Abstract
Objectives:
To evaluate the plasma and salivary total antioxidant capacity (TAOC) in patients with generalized chronic periodontitis (CP), generalized aggressive periodontitis (AgP), and periodontally healthy controls.
Methods:
This cross-sectional study includes of 88 individuals seeking dental treatment at the Faculty of Dentistry, Istanbul University, Istanbul, Turkey between January 2011 and March 2012. Fifteen AgP patients were compared with 21 healthy controls (C1), while 36 CP patients were compared with 16 healthy controls (C2). Clinical periodontal measurements were recorded, and plasma and saliva samples were collected. The TAOC of the plasma and saliva samples were determined using a commercially available colorimetric kit.
Results:
The plasma TAOC of both AgP and CP patients was significantly lower for C1 and C2. The salivary TAOC of CP patients was significantly lower for C2, but there was no significant difference between AgP patients and C1.
Conclusion:
Our results demonstrate that severe periodontitis may be associated with a lower plasma antioxidant capacity. The reduced antioxidant capacity in patients with severe periodontitis, especially with aggressive forms may be an important contributing factor to severe tissue destruction.
Periodontitis is a multifactorial degenerative disease associated with inflammation. It is characterized by the colonization of pathogenic bacteria, and the progressive destruction of the alveolar bone and connective tissues leading to possible tooth loss.1,2 Although aggressive periodontitis (AgP) and chronic periodontitis (CP) have many similar clinical manifestations, there are several differences including: rate of progression, pattern of destruction, and clinical signs of inflammation.3 It suggested that in periodontitis, activated neutrophils release several mediators, including reactive oxygen species (ROS) and proteolytic enzymes, which subsequently induce the secretion of several immunomodulatory compounds involved in the elimination of pathogens. If the excessive ROS produced during the immune response are not effectively neutralized by antioxidants, host-tissue damage may result.4-6 The contribution of ROS to the tissue destruction in periodontitis has been known for some time, but the role of antioxidants in this process remains unclear.7,8 Reactive oxygen species include a variety of chemical species with one unpaired electron in the outer shell and are derived predominantly from molecular oxygen.9 Under physiological conditions, a small portion of the oxygen consumed by the body is constantly converted to ROS (such as, superoxide anions, hydrogen peroxide, hydroxyl radicals, and hypochlorous acid). The main sources of ROS include the mitochondrial respiratory electron transport chain, the respiratory burst associated with neutrophil activation, xanthine oxidase activation from ischemia-reperfusion injury, and arachidonic acid metabolism.10 Up to a threshold concentration, ROS modulate some cellular functions, and they are recognized as important mediators of cell growth, adhesion, differentiation, protection against environmental pathogens, senescence, and apoptosis.11 However, when these species exceed a threshold level they cause cellular damage either directly or by modulating the effectors of various signaling pathways. Cells, tissues, and body fluids have powerful antioxidant defense systems that counteract oxidative damage, such as superoxide dismutases, catalase, glutathione peroxidase, uric acid, ascorbic acid, glutathione, lipoic acid, carotenoids, vitamin E, and ubiquinol.12 Oxidative stress occurs in tissues when there is an imbalance between ROS generation and antioxidant defenses. The total antioxidant capacity (TAOC) is an integrated parameter that reflects the cumulative action of mainly non-enzymatic antioxidants present in the plasma and body fluids.13,14 It is suggested that measurement of the TAOC may provide information on the balance between oxidants and antioxidant systems.15 An inadequate antioxidant capacity may play a role in the excessive tissue destruction with AgP. Our hypothesis was that AgP patients would have a lower antioxidant capacity than CP patients and the controls. The aim of this cross-sectional study was to examine the salivary and plasma TAOC, as well as the clinical findings in patients with CP and AgP compared with age- and gender-matched healthy controls.
Methods
Study population
A total of 88 individuals seeking dental treatment at the Faculty of Dentistry, Istanbul University, Istanbul, Turkey between January 2011 and March 2012 were included in the present study. Fifteen otherwise healthy, untreated patients with generalized AgP (2 males and 13 females; age range: 22-29 years), and 36 systemically healthy, untreated patients with generalized CP (11 males and 25 females; age range: 31-50 years) were recruited. Two groups of systemically and periodontally healthy individuals were also recruited: Control group 1 (C1) included 21 individuals (4 males and 17 females; age range: 21-31 years) matched in age to the AgP group; and Control group 2 (C2) included 16 individuals (10 females and 6 males; age range: 32- 50 years) matched in age to the CP group. The study was approved by the Ethics Committee of Istanbul University Faculty of Medicine, and conducted in full accordance with ethical principles including the Declaration of Helsinki, as revised in 2002. Written informed consent was obtained from all participants before the clinical periodontal examinations and specimen collection. The TAOC varies from each geographic region, gender, and diet. Therefore, we tried the recruited, test, and control groups similarly. There is no difference between the corresponding control groups and the test groups in terms of gender, and they are all Caucasian. Individuals were systemically healthy and exclusion criteria included: pregnancy, use of non-steroidal anti-inflammatory drugs, use of antimicrobial drugs, or use of mouthwashes and vitamin supplements in the 3 months preceeding the study. Individuals with obvious oral mucosal lesions, periodontal abscesses, or acute necrotizing gingivitis-like conditions were also excluded from the study. All volunteers did not have a history of smoking or recreational drug use. Individuals with generalized AgP and CP were diagnosed in accordance with the clinical criteria stated in the consensus report of the 1999 American Academy of Periodontology Workshop.16 The enrollment criteria for the groups were as follows: CP - a minimum of 20 natural teeth (excluding third molars), ≥30% of periodontal sites with ≥4 mm probing depth (PD), ≥50% of sites bleed on probing (BOP), ≥20% of periodontal sites with >2 mm interproximal clinical attachment level (CAL), and radiographic evidence of bone loss; AgP - the presence of any other systemic infection or disease, having at least 20 teeth, attachment loss of at least 5 mm affecting at least 8 teeth, and bone loss around at least 3 teeth other than incisors and first molars; and C1 & C2 - individuals with clinically healthy periodontium, <20% of sites with BOP, none of the sites with ≥4 mm PD, none of the sites with >2 mm CAL, and no radiographic evidence of bone loss. Periodontal disease severity was defined as follows; 2 or more interproximal sites with CAL ≥6 mm, not on the same tooth, and one or more interproximal sites with PD ≥5 mm.
Clinical examination
Clinical periodontal measurements were obtained at 6 sites (mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual, and disto-lingual locations) on each tooth, except the third molars, using a periodontal probe (Williams periodontal probe, Hu-Friedy, Chicago, IL, USA) and included the following: the dichotomous plaque index (PI) (positive or negative), gingival index (GI),17 PD, CAL, and the presence of BOP (positive or negative). The CAL was assessed from the cement-enamel junction (CEJ) to the base of the probable pocket. The BOP (deemed positive if it occurred within 15 seconds after periodontal probing), and visible plaque accumulation was recorded dichotomously by visual examination. All measurements were performed by a single calibrated examiner. The intraexaminer reliability was high, as revealed by an intraclass correlation coefficient of 0.83 for PD measurements.
Saliva and blood sampling
Saliva and blood samples were collected one week after the clinical periodontal measurements. Participants were instructed to refrain from eating, drinking, and performing oral hygiene procedures 12 hours before saliva collection. Saliva samples were collected according to the unstimulated whole saliva method between 8:00-11:00 am. After rinsing the mouth with tap water, subjects were asked to expectorate saliva into sterile plastic cups for 10 minutes. Following collection, sample cups were immediately sealed with rubber stoppers and stored at -80°C. Fasting blood samples were collected in heparinized tubes by trained assistants. After collection, samples were immediately transported to the laboratory for plasma separation and were stored at -80°C.
Saliva and plasma TAOC analysis
The TAOC was measured using a commercially available colorimetric kit (Cat. No: 709001, Cayman Chemical Company, MI, USA) which is based on the ability of antioxidants to inhibit the oxidation of ABTS® (2,2’-azino-di-[3-ethylbenzthiazoline sulphonate]) to ABTS®·+ by metmyoglobin. The capacity of the sample antioxidants to prevent ABTS oxidation is compared with that of Trolox, a water-soluble tocopherol analogue, and is quantified as molar Trolox equivalents. After saliva samples were diluted 1:2 and plasma samples 1:20, all samples were analyzed according to the manufacturer’s instructions. The absorbance was read at 405 nm, and the results were expressed as µM.
Statistical analysis
The Number Cruncher Statistical System (NCSS) 2007 and PASS 2008 Statistical Software (Kaysville, Utah, USA) packages were used for data analyses. Demographic and diagnostic clinical data were performed using Pearson’s chi-square and independent-sample t-test. Each patient was considered an observational unit for all clinical measurements, and for each parameter, the average of the whole mouth measurements (excluding the third molars) was used for calculations. The normality of data distribution was assessed using the Kolmogorov-Smirnov test, which indicated that the data were not normally distributed. Kruskal-Wallis multiple-comparison test was used to determine the differences in parameters among the four groups. If a difference was detected, Bonferroni (all-pairwise) multiple-comparison test were used to determine which of the groups were different. Pearson’s correlation analysis was performed to investigate possible relationships between the biochemical and clinical parameters.
Results
Clinical findings
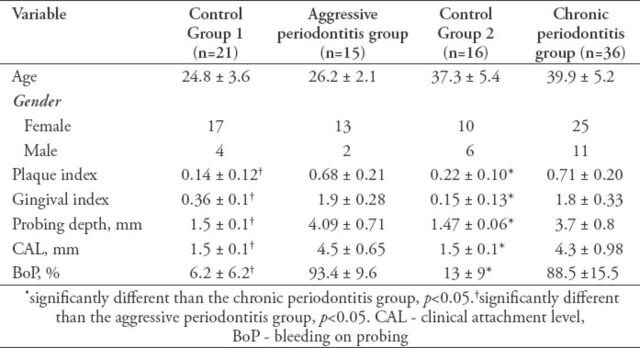
Table 1 shows the demographic variables and clinical parameters of the subjects. There were no significant differences between the mean age of the AgP or CP patients, and their respective controls. The CP patients were significantly older than the AgP patients (p<0.05). The PI, GI, PD, CAL, and BOP were significantly higher in the AgP and the CP group than in C1 and C2 (p<0.005 for all parameters). There was no significant difference between the periodontal clinical parameters of AgP and CP patients.
Table 1.
Demographic characteristics and diagnostic clinical parameters of dental subjects (mean ± standard deviation).
Saliva and plasma TAOC
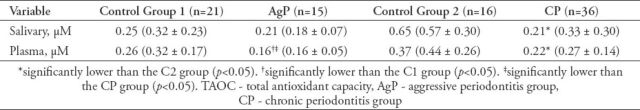
Table 2 indicates the salivary and plasma TAOC for all subjects. The plasma TAOC for AgP and CP patients were lower than those of the C1 and C2 groups (p<0.05). The plasma TAOC of the CP group was higher than that of the AgP group (p<0.05). The only significant difference in the saliva TAOC was between CP patients and the C2 group, with the capacity being lower in periodontitis patients (p<0.05). The saliva TAOC was negatively associated with the PD (r=0.37, p<0.001), BOP (r=0.36, p<0.001), and CAL (r=0.32, p<0.001). There were also negative correlations between the plasma TAOC and the PD (r=0.30, p<0.01), BOP (r=0.26, p=0.01), and CAL (r=0.25, p<0.01). When the correlation between saliva and plasma TAOC levels were evaluated, only KP group have shown a correlation (r=0.48, p<0.0001).
Table 2.
Salivary and plasma TAOC of patients with CP and AgP and the respective controls (median, mean ± standard deviation).
Discussion
The present study compared saliva and plasma TAOC levels in non-smoking patients with generalized CP or AgP, and clinically healthy individuals. Several reports associate periodontal diseases with an imbalance between oxidants and antioxidants. This can be the result of an increase in free radical production and/or a defect in antioxidant activity.7,18 It is suggested that the severity of periodontitis is independently associated with increased oxidative stress and a reduced antioxidant capacity.8,19 This study demonstrated that plasma TAOC levels were significantly lower in both severe CP and AgP patients than age- and gender-matched control groups.
Periodontal disease is caused by bacteria that populates the gingival crevices and periodontal pockets. The tissue destruction is mediated by chronic and progressive inflammation in response to bacterial colonization.1 Inflammation and infection in periodontal disease exhibit a low-grade systemic state, which may reduce the plasma total antioxidant capacity. Such inflammation may also be an innate response to periodontal bacteria.7,20 The AgP and CP have been associated with an enhanced host response characterized by higher serum levels of inflammatory markers. The AgP causes more rapid damage to the supporting dental tissues than CP.3,19,21 Our findings indicated that individuals with AgP had a lower plasma TAOC than those with CP. Oxidative stress has been implicated in the pathogenesis of aging, and the degree of oxidative damage increases with age.22,23 Lower TAOC levels and younger age could be associated with AgP. In contrast to our findings, D’Aiuto et al19 failed to find a significant difference between individuals with AgP and CP. However, the data for CP and AgP patients were not presented separately. Discrepancies among studies may arise from differing methodologies, or the lack of a specific diagnosis. Strong evidence suggests an association between plasma TAOC and CP. In the present study, individuals with severe CP had a lower plasma TAOC, in accordance with previous reports.18,24 Chapple et al18 concluded that the plasma TAOC is inversely associated with periodontitis, and the association is stronger for severe disease. Both Abou Sulaiman et al24 and Brock et al7 concluded that CP patients had a lower plasma TAOC than controls.
Our results revealed that plasma TAOC is significantly lower in AgP patients than in age-matched controls. Unfortunately, few studies have compares these 2 patient groups. Lower saliva TAOC in CP patients has been reported previously.7,25-27 Guentsch et al27 reported that the TAOC flow rate (antioxidant delivery in saliva) was significantly lower in patients with periodontitis (0.34 ± 0.26 µM) compared with controls (0.62 ± 0.24 µM; p<0.05). Miricescu et al25 reported that the salivary antioxidant activity was decreased significantly in patients with CP (p<0.05). Our results are consistent with these studies. We also found a significant difference between CP patients and controls. Brock et al7 reported that the salivary TAOC was lower with periodontitis, but this difference was not significant. Conflicting results between studies might be due to differences in disease severity among subjects. All patients in this study had severe periodontitis. In contrast, Novakovic et al26 found similar salivary antioxidant profiles between periodontitis patients and controls. In their study, the mean PD levels were similar between groups. This could explain the similar salivary TAOC. We found that the salivary TAOC of AgP patients was lower than that of periodontally healthy individuals, albeit not significantly so. Similar to our results, Baltacioglu et al28 reported that the salivary TAOC concentrations were significantly lower in the AgP group than CP group and controls. To our knowledge, only a few studies focused on saliva TAOC of AgP patients. Further investigation of the salivary TAOC in AgP patients is needed.
Study limitations
Inclusion of only non-smokers and the use of separate age- and gender-matched control groups for both CP and AgP strengthen our findings. To eliminate the overlap between CP and AgP patients, subjects older than 30 years were not included in the AgP group. A limitation of our study was the lack of plasma and saliva oxidant parameter measurements. This could explain the lower antioxidant capacity in severe periodontitis patients, particularly with AgP. However, evaluation of the plasma and salivary TAOC in plasma and saliva was the main objective of the present study.
In conclusion, this study shows that severe periodontitis is associated with the plasma and salivary antioxidant capacity. The reduced antioxidant capacity in patients with severe periodontitis, especially an aggressive form may be an important contributor to severe tissue destruction. Longitudinal studies with a larger sample size are needed to define the role played by antioxidants in the progression of periodontal disease.
Acknowledgment
The authors gratefully acknowledge Professor Halim Issever, PhD, Department of Public Health, Istanbul Medical Faculty, Istanbul University for his invaluable contribution to the statistical analyses. We are also extremely grateful to Dr. Sami Fatayel, PhD, Department of Periodontology, Istanbul University, Istanbul, Turkey for the Arabic translation.
Footnotes
Related Articles
Al-Zahrani MS, Alghamdi HS. Effect of periodontal treatment on serum C-reactive protein level in obese and normal-weight women affected with chronic periodontitis. Saudi Med J 2012; 33: 309-314.
Asemi Z, Jazayeri S, Najafi M, Samimi M, Tabasi Z, Shidfar F, et al. Plasma total antioxidant capacity and its related factors in Iranian pregnant women. Saudi Med J 2011; 32: 1246-1250.
References
- 1.Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 2.Goodson JM, Tanner ACR, Haffajee AD, Sornberger GC, Socransky SS. Patterns of progression and regression of advanced destructive periodontal disease. J Clin Periodontol. 1982;9:472–481. doi: 10.1111/j.1600-051x.1982.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 3.Armitage GC, Cullinan MP. Comparison of the clinical features of chronic and aggressive periodontitis. Periodontol 2000. 2010;53:12–27. doi: 10.1111/j.1600-0757.2010.00353.x. [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson A, Asman B. Increased release of free oxygen radicals from peripheral neutrophils in adult periodontitis after Fc delta-receptor stimulation. J Clin Periodontol. 1996;23:38–44. doi: 10.1111/j.1600-051x.1996.tb00502.x. [DOI] [PubMed] [Google Scholar]
- 5.Sheikhi M, Gustafsson A, Jarstrand C. Cytokine, elastase and oxygen radical release by Fusobacterium nucleatum-activated leukocytes: a possible pathogenic factor in periodontitis. J Clin Periodontol. 2000;27:758–762. doi: 10.1034/j.1600-051x.2000.027010758.x. [DOI] [PubMed] [Google Scholar]
- 6.Kantarci A, Oyaizu K, Van Dyke TE. Neutrophil-mediated tissue injury in periodontal disease pathogenesis: findings from localized aggressive periodontitis. J Periodontol. 2003;74:66–75. doi: 10.1902/jop.2003.74.1.66. [DOI] [PubMed] [Google Scholar]
- 7.Brock GR, Butterworth CJ, Matthews JB, Chapple IL. Local and systemic total antioxidant capacity in periodontitis and health. J Clin Periodontol. 2004;31:515–521. doi: 10.1111/j.1600-051X.2004.00509.x. [DOI] [PubMed] [Google Scholar]
- 8.Chapple IL, Brock GR, Milward MR, Ling N, Matthews JB. Compromised GCF total antioxidant capacity in periodontitis: cause or effect? J Clin Periodontol. 2007;34:103–110. doi: 10.1111/j.1600-051X.2006.01029.x. [DOI] [PubMed] [Google Scholar]
- 9.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;15:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tahara EB, Navarete FD, Kowaltowski AJ. Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic Biol Med. 2009;46:1283–1297. doi: 10.1016/j.freeradbiomed.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 12.Halliwell B. Oral inflammation and reactive species: a missed opportunity? Oral Dis. 2000;6:136–137. doi: 10.1111/j.1601-0825.2000.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 13.Ghiselli A, Serafini M, Natella F, Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med. 2000;29:1106–1114. doi: 10.1016/s0891-5849(00)00394-4. [DOI] [PubMed] [Google Scholar]
- 14.Sies H. Total antioxidant capacity: appraisal of a concept. J Nutr. 2007;137:1493–1495. doi: 10.1093/jn/137.6.1493. [DOI] [PubMed] [Google Scholar]
- 15.Collins AR. Assays for oxidative stress and antioxidant status: applications to research into the biological effectiveness of polyphenols. Am J Clin Nutr. 2005;81(Suppl 1):261–267. doi: 10.1093/ajcn/81.1.261S. [DOI] [PubMed] [Google Scholar]
- 16.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Löe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;25:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 18.Chapple IL, Milward MR, Dietrich T. The prevalence of inflammatory periodontitis is negatively associated with serum antioxidant concentrations. J Nutr. 2007;137:657–664. doi: 10.1093/jn/137.3.657. [DOI] [PubMed] [Google Scholar]
- 19.D’Aiuto F, Nibali L, Parkar M, Patel K, Suvan J, Donos N. Oxidative stress, systemic inflammation, and severe periodontitis. J Dent Res. 2010;89:1241–1246. doi: 10.1177/0022034510375830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76(Suppl 11):2106–2115. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 21.Salzberg TN, Overstreet BT, Rogers JD, Califano JV, Best AM, Schenkein HA. C-reactive protein levels in patients with aggressive periodontitis. J Periodontol. 2006;77:933–939. doi: 10.1902/jop.2006.050165. [DOI] [PubMed] [Google Scholar]
- 22.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 23.Golden TR, Melov S. Mitochondrial DNA mutations, oxidative stress, and aging. Mech Ageing Dev. 2001;122:1577–1589. doi: 10.1016/s0047-6374(01)00288-3. [DOI] [PubMed] [Google Scholar]
- 24.Abou Sulaiman AE, Shehadeh RM. Assessment of total antioxidant capacity and the use of vitamin C in the treatment of non-smokers with chronic periodontitis. J Periodontol. 2010;81:1547–1554. doi: 10.1902/jop.2010.100173. [DOI] [PubMed] [Google Scholar]
- 25.Miricescu D, Totan A, Calenic B, Mocanu B, Didilescu A, Mohora M, et al. Salivary biomarkers: relationship between oxidative stress and alveolar bone loss in chronic periodontitis. Acta Odontol Scand. 2014;72:42–47. doi: 10.3109/00016357.2013.795659. [DOI] [PubMed] [Google Scholar]
- 26.Novakovic N, Todorovic T, Rakic M, Milinkovic I, Dozic I, Jankovic S, et al. Salivary antioxidants as periodontal biomarkers in evaluation of tissue status and treatment outcome. J Periodontal Res. 2014;49:129–136. doi: 10.1111/jre.12088. [DOI] [PubMed] [Google Scholar]
- 27.Guentsch A, Preshaw PM, Bremer-Streck S, Klinger G, Glockmann E, Sigusch BW. Lipid peroxidation and antioxidant activity in saliva of periodontitis patients: effect of smoking and periodontal treatment. Clin Oral Investig. 2008;12:345–352. doi: 10.1007/s00784-008-0202-z. [DOI] [PubMed] [Google Scholar]
- 28.Baltacioglu E, Yuva P, Aydin G, Alver A, Kahraman C, Karabulut E, Akalin FA. Lipid peroxidation levels and total oxidant/antioxidant status in serum and saliva from patients with chronic and aggressive periodontitis. Oxidative stress index: a new biomarker for periodontal disease? J Periodontol. 2014;85:1432–1441. doi: 10.1902/jop.2014.130654. [DOI] [PubMed] [Google Scholar]