Abstract
β-thalassemia is the most common single gene disorder worldwide, in which hemoglobin β-chain production is decreased. Today, the life expectancy of thalassemic patients is increased because of a variety of treatment methods; however treatment related complications have also increased. The most common side effect is osteoporosis, which usually occurs in early adulthood as a consequence of increased bone resorption. Increased bone resorption mainly results from factors such as delayed puberty, diabetes mellitus, hypothyroidism, ineffective hematopoiesis as well as hyperplasia of the bone marrow, parathyroid gland dysfunction, toxic effect of iron on osteoblasts, growth hormone (GH) and insulin-like growth factor-1 (IGF-1) deficiency. These factors disrupt the balance between osteoblasts and osteoclasts by interfering with various molecular mechanisms and result in decreased bone density.
Given the high prevalence of osteopenia and osteoporosis in thalassemic patients and complexity of their development process, the goal of this review is to evaluate the molecular aspects involved in osteopenia and osteoporosis in thalassemic patients, which may be useful for therapeutic purposes.
Keywords: β-thalassemia, Bone Resorption, Bone Marrow, Osteoblasts, Osteoclasts
Introduction
β-thalassemia is the most common single gene disorder worldwide, in which synthesis of the β-globin chain is decreased, leading to ineffective erythropoiesis (1, 2). Frequent blood transfusions increase the life expectancy of thalassemic patients. However osteopenia and osteoporosis are significant complications that contribute to morbidity of these patients. These two complications are observed in approximately 50% of β-thalassemia patients (3). Subsequent to this complication, bone fracture is noted in 36% of thalassemic patients (4). Osteopenia and osteoporosis are detected by the presence of reduced bone mineral density (BMD), reflecting decreased bone turnover (4).
In thalassemia, osteoporosis is a complicated process affected by several factors. The most important factors for osteoporosis in thalassemia included elayed puberty, diabetes mellitus, hypothyroidism, ineffective hematopoiesis with bone marrow hyperplasia, parathyroid gland dysfunction, toxic effect of iron on osteoblasts and deficiency of growth hormone/insulin-like growth factor-1 (GH/IGF-1). In general, decreased bone density and osteoporosis are the result of a disrupted balance between osteoblasts and osteoclasts (5, 6).
Osteoblasts
Osteoblasts originate from mesenchymal stem cells (MSCs). Their production is increased by transforming growth factor-beta (TGF-β), basic fibroblast growth factor (bFGF) and bone morphogenetic protein (BMP) (7-9). These cells secrete macrophagecolony stimulating factor (M-CSF), granulocyte macrophage-CSF (GM-CSF), interleukin-1 (IL-1), IL-6 and TGF-β (10). These cytokines are also involved in bone formation because they release alkaline phosphatase (ALP), osteopontin, osteocalcin, collagen and fibronectin (4, 7, 11).
Osteoclasts
These are multinucleate cells that originate from hematopoietic stem cells (HSC) under the effect of MCSF and the receptor activator of nuclear factor κB (NF-κB) ligand (RANKL), causing bone resorption by secretion of matrix metalloproteinase and cathepsin (4, 7, 12, 13).
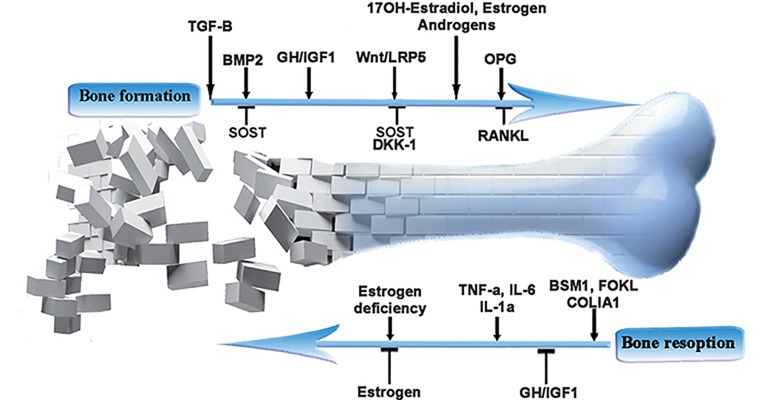
The RANK/RANKL cytokine system, parathyroid hormone (PTH), sex hormones (such as estrogen and testosterone), inflammatory cytokines, GH/ IGF-1, BMP2 protein as well as the wingless related protein/β-catenin (Wnt/β-catenin) signaling pathway and iron deposition in the bone marrow are the most common factors that affect the balance between these two cell types (14, 15) (Fig.1).
Fig.1.
Important molecules involved in bone resorption in thalassemia. TGF-β; Transforming growth factor-beta, SOST; Sclerostin, DKK-1; Dickkopf, BMP2; Bone morphogenetic protein-2, GH; Growth hormone, IGF-1; Insulin-like growth factor-1, Wnt; Wingless related protein, LRP5; Low density lipoprotein (LDL)-related protein 5, OPG; Osteoprotegrin, RANKL; Activator of NF-κB receptor ligand, TNF-α; Tumor necrotic factor-alpha, IL-6; Interlukin-6, IL-1α; Interlukin-1 alpha and COLIA1; Collagen type I alpha 1 gene.
Cytokine system
Osteoprotegrin/RANKL/RANK
This cytokine system is among the most effective mechanisms in bone reabsorption, which regulates bone density by several factors explained later (16) (Table 1). RANKL has three isoforms and its soluble form is secreted from osteoblasts. RANKL binding to its receptor on precursors and mature osteoclasts triggers the NF-κB pathway, resulting in differentiation, activation and survival of osteoclasts as well as bone turnover (17, 18). Osteoprotegrin (OPG), an antagonist of RANKL and member of the tumor necrosis factor (TNF) receptor superfamily, is secreted by osteoblasts and prevents the differentiation and activity of osteoclasts (14, 19-22).
Table 1.
Overview of molecular mechanisms in bone resorption in thalassemia
| Factors | Target (pathway/gene) | Role |
|---|---|---|
| PTH | PKA activation and down regulation ofOPG/RANKL ratio | Osteoclast activation |
| 17OH-estradiol | Down regulation of JNK pathway onRANKL downstream | Reduction of osteoclast differentiation |
| Estrogen andtestosterone | Influence on OPG and RANKL mRNA | Up regulation of OPG/RANKL ratio |
| Estrogen | RUNX2 activation | Osteoblastic differentiation |
| Fasl activation | Osteoclast apoptosis | |
| IL-1α, IL-6, TNF-α | Initiation of the NF-κB pathway | Increased osteoclast differentiationand activation |
| TGF-β | Activation of Smad pathway andinduced RUNX2 production | Osteoblast differentiation |
| IGF-1 | Increased OPG, collagen type I, RUNX2,ALP production in HMSC | Osteoblastic differentiation |
| GH | Increased OPG | Inhibition of osteoclasto-genesis |
| Increased BMP2 | Induction of osteoblastic differentiation | |
| BMP2 | Elevated β-catenin level thatresults in CBF-α transcription | Osteoblasticdifferentiation |
| RUNX2 | Wnt canonical pathway | Osteoblastic differentiation |
| Wnt/βcateninsignaling pathway | β-catenin stabilization | Osteoblastic differentiation |
| Up regulation of OPG/RANKL ratio | Reduction of osteoclastic differentiation | |
| DKK-1 | Antagonizes canonical Wnt signaling byinhibiting LRP5/6 interaction with Wnt | Inhibition of osteoblastic differentiation |
| SOST | Inhibition of Wnt signaling | Inhibition of osteoblastic differentiation |
PTH; Parathyroid hormone, PKA; Protein kinase A, TGF-β; Transforming growth factor-beta, SOST; Sclerostin, DKK-1; Dickkopf, BMP2; Bone morphogenetic protein 2, GH; Growth hormone, IGF-1; Insulin-like growth factor 1, Wnt; Wingless related protein, LRP5; Low density lipoprotein (LDL)-related protein 5, OPG; Osteoprotegrin, RANX2; Runt-related transcription factor 2, JNK; Janus kinase, ALP; alkaline phosphatase, HMSC; Human mesenchymal stem cells, NF-κB; Nuclear factor kappa B, RANKL; Activator of NF-κB receptor ligand, TNF-α; Tumor necrotic factor-alpha, IL-6; Interlukin-6 and IL-1α; Interlukin-1 alpha.
In patients with thalassemia, toxicity of iron for osteoblasts along with endocrine effects increase RANKL and decrease OPG, thereby increasing the risk of osteoporosis (23, 24). In chronic diseases, disorders of iron deposition in body organs, endocrine disorders, malignant bone tumors and rheumatoid arthritis disrupt the OPG/RANKL balance which results in inhibition of bone reabsorption (25). Therefore, antibodies against OPG and RANKL (which have an inhibitory effect on osteoclasts) are used in the treatment of bone complications (4, 5).
Inflammatory cytokines
Increased levels of inflammatory cytokines such as IL-1α, IL-6 and TNF-α (due to iron overload) (3, 10, 11) in the serum of thalassemic patients is inversely related to their bone density (26). The sepro-osteoclastogenic cytokines exert their effects predominantly via the OPG/RANKL system (3, 27).Thus, IL-6, IL-1α and TNF-α induce cyclooxygenase2 (COX2) and prostaglandin E2 (PGE2) which cause an increase in RANKL and a decrease in OPG, resulting in increased bone resorption (3, 19, 28). Moreover, these cytokines trigger the NF-κB and Janus kinase (JNK) pathways, ultimately increasing activation and differentiation of osteoclasts (10). IL-6 and TNF-α are also involved in pathogenesis of bone resorption in acute abdominal disease, rheumatoid arthritis and menopause-associated osteoporosis (14). Anti-TNF-α is used to improve bone metabolism in patients with rheumatoid arthritis (29).
Transforming growth factor-beta
Local reduction of TGF-β in the bone marrow is a likely risk factor of osteoporosis in patients with thalassemia (26). TGF-β is a type of receptor tyrosine kinase that phosphorylates Smad and induces the production of Runt-related transcription factor 2 (RUNX2) in mesenchymal precursors, resulting in osteoblastic differentiation in vitro (30, 31). TGF-β causes the death of osteoclasts by reducing the activity of C-JUN factorin the RANKL pathway (7, 32). Most cytokines have paracrine effects. The majority of studies have found no correlation between their circulating concentrations and bone resorption markers (33). Therefore, the best method is to analyze these cytokines in the tissues.
Bone morphogenetic protein 2
Expression of BMP2 is decreased in thalassemic patients with osteoporosis (34). BMP2 is acytokine from the TGF-β family involved in commitment of mesenchymal precursors to osteoblasts. Local production of BMP2 and TGF-β is associated with increased proliferation and differentiation of osteoblasts (20). BMP2 binding with serine/threonine kinase receptors on the cell surface phosphorylates Smad complex and activates transcription factors effective in osteoblastic differentiation such as RUNX2 and Ostrix (35-37). BMP2 also increases RUNX2 gene transcription by increasing the level of β-catenin, thereby differentiating mesenchymal cells to osteoblasts (38, 39). In addition, BMP2 plays a role in Ostrix activation and mesenchymal differentiation to osteoblasts by activating JNK and P38 factors and triggering the mitogenactivated protein kinase (MAPK) cascade (40-42). P53 inhibits BMP2 and exerts its inhibitory effect by suppressing Ostrix (43).
Endocrine disorders
Growth hormone/insulin-like growth factor-1
Disruption of the GH/IGF-1 pathway is another mechanism in reducing bone density in thalassemic patients (5). GH stimulates the liver to secrete IGF- 1. Both hormones have an anabolic role in the bone marrow (16). IGF-1 is mainly released in the liver and GH in the anterior pituitary. In thalassemic patients, iron toxicity for the liver and anterior pituitary possibly reduce serum levels of IGF-1 and GH, respectively (16, 44). However, IGF-1 deficiency is prominently caused by hepatitis C virus (HCV) infection in these patients (45). IGF-1 increases the level of OPG, type I collagen, RUNX2 and ALP in human MSCs (hMSCs) (46) along with inducing the expression of Ostrix (via the MAPK pathway), which result in osteoblastic differentiation. Therefore, there is a positive relationship between the level of IGF-1 and BMD in thalassemic patients (47). According to research, reduction of IGF-1 plays a role in glucocorticoid-induced osteoporosis (48). GH stimulates the production of BMP and OPG, causing increased proliferation of osteoblasts and inhibition of osteoclast production, respectively (6, 8). GH deficiency has been reported in only 8% of β-thalassemic patients, and is mainly caused by iron overload. In contrast, IGF-1 production is impaired in 72% of patients (45). As a result, introduction of these hormones in thalassemic patients with hormone deficiency is recommended to prevent osteoporosis (3).
Parathyroid hormone
Long-term increase in PTH causes reduction of OPG/RANKL by activating osteoblastic protein kinase A (PKA), thereby increasing the activity of osteoclasts (49, 50).
Sex hormones
A reduced level of sex hormones in thalassemic men with hypogonadism and postmenopausal women can cause osteoporosis. In thalassemic patients, iron deposition in the anterior pituitary disrupts the release of sex hormones and delays puberty in 50% of patients (6, 15).
17OH-estradiol binds its alpha receptor on osteoclasts, decreasing the activity of JNK downstream of RANKL and inducing the production of OPG which results in inhibition of osteoclasts. Therefore, there is a strong correlation between 17OH-estradiol and serum concentrations of OPG and RANKL in thalassemic patients (6, 12, 18). Free estrogen and testosterone in thalassemic patients increase OPG mRNA and decrease RANKL (6, 8, 50). Estrogen also binds the alpha receptor on osteoblasts and osteoclasts, activating RUNX2 in osteoblasts and Fas ligand (Fasl) inosteoclasts, which results in increased osteoblastic differentiation and death of osteoclasts (21, 51-54). In addition, androgens and estrogens regulate resorption in bone by regulating cytokines secreted by osteoblasts and stromal cells such as IL1-α, IL-6, TGF-β and PGE2, which control the activity of osteoclasts through paracrine effects (33). Hormone therapy is an approach to prevent osteoporosis in thalassemic patients (3).
Transcription factors
Runt-related transcription factor 2
RUNX2 is an early transcription factor in osteoblastic differentiation. This factor is decreased in thalassemic patients affected by iron deposition in the bone marrow (55). RUNX2 prevents differentiation of MSCs into adipocytes and chondrocytes through the wingless related protein canonical pathway (Wnt-1 pathway), and plays an important role in osteoblastic differentiation and bone formationby increasing BMP2 and induction of Ostrix expression (54, 56).
Ostrix
Reduction of Ostrix in thalassemia is associated with decreased BMD, and it is involved in the pathogenesis of osteoporosis in thalassemic patients (34). It is an essential transcription factor for differentiation of osteoblasts, which is activated by IGF-1, TGF-β and RUNX2, resulting in osteoblastic differentiation (40, 57).
Wnt/β-catenin signaling pathway proteins
Wnt proteins play an important role in regulating bone massby affecting osteoblastic maturation and activity. Wnt protein binding with Frizzled (Fz) receptor (a member of the G protein coupled receptors) and LDL related protein co-receptor (LRP) results in signal transduction, stability of β-catenin and its transfer to the nucleus, and eventual transcription of genes associated with osteoblastic differentiation (58-60). Wnt proteins increase OPG/RANKL through the β-catenin dependent canonical pathway (Wnt3a) in osteoblasts, causing an increase in osteoblastic differentiation and suppression of osteoclast production (17, 18, 51).
Dickkopf
DKK-1 is increased in serum of thalassemic patients who have osteoporosis (6), and is associated with reduced BMD in the lumbar vertebrae and end of the radius (59). Secreted molecular DKK-1 has a cysteine-rich domain at its carboxyl end, which binds LRP5/LRP6 co-receptors and inhibits Wnt binding with these cofactors, preventing osteoblastic differentiation as an antagonist of the canonical Wnt pathway (40, 61, 62). In addition, serum DKK-1 is increased in multiple myeloma (MM) patients with lytic bone lesions, menopause induced osteoporosis, Paget’s disease, glucocorticoid induced osteoporosis and estrogen deficiency (39, 63-65). Anti-DKK-1is used to treat bone loss in patients with MM (66).
Sclerostin
This factor is involved in the incidence of osteoporosis in thalassemic patients. Increased serum levels of this molecule are associated with reduced BMD in thalassemic patients (59). Sclerostin is a secretory molecule and product of the SOST gene which antagonizes LRP4, LRP5 and LRP6 co-receptors, resulting in inhibition of the Wnt canonical pathway and differentiation of osteoblasts (46, 62, 67) (Table 1). Sclerostin is also anantagonist of BMP2 (68), which is increased in MM (in which it is released by plasma cells) and in cancer-induced bone loss (69). Anti-sclerostin is used to treat menopause-related osteoporosis (59).
Genetic factors
Genetic factors play an important role in reduction of bone density and development of osteoporosis in thalassemia (Table 2). One factor is polymorphism in the SP1 region of the collagen type I alpha 1 gene (COLIA1), which has an incidence of 90% in thalassemic patients (14, 20). However, it plays no role in osteoporosis in sickle cell patients (70). Polymorphisms in the gene region of vitamin D such as BSM1 (in intron 8) and FOKL (in exon 2) are also associated with reduced bone density in patients with thalassemia and sickle cell anemia (71, 72).
Table 2.
Genetic factors involved in osteoporosis in thalassemia
| COLIA-1 polymorphism | Down regulation in procollagen production | |
|---|---|---|
| Genetic factors | BSM1 polymorphism | |
| FOKL polymorphism | Down regulation in vitamin D absorption | |
COLIA-1; Collagen type 1 alpha 1.
Discussion
Although increased osteoclastogenesis and in adequate osteoblastogenesis can cause an imbalance between bone formation and resorption with a possible decrease in BMD (7), osteoporosis in thalassemic patients is a complicated process influenced by multiple genetic and acquired factors. These factors not only affect the proliferation and activity of osteoblasts and osteoclasts, some such as gastrointestinal absorption disorder play an important role in providing the resources necessary for bone formation. In addition, the person’s age, nutritional and physiological conditions are effective in development of bone lesions. Therefore, the presence of a single factor cannot be considered a risk factor for osteoporosis in patients with thalassemia. Rather, a variety of factors must be examined together. In addition, as an osteoporotic skeleton has not been reported to be restored to healthy status in thalassemia, prevention of osteoporosis is of utmost importance in managing β-thalassemic patients. Considering the fact that bone resorption is caused by multiple factors, healthcare vigilance in these patients should be multifactorial. Prescription of calcium can provide adequate calcium levels during skeleton development and can increase bone mass (73-75). Moreover, prescription of vitamin D supplements plays an important role in this process (74, 76). Therefore, thalassemic patients should observe a proper diet as part of their preventive program. Early hormone replacement is the most effective strategy to prevent gonadal deficiency-induced bone loss (77, 78).
In clinical trials, novel therapeutic agents are very promising treatments for bone diseases. These agents include calcitonin and bisphosphonates. Calcitonin is a hormone secreted by the thyroid that inhibits osteoclastic activity. It causes bone pain relief and radiographic improvement after a one year administration in thalassemic patients (79). Also, bisphosphonates inhibit bone resorption and are as beneficial as estrogen, in preventing loss of bone mass (80). Anti-DKK-1 has a pivotal role in bone health for management of bone lesions in MM patients and is a novel therapeutic agent for these patients. However, the clinical trial role of anti-DKK-1 in thalassemic patients should be elucidated (81). Further studies are required to evaluate its effect as well as that of anti-sclerostin on β-thalassemic patients.
Conclusion
This review has discussed a number of genetic and acquired factors that affect bone density in patients with thalassemia. Considering the above factors, hormone therapy, optimal transfusion (preventing precipitation of iron), calcium and vitamin D prescription can be effective in preventing bone lesions.
Given the high prevalence of musculoskeletal disorders in patients with thalassemia and considering the fact that osteopenia and osteoporosis are progressive disorders in these patients, early screening and preventive intervention are of utmost importance. In addition, annual bone density screenings in these patients is recommended. Although the factors mentioned in this article can be important to manage this process, further research in this field is needed.
Acknowledgments
We wish to thank our colleagues at Shafa Hospital and the Research Center of Thalassemia and Hemoglobinopathy of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The authors declare no conflict of interest.
References
- 1.Chen FE, Ooi C, Ha SY, Cheung BM, Todd D, Liang R, et al. Genetic and clinical features of hemoglobin H disease in Chinese patients. N Engl J Med. 2000;343(8):544–550. doi: 10.1056/NEJM200008243430804. [DOI] [PubMed] [Google Scholar]
- 2.Ehteram H, Bavarsad MS, Mokhtari M, Saki N, Soleimani M, Parizadeh SM, et al. Prooxidant-antioxidant balance and hs-CRP in patients with beta-thalassemia major. Clin Lab. 2014;60(2):207–215. doi: 10.7754/clin.lab.2013.130132. [DOI] [PubMed] [Google Scholar]
- 3.Haidar R, Musallam KM, Taher AT. Bone disease and skeletal complications in patients with β thalassemia major. Bone. 2011;48(3):425–432. doi: 10.1016/j.bone.2010.10.173. [DOI] [PubMed] [Google Scholar]
- 4.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 5.Terpos E, Voskaridou E. Treatment options for thalassemia patients with osteoporosis. Ann N Y Acad Sci. 2010;1202:237–243. doi: 10.1111/j.1749-6632.2010.05542.x. [DOI] [PubMed] [Google Scholar]
- 6.Voskaridou E, Christoulas D, Xirakia C, Varvagiannis K, Boutsikas G, Bilalis A, et al. Serum Dickkopf-1 is increased and correlates with reduced bone mineral density in patients with thalassemia-induced osteoporosis.Reduction post-zoledronic acid administration. Haematologica. 2009;94(5):725–728. doi: 10.3324/haema-tol.2008.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling.Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332(5):305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 8.Komori T. Regulation of osteoblast differentiation by Runx2. Adv Exp Med Biol. 2010;658:43–49. doi: 10.1007/978-1-4419-1050-9_5. [DOI] [PubMed] [Google Scholar]
- 9.Azizidoost S, Babashah S, Rahim F, Shahjahani M, Saki N. Bone marrow neoplastic niche in leukemia. Hematology. 2014;19(4):232–238. doi: 10.1179/1607845413Y.0000000111. [DOI] [PubMed] [Google Scholar]
- 10.Riggs BL. The mechanisms of estrogen regulation of bone resorption. J Clin Invest. 2000;106(10):1203–1204. doi: 10.1172/JCI11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, et al. PDGF, TGF-β, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112(2):295–307. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi M, Hayashi M, Fujita S, Yoshida T, Utsunomiya T, Yamamoto H, et al. Low-energy laser irradiation facilitates the velocity of tooth movement and the expressions of matrix metalloproteinase-9, cathepsin K, and alpha (v) beta (3) integrin in rats. Eur J Orthod. 2010;32(2):131–139. doi: 10.1093/ejo/cjp078. [DOI] [PubMed] [Google Scholar]
- 13.Saba F, Soleimani M, Atashi A, Mortaz E, Shahjahani M, Roshandel E, et al. The role of the nervous system in hematopoietic stem cell mobilization. Lab Hematol. 2013;19(3):8–16. doi: 10.1532/LH96.12013. [DOI] [PubMed] [Google Scholar]
- 14.Saki N, Abroun S, Farshdousti Hagh M, Asgharei F. Neoplastic bone marrow niche: hematopoietic and mesenchymal stem cells. Cell J. 2011;13(3):131–136. [PMC free article] [PubMed] [Google Scholar]
- 15.Weinberg ED. Role of iron in osteoporosis. Pediatr Endocrinol Rev. 2008;6(Suppl 1):81–85. [PubMed] [Google Scholar]
- 16.Toumba M, Skordis N. Osteoporosis syndrome in thalassaemia major: an overview. J osteoporos. 2010;2010:537673–537673. doi: 10.4061/2010/537673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srivastava S, Toraldo G, Weitzmann MN, Cenci S, Ross FP, Pacifici R. Estrogen decreases osteoclast formation by down-regulating receptor activator of NF-kappa B ligand (RANKL)-induced JNK activation. J Biol Chem. 2001;276(12):8836–8840. doi: 10.1074/jbc.M010764200. [DOI] [PubMed] [Google Scholar]
- 18.Pietrapertosa AC, Minenna G, Colella SM, Santeramo TM, Renni R, DAmore M. Osteoprotegerin and RANKL in the pathogenesis of osteoporosis in patients with thalassaemia major. Panminerva Med. 2009;51(1):17–23. [PubMed] [Google Scholar]
- 19.Perifanis V, Vyzantiadis T, Vakalopoulou S, Tziomalos K, Garypidou V, Athanassiou-Metaxa M, et al. Treatment of beta-thalassaemia-associated osteoporosis with zoledronic acid. Br J Haematol. 2004;125(1):91–92. doi: 10.1111/j.1365-2141.2004.04871.x. [DOI] [PubMed] [Google Scholar]
- 20.Voskaridou E, Terpos E. New insights into the pathophysiology and management of osteoporosis in patients with beta thalassaemia. Br J Haematol. 2004;127(2):127–139. doi: 10.1111/j.1365-2141.2004.05143.x. [DOI] [PubMed] [Google Scholar]
- 21.Waalen J. Current and emerging therapies for the treatment of osteoporosis. J Exp Pharmacol. 2010;2:121–134. doi: 10.2147/JEP.S7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abroun S, Saki N, Fakher R, Asghari F. Biology and bioinformatics of myeloma cell. Lab Hematol. 2012;18(4):30–41. doi: 10.1532/LH96.11003. [DOI] [PubMed] [Google Scholar]
- 23.Morabito N, Gaudio A, Lasco A, Atteritano M, Pizzoleo MA, Cincotta M, et al. Osteoprotegerin and RANKL in the pathogenesis of thalassemia-induced osteoporosis: new pieces of the puzzle. J Bone Miner Res. 2004;19(5):722–727. doi: 10.1359/JBMR.040113. [DOI] [PubMed] [Google Scholar]
- 24.Salah H, Atfy M, Fathy A, Atfy M, Mansor H, Saeed J. The clinical significance of OPG/sRANKL ratio in thalassemia patients suffering from osteopenia or osteoporosis in Egyptian patients. Immunol Invest. 2010;39(8):820–832. doi: 10.3109/08820139.2010.498492. [DOI] [PubMed] [Google Scholar]
- 25.Skordis N, Toumba M. Bone disease in thalassaemia major: recent advances in pathogenesis and clinical aspects. Pediatr Endocrinol Rev. 2011;8(Suppl 2):300–306. [PubMed] [Google Scholar]
- 26.Salsaa B, Zoumbos NC. A distinct pattern of cytokine production from blood mononuclear cells in multitransfused patients with beta-thalassaemia. Clin Exp Immunol. 1997;107(3):589–592. doi: 10.1046/j.1365-2249.1997.d01-962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morabito N, Russo GT, Gaudio A, Lasco A, Catalano A, Morini E, et al. The "lively" cytokines network in beta-thalassemia major-related osteoporosis. Bone. 2007;40(6):1588–1594. doi: 10.1016/j.bone.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Park YG, Kang SK, Kim WJ, Lee YC, Kim CH. Effects of TGF-β, TNF-α, IL-β and IL-6 alone or in combination, and tyrosine kinase inhibitor on cyclooxygenase expression, prostaglandin E2 production and bone resorption in mouse calvarial bone cells. Int J Biochem Cell Biol. 2004;36(11):2270–2280. doi: 10.1016/j.biocel.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 29.Kastelan D, Kastelan M, Massari LP, Korsic M. Possible association of psoriasis and reduced bone mineral density due to increased TNF-alpha and IL-6 concentrations. Med Hypotheses. 2006;67(6):1403–1405. doi: 10.1016/j.mehy.2006.04.069. [DOI] [PubMed] [Google Scholar]
- 30.Lee KS, Kim HJ, Li QL, Chi XZ, Ueta C, Komori T, et al. Runx2 is a common target of transforming growth factor β1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal Precursor cell line C2C12. Mol Cell Biol. 2000;20(23):8783–8792. doi: 10.1128/mcb.20.23.8783-8792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee KS, Hong SH, Bae SC. Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein. Oncogene. 2002;21(47):7156–7163. doi: 10.1038/sj.onc.1205937. [DOI] [PubMed] [Google Scholar]
- 32.Khosla S, Riggs BL. Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin North Am. 2005;34(4):1015–1030. doi: 10.1016/j.ecl.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 33.McLean RR. Proinflammatory cytokines and osteoporosis. Curr Osteoporos Rep. 2009;7(4):134–139. doi: 10.1007/s11914-009-0023-2. [DOI] [PubMed] [Google Scholar]
- 34.Tanno T, Porayette P, Sripichai O, Noh SJ, Byrnes C, Bhupatiraju A, et al. Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood. 2009;114(1):181–186. doi: 10.1182/blood-2008-12-195503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita K, Janz S. Attenuation of WNT signaling by DKK-1 and-2 regulates BMP2-induced osteoblast differentiation and expression of OPG, RANKL and M-CSF. Mol Cancer. 2007;6:71–71. doi: 10.1186/1476-4598-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423(6937):349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 37.Jeon EJ, Lee KY, Choi NS, Lee MH, Kim HN, Jin YH, et al. Bone morphogenetic protein-2 stimulates Runx2 acetylation. J Biol Chem. 2006;281(24):16502–16511. doi: 10.1074/jbc.M512494200. [DOI] [PubMed] [Google Scholar]
- 38.Weinstein RS, Manolagas SC. Apoptosis and osteoporosis. Am J Med. 2000;108(2):153–164. doi: 10.1016/s0002-9343(99)00420-9. [DOI] [PubMed] [Google Scholar]
- 39.Anastasilakis AD, Polyzos SA, Avramidis A, Toulis KA, Papatheodorou A, Terpos E. The effect of teriparatide on serum Dickkopf-1 levels in postmenopausal women with established osteoporosis. Clin Endocrinol (Oxf) 2010;72(6):752–757. doi: 10.1111/j.1365-2265.2009.03728.x. [DOI] [PubMed] [Google Scholar]
- 40.Celil AB, Campbell PG. BMP-2 and insulin-like growth factor-I mediate Osterix (Osx) expression in human mesenchymal stem cells via the MAPK and protein kinase D signaling pathways. J Biol Chem. 2005;280(36):31353–31359. doi: 10.1074/jbc.M503845200. [DOI] [PubMed] [Google Scholar]
- 41.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127(6 Pt 1):1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee MH, Kwon TG, Park HS, Wozney JM, Ryoo HM. BMP-2-induced Osterix expression is mediated by Dlx5 but is independent of Runx2. Biochem Biophys Res Commun. 2003;309(3):689–694. doi: 10.1016/j.bbrc.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Kua HY, Hu Y, Guo K, Zeng Q, Wu Q, et al. p53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J Cell Biol. 2006;172(1):115–125. doi: 10.1083/jcb.200507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vidergor G, Goldfarb AW, Glaser B, Dresner-Pollak R. Growth hormone reserve in adult beta thalassemia patients. Endocrine. 2007;31(1):33–37. doi: 10.1007/s12020-007-0018-7. [DOI] [PubMed] [Google Scholar]
- 45.Pincelli A, Masera N, Tavecchia L, Perotti M, Perra S, Mariani R, et al. GH deficiency in adult B-thalassemia major patients and its relationship with IGF-1 production. Pediatr Endocrinol Rev. 2011;8(Suppl 2):284–289. [PubMed] [Google Scholar]
- 46.Koch H, Jadlowiec JA, Campbell PG. Insulin-like growth factor-I induces early osteoblast gene expression in hu man mesenchymal stem cells. Stem Cells Dev. 2005;14(6):621–631. doi: 10.1089/scd.2005.14.621. [DOI] [PubMed] [Google Scholar]
- 47.Merchant R, Udani A, Puri V, Dcruz V, Patkar D, Karkera A. Evaluation of osteopathy in thalassemia by bone mineral densitometry and biochemical indices. Indian J Pediatr. 2010;77(9):987–991. doi: 10.1007/s12098-010-0158-2. [DOI] [PubMed] [Google Scholar]
- 48.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115(12):3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hofbauer L, Kuhne C, Viereck V. The OPG/RANKL/RANK system in metabolic bone diseases. J Musculoskelet Neuronal Interact. 2004;4(3):268–275. [PubMed] [Google Scholar]
- 50.Szulc P, Hofbauer LC, Heufelder AE, Roth S, Delmas PD. Osteoprotegerin serum levels in men: correlation with age, estrogen, and testosterone status. J Clin Endocrinol Metab. 2001;86(7):3162–3165. doi: 10.1210/jcem.86.7.7657. [DOI] [PubMed] [Google Scholar]
- 51.Khalid O, Baniwal SK, Purcell DJ, Leclerc N, Gabet Y, Stallcup MR, et al. Modulation of Runx2 activity by estrogen receptor-alpha: implications for osteoporosis and breast cancer. Endocrinology. 2008;149(12):5984–5995. doi: 10.1210/en.2008-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trivedi R, Goswami R, Chattopadhyay N. Investigational anabolic therapies for osteoporosis. Expert Opin Investig Drugs. 2010;19(8):995–1005. doi: 10.1517/13543784.2010.501077. [DOI] [PubMed] [Google Scholar]
- 53.Gudjonsson JE, Johnston A, Sigmundsdottir H, Valdimarsson H. Immunopathogenic mechanisms in psoriasis. Clin Exp Immunol. 2004;135(1):1–8. doi: 10.1111/j.1365-2249.2004.02310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Komori T. Regulation of bone development and maintenance by Runx2. Front Biosci. 2008;13:898–903. doi: 10.2741/2730. [DOI] [PubMed] [Google Scholar]
- 55.Doyard M, Fatih N, Monnier A, Island ML, Aubry M, Leroyer P, et al. Iron excess limits HHIPL-2 gene expression and decreases osteoblastic activity in human MG-63 cells. Osteoporos Int. 2012;23(10):2435–2445. doi: 10.1007/s00198-011-1871-z. [DOI] [PubMed] [Google Scholar]
- 56.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473(2):139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGee S, Havens AM, Shiozawa Y, Jung Y, Taichman RS. Effects of erythropoietin on the bone microenvironment. Growth Factors. 2012;30(1):22–28. doi: 10.3109/08977194.2011.637034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 59.Voskaridou E, Christoulas D, Plata E, Bratengeier C, Anastasilakis AD, Komninaka V, et al. High circulating sclerostin is present in patients with thalassemia-associated osteoporosis and correlates with bone mineral density. Horm Metab Res. 2012;44(12):909–913. doi: 10.1055/s-0032-1312618. [DOI] [PubMed] [Google Scholar]
- 60.Rawadi G, Vayssiere B, Dunn F, Baron R, Roman-Roman S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res. 2003;18(10):1842–1853. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- 61.Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11(12):951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 62.Semenov M, Tamai K, He X. SOST is a ligand for LRP5/ LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280(29):26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 63.Daoussis D, Andonopoulos AP. The emerging role of Dickkopf- 1 in bone biology: is it the main switch controlling bone and joint remodeling? Semin Arthritis Rheum. 2011;41(2):170–177. doi: 10.1016/j.semarthrit.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 64.Gaudio A, Pennisi P, Bratengeier C, Torrisi V, Lindner B, Mangiafico RA, et al. Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab. 2010;95(5):2248–2253. doi: 10.1210/jc.2010-0067. [DOI] [PubMed] [Google Scholar]
- 65.Anastasilakis AD, Polyzos SA, Avramidis A, Toulis K, Giomisi A, Papatheodorou A, et al. Serum Dikkopf-1 levels in postmenopausal women with established osteoporosis before and after treatment with teriparatide. Bone. 2009;44(Suppl 2):S294–S294. [Google Scholar]
- 66.Heider U, Fleissner C, Zavrski I, Kaiser M, Hecht M, Jakob C, et al. Bone markers in multiple myeloma. Eur J Cancer. 2006;42(11):1544–1553. doi: 10.1016/j.ejca.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 67.Leupin O, Piters E, Halleux C, Hu S, Kramer I, Morvan F, et al. Bone overgrowth-associated mutations in the LRP4 gene impair sclerostin facilitator function. J Biol Chem. 2011;286(22):19489–19500. doi: 10.1074/jbc.M110.190330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robey PG, Young MF, Flanders KC, Roche NS, Kondaiah P, Reddi AH, et al. Osteoblasts synthesize and respond to transforming growth factor-type beta (TGF-beta) in vitro. J Cell Biol. 1987;105(1):457–463. doi: 10.1083/jcb.105.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brunetti G, Oranger A, Mori G, Specchia G, Rinaldi E, Curci P, et al. Sclerostin is overexpressed by plasma cells from multiple myeloma patients. Ann N Y Acad Sci. 2011;1237:19–23. doi: 10.1111/j.1749-6632.2011.06196.x. [DOI] [PubMed] [Google Scholar]
- 70.Ballas SK, Kesen MR, Goldberg MF, Lutty GA, Dampier C, Osunkwo I, et al. Beyond the definitions of the phenotypic complications of sickle cell disease: an update on management. ScientificWorldJournal. 2012;2012:949535–949535. doi: 10.1100/2012/949535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamed HM, Galal A, Ghamrawy MEL, Abd El Azeem K, El Ghoroury EA, Rasheed MA, et al. Vitamin D receptor polymorphisms and indices of bone turnover and bone mass in egyptian children with sickle cell disease. Res J Med Med Sci. 2010;5(1):1–7. [Google Scholar]
- 72.Perrotta S, Cappellini MD, Bertoldo F, Servedio V, Iolascon G, DAgruma L, et al. Osteoporosis in beta-thalassaemia major patients: analysis of the genetic background. Br J Haematol. 2000;111(2):461–466. doi: 10.1046/j.1365-2141.2000.02382.x. [DOI] [PubMed] [Google Scholar]
- 73.Lindsay R. Hormone replacement therapy for prevention and treatment of osteoporosis. Am J Med. 1993;95(5A):37S–39S. doi: 10.1016/0002-9343(93)90380-8. [DOI] [PubMed] [Google Scholar]
- 74.Heaney RP. Nutritional factors in osteoporosis. Annu Rev Nutr. 1993;13:287–316. doi: 10.1146/annurev.nu.13.070193.001443. [DOI] [PubMed] [Google Scholar]
- 75.Anapllotou ML, Kastanias IT, Psara P, Evangelou EA, Liparaki M, Dimitriou P. The contribution of hypogonadism to the development of osteoporosis in thalassaemia major: new therapeutic approaches. Clin Endocrinol (Oxf) 1995;42(3):279–287. doi: 10.1111/j.1365-2265.1995.tb01876.x. [DOI] [PubMed] [Google Scholar]
- 76.Lindsay R. Prevention and treatment of osteoporosis. Lancet. 1993;341(8848):801–805. doi: 10.1016/0140-6736(93)90571-w. [DOI] [PubMed] [Google Scholar]
- 77.Filosa A, Di Maio S, Saviano A, Vocca S, Esposito G. Can adrenarche influence the degree of osteopenia in thalassemic children? J Pediatr Endocrinol Metab. 1996;9(3):401–406. doi: 10.1515/jpem.1996.9.3.401. [DOI] [PubMed] [Google Scholar]
- 78.Murphy S, Khaw KT, Sneyd MJ, Compston JE. Endogenous sex hormones and bone mineral density among community-based postmenopausal women. Postgrad Med J. 1992;68(805):908–913. doi: 10.1136/pgmj.68.805.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Canatan D, Akar N, Arcasoy A. Effects of calcitonin therapy on osteoporosis in patients with thalassemia. Acta Haematol. 1995;93(1):20–24. doi: 10.1159/000204084. [DOI] [PubMed] [Google Scholar]
- 80.Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, et al. Bisphosphonate action.Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest. 1991;88(6):2095–2105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gavriatopoulou M, Dimopoulos MA, Christoulas D, Migkou M, Iakovaki M, Gkotzamanidou M, et al. Dickkopf-1: a suitable target for the management of myeloma bone disease. Expert Opin Ther Targets. 2009;13(7):839–848. doi: 10.1517/14728220903025770. [DOI] [PubMed] [Google Scholar]