Abstract
IN BRIEF Insulin therapy is challenging for providers as well as for patients. This article describes a set of principles underlying appropriate insulin treatment and a detailed discussion of how to use them.
The most recent information from 2014 indicates that 29.1 million Americans have diabetes (1). Of these, 25–30% are taking insulin (2,3). Primary care providers are reluctant to start patients on insulin (4). Numerous studies have shown that, typically, 3–7 years elapse between failure of oral antidiabetic medications and insulin initiation (5–8). A1C levels at the time of insulin initiation in these studies ranged from 8.9 to 9.7% (5,9). Furthermore, once insulin is started, many primary care providers are uncomfortable adjusting doses and further intensifying insulin regimens. Mean A1C levels in patients receiving insulin have been found to range from 7.9 to 9.3% (9–11).
In a recent article in Clinical Diabetes (12), Galdo et al. nicely described the available insulin preparations and their pharmacokinetic and pharmacodynamic properties. They summarized the guidelines for using insulin published by several diabetes organizations and discussed various articles that described (to some extent) how to start insulin and adjust doses. They concluded that available instructions regarding how to accomplish this were not very specific. Although there is no one right way to use insulin, there are some important principles and relationships that underlie the appropriate use of insulin. This article will summarize that information and provide a more detailed (personal) approach to starting insulin, intensifying insulin regimens when necessary, and adjusting insulin doses.
Principles of Adjusting Insulin Doses
Table 1 summarizes the most important relationships among various components of the insulin prescription, their injection times, and the most appropriate self-monitoring of blood glucose (SMBG) test to judge their effects. Preprandial testing is preferred because it is much easier for patients to remember to test when they are stopping for a meal (and usually an insulin injection) rather than when they are busy 1–2 hours after eating. If preprandial glucose values are at target but A1C levels remain too high, postprandial SMBG is indicated to determine whether high glucose levels after meals may explain the above-target A1C.
TABLE 1.
Relationships Among Components of the Insulin Prescription, Injection Times, and Blood Glucose Testing
| Insulin Preparation | Time Injected | Period of Activity | Preprandial Test Best Reflecting Insulin Effect |
| Regular | Before a meal | Between that meal and either the next one or bedtime (snack) if injected before supper | Before next meal or bedtime (snack) if injected before supper |
| Lispro | |||
| Aspart | |||
| Glulisine | |||
| NPH | Before breakfast | Between lunch and supper | Before supper |
| U-500 Regular | |||
| NPH | Before supper or bedtime | Overnight | Before breakfast |
| U-500 Regular | |||
| Glargine | Any time | Peakless: 24 hours for glargine, ∼18 hours for detemir | Before breakfast |
| Detemir |
Each component of the insulin prescription is changed (or not) depending on the pattern of SMBG results that reflects its maximal activity. How should this be done? First, one must decide on the appropriate target ranges for each of these times. The American Diabetes Association (ADA) in 2015 changed its recommended preprandial target range of 70–130 mg/dL to 80–130 mg/dL “to better reflect new data comparing actual average glucose levels with A1C targets” (13). However, using this preprandial target range defines glucose values of 70–79 mg/dL as hypoglycemia, which contradicts the previous ADA definition of hypoglycemia as <70 mg/dL. Therefore, I continue to use the older preprandial target range of 70–130 mg/dL. The ADA kept its previous postprandial target of <180 mg/dL 1–2 hours after eating (13). I feel that their postprandial target is too high; instead, I use a postprandial target of 100–160 mg/dL.
The next step is to decide under what circumstances changes in insulin doses should be made. If a test at a specific time of day (usually before a meal or bedtime snack) is consistently too high or too low, raise or lower the appropriate insulin dose by 2 units in lean patients or 4 units in overweight or obese patients, or 10% of the current dose, whichever is higher. For insulin dose adjustments, I consider people to be overweight if they are ≥120% of their desirable body weight (DBW). I chose this value because mortality starts to increase at weights exceeding this level. The calculation of DBW (14) is described in Table 2. Alternatively, one could use the BMI for the designation of overweight. I would suggest using the older BMI definition of obesity (≥27 kg/m2) because it captures the upper cohort of the current definition of overweight (25.1–29.9 kg/m2).
TABLE 2.
Calculating Desirable Body Weight (DBW)
| Weight for Females (lb) | Weight for Males (lb) | |
| Initial Calculation | ||
| First 5 feet | 100 | 106 |
| Each inch over 5 feet | 5 | 6 |
| Subsequent Adjustment for Frame Size | Add 10% to DBW for large-framed individuals or subtract 10% for small-framed individuals. Frame size can be estimated by having the patient’s predominant hand grasp the other wrist and oppose the thumb and middle finger. If the two fingers meet, the patient has a medium frame (and thus no DBW adjustment is necessary). If they overlap appreciably, the patient is small framed, and if they fail to meet, the patient is large framed. | |
| Interpretation | Patients who are ≥120% of their DBW are considered overweight/obese, whereas those <120% of their DBW are considered lean. | |
Glucose concentrations at a specific time are considered too high if the number of values that exceed the upper target level minus the number of values that are less than the lower target level plus bona fide episodes of unexplained hypoglycemia for which no measured low glucose level is available constitute 50% or more of the glucose concentrations at that time of day during the period being considered. As examples, that would mean ≥2 such values over 3 days, ≥4/7 days, ≥5/10 days, ≥7/14 days, ≥11/21 days, ≥14/28 days, ≥18/35 days, ≥21/42 days, or ≥50% of the values during any number of days between 1 and 6 weeks. I prefer to analyze SMBG results at least every 6 weeks once insulin doses are reasonably stable because, over longer periods, patients (at least those in my practice) often become less adherent to the recommended insulin doses and SMBG testing frequency. However, if longer periods supervene, the principle that ≥50% of the glucose concentrations at that time of day during the time period being considered should be measured before an adjustment of an insulin dose would still hold. On the other hand, 50% may be too stringent a criterion for some, and individual providers may be comfortable with making decisions based on a smaller number of glucose results per time interval, perhaps as few as one-third.
Conversely, glucose concentrations at a specific time are considered too low if the number of values that are less than the lower end of the target range, plus bona fide episodes of unexplained hypoglycemia for which no measured low glucose value is available, minus the number of values that exceed the upper end of the target range constitute ≥50% of the glucose concentrations at that time of day during a 1- to 6-week period. If the glucose concentrations at a specific time of day are neither too high nor too low, no change is needed in the component of the insulin prescription that primarily affects the test at that time of day. Of course, no adjustment is made in that component of the insulin regimen for which there are too few available test results (i.e., the patient did not perform SMBG at that time on enough days during the period under review). On the other hand, episodes of unexplained severe hypoglycemia warrant serious consideration to reduce the dose of the appropriate component of the insulin regimen, especially if the patient has hypoglycemia unawareness.
The desired frequency of SMBG testing for insulin dose adjustments is an important issue. Patients taking only bedtime NPH insulin or only a basal insulin need only test in the fasting state (i.e., before breakfast). Once two or more insulin injections are required, testing before each meal and before the bedtime snack would be ideal. However, it is usually unrealistic to expect this frequency of testing for most patients. I strongly recommend that patients perform SMBG at least twice daily, alternating before breakfast and before supper with before lunch and before the bedtime snack.
I even more strongly recommend that all patients taking insulin eat a small bedtime snack containing some protein to decrease the chances of overnight hypoglycemia. The key to effective insulin dosing is a fairly consistent eating pattern around which to fit the insulin regimen. If a patient always foregoes a bedtime snack, one might not require the patient to eat one. However, for a patient in the habit of eating bedtime snacks intermittently, it would be impossible to safely adjust the basal or evening NPH insulin doses without knowing whether there would be a snack on any given evening. Furthermore, the longer the patient refrains from eating, the more likely (nocturnal) hypoglycemia is to occur. Therefore, I recommend eating a bedtime snack every night to maximize the chance of appropriate basal and evening NPH insulin dosing and to minimize the possibility of nocturnal hypoglycemia.
In my experience, it is unusual for patients taking a bedtime snack to experience overnight hypoglycemia. They are instructed to decrease their supper calories to accommodate the bedtime calories. Even if they should gain a few pounds, the additional cardiovascular risk in these obese individuals is minimal, especially compared to avoiding a potentially serious episode of overnight hypoglycemia. For example, the increase in the risk of cardiovascular disease in a 250-lb patient who may gain 10 lb more is minimal. On the other hand, patients experiencing hypoglycemia, especially overnight, may be reluctant to increase insulin doses when necessary. A few discontinue insulin altogether, and convincing them to re-start it may be difficult.
Hypoglycemia is another important factor affecting insulin dose adjustments. Frankly, few patients perform SMBG when they are experiencing hypoglycemia; treating it typically overrides testing. Therefore, deciding whether the episode described was bona fide hypoglycemia is important for making appropriate insulin dose adjustments. Readers of this journal will certainly be familiar with the symptoms of hypoglycemia, but, as is well known, a number of the symptoms are nonspecific. The key question to ask is what the patient did to treat the episode and how long it took for the symptoms to start to improve. If >20 minutes or so passed before improvement started (assuming some simple carbohydrate was ingested for treatment), this was probably not hypoglycemia, but instead possibly anxiety, the symptoms of which can mimic hypoglycemia. If hypoglycemia is either documented by SMBG or the episode seems bona fide by history, one must next determine whether it was explained or unexplained.
The principle behind prescribing an insulin regimen is to formulate it around the patient’s usual practiced lifestyle. Prescribing an insulin regimen and expecting the patient to change his or her lifestyle to accommodate it is seldom successful. This is not to say that one should not encourage patients to adopt a healthier lifestyle, but rather that one has to base the insulin regimen on what a patient is actually doing and not what one hopes he or she will do. From the patient’s perspective, this requires relatively consistent eating and exercise patterns. (Carbohydrate counting is an exception and will be discussed later.) If hypoglycemia occurs because the patient has altered the usual eating or exercise pattern (most commonly by missing a meal, eating a meal later than usual, or eating less carbohydrate than usual), this would be explained hypoglycemia and therefore should be ignored in the analysis. (Of course, one would point this out and educate the patient to avoid a similar episode in the future.) On the other hand, if hypoglycemia occurs in the presence of the patient’s usual lifestyle routine, this would be unexplained hypoglycemia and, if it occurs frequently enough, one must consider lowering the appropriate insulin dose accordingly. Often, patients perform SMBG after starting to treat an episode of hypoglycemia to determine if more carbohydrate is needed. These results (often high because of overtreatment) should be ignored. Only bona fide episodes of hypoglycemia need to be considered as low glucose values and entered into the analysis as such.
When and How to Initiate Insulin Therapy
Patients with type 1 diabetes, of course, need insulin immediately after diagnosis. An exception is patients with latent autoimmune diabetes of the adult (LADA), whose glucose can be controlled on noninsulin medications for a while, although for a much shorter period than for patients with type 2 diabetes. Because of the difficulties of using insulin (from both the patient and provider perspectives), as long as patients with LADA meet A1C targets, I do not use insulin. As soon as noninsulin medications fail, I start insulin. Although many guidelines recommend insulin in type 2 diabetes patients with high A1C levels, this is not always necessary. Almost all patients with newly diagnosed type 2 diabetes will respond quickly to maximal doses of a sulfonylurea with reduction of the initial dose often necessary to avoid hypoglycemia (15,16). This avoids the hassle of starting insulin, making frequent down-titrations, and—not uncommonly—discontinuing insulin in patients with newly diagnosed type 2 diabetes. Because of the progressive loss of insulin secretion in type 2 diabetes, however, patients with a longer duration of disease and very high A1C levels do require insulin.
Bedtime NPH and Basal Insulin Regimens
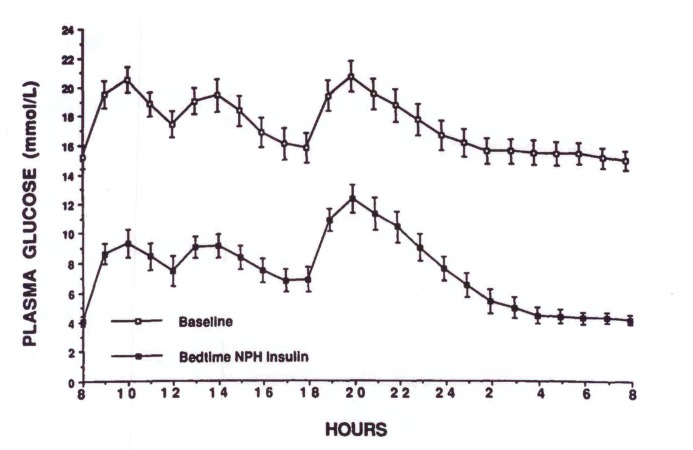
Almost all people with type 2 diabetes (with the exception of those who are newly diagnosed, in whom insulin may have been unnecessarily initiated) will have been treated with a combination of two to four noninsulin medications before insulin is started. Several older studies have shown that, when patients’ blood glucose fails to be controlled with maximal doses of metformin plus a sulfonylurea, multiple-dose insulin regimens do not yield better control than bedtime NPH insulin plus oral antidiabetic drugs when compared for up to 1 year (17–20). Therefore, if the patient’s fasting plasma glucose (FPG) concentration can be lowered appreciably, overall diabetes control is often significantly improved (Figure 1).
FIGURE 1.
One- to two-hour plasma glucose concentrations over a 24-hour period in patients taking maximal doses of either glyburide (20 mg/day) or glipizide (40 mg/day) (upper curve) or bedtime NPH insulin (lower curve). Reprinted with permission from Ref. 40.
Bedtime NPH or basal insulin is an easy way to introduce insulin therapy to patients with type 2 diabetes that is not well controlled with oral antidiabetic medications. Such a regimen involves only one daily insulin injection and, initially, requires only one SMBG test per day. Because the peak effect of NPH insulin occurs around breakfast time and glargine and detemir insulins are peakless, there is a much lower chance of daytime hypoglycemia mediated by exogenous insulin compared to a regimen in which NPH is injected before breakfast or a short- or rapid-acting insulin is given before meals. Bedtime NPH or basal insulin gives patients much more flexibility in their eating and exercise patterns during the day. Nocturnal hypoglycemia occurs somewhat less frequently with glargine and detemir compared to NPH. However, I have had little difficulty with overnight hypoglycemia from NPH when employing gradual dose increases, especially when patients eat a small bedtime snack.
Although the ADA A1C target is <7.0% for most patients, I do not start insulin until the A1C exceeds 7.4% because the risk of microvascular complications is only slightly increased between the A1C levels of 7.0 and 7.5% (21), and starting insulin is a big change in a person’s lifestyle (e.g., taking injections, performing more frequent SMBG, and being mindful of the risk of hypoglycemia). For patients whose A1C target should be less stringent (13), I use an A1C level of ≥8.0% before introducing insulin.
Overweight and obese patients are started on 16 units, and lean patients are started on 10 units of bedtime NPH insulin or one of the peakless basal insulins. With the exception of thiazolidinediones (TZDs), I maintain patients on their noninsulin medications to maximize the chances that daytime glycemia will be controlled after the FPG target is reached. TZDs are discontinued because they will enhance the weight gain seen with insulin, increase the degree of fluid retention, and thereby increase the risk of heart failure. The initial insulin doses are almost always less than patients eventually require but do prevent overnight hypoglycemia early on, which could discourage patients from remaining on insulin therapy. Doses are increased as described above (i.e., if ≥50% of the SMBG values before breakfast exceed the target, the dose is increased by 4 units for overweight or obese patients or 2 units for lean patients, or by 10% of the current dose, whichever is higher). Ideally, test values should be acted on frequently (every 3–7 days) when insulin is started; as values approach target levels, longer intervals between adjustments are reasonable. Ideally, I prefer adjusting the dose at least once per week for several weeks after starting insulin, every 2 weeks as the dose is titrated upward, every 3 weeks as the dose becomes stabilized, and every 6 weeks on an ongoing basis. Of course, one’s practice situation will largely determine the frequency of adjustments.
Many patients with type 2 diabetes are very obese and consequently require large doses of insulin to reach their target FPG levels. Because this can take many months to achieve, I attempt to teach these patients to self-titrate their insulin dose based on their FPG levels. Overweight or obese patients are instructed to increase their bedtime dose by 2 units and lean patients by 1 unit each evening if that morning’s SMBG value exceeded 130 mg/dL. Conversely, if the value was <70 mg/dL, the dose would be decreased by the same amount. Patients on high doses of a basal insulin sometimes inject these twice daily. In that case, overweight or obese patients are instructed to increase each dose by 2 units and lean patients by 1 unit. Self-titration ceases when there have been no dose changes for 1 week.
Self-titration instructions for patients are provided in English and Spanish on p. 128 and p. 129, respectively, of this issue of Clinical Diabetes. Providers can copy these instructions and simply circle the appropriate section for each patient.
When should a regimen including bedtime NPH or basal insulin plus daytime oral antidiabetic drugs be judged ineffective and replaced with a multiple-dose insulin regimen? This should not occur until target FPG concentrations are achieved and A1C levels 3 months later are still too high. A common error is to give up before reaching the target FPG level. This step usually occurs in obese patients for whom not enough insulin is prescribed, and the FPG levels hover around the mid- to high-100 mg/dL range. Many of these patients are extremely obese with resulting severe insulin resistance. They often require more insulin in a dose than can be given in a single syringe (100 units) or pen injection of glargine (80 units) or detemir (60 units). I have sometimes prescribed >100 units of insulin at bedtime to achieve the target FPG range. However, I usually prescribe basal insulin twice daily when the dose exceeds a single injection because large volumes of injectate impair absorption. (See discussion of U-500 insulin below.) Overnight hypoglycemia is seldom a problem because, as previously mentioned, the insulin doses are increased gradually; NPH peaks around breakfast time (instead of in the middle of the night when it is injected before supper); glargine and detemir are peakless; and patients are strongly encouraged to eat a small bedtime snack.
Once >50% of the fasting SMBG values are within the FPG target range, the A1C level determines whether the regimen of bedtime NPH or basal insulin is sufficient to control the patient’s diabetes. Because of the delay of A1C levels to plateau (22), one must wait 3 months to make this decision.
Soon after reaching the FPG target range, there is reason to perform SMBG a few times before supper. With FPG levels this much lower, the maximal dose of the patient’s sulfonylurea may cause daytime hypoglycemia. Before-supper values <80 mg/dL may presage daytime hypoglycemia, and the sulfonylurea dose should be halved. If this persists, the sulfonylurea should be discontinued. Halving and possibly subsequently discontinuing the sulfonylurea dose should also occur with bona fide episodes of unexplained (undocumented) daytime hypoglycemia, as well. Conversely, once FPG levels reach the target range, before-supper glucose values that consistently exceed 180 mg/dL strongly suggest that the target A1C level of <7.5% will not be attained 3 months hence, and intensification of the insulin regimen could be considered at this point.
Multiple-Injection Insulin Regimens
As previously mentioned, I use an A1C level ≥7.5% to signal the need to switch to two or more injections of insulin because multiple-dose insulin regimens, when followed appropriately, are much more difficult for patients in that they require more frequent SMBG; increase the risk of hypoglycemia, especially during the daytime; and, depending on the regimen implemented, may reduce flexibility with regard to eating and exercise patterns. There are three possible intensified insulin regimens that could be used: basal/bolus, self-mixed/split, and premixed insulin. I give patients the choice of the basal/bolus or the self-mixed split regimen, explaining the pros and cons of each. I do not recommend premixed insulins for reasons explained below.
Basal/Bolus Insulin Regimen
The basal/bolus insulin regimen usually consists of a basal insulin plus a short- or rapid-acting insulin before meals. In patients with type 2 diabetes, NPH insulin at bedtime would also be appropriate because these patients retain some insulin secretion. NPH insulin at bedtime would not be appropriate for patients with type 1 diabetes because, if there is a long period of time between lunch and supper, the effect of the pre-lunch dose might wane, and patients would experience high pre-supper glucose because they have little or no endogenous insulin secretion. This potential problem is much more likely if a rapid-acting insulin is the formulation used before lunch.
The basal/bolus approach requires four daily injections of insulin, which is a problem for some patients. On the other hand, it should be strongly considered for patients with irregular eating and exercise patterns.
Some investigators have recommended that, initially upon switching to a basal/bolus regimen, short- or rapid-acting insulin is only needed before the largest meal of the day (23,24). If the subsequent preprandial (or before bedtime snack, if the largest meal is supper) target is reached and the A1C remains above target, short- or rapid-acting insulin is introduced before the next largest meal. This is repeated if the second preprandial injection of short- or rapid-acting insulin achieves the subsequent preprandial target but the A1C target is still not attained; at this point, short- or rapid-acting insulin is taken before all three meals.
However, there are two potential problems with this approach. Although logically appealing, it can lead to long delays in reaching target A1C levels. First, the target glucose level, either postprandially or before the subsequent meal (or bedtime snack when supper is the meal in question), must be achieved. Then, at least another 3 months must elapse before the A1C level will accurately reflect overall glycemia. This period will be doubled if an injection before a second meal is required, and tripled if injections before all three meals are deemed necessary. Because only 25–30% of patients who start by adding a single preprandial dose of rapid-acting insulin to optimized basal insulin achieve an A1C <7.0% (25,26), there will be long delays in reaching A1C goals for most patients. Indeed, a recent study showed that this can take 8 months (27).
The second potential problem with regard to adding prandial insulin one meal at a time is that the most important determinant of postprandial glucose (and therefore subsequent preprandial glucose) is the preprandial value. The increases in postprandial over preprandial glucose values are similar regardless of the starting preprandial glucose level (28–30). Therefore, postprandial hyperglycemia is best treated initially by lowering preprandial glucose levels. When preprandial insulin before a single meal has controlled postprandial glucose concentrations but A1C is still not within the target range, a second preprandial injection must be introduced. However, when the second injection lowers the preprandial values before the initial meal that had just been successfully treated, the short- or rapid-acting insulin dose given before that first meal may be too high, leading to postprandial hypoglycemia. The same potential problem occurs when a third preprandial injection is introduced. For these reasons, I usually start preprandial insulin before all three meals when instituting a basal/bolus regimen. Because the patient’s current bedtime NPH or basal insulin dose has already achieved the FPG target, I continue it. The initial preprandial insulin dose is 2–4 units in lean patients and 4–6 units in overweight or obese patients. These doses almost always need to be increased, but starting at this level avoids hypoglycemia, which may discourage patients from continuing their intensified regimen.
Self-Mixed/Split Insulin Regimen
In the self-mixed/split insulin regimen, the insulin preparations are mixed in the same syringe and injected twice daily. If used properly, this approach can yield as tight a level of control as a basal/bolus regimen (31,32). However, patients have less flexibility with their eating and exercise patterns. To switch from a bedtime NPH or basal insulin regimen, I take 80% of that dose as the total NPH dose of the self-mixed split regimen and give two-thirds before breakfast and one-third before supper. This is most often less than the patient will eventually require but, again, avoids hypoglycemia, which can be discouraging to patients. Because almost all patients will require prandial insulin to achieve their A1C target, I routinely add a small amount of short- or rapid-acting insulin to each NPH injection after several visits. I delay adding the short- or rapid-acting insulin to allow patients to more gradually accommodate themselves to the lifestyle changes that taking insulin entails. Initially, patients are asked to perform SMBG before breakfast and supper to adjust their NPH doses. When these results are <200 mg/dL, patients are asked to alternate their twice-daily testing to before breakfast and supper one day and before lunch and bedtime snack the next day. The latter two tests allow for adjusting the short- or rapid-acting insulin doses.
The pre-supper and pre-breakfast glucose levels are brought down to <200 mg/dL with the appropriate NPH doses before the short- or rapid-acting insulin is increased. This is because larger doses of the short- or rapid-acting insulin will be needed to lower glucose levels to target before lunch and the bedtime snack if the pre-breakfast and pre-supper glucose levels, respectively, are higher than if they are closer to target. For example, reaching a pre-lunch glucose concentration of <130 mg/dL will require more short- or rapid-acting insulin when the FPG is 280 mg/dL than when it is 120 mg/dL. Delaying the increase of the short- or rapid-acting insulin until the doses of NPH have started to achieve better control reduces the possibility of hypoglycemic reactions. On the other hand, if one waits until target levels before breakfast and supper are reached before adjusting the appropriate doses of prandial insulin, too much time often elapses before better overall control is achieved.
Given that the peak effect of NPH taken before supper occurs between 6 and 12 hours later, as one increases this dose to control FPG, hypoglycemia may occur overnight before the target is reached. Decreasing the pre-supper dose may prevent the overnight hypoglycemia, but will hinder efforts to achieve the FPG target. If the patient is already eating a bedtime snack, adding a snack is not an available option to help prevent overnight hypoglycemia. In that event, moving the NPH from before supper to bedtime will almost always take care of the problem because the peak effect of the evening NPH will now occur just before breakfast when the patient is about to eat. This approach converts the two-injection self-mixed/split regimen to a three-injection regimen. However, it is often the only way to both avoid overnight hypoglycemia and meet the FPG target without converting to a four-injection basal/bolus regimen.
Premixed Insulin Regimens
Although premixed insulin preparations obviate the need for patients to mix two different insulin preparations in the same syringe before injection, they have a major drawback: one cannot adjust the doses of the intermediate-acting and short- or rapid-acting insulin separately. For example, a common SMBG pattern in patients taking a premixed insulin preparation is high glucose before lunch but acceptable or low glucose before supper. Raising the morning dose to lower the pre-lunch levels would jeopardize the pre-supper situation. Another common pattern is high glucose at bedtime but acceptable or low levels before breakfast. Raising the pre-supper dose to lower the bedtime results would increase the likelihood of overnight hypoglycemia. Therefore, achieving near-euglycemia with premixed insulins usually is not possible, and these preparations should be used only by patients who cannot be taught to mix insulins themselves and for whom no family or other caregiver is available. In my experience, most patients can be taught to mix insulin with persistent instruction.
Short- or Rapid-Acting Insulin
There are two ways to decide on the usual dose of short- or rapid-acting insulin. More sophisticated patients can learn carbohydrate counting and then determine the preprandial doses of these insulins based on the amount of carbohydrate to be ingested, usually 1 unit per 10 or 15 g of carbohydrate (33). In a self-mixed/split regimen, this would work for breakfast and supper but, in my view, would not be suitable for lunch. This is because, as mentioned previously, both the morning NPH insulin and the short- or rapid-acting insulin before lunch have their maximal effects between lunch and supper. It would not be clear which of these insulin doses to adjust in response to the pre-supper SMBG values. It would also mean a third daily injection, negating one of the advantages of the two-injection self-mixed/split regimen.
The other dietary approach is a constant-carbohydrate eating pattern, in which patients are instructed about the carbohydrate content of various food products and asked to ingest a relatively consistent amount of carbohydrate at each meal (i.e., a similar amount at every breakfast, lunch, and supper from day to day—not the same amount at every meal of the day). There is no evidence that carbohydrate counting leads to better glycemic control than the constant-carbohydrate approach (34), which is similar to the older “Exchange” system, recently termed “experience-based estimation” (35).
With experience-based estimation, the preprandial short- or rapid-acting insulin dose (in either a basal/bolus or a self-mixed/split regimen) can be broken down into three components. The “basic dose” is the amount prescribed to be taken before the meal and is the dose that is adjusted based on the pattern of SMBG values, as described earlier. The “correction dose” or “supplemental dose” depends on the preprandial glucose level. Some patients are also able to incorporate an “anticipatory dose” that depends on anticipated activities to occur with the meal or within several hours after the meal. For example, if a patient is eating at a Chinese restaurant, he or she may add a few units (in addition to any correction dose) in anticipation of a meal that is higher than usual in carbohydrate. Alternatively, if a patient is going to engage in more exercise than usual after supper, he or she may reduce the short- or rapid-acting insulin dose taken before the meal that would otherwise have been dictated by the calculations for basic and correction doses. There is no formula for anticipatory doses. They must be arrived at empirically based on the patient’s ongoing experiences.
Correction (Supplemental) Doses
Assuming no change in the carbohydrate content of the anticipated meal, high preprandial glucose concentrations require additional short- or rapid-acting insulin to lower the subsequent preprandial SMBG value into the target range. Table 3 shows the extra amounts of these insulins I start with to add to (or subtract from) the basic dose to correct elevated preprandial glucose concentrations. To evaluate the responses to these corrections doses, I use a target range for the subsequent preprandial SMBG value of 100–150 mg/dL. If the majority of responses to a specific correction dose are >150 mg/dL, the correction dose needs to be increased; if the majority are <100 mg/dL (plus episodes of undocumented, unexplained hypoglycemia), the correction dose should be decreased. A minimum of three responses to a specific correction dose is necessary before it can be evaluated. For example, if there are five instances in which a patient added 2 units of regular insulin for preprandial SMBG values between 201 and 250 mg/dL and the subsequent preprandial results were >150 mg/dL on four of those occasions, the correction dose for this preprandial range should be increased to +3 units. Moving forward, +3 units of short- or rapid-acting insulin would be added for the wider preprandial glucose range of 201–300 mg/dL. If subsequent experience shows that this correction dose is inadequate for the upper part of that range (251–300 mg/dL), the correction dose should be increased to +4 units for that range of preprandial values. To simplify the evaluation, it is helpful if patients record their usual prandial insulin dose and their correction dose separately, e.g., 3 + 2, before the meal in their logbooks.
TABLE 3.
Initial Correction Doses of Short- or Rapid-Acting Insulin
U-500 Regular Insulin
It is not uncommon for very obese patients with type 2 diabetes to require hundreds of units of insulin to achieve satisfactory control. Some, despite testing appropriately and increasing insulin doses as recommended, are unable to achieve A1C levels <8.0% because large volumes of injectate impair insulin absorption (36). In my practice, patients requiring >200 units of U-100 insulin per day are switched to injections of U-500 regular insulin before breakfast and before supper. The action profile of U-500 regular insulin is similar to that of NPH (37).
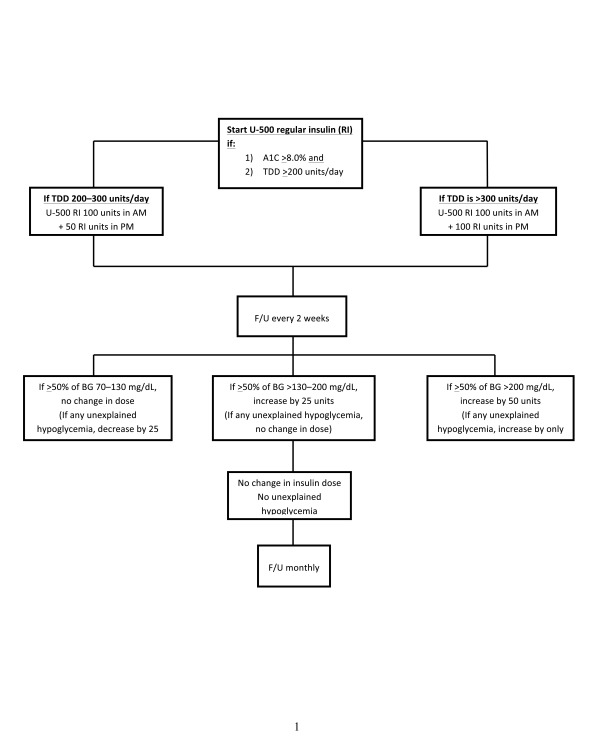
Initial doses and dose adjustments for U-500 insulin are shown in Figure 2. If A1C levels remain ≥7.5% after the pre-breakfast and pre-supper SMBG values reach target levels, separate injections of short- or rapid-acting insulin are given along with the U-500 insulin. I usually start with 6 units, and, inexplicably, pre-lunch and bedtime SMBG values show that these patients respond to lower doses of these U-100 insulins (10–30 units). A1C levels often can be lowered to <7.5% and routinely to <8.0% with U-500 regular insulin (36).
FIGURE 2.
Initiation and dose adjustments of U-500 regular insulin. BG, blood glucose; F/U, follow-up; RI, regular insulin; TDD, total daily dose. Reprinted with permission from Ref. 41.
Single Pre-Breakfast Injection of NPH Insulin
If a single morning injection of NPH is used, near-euglycemia is seldom achieved. This approach requires one injection of NPH to control both the pre-supper glucose concentration and the next morning’s fasting glucose value. In the typical scenario, the morning NPH dose is increased, and pre-supper glucose levels become acceptable before fasting values do. As the NPH dose is increased further to lower the fasting values, late-afternoon hypoglycemia occurs, and the NPH dose must be stabilized or decreased before target fasting levels have been reached. At this point, an evening dose of NPH must be introduced to further improve control. This means that the patient is now on a self-mixed/split regimen, which should have been the initial approach.
There are two exceptions to this typical scenario, however. A few patients with type 2 diabetes who are not well controlled with non-insulin medications have FPG values at or very near the target range. Their elevated A1C levels are the result of daytime hyperglycemia, most often manifested by high pre-supper SMBG results. In this case, pre-breakfast NPH insulin is indicated, initially 10 units in lean patients and 16 units in those who are overweight or obese. Eventually, as insulin secretion continues to decrease, evening NPH insulin becomes necessary.
A second rare exception to the usual morning NPH scenario is a patient who has a delayed response to NPH insulin (i.e., the peak effect of a morning injection occurs overnight, such that the morning injection affects the next day’s FPG more than it influences the pre-supper glucose value (38). In this very unusual situation, a single morning injection of NPH insulin is appropriate, with short- or rapid-acting insulin given preprandially as necessary. Interestingly, the pharmacodynamic response to short- and rapid-acting insulin remains normal. This suggests that the reason for the delayed response involves a slowed release of insulin from the protamine in the NPH preparation rather than a general delay in the egress of insulin from the subcutaneous space into the circulation.
Patients with a delayed response to NPH insulin are usually identified by recognizing that the FPG concentrations remain low as the evening NPH insulin dose in a self-mixed/split regimen continues to be reduced and remains normal or low even when no evening NPH insulin is injected. These patients can be challenging to control. Fortunately, this delayed response to NPH insulin does not occur very often.
Intensified Insulin Regimens as Initial Therapy
As discussed above, most patients with type 2 diabetes will transition to insulin therapy via bedtime NPH or a basal insulin. A few newly diagnosed patients with markedly elevated glucose concentrations will (unnecessarily) be treated initially with insulin, and the usual subsequent scenario then involves decreasing the insulin doses and often safely discontinuing insulin in favor of oral medications. Patients with type 1 diabetes who present with diabetic ketoacidosis (DKA) will be discharged from the hospital on insulin. However, there are some patients who may benefit from an initial intensified insulin regimen (e.g., patients with type 1 diabetes diagnosed before DKA supervenes, those with LADA who are not controlled with noninsulin medications, and those with type 2 diabetes of long duration who are poorly controlled on noninsulin medications). Initial doses for lean and overweight or obese patients with regimens involving twice-daily or multiple injections are presented in Table 4. As with other regimens, these initial doses most often are set much lower than eventually will be required to reduce the likelihood of hypoglycemia, which may discourage patients from continuing with insulin therapy.
TABLE 4.
Doses for Initial Intensified Insulin Therapy (Twice-Daily or Multiple-Injection Regimens)
| Pre-Breakfast | Pre-Lunch | Pre-Supper | Bedtime | |
| Lean patients | ||||
| Twice-daily injection regimen | 10 units NPH/2–4 units short- or rapid-acting insulin | — | 6 units NPH/2–4 short- or rapid-acting insulin | — |
| Multiple-injection regimen | 4 units short- or rapid-acting insulin | 4 units short- or rapid-acting insulin | 4 units short- or rapid-acting insulin | 10 units NPH, glargine, or detemir insulin |
| Overweight or obese patients | ||||
| Twice-daily injection regimen | 20 units NPH/4–6 units short- or rapid-acting insulin | — | 10 units NPH/4–6 units short- or rapid-acting insulin | — |
| Multiple-injection regimen | 6–8 units short- or rapid-acting insulin | 6–8 units short- or rapid-acting insulin | 6–8 units short- or rapid-acting insulin | 16 units NPH, glargine, or detemir insulin |
Conclusion
Although there is no one right way to implement insulin therapy for patients with diabetes, this approach, taught to more than 40 mid-level health care professionals (registered nurses, nurse practitioners, physician assistants, and clinical pharmacists), has been very effective. For example, a registered nurse trained in this approach and placed in a family medicine clinic serving Latino and African-American patients lowered A1C levels in 111 insulin-requiring patients referred to her by the clinic physicians, from an average 11.1 to 7.3% within 9–12 months (39). Currently, three mid-level professionals supervised by the author treat ∼800 racial/ethnic minority patients with diabetes, approximately half of whom are taking insulin. The average A1C level in these insulin-requiring patients is in the mid-7% range.
Duality of Interest
M.B.D. serves on an advisory board for Sanofi. No other potential conflicts of interest relevant to this article were reported.
References
- 1.Centers for Disease Control and Prevention National diabetes statistics report—2014. Available from http://www.cdc.gov/diabetes/pubs/statsreport14.htm. Accessed 20 March 2015
- 2.Centers for Disease Control and Prevention Age-adjusted percentage of adults with diabetes using diabetes medication, by type of medication, United States, 1997–2011. Available from http://www.cdc.gov/diabetes/statistics/meduse/fig2.htm. Accessed 20 March 2015
- 3.Turner LW, Nortey D, Stafford RS, Singh S, Alexander GC. Ambulatory treatment of type 2 diabetes in the U.S., 1997. –2012. Diabetes Care 2014;37:985–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeavons D, Hungin APS, Cornford CS. Patients with poorly controlled diabetes in primary care: healthcare clinicians’ beliefs and attitudes. Postgrad Med J 2006;82:347–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khunti K, Wolden MI, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80.000 people. Diabetes Care 2013;36:3411–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvert MJ, McManis RJ, Freemantle N. Management of type 2 diabetes with multiple oral hyperglycaemic agents or insulin in primary care retrospective cohort study. Br J Gen Pract 2007;57:455–460 [PMC free article] [PubMed] [Google Scholar]
- 7.Rubino A, McQuay LJ, Gough SC, Kvasz V, Tennis P. Delayed initiation of subcutaneous insulin therapy after failure of oral glucose-lowering agents in patients with type 2 diabetes: a population-based analysis in the UK. Diabet Med 2007;24:1412–1418 [DOI] [PubMed] [Google Scholar]
- 8.Nichols GA, Koo YH, Shah SN. Delay of insulin addition to oral combination therapy despite inadequate glycemic control. J Gen Intern Med 2007;22:453–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris SB, Kapor J, Lank CN, Willan A, Houston T. Clinical inertia in patients with T2DM requiring insulin in family practice. Can Fam Phys 2010;56:e418–e424 [PMC free article] [PubMed] [Google Scholar]
- 10.Ziemer DC, Miller CD, Rhee MK, et al. . Clinical inertia contributes to poor diabetes control in a primary care setting. Diabetes Educ 2005;31:564–571 [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Abbott S, Nguyen M, Grabner M, Dumbo R. Glycemic control of insulin treated patients across the U.S.: epidemiologic analysis of a commercially insured population. Diabetes 2013;62(Suppl. 1):A704 [Google Scholar]
- 12.Galdo JA, Thurston MM, Bourg CA. Clinical considerations for insulin pharmacotherapy in ambulatory care, part one: introduction and review of current products and guidelines. Clinical Diabetes 2014;32:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association Glycemic targets. Sec. 6 in Standards of Medical Care in Diabetes—2015. Diabetes Care 2015;38(Suppl. 1):S33–S40 [DOI] [PubMed] [Google Scholar]
- 14.Hamwi GJ. Therapy: changing dietary concepts. In Diabetes Mellitus: Diagnosis and Treatment. Vol. 1 Danowski TS, Ed. New York, American Diabetes Association, 1964, p. 73–78 [Google Scholar]
- 15.Peters AL, Davidson MB. Maximal dose glyburide in markedly symptomatic patients with type 2 diabetes: a new use for an old friend. J Clin Endocrinol Metab 1996;81:2423–2427 [DOI] [PubMed] [Google Scholar]
- 16.Babu A, Mehta A, Guerrero P, et al. . Safe and simple emergency department discharge therapy for patients with type 2 diabetes mellitus and severe hyperglycemia. Endocr Pract 2009;15:696–704 [DOI] [PubMed] [Google Scholar]
- 17.Yki-Jarvinen H, Kauppila M, Kujansuu E, et al. . Comparison of insulin regimens in patients with non-insulin-dependent diabetes mellitus. N Engl J Med 1992;327:1426–1433 [DOI] [PubMed] [Google Scholar]
- 18.Wolffenbuttal BH, Sets JP, Rondas-Colbers GJ, Menheere PP, Nieuwenhuijzen-Kruseman AC. Comparison of different regimens in elderly patients with NIDDM. Diabetes Care 1996;19:1326–1332 [DOI] [PubMed] [Google Scholar]
- 19.Yki-Jarvinen H, Ryysy L, Kauppila M, et al. . Effect of obesity on the response to insulin therapy in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1997;82:4037–4043 [DOI] [PubMed] [Google Scholar]
- 20.Yki-Jarvinen H, Ryysy L, Nikkila K, Tulokas T, Vanamo R, Heikkila M. Comparison of bedtime insulin in patients with type 2 diabetes mellitus: a randomized, controlled trial. Ann Intern Med 1999;130:389–396 [DOI] [PubMed] [Google Scholar]
- 21.Skyler JS. Diabetic complications: the importance of control. Endocrinol Metab Clin North Am 1996;25:243–254 [DOI] [PubMed] [Google Scholar]
- 22.Tahara Y, Shima K. The response of GHb to stepwise plasma glucose change over time in diabetic patients. Diabetes Care 1993;16:1313–1314 [DOI] [PubMed] [Google Scholar]
- 23.Raccah D, Bretzel RG, Owens D, Riddle M. When basal insulin therapy in type 2 diabetes mellitus is not enough—what next? Diabetes Metab Res Rev 2007;23:257–264 [DOI] [PubMed] [Google Scholar]
- 24.Nathan DM, Buse JB, Davidson MB, et al. ; American Diabetes Association; European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owens DR, Luzio SD, Sert-Langeron C, Riddle MC. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6 month “proof of concept” study. Diabetes Obes Metab 2011;13:1020–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson MB, Raskin P, Tanenberg RJ, Vlajnic A, Hollander P. A stepwise approach to insulin therapy in patients with type 2 diabetes and basal insulin treatment failure. Endocr Pract 2011;17:395–403 [DOI] [PubMed] [Google Scholar]
- 27.Rodbard H, Visco VE, Andersen H, Hiort LC, Shu DHW. Treatment intensification with stepwise addition of prandial insulin aspart boluses compared with full basal-bolus therapy (FullSTEP Study): a randomized, treat-to-target clinical trial. Lancet Diabetes Endocrinol 2014;2:30–37 [DOI] [PubMed] [Google Scholar]
- 28.Cusi K, Cunningham GR, Comstock JP. Safety and efficacy of normalizing fasting glucose with bedtime insulin alone in NIDDM. Diabetes Care 1995;18:843–851 [DOI] [PubMed] [Google Scholar]
- 29.Monnier L, Colette C, Dunseath GJ, Owens DR. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care 2007;30:263–269 [DOI] [PubMed] [Google Scholar]
- 30.Bonomo K, DeSalve A, Fiova E, et al. . Evaluation of a simple policy for pre- and post-prandial blood glucose self-monitoring in people with type 2 diabetes not on insulin. Diabetes Res Clin Pract 2010;87:246–251 [DOI] [PubMed] [Google Scholar]
- 31.Reeves ML, Seigler DE, Ryan EA, Skyler JS. Glycemic control in insulin-dependent diabetes mellitus: comparison of outpatient intensified conventional therapy with continuous subcutaneous insulin infusion. Am J Med 1982;72:673–680 [DOI] [PubMed] [Google Scholar]
- 32.Umpierrez GE, Hor T, Smiley D, et al. . Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine Hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab 2009;94:564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulkarni K. Carbohydrate counting for pump therapy. In A Core Curriculum for Diabetes Education: Diabetes Management Therapies. 5th ed. Franz MJ, Ed. Chicago, Ill, American Association of Diabetes Educators, 2003, p. 265–276 [Google Scholar]
- 34.Bergenstal RM, Johnson M, Powers MA, et al. . Adjust to target in type 2 diabetes: comparison of a simple algorithm with carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care 2008;31:1305–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American Diabetes Association Standards of medical care in diabetes—2014. Diabetes Care 2014;37 (Suppl. 1):S14–S80 [DOI] [PubMed] [Google Scholar]
- 36.Binder C. Absorption of injected insulin: a clinical-pharmacological study. Acta Pharmacol Toxicol (Copenh) 1969;27 (Suppl. 2):11–87 [DOI] [PubMed] [Google Scholar]
- 37.Davidson MB, Navar MD, Echeverry D, Duran P. U-500 regular insulin: clinical experience and pharmacokinetics in obese, severely insulin-resistant type 2 diabetic patients. Diabetes Care 2010;33:281–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davidson MB. Letter to the editor. Diabetes Res Clin Pract 2004;64:229. [DOI] [PubMed] [Google Scholar]
- 39.Davidson MB, Blanco-Castellanos M, Duran P. Integrating nurse-directed diabetes management into a primary care setting. Am J Manag Care 2010;16:652–656 [PubMed] [Google Scholar]
- 40.Cusi K, Cunningham GR, Comstock JP. Safety and efficacy of normalizing fasting glucose with bedtime NPH insulin alone in NIDDM. Diabetes Care 1995;18;843–851 [DOI] [PubMed] [Google Scholar]
- 41.Ballani P, Tran MT, Navar MD, Davidson MB. Clinical experience with U-500 regular insulin in obese, markedly insulin resistant type 2 diabetic patients. Diabetes Care 2006;29:2504–2505; erratum 2007;30:455 [DOI] [PubMed] [Google Scholar]