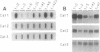Abstract
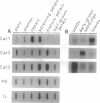
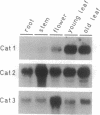
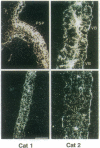
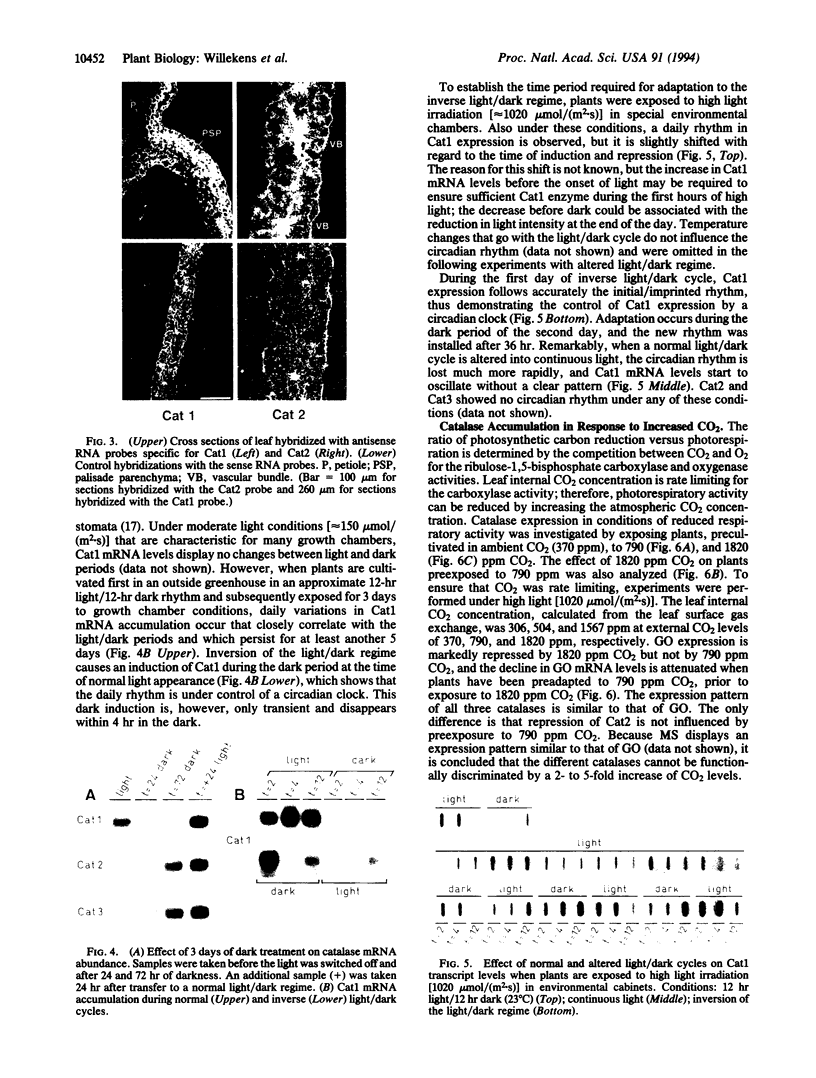
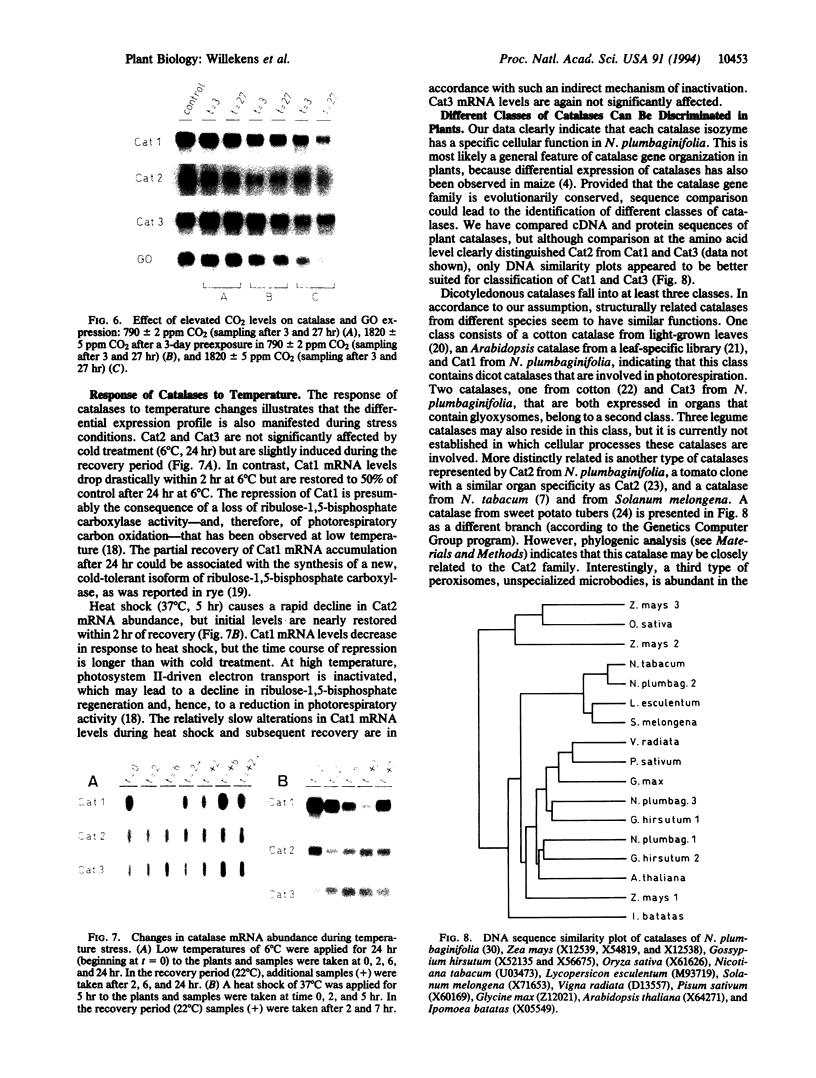
We have analyzed the expression of three catalase (Cat; EC 1.11.1.6) genes from Nicotiana plumbaginifolia by means of RNA blot and in situ hybridizations. Our data demonstrate that the expression of each catalase is associated with a particular H2O2-producing process. Cat1 appears to be specifically involved in the scavenging of photorespiratory H2O2 and is under control of a circadian rhythm, Cat2 is uniformly expressed in different organs with a cellular preference for vascular tissues, and the expression profile of Cat3 points to a role in glyoxysomal processes. Differential expression of these catalases is also manifested in response to temperature changes. DNA sequence comparison with other dicotyledonous catalases led to the identification of at least three distinct classes, which indicates that the functional organization of catalases is generally conserved in dicotyledonous plants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breidenbach R. W., Kahn A., Beevers H. Characterization of glyoxysomes from castor bean endosperm. Plant Physiol. 1968 May;43(5):705–713. doi: 10.1104/pp.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Silva H., Klessig D. F. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science. 1993 Dec 17;262(5141):1883–1886. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- Chevalier C., Yamaguchi J., McCourt P. Nucleotide Sequence of a cDNA for Catalase from Arabidopsis thaliana. Plant Physiol. 1992 Aug;99(4):1726–1728. doi: 10.1104/pp.99.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Dietrich R. A., Maslyar D. J., Baden C. S., Harada J. J. Coordinate expression of transcriptionally regulated isocitrate lyase and malate synthase genes in Brassica napus L. Plant Cell. 1989 Mar;1(3):293–300. doi: 10.1105/tpc.1.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Elzen P., Tamaki S., Dunsmuir P., Bedbrook J. Differential expression of the eight genes of the petunia ribulose bisphosphate carboxylase small subunit multi-gene family. EMBO J. 1985 Dec 1;4(12):3055–3061. doi: 10.1002/j.1460-2075.1985.tb04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droillard M. J., Paulin A. Isozymes of Superoxide Dismutase in Mitochondria and Peroxisomes Isolated from Petals of Carnation (Dianthus caryophyllus) during Senescence. Plant Physiol. 1990 Nov;94(3):1187–1192. doi: 10.1104/pp.94.3.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drory A., Woodson W. R. Molecular cloning and nucleotide sequence of a cDNA encoding catalase from tomato. Plant Physiol. 1992 Nov;100(3):1605–1606. doi: 10.1104/pp.100.3.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eising R., Trelease R. N., Ni W. T. Biogenesis of catalase in glyoxysomes and leaf-type peroxisomes of sunflower cotyledons. Arch Biochem Biophys. 1990 Apr;278(1):258–264. doi: 10.1016/0003-9861(90)90256-x. [DOI] [PubMed] [Google Scholar]
- Gorton H. L., Williams W. E., Assmann S. M. Circadian Rhythms in Stomatal Responsiveness to Red and Blue Light. Plant Physiol. 1993 Oct;103(2):399–406. doi: 10.1104/pp.103.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerche P., Tire C., De Sa F. G., De Clercq A., Van Montagu M., Krebbers E. Differential Expression of the Arabidopsis 2S Albumin Genes and the Effect of Increasing Gene Family Size. Plant Cell. 1990 May;2(5):469–478. doi: 10.1105/tpc.2.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havir E. A., McHale N. A. Enhanced-peroxidatic activity in specific catalase isozymes of tobacco, barley, and maize. Plant Physiol. 1989 Nov;91(3):812–815. doi: 10.1104/pp.91.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huner N. P., Macdowall F. D. Changes in the net charge and subunit properties of ribulose bisphosphate carboxylase--oxygenase during cold hardening of Puma rye. Can J Biochem. 1979 Feb;57(2):155–164. doi: 10.1139/o79-019. [DOI] [PubMed] [Google Scholar]
- Logemann J., Schell J., Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987 May 15;163(1):16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Ni W. T., Trelease R. N. Two genes encode the two subunits of cottonseed catalase. Arch Biochem Biophys. 1991 Sep;289(2):237–243. doi: 10.1016/0003-9861(91)90467-w. [DOI] [PubMed] [Google Scholar]
- Ni W., Trelease R. N. Post-Transcriptional Regulation of Catalase Isozyme Expression in Cotton Seeds. Plant Cell. 1991 Jul;3(7):737–744. doi: 10.1105/tpc.3.7.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W., Turley R. B., Trelease R. N. Characterization of a cDNA encoding cottonseed catalase. Biochim Biophys Acta. 1990 Jun 21;1049(2):219–222. doi: 10.1016/0167-4781(90)90044-3. [DOI] [PubMed] [Google Scholar]
- Prasad T. K., Anderson M. D., Martin B. A., Stewart C. R. Evidence for Chilling-Induced Oxidative Stress in Maize Seedlings and a Regulatory Role for Hydrogen Peroxide. Plant Cell. 1994 Jan;6(1):65–74. doi: 10.1105/tpc.6.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbaugh M. G., Sabre M., Scandalios J. G. The distribution of catalase activity, isozyme protein, and transcript in the tissues of the developing maize seedling. Plant Physiol. 1990 Feb;92(2):375–380. doi: 10.1104/pp.92.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakajo S., Nakamura K., Asahi T. Molecular cloning and nucleotide sequence of full-length cDNA for sweet potato catalase mRNA. Eur J Biochem. 1987 Jun 1;165(2):437–442. doi: 10.1111/j.1432-1033.1987.tb11457.x. [DOI] [PubMed] [Google Scholar]
- Scandalios J. G. The antioxidant enzyme genes Cat and Sod of maize: regulation, functional significance, and molecular biology. Isozymes Curr Top Biol Med Res. 1987;14:19–44. [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Volokita M., Somerville C. R. The primary structure of spinach glycolate oxidase deduced from the DNA sequence of a cDNA clone. J Biol Chem. 1987 Nov 25;262(33):15825–15828. [PubMed] [Google Scholar]