Abstract
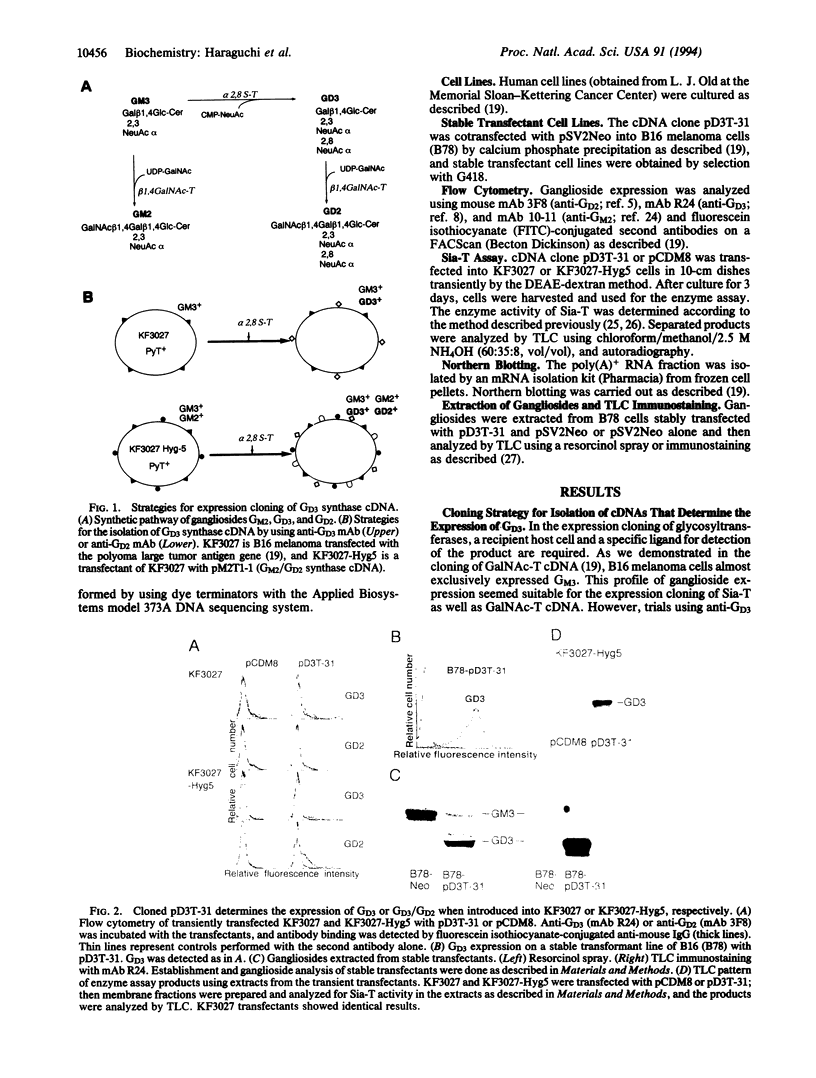
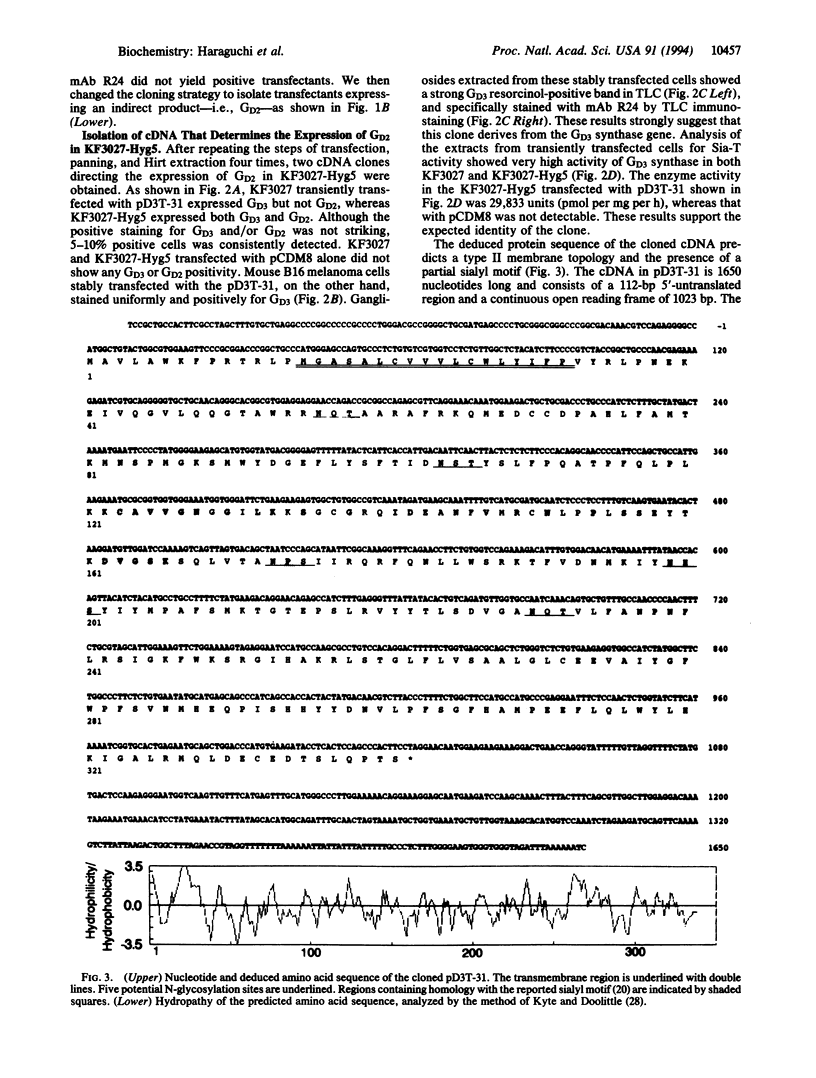
For the isolation of ganglioside GD3 synthase (EC 2.4.99.8) cDNA, we developed an expression cloning approach that used an anti-GD2 monoclonal antibody for selection. A host recipient cell line that we have named KF3027-Hyg5 was also utilized. This cell line expresses high levels of GM2 as well as GM3 but no GD3 or GD2 and was constructed from mouse B16 melanoma cells transfected with the polyoma large tumor antigen gene (KF3027) and the previously cloned beta-1,4-N-acetylgalactosaminyltransferase (EC 2.4.1.92) cDNA. Four rounds of transfection, monoclonal antibody 3F8 panning, and Hirt extraction resulted in the isolation of two cDNA clones, transfection of which directed the expression of GD3 in KF3027 and B16 melanoma cells and GD3 and GD2 in KF3027-Hyg5 cells. The cDNA contained a 1650-bp insert and a single open reading frame. The deduced amino acid predicted a type II membrane topology consisting of cytoplasmic (14 aa), transmembrane (18 aa), and catalytic (309 aa) domains. The sequence also predicted the presence of a sialyl motif similar to that found in the other sialyltransferases cloned so far. As expected, mRNA of this gene (2.6 kb) was strongly expressed in human melanoma lines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung N. K., Saarinen U. M., Neely J. E., Landmeier B., Donovan D., Coccia P. F. Monoclonal antibodies to a glycolipid antigen on human neuroblastoma cells. Cancer Res. 1985 Jun;45(6):2642–2649. [PubMed] [Google Scholar]
- Cortassa S., Panzetta P., Maccioni H. J. Biosynthesis of gangliosides in the developing chick embryo retina. J Neurosci Res. 1984;12(2-3):257–267. doi: 10.1002/jnr.490120213. [DOI] [PubMed] [Google Scholar]
- Dippold W. G., Lloyd K. O., Li L. T., Ikeda H., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: definition of six antigenic systems with mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6114–6118. doi: 10.1073/pnas.77.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Akagi T., Nagata Y., Yamada Y., Shimotohno K., Cheung N. K., Shiku H., Furukawa K. GD2 ganglioside on human T-lymphotropic virus type I-infected T cells: possible activation of beta-1,4-N-acetylgalactosaminyltransferase gene by p40tax. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1972–1976. doi: 10.1073/pnas.90.5.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Clausen H., Hakomori S., Sakamoto J., Look K., Lundblad A., Mattes M. J., Lloyd K. O. Analysis of the specificity of five murine anti-blood group A monoclonal antibodies, including one that identifies type 3 and type 4 A determinants. Biochemistry. 1985 Dec 17;24(26):7820–7826. doi: 10.1021/bi00347a047. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Hersey P., Schibeci S. D., Townsend P., Burns C., Cheresh D. A. Potentiation of lymphocyte responses by monoclonal antibodies to the ganglioside GD3. Cancer Res. 1986 Dec;46(12 Pt 1):6083–6090. [PubMed] [Google Scholar]
- Joziasse D. H. Mammalian glycosyltransferases: genomic organization and protein structure. Glycobiology. 1992 Aug;2(4):271–277. doi: 10.1093/glycob/2.4.271. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa N., Hamamoto T., Lee Y. C., Nakaoka T., Kojima N., Tsuji S. Molecular cloning and expression of GalNAc alpha 2,6-sialyltransferase. J Biol Chem. 1994 Jan 14;269(2):1402–1409. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Ledeen R. W., Yu R. K. Gangliosides: structure, isolation, and analysis. Methods Enzymol. 1982;83:139–191. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- Livingston B. D., Paulson J. C. Polymerase chain reaction cloning of a developmentally regulated member of the sialyltransferase gene family. J Biol Chem. 1993 Jun 5;268(16):11504–11507. [PubMed] [Google Scholar]
- Merritt W. D., Casper J. T., Lauer S. J., Reaman G. H. Expression of GD3 ganglioside in childhood T-cell lymphoblastic malignancies. Cancer Res. 1987 Mar 15;47(6):1724–1730. [PubMed] [Google Scholar]
- Nagata Y., Yamashiro S., Yodoi J., Lloyd K. O., Shiku H., Furukawa K. Expression cloning of beta 1,4 N-acetylgalactosaminyltransferase cDNAs that determine the expression of GM2 and GD2 gangliosides. J Biol Chem. 1992 Jun 15;267(17):12082–12089. [PubMed] [Google Scholar]
- Nakakuma H., Sanai Y., Shiroki K., Nagai Y. Gene-regulated expression of glycolipids: appearance of GD3 ganglioside in rat cells on transfection with transforming gene E1 of human adenovirus type 12 DNA and its transcriptional subunits. J Biochem. 1984 Nov;96(5):1471–1480. doi: 10.1093/oxfordjournals.jbchem.a134976. [DOI] [PubMed] [Google Scholar]
- Natoli E. J., Jr, Livingston P. O., Pukel C. S., Lloyd K. O., Wiegandt H., Szalay J., Oettgen H. F., Old L. J. A murine monoclonal antibody detecting N-acetyl- and N-glycolyl-GM2: characterization of cell surface reactivity. Cancer Res. 1986 Aug;46(8):4116–4120. [PubMed] [Google Scholar]
- Norihisa Y., McVicar D. W., Ghosh P., Houghton A. N., Longo D. L., Creekmore S. P., Blake T., Ortaldo J. R., Young H. A. Increased proliferation, cytotoxicity, and gene expression after stimulation of human peripheral blood T lymphocytes through a surface ganglioside (GD3) J Immunol. 1994 Jan 15;152(2):485–495. [PubMed] [Google Scholar]
- Old L. J. Cancer immunology: the search for specificity--G. H. A. Clowes Memorial lecture. Cancer Res. 1981 Feb;41(2):361–375. [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Portoukalian J., Zwingelstein G., Doré J. F. Lipid composition of human malignant melanoma tumors at various levels of malignant growth. Eur J Biochem. 1979 Feb 15;94(1):19–23. doi: 10.1111/j.1432-1033.1979.tb12866.x. [DOI] [PubMed] [Google Scholar]
- Pukel C. S., Lloyd K. O., Travassos L. R., Dippold W. G., Oettgen H. F., Old L. J. GD3, a prominent ganglioside of human melanoma. Detection and characterisation by mouse monoclonal antibody. J Exp Med. 1982 Apr 1;155(4):1133–1147. doi: 10.1084/jem.155.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan S., Lloyd K. O. Glycosylation pathways in the biosynthesis of gangliosides in melanoma and neuroblastoma cells: relative glycosyltransferase levels determine ganglioside patterns. Cancer Res. 1992 Oct 15;52(20):5725–5731. [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Sanai Y., Oda K., Nagai Y. Induction of GD3 ganglioside by adenovirus E1A gene 13S- and 12S-mRNA products in rat 3Y1 cells. J Biochem. 1990 May;107(5):740–742. doi: 10.1093/oxfordjournals.jbchem.a123118. [DOI] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui B., Buehler J., DeGregorio M. W., Macher B. A. Differential expression of ganglioside GD3 by human leukocytes and leukemia cells. Cancer Res. 1984 Nov;44(11):5262–5265. [PubMed] [Google Scholar]
- Suzaki K. The pattern of mammalian brain gangliosides. 3. Regional and developmental differences. J Neurochem. 1965 Dec;12(12):969–979. doi: 10.1111/j.1471-4159.1965.tb10256.x. [DOI] [PubMed] [Google Scholar]
- Welte K., Miller G., Chapman P. B., Yuasa H., Natoli E., Kunicka J. E., Cordon-Cardo C., Buhrer C., Old L. J., Houghton A. N. Stimulation of T lymphocyte proliferation by monoclonal antibodies against GD3 ganglioside. J Immunol. 1987 Sep 15;139(6):1763–1771. [PubMed] [Google Scholar]
- Wen D. X., Livingston B. D., Medzihradszky K. F., Kelm S., Burlingame A. L., Paulson J. C. Primary structure of Gal beta 1,3(4)GlcNAc alpha 2,3-sialyltransferase determined by mass spectrometry sequence analysis and molecular cloning. Evidence for a protein motif in the sialyltransferase gene family. J Biol Chem. 1992 Oct 15;267(29):21011–21019. [PubMed] [Google Scholar]
- Yamashiro S., Ruan S., Furukawa K., Tai T., Lloyd K. O., Shiku H., Furukawa K. Genetic and enzymatic basis for the differential expression of GM2 and GD2 gangliosides in human cancer cell lines. Cancer Res. 1993 Nov 15;53(22):5395–5400. [PubMed] [Google Scholar]
- Yu R. K., Macala L. J., Taki T., Weinfield H. M., Yu F. S. Developmental changes in ganglioside composition and synthesis in embryonic rat brain. J Neurochem. 1988 Jun;50(6):1825–1829. doi: 10.1111/j.1471-4159.1988.tb02484.x. [DOI] [PubMed] [Google Scholar]