Severe Streptococcus pneumoniae infections are frequent complications after hematopoietic stem cell transplant (HSCT). A 3-dose regimen of 13-valent pneumococcal conjugate vaccine, starting 3–6 months after HSCT and followed by a booster dose, may be required for adequate protection.
Keywords: 13-valent pneumococcal conjugate vaccine, 23-valent pneumococcal polysaccharide vaccine, hematopoietic stem cell transplant, Streptococcus pneumoniae infections
Abstract
Background. Life-threatening Streptococcus pneumoniae infections often occur after hematopoietic stem cell transplant (HSCT); vaccination is important for prevention.
Methods. In an open-label study, patients (n = 251) 3–6 months after allogeneic HSCT received 3 doses of 13-valent pneumococcal conjugate vaccine (PCV13) at 1-month intervals, a fourth dose 6 months later, and 1 dose of 23-valent pneumococcal polysaccharide vaccine (PPSV23) 1 month later. Immunogenicity at prespecified time points and vaccine safety were assessed.
Results. In the evaluable immunogenicity population (N = 216; mean age, 37.8 years), geometric mean fold rises (GMFRs) of immunoglobulin G geometric mean concentrations from baseline to postdose 3 showed significant increases in antibody levels across all PCV13 serotypes (GMFR range, 2.99–23.85; 95% confidence interval lower limit, >1); there were significant declines over the next 6 months, significant increases from predose 4 to postdose 4 (GMFR range, 3.00–6.97), and little change after PPSV23 (GMFR range, 0.86–1.12). Local and systemic reactions were more frequent after dose 4. Six patients experienced serious adverse events possibly related to PCV13 (facial diplegia, injection-site erythema and pyrexia, autoimmune hemolytic anemia, and suspected lack of vaccine efficacy after dose 3 leading to pneumococcal infection), PCV13 and PPSV23 (Guillain-Barré syndrome), or PPSV23 (cellulitis). There were 14 deaths, none related to study vaccines.
Conclusions. A 3-dose PCV13 regimen followed by a booster dose may be required to protect against pneumococcal disease in HSCT recipients. Dose 4 was associated with increased local and systemic reactions, but the overall safety profile of a 4-dose regimen was considered acceptable.
Clinical Trials Registration. NCT00980655.
Recipients of hematopoietic stem cell transplant (HSCT) are at high risk of developing life-threatening Streptococcus pneumoniae infections, especially after allogeneic HSCT and when complicated by chronic graft-vs-host disease (GVHD) [1–3]. Studies have shown a 20- to 30-fold, or higher, rate of invasive pneumococcal disease (IPD) compared with the general population [1–3].
Vaccination is an important preventive strategy [4]. In the past, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) was recommended [5]. However, PPSVs have limited efficacy during the first year after HSCT because of the time to regeneration of the T- and B-cell responses [4, 6]. During this period and thereafter, especially in patients with chronic GVHD, patients are at increased risk of infection. Across studies, IPD was reported within 100 days of HSCT in 4%–20% of patients, with a median onset of 14–28 months [1–3, 7]. Memory T cells are the first to expand after HSCT [4], suggesting that conjugate vaccines may be of advantage in this population. The 13-valent pneumococcal conjugate vaccine (PCV13) elicits a T-cell–dependent immune response. T cells provide the signals required for the generation of B-cell memory [8, 9]. Thus, PCVs have the potential to elicit a memory response on subsequent natural exposure to vaccine-type strains and allow revaccination if required, and therefore could be more immunogenic in immunocompromised hosts. Studies with the 7-valent pneumococcal conjugate vaccine (PCV7) demonstrated favorable immune responses [10–13], with responses at 3 months noninferior to those at 9 months [10], so that vaccination recommendations were updated to include PCV7 [4, 14, 15] and, later, PCV13 [16]. PCVs are efficacious against IPD and pneumonia in children [17–20]. The Community-Acquired Pneumonia Immunization Trial in Adults aged ≥65 years, with approximately 85 000 participants, demonstrated that PCV13 is efficacious against vaccine-type community-acquired pneumonia (including nonbacteremic) and IPD [21–23].
The main aim of this study was to assess the immunogenicity and safety of 4 doses of PCV13 in allogeneic HSCT recipients. Until now, no data were available on PCV13 after allogeneic HSCT. When the study was designed, PPSV23 was recommended 1 year after HSCT [5, 14] and thus was included in this study. PPSV23 has the potential to extend serotype coverage.
METHODS
Study Design
This open-label study was conducted at 37 centers in Europe, Canada, and the United States between January 2010 and May 2013. Approximately 3–6 months after HSCT, 3 doses of PCV13 were administered monthly, a fourth dose of PCV13 was administered 6 months later, and a dose of PPSV23 was administered 1 month later. The study was conducted in compliance with the Declaration of Helsinki and was approved by the responsible institutional review boards and independent ethics committees.
Participants
Eligible participants were patients aged ≥2 years with hematologic disorders who had received, 91–203 days before enrollment, an allogeneic HSCT following myeloablative or reduced-intensity conditioning; had stable engraftment with an absolute neutrophil count >1000/µL and a platelet count >50 000/µL; and had complete or (for lymphoma and myeloma) very good partial hematologic remission of underlying disease. Main exclusion criteria included donor lymphocyte infusions within 28 days; plasma products or immunoglobulins within 60 days; rituximab, advanced therapy medicinal products, chemotherapy for relapse of underlying malignancy, or any pneumococcal vaccine other than study vaccines since HSCT. Concomitant treatments and other licensed nonstudy vaccines, which reflect the standard of care at each site, were permitted.
Vaccines and Administration
PCV13 (Prevnar 13/Prevenar 13, Wyeth Vaccines, acquired by Pfizer Inc in 2009) contains saccharides from serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F individually conjugated to nontoxic diphtheria cross-reactive material. PPSV23 (Pneumovax 23, Merck & Co, Inc) contains purified capsular polysaccharides from all PCV13 serotypes except 6A, as well as 11 additional serotypes (2, 8, 9N, 10A, 11A, 12F, 15B, 17F, 20, 22F, and 33F). Vaccines were administered intramuscularly in a dose of 0.5 mL.
Immunogenicity Assessment
Immunoglobulin G (IgG) concentrations were assessed from blood samples taken immediately before and 1 month after each vaccination using enzyme-linked immunosorbent assay (ELISA) [24]. After study completion, functional antibody titers using opsonophagocytic activity (OPA) assays were assessed in any available sera based on previously described methods [25–27]. Assays (ELISA and OPA) were conducted at a central laboratory by the sponsor.
Safety Assessment
For all 4 doses of PCV13, participants reported local and systemic reactions in an electronic diary (e-diary) for 14 days postvaccination. For PPSV23, no e-diary was used. All adverse events (AEs) not collected in e-diaries were collected on the case report form for approximately 1 month after each vaccination; serious AEs (SAEs) were collected from enrollment through telephone follow-up contact 6 months after the last PCV13 vaccination.
Statistical Methods
Objectives of the Study
The primary objective was to evaluate immune responses to the PCV13 serotypes 1 month after dose 3 of PCV13, as measured by geometric mean fold rises (GMFRs) of the IgG geometric mean concentrations (GMCs) (GMFR postdose 3/baseline). Other objectives included GMFR assessments 1 month after dose 4 and after PPSV23 relative to baseline or predose levels. In addition, the proportion of patients achieving IgG concentration ≥0.35 µg/mL postdose 3 and postdose 4 was assessed. This reference antibody concentration of 0.35 µg/mL was defined by the World Health Organization [28] to estimate efficacy of PCVs against IPD for infants and is not a defined threshold of protection for other age groups or for this immunocompromised population. The safety objective was to evaluate the safety profile of PCV13. Objectives were evaluated in the pediatric (2–17 years) and adult (≥18 years) age groups.
Sample Size
Planned recruitment was up to 300 patients (≤150 adults), with a minimum of 200, which would provide at least 134 evaluable patients based on an estimated 33% rate of dropouts and exclusions from the evaluable immunogenicity population between visits 1 and 6. Owing to slow recruitment, particularly for the pediatric population, the protocol was amended to allow for enrollment of >150 adults to ensure the sample size.
Analyses Populations
The primary analysis population was the evaluable immunogenicity population and consisted of eligible patients who received ≥3 PCV13 doses as assigned, had blood drawn within the required time frames, had ≥1 valid and determinate assay result, had received no prohibited vaccines, and had no other major protocol violation. Patients who received plasma products and/or immunoglobulins during the study as part of the standard of care were excluded from the evaluable population. The safety population included all patients who received ≥1 vaccination.
Statistical Analyses
For each PCV13 serotype, IgG concentrations were logarithmically transformed for analysis; IgG GMCs were calculated at each time point. Two-sided 95% confidence intervals (CIs) were constructed by back-transformation of the CIs for the mean of the logarithmically transformed assay results computed using the Student t distribution. GMFRs were computed and 2-sided 95% CIs constructed using logarithmically transformed assay results. A statistically significant increase in response was observed when the lower limit of the GMFR 95% CI was >1; a statistically significant decrease was observed when the upper limit of the GMFR 95% CI was <1. Reverse cumulative distribution curves were constructed to show the percentage of patients achieving IgG concentrations ranging from approximately 0.001 µg/mL to 1000 µg/mL.
For the post hoc analyses of OPA titers, the same statistical rules were applied as for the IgG concentrations. For the correlations between IgG concentration and OPA titer after dose 3 and after dose 4 of PCV13, the Pearson correlation coefficient and the corresponding 95% CI were calculated for each serotype. Positive correlations were significant if the lower limit of the 95% CI was >0.
Post hoc, the impact of 12 independent variables on IgG response after PCV13 dose 3 was assessed using a stepwise regression method. The independent variables were continuous (age, and time between HSCT and PCV13 dose 1), categorical (donor type [human leukocyte antigen {HLA}–identical sibling vs HLA-matched unrelated donor vs others], recipient sex, underlying disease [acute myeloid or lymphocytic leukemia vs aplastic anemia vs myelodysplastic syndrome vs others], type of conditioning [myeloablative vs reduced intensity conditioning], and source of stem cell [bone marrow vs peripheral blood vs umbilical cord blood]), or binary (yes/no to the threshold lymphocyte count [≤1.0 × 109/L] at baseline, the threshold serum γ-globulin level [≥4 g/L] at baseline, and to each of the following before or at the time of the dose 1 to dose 3 blood sample: any type of GVHD, steroids, and other immunosuppressive therapy).
For safety, the incidence of local and systemic reactions and AEs were summarized. AEs were categorized according to the Medical Dictionary for Regulatory Activities.
RESULTS
Of 251 eligible patients, 247 received ≥1 study vaccine; 184 (73%) completed the study.
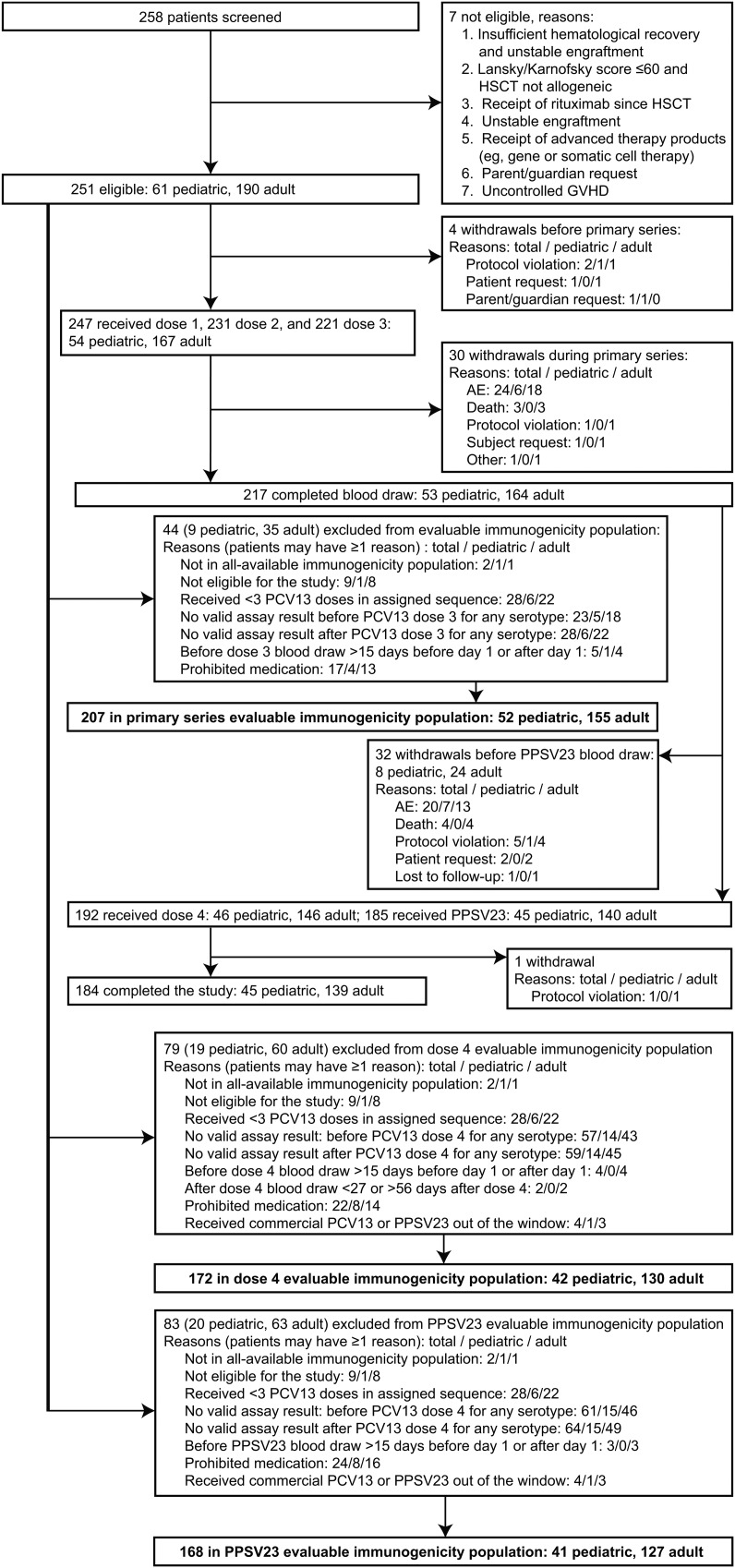
The evaluable immunogenicity population for analysis of the primary objective included 207 patients (155 adults and 52 children); the most common reason for exclusion from this population was receipt of <3 doses of PCV13 in the sequence assigned (Figure 1). A detailed description of the evaluable immunogenicity population at baseline (visit 1) before administration of the first PCV13 dose (N = 216) is shown in Table 1.
Figure 1.
Patient disposition. The term “prohibited medication” refers to predefined medication that excluded patients from the evaluable immunogenicity population: immunoglobulins (n = 18), rituximab (n = 4), both rituximab and immunoglobulins (n = 1), and donor lymphocyte infusions (n = 1). Abbreviations: AE, adverse event; GVHD, graft-vs-host disease; HSCT, hematopoietic stem cell transplant; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
Table 1.
Characteristics of the Evaluable Immunogenicity Population Reported at Baseline (at Dose 1) or Present Between Dose 1 and Dose 3
| Characteristic | Pediatric (n = 54) | Adult (n = 162) | Total (N = 216) |
|---|---|---|---|
| Age, y | |||
| Mean ± SD | 10.1 ± 4.42 | 47.1 ± 12.24 | 37.8 ± 19.34 |
| Median | 10.0 | 47.0 | 42.0 |
| Min, max | 2, 17 | 18, 71 | 2, 71 |
| HSCT to PCV13 dose 1 in days (n = 203) | |||
| Mean ± SD | 165.7 ± 31.40 | 151.1 ± 33.27 | 154.6 ± 33.35 |
| Median | 170.0 | 147.5 | 153.0 |
| Min, max | 97, 209 | 97, 204 | 97, 209 |
| Male, No. (%) | 28 (51.9) | 99 (61.1) | 127 (58.8) |
| Race, No. (%) | |||
| White | 35 (64.8) | 129 (79.6) | 164 (75.9) |
| Black or African American | 4 (7.4) | 0 | 4 (1.9) |
| Asian | 3 (5.6) | 1 (0.6) | 4 (1.9) |
| Othera | 12 (22.3) | 32 (19.8) | 44 (20.4) |
| Underlying disease, No. (%) | |||
| Acute myeloid leukemia | 5 (9.3) | 78 (48.1) | 83 (38.4) |
| Acute lymphocytic leukemia | 13 (24.1) | 13 (8.0) | 26 (12.0) |
| Aplastic anemia | 11 (20.4) | 7 (4.3) | 18 (8.3) |
| Myelodysplastic syndrome | 3 (5.6) | 11 (6.8) | 14 (6.5) |
| Chronic myeloid leukemia | 3 (5.6) | 6 (3.7) | 9 (4.2) |
| Multiple myeloma | 0 | 7 (4.3) | 7 (3.2) |
| Other hematologic conditions | 19 (35.0) | 40 (24.8) | 59 (27.4) |
| Conditioning regimen, No. (%) | |||
| Myeloablative | 43 (79.6) | 89 (54.9) | 132 (61.1) |
| Reduced intensity | 11 (20.4) | 73 (45.1) | 84 (38.9) |
| Donor HLA type, No. (%) | |||
| Haploidentical | 2 (3.7) | 2 (1.2) | 4 (1.9) |
| Identical sibling | 24 (44.4) | 74 (45.7) | 98 (45.4) |
| Matched family | 3 (5.6) | 7 (4.3) | 10 (4.6) |
| Matched unrelated | 20 (37) | 68 (42) | 88 (40.7) |
| Mismatched family | 1 (1.9) | 0 | 1 (0.5) |
| Mismatched unrelated | 4 (7.4) | 11 (6.8) | 15 (6.9) |
| Stem cell source, No. (%) | |||
| Bone marrow | 31 (57.4) | 17 (10.5) | 48 (22.2) |
| Peripheral blood | 17 (31.5) | 141 (87.0) | 158 (73.2) |
| Umbilical cord blood | 6 (11.1) | 4 (2.5) | 10 (4.6) |
| T-cell depletion, No. (%) | |||
| In vitro | 8 (14.8) | 3 (1.9) | 11 (5.1) |
| In vivo | 21 (38.9) | 56 (34.6) | 77 (35.6) |
| Baseline IgG, g/L (n = 211), mean ± SD | 8.1 ± 3.19 | 7.3 ± 3.18 | 7.5 ± 3.19 |
| Baseline absolute lymphocytes × 109/L (n = 208), mean ± SD | 1.7 ± 1.29 | 1.3 ± 0.87 | 1.4 ± 1.00 |
| GVHD at baseline, No. (%) | 16 (29.6) | 57 (35.2) | 73 (33.8) |
| Acute | 4 (7.4) | 7 (4.3) | 11 (5.1) |
| Chronic | 4 (7.4) | 11 (6.8) | 15 (6.9) |
| Unspecified | 8 (14.8) | 43 (26.5) | 51 (23.6) |
| GVHD present between dose 1 and dose 3, No. (%) | 7 (13.0) | 64 (39.4) | 71 (32.9) |
| Acute | 1 (1.9) | 2 (1.2) | 3 (1.4) |
| Chronic | 5 (9.3) | 21 (13.0) | 26 (12.0) |
| Unspecified | 1 (1.9) | 46 (28.4) | 47 (21.8) |
| Immunosuppressives at baseline, No. (%) | |||
| Systemic steroid (≥1 product) | 10 (18.5) | 38 (23.5) | 48 (22.2) |
| Cyclosporine | 24 (44.4) | 98 (60.5) | 122 (56.5) |
| Tacrolimus | 3 (5.6) | 27 (16.7) | 30 (13.9) |
| Sirolimus | 0 | 3 (1.9) | 3 (1.4) |
| Immunosuppressives administered between dose 1 and dose 3, No. (%) | |||
| Systemic steroid (≥1 product) | 12 (22.2) | 59 (36.4) | 71 (32.9) |
| Cyclosporine | 25 (46.3) | 103 (63.6) | 128 (59.3) |
| Tacrolimus | 3 (5.6) | 29 (17.9) | 32 (14.8) |
| Sirolimus | 0 | 3 (1.9) | 3 (1.4) |
Abbreviations: GVHD, graft-vs-host disease; HLA, human leukocyte antigen; HSCT, hematopoietic stem cell transplant; IgG, immunoglobulin G; No., total number of patients assessed; PCV13, 13-valent pneumococcal conjugate vaccine; SD, standard deviation.
a All others including American Indian or Alaska Native.
Immunogenicity
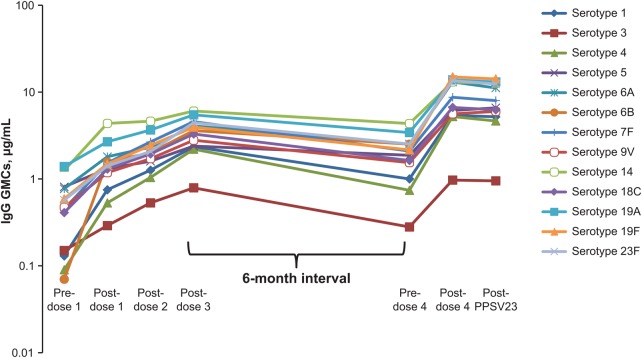
IgG GMCs for all PCV13 serotypes generally increased after each of the first 3 PCV13 doses. GMCs decreased before PCV13 dose 4 but remained similar or higher than after dose 1 and, for serotypes 5, 6A, 6B, and 23F, higher than after dose 2. GMCs increased after dose 4 compared with after dose 3 (except for serotype 3 in the pediatric group) and generally remained stable after PPSV23 (Figure 2; Supplementary Table 1). Except for a few serotypes after dose 1 and dose 2, pediatric patients had numerically higher GMCs than adults after each PCV13 dose (Supplementary Table 1).
Figure 2.
Pneumococcal immunoglobulin G (IgG) geometric mean concentrations (GMCs) in the evaluable immunogenicity population after 3 doses of 13-valent pneumococcal conjugate vaccine (monthly), a booster dose (6 months later), and a dose of 23-valent pneumococcal polysaccharide vaccine (PPSV23) (1 month later).
GMFRs demonstrated statistically significant increases (lower limit of the GMFR 95% CI >1) in IgG GMCs for all serotypes from before dose 1 (baseline) to after dose 3 and dose 4 of PCV13, and to after PPSV23; from after dose 3 to after dose 4 of PCV13 (except for serotype 3 in the pediatric group, for which responses were similar); and from before to after dose 4 of PCV13. GMFRs demonstrated that IgG GMCs after PCV13 dose 4 compared with after PPSV23 were similar for most of the PCV13 serotypes; exceptions were serotype 9V (total and adult populations), with significantly higher GMCs after PPSV23; and serotypes 6B (total and pediatric populations) and 6A (all groups), with significantly lower GMCs after PPSV23 (Table 2; Supplementary Table 2). Serotype 6A is not included in PPSV23.
Table 2.
Pneumococcal Geometric Mean Fold Rise of Immunoglobulin G Geometric Mean Concentrations, Evaluable Immunogenicity Population ≥2 Years of Age
| PCV13 Serotypes |
Geometric Mean Fold Rise (95% Confidence Interval) |
|||||
|---|---|---|---|---|---|---|
| Postdose 3/ Baseline (n = 191–197) | Postdose 4/ Baseline (n = 156–161) | Post PPSV23/Baseline (n = 146–151) | Postdose 4/Postdose 3 (n = 118–121) | Postdose 4/Predose 4 (n = 159–162) | Post PPSV23/Postdose 4 (n = 153–155) | |
| 1 | 17.96 (13.63–23.66) | 42.03 (30.94–57.11) | 38.64 (28.64–52.12) | 2.30 (1.85–2.86) | 5.64 (4.56–6.98) | 0.99 (.89–1.10) |
| 3 | 5.07 (3.98–6.46) | 6.28 (4.81–8.19) | 5.80 (4.48–7.51) | 1.20 (1.01–1.43) | 3.55 (2.93–4.31) | 1.03 (.94–1.13) |
| 4 | 23.85 (17.61–32.30) | 55.02 (39.39–76.85) | 46.67 (33.45–65.12) | 2.29 (1.91–2.74) | 6.97 (5.66–8.58) | 0.92 (.84–1.00) |
| 5 | 2.99 (2.46–3.63) | 7.40 (5.69–9.63) | 7.62 (5.94–9.78) | 2.39 (1.99–2.87) | 3.29 (2.76–3.93) | 1.06 (.98–1.15) |
| 6A | 5.35 (4.18–6.85) | 16.69 (12.47–22.34) | 13.66 (10.17–18.35) | 2.88 (2.34–3.55) | 5.29 (4.33–6.47) | 0.86 (.81–0.91) |
| 6B | 5.18 (3.99–6.74) | 19.29 (14.22–26.18) | 17.63 (12.94–24.02) | 3.07 (2.51–3.75) | 5.42 (4.46–6.60) | 0.93 (.87–0.99) |
| 7F | 10.28 (8.15–12.96) | 18.81 (14.48–24.43) | 16.86 (13.11–21.67) | 1.75 (1.46–2.11) | 4.17 (3.43–5.08) | 1.01 (.94–1.08) |
| 9V | 5.76 (4.63–7.17) | 11.90 (9.27–15.27) | 12.19 (9.40–15.82) | 1.88 (1.57–2.24) | 3.68 (3.08–4.40) | 1.12 (1.03–1.22) |
| 14 | 4.95 (3.72–6.58) | 10.30 (7.55–14.03) | 9.48 (6.85–13.11) | 1.84 (1.50–2.26) | 3.00 (2.49–3.61) | 1.01 (.93–1.10) |
| 18C | 8.22 (6.36–10.62) | 15.80 (11.76–21.24) | 14.66 (10.93–19.66) | 1.81 (1.54–2.13) | 4.16 (3.50–4.94) | 0.95 (.88–1.03) |
| 19A | 3.90 (3.10–4.89) | 9.94 (7.61–12.98) | 8.79 (6.67–11.59) | 2.31 (1.95–2.74) | 4.04 (3.35–4.87) | 0.97 (.90–1.05) |
| 19F | 6.73 (5.15–8.78) | 26.47 (19.31–36.29) | 23.10 (16.80–31.75) | 3.61 (2.94–4.44) | 6.95 (5.63–8.56) | 0.99 (.90–1.09) |
| 23F | 8.01 (6.11–10.51) | 23.56 (17.11–32.45) | 20.69 (14.95–28.64) | 2.57 (2.10–3.14) | 5.42 (4.41–6.67) | 0.96 (.88–1.03) |
Abbreviations: n, number of patients with valid and determinate assay results for the specified serotype for both of the specified blood draws; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
The reverse cumulative distribution curves for all serotypes for the total population after each PCV13 dose also demonstrated the increasing IgG responses from dose to dose over the entire range of measurable concentrations, which were not observed after PPSV23 (Supplementary Figure 1). The percentage of patients achieving a serotype-specific IgG concentration ≥0.35 µg/mL increased from before dose 1 (range, 23.5%–89.7%) to 1 month after dose 3 (89.7%–98.0%), decreased in the 6-month period to before dose 4 (46.3%–93.2%), and increased 1 month after dose 4 (82.6%–98.8%).
For the post hoc OPA assessments, OPA geometric mean titers (GMTs) showed a similar pattern of response in both age groups for all PCV13 serotypes as that observed by IgG GMCs after each vaccination, with 1 exception. The OPA GMT for serotype 3 in the pediatric group was numerically higher after dose 4 than after dose 3, whereas by ELISA, IgG GMC was numerically lower after dose 4 than after dose 3 (Supplementary Table 1). In addition, a significant positive correlation between IgG GMCs and OPA GMTs for all serotypes was observed in both age groups after dose 3 of the primary series and after dose 4, with the lower limit of the 95% CI >0 for all correlations (Supplementary Tables 3 and 4).
The stepwise regression method yielded 5 of the 12 independent risk factors studied to be useful predictors of the immune response when all PCV13 serotypes were combined (Table 3) and for single serotypes (Supplementary Table 5). However, the only risk factors that significantly (P ≤ .05) impaired the immune response for all the PCV13 serotypes combined and most single serotypes were baseline γ-globulin levels <4 g/L, donor type other than an HLA-identical sibling, and increasing recipient age. In addition, only for serotype 18C was time between HSCT and dose 1 of PCV13 significant (Supplementary Table 5).
Table 3.
Influence of the Selected Risk Factors on Pneumococcal Immunoglobulin G Antibody Immune Response After 13-Valent Pneumococcal Conjugate Vaccine Dose 3 for All Serotypes Combined, Evaluable Immunogenicity Population
| Predictor Variable, Selected From Stepwise Regressiona | Category | LS Mean/Regression Coefficientb | P Valuec |
|---|---|---|---|
| Donor type | .001 | ||
| HLA-identical sibling | 2.493 | ||
| HLA-matched unrelated | 2.012 | ||
| Other | 1.094 | ||
| Recipient age | –0.015 | <.001 | |
| Recipient sex | .221 | ||
| Female | 1.925 | ||
| Male | 1.616 | ||
| Serum γ-globulin, baseline | <.001 | ||
| <4 g/L | 0.983 | ||
| ≥4 g/L | 3.165 | ||
| Time, d from HSCT and PCV13 dose 1 | 0.003 | .152 |
Abbreviations: HLA, human leukocyte antigen; HSCT, hematopoietic stem cell transplant; IgG, immunoglobulin G; LS, least squares; PCV13, 13-valent pneumococcal conjugate vaccine.
a The forward stepwise regression predicts the IgG antibody immune response after PCV13 dose 3 for all serotypes on the basis of 12 independent variables in all models fitted. A significance level of 0.2 is required to allow a variable into the model, and a significance level of 0.25 is required for a variable to stay in the model.
b Least squares mean for categorical variables and regression coefficient for continuous variables.
c Independent risk factors are significant if P ≤ .05.
Safety
Local reactions (Supplementary Figure 2) and systemic reactions (Table 4) were more frequent after PCV13 dose 4, with frequencies of redness, swelling, fever ≥38°C, fatigue, and muscle pain significantly higher compared with after dose 3 in the total and adult populations; for pediatric patients the numbers available for comparison were too few to draw inferences (Supplementary Table 6). After each PCV13 dose, local reactions were more frequent in the pediatric group; systemic reactions were generally similar in frequency across age groups except for fever, which was more common in the pediatric group. The mean durations were generally similar between age groups after each dose and did not exceed 4.5 days for local reactions in the pediatric group and 7.2 days for systemic reactions for the total population.
Table 4.
Systemic Reactions Within 14 Days of 13-Valent Pneumococcal Conjugate Vaccination, Safety Population
| Event | Patients With Events, % (no./No.) |
|||
|---|---|---|---|---|
| Dose 1 | Dose 2 | Dose 3 | Dose 4 | |
| Fever ≥38°C | ||||
| Total | 8 (13/169) | 10 (13/131) | 7 (8/120) | 18 (17/96) |
| Pediatric | 13 (5/39) | 23 (6/26) | 15 (4/27) | 28 (5/18) |
| Adult | 6 (8/130) | 7 (7/105) | 4 (4/93) | 15 (12/78) |
| Fever 39°C–40°Ca | ||||
| Total | 2 (3/167) | 2 (3/126) | 2 (2/116) | 2 (2/87) |
| Pediatric | 8 (3/39) | 8 (2/24) | 4 (1/24) | 0 (0/15) |
| Adult | 0 (0/128) | 1 (1/102) | 1 (1/92) | 3 (2/72) |
| Fatigue | ||||
| Total | 58 (119/204) | 57 (98/171) | 49 (77/157) | 67 (86/128) |
| Pediatric | 53 (24/45) | 61 (23/38) | 49 (17/35) | 68 (19/28) |
| Adult | 60 (95/159) | 56 (75/133) | 49 (60/122) | 67 (67/100) |
| Headache | ||||
| Total | 44 (84/189) | 35 (53/152) | 37 (53/142) | 47 (53/114) |
| Pediatric | 45 (19/42) | 32 (10/31) | 38 (12/32) | 52 (11/21) |
| Adult | 44 (65/147) | 36 (43/121) | 37 (41/110) | 45 (42/93) |
| Vomiting | ||||
| Total | 21 (36/173) | 15 (20/135) | 11 (13/120) | 6 (5/89) |
| Pediatric | 21 (8/39) | 21 (6/28) | 8 (2/25) | 6 (1/16) |
| Adult | 21 (28/134) | 13 (14/107) | 12 (11/95) | 6 (4/73) |
| Diarrhea | ||||
| Total | 35 (66/189) | 29 (44/150) | 23 (30/130) | 29 (29/101) |
| Pediatric | 32 (14/44) | 31 (9/29) | 15 (4/26) | 22 (4/18) |
| Adult | 36 (52/145) | 29 (35/121) | 25 (26/104) | 30 (25/83) |
| Muscle pain | ||||
| Total | 51 (103/201) | 45 (72/160) | 40 (61/152) | 60 (74/123) |
| Pediatric | 55 (26/47) | 44 (16/36) | 46 (16/35) | 58 (14/24) |
| Adult | 50 (77/154) | 45 (56/124) | 39 (45/117) | 61 (60/99) |
| Joint pain | ||||
| Total | 27 (48/181) | 25 (37/147) | 21 (28/132) | 31 (31/99) |
| Pediatric | 26 (10/38) | 32 (10/31) | 25 (7/28) | 25 (4/16) |
| Adult | 27 (38/143) | 23 (27/116) | 20 (21/104) | 33 (27/83) |
a There was no fever >40°C.
The frequencies of AEs were similar between age groups after each PCV13 dose. Infections were most common. Frequencies of AEs possibly related to PCV13 were numerically similar after dose 4 (total 6.8% [13/192 patients]) compared with from dose 1 to after dose 3 (6.1% [15/247 patients]).
After PPSV23, 15.8% (29/184) of patients experienced possibly related AEs. These were mainly local reactions and fever (n = 4), which were not collected with an e-diary but on the case report form and classified as general disorders and administration site conditions (14.1% [26/184 patients]); other AEs included cellulitis (n = 2), myalgia (n = 2), arthralgia (n = 1), pain in extremity (n = 1), Guillain-Barré syndrome (n = 1), cellulitis (n = 2), and hematoma (n = 1).
Six patients experienced SAEs that were reported by the investigator as possibly related to study vaccination. These SAEs included facial diplegia (14 days after PCV13 dose 1), injection-site erythema and pyrexia (1 day after PCV13 dose 2), 2 episodes of autoimmune hemolytic anemia in a single patient (18 days and 116 days after PCV13 dose 3), 1 Guillain-Barré syndrome (29 days after PCV13 dose 4 and 1 day after PPSV23), and 1 cellulitis (2 days after PPSV23). Additionally, an investigator reported as possibly related 1 episode of suspected lack of vaccine efficacy resulting in a case of bilateral pneumonia (36 days after PCV13 dose 3), for which a urinary antigen detection assay, which does not identify the serotype, tested positive for S. pneumoniae. Fifty-one AEs led to study withdrawal, and were generally due to disease relapse or to complications of HSCT. There were 14 deaths; none was related to study vaccines.
DISCUSSION
The study showed significant increases in IgG binding and OPA functional antibodies for all PCV13 serotypes after a 3-dose primary series of PCV13 administered at monthly intervals starting approximately 5 months after HSCT (mean ± standard deviation, 154.6 ± 33.35 days). PCV13 dose 4, administered approximately 6 months after dose 3, also led to significant increases in antibody levels compared with before dose 4, and relative to after dose 3 for all serotypes (including serotype 3 when measured by OPA assay), indicative of a memory response on reexposure to the vaccine. Higher immune responses were elicited in pediatric than in adult patients. After the 3-dose primary series and after dose 4 (booster dose), OPA and IgG responses correlated significantly for each of the PCV13 serotypes. OPA titers, which measure serotype-specific killing of S. pneumoniae, provide the best functional correlate of vaccine-induced protection in humans [29, 30].
PCV13 dose 4 was associated with increased reactogenicity compared with after dose 3. However, with the exception of fever, reactogenicity was not higher than that observed in other studies where participants received a single dose of PCV13 [31, 32]. The possibly related SAEs in 6 patients occurred across doses. Of note, the patients who experienced facial diplegia, autoimmune hemolytic anemia, and Guillain-Barré syndrome had a complex constellation of comorbid conditions, received concomitant medications, and were exposed to multiple infections and GVHD, so that it was difficult to establish a clear causal relationship between these events and PCV13. Considering the risk/benefit of vaccination, the overall safety profile of a 4-dose regimen was considered acceptable.
Vaccination guidelines for HSCT patients currently recommend a fourth PCV13 dose in patients with chronic GVHD (because of the lack of efficacy of PPSV23 in this subpopulation) [33]. However, for patients without GVHD, a booster dose of PPSV23 is recommended, with the aim of broadening serotype coverage [33]. A fourth dose of PCV13 administered during the first year after HSCT in this study elicited, for the majority of serotypes, antibody levels similar to those observed after a single dose of PCV13 in healthy individuals of comparable age [31, 32, 34, 35]. This study did not assess a booster dose of PPSV23 as an alternative to PCV13 dose 4; nor were the responses elicited by the 11 additional serotypes unique to PPSV23 evaluated; nor was the reactogenicity of PPSV23 assessed daily using an e-diary, with the possibility of underreporting AEs. Therefore, no conclusions can be drawn from this study on potential benefits of PPSV23 as an alternative to PCV13 dose 4. In a previous study in HSCT recipients, a dose of PPSV23 given 6 months after 3 PCV7 doses increased immune responses elicited by specific serotypes (1 and 5) unique to PPSV23, but also increased responses to the PCV7 serotypes included in PPSV23 [36].
In this study, PPSV23 was administered at approximately 1 year after HSCT as recommended [5, 14]. It was administered to broaden serotype coverage and not to assess an appropriate vaccination interval. PPSV23 did not further increase the immune response for the majority of PCV13 serotypes included in PPSV23. We hypothesize that this was due to the short time interval of 1 month between PCV13 and PPSV23 administration.
The long-term effects on immunity after vaccination with either 3 doses of PCV13 followed by 1 dose of PPSV23 or 4 doses of PCV13 are not known and will require additional studies.
In the current study, the main risk factors that correlated with lower serum antibody responses to PCV13 (when PCV13 serotypes were combined, and for most of the single PCV13 serotypes) included donor type other than an HLA-identical sibling, increasing recipient age, and baseline serum γ-globulin level <4 g/L. These results confirm, in part, previous reports [10, 13]. A possible limitation of the study is that, considering the fewer than anticipated number of cases of GVHD and the number of deaths (14/247), the inclusion/exclusion criteria likely selected a “lower-risk” population than anticipated for the allogeneic HSCT population. This may explain the lack of significant impact of GVHD on the immune response of all PCV13 serotypes.
In conclusion, given the marked increase in antibody levels after dose 4 of PCV13, this 4-dose regimen may provide better protection than a 3-dose series against pneumococcal disease in HSCT recipients. This finding must be balanced against a higher reactogenicity. In patients without GVHD, whether this fourth PCV13 dose provides a clear benefit compared with the recommended schedule of PPSV23 as the fourth dose cannot be determined from this study.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the patients who participated in this study (and the parents and guardians of the children), the principal investigators and their study teams, and the members of the safety advisory board. We also honor the memory of Dr Trudy Small, who contributed to the design and conduct of the study from a US perspective.
Financial support. This work was supported by Pfizer Inc. Medical writing assistance was provided by Deborah M. Campoli-Richards, BSPharm, RPh, of Complete Healthcare Communications, Inc, and was funded by Pfizer Inc.
Potential conflicts of interest. C. C. served on advisory boards and speaker bureaus for Pfizer; she received research funding for her department and travel grants from Pfizer. P. L. served on an advisory board for Pfizer for a different product. His institution received research funding for the study. D. S. received research funding, served on advisory boards, and received travel grants from Pfizer. His institution received research funding for the study. W. C. G., D. A. S., B. S.-T., K. C., P. C. G., and C. J. are employees and shareholders of Pfizer. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
APPENDIX
Institutional review boards and independent ethics committees: Commissie Medische Ethiek van de Universitaire Ziekenhuizen KU, U.Z. Belgium; Comite D'Ethique de la Recherche de l'Hopital Maisonneuve-Rosemont, Montreal, Canada; Comite d'Ethique de la Recherche du Centre Hospitalier Universitaire Sainte-Justine, Montreal, Canada; University of Manitoba Biomedical Research Ethics Board, Winnipeg, Canada; Fakultni nemocnice v Motole Lokalni eticka komise, Prague, Czech Republic; Multicentricka eticka komise, Brno, Czech Republic; Comite de Protection des Personnes, IIe-de-France I, Paris, France; Comité de protection des personnes, Ile-de-France IX, Créteil, France; Ethik-Kommission der Medizinischen Fakultaet der Heinrich-Heine-Universitaet Duesseldorf, Duesseldorf, Germany; Centrale Commissie Mensgebonden, Den Haag, Netherlands; Komisja Bioetyczna przy, Uniwersytecie Medycznym im., Wroclaw, Poland; Komisja Bioetyczna przy, Akademii Medycznej we Wroclawiu, Wroclaw, Poland; Hospital Santa Creu i Sant Pau, Comite Etico de Investigacion Clinica, Barcelona, Spain; Hospital Universitario La Fe, Comite Etico de Investigacion Clinica, Valencia, Spain; Regionala etikprovningsnamnden i Stockholm, Stockholm, Sweden; Memorial Sloan Kettering Cancer Center, Institutional Review Board, New York, New York, USA; Duke University Health System Institutional Review Board, Durham, North Carolina, USA; Western Institutional Review Board, Olympia, Washington, USA; Baylor College of Medicine Institutional Review Board for Human Subjects, Houston, Texas, USA; Committees for Protection of Human Subjects, Philadelphia, Pennsylvania, USA; Cincinnati Children's Hospital Medical Center Institutional Review Board, Cincinnati, Ohio, USA; BRANY, LLC, Lake Success, New York, USA; University of Utah Institutional Review Board, Salt Lake City, Utah, USA; University of Kentucky Office of Research Integrity, Lexington, Kentucky, USA.
References
- 1.Engelhard D, Cordonnier C, Shaw PJ et al. Early and late invasive pneumococcal infection following stem cell transplantation: a European Bone Marrow Transplantation survey. Br J Haematol 2002; 117:444–50. [DOI] [PubMed] [Google Scholar]
- 2.Kumar D, Humar A, Plevneshi A et al. Invasive pneumococcal disease in adult hematopoietic stem cell transplant recipients: a decade of prospective population-based surveillance. Bone Marrow Transplant 2008; 41:743–7. [DOI] [PubMed] [Google Scholar]
- 3.Torda A, Chong Q, Lee A et al. Invasive pneumococcal disease following adult allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis 2014; 16:751–9. [DOI] [PubMed] [Google Scholar]
- 4.Tomblyn M, Chiller T, Einsele H et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009; 15:1143–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients: recommendations of CDC, the Infectious Disease Society of America, and the American Society of Blood and Marrow Transplantation. MMWR Recomm Rep 2000; 49(RR-10):1–127. [PubMed] [Google Scholar]
- 6.D'Orsogna LJ, Wright MP, Krueger RG et al. Allogeneic hematopoietic stem cell transplantation recipients have defects of both switched and igm memory B cells. Biol Blood Marrow Transplant 2009; 15:795–803. [DOI] [PubMed] [Google Scholar]
- 7.Youssef S, Rodriguez G, Rolston KV, Champlin RE, Raad II, Safdar A. Streptococcus pneumoniae infections in 47 hematopoietic stem cell transplantation recipients: clinical characteristics of infections and vaccine-breakthrough infections, 1989–2005. Medicine (Baltimore) 2007; 86:69–77. [DOI] [PubMed] [Google Scholar]
- 8.Ada G. Vaccines and vaccination. N Engl J Med 2001; 345:1042–53. [DOI] [PubMed] [Google Scholar]
- 9.Clutterbuck EA, Lazarus R, Yu LM et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis 2012; 205:1408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cordonnier C, Labopin M, Chesnel V et al. Randomized study of early versus late immunization with pneumococcal conjugate vaccine after allogeneic stem cell transplantation. Clin Infect Dis 2009; 48:1392–401. [DOI] [PubMed] [Google Scholar]
- 11.Meisel R, Kuypers L, Dirksen U et al. Pneumococcal conjugate vaccine provides early protective antibody responses in children after related and unrelated allogeneic hematopoietic stem cell transplantation. Blood 2007; 109:2322–6. [DOI] [PubMed] [Google Scholar]
- 12.Molrine DC, Antin JH, Guinan EC et al. Donor immunization with pneumococcal conjugate vaccine and early protective antibody responses following allogeneic hematopoietic cell transplantation. Blood 2003; 101:831–6. [DOI] [PubMed] [Google Scholar]
- 13.Pao M, Papadopoulos EB, Chou J et al. Response to pneumococcal (PNCRM7) and haemophilus influenzae conjugate vaccines (HIB) in pediatric and adult recipients of an allogeneic hematopoietic cell transplantation (alloHCT). Biol Blood Marrow Transplant 2008; 14:1022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ljungman P, Engelhard D, de la Camara R et al. Vaccination of stem cell transplant recipients: recommendations of the Infectious Diseases Working Party of the EBMT. Bone Marrow Transplant 2005; 35:737–46. [DOI] [PubMed] [Google Scholar]
- 15.Ljungman P, Cordonnier C, Einsele H et al. Vaccination of hematopoietic cell transplant recipients. Bone Marrow Transplant 2009; 44:521–6. [DOI] [PubMed] [Google Scholar]
- 16.Ljungman P, Small TN; Vaccination Recommendations Writing Group. Update to vaccination guidelines. Biol Blood Marrow Transplant 2010; 16:1608–9. [DOI] [PubMed] [Google Scholar]
- 17.Pilishvili T, Lexau C, Farley MM et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201:32–41. [DOI] [PubMed] [Google Scholar]
- 18.Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine. Vaccine 2011; 29:9127–31. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan SL, Barson WJ, Lin PL et al. Early trends for invasive pneumococcal infections in children after the introduction of the 13-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2013; 32:203–7. [DOI] [PubMed] [Google Scholar]
- 20.Singleton R, Wenger J, Klejka JA et al. The 13-valent pneumococcal conjugate vaccine for invasive pneumococcal disease in Alaska native children: results of a clinical trial. Pediatr Infect Dis J 2013; 32:257–63. [DOI] [PubMed] [Google Scholar]
- 21.Hak E, Grobbee DE, Sanders EA et al. Rationale and design of CAPITA: a RCT of 13-valent conjugated pneumococcal vaccine efficacy among older adults. Neth J Med 2008; 66:378–83. [PubMed] [Google Scholar]
- 22.Bonten MJM, Huijts SM, Bolkenbaas M et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114–25. [DOI] [PubMed] [Google Scholar]
- 23.Tomczyk S, Bennett NM, Stoecker C et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged >/=65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014; 63:822–5. [PMC free article] [PubMed] [Google Scholar]
- 24.Wernette CM, Frasch CE, Madore D et al. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin Diagn Lab Immunol 2003; 10:514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero-Steiner S, Libutti D, Pais LB et al. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol 1997; 4:415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper D, Yu X, Sidhu M, Nahm MH, Fernsten P, Jansen KU. The 13-valent pneumococcal conjugate vaccine (PCV13) elicits cross-functional opsonophagocytic killing responses in humans to Streptococcus pneumoniae serotypes 6C and 7A. Vaccine 2011; 29:7207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Wang S, Sendi L, Caulfield MJ. High-throughput imaging of bacterial colonies grown on filter plates with application to serum bactericidal assays. J Immunol Methods 2004; 292:187–93. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Recommendations to assure the quality, safety and efficacy of pneumococcal conjugate vaccines—proposed replacement of TRS 927, Annex 2, ECBS, 19–23 October 2009. Geneva, Switzerland: WHO, 2009. [Google Scholar]
- 29.Hu BT, Yu X, Jones TR et al. Approach to validating an opsonophagocytic assay for Streptococcus pneumoniae. Clin Diagn Lab Immunol 2005; 12:287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vernacchio L, Romero-Steiner S, Martinez JE et al. Comparison of an opsonophagocytic assay and IgG ELISA to assess responses to pneumococcal polysaccharide and pneumococcal conjugate vaccines in children and young adults with sickle cell disease. J Infect Dis 2000; 181:1162–6. [DOI] [PubMed] [Google Scholar]
- 31.Bryant KA, Frenck RW, Gurtman A et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults aged 18 to 49 years, naive to 23-valent pneumococcal polysaccharide vaccine. In: European Congress of Clinical Microbiology and Infectious Diseases, Berlin, Germany, 27–30 April 2013. [Google Scholar]
- 32.Jackson LA, Gurtman A, van Cleeff M et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine 2013; 31:3577–84. [DOI] [PubMed] [Google Scholar]
- 33.Rubin LG, Levin MJ, Ljungman P et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:309–18. [DOI] [PubMed] [Google Scholar]
- 34.Frenck R Jr, Thompson A, Senders S et al. 13-Valent pneumococcal conjugate vaccine in older children and adolescents either previously immunized with or naive to 7-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2014; 33:183–9. [DOI] [PubMed] [Google Scholar]
- 35.Schwarz TF, Flamaing J, Rumke HC et al. A randomized, double-blind trial to evaluate immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given concomitantly with trivalent influenza vaccine in adults aged >/=65 years. Vaccine 2011; 29:5195–202. [DOI] [PubMed] [Google Scholar]
- 36.Cordonnier C, Labopin M, Chesnel V et al. Immune response to the 23-valent polysaccharide pneumococcal vaccine after the 7-valent conjugate vaccine in allogeneic stem cell transplant recipients: results from the EBMT IDWP01 trial. Vaccine 2010; 28:2730–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.