Highlights
-
•
First complete sequence of a floR plasmid from Actinobacillus pleuropneumoniae
-
•
Extended similarity to floR plasmids in other Pasteurellaceae species
-
•
Conjugal transfer between between species confirmed
Abstract
The complete nucleotide sequence of a 7.7 kb mobilisable plasmid (pM3446F), isolated from a florfenicol resistant isolate of Actinobacillus pleuropneumoniae, showed extended similarity to plasmids found in other members of the Pasteurellaceae containing the floR gene as well as replication and mobilisation genes. Mobilisation into other Pasteurellaceae species confirmed that this plasmid can be transferred horizontally.
1. Introduction
Actinobacillus pleuropneumoniae, one of the main pathogens contributing to swine respiratory disease throughout the world, has been used as an indicator organism in surveillance studies of antimicrobial resistance in bacteria of animal origin (de Jong et al., 2014; Hendriksen et al., 2008). Tetracyclines, beta-lactams, and trimethoprim/sulphonamides are the commonest frontline treatments for porcine pleuropnuemonia in Europe (European Medicines Agency, 2012), however, levels of resistance are increasing (de Jong et al., 2014; Vanni et al., 2012). To a lesser extent, florfenicol, a fluorinated thiamphenicol derivative licensed to treat respiratory diseases of pigs and cattle in Europe since 2000 and 1995, respectively (Kehrenberg et al., 2004), has been used. Most surveys of A. pleuropneumoniae antimicrobial susceptibility have shown that nearly all isolates are susceptible to florfenicol, except in Korea where resistance levels of 34% were recently reported (Gutiérrez-Martín et al., 2006; Kucerova et al., 2011; Priebe and Schwarz, 2003; Shin et al., 2005; Vanni et al., 2012; Yoo et al., 2014).
A chloramphenicol/florfenicol efflux pump, encoded by floR (Schwarz et al., 2004), has been detected by PCR in florfenicol-resistant A. pleuropneumoniae isolates (Kucerova et al., 2011; Yoo et al., 2014), and this resistance was transferrable to Escherichia coli (Yoo et al., 2014), however no plasmid was characterised.
Here we describe the isolation and characterisation of a 7.7 kb florfenicol resistance plasmid from a clinical isolate of A. pleuropneumoniae. Comparative sequence analysis of this plasmid with others reported as mediating florfenicol resistance in bovine isolates of Pasteurella multocida (Kehrenberg and Schwarz, 2005; Kehrenberg et al., 2008) and Mannheimia haemolytica (Katsuda et al., 2012) is also described.
1.1. Material and methods
1.1.1. Bacterial isolates and MIC measurements
A. pleuropneumoniae MIDG3446, a serovar 2 clinical isolate, was originally cultured from pneumonic lungs of a pig in Greece in 2010 and archived at the then Animal Health and Veterinary Laboratory Agency (now Animal and Plant Health Agency) in England. Minimum inhibitory concentrations (MIC) for florfenicol and chloramphenicol were determined by agar dilution using Chocolate Mueller-Hinton plates, with A. pleuropneumoniae ATCC 27090, Histophilus somni ATCC 70025 and Staphylococcus aureus ATCC 29213 used as quality control strains (CLSI, 2008).
1.1.2. Plasmid isolation and confirmation of floR by PCR
Plasmid DNA was isolated using a Qiaprep Spin Miniprep kit (Qiagen) according to the manufacturer's protocol. The presence of the floR gene was confirmed by PCR using primers floR_for (CGACGCCCGCTATGATCCAACTC) and floR_rev (CCCAAAAAGCCGGACTCGCGAAG). Initially, florfenicol resistance was transferred to E. coli Stellar cells (Clontech) by heat shock with the MIDG3446 plasmid extract, according to the manufacturer's protocol, with selection of transformants on LB agar supplemented with 10 μg/ml florfenicol. Subsequently, mobilisation into selected Pasteurellaceae strains was assessed using a mating protocol previously described (Bossé et al., 2009; Bossé et al., 2015). Recipient strains included nalidixic acid resistant derivatives of M. haemolytica (MIDG1579NalR), P. multocida (MIDG1570 NalR), and Haemophilus parasuis (MIDG3176NalR), as well as the NAD-independent A. pleuropneumoniae isolate, MIDG2331ΔureC::nadV, previously described (Bossé et al., 2009; Bossé et al., 2014). Transconjugants were selected on Brain Heart Infusion agar (with or without 0.01% NAD or 20 μg/ml nalidixic acid, as appropriate) supplemented with 2 μg/ml florfenicol. Transformants and transconjugants were tested by PCR for the presence of floR, as above, and for the nadV gene, as previously described (Bossé et al., 2015), where appropriate. MICs for florfenicol and chloramphenicol were determined, as above, for recipient strains +/− plasmid.
1.2. Plasmid sequence
A plasmid carrying the floR gene was isolated from a selected E. coli transformant (above) and the complete nucleotide sequence was determined using a primer walking strategy. Sequence analysis was carried out using BLASTn and BLASTx. Alignments with other floR plasmids were done using ClustalW. The sequence of the A. pleuropneumoniae floR plasmid (pM3446F) has been deposited to Genbank (accession number KP696484).
2. Results and discussion
A serovar 2 isolate of A. pleuropneumoniae, MIDG3446, was found to be resistant to florfenicol and chloramphenicol (Table 1). Analysis of DNA from MIDG3446 revealed multiple small plasmids, one of which when transformed into E. coli Stellar cells (Clontech), conferred resistance to florfenicol and chloramphenicol (Fig. 1; Table 1). Additionally, mobilisation from MIDG3446 was successful into A. pleuropneumoniae MIDG2331ΔureC::nadV and M. haemolytica MIDG1579NalR, but not P. multocida MIDG1570NalR nor H. parasuis MIDG3176NalR. The location of the genes encoding the conjugation machinery was not determined, and no attempts were made to optimise mating conditions, nor were other isolates of these species tested. PCR confirmed the presence of floR in transformants and transconjugants (Fig. 1), and MICs for florfenicol and chloramphenicol, determined as above, for recipient strains +/− plasmid indicated transfer of resistance with the plasmid (Table 1).
Table 1.
Antimicrobial susceptibility for strains with and without plasmids.
| Strain | Species | MIC (μg/ml) |
Source or Reference | |
|---|---|---|---|---|
| Florfenicol | Chloramphenicol | |||
| MIDG3446 | Apa | 8 | 8 | This study |
| MIDG2331ΔureC::nadV | Ap | 2 | 2 | (Bossé et al., 2014) |
| MIDG2331ΔureC::nadV + pM3446F | Ap | 8 | 8 | This study |
| MIDG1579 | Mhb | 1 | 1 | (Bossé et al., 2009) |
| MIDG1579 + pM3446F | Mh | 16 | 16 | This study |
| Stellar | Ecc | 2 | 4 | Clontech |
| Stellar + pM3446F | Ec | 16 | 32 | This study |
Ap = A. pleuropneumoniae.
Mh = M. haemolytica.
Ec = E. coli.
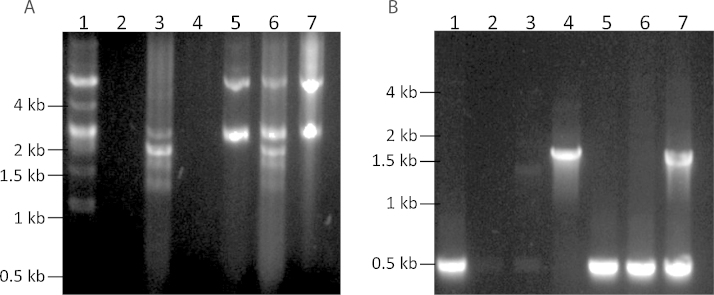
Fig. 1.
Transfer of florfenicol resistance plasmid from A. pleuropneumoniae MIDG3446 by transformation into E. coli Stellar, or mating into M. heamolytica MIDG1579 and A. pleuropneumoniae MIDG2331 ΔureC::nadV.
(A) Comparison of plasmid extracts from donor strain MIDG3446 (lane 1), recipient strains E. coli Stellar, M. heamolytica MIDG1579 and A. pleuropneumoniae MIDG2331 ΔureC::nadV (lanes 2-4), and respective florfenicol resistant transformant/transconjugants showing transfer of plasmid pM3446F into E. coli Stellar, M. heamolytica MIDG1579, and A. pleuropneumoniae MIDG2331 ΔureC::nadV (lanes 5-7)
(B) Multiplex PCR amplification of floR (510-bp amplicon) and nadV (1.5 kb amplicon) from A. pleuropneumoniae MIDG3446 (lane 1), E. coli Stellar (lane 2), M. heamolytica MIDG1579 (lane 3), A. pleuropneumoniae MIDG2331 ΔureC::nadV (lane 4), E. coli Stellar + pM3446F (lane 5), M. heamolytica MIDG1579 + pM3446F (lane 6), A. pleuropneumoniae MIDG2331 ΔureC::nadV + pM3446F (lane 7).
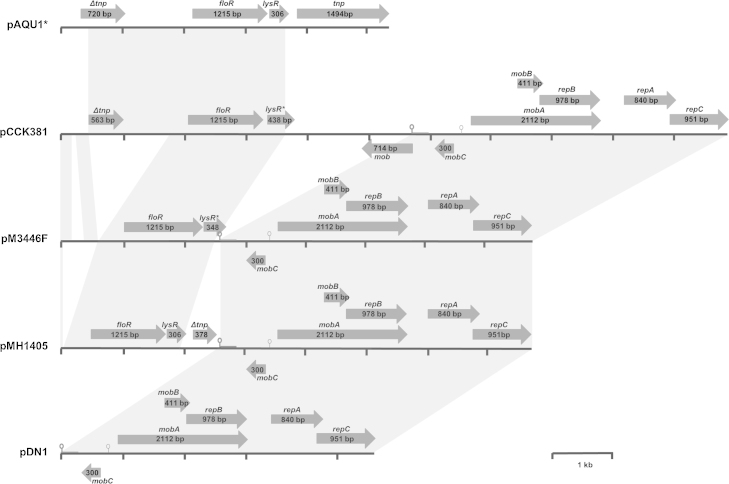
The complete 7,709-bp sequence of pM3446F, isolated from a selected E. coli transformant, was determined using a primer walking strategy. Sequence analysis using BLASTn and BLASTx (http://blast.ncbi.nlm.nih.gov/Blast.cgi) revealed extended similarity to pMh1405 (AB621552), a 7,674-bp plasmid isolated in 2009 from an M. haemolytica strain recovered from a calf in Japan (Katsuda et al., 2012), and to a lesser extent to pCCK381 (AJ871969), a 10,874-bp plasmid isolated in 2005 from a P. multocida strain recovered from a calf in Thirsk, England (Kehrenberg and Schwarz, 2005), and subsequently from bovine and porcine isolates of P. multocida in Germany (Kehrenberg et al., 2008). All three Pasteurellaceae-derived plasmids (Fig. 2) have virtually identical replication (repA-C) and mobilisation genes (mobA-C), as well as oriV and oriT sequences, as those comprising pDN1 (Fig. 2), a 5,112-bp RSF1010-like IncQ broad-host-range plasmid (Y19120) isolated from Dichelobacter nodosus, the causative agent of ovine footrot (Kehrenberg and Schwarz, 2005; Rawlings and Tietze, 2001; Whittle et al., 2000). However, whereas pDN1 does not encode resistance genes, these Pasteurellaceae plasmids contain differing fragments of what was initially described as TnfloR, a transposable element encoding floR, a lysR transcriptional regulator, and the tnpA transposase (Doublet et al., 2005), and subsequently shown to be an ISCR2-floR element (Toleman et al., 2006). Similar sequences have been found in various plasmids and chromosomes of different bacterial species, suggesting recombination and/or deletion events following integration have given rise to different truncated forms of the genes flanking floR (Doublet et al., 2005; Schwarz et al., 2004; Toleman et al., 2006).
Fig. 2.
Schematic comparison of florfenicol resistance plasmid pM3446F from A. pleuropneumoniae with plasmids pAQU1 (from Photobacterium damselae, subsp. damselae), pCCK381 and pMH1405 (from related Pasteurellaceae), and pDN1 (from Dichelobacter nodosus). Note: only 5.3 kb (from bases 138014–143017 of 204052 total) of pAQU1 is shown. Reading frames are indicated by arrows, with arrowheads showing direction of transcription (floR: florfenicol resistance; lysR: transcriptional regulator; lysR*: partial lysR; tnp: transposase; Δtnp: truncated transposase; mob, mobA, mobB, mobC: plasmid mobilisation; repA, repB, repC: plasmid replication). The predicted origins of replication (oriV;  ) and transfer (oriT;
) and transfer (oriT;  ) are shown. Grey blocks between sequences indicate ≥ 98% nucleotide sequence identity. A distance scale in kb is shown.
) are shown. Grey blocks between sequences indicate ≥ 98% nucleotide sequence identity. A distance scale in kb is shown.
The sequences flanking floR in these Pasteurellaceae plasmids show greatest similarity to those found in pAQU1 (AB571865), a 200 kb conjugative plasmid isolated from Photobacterium damselae, subsp. damselae in Japan (Nonaka et al., 2012). In Figure 2, only the sequence around the ISCR2-floR element of pAQU1 is shown (from bases 138014 to 143017 in the Genbank annotated sequence), with the Δtnp gene upstream of floR almost identical to the last 720 bases of the tnp gene downstream. In pCCK381, in addition to the partial ISCR2-floR (99% identity with pAQU1 bases 138160–141381), the 2062 bases comprising the end of the lysR gene to the start of the oriV are 99% identical to bases 146509–148591 of pAQU1. Whereas, upstream of the Δtnp gene in pCCK381, there are sequences 99-100% identity with other regions (bases 148616–148778 and bases 148992–149190) of pAQU1. In pMh1405, the 2.5 kb upstream of oriV shares 99% identity with bases 139426–141904 of pAQU1 (a different deletion fragment of ISCR2-floR than that seen in pCCK381); and in pM3446F no tnp sequences remain (with bases 622–2522 of pM3446F being 99% identical to bases 139426–141904 of pAQU1). An orf downstream of floR in pM3446F shows partial identity with lysR, with the first 223/348 bp being 100% identical to bases 141107–141329, and bases 214–334 of the orf having 98% identity with bases 148514–148633, of pAQU1. In addition, bases 25– 597 of pM3446F are 99% identical to bases 148616–149190 of pAQU1, parts of which are conserved in the sequence upstream of the Δtnp gene in pCCK381. These data suggest that these Pasteurellaceae plasmids share a common origin, with sequences from pAQU1 (or a related plasmid) having integrated into pDN1, and subsequent deletions/rearrangements of the inserted sequences.
Two further Pasteurellaceae floR plasmids have been identified: pCCK1900 (NC_011378), a 10,226-bp plasmid from a porcine isolate of P. multocida in Germany (Kehrenberg et al., 2008) that in encodes resistance to florfenicol (floR), sulphonamide (sul2), and streptomycin (strA, strB); pCCK13698 (NC_007800), a 14,969-bp plasmid from a bovine isolate of Bibersteinia trehalosi in France (Kehrenberg et al., 2006) that encodes resistance to florfenicol (floR), chloramphenicol (catA3) and sulphonamide (sul2). The sequence of pCCK1900 shows simple integration of the floR and lysR genes into an RSF1010 backbone (Kehrenberg et al., 2008). Although pCCK13698 shares some rep and mob gene sequences, as well as the sul2 gene, with RSF1010, it shows more extensive rearrangements with sequences from other plasmids including pHS-rec from H. parasuis (Lancashire et al., 2005) and pMVSCS1 from Mannheimia varigena (Kehrenberg and Schwarz, 2002), and the floR and lysR genes from ISCR2-floR (Kehrenberg et al., 2006). Both pCCK1900 and pCCK13698 appear to have arisen separately from the pDN1-like plasmids described above.
3. Conclusion
In summary, to our knowledge, this is the first report of a complete sequence of a mobilisable florfenicol resistance plasmid from A. pleuropneumoniae. Structural analysis of pM3446F revealed extensive similarity to two florfenicol resistance plasmids found in other members of the Pasteurellaceae, and mating experiments confirmed the ability to mobilise between species. This highlights the importance of continued surveillance of florfenicol susceptibility in A. pleuropneumoniae and other Gram-negative pathogens that may co-exist within the respiratory tract of pigs.
3.1. Funding
This work was supported by a Longer and Larger (LoLa) grant from the Biotechnology and Biological Sciences Research Council (grant numbers BB/G020744/1, BB/G019177/1, BB/G019274/1 and BB/G018553/1), the UK Department for Environment, Food and Rural Affairs, and Zoetis (formerly Pfizer Animal Health) awarded to the Bacterial Respiratory Diseases of Pigs-1 Technology (BRaDP1T) consortium. MTGH was supported by Wellcome Trust grant 98051. The MIC work was funded from the former AHVLA's Research and Development Internal Investment Fund RD0030c . The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
The BRaDP1T Consortium comprises: Duncan J. Maskell, Alexander W. (Dan) Tucker, Sarah E. Peters, Lucy A. Weinert, Jinhong (Tracy) Wang, Shi-Lu Luan, Roy R. Chaudhuri (University of Cambridge; present address for R. Chaudhuri is: Department of Molecular Biology and Biotechnology, University of Sheffield, Firth Court, Western Bank, Sheffield, S10 2TN, UK), Andrew N. Rycroft, Gareth A. Maglennon, Dominic Matthews (Royal Veterinary College); Brendan W. Wren, Jon Cuccui, Vanessa S. Terra (London School of Hygiene and Tropical Medicine); and Paul R. Langford, Janine T. Bossé, Yanwen Li (Imperial College London). We wish to thank Susanna Williamson and Chris Teale from the APHA for their advice and input.
Contributor Information
Janine T Bossé, Email: j.bosse@imperial.ac.uk.
Paul R Langford, Email: p.langford@imperial.ac.uk.
References
- Bossé J.T., Durham A.L., Rycroft A.N., Kroll J.S., Langford P.R. New plasmid tools for genetic analysis of Actinobacillus pleuropneumoniae and other Pasteurellaceae. Appl. Environ. Microbiol. 2009;75:6124–6131. doi: 10.1128/AEM.00809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossé J.T., Soares-Bazzolli D.M., Li Y., Wren B.W., Tucker A.W., Maskell D.J., Rycroft A.N., Langford P.R., BRaDP1T consortium The generation of successive unmarked mutations and chromosomal insertion of heterologous genes in Actinobacillus pleuropneumoniae using natural transformation. PLoS ONE. 2014;9:e111252. doi: 10.1371/journal.pone.0111252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossé J.T., Li Y., Walker S., Atherton T., Fernandez Crespo R., Williamson S.M., Rogers J., Chaudhuri R.R., Weinert L.A., Oshota O., Holden M.T.G., Maskell D.J., Tucker A.W., Wren B.W., Rycroft A.N., Langford P.R. BRaDP1T consortium, 2015. Identification of dfrA 14 in two distinct plasmids conferring trimethoprim resistance in Actinobacillus pleuropneumoniae. J. Antimicrob. Chemother. 2015 doi: 10.1093/jac/dkv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong A., Thomas V., Simjee S., Moyaert H., Garch, El F., Maher K., Morrissey I., Butty P., Klein U., Marion H., Rigaut D., Vallé M. Antimicrobial susceptibility monitoring of respiratory tract pathogens isolated from diseased cattle and pigs across Europe: the vetPath study. Vet. Microbiol. 2014;172:202–215. doi: 10.1016/j.vetmic.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Clinical Laboratory Standards Institute C.L.S.I. CLSI; Wayne, PA: 2008. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals - Third Edition: Approved Standard M31-A3. [Google Scholar]
- Doublet B., Schwarz S., Kehrenberg C., Cloeckaert A. Florfenicol resistance gene floR is part of a novel transposon. Antimicrob. Agents Chemother. 2005;49:2106–2108. doi: 10.1128/AAC.49.5.2106-2108.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency, 2012. Sales of veterinary antimicrobial agents in 19 EU/EEA countries in 2010. (EMA/88728/2012).
- Gutiérrez-Martín C.B., del Blanco N.G., Blanco M., Navas J., Rodríguez-Ferri E.F. Changes in antimicrobial susceptibility of Actinobacillus pleuropneumoniae isolated from pigs in Spain during the last decade. Vet. Microbiol. 2006;115:218–222. doi: 10.1016/j.vetmic.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Hendriksen R.S., Mevius D.J., Schroeter A., Teale C., Jouy E., Butaye P., Franco A., Utinane A., Amado A., Moreno M., Greko C., Stärk K.D., Berghold C., Myllyniemi A.-L., Hoszowski A., Sunde M., Aarestrup F.M. Occurrence of antimicrobial resistance among bacterial pathogens and indicator bacteria in pigs in different European countries from year 2002–2004: the ARBAO-II study. Acta. Vet. Scand. 2008;50:19. doi: 10.1186/1751-0147-50-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuda K., Kohmoto M., Mikami O., Tamamura Y., Uchida I. Plasmid-mediated florfenicol resistance in Mannheimia haemolytica isolated from cattle. Vet. Microbiol. 2012;155:444–447. doi: 10.1016/j.vetmic.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Kehrenberg C., Schwarz S. Plasmid-borne florfenicol resistance in Pasteurella multocida. J. Antimicrob. Chemother. 2005;55:773–775. doi: 10.1093/jac/dki102. [DOI] [PubMed] [Google Scholar]
- Kehrenberg C., Meunier D., Targant H., Cloeckaert A., Schwarz S., Madec J.-Y. Plasmid-mediated florfenicol resistance in Pasteurella trehalosi. J. Antimicrob. Chemother. 2006;58:13–17. doi: 10.1093/jac/dkl174. [DOI] [PubMed] [Google Scholar]
- Kehrenberg C., Mumme J., Wallmann J., Verspohl J., Tegeler R., Kühn T., Schwarz S. Monitoring of florfenicol susceptibility among bovine and porcine respiratory tract pathogens collected in Germany during the years 2002 and 2003. J. Antimicrob. Chemother. 2004;54:572–574. doi: 10.1093/jac/dkh371. [DOI] [PubMed] [Google Scholar]
- Kehrenberg C., Schwarz S. Nucleotide sequence and organization of plasmid pMVSCS1 from Mannheimia varigena: identification of a multiresistance gene cluster. J. Antimicrob. Chemother. 2002;49:383–386. doi: 10.1093/jac/49.2.383. [DOI] [PubMed] [Google Scholar]
- Kehrenberg C., Wallmann J., Schwarz S. Molecular analysis of florfenicol-resistant Pasteurella multocida isolates in Germany. J. Antimicrob. Chemother. 2008;62:951–955. doi: 10.1093/jac/dkn359. [DOI] [PubMed] [Google Scholar]
- Kucerova Z., Hradecka H., Nechvatalova K., Nedbalcova K. Antimicrobial susceptibility of Actinobacillus pleuropneumoniae isolates from clinical outbreaks of porcine respiratory diseases. Vet. Microbiol. 2011;150:203–206. doi: 10.1016/j.vetmic.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Lancashire J.F., Terry T.D., Blackall P.J., Jennings M.P. Plasmid-encoded Tet B tetracycline resistance in Haemophilus parasuis. Antimicrob. Agents Chemother. 2005;49:1927–1931. doi: 10.1128/AAC.49.5.1927-1931.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka L., Maruyama F., Miyamoto M., Miyakoshi M., Kurokawa K., Masuda M. Novel conjugative transferable multiple drug resistance plasmid pAQU1 from Photobacterium damselae subsp. damselae isolated from marine aquaculture environment. Microbes Environ. 2012;27:263–272. doi: 10.1264/jsme2.ME11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe S., Schwarz S. In vitro activities of florfenicol against bovine and porcine respiratory tract pathogens. Antimicrob. Agents Chemother. 2003;47:2703–2705. doi: 10.1128/AAC.47.8.2703-2705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings D.E., Tietze E. Comparative biology of IncQ and IncQ-like plasmids. Microbiol. Mol. Biol. Rev. 2001;65:481–496. doi: 10.1128/MMBR.65.4.481-496.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S., Kehrenberg C., Doublet B., Cloeckaert A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004;28:519–542. doi: 10.1016/j.femsre.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Shin S.J., Kang S.G., Nabin, Kang R., Yoo M.L., HS Evaluation of the antimicrobial activity of florfenicol against bacteria isolated from bovine and porcine respiratory disease. Vet. Microbiol. 2005;106:73–77. doi: 10.1016/j.vetmic.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Toleman M.A., Bennet P.M., Walsh T.R. ISCR Elements: novel gene-capturing systems of the 21st century. Micriobiol. Mol. Biol. Rev. 2006;70:296–316. doi: 10.1128/MMBR.00048-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanni M., Merenda M., Barigazzi G., Garbarino C., Luppi A., Tognetti R., Intorre L. Antimicrobial resistance of Actinobacillus pleuropneumoniae isolated from swine. Vet. Microbiol. 2012;156:172–177. doi: 10.1016/j.vetmic.2011.10.022. [DOI] [PubMed] [Google Scholar]
- Whittle G., Katz M.E., Clayton E.H., Cheetham B.F. Identification and characterization of a native Dichelobacter nodosus plasmid, pDN1. Plasmid. 2000;43:230–234. doi: 10.1006/plas.1999.1456. [DOI] [PubMed] [Google Scholar]
- Yoo A.N., Cha S.B., Shin M.K., Won H.K., Kim E.H., Choi H.W., Yoo H.S. Serotypes and antimicrobial resistance patterns of the recent Korean Actinobacillus pleuropneumoniae isolates. Vet. Rec. 2014;174:223. doi: 10.1136/vr.101863. [DOI] [PubMed] [Google Scholar]