Abstract
Human congenital central hypoventilation syndrome (CCHS), resulting from mutations in transcription factor PHOX2B, manifests with impaired responses to hypoxemia and hypercapnia especially during sleep. To identify brainstem structures developmentally affected in CCHS, we analyzed two postmortem neonatal-lethal cases with confirmed polyalanine repeat expansion (PARM) or Non-PARM (PHOX2B∆8) mutation of PHOX2B. Both human cases showed neuronal losses within the locus coeruleus (LC), which is important for central noradrenergic signaling. Using a conditionally active transgenic mouse model of the PHOX2B∆8 mutation, we found that early embryonic expression (<E10.5) caused failure of LC neuronal specification and perinatal respiratory lethality. In contrast, later onset (E11.5) of PHOX2B∆8 expression was not deleterious to LC development and perinatal respiratory lethality was rescued, despite failure of chemosensor retrotrapezoid nucleus formation. Our findings indicate that early-onset mutant PHOX2B expression inhibits LC neuronal development in CCHS. They further suggest that such mutations result in dysregulation of central noradrenergic signaling, and therefore, potential for early pharmacologic intervention in humans with CCHS.
Electronic supplementary material
The online version of this article (doi:10.1007/s00401-015-1441-0) contains supplementary material, which is available to authorized users.
Keywords: Congenital central hypoventilation syndrome, Locus coeruleus, Noradrenergic system, PHOX2B
Introduction
Congenital central hypoventilation syndrome (CCHS) is a classic disorder of autonomic respiratory control characterized by alveolar hypoventilation and monotonous respiratory rates despite abnormal pCO2 and pH concentrations, especially during non-rapid eye movement (NREM) sleep [57]. Patients with CCHS lack behavioral responsiveness to hypoxemia and hypercarbia without symptoms of shortness of breath or respiratory distress [8], and will not automatically adjust spontaneous ventilation or awaken from sleep despite progressive physiologic compromise [57]. A subset of CCHS patients have Hirschsprung disease (HSCR; absence of ganglion cells from variable lengths of distal bowel), and/or solid extracranial tumors of neural crest origin [6, 53], and additional symptoms of autonomic nervous system dysregulation are reported [18, 20, 42, 48].
Autonomic respiratory networks are stimulated when specialized neuronal sensors (chemosensors) detect low levels of O2 and/or high levels of CO2 in the blood. These chemosensors include the carotid bodies (CB), located in the peripheral nervous system (PNS) near the bifurcation of the carotid artery, and several central nervous system (CNS) nuclei [22]. Specialized neurons and astrocytic populations in brain stem contribute to central CO2 chemosensation [9, 19]. Classical pharmacological studies show that catecholaminergic neuron depletion in the brain stem results in decreased ventilatory response to elevated CO2 levels [31]. The rodent retrotrapezoid nucleus (RTN), located ventral to the facial nerve nucleus, drives respiration in response to decreased pH, resulting from elevated blood CO2 concentrations [34]. Interestingly, such hypercapnic ventilatory responses were diminished in adult rodents after chemical ablation of the locus coeruleus (LC) [7], a major central noradrenergic structure that is located in dorsal brainstem with extensive connections to other local nuclei as well as forebrain.
The human paired-like homeodomain transcription factor PHOX2B contains C-terminal 9- and 20-alanine repeats (Fig. 1a). Heterozygous mutations of PHOX2B are etiologic in CCHS [1, 59], comprising polyalanine repeat expansion mutations (PARMs; 90–92 % of cases) in exon 3, as well as non-PARMs with missense, frameshift, nonsense and stop-codon mutations (NPARMs; 8–10 % of cases) throughout the coding region, and whole- or partial-gene deletions (<1 % of cases) [27]. A genotype–phenotype correlation has been shown between PHOX2B polyalanine repeat length and severity of the respiratory phenotype, associated symptoms, and the age of onset [2, 33, 58, 59]. Large heterozygous NPARM deletions within exon 3 are correlated with the most severe CCHS phenotype with complete apnea and/or profound hypoventilation during sleep, severe hypoventilation during wakefulness, and intestinal aganglionosis from duodenum to anus [6, 33, 53].
Fig. 1.

Genotype and respiratory physiological phenotypes of the proband and transgenic mouse model. a PHOX2B contains three exons and proband 8-nucleotide frameshift mutation in exon 3 is shown. Nucleotide number refers to nucleotide position of GenBank accession number CCDS 3463.1. Wild-type PHOX2B protein is 314 amino acids long; PHOX2B∆8 mutation results in loss of the 20-alanine repeat domain and generates a protein of 355 amino acids. b Polysomnographic recording from the proband while on Synchronized Intermittent Mandatory Ventilation (SIMV) and after switching to continuous positive airway pressure (CPAP). Note the rapid decrease in oxygen saturation (blue arrow) and increase in carbon dioxide (pink arrow) levels. Values of ETCO2 at each epoch shown. When challenged with persistent hypercarbia and hypoxemia during CPAP, the proband showed no increase in respiratory effort. Heart rate variability during the challenge was minimal. EKG electrocardiogram, SpO 2 oxygen saturation, E T CO 2 end-tidal CO2, Nasal nasal airflow, Chest chest wall movements from respiratory inductance plethysmography. CPAP pressure of 5 cm H2O was used. SIMV rate was 45 breaths/min. “Early” refers to 45 s after switching to CPAP, “late” refers to 75 s after switching to CPAP. Time scale is shown. c Targeting construct of patient-specific mouse model. Human PHOX2B exon 3 containing patient-specific PHOX2B mutation (denoted in blue color) is inserted following unmodified, non-mutated mouse Phox2b exon 3 flanked by loxP sites, to allow conditional expression of mutant gene by cre recombinase. For detailed generation of transgenic mouse line see Figure S4. d Endogenous respiratory output. Integrated C4 inspiratory activity from E18.5 control and Hprt-cre, Phox2b∆8 mutant mice under baseline (left) and stimulated (1 μM substance P, right) conditions. Note lack of response in Phox2b∆8 mouse brainstem (n = 4, a representative recording shown)
In rodents, RTN development requires Phox2b function [12], and mouse models of CCHS, either expressing the 27-polyalanine repeat PARM or NPARM mutations of Phox2b, prevent RTN formation [13, 35]. Although Phox2b is a well-known regulator of motor and noradrenergic neuronal specification [39, 40], the precise basis of respiratory control in CCHS remains incompletely understood. Indeed, abnormal development or injury to central noradrenergic structures is suggested by prior work. The LC is the major source of central noradrenergic signaling [5]; it is thus a major regulator of arousal state and is also thought to function in cognition [49]. Several lines of evidence support the role of LC as a central chemosensor [11, 17], but owing to the lack of neuropathological information from CCHS patients with confirmed PHOX2B mutations, it remains unclear whether CCHS-associated PHOX2B mutations primarily affect LC development.
To achieve further insight into the pathobiology of CCHS, we analyzed two postmortem cases of neonatal-lethal CCHS with confirmed PHOX2B mutations. Proband 1 was a full-term neonate with a heterozygous NPARM deletion/frameshift mutation (PHOX2B∆8), resulting in severe hypoventilation and total intestinal aganglionosis. Proband 2 was born preterm and had the most common heterozygous CCHS PHOX2B 20/27 mutation (PARM) with less severe phenotype. Interestingly, both cases showed loss or severe diminishment of noradrenergic LC neurons. We modeled the NPARM case in vivo by generating a cognate conditional transgenic mouse line. Early embryonic conditional activation of Phox2b∆8 in mouse brainstem (<E10.5) caused loss of a functional LC and abnormalities in all central noradrenergic (NA) neurons (e.g., A1/C2, forebrain projections to hypothalamus) tested. In contrast, later-onset (E11.5) activation of Phox2b∆8 expression spared NA neuron development and was not perinatal respiratory-lethal, despite loss of the RTN. Our findings demonstrate that LC development is compromised, and suggest abnormal central noradrenergic signaling, as a component of human CCHS.
Materials and methods
Human neuropathological studies
Postmortem human samples (proband 1) were obtained using University of California, San Francisco (UCSF) guidelines with oversight of the Committee for Human Research and Gamete, Embryo and Stem Cell Research (GESCR) committee. Tissue from PHOX2B 20/27 proband 2 was obtained from Rainbow Babies and Children’s Hospital (Cleveland, OH, USA) under an IRB-approved protocol at The Ohio State University. The entire formalin-fixed brainstems were serially sectioned for microscopic evaluation and compared to samples obtained from four roughly age-matched (control) patients that expired from other diseases. For case histories of controls, histological and immunohistochemical (IHC) procedures, see Suppl. Data.
Phox2b∆8 mouse model generation and animal husbandry
We generated a transgenic mouse line carrying a cre–loxP-inducible allele of human PHOX2B∆8 exon 3 by BAC recombineering and homologous recombination in embryonic stem cells (ES cells; See Suppl. Data). For early-onset (germline) activation of Phox2b∆8 allele, Hprt-cre mice (JAX 004302 on C57/Bl6 background) were intercrossed to Phox2b∆8 heterozygotes. For late-onset CNS activation of Phox2b∆8 allele, we used Blbp-cre [23]. Mutant mice in each genotype were compared to cre-negative littermate controls. Animal procedures were approved by Institutional Animal Care and Use Committees at Washington University (St. Louis, MO, USA), University of Connecticut Health Center (Farmington, CT, USA) and UCSF (San Francisco, CA, USA).
Mouse tissue processing, and histology
Embryos were collected from time-pregnant females (E0.5 at time of plug recognition) under deep anesthesia; those older than E16.5 were perfused with PBS followed by 4 % PFA under hypothermia anesthesia. For IHC, tissues were post-fixed in 4 % PFA and cryoprotected, frozen in OCT and sectioned at 14 μm. Cryosections were subjected to antigen retrieval in citrate buffer, pH 6.0 for 10 min at 90 °C as necessary, blocked with 5 % donkey serum in PBS with 0.3 % Triton X-100, incubated with primary antibodies overnight at 4 °C, followed by appropriate secondary antibodies (see Suppl. Data) for 1 h at room temperature prior to imaging on a Nikon 80i microscope equipped with Hamamatsu CCD camera.
In vitro mouse explant respiratory physiology
In vitro explant respiratory physiology was performed as described [26]. Brainstem–spinal (en bloc) preparations with an anterior transection near diencephalon–midbrain junction were made using E18.5 embryos as detailed in Suppl. Data.
Quantifications and statistical analysis
All statistical analysis was performed using Microsoft Excel (Mac Office 2008) or R version 2.11.1. Transgenic mouse phenotypes were analyzed with Student’s t test.
Results
Human CCHS proband 1: clinical history and neuropathological findings
A full-term male presented with respiratory depression at birth, apnea and oxygen desaturation that required mechanical ventilation. Polysomnography demonstrated normal baseline oxygen saturation (SpO2) and end-tidal CO2 (ETCO2) while mechanically ventilated (Fig. 1b, Figure S1). However, when challenged by removal of the ventilator-generated respiratory rate, proband 1 showed hypoventilation resulting in oxygen desaturation (nadir 57 %) and rise of ETCO2 (peak 82 mmHg) during both wakefulness and sleep. Persistent and profound hypoxemia and hypercapnia failed to induce chemoreceptor reflexes (i.e., increased breathing rate/effort or a variation of heart rate); there was no arousal response from sleep (Fig. 1b, Figure S1). Magnetic resonance imaging and spectroscopy of the brain were normal. The electroencephalogram showed normal brain activity, both during wakefulness and sleep, without seizures. Proband 1 had permanently dilated pupils with non-measurable response to light, suggesting autonomic dysfunction. Because of enteral feeding intolerance, he was dependent on total parenteral nutrition. The intestine showed pervasive aganglionosis from 10 cm distal to the ligament of Treitz to the rectum (Figure S2), indicating HSCR disease.
Genomic DNA analysis demonstrated an eight-nucleotide deletion in exon 3 of PHOX2B (Fig. 1a; cDNA position 691-GGCCCGGG-698; heretofore called PHOX2B∆8). This caused a frameshift that removed the alanine repeat-generated elongated aberrant residues from amino acid 230 to the C-terminus (Figure S3a). Maternal DNA showed intact copies of PHOX2B; however, our analysis does not rule out possible low-level somatic mosaicism [28]. Paternal DNA was unavailable. Together, these clinical, genetic, and pathological findings confirmed a NPARM PHOX2B mutation and diagnosis of CCHS with intestinal aganglionosis. Following withdrawal of life support at 6 weeks of age, an autopsy was performed.
Postmortem examination showed a dramatic loss of LC neurons that express dopamine β-hydroxylase (DBH) (Fig. 2a, b). The dorsal median raphe (dMnR), a major source of central serotonergic innervation, was severely diminished. We found additional losses of the hindbrain mesencephalic trigeminal nucleus (MesV) and dorsal motor nucleus of the vagus (DMNV), which derive from Phox2b+ progenitors [12, 24] (Fig. 2a, b; Table S1). In contrast, there were no gross or microscopic abnormalities detectable in cerebral cortex, striatum or thalamus (not shown), or significant abnormalities of the medullary arcuate nucleus, CNVII (facial nucleus), or surrounding areas including inferior olive, area postrema or nucleus prepositus (Figure S3b).
Fig. 2.
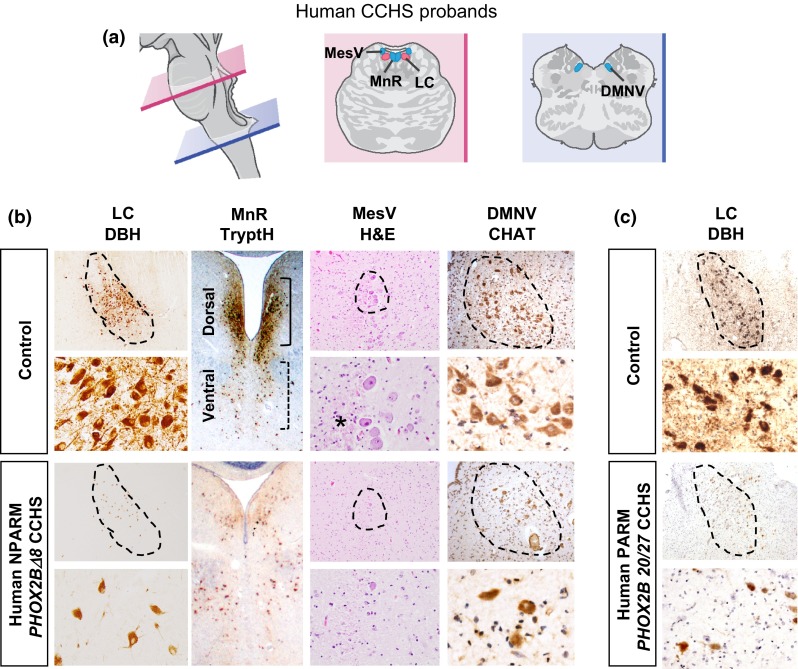
Brainstem pathology in CCHS probands. a Cartoon of human hindbrain at levels of pons (pink) and medulla (blue) is shown. b Dramatic losses were observed in NPARM PHOX2BΔ8 proband locus coeruleus (LC), dorsal median raphe (MnR), mesencephalic trigeminal nucleus (MesV) and dorsal motor nucleus of vagus (DMNV). Note lack of dopamine β-hydroxylase (DBH) expression in LC and tryptophan hydroxylase (TrypH) in dorsal MnR indicating defects in synthesis of noradrenaline and serotonin production. DMNV showed diminished cholinergic neurons, indicated by choline acetyltransferase (CHAT) expression. Asterisk indicates diminished MesV fibers originating from the nucleus in the proband. H&E hematoxylin and eosin. c Dramatic loss was observed in PARM PHOX2B 20/27 proband LC. Note a significant reduction of DBH expression
Dysregulated brain stem development in PHOX2B∆8 conditional mouse model
To assess function of PHOX2B∆8 during development in vivo, we generated a conditional transgenic mouse line by targeted homologous recombination in ES cells (Fig. 1c). Note because human and mouse exon 3 are identical at the amino acid level we used mutant human exon 3 for conditional expression of PHOX2B∆8 in mouse. In this line, the engineered PHOX2B∆8 allele is activated by bacteriophage P1 cre recombinase, which initiates expression of PHOX2B∆8 proteins and a downstream green fluorescent protein (GFP) reporter gene, as shown in Figure S4a.
We crossed this line with the germline driver Hprt-cre to activate recombination in all tissues from early embryonic stages. In PHOX2B∆8 mutant mice, we first confirmed faithful reporter GFP expression in all known Phox2b-expressing regions (e.g., hindbrain nuclei, enteric neurons) using co-labeling with an antibody for the unmutated N-terminal region of Phox2b protein (Figure S4b). No GFP expression was observed in WT littermates. Finally, we used IHC with an antibody for the C-terminal region of Phox2b protein (a region affected by PHOX2B∆8 mutation) to confirm expression of the non-mutant allele in heterozygotes (not shown). These findings confirmed all expected characteristics of PHOX2B∆8 expression in vivo.
Heterozygous Hprt-cre, Phox2b∆8 pups showed perinatal lethality and died before P1. Harvest just prior to birth at embryonic day 18.5 (E18.5) revealed that only 33 % of mutants took one spontaneous breath (vs. 100 % in control, n = 8 mutants, 15 controls). No mutants showed further spontaneous respiratory effort; thus, all died within minutes of delivery. Electrophysiological recording from E18.5 ex vivo brain stem preparations showed depression of endogenous respiratory motor root output under baseline conditions and in response to the excitatory neuropeptide, substance P, confirming abnormal respiratory phenotype in Hprt-cre, Phox2b∆8 mice (Fig. 1d), in keeping with other mouse models of CCHS [14, 26, 44, 54].
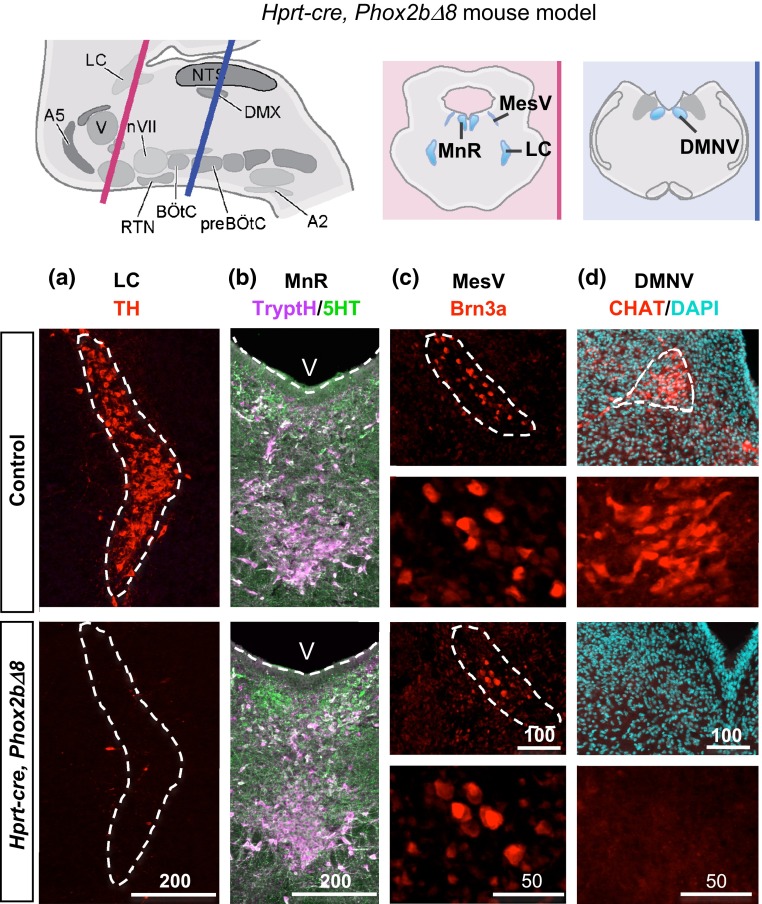
Abnormal noradrenergic structures in brainstem of Hprt-cre, Phox2b∆8 mice
Generation of a patient-specific NPARM CCHS mouse model and findings from our human proband provided an opportunity for cross-species analysis to identify conserved neuropathological features (Table S1, Figs. 2, 3). In the mouse, we observed that the LC was also abnormal and failed to express tyrosine hydroxylase (TH), indicating a synthetic defect in noradrenergic pathway (Fig. 3a). Absence of TH neurons within the LC was correlated with sparse and small neuronal cell bodies, suggesting cellular loss/attrition rather than selective reduction of TH expression (Figure S5a). In addition, we observed consistent losses in the DMNV and mesencephalic trigeminal nucleus nuclei (MesV) (Fig. 3c, d). Neuronal precursors of the DMNV were detectable at E13.5 (Figure S5b) in the mouse model, suggesting PHOX2B∆8 prevents DMNV formation despite progenitor specification. In contrast, while the dMnR showed severe attrition in the human proband (Fig. 2b), we found normal-appearing populations of serotonergic neurons that expressed tryptophan hydroxylase (17.00 ± 2.81 SEM (mutant) vs. 16.75 ± 2.79 SEM (control) cells per area, p = 0.812, n = 3) and 5HT (25.58 ± 9.13 vs 23.08 ± 1.88 cells per area, p = 0.952, n = 3, Student’s t test) spanning murine dMnR (Fig. 3b). No gross abnormalities in forebrain were observed. In summary, abnormal development of the LC was prominent consistently across species.
Fig. 3.
Brain pathology in Hprt-cre, Phox2b∆8 mouse. Top Cartoon of mouse hindbrain at levels of rostral (pink) and caudal (blue) hindbrain is shown. a–d The Hprt-cre, Phox2b∆8 mutant mouse showed profoundly abnormal differentiation of LC, characterized by absent expression of tyrosine hydroxylase (TH). Additional abnormalities were diminished MesV (Brn3) and DMNV Choline Acetyltransferase (CHAT) neurons. In contrast to NPARM PHOX2BΔ8 proband (see Fig. 2b), the dorsal MnR in Hprt-cre, Phox2b∆8 mouse showed non-significant reduction in counts of TrypH and 5HT cells compared with controls (n = 3). V, 4th ventricle. Scale bar unit µm
PHOX2B∆8 inhibits LC noradrenergic neuronal specification
The LC is the major source of noradrenergic neurotransmitters in the CNS [5], and it projects to circuits in forebrain, midbrain and hindbrain [45]. As shown (Figs. 3a, 4b), early activation of PHOX2B∆8 in the brainstem of Hprt-cre, Phox2b∆8 mice resulted in developmental failure of TH + LC neurons. As we observed normal-sized populations of Phox2b-GFP+ precursors (Fig. 4a, discussed below), we conclude that early-onset PHOX2B∆8 expression inhibits LC specification. Consistent with this, we observed widespread abnormalities in noradrenergic circuits, including caudal hindbrain nuclei A1/C2 and the forebrain projections of LC to hypothalamus (Fig. 4b, A1/C2 in Figure S5c).
Fig. 4.
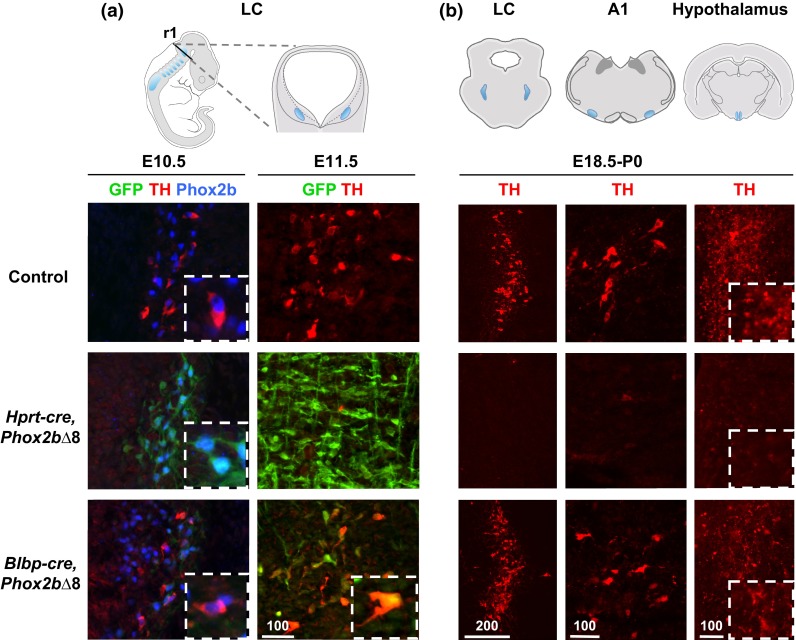
Noradrenergic neurons are affected by stage-specific activation of PHOX2B∆8. a Left panels the specification of noradrenergic neurons in LC appears by E10.5 in control, which express TH in addition to Phox2b. In Hprt-cre fate-mapped early-onset mice, the TH expression is diminished despite available Phox2b+ precursors. Note expression of PHOX2B∆8 protein (GFP+) in the same cells. Blbp-cre fate-mapped late-onset mice showed normal TH expressions and PHOX2B∆8 is not expressed. Right panels by E11.5, cre-activation in late-onset mice commences in noradrenergic neurons, shown by GFP+ (n = 3–4). Note that mouse E10.5 and E11.5 are roughly equivalent to human gestational age week 6 and week 7, respectively. b Central noradrenergic neurons affected by early-onset Hprt-cre, Phox2b∆8 were pervasive at E18.5 C-section including hindbrain nuclei LC and A1 and forebrain projection to periventricular nucleus of the hypothalamus. In the late-onset Blbp-cre, Phox2b∆8, noradrenergic neurons were intact in all areas at P0 (n = 3)
Our conditional Phox2b∆8 mouse model and cre-driver lines permitted introduction of PHOX2B∆8 at two distinct time points in noradrenergic neuronal development. Whereas Hprt-cre introduces the mutation in the early embryo (<E10.5), Blbp-cre [23] results in CNS-restricted activation of the conditional PHOX2B∆8 allele at E10.5 and later, after most neurogenesis. Fate mapping, using the conditional reporter function of the PHOX2B∆8 mutant locus (Fig. 1c), showed robust onset of GFP expression in Phox2b+ cells at E10.5 with Hprt-cre, but only confined GFP+ population in Blbp-cre fate-mapped hindbrain (Figure S5d). In contrast, Blbp-cre targeting in brainstem was robust after E11.5 (Fig. 4a). Differences in prenatal viability between these two lines were noted (Figure S5e).
We next assessed consequences of early (<E10.5) versus later onset (>E11.5) of PHOX2B∆8 expression for LC development. As shown (Fig. 4a), at E10.5 TH+, presumptive noradrenergic neurons were detectable in LC of control mice; moreover, these cells co-expressed Phox2b, consistent with previous findings [24]. Such TH+ populations were absent in the early-onset Hprt-cre, Phox2b∆8 mice. Contrasting this, the late-onset Blbp-cre, Phox2b∆8 model showed normal LC TH+ populations co-expressing Phox2b (Fig. 4a, b). The TH+, Phox2b+ populations did not express GFP, suggesting these cells expressed wild-type Phox2b allele. Together, these findings suggest that PHOX2B∆8 inhibits LC noradrenergic differentiation in a stage-specific manner. That is, early-onset mutant protein expression derails LC differentiation in a dominant-toxic manner, whereas later-stage expression of the PHOX2B∆8 allele does not interfere with acquisition of TH expression in Phox2b+-derived LC neurons.
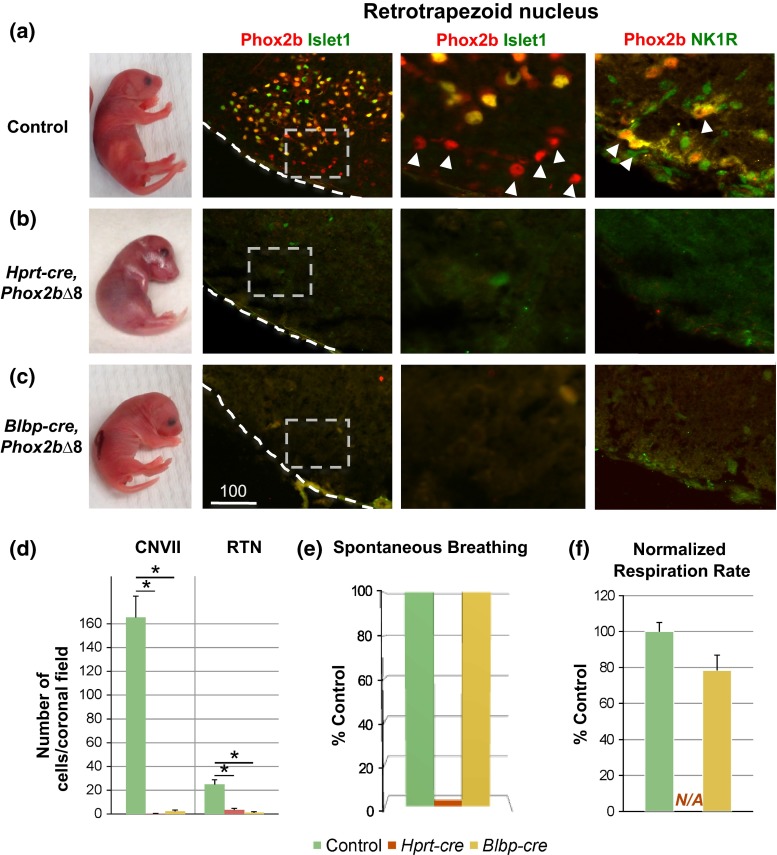
RTN development is uncoupled from the perinatal respiratory-lethal phenotype of PHOX2B∆8 mice
We observed striking differences in the perinatal respiratory phenotype of early-onset Hprt-cre, Phox2b∆8 versus late-onset Blbp-cre, Phox2b∆8 models (Fig. 5). As mentioned above, Hprt-cre, Phox2b∆8 animals showed little/no spontaneous respiratory effort, rapidly became cyanotic (dark violet skin tone) and died minutes after birth (Fig. 5b, e–f). In contrast, Blbp-cre, Phox2b∆8 mice were typically pink (non-cyanotic) and showed spontaneous/continuous breathing (albeit at slightly reduced frequency; 78 ± 8.5 % of control respiratory rate, n = 3, p > 0.1, Student’s t test) (Fig. 5c, e–f). Longitudinal observation of Blbp-cre, Phox2b∆8 mice past P1 was not possible, as the mutants failed to nurse and gain adequate body weight.
Fig. 5.
Central chemosensor RTN and CNVII are dispensable for perinatal respiratory regulation. a–c Respiratory phenotype and central chemosensor of a control, b early-onset Hprt-cre, Phox2b∆8, and c late-onset Blbp-cre, Phox2b∆8 mice. Whereas Hprt-cre, Phox2b∆8 mice failed to show any respiratory effort, were cyanotic (dark violet skin tone), and died in the immediate perinatal period, Blbp-cre, Phox2b∆8 mice showed spontaneous respiration and were pink (n = 7–11 from at least 3 litters/genotype). Both Hprt-cre, Phox2b∆8 and Blbp-cre, Phox2b∆8 mice lacked the central chemosensor RTN (Phox2b+/Islet−, and NK1R+/Phox2b+) and CNVII (Phox2b+/Islet+), despite continuous respiration in Blbp-cre, Phox2b∆8 and lack of respiration in Hprt-cre, Phox2b∆8 (n = 3). d Quantification of RTN (Phox2b+/Islet−) and CNVII (Phox2b+/Islet+) showed significant losses of both nuclei at E18.5 (n = 3). e When mice were harvested at E18.5, no Hprt-cre, Phox2b∆8 mice showed continuous respiration in contrast to Blbp-cre, Phox2b∆8 and control mice (n = 7–11 from at least 3 litters/genotype). f Quantification of breathing rate at birth showed spontaneous/continuous breathing in Blbp-cre, Phox2b∆8 mice albeit at reduced frequency. Respiratory rate ranged 20–132 breaths/min depending on time post-birth, normalized to control within the same litter (n = 3–5). N/A not assessed due to absence of respiration
One possibility to account for these differences was differential effects of early versus late Phox2b∆8 expression on RTN development [34]. However, histological examination of the brainstems from both Hprt-cre- and Blbp-cre-driven models showed loss of RTN and CNVII nuclei, as shown by IHC and quantitative analysis of the markers Phox2b, Islet1 and neurokinin 1 receptor (NK1R) (Fig. 5a–d). Moreover, developmental analysis of embryonic brainstem confirmed that abnormal formation of CNVII was due to failure of precursor migration in the Hprt-cre, Phox2b∆8 mouse model (Fig. 6a–c), in keeping with reported findings in other CCHS mouse models [10, 13, 26, 35]. A similarly mis-located putative CNVII was found in Blbp-cre, Phox2b∆8 mouse (Figure S5f). Analysis of MnR in Blbp-cre, Phox2b∆8 mouse showed normal number of serotonergic neurons expressing TryptH (17.833 ± 1.815 SEM cells per area, p = 0.682, n = 3, and 5HT (Figure S5g) (27.166 ± 1.249 SEM cells per area, p = 0.793, n = 3, Student’s t test). Thus, our findings in late-onset Blbp-cre, Phox2b∆8 mice indicate that the RTN is dispensable for generation of minimal perinatal respiratory rhythm, in keeping with the proposal that self-evoked respiration is possible without the RTN [26, 44].
Fig. 6.
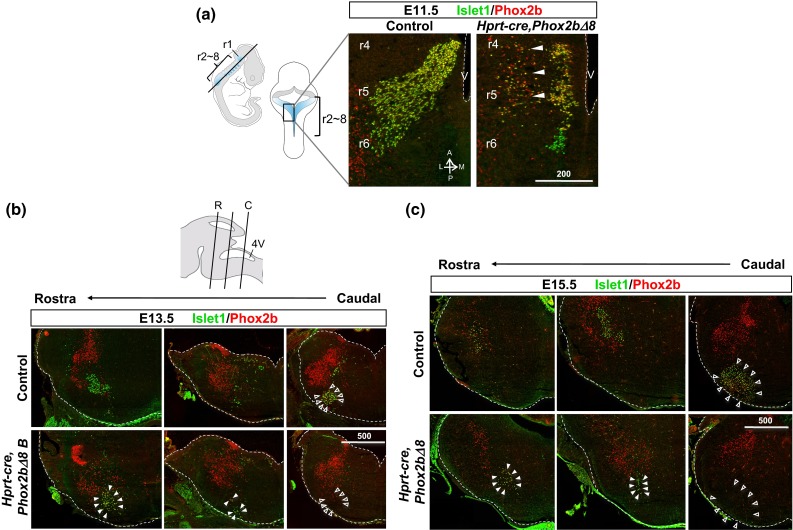
Abnormal migration of facial nucleus CNVII in Hprt-cre, Phox2b∆8 mouse. a Neurons of CNVII migrate caudally from their origin in rhombomere (r)4 toward r6 followed by lateral migration toward ventral surface. Horizontal sections at E11.5 to visualize trans-rhombomere migration showed in Hprt-cre, Phox2b∆8 mouse, precocious termination of rostral-to-caudal migration (note accumulation of Phox2b+ Islet1+ cells at r4–5), and abnormal lateral migration within r4–5 (arrowheads) (V 4th ventricle, A anterior, P posterior, L lateral, M medial). b Top cartoon of mouse hindbrain levels of rostral (R) and caudal (C) at E14 is shown. At E13.5, stalled migration of CNVII/RTN, detected by Islet1 and Phox2b antibodies, was found at rostral levels (denoted by solid arrowheads) of hindbrain than normally found at caudal level (denoted by empty arrowheads) in control littermates. c The same pattern described in b was observed at E15.5, implicating permanent migration defect of CNVII. The number of putative Phox2b+ Islet1+ CNVII cells found in the mutants declined from 37.6 to 16.2 % of WT at E13.5 and E15.5, respectively. Scale bar unit µm
Human CCHS proband 2: clinical history and neuropathological findings
Proband 2 was a 27 5/7-week gestation age preterm infant male. He was intubated at birth due to poor respiratory effort and received surfactant. Although there was no evidence for chronic lung disease of prematurity, the patient remained ventilator dependent. At approximately 4 weeks of age, lack of respiratory drive despite persistent hypercapnia prompted genetic testing for CCHS. Proband 2 carried a heterozygous PARM PHOX2B mutation, resulting in the most common, 7-residue alanine expansion (PHOX2B 20/27 genotype). After withdrawal of life support at ~41-week corrected gestational age, autopsy was performed with postmortem analysis of brainstem.
While the pons contained a cluster of cells morphologically and anatomically consistent with the LC, as shown in Figs. 2c and S6, they failed to express normal levels of DBH or TH, indicating LC dysfunction and noradrenergic synthesis. We did not observe gliosis or other signs indicative of hypoxic damage in the brain stem. In contrast to the NPARM PHOX2BΔ8 CCHS proband 1 (Fig. 2b), the MnR, MesV, and DMNV hindbrain nuclei in the PARM subject appeared normal (Figure S6). Taken together, our findings indicate that both NPARM and PARM PHOX2B mutations result in defects of LC neuronal populations in human neonates with CCHS.
Discussion
The underlying pathobiology of CCHS remains unclear, reflecting in part the complexity of central and peripheral centers that interact to control respiratory drive. We used a combinatorial approach incorporating human neuropathological analysis from two (NPARM and PARM) human probands and a conditionally activated NPARM patient-specific transgenic mouse model to study temporal effects of mutant protein on brainstem development. Our study is the first to describe CNS neuropathological findings in two human cases of CCHS with confirmed PARM and NPARM mutations of PHOXB2B, which are summarized in Table S1. Further, we modeled the proband-specific NPARM mutation introduced at several stages of mouse hindbrain development. While several structures are affected variously in these cases, a focus on conserved abnormalities between species revealed abnormalities in LC populations, which probably result from failure to specify LC neurons in the embryonic brain. Our findings suggest that disruption of LC noradrenergic neuron development and function may be a common pathobiological feature of CCHS.
Brainstem pathological findings in two human CCHS cases with confirmed NPARM and PARM PHOX2B mutations
The LC is the major source of noradrenergic innervation to both rostral brain regions as well as the brainstem. Abnormal noradrenergic signaling has been implicated in clinical features of CCHS [32, 55]. Studies of CCHS by diffusion MRI (albeit without confirmed PHOX2B mutations) showed altered diffusivity (decreased fractional anisotropy and increased axial and radial diffusivity) in several brainstem regions as well as other potentially connected regions of the mid-hindbrain [41]. Such late structure/function studies in adolescents carry the caveat that injury to LC or dMnR could have accrued from cumulative effects of repeated episodes of hypoxemia.
In contrast, the two NPARM and PARM probands we studied were neonates that were intubated and/or managed in an NICU from birth with stringent monitoring and interventions to prevent hypoxemic events after birth. Proband 1 carried a NPARM mutation of (PHOX2B∆8) and demonstrated severe CCHS and intestinal aganglionosis (Haddad syndrome). Postmortem analysis showed several brainstem nuclei were abnormal (Table S1) including almost total absence of LC neurons. Furthermore, a second PHOX2B 20/27 PARM proband 2 also showed defects in DBH and TH expression in the context of a well-formed LC, indicating deficient numbers of functioning noradrenergic neurons. The correspondence of abnormalities in the LC in both human cases is striking. While further confirmation in additional cases of CCHS would be useful, such pathological specimens in confirmed cases of neonatal CCHS with PHOX2B mutation are extremely rare. We note these data are consistent with a previously reported case of CCHS (albeit without a confirmed PHOX2B mutation), showing significant defects in noradrenergic cell number [52]. Together, these findings suggest that abnormal development of the LC is common in CCHS.
NPARM PHOX2B∆8 permits brainstem noradrenergic neuron precursor allocation but inhibits differentiation in a stage-restricted manner
Our findings demonstrate that inhibitory effects of PHOX2B∆8 proteins during LC development are stage restricted. The murine LC is formed during E9–E11 [50] and by E11.5 it is a clearly identifiable structure [3]. Human equivalent developmental stage for mesencephalic TH neurons occurs 6.5–8 weeks post-conception [16, 36]. We found that early mutant protein expression (using Hprt-cre) prevented LC neuron specification/differentiation to a TH+ state. In contrast, delayed expression of the mutation (with Blbp-cre) permitted LC neuron differentiation to the TH+ stage. Together, these findings indicate PHOX2B∆8 proteins inhibit early LC neuronal specification, rather than the program of expression characteristic of mature LC neurons.
Abnormal respiratory arousal during NREM sleep is associated with dysregulation of central adrenergic [38] and serotonergic [25, 43] signaling. Caudally, the LC densely innervates the serotonergic dorsal raphe nucleus [30]. The dorsal raphe nucleus does not express PHOX2B. Therefore, the finding in human proband 1 that the dorsal raphe was lost is consistent with the possibility of long-term failure of normal feedback mechanisms. For example, classical ultrastructural studies have demonstrated in experimental animals innervation of serotonergic neurons by noradrenergic locus coeruleus neurons [4]. This circuitry between noradrenergic and serotonergic systems raise the possibility that disease affecting the noradrenergic system could cause secondary effects to the serotonergic neurons through transneuronal degeneration mechanisms similar to neurodegenerative diseases [15]. In keeping with this possibility, the NPARM early-onset mouse model did not show defects in the dMnR. Moreover, we observed that noradrenergic circuit formation in hypothalamus and A1/C2 nuclei of brain stem were also rescued in the Blbp-cre, Phox2b∆8 mice, suggesting that PHOX2B∆8 generally exerts its impact on noradrenergic neuron development at an early stage. Further work is needed to identify precise gene targets affected in the early-onset phenotype (see discussion below).
Evidence that RTN is dispensable for perinatal respiratory drive in the NPARM CCHS model
The RTN is generally thought to have critical roles in perinatal respiratory control in rodents [21]. However, while conditional targeting of Phox2b function resulted in failure of RTN development and lethal respiratory compromise in one study [14], another study that selectively targeted disruption of the RTN with Egr2-cre did not cause perinatal respiratory lethality and suggested its importance might be specific to chemosensation [44]. We found that while late-onset PHOX2B∆8 expression caused failure of RTN and CNVII development, animals showed near-normal perinatal respiration, indicating dispensability of the RTN for this function. Unfortunately, such Blbp-cre, PHOX2B∆8 animals did not survive past P1 due to oropharyngeal problems preventing feeding and so further testing was not performed. Nevertheless, our studies suggest the RTN is dispensable as an early regulator of respiratory drive. In the human, the equivalent structure to RTN has been suggested [29, 47], but its existence remains controversial. Despite exhaustive efforts, we failed to identify an RTN-like structure in our proband cases or specimens from five age-matched unaffected subjects, and the facial nucleus (CNVII) was normal in appearance in the human PHOX2BΔ8 proband (Figure S3b).
Dysregulation of locus coeruleus development might be general feature of human CCHS
Understanding mechanisms that underlie CCHS has general implications for development of human respiratory control [22] and other disorders of respiratory and autonomic regulation including Rett Syndrome [37], sudden infant death syndrome (SIDS) and apnea of prematurity. Findings from two human CCHS cases indicate that NPARM and PARM PHOX2B mutations disrupt development of LC noradrenergic populations, a finding that is phenocopied in the NPARM CCHS mouse model we generated, but not in another previously reported mouse model of PHOX2B 20/27 PARM [13], which failed to show defects in the LC. Why the mouse PHOX2B 20/27 model fails to capture aberrant LC neuron differentiation is unclear, but might reflect differences between mouse and human development in the context of 20/27 PARM mutations.
Several lines of evidence support the role of LC as a central chemosensor [17]. First, Phox2a function is required for differentiation of LC but leaves other noradrenergic centers (locus subcoeruleus and groups A7, A5, A2 and A1) intact, and loss of Phox2a function results in depressed central respiratory drive [60]. Thus, it is possible that mutant PHOX2B proteins act in a “dominant-negative” fashion to inhibit LC neuron specification. Second, loss of the LC is associated with markedly decreased breathing frequency [56]. Third, in a mouse model of Rett syndrome, caused by mutations of methyl-CpG-binding protein 2 (MECP2), there is loss of LC neurons [46, 51], and breathing dysfunction with decreased CO2 chemosensitivity [61]. Together, these findings suggest that LC dysfunction might explain, at least in part, central CO2 chemo-insensitivity in CCHS as well as failure of normal respiratory arousal. Further research is needed to evaluate the utility of pharmacological approaches that might target noradrenergic signaling imbalance so that the deleterious effects of PHOX2B mutations on CCHS patients are attenuated. Indeed, maturational decrement in ventilatory slope in response to hypercarbia/hypoxia is observed in CCHS [8] suggesting that early pharmacologic noradrenergic stimulation might assuage disease progression.
Electronic supplementary material
Supplementary material 2 (PPTX 186,429 kb)
Acknowledgments
The authors thank Arturo Alvarez-Buylla, A. James Barkovich and Bernard Lo for comments on the manuscript, Marta Couce for facilitating procurement of CCHS autopsy tissue, Kanji Nobuta for artistic assistance, and the Gladstone Stem Cell Core Facility for technical assistance. H.N. is grateful to the European Leukodystrophy Association for a postdoctoral fellowship. J.J.O. was supported by California Institute for Regenerative Medicine Clinical Scholar Award (TG2-01153), UCSF-CTSI Grant Number UL1RR024131, and OSU CRMCBT Grant Number 8UL1TR000090-05. This work was funded by NIH Grants HL098179, HL108677, HL100406 (to B.C.), HL089742 (to P.A.G., S.T.), OD010927 (to E.J. H.) and NS083513 (to D.H.R.). E.J.H acknowledges the University of California Pediatric Neuropathology Consortium. D.H.R. is a Howard Hughes Medical Institute Investigator.
Conflict of interest
The authors have declared that no conflict of interest exists.
Footnotes
H. Nobuta and J. J. Otero contributed equally.
References
- 1.Amiel J, Laudier B, Attie-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, et al. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- 2.Antic NA, Malow BA, Lange N, McEvoy RD, Olson AL, Turkington P, Windisch W, Samuels M, Stevens CA, Berry-Kravis EM, et al. PHOX2B mutation-confirmed congenital central hypoventilation syndrome: presentation in adulthood. Am J Respir Crit Care Med. 2006;174:923–927. doi: 10.1164/rccm.200605-607CR. [DOI] [PubMed] [Google Scholar]
- 3.Aroca P, Lorente-Canovas B, Mateos FR, Puelles L. Locus coeruleus neurons originate in alar rhombomere 1 and migrate into the basal plate: studies in chick and mouse embryos. J Comp Neurol. 2006;496:802–818. doi: 10.1002/cne.20957. [DOI] [PubMed] [Google Scholar]
- 4.Baraban JM, Aghajanian GK. Noradrenergic innervation of serotonergic neurons in the dorsal raphe: demonstration by electron microscopic autoradiography. Brain Res. 1981;204:1–11. doi: 10.1016/0006-8993(81)90646-6. [DOI] [PubMed] [Google Scholar]
- 5.Berridge CW, Waterhouse BD. The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/S0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 6.Berry-Kravis EM, Zhou L, Rand CM, Weese-Mayer DE. Congenital central hypoventilation syndrome: PHOX2B mutations and phenotype. Am J Respir Crit Care Med. 2006;174:1139–1144. doi: 10.1164/rccm.200602-305OC. [DOI] [PubMed] [Google Scholar]
- 7.Biancardi V, Bicego KC, Almeida MC, Gargaglioni LH. Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflugers Arch. 2008;455:1119–1128. doi: 10.1007/s00424-007-0338-8. [DOI] [PubMed] [Google Scholar]
- 8.Carroll MS, Patwari PP, Kenny AS, Brogadir CD, Stewart TM. Weese-Mayer DE (2014) Residual chemosensitivity to ventilatory challenges in genotyped congenital central hypoventilation syndrome. J Appl Physiol. 1985;116:439–450. doi: 10.1152/japplphysiol.01310.2013. [DOI] [PubMed] [Google Scholar]
- 9.Coates EL, Li A, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol. 1985;75:5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- 10.Coppola E, d’Autreaux F, Rijli FM, Brunet JF (2010) Ongoing roles of Phox2 homeodomain transcription factors during neuronal differentiation. Development 137:4211–4220. doi:10.1242/dev.056747 [DOI] [PubMed]
- 11.de Carvalho D, Patrone LG, Taxini CL, Biancardi V, Vicente MC, Gargaglioni LH. Neurochemical and electrical modulation of the locus coeruleus: contribution to CO2drive to breathe. Front Physiol. 2014;5:288. doi: 10.3389/fphys.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubreuil V, Barhanin J, Goridis C, Brunet JF. Breathing with phox2b. Philos Trans R Soc Lond B Biol Sci. 2009;364:2477–2483. doi: 10.1098/rstb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C (2008) A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci USA 105:1067–1072. doi:10.1073/pnas.0709115105 [DOI] [PMC free article] [PubMed]
- 14.Dubreuil V, Thoby-Brisson M, Rallu M, Persson K, Pattyn A, Birchmeier C, Brunet JF, Fortin G, Goridis C (2009) Defective respiratory rhythmogenesis and loss of central chemosensitivity in Phox2b mutants targeting retrotrapezoid nucleus neurons. J Neurosci Off J Soc Neurosci 29:14836–14846. doi:10.1523/JNEUROSCI.2623-09.2009 [DOI] [PMC free article] [PubMed]
- 15.Fornito A, Zalesky A, Breakspear M. The connectomics of brain disorders. Nat Rev Neurosci. 2015;16:159–172. doi: 10.1038/nrn3901. [DOI] [PubMed] [Google Scholar]
- 16.Freeman TB, Spence MS, Boss BD, Spector DH, Strecker RE, Olanow CW, Kordower JH. Development of dopaminergic neurons in the human substantia nigra. Exp Neurol. 1991;113:344–353. doi: 10.1016/0014-4886(91)90025-8. [DOI] [PubMed] [Google Scholar]
- 17.Gargaglioni LH, Hartzler LK, Putnam RW. The locus coeruleus and central chemosensitivity. Respir Physiol Neurobiol. 2010;173:264–273. doi: 10.1016/j.resp.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg DS, Ludwig IH. Congenital central hypoventilation syndrome: ocular findings in 37 children. J Pediatr Ophthalmol Strabismus. 1996;33:175–180. doi: 10.3928/0191-3913-19960501-11. [DOI] [PubMed] [Google Scholar]
- 19.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gronli JO, Santucci BA, Leurgans SE, Berry-Kravis EM, Weese-Mayer DE. Congenital central hypoventilation syndrome: PHOX2B genotype determines risk for sudden death. Pediatr Pulmonol. 2008;43:77–86. doi: 10.1002/ppul.20744. [DOI] [PubMed] [Google Scholar]
- 21.Guyenet PG, Stornetta RL, Abbott SB, Depuy SD, Kanbar R. The retrotrapezoid nucleus and breathing. Adv Exp Med Biol. 2012;758:115–122. doi: 10.1007/978-94-007-4584-1_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyenet PG, Stornetta RL, Bayliss DA. Central respiratory chemoreception. J Comp Neurol. 2010;518:3883–3906. doi: 10.1002/cne.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hegedus B, Dasgupta B, Shin JE, Emnett RJ, Hart-Mahon EK, Elghazi L, Bernal-Mizrachi E, Gutmann DH. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell Stem Cell. 2007;1:443–457. doi: 10.1016/j.stem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch MR, d’Autreaux F, Dymecki SM, Brunet JF, Goridis C. A Phox2b::FLPo transgenic mouse line suitable for intersectional genetics. Genesis. 2013;51(7):506–514. doi: 10.1002/dvg.22393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci Off J Soc Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang WH, Tupal S, Huang TW, Ward CS, Neul JL, Klisch TJ, Gray PA, Zoghbi HY. Atoh1 governs the migration of postmitotic neurons that shape respiratory effectiveness at birth and chemoresponsiveness in adulthood. Neuron. 2012;75:799–809. doi: 10.1016/j.neuron.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jennings LJ, Yu M, Rand CM, Kravis N, Berry-Kravis EM, Patwari PP, Weese-Mayer DE. Variable human phenotype associated with novel deletions of the PHOX2B gene. Pediatr Pulmonol. 2012;47:153–161. doi: 10.1002/ppul.21527. [DOI] [PubMed] [Google Scholar]
- 28.Jennings LJ, Yu M, Zhou L, Rand CM, Berry-Kravis EM, Weese-Mayer DE. Comparison of PHOX2B testing methods in the diagnosis of congenital central hypoventilation syndrome and mosaic carriers. Diag Mol Pathol Am J Surg Pathol Part B. 2010;19:224–231. doi: 10.1097/PDM.0b013e3181eb92ff. [DOI] [PubMed] [Google Scholar]
- 29.Lavezzi AM, Weese-Mayer DE, Yu MY, Jennings LJ, Corna MF, Casale V, Oneda R, Matturri L. Developmental alterations of the respiratory human retrotrapezoid nucleus in sudden unexplained fetal and infant death. Auton Neurosci. 2012;170:12–19. doi: 10.1016/j.autneu.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Lechin F, van der Dijs B, Hernandez-Adrian G (2006) Dorsal raphe vs. median raphe serotonergic antagonism. Anatomical, physiological, behavioral, neuroendocrinological, neuropharmacological and clinical evidences: relevance for neuropharmacological therapy. Prog Neuropsychopharmacol Biol Psychiatry 30:565–585. doi:10.1016/j.pnpbp.2005.11.025 [DOI] [PubMed]
- 31.Li A, Nattie E. Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J Physiol. 2006;570:385–396. doi: 10.1113/jphysiol.2005.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li A, Zhou S, Nattie E (2006) Simultaneous inhibition of caudal medullary raphe and retrotrapezoid nucleus decreases breathing and the CO2 response in conscious rats. J Physiol 577:307–318. doi:10.1113/jphysiol.2006.114504 [DOI] [PMC free article] [PubMed]
- 33.Matera I, Bachetti T, Puppo F, Di Duca M, Morandi F, Casiraghi GM, Cilio MR, Hennekam R, Hofstra R, Schober JG, et al. PHOX2B mutations and polyalanine expansions correlate with the severity of the respiratory phenotype and associated symptoms in both congenital and late onset central hypoventilation syndrome. J Med Genet. 2004;41:373–380. doi: 10.1136/jmg.2003.015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG (2004) Respiratory control by ventral surface chemoreceptor neurons in rats. Nature Neurosci 7:1360–1369. doi:10.1038/nn1357 [DOI] [PubMed]
- 35.Nagashimada M, Ohta H, Li C, Nakao K, Uesaka T, Brunet JF, Amiel J, Trochet D, Wakayama T, Enomoto H. Autonomic neurocristopathy-associated mutations in PHOX2B dysregulate Sox10 expression. J Clin Invest. 2012;122:3145–3158. doi: 10.1172/JCI63401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelander J, Hebsgaard JB, Parmar M. Organization of the human embryonic ventral mesencephalon. Gene Express Patterns GEP. 2009;9:555–561. doi: 10.1016/j.gep.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Ogier M, Katz DM. Breathing dysfunction in Rett syndrome: understanding epigenetic regulation of the respiratory network. Respir Physiol Neurobiol. 2008;164:55–63. doi: 10.1016/j.resp.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouyang M, Hellman K, Abel T, Thomas SA. Adrenergic signaling plays a critical role in the maintenance of waking and in the regulation of REM sleep. J Neurophysiol. 2004;92:2071–2082. doi: 10.1152/jn.00226.2004. [DOI] [PubMed] [Google Scholar]
- 39.Pattyn A, Hirsch M, Goridis C, Brunet JF. Control of hindbrain motor neuron differentiation by the homeobox gene Phox2b. Development. 2000;127:1349–1358. doi: 10.1242/dev.127.7.1349. [DOI] [PubMed] [Google Scholar]
- 40.Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;399:366–370. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
- 41.Patwari PP, Carroll MS, Rand CM, Kumar R, Harper R, Weese-Mayer DE. Congenital central hypoventilation syndrome and the PHOX2B gene: a model of respiratory and autonomic dysregulation. Respir Physiol Neurobiol. 2010;173:322–335. doi: 10.1016/j.resp.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patwari PP, Stewart TM, Rand CM, Carroll MS, Kuntz NL, Kenny AS, Brogadir CD, Weese-Mayer DE. Pupillometry in congenital central hypoventilation syndrome (CCHS): quantitative evidence of autonomic nervous system dysregulation. Pediatr Res. 2012;71:280–285. doi: 10.1038/pr.2011.38. [DOI] [PubMed] [Google Scholar]
- 43.Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci Off J Soc Neurosci. 2009;29:3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramanantsoa N, Hirsch MR, Thoby-Brisson M, Dubreuil V, Bouvier J, Ruffault PL, Matrot B, Fortin G, Brunet JF, Gallego J, et al. Breathing without CO(2) chemosensitivity in conditional Phox2b mutants. J Neurosci Off J Soc Neurosci. 2011;31:12880–12888. doi: 10.1523/JNEUROSCI.1721-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robertson SD, Plummer NW, de Marchena J, Jensen P. Developmental origins of central norepinephrine neuron diversity. Nat Neurosci. 2013;16:1016–1023. doi: 10.1038/nn.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roux JC, Panayotis N, Dura E, Villard L. Progressive noradrenergic deficits in the locus coeruleus of Mecp2 deficient mice. J Neurosci Res. 2010;88:1500–1509. doi: 10.1002/jnr.22312. [DOI] [PubMed] [Google Scholar]
- 47.Rudzinski E, Kapur RP. PHOX2B immunolocalization of the candidate human retrotrapezoid nucleus. Pediatric Dev Pathol Off J Soc Pediatr Pathol Paediatr Pathol Soc. 2010;13:291–299. doi: 10.2350/09-07-0682-OA.1. [DOI] [PubMed] [Google Scholar]
- 48.Saiyed R, Rand CM, Patwari PP, Koliboski CM, Stewart TH, Peters P, Carroll MS, Weese-Mayer DE. Altered temperature regulation in respiratory and autonomic disorders of infancy, childhood, and adulthood (RADICA) Am J Respir Crit Care Med. 2011;183A:6394. [Google Scholar]
- 49.Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- 50.Steindler DA, Trosko BK. Two types of locus coeruleus neurons born on different embryonic days in the mouse. Anat Embryol (Berl) 1989;179:423–434. doi: 10.1007/BF00319584. [DOI] [PubMed] [Google Scholar]
- 51.Taneja P, Ogier M, Brooks-Harris G, Schmid DA, Katz DM, Nelson SB. Pathophysiology of locus ceruleus neurons in a mouse model of Rett syndrome. J Neurosci. 2009;29:12187–12195. doi: 10.1523/JNEUROSCI.3156-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomycz ND, Haynes RL, Schmidt EF, Ackerson K, Kinney HC. Novel neuropathologic findings in the Haddad syndrome. Acta Neuropathol. 2010;119:261–269. doi: 10.1007/s00401-009-0599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trochet D, O’Brien LM, Gozal D, Trang H, Nordenskjold A, Laudier B, Svensson PJ, Uhrig S, Cole T, Niemann S et al (2005) PHOX2B genotype allows for prediction of tumor risk in congenital central hypoventilation syndrome. American journal of human genetics 76:421–426. doi:10.1086/428366 [DOI] [PMC free article] [PubMed]
- 54.Tupal S, Huang WH, Picardo MC, Ling GY, Del Negro CA, Zoghbi HY, Gray PA (2014) Atoh1-dependent rhombic lip neurons are required for temporal delay between independent respiratory oscillators in embryonic mice. eLife 3:e02265. doi:10.7554/eLife.02265 [DOI] [PMC free article] [PubMed]
- 55.Viemari JC (2008) Noradrenergic modulation of the respiratory neural network. Respir Physiol Neurobiol 164:123–130. doi:10.1016/j.resp.2008.06.016 [DOI] [PubMed]
- 56.Viemari JC, Bevengut M, Burnet H, Coulon P, Pequignot JM, Tiveron MC, Hilaire G. Phox2a gene, A6 neurons, and noradrenaline are essential for development of normal respiratory rhythm in mice. J Neurosci. 2004;24:928–937. doi: 10.1523/JNEUROSCI.3065-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Keens TG, Loghmanee DA, Trang H (2010) An official ATS clinical policy statement: congenital central hypoventilation syndrome: genetic basis, diagnosis, and management. Am J Respir Crit Care Med 181:626–644. doi:10.1164/rccm.200807-1069ST [DOI] [PubMed]
- 58.Weese-Mayer DE, Berry-Kravis EM, Zhou L. Adult identified with congenital central hypoventilation syndrome–mutation in PHOX2b gene and late-onset CHS. Am J Respir Crit Care Med. 2005;171:88. doi: 10.1164/ajrccm.171.1.950. [DOI] [PubMed] [Google Scholar]
- 59.Weese-Mayer DE, Berry-Kravis EM, Zhou L, Maher BS, Silvestri JM, Curran ME, Marazita ML. Idiopathic congenital central hypoventilation syndrome: analysis of genes pertinent to early autonomic nervous system embryologic development and identification of mutations in PHOX2b. Am J Med Genet Part A. 2003;123A:267–278. doi: 10.1002/ajmg.a.20527. [DOI] [PubMed] [Google Scholar]
- 60.Wrobel LJ, Ogier M, Chatonnet F, Autran S, Mezieres V, Thoby-Brisson M, McLean H, Taeron C, Champagnat J. Abnormal inspiratory depth in Phox2a haploinsufficient mice. Neuroscience. 2007;145:384–392. doi: 10.1016/j.neuroscience.2006.11.055. [DOI] [PubMed] [Google Scholar]
- 61.Zhang X, Su J, Cui N, Gai H, Wu Z, Jiang C. The disruption of central CO2 chemosensitivity in a mouse model of Rett syndrome. Am J Physiol Cell Physiol. 2011;301:C729–C738. doi: 10.1152/ajpcell.00334.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 2 (PPTX 186,429 kb)