.1056/NEJM200104263441701. [DOI] [PubMed] [Google Scholar]
- 102.Bhutta ZA, Capeding MR, Bavdekar A, Marchetti E, Ariff S, Soofi SB, Anemona A, Habib MA, Alberto E, Juvekar S, Khan RM, Marhaba R, Ali N, Malubay N, Kawade A, Saul A, Martin LB, Podda A. 2014. Immunogenicity and safety of the Vi-CRM197 conjugate vaccine against typhoid fever in adults, children, and infants in south and southeast Asia: results from two randomised, observer-blind, age de-escalation, phase 2 trials. Lancet Infect Dis 14:119–129. doi: 10.1016/S1473-3099(13)70241-X. [DOI] [PubMed] [Google Scholar]
- 103.Levine MM, Ferreccio C, Black RE, Lagos R, San Martin O, Blackwelder WC. 2007. Ty21a live oral typhoid vaccine and prevention of paratyphoid fever caused by Salmonella enterica serovar Paratyphi B. Clin Infect Dis 45(Suppl 1):S24–S28. doi: 10.1086/518141. [DOI] [PubMed] [Google Scholar]
- 104.Simanjuntak CH, Paleologo FP, Punjabi NH, Darmowigoto R, Soeprawoto Totosudirjo H, Haryanto P, Suprijanto E, Witham ND, Hoffman SL. 1991. Oral immunisation against typhoid fever in Indonesia with Ty21a vaccine. Lancet 338:1055–1059. doi: 10.1016/0140-6736(91)91910-M. [DOI] [PubMed] [Google Scholar]
- 105.Vugia DJ, Samuel M, Farley MM, Marcus R, Shiferaw B, Shallow S, Smith K, Angulo FJ, for the Emerging Infections Program FoodNet Working Group. 2004. Invasive Salmonella infections in the United States, FoodNet, 1996-1999: incidence, serotype distribution, and outcome. Clin Infect Dis 38(Suppl 3):S149–S156. doi: 10.1086/381581. [DOI] [PubMed] [Google Scholar]
- 106.Varma JK, Molbak K, Barrett TJ, Beebe JL, Jones TF, Rabatsky-Ehr T, Smith KE, Vugia DJ, Chang H-GH, Angulo FJ. 2005. Antimicrobial-resistant nontyphoidal Salmonella is associated with excess bloodstream infections and hospitalizations. J Infect Dis 191:554–561. doi: 10.1086/427263. [DOI] [PubMed] [Google Scholar]
- 107.Crump JA, Medalla FM, Joyce KW, Krueger AL, Hoekstra RM, Whichard JM, Barzilay EJ, Emerging Infections Program NARMS Working Group . 2011. Antimicrobial resistance among invasive nontyphoidal Salmonella enterica isolates in the United States: National Antimicrobial Resistance Monitoring System, 1996 to 2007. Antimicrob Agents Chemother 55:1148–1154. doi: 10.1128/AAC.01333-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parry CM, Thomas S, Aspinall EJ, Cooke RP, Rogerson SJ, Harries AD, Beeching NJ. 2013. A retrospective study of secondary bacteraemia in hospitalised adults with community acquired non-typhoidal Salmonella gastroenteritis. BMC Infect Dis 13:107. doi: 10.1186/1471-2334-13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jones TF, Ingram LA, Cieslak PR, Vugia DJ, Tobin-D'Angelo M, Hurd S, Medus C, Cronquist A, Angulo FJ. 2008. Salmonellosis outcomes differ substantially by serotype. J Infect Dis 198:109–114. doi: 10.1086/588823. [DOI] [PubMed] [Google Scholar]
- 110.Gal-Mor O, Boyle EC, Grassl GA. 2014. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front Microbiol 5:391. doi: 10.3389/fmicb.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Song J, Gao X, Galán JE. 2013. Structure and function of the Salmonella Typhi chimaeric A2B5 typhoid toxin. Nature 499:350–354. doi: 10.1038/nature12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Majowicz SE, Musto J, Scallan E, Angulo FJ, O'Brein SJ, Jones TF, Fazil A, Hoekstra RM, for the International Collaboration on Enteric Disease ‘Burden of Illness’ Studies. 2010. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis 50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 113.Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. 2015. Global burden of invasive non-typhoidal Salmonella disease. Emerg Infect Dis 21:941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gordon MA, Graham SM, Walsh AL, Wilson L, Phiri A, Molyneux E, Zijlstra EE, Heyderman RS, Hart CA, Molyneux ME. 2008. Epidemics of invasive Salmonella enterica serovar Enteritidis and S. enterica serovar Typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis 46:963–969. doi: 10.1086/529146. [DOI] [PubMed] [Google Scholar]
- 115.Gordon MA, Walsh AL, Chaponda M, Soko D, Mbvwinji M, Molyneux ME, Gordon SB. 2001. Bacteremia and mortality among adult medical admissions in Malawi: predominance of non-Typhi salmonellae and Streptococcus pneumoniae. J Infect 42:44–49. doi: 10.1053/jinf.2000.0779. [DOI] [PubMed] [Google Scholar]
- 116.Brent AJ, Oundo JO, Mwangi I, Ochola L, Lowe B, Berkley JA. 2006. Salmonella bacteremia in Kenyan children. Pediatr Infect Dis J 25:230–236. doi: 10.1097/01.inf.0000202066.02212.ff. [DOI] [PubMed] [Google Scholar]
- 117.Mabey DCW, Brown A, Greenwood BM. 1987. Plasmodium falciparum malaria and Salmonella infections in Gambian children. J Infect Dis 155:1319–1321. doi: 10.1093/infdis/155.6.1319. [DOI] [PubMed] [Google Scholar]
- 118.Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, Hart CA. 2006. Characterisation of community acquired non-typhoidal Salmonella from bacteraemia and diarrhoeal infections in children admitted to hospital in Nairobi, Kenya. BMC Microbiol 6:101. doi: 10.1186/1471-2180-6-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sigauque B, Roca A, Mandomando I, Morais L, Quinto L, Sacarlal J, Macete E, Nhamposa T, Machevo S, Aide P, Bassat Q, Bardaji A, Nhalungo D, Soriano-Gabarro M, Flannery B, Menendez C, Levine MM, Alonso PL. 2009. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J 28:108–113. doi: 10.1097/INF.0b013e318187a87d. [DOI] [PubMed] [Google Scholar]
- 120.O'Dempsey TJ, McArdle TF, Lloyd-Evans N, Baldeh I, Laurence BE, Secka O, Greenwood BM. 1994. Importance of enteric bacteria as a cause of pneumonia, meningitis and septicemia among children in a rural community in The Gambia, West Africa. Pediatr Infect Dis J 13:122–128. doi: 10.1097/00006454-199402000-00009. [DOI] [PubMed] [Google Scholar]
- 121.Lepage P, Bogaerts J, Van Goethem C, Ntahorutaba M, Nsengumuremyi F, Hitimana DG, Vandepitte J, Butzler J-P, Levy J. 1987. Community-acquired bacteremia in African children. Lancet i:1458–1461. [DOI] [PubMed] [Google Scholar]
- 122.Gilks CF, Brindle RJ, Otieno LS, Simani PM, Newnham RS, Bhatt SM, Lule GN, Okelo GBA, Watkins WM, Waiyaki PG, Were JBO, Warrell DA. 1990. Life-threatening bacteremia in HIV-1 seropositive adults admitted to hospital in Nairobi, Kenya. Lancet 336:545–549. doi: 10.1016/0140-6736(90)92096-Z. [DOI] [PubMed] [Google Scholar]
- 123.Green SDR, Cheesbrough JS. 1993. Salmonella bacteraemia among young children at a rural hospital in western Zaire. Ann Trop Paediatr 13:45–54. [DOI] [PubMed] [Google Scholar]
- 124.Beyene G, Nair S, Asrat D, Mengistu Y, Engers H, Wain J. 2011. Multidrug resistant Salmonella Concord is a major cause of salmonellosis in children in Ethiopia. J Infect Dev Ctries 5:23–33. [DOI] [PubMed] [Google Scholar]
- 125.Bronowski C, Fookes MC, Gilderthorp R, Ashelford KE, Harris SR, Phiri A, Hall N, Gordon MA, Wain J, Hart CA, Wigley P, Thomson NR, Winstanley C. 2013. Genomic characterisation of invasive non-typhoidal Salmonella enterica subspecies enterica serovar Bovismorbificans isolates from Malawi. PLoS Negl Trop Dis 7:e2557. doi: 10.1371/journal.pntd.0002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tennant SM, Diallo S, Levy H, Livio S, Sow SO, Tapia M, Fields PI, Mikoleit M, Tamboura B, Kotloff KL, Nataro JP, Galen JE, Levine MM. 2010. Identification by PCR of non-typhoidal Salmonella enterica serovars associated with invasive infections among febrile patients in Mali. PLoS Negl Trop Dis 4:e621. doi: 10.1371/journal.pntd.0000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wadula J, von Gottberg A, Kilner D, de Jong G, Cohen C, Khoosal M, Keddy K, Crewe-Brown H. 2006. Nosocomial outbreak of extended-spectrum beta-lactamase-producing Salmonella Isangi in pediatric wards. Pediatr Infect Dis J 25:843–844. doi: 10.1097/01.inf.0000233543.78070.a2. [DOI] [PubMed] [Google Scholar]
- 128.Kingsley RA, Msefula CL, Thomson NR, Kariuki S, Holt KE, Gordon MA, Harris D, Clarke L, Whitehead S, Sangal V, Marsh K, Achtman M, Molyneux ME, Cormican M, Parkhill J, MacLennan CA, Heyderman RS, Dougan G. 2009. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res 19:2279–2287. doi: 10.1101/gr.091017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Herrero-Fresno A, Wallrodt I, Leekitcharoenphon P, Olsen JE, Aarestrup FM, Hendriksen RS. 2014. The role of the st313-td gene in virulence of Salmonella Typhimurium ST313. PLoS One 9:e84566. doi: 10.1371/journal.pone.0084566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Preziosi MJ, Kandel SM, Guiney DG, Browne SH. 2012. Microbiological analysis of nontyphoidal Salmonella strains causing distinct syndromes of bacteremia or enteritis in HIV/AIDS patients in San Diego, California. J Clin Microbiol 50:3598–3603. doi: 10.1128/JCM.00795-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Painter JA, Hoekstra RM, Ayers T, Tauxe RV, Braden CR, Angulo FJ, Griffin PM. 2013. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998-2008. Emerg Infect Dis 19:407–415. doi: 10.3201/eid1903.111866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morpeth SC, Ramadhani HO, Crump JA. 2009. Invasive non-Typhi Salmonella disease in Africa. Clin Infect Dis 49:606–611. doi: 10.1086/603553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Centers for Disease Control and Prevention. 2011. Compendium of measures to prevent disease associated with animals in public settings, 2011. MMWR Morb Mortal Wkly Rep 60:1–24. [PubMed] [Google Scholar]
- 134.Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, Muyodi J, Githinji JW, Kagendo D, Munyalo A, Hart CA. 2006. Invasive multidrug-resistant non-typhoidal Salmonella infections in Africa: zoonotic or anthroponotic transmission? J Med Microbiol 55:585–591. doi: 10.1099/jmm.0.46375-0. [DOI] [PubMed] [Google Scholar]
- 135.Kariuki S, Revathi G, Gayuka F, Yamo V, Muyodi J, Hart CA. 2002. Lack of clonal relationship between non-typhi Salmonella strain types from humans and those isolated from animals living in close contact. FEMS Immunol Med Microbiol 33:165–171. doi: 10.1111/j.1574-695X.2002.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 136.Parsons BN, Humphrey S, Salisbury AM, Mikoleit J, Hinton JC, Gordon MA, Wigley P. 2013. Invasive non-typhoidal Salmonella Typhimurium ST313 are not host-restricted and have an invasive phenotype in experimentally infected chickens. PLoS Negl Trop Dis 7:e2487. doi: 10.1371/journal.pntd.0002487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Okoro CK, Kingsley RA, Connor TR, Harris SR, Parry CM, Al-Mashhadani MN, Kariuki S, Msefula CL, Gordon MA, de Pinna E, Wain J, Heyderman RS, Obaro S, Alonso PL, Mandomando I, MacLennan CA, Tapia MD, Levine MM, Tennant SM, Parkhill J, Dougan G. 2012. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat Genet 44:1215–1221. doi: 10.1038/ng.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 139.Paglietti B, Falchi G, Mason P, Chitsatso O, Nair S, Gwanzura L, Uzzau S, Cappuccinelli P, Wain J, Rubino S. 2013. Diversity among human non-typhoidal salmonellae isolates from Zimbabwe. Trans R Soc Trop Med Hyg 107:487–492. doi: 10.1093/trstmh/trt046. [DOI] [PubMed] [Google Scholar]
- 140.Waddell WR, Kunz LJ. 1956. Association of Salmonella enteritis with operations on the stomach. N Engl J Med 255:555–559. doi: 10.1056/NEJM195609202551203. [DOI] [PubMed] [Google Scholar]
- 141.Giannella RA, Broitman SA, Zamcheck N. 1972. Gastric acid barrier to ingested microorganisms in man: studies in vivo and in vitro. Gut 13:251–256. doi: 10.1136/gut.13.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Giannella RA, Broitman SA, Zamcheck N. 1973. Influence of gastric acidity on bacterial and parasitic enteric infections: a perspective. Ann Intern Med 78:271–276. doi: 10.7326/0003-4819-78-2-271. [DOI] [PubMed] [Google Scholar]
- 143.Neal RK, Briji SO, Slack RC, Hawkey CJ, Logan RF. 1994. Recent treatment with H2 antagonists and antibiotics and gastric surgery as risk factors for Salmonella infection. Br Med J 308:176. doi: 10.1136/bmj.308.6922.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bavishi C, Dupont HL. 2011. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther 34:1269–1281. doi: 10.1111/j.1365-2036.2011.04874.x. [DOI] [PubMed] [Google Scholar]
- 145.Delarocque-Astagneau E, Bouillant C, Vaillant V, Bouvet P, Grimont PA, Desenclos JC. 2000. Risk factors for the occurrence of sporadic Salmonella enterica serotype Typhimurium infections in children in France: a national case-control study. Clin Infect Dis 31:488–492. doi: 10.1086/313990. [DOI] [PubMed] [Google Scholar]
- 146.Feasey NA, Archer BN, Heyderman RS, Sooka A, Dennis B, Gordon MA, Keddy KH. 2010. Typhoid fever and invasive nontyphoidal salmonellosis, Malawi and South Africa. Emerg Infect Dis 16:1448–1451. doi: 10.3201/eid1609.100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.MacLennan CA, Gondwe EN, Msefula CL, Kingsley RA, Thomson NR, White SA, Goodall M, Pickard DJ, Graham SM, Dougan G, Hart CA, Molyneux ME, Drayson MT. 2008. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J Clin Invest 118:1553–1562. doi: 10.1172/JCI33998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Milledge J, Calis JCJ, Graham SM, Phiri A, Wilson LK, Soko D, Mbvwinji M, Walsh AL, Rogerson SJ, Molyneux ME, Molyneux E. 2005. Aetiology of neonatal sepsis in Blantyre, Malawi: 1996-2001. Ann Trop Paediatr 25:101–110. doi: 10.1179/146532805X45692. [DOI] [PubMed] [Google Scholar]
- 149.Talbert AW, Mwaniki M, Mwarumba S, Newton CR, Berkley JA. 2010. Invasive bacterial infections in neonates and young infants born outside hospital admitted to a rural hospital in Kenya. Pediatr Infect Dis J 29:945–949. doi: 10.1097/INF.0b013e3181dfca8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gordon MA. 2008. Salmonella infections in immunocompromised adults. J Infect 56:413–422. doi: 10.1016/j.jinf.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 151.van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, Corbeel L, Español T, Fischer A, Kurenko-Deptuch M, Mouy R, Petropoulou T, Roesler J, Seger R, Stasia MJ, Valerius NH, Weening RS, Wolach B, Roos D, Kuijpers TW. 2009. Chronic granulomatous disease: the European experience. PLoS One 4:e5234. doi: 10.1371/journal.pone.0005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sommet J, Missud F, Holvoet L, Ithier G, Lorrot M, Benkerrou M, Faye A. 2013. Morbidity among child travellers with sickle-cell disease visiting tropical areas: an observational study in a French tertiary care centre. Arch Dis Child 98:533–536. doi: 10.1136/archdischild-2012-302500. [DOI] [PubMed] [Google Scholar]
- 153.MacLennan C, Fieschi C, Lammas DA, Picard C, Dorman SE, Sanal O, MacLennan JM, Holland SM, Ottenhoff TH, Casanova JL, Kumararatne DS. 2004. Interleukin (IL)-12 and IL-23 are key cytokines for immunity against Salmonella in humans. J Infect Dis 190:1755–1757. doi: 10.1086/425021. [DOI] [PubMed] [Google Scholar]
- 154.Gordon MA, Banda HT, Gondwe M, Gordon SB, Boeree MJ, Walsh AL, Corkill JE, Hart CA, Gilks CF, Molyneux ME. 2002. Non-typhoidal Salmonella bacteremia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS 16:1633–1641. doi: 10.1097/00002030-200208160-00009. [DOI] [PubMed] [Google Scholar]
- 155.Watera C, Nakiyingi J, Miiro G, Muwonge R, Whitworth JA, Gilks CF, French N. 2004. 23-Valent pneumococcal polysaccharide vaccine in HIV-infected Ugandan adults: 6-year follow-up of a clinical trial cohort. AIDS 18:1210–1213. doi: 10.1097/00002030-200405210-00018. [DOI] [PubMed] [Google Scholar]
- 156.Profeta S, Forrester C, Eng Liu RHR, Johnson E, Palinkas R, Smith SM. 1985. Salmonella infections in patients with acquired immunodeficiency syndrome. Arch Intern Med 145:670–672. [PubMed] [Google Scholar]
- 157.Kankwatira AM, Mwafulirwa GAK, Gordon MA. 2004. Non-typhoidal Salmonella bacteremia: an under-recognized feature of AIDS in African adults. Trop Doctor 34:198–200. [DOI] [PubMed] [Google Scholar]
- 158.Berkley JA, Lowe BS, Mwangi I, Williams T, Banui E, Mwarumba S, Ngetsa C, Slack MPE, Njenga S, Hart CA, Maitland K, English M, Marsh K, Scott JAG. 2005. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med 352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 159.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Baumler AJ. 2008. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med 14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.MacLennan CA, Gilchrist JJ, Gordon MA, Cunningham AF, Cobbold M, Goodall M, Kingsley RA, van Oosterhout JJ, Msefula CL, Mandala WL, Leyton DL, Marshall JL, Gondwe EN, Bobat S, López Macías C, Doffinger R, Henderson IR, Zijlstra EE, Dougan G, Drayson MT, MacLennan IC, Molyneux ME. 2010. Dysregulated humoral immunity to nontyphoidal Salmonella in HIV-infected African adults. Science 328:508–512. doi: 10.1126/science.1180346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Trebicka E, Shanmugam NK, Mikhailova A, Alter G, Cherayil BJ. 2014. Effect of human immunodeficiency virus infection on plasma bactericidal activity against Salmonella enterica serovar Typhimurium. Clin Vaccine Immunol 21:1437–1442. doi: 10.1128/CVI.00501-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gordon MA, Kankwatira AMK, Mwafulirwa G, Walsh AL, Hopkins MJ, Parry CM, Faragher EB, Zijlstra EE, Heyderman RS, Molyneux ME. 2010. Invasive non-typhoid salmonellae establish systemic intracellular infection in HIV-infected adults: an emerging disease pathogenesis. Clin Infect Dis 50:953–962. doi: 10.1086/651080. [DOI] [PubMed] [Google Scholar]
- 163.Siggins MK, O'Shaughnessy CM, Pravin J, Cunningham AF, Henderson IR, Drayson MT, MacLennan CA. 2014. Differential timing of antibody-mediated phagocytosis and cell-free killing of invasive African Salmonella allows immune evasion. Eur J Immunol 44:1093–1098. doi: 10.1002/eji.201343529. [DOI] [PubMed] [Google Scholar]
- 164.Schreiber F, Lynn DJ, Houston A, Peters J, Mwafulirwa G, Finlay BB, Brinkman FS, Hancock RE, Heyderman RS, Dougan G, Gordon MA. 2011. The human transcriptome during nontyphoid Salmonella and HIV coinfection reveals attenuated NFkappaB-mediated inflammation and persistent cell cycle disruption. J Infect Dis 204:1237–1245. doi: 10.1093/infdis/jir512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gordon MA, Gordon SB, Musaya L, Zijlstra EE, Molyneux ME, Read RC. 2007. Primary macrophages from HIV-infected adults show dysregulated cytokine responses to Salmonella, but normal internalization and killing. AIDS 21:2399–2408. doi: 10.1097/QAD.0b013e3282f25107. [DOI] [PubMed] [Google Scholar]
- 166.Okoro CK, Kingsley RA, Quail MA, Kankwatira AM, Feasey NA, Parkhill J, Dougan G, Gordon MA. 2012. High-resolution single nucleotide polymorphism analysis distinguishes recrudescence and reinfection in recurrent invasive nontyphoidal Salmonella Typhimurium disease. Clin Infect Dis 54:955–963. doi: 10.1093/cid/cir1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nyirenda TS, Gilchrist JJ, Feasey NA, Glennie SJ, Bar-Zeev N, Gordon MA, MacLennan CA, Mandala WL, Heyderman RS. 2014. Sequential acquisition of T cells and antibodies to nontyphoidal Salmonella in Malawian children. J Infect Dis 210:56–64. doi: 10.1093/infdis/jiu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gondwe EN, Molyneux ME, Goodall M, Graham SM, Mastroeni P, Drayson MT, MacLennan CA. 2010. Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proc Natl Acad Sci U S A 107:3070–3075. doi: 10.1073/pnas.0910497107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Berkley JA, Bejon P, Mwangi T, Gwer S, Maitland K, Williams TN, Mohammed S, Osier F, Kinyanjui S, Fegan G, Lowe BS, English M, Peshu N, Marsh K, Newton CRJC. 2009. HIV infection, malnutrition, and invasive bacterial infection among children with severe malaria. Clin Infect Dis 49:336–343. doi: 10.1086/600299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bronzan RN, Taylor TE, Mwenechanya J, Tembo M, Kayira K, Bwanaisa L, Njobvu A, Kondowe W, Chalira C, Walsh AL, Phiri A, Wilson LK, Molyneux ME, Graham SM. 2007. Bacteremia in Malawian children with severe malaria: prevalence, etiology, HIV co-infection, and outcome. J Infect Dis 195:895–904. doi: 10.1086/511437. [DOI] [PubMed] [Google Scholar]
- 171.Biggs HM, Lester R, Nadjm B, Mtove G, Todd JE, Kinabo GD, Philemon R, Amos B, Morrissey AB, Reyburn H, Crump JA. 2014. Invasive Salmonella infections in areas of high and low malaria transmission intensity in Tanzania. Clin Infect Dis 58:638–647. doi: 10.1093/cid/cit798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tabu C, Breiman RF, Ochieng B, Aura B, Cosmas L, Audi A, Olack B, Bigogo G, Ongus JR, Fields P, Mintz E, Burton D, Oundo J, Feikin DR. 2012. Differing burden and epidemiology of non-Typhi Salmonella bacteremia in rural and urban Kenya, 2006-2009. PLoS One 7:e31237. doi: 10.1371/journal.pone.0031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.MacKenzie G, Ceesay SJ, Hill PC, Walther M, Bojang KA, Satoguina J, Enwere G, D'Alessandro U, Saha D, Ikumapayi UN, O'Dempsey T, Mabey DC, Corrah T, Conway DJ, Adegbola RA, Greenwood BM. 2010. A decline in the incidence of invasive non-typhoidal Salmonella infection in the Gambia temporally associated with a decline in malaria infection. PLoS One 5:e10568. doi: 10.1371/journal.pone.0010568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mtove G, Amos B, Nadjm B, Hendriksen IC, Dondorp AM, Mwambuli A, Kim DR, Ochiai RL, Clemens JD, von Seidlein L, Reyburn H, Deen J. 2011. Decreasing incidence of severe malaria and community-acquired bacteraemia among hospitalized children in Muheza, north-eastern Tanzania, 2006-2010. Malar J 10:320. doi: 10.1186/1475-2875-10-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Scott JA, Berkley JA, Mwangi I, Ochola L, Uyoga S, Macharia A, Ndila C, Lowe BS, Mwarumba S, Bauni E, Marsh K, Williams T. 2011. Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case-control study and a longitudinal study. Lancet 378:1316–1323. doi: 10.1016/S0140-6736(11)60888-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Graham SM, Molyneux EM, Walsh AL, Cheesbrough JS, Molyneux ME, Hart CA. 2000. Nontyphoidal Salmonella infections of children in tropical Africa. Pediatr Infect Dis J 19:1189–1196. doi: 10.1097/00006454-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 177.Walsh AL, Phiri AJ, Graham SM, Molyneux EM, Molyneux ME. 2000. Bacteremia in febrile Malawian children: clinical and microbiologic findings. Pediatr Infect Dis J 19:312–318. doi: 10.1097/00006454-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 178.Mandomando I, Macete E, Sigaúque B, Morais L, Quintó L, Sacarlal J, Espasa M, Vallès X, Bassat Q, Aide P, Nhampossa T, Machevo S, Ruiz J, Nhacolo A, Menéndez C, Kotloff KL, Roca A, Levine MM, Alonso PL. 2009. Invasive non-typhoidal Salmonella in Mozambican children. Trop Med Int Health 14:1467–1474. doi: 10.1111/j.1365-3156.2009.02399.x. [DOI] [PubMed] [Google Scholar]
- 179.Peters RPH, Zijlstra EE, Schijffelen MJ, Walsh AL, Joaki GRF, Kumwenda JJ, Kublin JG, Molyneux ME, Lewis DK. 2004. A prospective study of bloodstream infections as cause of fever in Malawi: clinical predictors and implications for management. Trop Med Int Health 9:928–934. doi: 10.1111/j.1365-3156.2004.01288.x. [DOI] [PubMed] [Google Scholar]
- 180.Brown M, Eykyn SJ. 2000. Non-typhoidal Salmonella bacteraemia without gastroenteritis: a marker of underlying immunosuppression: review of cases at St. Thomas' Hospital 1970-1999. J Infect 41:256–259. [DOI] [PubMed] [Google Scholar]
- 181.Ramos JM, García-Corbeira P, Aguado JM, Arjona R, Alés JM, Soriano F. 1994. Clinical significance of primary vs. secondary bacteremia due to nontyphoid Salmonella in patients without AIDS. Clin Infect Dis 19:777–780. doi: 10.1093/clinids/19.4.777. [DOI] [PubMed] [Google Scholar]
- 182.Graham SM, English M. 2009. Non-typhoidal salmonellae: a management challenge for children with community-acquired invasive disease in tropical African countries. Lancet 373:267–269. doi: 10.1016/S0140-6736(09)60073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schwarz NG, Sarpong N, Hünger F, Marks F, Acquah SE, Agyekum A, Nkrumah B, Loag W, Hagen RM, Evans JA, Dekker D, Fobil JN, Meyer CG, May J, Adu-Sarkodie Y. 2010. Systemic bacteraemia in children presenting with clinical pneumonia and the impact of non-typhoid Salmonella (NTS). BMC Infect Dis 10:319. doi: 10.1186/1471-2334-10-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nadjm B, Mtove G, Amos B, Walker NF, Diefenthal H, Reyburn H, Whitty CJ. 2012. Severe febrile illness in adult hospital admissions in Tanzania: a prospective study in an area of high malaria transmission. Trans R Soc Trop Med Hyg 106:688–695. doi: 10.1016/j.trstmh.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 185.Nadjm B, Amos B, Mtove G, Ostermann J, Chonya S, Wangai H, Kimera J, Msuya W, Mtei F, Dekker D, Malahiyo R, Olomi R, Crump JA, Whitty CJM, Reyburn H. 2010. WHO guidelines for antimicrobial treatment in children admitted to hospital in an area of intense Plasmodium falciparum transmission: prospective study. Br Med J 340:c1350. doi: 10.1136/bmj.c1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Molyneux EM, Mankhambo LA, Phiri A, Graham SM, Forsyth H, Phiri A, Walsh AL, Wilson LK, Molyneux ME. 2009. The outcome of non-typhoidal Salmonella meningitis in Malawian children, 1997-2006. Ann Trop Paed 29:13–22. doi: 10.1179/146532809X401980. [DOI] [PubMed] [Google Scholar]
- 187.Mankhambo LA, Chiwaya KW, Phiri A, Graham SM. 2006. Lobar pneumonia caused by nontyphoidal Salmonella in a Malawian child. Pediatr Infect Dis 25:1190–1192. doi: 10.1097/01.inf.0000245098.82276.d6. [DOI] [PubMed] [Google Scholar]
- 188.Hung C-C, Hung M-N, Hseuh P-R, Chang S-Y, Chen M-Y, Hsieh S-M, Sheng W-H, Sun H-Y, Lo Y-C, Hsiao C-F, Chang S-C. 2007. Risk of recurrent nontyphoid Salmonella bacteremia in HIV-infected patients in the era of highly active antiretroviral therapy and an increasing trend of fluoroquinolone resistance. Clin Infect Dis 45:e60–67. doi: 10.1086/520681. [DOI] [PubMed] [Google Scholar]
- 189.Cohen JI, Bartlett JA, Corey GR. 1987. Extra-intestinal manifestations of Salmonella infections. Medicine 66:349–388. [DOI] [PubMed] [Google Scholar]
- 190.Thamlikitkul V, Dhiraputra C, Paisarnsinsup T, Chareandee C. 1996. Non-typhoidal Salmonella bacteraemia: clinical features and risk factors. Trop Med Int Health 1:443–448. doi: 10.1046/j.1365-3156.1996.d01-92.x. [DOI] [PubMed] [Google Scholar]
- 191.Arthur G, Nduba VN, Kariuki SM, Kimari J, Bhatt SM, Gilks CF. 2001. Trends in bloodstream infections among human immunodeficiency virus-infected adults admitted to a hospital in Nairobi, Kenya, during the last decade. Clin Infect Dis 33:248–256. doi: 10.1086/321820. [DOI] [PubMed] [Google Scholar]
- 192.Feasey NA, Houston A, Mukaka M, Komrower D, Mwalukomo T, Tenthani L, Jahn A, Moore M, Peters RP, Gordon MA, Everett DB, French N, van Oosterhout JJ, Allain TJ, Heyderman RS. 2014. A reduction in adult blood stream infection and case fatality at a large African hospital following antiretroviral therapy roll-out. PLoS One 9:e92226. doi: 10.1371/journal.pone.0092226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Anglaret X, Chene G, Attia A, Toure S, Lafont S, Combe P, Manlan K, N′Dri-Yoman T, Salamon R. 1999. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d'Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet 353:1463–1468. doi: 10.1016/S0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 194.Wiktor SZ, Sassan-Moroko M, Grant AD, Abouya L, Karon JM, Maurice C, Djomand G, Ackah A, Domoua K, Kadio A, Yapi A, Combe P, Tossou O, Roels TH, Lackritz EM, Coulibaly D, De Cock KM, Coulibaly I-M, Greenberg AE. 1999. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d'Ivoire: a randomised controlled trial. Lancet 353:1469–1475. doi: 10.1016/S0140-6736(99)03465-0. [DOI] [PubMed] [Google Scholar]
- 195.Larsen IK, Gradel KO, Helms M, Hornstrup MK, Jürgens G, Mens H, Rosager CL, Clausen TH, Kronborg G, Nielsen H. 2011. Non-typhoidal Salmonella and Campylobacter infections among HIV-positive patients in Denmark. Scand J Infect Dis 43:3–7. doi: 10.3109/00365548.2010.517780. [DOI] [PubMed] [Google Scholar]
- 196.MacLennan CA, Saul A. 2014. Vaccines against poverty. Proc Natl Acad Sci U S A 111:12307–12312. doi: 10.1073/pnas.1400473111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tennant SM, Wang JY, Galen JE, Simon R, Pasetti MF, Gat O, Levine MM. 2011. Engineering and preclinical evaluation of attenuated nontyphoidal Salmonella strains serving as live oral vaccines and as reagent strains. Infect Immun 79:4175–4185. doi: 10.1128/IAI.05278-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dougan G, John V, Palmer S, Mastroeni P. 2011. Immunity to salmonellosis. Immunol Rev 240:196–210. doi: 10.1111/j.1600-065X.2010.00999.x. [DOI] [PubMed] [Google Scholar]
- 199.Ross IN, Abraham T. 1987. Predicting enteric fever without bacteriological culture results. Trans R Soc Trop Med Hyg 81:374–377. doi: 10.1016/0035-9203(87)90139-8. [DOI] [PubMed] [Google Scholar]
- 200.Richens J, Smith T, Mylius T, Spooner V. 1992. An algorithm for the clinical differentiation of malaria and typhoid: a preliminary communication. P N G Med J 35:298–302. [PubMed] [Google Scholar]
- 201.Vollaard AM, Ali S, Widjaja S, Asten HA, Visser LG, Surjadi C, van Dissel JT. 2005. Identification of typhoid fever and paratyphoid fever cases at presentation in outpatient clinics in Jakarta, Indonesia. Trans R Soc Trop Med Hyg 99:440–450. doi: 10.1016/j.trstmh.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 202.Hosoglu S, Geyik MF, Akalin S, Ayaz C, Kokoglu OF, Loeb M. 2006. A simple validated prediction rule to diagnose typhoid fever in Turkey. Trans R Soc Trop Med Hyg 100:1068–1074. doi: 10.1016/j.trstmh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 203.Baker S, Favorov M, Dougan G. 2010. Searching for the elusive typhoid diagnostic. BMC Infect Dis 10:45. doi: 10.1186/1471-2334-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shaw AB, MacKay HA. 1951. Factors influencing the results of blood culture in enteric fever. J Hyg (London) 49:315–323. doi: 10.1017/S0022172400044181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Watson KC, Laurie W. 1956. The laboratory diagnosis of typhoid fever in areas of endemicity. Am J Trop Med Hyg 5:1051–1057. [DOI] [PubMed] [Google Scholar]
- 206.Wang SK, Chu CJ, Sun PS, Shan DS, Kong FL, Liu HY, Wu Q, Yang RS, Yao YB. 2009. Study on blood cultures and bacteria counts in the blood of paratyphoid fever A patients. Eur J Clin Microbiol Infect Dis 28:1259–1261. doi: 10.1007/s10096-009-0766-9. [DOI] [PubMed] [Google Scholar]
- 207.Watson KC. 1955. Isolation of Salmonella Typhi from the bloodstream. J Lab Clin Med 46:128–134. [PubMed] [Google Scholar]
- 208.Butler T, Bell WR, Levin J, Linh NN, Arnold K. 1978. Typhoid fever: studies of blood coagulation, bacteremia, and endotoxemia. Arch Intern Med 138:407–410. [DOI] [PubMed] [Google Scholar]
- 209.Rubin FA, McWhirter PD, Burr D, Punjabi NH, Lane E, Kumala S, Sudarmono P, Pulungsih SP, Lesmana M, Tjaniadi P, Sukri N, Hoffman SL. 1990. Rapid diagnosis of typhoid fever through identification of Salmonella Typhi within 18 hours of specimen acquisition by culture of the mononuclear cell-platelet fraction of blood. J Clin Microbiol 28:825–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wain J, Diep TS, Ho VA, Walsh AM, Nguyen TT, Parry CM, White NJ. 1998. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J Clin Microbiol 36:1683–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cockerill FR, Wilson JW, Vetter EA, Goodman KM, Torgerson CA, Harmsen WS, Schleck CD, Ilstrup DM, Washington JA, Wilson WR. 2004. Optimal testing parameters for blood cultures. Clin Infect Dis 38:1724–1730. doi: 10.1086/421087. [DOI] [PubMed] [Google Scholar]
- 212.Kaye D, Palmieri M, Eyckmans L, Rocha H, Hook EW. 1966. Comparison of bile and trypticase soy broth for isolation of Salmonella Typhi from blood. Am J Clin Pathol 46:408–410. [DOI] [PubMed] [Google Scholar]
- 213.Escamilla J, Santiago LT, Sangalang RP, Ranoa CP, Cross JH. 1984. Comparative study of three blood culture systems for isolation of enteric fever Salmonella. Southeast Asian J Trop Med Pub Health 15:161–166. [PubMed] [Google Scholar]
- 214.Wain J, Diep TS, Bay PV, Walsh AL, Vinh H, Duong NM, Ho VA, Hien TT, Farrar J, White NJ, Parry CM, Day NP. 2008. Specimens and culture media for the laboratory diagnosis of typhoid fever. J Infect Dev Ctries 2:469–474. [DOI] [PubMed] [Google Scholar]
- 215.Hoffman SL, Punjabi NH, Rockhill RC, Sutomo A, Rivai AR, Pulungsih SP. 1984. Duodenal string-capsule culture compared with bone-marrow, blood, and rectal-swab cultures for diagnosing typhoid and paratyphoid fever. J Infect Dis 149:157–161. doi: 10.1093/infdis/149.2.157. [DOI] [PubMed] [Google Scholar]
- 216.Lee A, Mirrett S, Reller LB, Weinstein MC. 2007. Detection of bloodstream infections in adults: how many blood cultures are needed? J Clin Microbiol 45:3546–3548. doi: 10.1128/JCM.01555-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Duthie R, French GL. 1990. Comparison of methods for the diagnosis of typhoid fever. J Clin Pathol 43:863–865. doi: 10.1136/jcp.43.10.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Reller ME, Zaidi AK, Sultana S, Azeem S, Hanif B, Qureshi S, Hasan R, Bhutta Z, Akhter R, Goldmann DA. 2009. Controlled evaluation of Bactec Peds Plus/F and Bactec lytic/10 anaerobic/F media for isolation of Salmonella enterica serovars Typhi and Paratyphi A from blood. J Clin Microbiol 47:245–246. doi: 10.1128/JCM.01452-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Cummins SL. 1911. The anti-bactericidal action of bile salts. J Hyg (London) 11:373–380. doi: 10.1017/S002217240001682X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Escamilla J, Santiago LT, Uylangco CV, Cross JH. 1983. Evaluation of sodium polyanethanol sulfonate as a blood culture additive for recovery of Salmonella Typhi and Salmonella Paratyphi A. J Clin Microbiol 18:380–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Watson KC. 1978. Laboratory and clinical investigation of recovery of Salmonella Typhi from blood. J Clin Microbiol 7:122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Watson KC. 1955. Effect of chloramphenicol on the isolation of S. typhi from the blood stream. J Clin Pathol 8:55–57. doi: 10.1136/jcp.8.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Escamilla J, Florez-Ugarte H, Kilpatrick ME. 1986. Evaluation of blood clot cultures for isolation of Salmonella Typhi, Salmonella Paratyphi A, and Brucella melitensis. J Clin Microbiol 24:388–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Simanjuntak CH, Hoffman SL, Darmowigoto R, Lesmana M, Soeprawoto Edman DC. 1988. Streptokinase clot culture compared with whole blood culture for isolation of Salmonella typhi and S. paratyphi A from patients with enteric fever. Trans R Soc Trop Med Hyg 82:340–341. doi: 10.1016/0035-9203(88)90471-3. [DOI] [PubMed] [Google Scholar]
- 225.Mantur BG, Bidari LH, Akki AS, Mulimani MS, Tikare NV. 2007. Diagnostic yield of blood clot culture in the accurate diagnosis of enteric fever and human brucellosis. Clin Lab 53:57–61. [PubMed] [Google Scholar]
- 226.Edelman R, Levine MM. 1986. Summary of an international workshop on typhoid fever. Rev Infect Dis 8:329–349. doi: 10.1093/clinids/8.3.329. [DOI] [PubMed] [Google Scholar]
- 227.Saha SK, Darmstadt GL, Baqui AH, Hanif M, Ruhulamin M, Santosham M, Nagatake T, Black RE. 2001. Rapid identification and antibiotic susceptibility testing of Salmonella enterica serovar Typhi isolated from blood: implications for therapy. J Clin Microbiol 39:3583–3585. doi: 10.1128/JCM.39.10.3583-3585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gilman RH, Terminel M, Levine MM, Hernandez-Mendoza P, Hornick RB. 1975. Relative efficacy of blood, urine, rectal swab, bone-marrow, and rose-spot cultures for recovery of Salmonella Typhi in typhoid fever. Lancet i:1211–1213. [DOI] [PubMed] [Google Scholar]
- 229.Guerra-Caceres JG, Gotuzzo-Herencia E, Crosby-Dagnino E, Miro-Quesada M, Carrillo-Parodi C. 1979. Diagnostic value of bone marrow culture in typhoid fever. Trans R Soc Trop Med Hyg 73:680–683. doi: 10.1016/0035-9203(79)90020-8. [DOI] [PubMed] [Google Scholar]
- 230.Vallenas C, Hernandez H, Kay B, Black R, Gotuzzo E. 1985. Efficacy of bone marrow, blood, stool and duodenal contents cultures for bacteriologic confirmation of typhoid fever in children. Pediatr Infect Dis 4:496–498. doi: 10.1097/00006454-198509000-00011. [DOI] [PubMed] [Google Scholar]
- 231.Hoffman SL, Edman DC, Punjabi NH, Lesmana M, Cholid A, Sundah S, Harahap J. 1986. Bone marrow aspirate culture superior to streptokinase clot culture and 8 mL 1:10 blood-to-broth ratio blood culture for diagnosis of typhoid fever. Am J Trop Med Hyg 35:836–839. [DOI] [PubMed] [Google Scholar]
- 232.Farooqui BJ, Khurshid M, Ashfaq MK, Ata Khan M. 1991. Comparative yeild of Salmonella Typhi from blood and bone marrow cultures in patients with fever of unknown origin. J Clin Path 44:258–259. doi: 10.1136/jcp.44.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Akoh JA. 1991. Relative sensitivity of blood and bone marrow cultures in typhoid fever. Trop Doctor 21:174–176. [DOI] [PubMed] [Google Scholar]
- 234.Wain J, Bay PV, Vinh H, Duong NM, Diep TS, Walsh AL, Parry CM, Hasserjian RP, Ho VA, Hien TT, Farrar J, White NJ, Day NP. 2001. Quantitation of bacteria in bone marrow from patients with typhoid fever: relationship between counts and clinical features. J Clin Microbiol 39:1571–1576. doi: 10.1128/JCM.39.4.1571-1576.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.West B, Richens JE, Howard PF. 1989. Evaluation in Papua New Guinea of a urine coagglutination test and a Widal slide agglutination test for rapid diagnosis of typhoid fever. Trans R Soc Trop Med Hyg 83:715–717. doi: 10.1016/0035-9203(89)90407-0. [DOI] [PubMed] [Google Scholar]
- 236.Benavente L, Gotuzzo E, Guerra J, Grados O, Guerra H, Bravo N. 1984. Diagnosis of typhoid fever using a string capsule device. Trans R Soc Trop Med Hyg 78:404–406. doi: 10.1016/0035-9203(84)90134-2. [DOI] [PubMed] [Google Scholar]
- 237.Levine MM, Grados O, Gilman RH, Woodward WE, Solis-Plaza R, Waldman W. 1978. Diagnostic value of the Widal test in areas endemic for typhoid fever. Am J Trop Med Hyg 27:795–800. [DOI] [PubMed] [Google Scholar]
- 238.Ley B, Mtove G, Thriemer K, Amos B, von Seidlein L, Hendriksen I, Mwambuli A, Shoo A, Malahiyo R, Ame SM, Kim DR, Ochiai LR, Clemens JD, Reyburn H, Wilfing H, Magesa S, Deen JL. 2010. Evaluation of the Widal tube agglutination test for the diagnosis of typhoid fever among children admitted to a rural hdospital in Tanzania and a comparison with previous studies. BMC Infect Dis 10:180. doi: 10.1186/1471-2334-10-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Olopoenia LA, King AL. 2000. Widal agglutination test 100 years later: still plagued by controversy. Postgrad Med J 76:80–84. doi: 10.1136/pmj.76.892.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lunguya O, Phoba MF, Mundeke SA, Bonebe E, Mukadi P, Muyembe JJ, Verhaegen J, Jacobs J. 2012. The diagnosis of typhoid fever in the Democratic Republic of the Congo. Trans R Soc Trop Med Hyg 106:348–355. doi: 10.1016/j.trstmh.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 241.House D, Chinh NT, Diep TS, Parry CM, Wain J, Dougan G, White NJ, Hien TT, Farrar JJ. 2005. Use of paired serum samples for serodiagnosis of typhoid fever. J Clin Microbiol 43:4889–4890. doi: 10.1128/JCM.43.9.4889-4890.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Buck RL, Escamilla J, Sangalang RP, Cabanban AB, Santiago LT, Ranoa CP, Cross JH. 1987. Diagnostic value of a single, pre-treatment Widal test in suspected enteric fever cases in the Philippines. Trans R Soc Trop Med Hyg 81:871–873. doi: 10.1016/0035-9203(87)90056-3. [DOI] [PubMed] [Google Scholar]
- 243.Parry CM, Hoa NT, Diep TS, Wain J, Chinh NT, Vinh H, Hien TT, White NJ, Farrar JJ. 1999. Value of a single-tube Widal test in diagnosis of typhoid fever in Vietnam. J Clin Microbiol 37:2882–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Nardiello S, Pizzella T, Russo M, Galanti B. 1984. Serodiagnosis of typhoid fever by enzyme-linked immunosorbent assay determination of anti-Salmonella typhi lipopolysaccharide antibodies. J Clin Microbiol 20:718–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sarasombath S, Banchuin N, Sukosol T, Rungpitarangsi B, Manasatit S. 1987. Systemic and intestinal immunities after natural typhoid infection. J Clin Microbiol 25:10088–10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sippel J, Bukhtiari N, Awan MB, Krieg R, Duncan JF, Karamat KA, Malik IA, Igbal LM, Legters L. 1989. Indirect immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assays (ELISAs) and IgM capture ELISA for detection of antibodies to lipopolysaccharide in adult typhoid fever patients in Pakistan. J Clin Microbiol 27:1298–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mekara Y, Maneekarn N, Vithayasai V, Makonkawkeyoon S. 1990. Determination of antibody from typhoid patients against lipopolysaccharide and protein antigens of Salmonella Typhi. Asian Pac J Allergy Immunol 8:95–101. [PubMed] [Google Scholar]
- 248.Verdugo-Rodriguez A, Gam L-H, Devi S, Koh CL, Puthucheary SD, Calva E, Pang T. 1993. Detection of antibodies against Salmonella Typhi outer membrane (OMP) preparation in typhoid fever patients. Asian Pac J Allergy Immunol 11:45–52. [PubMed] [Google Scholar]
- 249.House D, Wain J, Ho VA, Diep TS, Chinh NT, Bay PV, Vinh H, Duc M, Parry CM, Dougan G, White NJ, Hien TH, Farrar JJ. 2001. Serology of typhoid fever in an area of endemicity and its relevance to diagnosis. J Clin Microbiol 39:1002–1007. doi: 10.1128/JCM.39.3.1002-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Shaheen HI, Girgis NI, Rodier GR, Kamal KA. 1995. Evaluation of the response of human humoral antibodies to Salmonella Typhi lipopolysaccharide in an area of endemic typhoid fever. Clin Infect Dis 21:1012–1013. doi: 10.1093/clinids/21.4.1012. [DOI] [PubMed] [Google Scholar]
- 251.Pulickal AS, Gautam S, Clutterbuck EA, Thorson S, Basynat B, Adhikari N, Makepeace K, Rijpkema S, Borrow R, Farrar JJ, Pollard AJ. 2009. Kinetics of the natural, humoral immune response to Salmonella enterica serovar Typhi in Kathmandu, Nepal. Clin Vaccine Immunol 16:1413–1419. doi: 10.1128/CVI.00245-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Chart H, Cheasty T, de Pinna E, Siorvanes L, Wain J, Alam D, Nizami Q, Bhutta Z, Threlfall EJ. 2007. Serodiagnosis of Salmonella enterica serovar Typhi and S. enterica serovars Paratyphi A, B and C human infections. J Med Microbiol 56:1161–1166. doi: 10.1099/jmm.0.47197-0. [DOI] [PubMed] [Google Scholar]
- 253.Parry CM, Wijedoru L, Arjyal A, Baker S. 2011. The utility of diagnostic tests for enteric fever in endemic locations. Expert Rev Anti Infect Ther 9:711–725. doi: 10.1586/eri.11.47. [DOI] [PubMed] [Google Scholar]
- 254.Choo KE, Davis TME, Ismail A, Tuan Ibrahim TA, Ghazali WN. 1999. Rapid and reliable serological diagnosis of enteric fever: comparative sensitivity and specificty of Typhidot and Typhidot-M tests in febrile Malaysian children. Acta Trop 72:175–183. doi: 10.1016/S0001-706X(98)00095-3. [DOI] [PubMed] [Google Scholar]
- 255.Bhutta ZA, Mansurali N. 1999. Rapid serologic diagnosis of paediatric typhoid fever in an endemic area: a prospective comparative evaluation of two dot-enzyme immunoassays and the Widal test. Am J Trop Med Hyg 61:654–657. [DOI] [PubMed] [Google Scholar]
- 256.Dutta S, Sur D, Manna B, Sen B, Deb AK, Deen JL, Wain J, Von Seidlein L, Ochiai L, Clemens JD, Bhattacharya SK. 2006. Evaluation of new-generation serologic tests for the diagnosis of typhoid fever: data from a community-based surveillance in Calcutta, India. Diagn Microbiol Infect Dis 56:359–365. doi: 10.1016/j.diagmicrobio.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 257.Kawano RL, Leano SA, Agdamag DM. 2007. Comparison of serological test kits for diagnosis of typhoid fever in the Philippines. J Clin Microbiol 45:246–247. doi: 10.1128/JCM.01403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Dong B, Galindo CM, Shin E, Acosta CJ, Page AL, Wang M, Kim D, Ochiai RL, Park J, Ali M, von Seidlein L, Xu Z, Yang J, Clemens JD. 2007. Optimizing typhoid fever case definitions by combining serological tests in a large population study in Hechi City, China. Epidemiol Infect 135:1014–1020. doi: 10.1017/S0950268806007801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Olsen SJ, Pruckler J, Bibb W, Thanh NT, Trinh TM, Minh NT, Sivapalasingam S, Gupta A, Phuong PT, Chinh NT, Chau NV, Cam PD, Mintz ED. 2004. Evaluation of rapid diagnostic tests for typhoid fever. J Clin Microbiol 42:1885–1889. doi: 10.1128/JCM.42.5.1885-1889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Naheed A, Ram PK, Brooks WA, Mintz ED, Hossain MA, Parsons MM, Luby SP, Breiman RF. 2008. Clinical value of Tubex and Typhidot rapid diagnostic tests for typhoid fever in an urban community clinic in Bangladesh. Diagn Microbiol Infect Dis 61:381–386. doi: 10.1016/j.diagmicrobio.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 261.Siba V, Horwood PF, Vanuga K, Wapling J, Sehuko R, Siba PM, Greenhill AR. 2012. Evaluation of serological diagnostic tests for typhoid fever in Papua New Guinea using a composite reference standard. Clin Vaccine Immunol 19:1833–1837. doi: 10.1128/CVI.00380-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Tarupiwa A, Tapera S, Mtapuri-Zinyowera S, Gumbo P, Ruhanya V, Gudza-Mugabe M, Majuru NX, Chin'ombe N. 2015. Evaluation of TUBEX-TF and OnSite Typhoid IgG/IgM Combo rapid tests to detect Salmonella enterica serovar Typhi infection during a typhoid outbreak in Harare, Zimbabwe. BMC Res Notes 8:50. doi: 10.1186/s13104-015-1015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lim P-K, Tam FCH, Cheong Y-M, Jegathesan M. 1998. One-step 2-minute test to detect typhoid specific antibodies based on particle separation tubes. J Clin Microbiol 36:2271–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Tam FC, Wang M, Dong B, Leung DT, Ma CH, Lim PL. 2008. New rapid test for paratyphoid a fever: usefulness, cross-detection, and solution. Diagn Microbiol Infect Dis 62:142–150. doi: 10.1016/j.diagmicrobio.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 265.Pastoor R, Hatta M, Abdoel TH, Smits HL. 2008. Simple, rapid, and affordable point-of-care test for the serodiagnosis of typhoid fever. Diagn Microbiol Infect Dis 61:129–134. doi: 10.1016/j.diagmicrobio.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 266.Moore CE, Pan-Ngum W, Wijedoru LP, Sona S, Nga TV, Duy PT, Vinh PV, Chheng K, Kumar V, Emary K, Carter M, White L, Baker S, Day NP, Parry CM. 2014. Evaluation of the diagnostic accuracy of a typhoid IgM flow assay for the diagnosis of typhoid fever in Cambodian children using a Bayesian latent class model assuming an imperfect gold standard. Am J Trop Med Hyg 90:114–120. doi: 10.4269/ajtmh.13-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Gopalakrishnan V, Sekhar WY, Soo EH, Vinsent RA, Devi S. 2002. Typhoid fever in Kuala Lumpur and a comparative evaluation of two commercial diagnostic kits for the detection of antibodies to Salmonella Typhi. Singapore Med J 43:354–358. [PubMed] [Google Scholar]
- 268.Anagha K, Deepika B, Shahriar R, Sanjeev K. 2012. The easy and early diagnosis of typhoid fever. J Clin Diagn Res 6:198–199. [Google Scholar]
- 269.Keddy KH, Sooka A, Letsoalo ME, Hoyland G, Chaignat C-L, Morrissey AB, Crump JA. 2011. Sensitivity and specificity of typhoid rapid antibody tests for laboratory diagnosis of typhoid fever at two sub-Saharan African sites. Bull World Health Organ 89:640–647. doi: 10.2471/BLT.11.087627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Thriemer K, Ley B, Menten J, Jacobs J, van den Ende J. 2013. A systematic review and meta-analysis of the performance of two point of care typhoid fever tests, Tubex TF and Typhidot, in endemic countries. PLoS One 8:e81263. doi: 10.1371/journal.pone.0081263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Song JH, Cho H, Park MY, Na DS, Moon HB, Pai CH. 1993. Detection of Salmonella Typhi in the blood of patients with typhoid fever by polymerase chain reaction. J Clin Microbiol 31:1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Nizami SQ, Bhutta ZA, Siddiqui AA, Lubbad L. 2006. Enhanced detection rate of typhoid fever in children in a periurban slum in Karachi, Pakistan using polymerase chain reaction technology. Scand J Clin Lab Invest 66:429–436. doi: 10.1080/00365510600791724. [DOI] [PubMed] [Google Scholar]
- 273.Ali A, Haque A, Haque A, Sarwar Y, Mohsin M, Bashir S, Tariq A. 2009. Multiplex PCR for differential diagnosis of emerging typhoidal pathogens directly from blood samples. Epidemiol Infect 137:102–107. doi: 10.1017/S0950268808000654. [DOI] [PubMed] [Google Scholar]
- 274.Zhu Q, Lim CK, Chan YN. 1996. Detection of Salmonella Typhi by polymerase chain reaction. J Appl Bacteriol 80:244–251. doi: 10.1111/j.1365-2672.1996.tb03216.x. [DOI] [PubMed] [Google Scholar]
- 275.Sénchez-Jiménez MM, Cardona-Castro N. 2004. Validation of a PCR for diagnosis of typhoid fever and salmonellosis by amplification of the hilA gene in clinical samples from Colombian patients. J Med Microbiol 53:875–878. doi: 10.1099/jmm.0.45630-0. [DOI] [PubMed] [Google Scholar]
- 276.Aziah I, Ravichandran M, Ismail A. 2007. Amplification of ST50 gene using dry-reagent-based polymerase chain reaction for the detection of Salmonella Typhi. Diagn Microbiol Infect Dis 59:373–377. doi: 10.1016/j.diagmicrobio.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 277.Kumar A, Balachandran Y, Gupta S, Khare S, Suman. 2010. Quick PCR based diagnosis of typhoid using specific genetic markers. Biotechnol Lett 32:707–712. doi: 10.1007/s10529-010-0211-2. [DOI] [PubMed] [Google Scholar]
- 278.Kumar A, Arora V, Bashamboo A, Ali S. 2002. Detection of Salmonella Typhi by polymerase chain reaction: implications in diagnosis of typhoid fever. Infect Genetics Evol 2:107–110. doi: 10.1016/S1567-1348(02)00090-4. [DOI] [PubMed] [Google Scholar]
- 279.Massi MN, Shirakawa T, Gotoh A, Bishnu A, Hatta M, Kawabata M. 2003. Rapid diagnosis of typhoid fever by PCR assay using one pair of primers from flagellin gene of Salmonella Typhi. J Infect Chemother 9:233–237. doi: 10.1007/s10156-003-0256-4. [DOI] [PubMed] [Google Scholar]
- 280.Prakash P, Mishra OP, Singh AK, Gulati AK, Nath G. 2005. Evaluation of nested PCR in diagnosis of typhoid fever. J Clin Microbiol 43:431–432. doi: 10.1128/JCM.43.1.431-432.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hatta M, Smits HL. 2007. Detection of Salmonella Typhi by nested polymerase chain reaction in blood, urine, and stool samples. Am J Trop Med Hyg 76:139–143. [PubMed] [Google Scholar]
- 282.Massi MN, Shirakawa T, Gotoh A, Bishnu A, Hatta M, Kawabata M. 2005. Quantitative detection of Salmonella enterica serovar Typhi from blood of suspected typhoid fever patients by real-time PCR. Int J Med Microbiol 295:117–120. doi: 10.1016/j.ijmm.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 283.Ambati SR, Nath G, Das BK. 2007. Diagnosis of typhoid fever by polymerase chain reaction. Indian J Pediatr 74:909–913. doi: 10.1007/s12098-007-0167-y. [DOI] [PubMed] [Google Scholar]
- 284.Nagarajan AG, Karnam G, Lahiri A, Allam US, Chakravortty D. 2009. Reliable means of diagnosis and serovar determination of blood-borne Salmonella strains: quick PCR amplification of unique genomic loci by novel primer sets. J Clin Microbiol 47:2435–2441. doi: 10.1128/JCM.00327-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Nandagopal B, Sankar S, Lingesan K, Appu KC, Padmini B, Sridharan G, Gopinath AK. 2010. Prevalence of Salmonella Typhi among patients with febrile illness in rural and peri-urban populations of Vellore district, as determined by nested PCR targeting the flagellin gene. Mol Diagn Ther 14:107–112. doi: 10.1007/BF03256360. [DOI] [PubMed] [Google Scholar]
- 286.Chaudhry R, Chandel DS, Verma N, Singh N, Singh P, Dey AB. 2010. Rapid diagnosis of typhoid fever by an in-house flagellin PCR. J Med Microbiol 59:1391–1393. doi: 10.1099/jmm.0.020982-0. [DOI] [PubMed] [Google Scholar]
- 287.Nga TV, Karkey A, Dongol S, Thuy HN, Dunstan S, Holt K, Tu LT, Campbell JI, Chau TT, Chau NV, Arjyal A, Koirala S, Basnyat B, Dolecek C, Farrar J, Baker S. 2010. The sensitivity of real-time PCR amplification targeting invasive Salmonella serovars in biological specimens. BMC Infect Dis 10:125. doi: 10.1186/1471-2334-10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kumar G, Pratap CB, Mishra OP, Kumar K, Nath G. 2012. Use of urine with nested PCR targeting the flagellin gene (fliC) for diagnosis of typhoid fever. J Clin Microbiol 50:1964–1967. doi: 10.1128/JCM.00031-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Rockhill RC, Rumans LW, Lesmana M, Dennis DT. 1980. Detection of Salmonella Typhi D, Vi, and d antigens, by slide coagglutination, in urine from patients with typhoid fever. J Clin Microbiol 11:213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Taylor DN, Harris JR, Barrett TJ, Hargrett NT, Prentzel I, Valdivieso C, Palomino C, Levine M, Blake PA. 1983. Detection of urinary Vi antigen as a diagnostic test for typhoid fever. J Clin Microbiol 18:872–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Fadeel MA, Crump JA, Mahoney FJ, Nakhla IA, Mansour AM, Reyad B, Melegi DE, Sultan Y, Mintz ED, Bibb WF. 2004. Rapid diagnosis of typhoid fever by enzyme-linked immunosorbent assay detection of Salmonella serotype Typhi antigens in urine. Am J Trop Med Hyg 70:323–328. [PubMed] [Google Scholar]
- 292.Chaicumpa W, Ruangkunaporn Y, Burr D, Chongsa-Nguan M, Echeverria P. 1992. Diagnosis of typhoid fever by detection of Salmonella Typhi antigen in urine. J Clin Microbiol 30:2513–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Nguyen NQ, Tapchaisri P, Chongsa-nguan M, Cao VV, Doan TT, Sakolvaree Y, Srimanote P, Chaicumpa W. 1997. Diagnosis of enteric fever caused by Salmonella spp. in Vietnam by a monoclonal antibody-based dot-blot ELISA. Asian Pac J Allergy Immunol 15:205–212. [PubMed] [Google Scholar]
- 294.Gupta AK, Rao KM. 1979. Simultaneous detection of Salmonella Typhi antigen and antibody and serum by counter-immunoelectrophoresis for an early and rapid diagnosis of typhoid fever. J Immunol Methods 30:349–353. doi: 10.1016/0022-1759(79)90017-6. [DOI] [PubMed] [Google Scholar]
- 295.Sundararaj T, Ilango B, Subramanian S. 1983. A study on the usefulness of counter immuno-electrophoresis for the detection of Salmonella Typhi antigen in the sera of suspected cases of enteric fever. Trans Royal Soc Trop Med Hyg 77:194–197. doi: 10.1016/0035-9203(83)90067-6. [DOI] [PubMed] [Google Scholar]
- 296.John JT, Sivadasan K, Kurien B. 1984. Evaluation of passive bacterial agglutination for the diagnosis of typhoid fever. J Clin Microbiol 20:751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Mukherjee C, Malik A, Khan HM, Malik A. 1993. Rapid diagnosis of typhoid fever by co-agglutination in an Indian hospital. J Med Microbiol 39:74–77. doi: 10.1099/00222615-39-1-74. [DOI] [PubMed] [Google Scholar]
- 298.Pandya M, Pillai P, Deb M. 1995. Rapid diagnosis of typhoid fever by detection of Barber protein and Vi antigen of Salmonella serotype Typhi. J Med Microbiol 43:185–188. doi: 10.1099/00222615-43-3-185. [DOI] [PubMed] [Google Scholar]
- 299.Rao PS, Prasad SV, Arunkumar G, Shivananda PG. 1999. Salmonella Typhi VI antigen co-agglutination test for the rapid diagnosis of typhoid fever. Indian J Med Sci 53:7–9. [PubMed] [Google Scholar]
- 300.Tracz DM, Tabor H, Jerome M, Ng LK, Gilmour MW. 2006. Genetic determinants and polymorphisms specific for human-adapted serovars of Salmonella enterica that cause enteric fever. J Clin Microbiol 44:2007–2018. doi: 10.1128/JCM.02630-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ou HY, Ju CT, Thong KL, Ahmad N, Deng Z, Barer MR, Rajakumar K. 2007. Translational genomics to develop a Salmonella enterica serovar Paratyphi A multiplex polymerase chain reaction assay. J Mol Diagn 9:624–630. doi: 10.2353/jmoldx.2007.070064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Levy H, Diallo S, Tennant SM, Livio S, Sow SO, Tapia M, Fields PI, Mikoleit M, Tamboura B, Kotloff KL, Lagos R, Nataro JP, Galen JE, Levine MM. 2008. PCR method to identify Salmonella enterica serovars Typhi, Paratyphi A, and Paratyphi B among Salmonella isolates from the blood of patients with clinical enteric fever. J Clin Microbiol 46:1861–1866. doi: 10.1128/JCM.00109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Ngan GJ, Ng LM, Lin RT, Teo JW. 2010. Development of a novel multiplex PCR for the detection and differentiation of Salmonella enterica serovars Typhi and Paratyphi A. Res Microbiol 161:243–248. doi: 10.1016/j.resmic.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 304.Zhou L, Pollard AJ. 2012. A novel method of selective removal of human DNA improves PCR sensitivity for detection of Salmonella Typhi in blood samples. BMC Infect Dis 12:164. doi: 10.1186/1471-2334-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Zhou L, Pollard AJ. 2010. A fast and highly sensitive blood culture PCR method for clinical detection of Salmonella enterica serovar Typhi. Ann Clin Microbiol Antimicrob 9:14. doi: 10.1186/1476-0711-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Herath HM. 2003. Early diagnosis of typhoid fever by the detection of salivary IgA. J Clin Pathol 56:694–698. doi: 10.1136/jcp.56.9.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Harris JB, Baresch-Bernal A, Rollins SM, Alam A, LaRocque RC, Bikowski M, Peppercorn AF, Handfield M, Hillman JD, Qadri F, Calderwood SB, Hohmann E, Breiman RF, Brooks WA, Ryan ET. 2006. Identification of in vivo-induced bacterial protein antigens during human infection with Salmonella enterica serovar Typhi. Infect Immun 74:5161–5168. doi: 10.1128/IAI.00488-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ansong C, Yoon H, Norbeck AD, Gustin JK, McDermott JE, Mottaz HM, Rue J, Adkins JN, Heffron F, Smith RD. 2008. Proteomics analysis of the causative agent of typhoid fever. J Proteome Res 7:546–557. doi: 10.1021/pr070434u. [DOI] [PubMed] [Google Scholar]
- 309.Sheikh A, Bhuiyan MS, Khanam F, Chowdhury F, Saha A, Ahmed D, Jamil KM, LaRocque RC, Harris JB, Ahmad MM, Charles R, Brooks WA, Calderwood SB, Cravioto A, Ryan ET, Qadri F. 2009. Salmonella enterica serovar Typhi-specific immunoglobulin A antibody responses in plasma and antibody in lymphocyte supernatant specimens in Bangladeshi patients with suspected typhoid fever. Clin Vaccine Immunol 16:1587–1594. doi: 10.1128/CVI.00311-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Charles RC, Sheikh A, Krastins B, Harris JB, Bhuiyan MS, LaRocque RC, Logvinenko T, Sarracino DA, Kudva IT, Eisenstein J, Podolsky MJ, Kalsy A, Brooks WA, Ludwig A, John M, Calderwood SB, Qadri F, Ryan ET. 2010. Characterization of anti-Salmonella enterica serotype Typhi antibody responses in bacteremic Bangladeshi patients by an immunoaffinity proteomics-based technology. Clin Vaccine Immunol 17:1188–1195. doi: 10.1128/CVI.00104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Liang L, Juarez S, Nga TV, Dunstan S, Nakajima-Sasaki R, Davies DH, McSorley S, Baker S, Felgner PL. 2013. Immune profiling with a Salmonella Typhi antigen microarray identifies new diagnostic biomarkers of human typhoid. Sci Rep 3:1043. doi: 10.1038/srep01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Thompson LJ, Dunstan SJ, Dolecek C, Perkins T, House D, Dougan G, Nguyen TH, Tran TP, Doan CD, Le TP, Nguyen TD, Tran TH, Farrar JJ, Monack D, Lynn DJ, Popper SJ, Falkow S. 2009. Transcriptional response in the peripheral blood of patients infected with Salmonella enterica serovar Typhi. Proc Natl Acad Sci U S A 106:22433–22438. doi: 10.1073/pnas.0912386106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Sheikh A, Charles RC, Rollins SM, Harris JB, Bhuiyan MS, Khanam F, Bukka A, Kalsy A, Porwollik S, Brooks WA, LaRocque RC, Hohmann EL, Cravioto A, Logvinenko T, Calderwood SB, McClelland M, Graham JE, Qadri F, Ryan ET. 2010. Analysis of Salmonella enterica serotype Paratyphi A gene expression in the blood of bacteremic patients in Bangladesh. PLoS Negl Trop Dis 4:e908. doi: 10.1371/journal.pntd.0000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Sheikh A, Charles RC, Sharmeen N, Rollins SM, Harris JB, Bhuiyan MS, Arifuzzaman M, Khanam F, Bukka A, Kalsy A, Porwollik S, Leung DT, Brooks WA, LaRocque RC, Hohmann EL, Cravioto A, Logvinenko T, Calderwood SB, McClelland M, Graham JE, Qadri F, Ryan ET. 2011. In vivo expression of Salmonella enterica serotype Typhi genes in the blood of patients with typhoid fever in Bangladesh. PLoS Negl Trop Dis 5:e1419. doi: 10.1371/journal.pntd.0001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Woodward TE, Smadel JE, Ley HL, Green R, Mankikar DS. 1948. Preliminary report on the beneficial effect of chloromycetin in the treatment of typhoid fever. Ann Intern Med 29:131–134. doi: 10.7326/0003-4819-29-1-131. [DOI] [PubMed] [Google Scholar]
- 316.El Ramli AH. 1950. Chloramphenicol in typhoid fever. Lancet i:618–620. [Google Scholar]
- 317.Watson KC. 1954. Chloramphenicol in typhoid fever: a review of 110 cases. Trans Royal Society Trop Med Hyg 48:526–532. doi: 10.1016/0035-9203(54)90089-9. [DOI] [PubMed] [Google Scholar]
- 318.Colquhoun J, Weetch RS. 1950. Resistance to chloramphenicol developing during treatment of typhoid fever. Lancet ii:621–623. [DOI] [PubMed] [Google Scholar]
- 319.Murti BR, Rajyalakshmi K, Bhaskaran CS. 1962. Resistance of Salmonella Typhi to chloramphenicol. I. A preliminary report. J Clin Pathol 15:544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Olarte J, Galindo E. 1973. Salmonella Typhi resistant to chloramphenicol, ampicillin, and other antimicrobial agents: strains isolated during an extensive typhoid fever epidemic in Mexico. Antimicrob Agents Chemother 4:597–601. doi: 10.1128/AAC.4.6.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Paniker CKJ, Vilma KN. 1972. Transferable chloramphenicol resistance in Salmonella Typhi. Nature 239:109–110. [DOI] [PubMed] [Google Scholar]
- 322.Butler T, Linh NN, Arnold K, Pollack M. 1973. Chloramphenicol-resistant typhoid fever in Vietnam associated with R factor. Lancet 302:983–985. doi: 10.1016/S0140-6736(73)91086-6. [DOI] [PubMed] [Google Scholar]
- 323.Brown JD, Duong Hong M, Rhoades ER. 1975. Chloramphenicol-resistant Salmonella Typhi in Saigon. JAMA 231:162–166. [PubMed] [Google Scholar]
- 324.Chun D, Seol SY, Cho DT, Tak R. 1977. Drug resistance and R plasmids in Salmonella Typhi isolated in Korea. Antimicrob Agents Chemother 11:209–213. doi: 10.1128/AAC.11.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Rowe B, Ward LR, Threlfall EJ. 1997. Multidrug-resistant Salmonella Typhi: a worldwide epidemic. Clin Infect Dis 24:S106–S109. doi: 10.1093/clinids/24.Supplement_1.S106. [DOI] [PubMed] [Google Scholar]
- 326.Anderson ES. 1975. The problem and implication of chloramphenicol resistance in the typhoid bacillus. J Hyg (London) 74:289–299. doi: 10.1017/S0022172400024360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Zaki SA, Karande S. 2011. Multidrug-resistant typhoid fever: a review. J Infect Dev Ctries 5:324–337. [DOI] [PubMed] [Google Scholar]
- 328.Rowe B, Ward LR, Threlfall EJ. 1991. Treatment of multiresistant typhoid fever. Lancet 337:1422. [DOI] [PubMed] [Google Scholar]
- 329.Wang F, Gu XJ, Zhang MF, Tai TY. 1989. Treatment of typhoid fever with ofloxacin. J Antimicrob Chemother 23:785–788. doi: 10.1093/jac/23.5.785. [DOI] [PubMed] [Google Scholar]
- 330.Mirza SH, Beeching NJ, Hart CA. 1996. Multi-drug resistant typhoid: a global problem. J Med Microbiol 44:317–319. doi: 10.1099/00222615-44-5-317. [DOI] [PubMed] [Google Scholar]
- 331.Sheorey HS, Kaundinya DV, Hulyalkar VS, Deshpande AK. 1993. Multi-drug resistant Salmonella Typhi in Bombay. Indian J Pathol Microbiol 36:8–12. [PubMed] [Google Scholar]
- 332.Ackers M-L, Puhr ND, Tauxe RV, Mintz ED. 2000. Laboratory-based surveillance of Salmonella serotype Typhi infections in the United States: antimicrobial resistance on the rise. JAMA 283:2668–2673. doi: 10.1001/jama.283.20.2668. [DOI] [PubMed] [Google Scholar]
- 333.Rao PS, Rajashekar V, Varghese GK, Shivananda PG. 1993. Emergence of multidrug-resistant Salmonella Typhi in rural southern India. Am J Trop Med Hyg 48:108–111. [DOI] [PubMed] [Google Scholar]
- 334.Chandra R, Srinivasan S, Nalini P, Rao RS. 1992. Multidrug resistant enteric fever. J Trop Med Hyg 95:284–287. [PubMed] [Google Scholar]
- 335.Nguyen TA, Ha Ba K, Nguyen TD. 1993. Typhoid fever in South Vietnam, 1990-1993. Bull Soc Pathol Exot 86:476–478. [PubMed] [Google Scholar]
- 336.Coovadia YM, Gathiram V, Bhamjee A, Garratt RM, Mlisana K, Pillay N, Madlalose T, Shorts M. 1992. An outbreak of multiresistant Salmonella Typhi in South Africa. Q J Med 82:91–100. [PubMed] [Google Scholar]
- 337.Cooke FJ, Day M, Wain J, Ward LR, Threlfall EJ. 2007. Cases of typhoid fever imported into England, Scotland and Wales (2000-2003). Trans R Soc Trop Med Hyg 101:398–404. doi: 10.1016/j.trstmh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 338.Mengo DM, Kariuki S, Muigai A, Revathi G. 2010. Trends in Salmonella enterica serovar Typhi in Nairobi, Kenya from 2004 to 2006. J Infect Dev Ctries 4:393–396. [PubMed] [Google Scholar]
- 339.Akinyemi KO, Smith SI, Oyefolu AO, Coker AO. 2005. Multidrug resistance in Salmonella enterica serovar Typhi isolated from patients with typhoid fever complications in Lagos, Nigeria. Public Health 119:321–327. doi: 10.1016/j.puhe.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 340.Kumar S, Rizvi M, Berry N. 2008. Rising prevalence of enteric fever due to multidrug-resistant Salmonella: an epidemiological study. J Med Microbiol 57:1247–1250. doi: 10.1099/jmm.0.2008/001719-0. [DOI] [PubMed] [Google Scholar]
- 341.Arjyal A, Basnyat B, Koirala S, Karkey A, Dongol S, Agrawaal KK, Shakya N, Shrestha K, Sharma M, Lama S, Shrestha K, Khatri NS, Shrestha U, Campbell JI, Baker S, Farrar J, Wolbers M, Dolecek C. 2011. Gatifloxacin versus chloramphenicol for uncomplicated enteric fever: an open-label, randomised, controlled trial. Lancet Infect Dis 11:445–454. doi: 10.1016/S1473-3099(11)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Wallace MR, Yousif AA, Mahroos GA, Mapes T, Threlfall EJ, Rowe B, Hyams KC. 1993. Ciprofloxacin versus ceftriaxone in the treatment of multiresistant typhoid fever. Eur J Clin Microbiol Infect Dis 12:907–910. doi: 10.1007/BF01992163. [DOI] [PubMed] [Google Scholar]
- 343.Umasankar S, Wall RA, Berger J. 1992. A case of ciprofloxacin-resistant typhoid fever. Commun Dis Rep 2:R139–R140. [PubMed] [Google Scholar]
- 344.Yoo S, Pai H, Byeon JH, Kang YH, Kim S, Lee BK. 2004. Epidemiology of Salmonella enterica serotype Typhi infections in Korea for recent 9 years: trends of antimicrobial resistance. J Korean Med Sci 19:15–20. doi: 10.3346/jkms.2004.19.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Chitnis V, Chitnis D, Verma S, Hemvani N. 1999. Multidrug-resistant Salmonella Typhi in India. Lancet 354:514–515. [DOI] [PubMed] [Google Scholar]
- 346.Murdoch DA, Banatvala NA, Bone A, Shoismatulloev BI, Ward LR, Threlfall EJ. 1998. Epidemic ciprofloxacin-resistant Salmonella Typhi in Tajikistan. Lancet 351:339. doi: 10.1016/S0140-6736(05)78338-0. [DOI] [PubMed] [Google Scholar]
- 347.Mahanta J. 1994. Drug sensitivity of Salmonella Paratyphi A isolated from a suspected outbreak of enteric fever in Duliajan. J Indian Med Assoc 92:49–50. [PubMed] [Google Scholar]
- 348.Adachi T, Sagara H, Hirose K, Watanabe H. 2005. Fluoroquinolone-resistant Salmonella Paratyphi A. Emerg Infect Dis 11:172–174. doi: 10.3201/eid1101.040145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Harish BN, Menezes GA, Sarangapani K, Parija SC. 2008. A case report and review of the literature: ciprofloxacin resistant Salmonella enterica serovar Typhi in India. J Infect Dev Ctries 2:324–327. [DOI] [PubMed] [Google Scholar]
- 350.Dutta S, Sur D, Manna B, Sen B, Bhattacharya M, Bhattacharya SK, Wain J, Nair S, Clemens JD, Ochiai RL. 2008. Emergence of highly fluoroquinolone-resistant Salmonella enterica serovar Typhi in a community-based fever surveillance from Kolkata, India. Int J Antimicrob Agents 31:387–389. doi: 10.1016/j.ijantimicag.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 351.Renuka K, Sood S, Das BK, Kapil A. 2005. High-level ciprofloxacin resistance in Salmonella enterica serotype Typhi in India. J Med Microbiol 54:999–1000. doi: 10.1099/jmm.0.45966-0. [DOI] [PubMed] [Google Scholar]
- 352.Gaind R, Paglietti B, Murgia M, Dawar R, Uzzau S, Cappuccinelli P, Deb M, Aggarwal P, Rubino S. 2006. Molecular characterization of ciprofloxacin-resistant Salmonella enterica serovar Typhi and Paratyphi A causing enteric fever in India. J Antimicrob Chemother 58:1139–1144. doi: 10.1093/jac/dkl391. [DOI] [PubMed] [Google Scholar]
- 353.Gupta V, Singla N, Bansal N, Kaistha N, Chander J. 2013. Trends in the antibiotic resistance patterns of enteric fever isolates—a three year report from a tertiary care centre. Malays J Med Sci 20:71–75. [PMC free article] [PubMed] [Google Scholar]
- 354.Jain S, Chugh TD. 2013. Antimicrobial resistance among blood culture isolates of Salmonella enterica in New Delhi. J Infect Dev Ctries 7:788–795. [DOI] [PubMed] [Google Scholar]
- 355.Menezes GA, Harish BN, Khan MA, Goessens WH, Hays JP. 2012. Antimicrobial resistance trends in blood culture positive Salmonella Typhi isolates from Pondicherry, India, 2005-2009. Clin Microbiol Infect 18:239–245. doi: 10.1111/j.1469-0691.2011.03546.x. [DOI] [PubMed] [Google Scholar]
- 356.Threlfall EJ, Ward LR. 2001. Decreased susceptibility to ciprofloxacin in Salmonella enterica serotype Typhi, United Kingdom. Emerg Infect Dis 7:448–450. doi: 10.3201/eid0703.017315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Demczuk WH, Finley R, Nadon C, Spencer A, Gilmour M, Ng LK, PulseNet Canada, Canadian Integrated Program for Antimicrobial Resistance Surveillance Public Health Partnership, Canadian Public Health Laboratory Network. 2010. Characterization of antimicrobial resistance, molecular and phage types of Salmonella enterica serovar Typhi isolations. Epidemiol Infect 138:1414–1426. doi: 10.1017/S0950268810000221. [DOI] [PubMed] [Google Scholar]
- 358.Centers for Disease Control and Prevention. 2007. National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS); human isolates final report, 2004. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 359.Threlfall EJ, de Pinna E, Day M, Lawrence J, Jones J. 2008. Alternatives to ciprofloxacin use for enteric fever, United Kingdom. Emerg Infect Dis 14:860–861. doi: 10.3201/eid1405.071184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Effa EE, Bukirwa H. 2011. Azithromycin for treating uncomplicated typhoid and paratyphoid fever (enteric fever). Cochrane Database Syst Rev 3:CD006083. doi: 10.1002/14651858. [DOI] [PubMed] [Google Scholar]
- 361.Ahmed D, Hoque A, Mazumder R, Nahar K, Islam N, Gazi SA, Hossain MA. 2012. Salmonella enterica serovar Typhi strain producing extended-spectrum beta-lactamases in Dhaka, Bangladesh. J Med Microbiol 61:1032–1033. doi: 10.1099/jmm.0.044065-0. [DOI] [PubMed] [Google Scholar]
- 362.Gokul BN, Menezes GA, Harish BN. 2010. ACC-1 beta-lactamase-producing Salmonella enterica serovar Typhi, India. Emerg Infect Dis 16:1170–1171. doi: 10.3201/eid1607.091643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Al Naiemi N, Zwart B, Rijnsburger MC, Roosendaal R, Debets-Ossenkopp YJ, Mulder JA, Fijen CA, Maten W, Vandenbroucke-Grauls CM, Savelkoul PH. 2008. Extended-spectrum-beta-lactamase production in a Salmonella enterica serotype Typhi strain from the Philippines. J Clin Microbiol 46:2794–2795. doi: 10.1128/JCM.00676-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Pfeifer Y, Matten J, Rabsch W. 2009. Salmonella enterica serovar Typhi with CTX-M beta-lactamase, Germany. Emerg Infect Dis 15:1533–1535. doi: 10.3201/eid1509.090567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Threlfall EJ. 2002. Antimicrobial drug resistance in Salmonella: problems and perspectives in food- and water-borne infections. FEMS Microbiol Rev 26:141–148. doi: 10.1111/j.1574-6976.2002.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 366.Parry CM. 2003. Antimicrobial drug resistance in Salmonella enterica. Curr Opin Infect Dis 16:467–472. doi: 10.1097/00001432-200310000-00014. [DOI] [PubMed] [Google Scholar]
- 367.Threlfall EJ. 2000. Epidemic Salmonella Typhimurium DT 104: a truly international multiresistant clone. J Antimicrob Chemother 46:7–10. doi: 10.1093/jac/46.1.7. [DOI] [PubMed] [Google Scholar]
- 368.Centers for Disease Control and Prevention. 2008. National Antimicrobial Resistance Monitoring System for enteric bacteria (NARMS); human isolates final report, 2005. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 369.Centers for Disease Control and Prevention. 2013. National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS); human isolates final report, 2011. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 370.Dimitrov T, Udo EE, Albaksami O, Kilani AA, el Shehab DM. 2007. Ciprofloxacin treatment failure in a case of typhoid fever caused by Salmonella enterica serotype Paratyphi A with reduced susceptibility to ciprofloxacin. J Med Microbiol 56:277–279. doi: 10.1099/jmm.0.46773-0. [DOI] [PubMed] [Google Scholar]
- 371.Stevenson JE, Gay K, Barrett TJ, Medalla F, Chiller TM, Angulo FJ. 2007. Increase in nalidixic acid resistance among non-Typhi Salmonella enterica isolates in the United States from 1996 to 2003. Antimicrob Agents Chemother 51:195–197. doi: 10.1128/AAC.00222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Threlfall EJ, Fisher IS, Berghold C, Gerner-Smidt P, Tschape H, Cormican M, Luzzi I, Schnieder F, Wannet W, Machado J, Edwards G. 2003. Antimicrobial drug resistance in isolates of Salmonella enterica from cases of salmonellosis in humans in Europe in 2000: results of international multi-centre surveillance. Euro Surveill 8:41–45. [DOI] [PubMed] [Google Scholar]
- 373.Lee HY, Su LH, Tsai MH, Kim SW, Chang HH, Jung SI, Park KH, Perera J, Carlos C, Tan BH, Kumarasinghe G, So T, Chongthaleong A, Hsueh PR, Liu JW, Song JH, Chiu CH. 2009. High rate of reduced susceptibility to ciprofloxacin and ceftriaxone among nontyphoid Salmonella clinical isolates in Asia. Antimicrob Agents Chemother 53:2696–2699. doi: 10.1128/AAC.01297-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Wannaprasat W, Padungtod P, Chuanchuen R. 2011. Class 1 integrons and virulence genes in Salmonella enterica isolates from pork and humans. Int J Antimicrob Agents 37:457–461. doi: 10.1016/j.ijantimicag.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 375.Miriagou V, Tassios PT, Legakis NJ, Tzouvelekis LS. 2004. Expanded-spectrum cephalosporin resistance in non-typhoid Salmonella. Int J Antimicrob Agents 23:547–555. doi: 10.1016/j.ijantimicag.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 376.Ben Hassen A, Bejaoui M, Lakhoua MR, Ben Redjeb S. 1993. Epidemiological pattern of the resistance of 153 Salmonella strains (S. Typhi excluded) isolated in a Tunisian pediatric unit from 1985 to 1990. Pathol Biol (Paris) 41:706–712. [PubMed] [Google Scholar]
- 377.Koh TH, Koh AE, Hamdan A, Khoo BC, Yu VY, Raymond RT, Tee NW. 2008. Ceftriaxone-resistant Salmonella spp. in Singapore. Ann Acad Med Singapore 37:900–901. [PubMed] [Google Scholar]
- 378.Pornruangwong S, Hendriksen RS, Pulsrikarn C, Bangstrakulnonth A, Mikoleit M, Davies RH, Aarestrup FM, Garcia-Migura L. 2011. Epidemiological investigation of Salmonella enterica serovar Kedougou in Thailand. Foodborne Pathog Dis 8:203–211. doi: 10.1089/fpd.2010.0626. [DOI] [PubMed] [Google Scholar]
- 379.Sirichote P, Hasman H, Pulsrikarn C, Schonheyder HC, Samulioniene J, Pornruangmong S, Bangtrakulnonth A, Aarestrup FM, Hendriksen RS. 2010. Molecular characterization of extended-spectrum cephalosporinase-producing Salmonella enterica serovar Choleraesuis isolates from patients in Thailand and Denmark. J Clin Microbiol 48:883–888. doi: 10.1128/JCM.01792-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Sirichote P, Bangtrakulnonth A, Tianmanee K, Unahalekhaka A, Oulai A, Chittaphithakchai P, Kheowrod W, Hendriksen RS. 2010. Serotypes and antimicrobial resistance of Salmonella enterica spp in central Thailand, 2001-2006. Southeast Asian J Trop Med Public Health 41:1405–1415. [PubMed] [Google Scholar]
- 381.Kulwichit W, Chatsuwan T, Unhasuta C, Pulsrikarn C, Bangtrakulnonth A, Chongthaleong A. 2007. Drug-resistant nontyphoidal Salmonella bacteremia, Thailand. Emerg Infect Dis 13:501–502. doi: 10.3201/eid1303.061059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Garbarg-Chenon A, Vu Thien H, Labia R, Ben-Yaghlane H, Godard V, Deny P, Bricout F, Nicolas JC. 1989. Characterization of a plasmid coding for resistance to broad-spectrum cephalosporins in Salmonella Typhimurium. Drugs Exp Clin Res 15:145–150. [PubMed] [Google Scholar]
- 383.Villa L, Mammina C, Miriagou V, Tzouvelekis LS, Tassios PT, Nastasi A, Carattoli A. 2002. Multidrug and broad-spectrum cephalosporin resistance among Salmonella enterica serotype Enteritidis clinical isolates in southern Italy. J Clin Microbiol 40:2662–2665. doi: 10.1128/JCM.40.7.2662-2665.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Tassios PT, Gazouli M, Tzelepi E, Milch H, Kozlova N, Sidorenko S, Legakis NJ, Tzouvelekis LS. 1999. Spread of a Salmonella Typhimurium clone resistant to expanded-spectrum cephalosporins in three European countries. J Clin Microbiol 37:3774–3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Herikstad H, Hayes P, Mokhtar M, Fracaro ML, Threlfall EJ, Angulo FJ. 1997. Emerging quinolone-resistant Salmonella in the United States. Emerg Infect Dis 3:371–372. doi: 10.3201/eid0303.970316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Fey PD, Safranek TJ, Rupp ME, Dunne EF, Ribot E, Iwen PC, Bradford PA, Angulo FJ, Hinrichs SH. 2000. Ceftriaxone-resistant Salmonella infection acquired by a child from cattle. N Engl J Med 342:1242–1249. doi: 10.1056/NEJM200004273421703. [DOI] [PubMed] [Google Scholar]
- 387.Mulvey MR, Soule G, Boyd D, Demczuk W, Ahmed R, Multi-Provincial Salmonella Typhimurium Case Control Sudy Group. 2003. Characterization of the first extended-spectrum beta-lactamase-producing Salmonella isolate identified in Canada. J Clin Microbiol 41:460–462. doi: 10.1128/JCM.41.1.460-462.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Pitout JD, Reisbig MD, Mulvey M, Chui L, Louie M, Crowe L, Church DL, Elsayed S, Gregson D, Ahmed R, Tilley P, Hanson ND. 2003. Association between handling of pet treats and infection with Salmonella enterica serotype Newport expressing the AmpC beta-lactamase, CMY-2. J Clin Microbiol 41:4578–4582. doi: 10.1128/JCM.41.10.4578-4582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Mataseje LF, Xiao J, Kost S, Ng LK, Doré K, Mulvey MR, Canadian Public Health Laboratory Network. 2009. Characterization of Canadian cefoxitin-resistant non-typhoidal Salmonella isolates, 2005-06. J Antimicrob Chemother 64:723–730. doi: 10.1093/jac/dkp249. [DOI] [PubMed] [Google Scholar]
- 390.Folster JP, Pecic G, Singh A, Duval B, Rickert R, Ayers S, Abbott J, McGlinchey B, Bauer-Turpin J, Haro J, Hise K, Zhao S, Fedorka-Cray PJ, Whichard J, McDermott PF. 2012. Characterization of extended-spectrum cephalosporin-resistant Salmonella enterica serovar Heidelberg isolated from food animals, retail meat, and humans in the United States 2009. Foodborne Pathog Dis 9:638–645. doi: 10.1089/fpd.2012.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Frye JG, Fedorka-Cray PJ. 2007. Prevalence, distribution and characterisation of ceftiofur resistance in Salmonella enterica isolated from animals in the USA from 1999 to 2003. Int J Antimicrob Agents 30:134–142. doi: 10.1016/j.ijantimicag.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 392.Sjölund M, Yam J, Schwenk J, Joyce K, Medalla F, Barzilay E, Whichard JM. 2008. Human Salmonella infection yielding CTX-M beta-lactamase, United States. Emerg Infect Dis 14:1957–1959. doi: 10.3201/eid1412.080494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Sjölund-Karlsson M, Howie R, Krueger A, Rickert R, Pecic G, Lupoli K, Folster JP, Whichard JM. 2011. CTX-M-producing non-Typhi Salmonella spp. isolated from humans, United States. Emerg Infect Dis 17:97–99. doi: 10.3201/eid1701.100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Sjöslund-Karlsson M, Rickert R, Matar C, Pecic G, Howie RL, Joyce K, Medalla F, Barzilay EJ, Whichard JM. 2010. Salmonella isolates with decreased susceptibility to extended-spectrum cephalosporins in the United States. Foodborne Pathog Dis 7:1503–1509. doi: 10.1089/fpd.2010.0607. [DOI] [PubMed] [Google Scholar]
- 395.Dunne EF, Fey PD, Kludt P, Reporter R, Mostashari F, Shillam P, Wicklund J, Miller C, Holland B, Stamey K, Barrett TJ, Rasheed JK, Tenover FC, Ribot EM, Angulo FJ. 2000. Emergence of domestically acquired ceftriaxone-resistant Salmonella infections associated with AmpC beta-lactamase. JAMA 284:3151–3156. doi: 10.1001/jama.284.24.3151. [DOI] [PubMed] [Google Scholar]
- 396.Allen KJ, Poppe C. 2002. Occurrence and characterization of resistance to extended-spectrum cephalosporins mediated by beta-lactamase CMY-2 in Salmonella isolated from food-producing animals in Canada. Can J Vet Res 66:137–144. [PMC free article] [PubMed] [Google Scholar]
- 397.Zhao S, Blickenstaff K, Glenn A, Ayers SL, Friedman SL, Abbott JW, McDermott PF. 2009. Beta-lactam resistance in Salmonella strains isolated from retail meats in the United States by the National Antimicrobial Resistance Monitoring System between 2002 and 2006. Appl Environ Microbiol 75:7624–7630. doi: 10.1128/AEM.01158-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Zhao S, Qaiyumi S, Friedman S, Singh R, Foley SL, White DG, McDermott PF, Donkar T, Bolin C, Munro S, Baron EJ, Walker RD. 2003. Characterization of Salmonella enterica serotype Newport isolated from humans and food animals. J Clin Microbiol 41:5366–5371. doi: 10.1128/JCM.41.12.5366-5371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Zhao S, White DG, McDermott PF, Friedman S, English L, Ayers S, Meng J, Maurer JJ, Holland R, Walker RD. 2001. Identification and expression of cephamycinase bla(CMY) genes in Escherichia coli and Salmonella isolates from food animals and ground meat. Antimicrob Agents Chemother 45:3647–3650. doi: 10.1128/AAC.45.12.3647-3650.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Butaye P, Michael GB, Schwarz S, Barrett TJ, Brisabois A, White DG. 2006. The clonal spread of multidrug-resistant non-typhi Salmonella serotypes. Microbes Infect 8:1891–1897. doi: 10.1016/j.micinf.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 401.Folster JP, Pecic G, Rickert R, Taylor J, Zhao S, Fedorka-Cray PJ, Whichard J, McDermott P. 2012. Characterization of multidrug-resistant Salmonella enterica serovar Heidelberg from a ground turkey-associated outbreak in the United States in 2011. Antimicrob Agents Chemother 56:3465–3466. doi: 10.1128/AAC.00201-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Tiong V, Thong KL, Yusof MY, Hanifah YA, Sam JI, Hassan H. 2010. Macrorestriction analysis and antimicrobial susceptibility profiling of salmonella enterica at a University Teaching Hospital, Kuala Lumpur. Jpn J Infect Dis 63:317–322. [PubMed] [Google Scholar]
- 403.Vo AT, van Duijkeren E, Gaastra W, Fluit AC. 2010. Antimicrobial resistance, class 1 integrons, and genomic island 1 in Salmonella isolates from Vietnam. PLoS One 5:e9440. doi: 10.1371/journal.pone.0009440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Benacer D, Thong KL, Watanabe H, Puthucheary SD. 2010. Characterization of drug resistant Salmonella enterica serotype Typhimurium by antibiograms, plasmids, integrons, resistance genes and PFGE. J Microbiol Biotechnol 20:1042–1052. doi: 10.4014/jmb.0910.10028. [DOI] [PubMed] [Google Scholar]
- 405.Le Hello S, Harrois D, Bouchrif B, Sontag L, Elhani D, Guibert V, Zerouali K, Weill FX. 2013. Highly drug-resistant Salmonella enterica serotype Kentucky ST198-X1: a microbiological study. Lancet Infect Dis 13:672–679. doi: 10.1016/S1473-3099(13)70124-5. [DOI] [PubMed] [Google Scholar]
- 406.Jure MA, Duprilot M, Musa HE, Lopez C, de Castillo MC, Weill FX, Arlet G, Decré D. 2014. Emergence of KPC-2-producing Salmonella enterica serotype Schwarzengrund in Argentina. Antimicrob Agents Chemother 58:6335–6336. doi: 10.1128/AAC.03322-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Rodríguez E, Bautista A, Barrero L. 2014. First report of a Salmonella enterica serovar Typhimurium isolate with carbapenemase (KPC-2) in Colombia. Antimicrob Agents Chemother 58:1263–1264. doi: 10.1128/AAC.02423-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Irfan S, Khan E, Jabeen K, Bhawan P, Hopkins KL, Day M, Nasir A, Meunier D, Woodford N. 2015. Clinical isolates of Salmonella enterica serovar Agona producing NDM-1 metallo-β-lactamase: first report from Pakistan. J Clin Microbiol 53:346–348. doi: 10.1128/JCM.02396-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Rasheed JK, Kitchel B, Zhu W, Anderson KF, Clark NC, Ferraro MJ, Savard P, Humphries RM, Kallen AJ, Limbago BM. 2013. New Delhi metallo-β-lactamase-producing Enterobacteriaceae, United States. Emerg Infect Dis 19:870–878. doi: 10.3201/eid1906.121515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Huang J, Wang M, Ding H, Ye M, Hu F, Guo Q, Xu X, Wang M. 2013. New Delhi metallo-β-lactamase-1 in carbapenem-resistant Salmonella strain, China. Emerg Infect Dis 19:2049–2051. doi: 10.3201/eid1912.130051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Arcangioli MA, Leroy-Setrin S, Martel JL, Chaslus-Dancla E. 2000. Evolution of chloramphenicol resistance, with emergence of cross-resistance to florfenicol, in bovine Salmonella Typhimurium strains implicates definitive phage type (DT) 104. J Med Microbiol 49:103–110. [DOI] [PubMed] [Google Scholar]
- 412.Glenn LM, Lindsey RL, Frank JF, Meinersmann RJ, Englen MD, Fedorka-Cray PJ, Frye JG. 2011. Analysis of antimicrobial resistance genes detected in multidrug-resistant Salmonella enterica serovar Typhimurium isolated from food animals. Microb Drug Resist 17:407–418. doi: 10.1089/mdr.2010.0189. [DOI] [PubMed] [Google Scholar]
- 413.Levings RS, Lightfoot D, Partridge SR, Hall RM, Djordjevic SP. 2005. The genomic island SGI1, containing the multiple antibiotic resistance region of Salmonella enterica serovar Typhimurium DT104 or variants of it, is widely distributed in other S. enterica serovars. J Bacteriol 187:4401–4409. doi: 10.1128/JB.187.13.4401-4409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Doublet B, Boyd D, Mulvey MR, Cloeckaert A. 2005. The Salmonella genomic island 1 is an integrative mobilizable element. Mol Microbiol 55:1911–1924. doi: 10.1111/j.1365-2958.2005.04520.x. [DOI] [PubMed] [Google Scholar]
- 415.Hall RM. 2010. Salmonella genomic islands and antibiotic resistance in Salmonella enterica. Future Microbiol 5:1525–1538. doi: 10.2217/fmb.10.122. [DOI] [PubMed] [Google Scholar]
- 416.Boyd D, Cloeckaert A, Chaslus-Dancla E, Mulvey MR. 2002. Characterization of variant Salmonella genomic island 1 multidrug resistance regions from serovars Typhimurium DT104 and Agona. Antimicrob Agents Chemother 46:1714–1722. doi: 10.1128/AAC.46.6.1714-1722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Hopkins KL, Davies RH, Threlfall EJ. 2005. Mechanisms of quinolone resistance in Escherichia coli and Salmonella: recent developments. Int J Antimicrob Agents 25:358–373. doi: 10.1016/j.ijantimicag.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 418.Turner AK, Nair S, Wain J. 2006. The acquisition of full fluoroquinolone resistance in Salmonella Typhi by accumulation of point mutations in the topoisomerase targets. J Antimicrob Chemother 58:733–740. doi: 10.1093/jac/dkl333. [DOI] [PubMed] [Google Scholar]
- 419.Wain J, Hoa NTT, Chinh NT, Vinh H, Everett MJ, Diep TS, Day NPJ, Solomon T, White NJ, Piddock LJV, Parry CM. 1997. Quinolone-resistant Salmonella Typhi in Viet Nam: molecular basis of resistance and clinical response to treatment. Clin Infect Dis 25:1404–1410. doi: 10.1086/516128. [DOI] [PubMed] [Google Scholar]
- 420.Renuka K, Kapil A, Kabra SK, Wig N, Das BK, Prasad VV, Chaudhry R, Seth P. 2004. Reduced susceptibility to ciprofloxacin and gyra gene mutation in North Indian strains of Salmonella enterica serotype Typhi and serotype Paratyphi A. Microb Drug Resist 10:146–153. doi: 10.1089/1076629041310028. [DOI] [PubMed] [Google Scholar]
- 421.Brown JC, Shanahan PM, Jesudason MV, Thomson CJ, Amyes SG. 1996. Mutations responsible for reduced susceptibility to 4-quinolones in clinical isolates of multi-resistant Salmonella Typhi in India. J Antimicrob Chemother 37:891–900. doi: 10.1093/jac/37.5.891. [DOI] [PubMed] [Google Scholar]
- 422.Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A. 2009. Plasmid-mediated quinolone resistance: a multifaceted threat. Clin Microbiol Rev 22:664–689. doi: 10.1128/CMR.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Martinez-Martinez L, Pascual A, Jacoby GA. 1998. Quinolone resistance from a transferable plasmid. Lancet 351:797–799. doi: 10.1016/S0140-6736(97)07322-4. [DOI] [PubMed] [Google Scholar]
- 424.Cavaco LM, Aarestrup FM. 2009. Evaluation of quinolones for use in detection of determinants of acquired quinolone resistance, including the new transmissible resistance mechanisms qnrA, qnrB, qnrS, and aac(6′)Ib-cr, in Escherichia coli and Salmonella enterica and determinations of wild-type distributions. J Clin Microbiol 47:2751–2758. doi: 10.1128/JCM.00456-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Sjölund-Karlsson M, Howie R, Rickert R, Newton A, Gonzalez-Aviles G, Crump JA. 2015. Plasmid-mediated quinolone resistance in isolates of Salmonella enterica serotype Typhi, USA. Int J Antimicrob Agents 45:88–90. doi: 10.1016/j.ijantimicag.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 426.Robicsek A, Jacoby GA, Hooper DC. 2006. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect Dis 6:629–640. doi: 10.1016/S1473-3099(06)70599-0. [DOI] [PubMed] [Google Scholar]
- 427.Sjölund-Karlsson M, Folster JP, Pecic G, Joyce K, Medalla F, Rickert R, Whichard JM. 2009. Emergence of plasmid-mediated quinolone resistance among non-Typhi Salmonella enterica isolates from humans in the United States. Antimicrob Agents Chemother 53:2142–2144. doi: 10.1128/AAC.01288-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Marshall WF, Blair JE. 1999. The cephalosporins. Mayo Clin Proc 74:187–195. doi: 10.4065/74.2.187. [DOI] [PubMed] [Google Scholar]
- 429.Paterson DL, Bonomo RA. 2005. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev 18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Philippon A, Arlet G, Jacoby GA. 2002. Plasmid-determined AmpC-type beta-lactamases. Antimicrob Agents Chemother 46:1–11. doi: 10.1128/AAC.46.1.1-11.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Folster JP, Rickert R, Barzilay EJ, Whichard JM. 2009. Identification of the aminoglycoside resistance determinants armA and rmtC among non-Typhi Salmonella isolates from humans in the United States. Antimicrob Agents Chemother 53:4563–4564. doi: 10.1128/AAC.00656-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Molloy A, Nair S, Cooke FJ, Wain J, Farrington M, Lehner PJ, Torok ME. 2010. First report of Salmonella enterica serotype Paratyphi A azithromycin resistance leading to treatment failure. J Clin Microbiol 48:4655–4657. doi: 10.1128/JCM.00648-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Takkar VP, Kumar R, Khurana S, Takkar R. 1994. Comparison of ciprofloxacin versus cephelexin and gentamicin in the treatment of multi-drug resistant typhoid fever. Indian Pediatr 31:200–201. [PubMed] [Google Scholar]
- 434.Edge W. 1950. Typhoid fever treated with chloramphenicol: review of 16 cases. Lancet 255:710–712. doi: 10.1016/S0140-6736(50)91950-7. [DOI] [PubMed] [Google Scholar]
- 435.Ti T-Y, Monteiro EH, Lam S, Lee HS. 1990. Chloramphenicol concentrations in sera of patients with typhoid fever being treated with oral or intravenous preparation. Antimicrob Agents Chemother 34:1809–1811. doi: 10.1128/AAC.34.9.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Bhutta ZA, Niazi SK, Suria A. 1992. Chloramphenicol clearance in typhoid fever: implications for therapy. Indian J Pediatr 59:213–219. doi: 10.1007/BF02759987. [DOI] [PubMed] [Google Scholar]
- 437.Acharya GP, Davis TM, Ho M, Harris S, Chataut C, Acharya S, Tuhladar N, Kafle KE, Pokhrel B, Nosten F, Dance DA, Smith A, Weber A, White NJ. 1997. Factors affecting the pharmacokinetics of parenteral chloramphenicol in enteric fever. J Antimicrob Chemother 40:91–98. doi: 10.1093/jac/40.1.91. [DOI] [PubMed] [Google Scholar]
- 438.Robertson RP, Wahab MF, Raasch FO. 1968. Evaluation of chloramphenicol and ampicillin in Salmonella enteric fever. N Engl J Med 278:171–176. doi: 10.1056/NEJM196801252780401. [DOI] [PubMed] [Google Scholar]
- 439.Snyder MJ, Gonzalez O, Palomino C, Music SI, Hornick RB, Perroni J, Woodward WE, Gonzalez C, DuPont HL, Woodward TE. 1976. Comparative efficacy of chloramphenicol, ampicillin, and co-trimoxazole in the treatment of typhoid fever. Lancet 308:1155–1157. doi: 10.1016/S0140-6736(76)91678-0. [DOI] [PubMed] [Google Scholar]
- 440.Moosa A, Rubidge CJ. 1989. Once daily ceftriaxone vs. chloramphenicol for treatment of typhoid fever in children. Pediatr Infect Dis J 8:696–699. doi: 10.1097/00006454-198910000-00007. [DOI] [PubMed] [Google Scholar]
- 441.Tanaka-Kido J, Ortega L, Santos JI. 1990. Comparative efficacies of aztreonam and chloramphenicol in children with typhoid fever. Pediatr Infect Dis J 9:44–48. doi: 10.1097/00006454-199001000-00010. [DOI] [PubMed] [Google Scholar]
- 442.Lasserre R, Sangalang RP, Santiago L. 1991. Three-day treatment of typhoid fever with two different doses of ceftriaxone, compared to 14-day therapy with chloramphenicol: a randomized trial. J Antimicrob Chemother 28:765–772. doi: 10.1093/jac/28.5.765. [DOI] [PubMed] [Google Scholar]
- 443.Cristiano P, Imparato L, Carpinelli C, Lauria F, Iovene MR, Corrado MF, Maio P, Imperatore C. 1995. Pefloxacin versus chloramphenicol in the therapy of typhoid fever. Infection 23:103–106. doi: 10.1007/BF01833875. [DOI] [PubMed] [Google Scholar]
- 444.Butler T, Sridhar CB, Daga MK, Pathak K, Pandit RB, Khakhria R, Potkar CN, Zelasky MT, Johnson RB. 1999. Treatment of typhoid fever with azithromycin versus chloramphenicol in a randomized multicentre trial in India. J Antimicrob Chemother 44:243–250. doi: 10.1093/jac/44.2.243. [DOI] [PubMed] [Google Scholar]
- 445.Liberti A, Loiacono L. 2000. Ciprofloxacin versus chloramphenicol in the treatment of Salmonella infection. Int J Antimicrob Agents 16:347–348. doi: 10.1016/S0924-8579(00)00262-4. [DOI] [PubMed] [Google Scholar]
- 446.Gasem MH, Keuter M, Dolmans WMV, van der Ven-Jongekrijg J, Djokomoeljanto R, van der Meer JWM. 2003. Persistence of salmonellae in blood and bone marrow: randomized controlled trial comparing ciprofloxacin and chloramphenicol treatments against enteric fever. Antimicrob Agents Chemother 47:1727–1731. doi: 10.1128/AAC.47.5.1727-1731.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Phongmany S, Phetsouvanh R, Sisouphone S, Darasavath C, Vongphachane P, Rattanavong O, Mayxay M, Ramsay AC, Blacksell SD, Thammavong C, Syhavong B, White NJ, Newton PN. 2005. A randomized comparison of oral chloramphenicol versus ofloxacin in the treatment of uncomplicated typhoid fever in Laos. Trans R Soc Trop Med Hyg 99:451–458. doi: 10.1016/j.trstmh.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 448.Wilcox MH. 2003. Chloramphenicol and thiamphenicol, p 279–283. In Finch RG, Greenwood D, Norrby SR, Whitley RJ (ed), Antibiotics and chemotherapy, 8th ed Churchill Livingstone, Edinburgh, United Kingdom. [Google Scholar]
- 449.Sardesai HV, Melinkere RD, Diwate AB. 1971. Trimethoprim-sulphamethoxazole in typhoid fever. Trans R Soc Trop Med Hyg 65:189–194. doi: 10.1016/0035-9203(71)90216-1. [DOI] [PubMed] [Google Scholar]
- 450.Scragg JN, Rubidge CJ. 1971. Trimethoprim and sulphamethoxazole in typhoid fever in children. Br Med J 3:738–741. doi: 10.1136/bmj.3.5777.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Scragg JN, Rubidge CJ. 1975. Amoxycillin in the treatment of typhoid fever in children. Am J Trop Med Hyg 24:860–865. [DOI] [PubMed] [Google Scholar]
- 452.Pillay N, Adams EB, North-Coombes D. 1975. Comparative trial of amoxycillin and chloramphenicol in treatment of typhoid fever in adults. Lancet 306:333–334. doi: 10.1016/S0140-6736(75)92776-2. [DOI] [PubMed] [Google Scholar]
- 453.Butler T, Linh NN, Arnold K, Adickman MD, Chau DM, Muoi MM. 1977. Therapy of antimicrobial-resistant typhoid fever. Antimicrob Agents Chemother 11:645–650. doi: 10.1128/AAC.11.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Gilman RH, Terminel M, Levine MM, Hernandez-Mendosa P, Calderone E, Vasquez V, Martinez E, Snyder MJ, Hornick RB. 1975. Comparison of trimethoprim-sulfamethoxazole and amoxicillin in therapy of chloramphenicol-resistant and chloramphenicol-sensitive typhoid fever. J Infect Dis 132:630–636. doi: 10.1093/infdis/132.6.630. [DOI] [PubMed] [Google Scholar]
- 455.Butler T, Rumans L, Arnold K. 1982. Response of typhoid fever caused by chloramphenicol-susceptible and chloramphenicol-resistant strains of Salmonella Typhi to treatment with trimethoprim-sulfamethoxazole. Rev Infect Dis 4:551–561. doi: 10.1093/clinids/4.2.551. [DOI] [PubMed] [Google Scholar]
- 456.Ball AP, Farrell ID, Gillett AP, Geddes AM, Clarke PD, Ellis CJ. 1979. Enteric fever in Birmingham: clinical features, laboratory investigation and comparison of treatment with pivmecillinam and co-trimoxazole. J Infect 1:353–365. doi: 10.1016/S0163-4453(79)90702-3. [DOI] [Google Scholar]
- 457.Mandal BK, Ironside AG, Brennand J. 1979. Mecillinam in enteric fever. Br Med J 1:586–587. doi: 10.1136/bmj.1.6163.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Jones DA, Kudlac H, Edwards IR. 1982. Pivmecillinam and relapse of typhoid fever. J Infect Dis 145:773. doi: 10.1093/infdis/145.2.773. [DOI] [PubMed] [Google Scholar]
- 459.Pape JW, Gerdes H, Oriol L, Johnson WD. 1986. Typhoid fever: successful therapy with cefoperazone. J Infect Dis 153:272–276. doi: 10.1093/infdis/153.2.272. [DOI] [PubMed] [Google Scholar]
- 460.Soe GB, Overturf GD. 1987. Treatment of typhoid fever and other systemic salmonelloses with cefotaxime, ceftriaxone, cefoperazone, and other newer cephalosporins. Rev Infect Dis 9:719–736. doi: 10.1093/clinids/9.4.719. [DOI] [PubMed] [Google Scholar]
- 461.Acharya G, Crevoisier C, Butler T, Ho M, Tiwari M, Stoeckel K, Bradley CA. 1994. Pharmacokinetics of ceftriaxone in patients with typhoid fever. Antimicrob Agents Chemother 38:2415–2418. doi: 10.1128/AAC.38.10.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Ti TY, Monteiro EH, Lam S, Lee HS. 1985. Ceftriaxone therapy in bacteremic typhoid fever. Antimicrob Agents Chemother 28:540–543. doi: 10.1128/AAC.28.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Chang HR, Vladoianu IR, Pechèe JC. 1990. Effects of ampicillin, ceftriaxone, chloramphenicol, pefloxacin and trimethoprim-sulphamethoxazole on Salmonella Typhi within human monocyte-derived macrophages. J Antimicrob Chemother 26:689–694. doi: 10.1093/jac/26.5.689. [DOI] [PubMed] [Google Scholar]
- 464.Ekinci B, Coban AY, Birinci A, Durupinar B, Erturk M. 2002. In vitro effects of cefotaxime and ceftriaxone on Salmonella Typhi within human monocyte-derived macrophages. Clin Microbiol Infect 8:810–813. doi: 10.1046/j.1469-0691.2002.00457.x. [DOI] [PubMed] [Google Scholar]
- 465.Bhutta ZA, Khan IA, Molla AM. 1994. Therapy of multidrug-resistant typhoid fever with oral cefixime vs. intravenous ceftriaxone. Pediatr Infect Dis J 13:990–994. doi: 10.1097/00006454-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 466.Bhutta ZA. 1996. Therapeutic aspects of typhoidal salmonellosis in childhood: the Karachi experience. Ann Trop Paediatr 16:299–306. [DOI] [PubMed] [Google Scholar]
- 467.Bhutta ZA, Khan IA, Shadmani M. 2000. Failure of short-course ceftriaxone chemotherapy for multidrug-resistant typhoid fever in children: a randomized controlled trial in Pakistan. Antimicrob Agents Chemother 44:450–452. doi: 10.1128/AAC.44.2.450-452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Islam A, Butler T, Nath SK, Alam NH, Stoeckel K, Houser HB, Smith AL. 1988. Randomized treatment of patients with typhoid fever by using ceftriaxone or chloramphenicol. J Infec Dis 158:742–747. doi: 10.1093/infdis/158.4.742. [DOI] [PubMed] [Google Scholar]
- 469.Frenck RW, Nakhla IA, Sultan Y, Bassily SB, Girgis FY, David J, Butler TC, Girgis NI, Morsy M. 2000. Azithromycin versus ceftriaxone for the treatment of uncomplicated typhoid fever in children. Clin Infect Dis 31:1134–1138. doi: 10.1086/317450. [DOI] [PubMed] [Google Scholar]
- 470.Tran TH, Nguyen MD, Huynh DH, Nguyen TT, To SD, Le TP, Arnold K. 1994. A randomized comparative study of fleroxacin and ceftriaxone in enteric fever. Trans Royal Soc Trop Med Hyg 88:464–465. doi: 10.1016/0035-9203(94)90435-9. [DOI] [PubMed] [Google Scholar]
- 471.Islam A, Butler T, Kabir I, Alam NH. 1993. Treatment of typhoid fever with ceftriaxone for 5 days or chloramphenicol for 14 days: a randomized clinical trial. Antimicrob Agents Chemother 37:1572–1575. doi: 10.1128/AAC.37.8.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Girgis NI, Sultan Y, Hammad O, Farid Z. 1995. Comparison of the efficacy, safety and cost of cefixime, ceftriaxone and aztreonam in the treatment of multidrug-resistant Salmonella Typhi septicemia in children. Pediatr Infect Dis J 14:603–605. doi: 10.1097/00006454-199507000-00010. [DOI] [PubMed] [Google Scholar]
- 473.Frenck RW, Mansour A, Nakhla I, Sultan Y, Putnam S, Wierzba T, Morsy M, Knirsch C. 2004. Short-course azithromycin for the treatment of uncomplicated typhoid fever in children and adolescents. Clin Infect Dis 38:951–957. doi: 10.1086/382359. [DOI] [PubMed] [Google Scholar]
- 474.Smith MD, Duong NM, Hoa NT, Wain J, Ha HD, Diep TS, Day NP, Hien TT, White NJ. 1994. Comparison of ofloxacin and ceftriaxone for short-course treatment of enteric fever. Antimicrob Agents Chemother 38:1716–1720. doi: 10.1128/AAC.38.8.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Acharya G, Butler T, Ho M, Sharma PR, Tiwari M, Adhikari RK, Khagda JB, Pokhrel B, Pathak UN. 1995. Treatment of typhoid fever: randomized trial of a three-day course of ceftriaxone versus a fourteen-day course of chloramphenicol. Am J Trop Med Hyg 52:162–165. [DOI] [PubMed] [Google Scholar]
- 476.Girgis NI, Kilpatrick ME, Farid Z, Sultan Y, Podgore JK. 1993. Cefixime in the treatment of enteric fever in children. Drugs Exp Clin Res 19:47–49. [PubMed] [Google Scholar]
- 477.Memon IA, Billoo AG, Memon HI. 1997. Cefixime: an oral option for the treatment of multidrug-resistant enteric fever in children. South Med J 90:1204–1207. doi: 10.1097/00007611-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 478.Rabbani MW, Iqbal I, Malik MS. 1998. A comparative study of cefixime and chloramphenicol in children with typhoid fever. J Pakistan Med Assoc 48:163–164. [PubMed] [Google Scholar]
- 479.Cao XT, Kneen R, Nguyen TA, Truong DL, White NJ, Parry CM. 1999. A comparative study of ofloxacin and cefixime for treatment of typhoid fever in children. The Dong Nai Pediatric Center Typhoid Study Group. Pediatr Infect Dis J 18:245–248. [DOI] [PubMed] [Google Scholar]
- 480.Pandit A, Arjyal A, Day JN, Paudyal B, Dangol S, Zimmerman MD, Yadav B, Stepniewska K, Campbell JI, Dolecek C, Farrar JJ, Basnyat B. 2007. An open randomized comparison of gatifloxacin versus cefixime for the treatment of uncomplicated enteric fever. PLoS One 2:e542. doi: 10.1371/journal.pone.0000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Trautmann M, Krause B, Birnbaum D, Wagner J, Lenk V. 1986. Serum bactericidal activity of two newer quinolones against Salmonella Typhi compared with standard therapeutic regimens. Eur J Clin Microbiol 5:297–302. doi: 10.1007/BF02017785. [DOI] [PubMed] [Google Scholar]
- 482.Bethell DB, Day NPJ, Dung NM, McMullin C, Loan HT, Tam DTH, Minh LTN, Linh NTM, Dung NQ, Vinh H, MacGowan AP, White LO, White NJ. 1996. Pharmacokinetics of oral and intravenous ofloxacin in children with multidrug-resistant typhoid fever. Antimicrob Agents Chemother 40:2167–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Usman M, Ashraf M, Khokhar MI, Ashiq B, Masood MI, Afzal S, Omer O, Ali M, Qadir MI. 2013. Comparative pharmacokinetics of levofloxacin in healthy volunteers and in patients suffering from typhoid fever. Iran J Pharm Res 12:147–154. [PMC free article] [PubMed] [Google Scholar]
- 484.Easmon CS, Crane JP, Blowers A. 1986. Effect of ciprofloxacin on intracellular organisms: in vitro and in vivo studies. J Antimicrob Chemother 18(Suppl D):43–48. [DOI] [PubMed] [Google Scholar]
- 485.Dan M, Verbin N, Gorea A, Nagar H, Berger SA. 1987. Concentrations of ciprofloxacin in human liver, gallbladder, and bile after oral administration. Eur J Clin Pharmacol 32:217–218. doi: 10.1007/BF00542200. [DOI] [PubMed] [Google Scholar]
- 486.Chau TT, Campbell JI, Galindo CM, Van Minh Hoang N, Diep TS, Nga TT, Van Vinh Chau N, Tuan PQ, Page AL, Ochiai RL, Schultsz C, Wain J, Bhutta ZA, Parry CM, Bhattacharya SK, Dutta S, Agtini M, Dong B, Honghui Y, Anh DD, Canh do, Naheed GA, Albert MJ, Phetsouvanh R, Newton PN, Basnyat B, Arjyal A, La TT, Rang NN, Phuong le, Van Be Bay TP, von Seidlein L, Dougan G, Clemens JD, Vinh H, Hien TT, Chinh NT, Acosta CJ, Farrar J, Dolecek C. 2007. Antimicrobial drug resistance of Salmonella enterica serovar Typhi in Asia and molecular mechanism of reduced susceptibility to the fluoroquinolones. Antimicrob Agents Chemother 51:4315–4323. doi: 10.1128/AAC.00294-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Ramirez CA, Bran JL, Mejia CR, Garcia JF. 1985. Open, prospective study of the clinical efficacy of ciprofloxacin. Antimicrob Agents Chemother 28:128–132. doi: 10.1128/AAC.28.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Mandal B, Flegg P, Dunbar E, Whale K, Brennand J. 1987. Ciprofloxacin in enteric fever. Chemioterapia 6(Suppl 2):492–493. [PubMed] [Google Scholar]
- 489.Hajji M, el Mdaghri N, Benbachir M, el Filali KM, Himmich H. 1988. Prospective randomized comparative trial of pefloxacin versus cotrimoxazole in the treatment of typhoid fever in adults. Eur J Clin Microbiol Infect Dis 7:361–363. doi: 10.1007/BF01962337. [DOI] [PubMed] [Google Scholar]
- 490.Stanley PJ, Flegg PJ, Mandal BK, Geddes AM. 1989. Open study of ciprofloxacin in enteric fever. J Antimicrob Chemother 23:789–791. doi: 10.1093/jac/23.5.789. [DOI] [PubMed] [Google Scholar]
- 491.Cristiano P, Morelli G, Briante V, Iovene MR, Simioli F, Altucci P. 1989. Clinical experience with pefloxacin in the therapy of typhoid fever. Infection 17:86–87. doi: 10.1007/BF01646882. [DOI] [PubMed] [Google Scholar]
- 492.Limson BM, Littaua RT. 1989. Comparative study of ciprofloxacin versus co-trimoxazole in the treatment of Salmonella enteric fever. Infection 17:105–106. doi: 10.1007/BF01646892. [DOI] [PubMed] [Google Scholar]
- 493.Sabbour MS, Osman LM. 1990. Experience with ofloxacin in enteric fever. J Chemother 2:113–115. [DOI] [PubMed] [Google Scholar]
- 494.Chew SK, Monteiro EH, Lim YS, Allen DM. 1992. A 7-day course of ciprofloxacin for enteric fever. J Infect 25:267–271. doi: 10.1016/0163-4453(92)91519-H. [DOI] [PubMed] [Google Scholar]
- 495.Uwaydah AK, al Soub H, Matar I. 1992. Randomized prospective study comparing two dosage regimens of ciprofloxacin for the treatment of typhoid fever. J Antimicrob Chemother 30:707–711. doi: 10.1093/jac/30.5.707. [DOI] [PubMed] [Google Scholar]
- 496.Meskin S, Jacob MS, Macaden R, Keystone JS, Kozarsky PE, Ramachadran AN, Metchock B. 1992. Short-course treatment of typhoid fever with ciprofloxacin in south India. Trans R Soc Trop Med Hyg 86:446–447. doi: 10.1016/0035-9203(92)90264-D. [DOI] [PubMed] [Google Scholar]
- 497.Arnold K, Hong CS, Nelwan R, Zavala-Trujillo I, Kadio A, Barros MA, de Garis S. 1993. Randomized comparative study of fleroxacin and chloramphenicol in typhoid fever. Am J Med 94:195S–200S. [PubMed] [Google Scholar]
- 498.Unal S, Hayran M, Tuncer S, Gür D, Uzun O, Akova M, Akalin HE. 1996. Treatment of enteric fever with pefloxacin for 7 days versus 5 days: a randomized clinical trial. Antimicrob Agents Chemother 40:2898–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Kalo T, Davachi F, Nushi A, Dedja S, Karapici L, Como N, Kraja D. 1997. Therapeutic efficacy of perfloxacin in treatment of ampicillin-resistant typhoid fever in 7 days versus 10 days. Int J Infect Dis 2:12–14. doi: 10.1016/S1201-9712(97)90004-7. [DOI] [Google Scholar]
- 500.Agalar C, Usubütün S, Tütüncü E, Türkyilmaz R. 1997. Comparison of two regimens for ciprofloxacin treatment of enteric infections. Eur J Clin Microbiol Infect Dis 16:803–806. doi: 10.1007/BF01700409. [DOI] [PubMed] [Google Scholar]
- 501.Girgis NI, Butler T, Frenck RW, Sultan Y, Brown FM, Tribble D, Khakhria R. 1999. Azithromycin versus ciprofloxacin for treatment of uncomplicated typhoid fever in a randomized trial in Egypt that included patients with multidrug resistance. Antimicrob Agents Chemother 43:1441–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Nelwan RH, Chen K, Nafrialdi Paramita D. 2006. Open study on efficacy and safety of levofloxacin in treatment of uncomplicated typhoid fever. Southeast Asian J Trop Med Pub Health 37:126–130. [PubMed] [Google Scholar]
- 503.Rizvi Q. 2007. Effectiveness of anti-typhoid drugs currently used in Pakistan. Pakistan J Surg 23:57–64. [Google Scholar]
- 504.Chandey M, Multani AS. 2012. A comparative study of efficacy and safety of azithromycin and ofloxacin in uncomplicated typhoid fever: a randomised, open labelled study. J Clin Diagn Res 6:1736–1739. doi: 10.7860/JCDR/2012/4702.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Effa EE, Lassi ZS, Critchley JA, Garner P, Sinclair D, Olliaro PL, Bhutta ZA. 2011. Fluoroquinolones for treating typhoid and paratyphoid fever (enteric fever). Cochrane Database Syst Rev 2011:CD004530. doi: 10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Carbon C, Weber P, Levy M, Boussougant Y, Cerf M. 1987. Short-term ciprofloxacin therapy for typhoid fever. J Infect Dis 155:833. doi: 10.1093/infdis/155.4.833. [DOI] [PubMed] [Google Scholar]
- 507.Tran TH, Bethell DB, Nguyen TT, Wain J, To SD, Le TP, Bul MC, Nguyen MD, Pham TT, Walsh AL, Day NP, White NJ. 1995. Short course ofloxacin for treatment of multidrug-resistant typhoid. Clin Infect Dis 20:917–923. doi: 10.1093/clinids/20.4.917. [DOI] [PubMed] [Google Scholar]
- 508.Vinh H, Wain J, Hanh VTN, Nga CN, Chinh MT, Bethell D, Hoa NTT, Diep TS, Dung NM, White NJ. 1996. Two or three days of ofloxacin treatment for uncomplicated multi-drug resistant typhoid fever in children. Antimicrob Agents Chemother 40:958–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Chinh NT, Solomon T, Thong MX, Ly NT, Hoa NTT, Wain J, Diep TS, Smith MD, Day NPJ, Phi LT, Parry C, White NJ. 1997. Short courses of ofloxacin for the treatment of enteric fever. Trans Royal Soc Trop Med Hyg 91:347–349. doi: 10.1016/S0035-9203(97)90102-4. [DOI] [PubMed] [Google Scholar]
- 510.Vinh H, Duong NM, Phuong le, Truong TNT, Bay PV, Wain J, Diep TS, Ho VA, White NJ, Day NP, Parry CM. 2005. Comparative trial of short-course ofloxacin for uncomplicated typhoid fever in Vietnamese children. Ann Trop Paediatr 25:17–22. doi: 10.1179/146532805X23308. [DOI] [PubMed] [Google Scholar]
- 511.Chinh NT, Parry CM, Ly NT, Ha HD, Thong MX, Diep TS, Wain J, White NJ, Farrar JJ. 2000. A randomized controlled comparison of azithromycin and ofloxacin for treatment of multidrug-resistant or nalidixic acid-resistant enteric fever. Antimicrob Agents Chemother 44:1855–1859. doi: 10.1128/AAC.44.7.1855-1859.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Dutta P, Mitra U, Dutta S, De A, Chatterjee MK, Bhattacharya SK. 2001. Ceftriaxone therapy in ciprofloxacin treatment failure typhoid fever in children. Indian J Med Res 113:210–213. [PubMed] [Google Scholar]
- 513.Aarestrup FM, Wiuff C, Molbak K, Threlfall EJ. 2003. Is it time to change fluoroquinolone breakpoints for Salmonella spp? Antimicrob Agents Chemother 47:827–829. doi: 10.1128/AAC.47.2.827-829.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Crump JA, Barrett TJ, Nelson JT, Angulo FJ. 2003. Reevaluating fluoroquinolone breakpoints for Salmonella enterica serotype Typhi and for non-Typhi salmonellae. Clin Infect Dis 37:75–81. doi: 10.1086/375602. [DOI] [PubMed] [Google Scholar]
- 515.Rupali P, Abraham OC, Jesudason MV, John TJ, Zachariah A, Sivaram S, Mathai D. 2004. Treatment failure in typhoid fever with ciprofloxacin susceptible Salmonella enterica serotype Typhi. Diagn Microbiol Infect Dis 49:1–3. doi: 10.1016/j.diagmicrobio.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 516.Parry CM, Ho VA, Phuong LT, Bay PV, Lanh MN, Tung LT, Tham NT, Wain J, Hien TT, Farrar JJ. 2007. Randomized controlled comparison of ofloxacin, azithromycin, and an ofloxacin-azithromycin combination for treatment of multidrug-resistant and nalidixic acid-resistant typhoid fever. Antimicrob Agents Chemother 46:819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Crump JA, Kretsinger K, Gay K, Hoekstra RM, Vugia DJ, Hurd S, Segler S, Megginson M, Luedeman LJ, Shiferaw B, Hanna SS, Joyce KW, Mintz ED, Angulo FJ, Emerging Infections Program FoodNet and NARMS Working Groups. 2008. Clinical response and outcome of infection with Salmonella enterica serotype Typhi with decreased susceptibility to fluoroquinolones: a United States FoodNet multicenter retrospective cohort study. Antimicrobial Agents Chemother 52:1278–1284. doi: 10.1128/AAC.01509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Parry CM, Vinh H, Chinh NT, Wain J, Campbell JI, Hien TT, Farrar JJ, Baker S. 2011. The influence of reduced susceptibility to fluoroquinolones in Salmonella enterica serovar Typhi on the clinical response to ofloxacin therapy. PLoS Negl Trop Dis 5:e1163. doi: 10.1371/journal.pntd.0001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Hassing RJ, Goessens WH, Mevius DJ, van Pelt W, Mouton JW, Verbon A, van Genderen PJ. 2013. Decreased ciprofloxacin susceptibility in Salmonella Typhi and Paratyphi infections in ill-returned travellers: the impact on clinical outcome and future treatment options. Eur J Clin Microbiol Infect Dis 32:1295–1301. doi: 10.1007/s10096-013-1878-9. [DOI] [PubMed] [Google Scholar]
- 520.Parry CM, Thuy CT, Dongol S, Karkey A, Vinh H, Chinh NT, Duy PT, Nga TT, Campbell JI, Van Minh Hoang N, Arjyal A, Bhutta ZA, Bhattacharya SK, Agtini MD, Dong B, Canh do, Naheed GA, Wain J, Hien TT, Basnyat B, Ochiai L, Clemens J, Farrar JJ, Dolecek C, Baker S. 2010. Suitable disk antimicrobial susceptibility breakpoints defining Salmonella enterica serovar Typhi isolates with reduced susceptibility to fluoroquinolones. Antimicrob Agents Chemother 54:5201–5208. doi: 10.1128/AAC.00963-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Sjölund-Karlsson M, Howie RL, Crump JA, Whichard JM. 2014. Fluoroquinolone susceptibility testing of Salmonella enterica: detection of acquired resistance and selection of zone diameter breakpoints for levofloxacin and ofloxacin. J Clin Microbiol 52:877–884. doi: 10.1128/JCM.02679-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Booker BM, Smith PF, Forrest A, Bullock J, Kelchlin P, Bhavnani SM, Jones RN, Ambrose PG. 2005. Application of an in vitro infection model and simulation for reevaluation of fluoroquinolone breakpoints for Salmonella enterica serotype Typhi. Antimicrob Agents Chemother 49:1775–1781. doi: 10.1128/AAC.49.5.1775-1781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing: 23rd informational supplement. CLSI document M100-S23 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 524.Humphries RM, Fang FC, Aarestrup FM, Hindler JA. 2012. In vitro susceptibility testing of fluoroquinolone activity against Salmonella: recent changes to CLSI standards. Clin Infect Dis 55:1107–1113. doi: 10.1093/cid/cis600. [DOI] [PubMed] [Google Scholar]
- 525.Tarr PE, Kuppens L, Jones TC, Ivanoff B, Aparin PG, Heymann DL. 1999. Considerations regarding mass vaccination against typhoid fever as an adjunct to sanitation and public health measures: potential use in an epidemic in Tajikistan. Am J Trop Med Hyg 61:163–170. [DOI] [PubMed] [Google Scholar]
- 526.Lewis MD, Serichantalergs O, Pitarangsi C, Chuanak N, Mason CJ, Regmi LR, Pandey P, Laskar R, Shrestha CD, Malla S. 2005. Typhoid fever: a massive, single-point source, multidrug-resistant outbreak in Nepal. Clin Infect Dis 40:554–561. doi: 10.1086/427503. [DOI] [PubMed] [Google Scholar]
- 527.Smith AM, Govender N, Keddy KH, Group for Enteric, Respiratory and Meningeal Disease Survellance in South Africa . 2010. Quinolone-resistant Salmonella Typhi in South Africa, 2003-2007. Epidemiol Infect 138:86–90. doi: 10.1017/S0950268809990331. [DOI] [PubMed] [Google Scholar]
- 528.Lunguya O, Lejon V, Phoba MF, Bertrand S, Vanhoof R, Verhaegen J, Smith AM, Keddy KH, Muyembe-Tamfum JJ, Jacobs J. 2012. Salmonella Typhi in the Democratic Republic of the Congo: fluoroquinolone decreased susceptibility on the rise. PLoS Negl Trop Dis 6:e1921. doi: 10.1371/journal.pntd.0001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.García C, Lejon V, Horna G, Astocondor L, Vanhoof R, Bertrand S, Jacobs J. 2014. Intermediate susceptibility to ciprofloxacin among Salmonella enterica serovar Typhi isolates in Lima, Peru. J Clin Microbiol 52:968–970. doi: 10.1128/JCM.02663-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Roumagnac P, Weill FX, Dolecek C, Baker S, Brisse S, Chinh NT, Le TA, Acosta CJ, Farrar J, Dougan G, Achtman M. 2006. Evolutionary history of Salmonella Typhi. Science 314:1301–1304. doi: 10.1126/science.1134933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Holt KE, Parkhill J, Mazzoni CJ, Roumagnac P, Weill FX, Goodhead I, Rance R, Baker S, Maskell DJ, Wain J, Dolecek C, Achtman M, Dougan G. 2008. High-throughput sequencing provides insights into genome variation and evolution in Salmonella Typhi. Nat Genet 40:987–993. doi: 10.1038/ng.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Kariuki S, Revathi G, Kiiru J, Mengo DM, Mwituria J, Muyodi J, Munyalo A, Teo YY, Holt KE, Kingsley RA, Dougan G. 2010. Typhoid in Kenya is associated with a dominant multidrug-resistant Salmonella enterica serovar Typhi haplotype that is also widespread in Southeast Asia. J Clin Microbiol 48:2171–2176. doi: 10.1128/JCM.01983-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Emary K, Moore CE, Chanpheaktra N, An KP, Chheng K, Sona S, Duy PT, Nga TV, Wuthiekanun V, Amornchai P, Kumar V, Wijedoru L, Stoesser NE, Carter MJ, Baker S, Day NP, Parry CM. 2012. Enteric fever in Cambodian children is dominated by multidrug-resistant H58 Salmonella enterica serovar Typhi with intermediate susceptibility to ciprofloxacin. Trans R Soc Trop Med Hyg 106:718–724. doi: 10.1016/j.trstmh.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 534.Baker S, Duy PT, Nga TV, Dung TT, Phat VV, Chau TT, Turner AK, Farrar J, Boni MF. 2013. Fitness benefits in fluoroquinolone-resistant Salmonella Typhi in the absence of antimicrobial pressure. eLife 2:e01229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Feasey NA, Gaskell K, Wong V, Msefula C, Selemani G, Kumwenda S, Allain TJ, Mallewa J, Kennedy N, Bennett A, Nyirongo JO, Nyondo PA, Zulu MD, Parkhill J, Dougan G, Gordon MA, Heyderman RS. 2015. Rapid emergence of multidrug resistant, H58-lineage Salmonella Typhi in Blantyre, Malawi. PLoS Negl Trop Dis 9:e0003748. doi: 10.1371/journal.pntd.0003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Wong VK, Baker S, Pickard DJ, Parkhill J, Page AJ, Feasey NA, Kingsley RA, Thomson NR, Keane JA, Weill F-X, Edwards DJ, Hawkey J, Harris SR, Mather AE, Cain AK, Hadfield J, Hart PJ, Thieu NTV, Klemm EJ, Glinos DA, Breiman RF, Watson CH, Kariuki S, Gordon MA, Heyderman RS, Okoro C, Jacobs J, Lunguya O, Edmunds WJ, Msefula C, Chabalgoity JA, Kama M, Jenkins K, Dutta S, Marks F, Campos J, Thompson C, Obaro S, MacLennan CA, Dolecek C, Keddy KH, Smith AM, Parry CM, Karkey A, Mulholland EK, Campbell JI, Dongol S, Basnyat B, Dufour M, Bandaranayake D, et al. 2015. Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella Typhi identifies inter- and intracontinental transmission events. Nat Genet 47:632–639. doi: 10.1038/ng.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Koirala S, Basnyat B, Arjyal A, Shilpakar O, Shrestha K, Shrestha R, Shrestha UM, Agrawal K, Koirala KD, Thapa SD, Karkey A, Dongol S, Giri A, Shakya M, Pathak KR, Campbell J, Baker S, Farrar J, Wolbers M, Dolecek C. 2013. Gatifloxacin versus ofloxacin for the treatment of uncomplicated enteric fever in Nepal: an open-label, randomized, controlled trial. PLoS Negl Trop Dis 7:e2523. doi: 10.1371/journal.pntd.0002523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Dolecek C, Tran TP, Nguyen NR, Le TP, Ha V, Phung QT, Doan CD, Nguyen TB, Duong TL, Luong BH, Nguyen TB, Nguyen TA, Pham ND, Mai NL, Phan VB, Vo AH, Nguyen VM, Tran TT, Tran TC, Schultsz C, Dunstan SJ, Stepniewska K, Campbell JI, To SD, Basnyat B, Nguyen VV, Nguyen VS, Nguyen TC, Tran TH, Farrar J. 2008. A multi-center randomised controlled trial of gatifloxacin versus azithromycin for the treatment of uncomplicated typhoid fever in children and adults in Vietnam. PLoS One 3:e2188. doi: 10.1371/journal.pone.0002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Saha SK, Darmstadt GL, Baqui AH, Crook DW, Islam MN, Islam M, Hossain M, El Arifeen S, Santosham M, Black RE. 2006. Molecular basis of resistance displayed by highly ciprofloxacin-resistant Salmonella enterica serovar Typhi in Bangladesh. J Clin Microbiol 44:3811–3813. doi: 10.1128/JCM.01197-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Joshi S, Amarnath SK. 2007. Fluoroquinolone resistance in Salmonella Typhi and S. Paratyphi A in Bangalore, India. Trans R Soc Trop Med Hyg 101:308–310. doi: 10.1016/j.trstmh.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 541.Morita M, Hirose K, Takai N, Terajima J, Watanabe H, Sagara H, Kurazono T, Yamaguchi M, Kanazawa Y, Oyaizu T, Izumiya H. 2010. Salmonella enterica serovar Typhi in Japan, 2001-2006: emergence of high-level fluoroquinolone-resistant strains. Epidemiol Infect 138:318–321. doi: 10.1017/S0950268809990380. [DOI] [PubMed] [Google Scholar]
- 542.Keddy KH, Smith AM, Sooka A, Ismail H, Oliver S. 2010. Fluoroquinolone-resistant typhoid, South Africa. Emerg Infect Dis 16:879–880. doi: 10.3201/eid1605.091917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Medalla F, Sjölund-Karlsson M, Shin S, Harvey E, Joyce K, Theobald L, Nygren BN, Pecic G, Gay K, Austin J, Stuart A, Blanton E, Mintz ED, Whichard JM, Barzilay EJ. 2011. Ciprofloxacin-resistant Salmonella enterica serotype Typhi, United States, 1999-2008. Emerg Infect Dis 17:1095–1098. doi: 10.3201/eid/1706.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Kubin R. 1993. Safety and efficacy of ciprofloxacin in paediatric patients: review. Infection 21:413–421. doi: 10.1007/BF01728929. [DOI] [PubMed] [Google Scholar]
- 545.Schaad UB, abdus Salam M, Aujard Y, Dagan R, Green SD, Peltola H, Rubio TT, Smith AL, Adam D. 1995. Use of fluoroquinolones in pediatrics: consensus report of an International Society of Chemotherapy commission. Pediatr Infect Dis J 14:1–9. doi: 10.1097/00006454-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 546.Burkhardt JE, Walterspiel JN, Schaad UB. 1997. Quinolone arthropathy in animals versus children. Clin Infect Dis 25:1196–1204. doi: 10.1086/516119. [DOI] [PubMed] [Google Scholar]
- 547.Bethell DB, Hien TT, Phi LT, Day NPJ, Vinh H, Duong NM, Len NV, Chuong LV, White NJ. 1996. Effects on growth of single short courses of fluoroquinolones. Arch Dis Child 74:44–46. doi: 10.1136/adc.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Doherty CP, Saha SK, Cutting WA. 2000. Typhoid fever, ciprofloxacin and growth in young children. Ann Trop Paediatr 20:297–303. [DOI] [PubMed] [Google Scholar]
- 549.Yee CL, Duffy C, Gerbino PG, Stryker S, Noel GJ. 2002. Tendon or joint disorders in children after treatment with fluoroquinolones or azithromycin. Pediatr Infect Dis J 21:525–529. doi: 10.1097/00006454-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 550.Forsythe CT, Ernst ME. 2007. Do fluoroquinolones commonly cause arthropathy in children? Can J Emerg Med 9:459–462. [DOI] [PubMed] [Google Scholar]
- 551.Adefurin A, Sammons H, Jacqz-Aigrain E, Choonara I. 2011. Ciprofloxacin safety in paediatrics: a systematic review. Arch Dis Child 96:874–880. doi: 10.1136/adc.2010.208843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Park-Wyllie LY, Juurlink DN, Kopp A, Shah BR, Stukel TA, Stumpo C, Dresser L, Low DE, Mamdani MM. 2006. Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med 354:1352–1361. doi: 10.1056/NEJMoa055191. [DOI] [PubMed] [Google Scholar]
- 553.Metchock B. 1990. In vitro activity of azithromycin compared with other macrolides and oral antibiotics against Salmonella Typhi. J Antimicrob Chemother 25(Suppl A):29–31. doi: 10.1093/jac/25.suppl_A.29. [DOI] [PubMed] [Google Scholar]
- 554.Gordillo ME, Singh KV, Murray BE. 1993. In vitro activity of azithromycin against bacterial enteric pathogens. Antimicrob Agents Chemother 37:1203–1205. doi: 10.1128/AAC.37.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Tribble D, Girgis N, Habib N, Butler T. 1995. Efficacy of azithromycin for typhoid fever. Clin Infect Dis 21:1045–1046. doi: 10.1093/clinids/21.4.1045. [DOI] [PubMed] [Google Scholar]
- 556.Butler T, Frenck RW, Johnson RB, Khakhria R. 2001. In vitro effects of azithromycin on Salmonella Typhi: early inhibition by concentrations less than the MIC and reduction of MIC by alkaline pH and small inocula. J Antimicrob Chemother 47:455–458. doi: 10.1093/jac/47.4.455. [DOI] [PubMed] [Google Scholar]
- 557.Foulds G, Shepard RM, Johnson RB. 1990. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother 25(Suppl A):73–82. [DOI] [PubMed] [Google Scholar]
- 558.Panteix G, Guillaumond B, Harf R, Desbos A, Sapin V, Leclercq M, Perrin-Fayolle M. 1993. In vitro concentration of azithromycin in human phagocytic cells. J Antimicrob Chemother 31(Suppl E):1–4. [DOI] [PubMed] [Google Scholar]
- 559.Rakita RM, Jacques-Palaz K, Murray BE. 1994. Intracellular activity of azithromycin against bacterial enteric pathogens. Antimicrob Agents Chemother 38:1915–1921. doi: 10.1128/AAC.38.9.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Kanoh S, Rubin BK. 2010. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev 23:590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Wallace MR, Yousif AA, Habib NF, Tribble DR. 1994. Azithromycin and typhoid. Lancet 343:1497–1498. doi: 10.1016/S0140-6736(94)92604-2. [DOI] [PubMed] [Google Scholar]
- 562.Capoor MR, Rawat D, Nair D, Hasan AS, Deb M, Aggarwal P, Pillai P. 2007. In vitro activity of azithromycin, newer quinolones and cephalosporins in ciprofloxacin-resistant Salmonella causing enteric fever. J Med Microbiol 56:1490–1494. doi: 10.1099/jmm.0.47353-0. [DOI] [PubMed] [Google Scholar]
- 563.Sjolund-Karlsson M, Joyce K, Blickenstaff K, Ball T, Haro J, Medalla FM, Fedorka-Cray P, Zhao S, Crump JA, Whichard JM. 2011. Antimicrobial susceptibility to azithromycin among Salmonella enterica isolates from the United States. Antimicrob Agents Chemother 55:3985–3989. doi: 10.1128/AAC.00590-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Gunell M, Kotilainen P, Jalava J, Huovinen P, Siitonen A, Hakanen AJ. 2010. In vitro activity of azithromycin against nontyphoidal Salmonella enterica. Antimicrob Agents Chemother 54:3498–3501. doi: 10.1128/AAC.01678-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Parry CM, Thieu NT, Dolecek C, Karkey A, Gupta R, Turner P, Dance D, Maude RR, Ha V, Tran CN, Thi PL, Be BP, Phi LT, Ngoc RN, Ghose A, Dongol S, Campbell JI, Thanh DP, Thanh TH, Moore CE, Sona S, Gaind R, Deb M, Anh HV, Van SN, Tinh HT, Day NP, Dondorp A, Thwaites G, Faiz MA, Phetsouvanh R, Newton P, Basnyat B, Farrar JJ, Baker S. 2015. Clinically and microbiologically derived azithromycin susceptibility breakpoints for Salmonella enterica serovars Typhi and Paratyphi A. Antimicrob Agents Chemother 59:2756–2764. doi: 10.1128/AAC.04729-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Clinical and Laboratory Standards Institute. 2008. Defining, establishing, and verifying reference intervals in the clinical laboratory: approved guideline, 3rd ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 567.Turnidge J, Paterson DL. 2007. Setting and revising antibacterial susceptibility breakpoints. Clin Microbiol Rev 20:391–408. doi: 10.1128/CMR.00047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Rai S, Jain S, Prasad KN, Ghoshal U, Dhole TN. 2012. Rationale of azithromycin prescribing practices for enteric fever in India. Indian J Med Microbiol 30:30–33. doi: 10.4103/0255-0857.93017. [DOI] [PubMed] [Google Scholar]
- 569.Hassing RJ, Goessens WH, van Pelt W, Mevius DJ, Stricker BH, Molhoek N, Verbon A, van Genderen PJ. 2014. Salmonella subtypes with increased MICs for azithromycin in travelers returned to The Netherlands. Emerg Infect Dis 20:705–708. doi: 10.3201/eid2004.131536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing: 25th informational supplement. CLSI document M100-S25 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 571.Capoor MR, Nair D, Posti J, Singhal S, Deb M, Aggarwal P, Pillai P. 2009. Minimum inhibitory concentration of carbapenems and tigecycline against Salmonella spp. J Med Microbiol 58:337–341. doi: 10.1099/jmm.0.47853-0. [DOI] [PubMed] [Google Scholar]
- 572.Bhutta ZA. 1997. Quinolone-resistant Salmonella Paratyphi B meningitis in a newborn: a case report. J Infect 35:308–310. doi: 10.1016/S0163-4453(97)93460-5. [DOI] [PubMed] [Google Scholar]
- 573.Meltzer E, Stienlauf S, Leshem E, Sidi Y, Schwartz E. 2014. A large outbreak of Salmonella Paratyphi A infection among israeli travelers to Nepal. Clin Infect Dis 58:359–364. doi: 10.1093/cid/cit723. [DOI] [PubMed] [Google Scholar]
- 574.Mandal S, Mandal MD, Pal NK. 2003. Combination effect of ciprofloxacin and gentamicin against clinical isolates of Salmonella enterica serovar Typhi with reduced susceptibility to ciprofloxacin. Jpn J Infect Dis 56:156–157. [PubMed] [Google Scholar]
- 575.Mandal S, Mandal MD, Pal NK. 2004. Synergism of ciprofloxacin and trimethoprim against Salmonella enterica serovar Typhi isolates showing reduced susceptibility to ciprofloxacin. Chemotherapy 50:152–154. doi: 10.1159/000077890. [DOI] [PubMed] [Google Scholar]
- 576.Gluck B, Ramin KD, Ramin SM. 1994. Salmonella Typhi and pregnancy: a case report. Infect Dis Obstet Gynecol 2:186–189. doi: 10.1155/S1064744994000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Koul PA, Wani JI, Wahid A. 1995. Ciprofloxacin for multiresistant enteric fever in pregnancy. Lancet 346:307–308. doi: 10.1016/S0140-6736(95)92191-5. [DOI] [PubMed] [Google Scholar]
- 578.Leung D, Venkatesan P, Boswell T, Innes JA, Wood MJ. 1995. Treatment of typhoid in pregnancy. Lancet 346:648. [DOI] [PubMed] [Google Scholar]
- 579.Lin KJ, Mitchell AA, Yau WP, Louik C, Hernández-Díaz S. 2013. Safety of macrolides during pregnancy. Am J Obstet Gynecol 208:221. doi: 10.1016/j.ajog.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Vaidya A, Supe A, Samsi AB, Ramakantan R. 1990. Continuous intra arterial vasopressin infusion for control of typhoid hemorrhage. Indian J Gastroenterol 9:225–226. [PubMed] [Google Scholar]
- 581.Lee JH, Kim JJ, Jung JH, Lee SY, Bae MH, Kim YH, Son HJ, Rhee PL, Rhee JC. 2004. Colonoscopic manifestations of typhoid fever with lower gastrointestinal bleeding. Dig Liver Dis 36:141–146. doi: 10.1016/j.dld.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 582.Shaikhani MA, Husein HA, Karbuli TA, Mohamed MA. 2013. Colonoscopic findings and management of patients with outbreak typhoid fever presenting with lower gastrointestinal bleeding. Indian J Gastroenterol 32:335–340. doi: 10.1007/s12664-013-0337-y. [DOI] [PubMed] [Google Scholar]
- 583.Simon HJ, Miller RC. 1966. Ampicillin in the treatment of chronic typhoid carriers: report on fifteen treated cases and a review of the literature. N Engl J Med 274:807–815. doi: 10.1056/NEJM196604142741501. [DOI] [PubMed] [Google Scholar]
- 584.Phillips WE. 1971. Treatment of chronic typhoid carriers with ampicillin. JAMA 217:913–915. [PubMed] [Google Scholar]
- 585.Johnson WD, Hook EW, Lindsey E, Kaye D. 1973. Treatment of chronic typhoid carriers with ampicillin. Antimicrob Agents Chemother 3:439–440. doi: 10.1128/AAC.3.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Pichler H, Knothe H, Spitzy KH, Vieliind G. 1973. Treatment of chronic carriers of Salmonella Typhi and Salmonella Paratyphi B with trimethoprim-sulfamethoxazole. J Infec Dis 128(Suppl):743–744. [DOI] [PubMed] [Google Scholar]
- 587.Geddes AM. 1975. Trimethoprim-sulfamethoxazole in the treatment of gastrointestinal infections, including enteric fever and typhoid carriers. Can Med Assoc J 112:35–36. [PMC free article] [PubMed] [Google Scholar]
- 588.Nolan CM, White PC. 1978. Treatment of typhoid carriers with amoxicillin: correlates of successful therapy. JAMA 239:2352–2354. doi: 10.1001/jama.239.22.2352. [DOI] [PubMed] [Google Scholar]
- 589.Hudson SJ, Ingham HR, Snow MH. 1985. Treatment of Salmonella Typhi carrier state with ciprofloxacin. Lancet 325:1047. [DOI] [PubMed] [Google Scholar]
- 590.Ferreccio C, Morris JG, Valdivieso C, Prenzel I, Sotomayor V, Drusano GL, Levine MM. 1988. Efficacy of ciprofloxacin in the treatment of chronic typhoid carriers. J Infect Dis 157:1235–1239. doi: 10.1093/infdis/157.6.1235. [DOI] [PubMed] [Google Scholar]
- 591.Gotuzzo E, Guerra JG, Benavente L, Palomino JC, Carrillo C, Lopera J, Delgado F, Nalin DR, Sabbaj J. 1988. Use of norfloxacin to treat chronic typhoid carriers. J Infect Dis 157:1221–1225. [DOI] [PubMed] [Google Scholar]
- 592.Münnich D, Békési S. 1979. Curing of typhoid carriers by cholecystectomy combined with amoxycillin plus probenecid treatment. Chemotherapy 25:362–366. doi: 10.1159/000237865. [DOI] [PubMed] [Google Scholar]
- 593.World Health Organization. 2003. Background document: the diagnosis, treatment and prevention of typhoid fever. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 594.Kundu R, Ganguly N, Ghosh TK, Yewale VN, Shah RC, Shah NK, IAP Task Force. 2006. IAP Task Force report: management of enteric fever in children. Indian Pediatr 43:884–887. [PubMed] [Google Scholar]
- 595.Bhutta ZA. 2006. Current concepts in the diagnosis and treatment of typhoid fever. Br Med J 333:78–82. doi: 10.1136/bmj.333.7558.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Butler T. 2011. Treatment of typhoid fever in the 21st century: promises and shortcomings. Clin Microbiol Infect 17:959–963. doi: 10.1111/j.1469-0691.2011.03552.x. [DOI] [PubMed] [Google Scholar]
- 597.Caudron JM, Ford N, Henkens M, Macé C, Kiddle-Monroe R, Pinel J. 2008. Substandard medicines in resource-poor settings: a problem that can no longer be ignored. Trop Med Int Health 13:1062–1072. doi: 10.1111/j.1365-3156.2008.02106.x. [DOI] [PubMed] [Google Scholar]
- 598.Trivedi NA, Shah PC. 2012. A meta-analysis comparing the safety and efficacy of azithromycin over the alternate drugs used for treatment of uncomplicated enteric fever. J Postgrad Med 58:112–118. doi: 10.4103/0022-3859.97172. [DOI] [PubMed] [Google Scholar]
- 599.Hirsowitz L, Cassel R. 1951. Sternal marrow cultures in typhoid fever. Br Med J 1:862–863. doi: 10.1136/bmj.1.4711.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Dance DA, Richens JE, Ho M, Acharya G, Pokhrel B, Tuladhar NR. 1991. Blood and bone marrow cultures in enteric fever. J Clin Pathol 44:1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Gasem MH, Dolmans WMV, Isbandrio BB, Wahyono H, Keuter M, Djokomoeljanto R. 1995. Culture of Salmonella Typhi and Salmonella Paratyphi from blood and bone marrow in suspected typhoid fever. Trop Geogr Med 47:164–167. [PubMed] [Google Scholar]
- 602.Gasem MH, Smits HL, Goris MG, Dolmans WM. 2002. Evaluation of a simple and rapid dipstick assay for the diagnosis of typhoid fever in Indonesia. J Med Microbiol 51:173–177. [DOI] [PubMed] [Google Scholar]
- 603.Parry CM, Basnyat B. 2010. Typhoid and paratyphoid fevers, p 75–82. In Warrell DA, Cox TM, Firth JD (ed), Oxford textbook of medicine, 5th ed Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 604.Wain J, Hendriksen RS, Mikoleit ML, Keddy KH, Ochiai RL. 2015. Typhoid fever. Lancet 385:1136–1145. doi: 10.1016/S0140-6736(13)62708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Mawatari M, Kato Y, Hayakawa K, Morita M, Yamada K, Mezaki K, Kobayashi T, Fujiya Y, Kutsuna S, Takeshita N, Kanagawa S, Ohnishi M, Izumiya H, Ohmagari N. 14 November 2013. Salmonella enterica serotype Paratyphi A carrying CTX-M-15 type extended-spectrum beta-lactamase isolated from a Japanese traveller returning from India, Japan, July 2013. Euro Surveill 18(46):pii=20632 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20632. [DOI] [PubMed] [Google Scholar]