There has been considerable concern regarding the rapidly growing prevalence of diabetes, particularly in resource-rich settings as a result of the shift toward more sedentary lifestyles that occurs with calorie-rich diets (1). Although much of the global attention to diabetes has focused on resource-rich settings and emerging markets, the diabetes epidemic has also been expanding in resource-constrained settings such as sub-Saharan Africa (2). Estimates from the International Diabetes Federation suggest that the prevalence of diabetes is expected to increase by 98% in sub-Saharan Africa by 2030, in contrast to an expected 54% increase in the rest of the world (3). Furthermore, there is an alarmingly high mortality rate attributable to diabetes in sub-Saharan Africa compared with all other parts of the world, with 76.4% of diabetes-related deaths occurring in people <60 years of age (4).
Despite these disturbing trends, there has been little effort to address this growing burden. Currently, most funding for international health care development focuses on communicable diseases, especially HIV and tuberculosis (5). However, the infrastructure that has been established to manage chronic infectious diseases such as HIV can be adapted to address many other chronic diseases, including diabetes (6–8).
The Academic Model Providing Access to Healthcare (AMPATH) disease management program based in western Kenya has provided comprehensive HIV care for >140,000 HIV-infected patients throughout a catchment area of >3.5 million people. AMPATH is a partnership between Moi Teaching and Referral Hospital (MTRH) and Moi University College of Health Sciences in Eldoret, Kenya, and a consortium of North American universities led by Indiana University (Indianapolis, Ind.). Its goal is to deliver comprehensive health care at all levels of the health care system, in partnership with the Kenyan government (9,10). AMPATH’s chronic disease management (CDM) program was formed to address the growing burden of diabetes in the Kenyan population (11).
Leveraging the many gains made in managing HIV, AMPATH is using its experience with communicable diseases to address the challenges posed by diabetes and other chronic diseases. An estimated 4.56% of the Kenyan population has diabetes (4). In response to increasing diabetes prevalence, AMPATH developed a comprehensive diabetes care program that provides community and home-based screening, medication and laboratory support at Kenyan Ministry of Health facilities, and referral services for patients suffering from long-term diabetes complications. Through partnerships with Abbott, the Abbott Fund, Eli Lilly and Company, several North American universities, and the Kenyan Ministry of Health, AMPATH has expanded to provide diabetes care for >5,000 patients in the 5 years since it first began providing care for chronic diseases other than HIV (6,12).
The introduction of A1C testing to monitor glycemic control has highlighted the poor state of diabetes care in the region. Initial A1C data from the first 641 tests performed at the main center of MTRH over 1.5 years revealed a high frequency of elevated A1C levels (mean 10.4%). This trend was largely influenced by persistently elevated A1C values among patients requiring insulin; 63% of A1C values >14% and 61% of A1C values between 10.1 and 14% were for patients who were using insulin (6). Most patients receiving diabetes care within the public sector do not have access to self-monitoring of blood glucose (SMBG) supplies and receive only a single point-of-care (POC) glucose test on the day of their clinic visit due to resource constraints. Although the initial focus of our activities in western Kenya was on increasing access to essential laboratory supplies and medications needed for the basic management of patients with diabetes, our experience has revealed that the infrastructure necessary to remotely monitor patients who require a higher level of care also needed to be developed to improve glucose control in these patients. Separate investigations of our chronic disease activities in western Kenya have revealed that only 42% of patients maintain continued follow-up after seeking care within the public sector. To improve retention of patients within the health care system, we need to ensure that patients have access to highly effective services, either remotely or in person during clinic visits (13). The low retention in care and limited infrastructure for diabetes care initially found in our rural, western Kenyan setting are consistent with findings in other rural areas throughout sub-Saharan Africa (3).
Although SMBG has consistently been proven to be an effective means of assessing glycemic control in resource-rich settings for patients at high risk of developing diabetes-related complications, there has been limited investigation of its feasibility and usability within resource-constrained settings (14,15). Many challenges exist for rural patients with diabetes in such locations, including but not limited to, inadequate health, care resources, lower literacy rates, long distances from clinics, and limited access to diabetes services. The feasibility of phone-based follow-up for SMBG support has not been tested previously, and this care modality has been largely unavailable to date for the majority of public sector patients in sub-Saharan Africa (16).
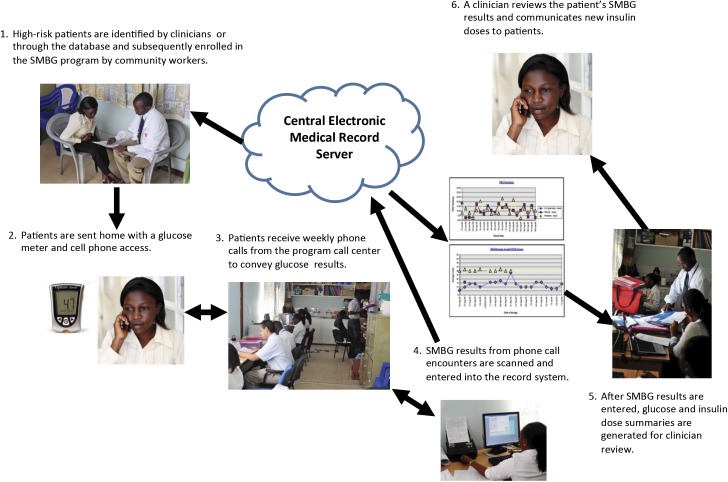
To address some of these deficiencies in the management of patients with diabetes, AMPATH’s CDM program created a phone-based SMBG program in partnership with Abbott and the Abbott Fund (Figure 1). This article describes the implementation and evaluation of the phone-based SMBG program for patients with diabetes on insulin therapy in two diabetes clinics in western Kenya (14,16–18).
FIGURE 1.
Steps in enrolling and following up with patients for the SMBG program.
The feasibility and impact of this program were determined by assessing the change in blood glucose and A1C among eligible enrollees after 6 months of intensive phone-based SMBG follow-up support. Secondary analyses included an assessment of potential predictors of response to this modality of care and a descriptive analysis of alterations in insulin dosing and loss to follow-up within the program.
Methods
This was a retrospective, observational cohort study of patients at high risk for diabetes complications in two outpatient diabetes clinics in western Kenya. The study was conducted within the central diabetes clinic at MTRH and at the diabetes clinic at Webuye District Hospital (WDH), both in western Kenya. MTRH is the primary referral hospital for all of western Kenya; its diabetes call center supports SMBG for patients receiving care at MTRH and at several rural clinics. WDH, located ∼75 km from Eldoret, serves a predominantly rural population and is the primary teaching center for the Moi University Family Medicine Department, whose faculty and trainees typically staff the diabetes clinics. This analysis includes the initial cohort of patients receiving this service at MTRH and WDH.
Patients included in this analysis were enrolled between January 2008 and March 2010. Patients receiving routine care within the specialty diabetes clinics at MTRH or WDH were eligible for inclusion in the SMBG program. All patients enrolled in the program for at least 6 months were included in the analysis. Patients who were enrolled in the program but did not have any follow-up A1C measurements were excluded.
To derive maximum benefit from this program, patients who require insulin and have a relatively higher risk for diabetes-related complications were preferentially enrolled. The majority of patients were enrolled because of their markedly elevated A1Cs (>12%). However, patients with other high-risk characteristics such as severe skin and soft tissue infections, repeated hospitalizations secondary to hyperglycemia or hypoglycemia, pregnancy, severe kidney disease, extreme poverty or food insecurity, or age <18 years were also eligible for enrollment. Peer educators and other staff members provided SMBG education to patients who met the predefined criteria.
All patients eligible for this service were provided with a free Optium Xceed glucose meter and free glucose testing strips manufactured and donated by Abbott Diabetes Care (19). Patients were instructed to perform at least two daily blood glucose checks—a fasting blood glucose test before breakfast and before dinner prior to administering insulin and eating. Patients were required to record their blood glucose readings in diabetes diaries and report their results to the call center via verbal phone calls on a weekly basis.
All calls originated from the central call center, and the program bore all associated costs. Patients had the option of calling the center as well and then receiving a call back to avoid incurring charges on their own phone lines. This phone-based system was designed to minimize the number of times patients had to be physically present in the clinic and incur transportation and encounter costs. During each weekly phone call, patients were asked to provide the time and date of all glucose results, the doses of all diabetes-related medications, any changes in diet, and any subjective symptoms of hypo- or hyperglycemia or intercurrent illness.
A multidisciplinary team of local volunteers, social workers, pharmacists, clinical officers, and physicians was responsible for completing all the steps shown in Figure 1. After collating weekly patient results, trained diabetes clinicians reviewed patient charts and advised on the appropriate dosage adjustments based on standardized protocols. Patients were then called back with the recommended dosage adjustments. In addition to the weekly phone calls, patients were required to bring in their glucose meter at every return visit and whenever they required additional strips to verify the self-reported glucose measurements with those stored on the meter. Based on patient progress and glucose strip availability, clinicians could also request more intensive glucose monitoring, up to four times per day.
Through a partnership with Eli Lilly and Company, a second-line insulin regimen became available halfway through the program. Patients not achieving adequate control with the first-line regimen of premixed 70/30 insulin, typically given before breakfast and dinner, were able to switch to a combination of intermediate-acting NPH insulin and rapid-acting lispro insulin, typically given three times daily (NPH + lispro before breakfast, lispro with lunch, and NPH + lispro before dinner) (20). Patients who no longer required frequent adjustments to their insulin doses were changed to a less intensive monitoring regimen involving SMBG three times per week and phone calls once every 2 weeks or were given the option to stop SMBG completely and return their meters to the program.
During their routine in-person clinic visits, patients had A1C tests at 3- to 6-month intervals using the POC DCA Analyzer (Siemens, Erlangen, Germany) (21). Whenever patients were registered to receive general diabetes care, diabetes staff and peer educators administered a comprehensive initial assessment form that captured a wide variety of demographic and personal characteristics. These included age, self-reported year of diabetes diagnosis, date of enrollment into SMBG care if applicable, self-reported history of tuberculosis, availability of caregiver assistance, assessment of alcohol use via the AUDIT-C (Alcohol Use Disorders Identification Test alcohol consumption questions) questionnaire (22), smoking history, assessment of food insecurity via the Household Food Insecurity Access Scale (23), and location of patients’ primary diabetes clinic.
All data were entered into a database from which reports were generated to facilitate weekly clinician review. Data required for this analysis were exported from the Access database (Microsoft, Redmond, Wash.) into the Stata Statistical Software package (StataCorp, College Station, Tex.). All data were verified by examination of the paper medical record and corrected where appropriate before performing the analysis.
Retention in the program was tracked to identify all patients who were lost to follow-up (LTFU). Patients were classified as LTFU if they could not be traced by phone or in-person visit for >3 months.
The primary analysis was to demonstrate the utility of this approach based on the change in the median A1C from baseline to 6 months after enrollment. Because of the variability and unpredictability of patient follow-up visits, the date of the A1C was rounded up or down to the closest 3-month interval for the purpose of analysis. For statistical analysis of the primary outcome, the A1C closest to the 6-month period and between 4 and 8 months after enrollment was used. All patients included in this investigation had an evaluable result for the primary outcome measure. For all other A1C analyses, the A1C was recorded under the closest 3-month period from the date of enrollment. The Wilcoxon signed rank test was used to compare baseline and 6-month A1C results and a P value <0.05 was considered statistically significant.
In addition to the primary outcome, the trends in SMBG changes from baseline were tracked and compared using the one-sample, paired Student’s t test. SMBG results from the first week, after testing supplies were received but before phone calls were initiated, were considered to be the baseline results. For patients who had prolonged enrollment beyond 6 months, SMBG results were analyzed descriptively to assess trends in results over an extended period of time and compared to the original baseline values to determine whether statistically significant differences in glucose control persisted. To identify potential trends in subpopulations within the program, univariate Wilcoxon rank sum analyses were performed to compare the percentage change in A1C at 6 months for the demographic and personal characteristics collected during enrollment. Any characteristic showing a P value <0.2 was then introduced into an adjusted multivariate linear regression model to provide adjusted 95% confidence intervals (CIs) for the percentage change in A1C from baseline to 6 months.
Results
A total of 137 patients met the eligibility criteria and were included in the study. Two patients were excluded from the analysis because they did not have the required A1C test at 6 months.
Analysis of the primary outcome across the full cohort (n = 137) found a statistically significant 4-point difference (31.6% difference, P <0.01) between the median baseline (13.3%) and 6-month A1C results (9.3%). For patients who were followed beyond 6 months, a statistically significant decline (P <0.01) was maintained compared to baseline at each subsequent 3-month period of evaluation. Similarly, the SMBG program yielded statistically significant declines at each 3-month interval compared to baseline (Figure 2 and Table 1). Further analysis of individual blood glucose readings revealed that 8.6% of results were <70 mg/dL and therefore indicative of hypoglycemia (24). All patients required adjustments to their original insulin dose, with patients requiring an average 16% increase in the total daily dose of premixed 70/30 insulin from baseline to 6 months (median 41.3 and 48.0 units of insulin, respectively). With the availability of NPH and lispro insulin midway through the study, 13 patients (9.3%) required a switch from the premixed insulin to separate injections of rapid- and intermediate-acting insulin, based on clinician discretion.
FIGURE 2.
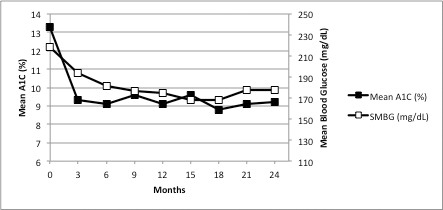
Changes in A1C and SMBG over the period of evaluable data.
TABLE 1.
Trends in Glycemic Control and Insulin Dosing During the Period of Evaluable Data
| Baseline | 3 Months | 6 Months | 9 Months | 12 Months | 15 Months | 18 Months | 21 Months | 24 Months | |
| Median A1C (%) | 13.3 | 9.3* | 9.1* | 9.6* | 9.1* | 9.6* | 8.8* | 9.1* | 9.2* |
| A1C decline from baseline (%) | 30.0 | 31.6 | 27.8 | 31.6 | 27.8 | 33.8 | 31.6 | 30.8 | |
| Evaluable A1C results since last visit (n) | 139 | 70 | 86 | 77 | 51 | 42 | 41 | 28 | 15 |
| Mean blood glucose (mg/dL ± SD) | 218.16 ± 8.46 | 193.68 ± 2.88† | 181.62 ± 1.44† | 176.58 ± 1.98† | 174.96 ± 3.6† | 168.3 ± 1.8† | 167.76 ± 6.48† | 177.66 ± 3.96† | 177.48 ± 11.52† |
| (days 0–7) | (days 8–90) | (days 91–180) | (days 181–270) | (days 27–360) | (days 361–450) | (days 451–540) | (days 541–630) | (days 631–730) | |
| Blood glucose results included (n) | 1,988 | 14,919 | 13,619 | 11,675 | 7,182 | 5,059 | 3,651 | 3,600 | 1,212 |
| Median total daily insulin dose (units [% increase from baseline]) | 41.3 | 45.9 (11.1) | 48.0 (16.2) | 51.0 (23.5) | 51.5 (24.7) | 53.3 (29.1) | 50.1 (21.3) | 50.8 (23.0) | 51.5 (24.7) |
| Median total daily insulin dose (units/kg) | 0.64 | 0.7 | 0.78 | 0.79 | 0.84 | 0.91 | 0.91 | 0.88 | 0.98 |
P <0.01 via Wilcoxon signed rank test compared to baseline A1C.
P <0.01 via paired Student’s t test compared to the first week of SMBG.
Further analysis of the follow-up of patients after 6 months revealed that only 16 patients (15%) were classified as LTFU. An additional three patients died during the period of evaluation, with one of the deaths attributed to a diabetes-related cause.
In the secondary analysis of factors that might be associated with response to this intervention, patients diagnosed with diabetes before the age of 25 years were statistically significantly more likely to have a lower percentage change in A1C after 6 months than the rest of the population, in both unadjusted and adjusted analyses (Table 2). A trend toward worse control among pediatric patients <15 years of age was also seen, but this was not statistically significant. Patients who were enrolled in the general diabetes care program for <5 years had a statistically significantly lower percentage improvement in A1C in both the adjusted (P <0.03) and unadjusted (P <0.001) analyses. Patients who received care in the rural clinic experienced a greater reduction in A1C than those who received care at the more urban MTRH clinic (P <0.01 in both adjusted and unadjusted analyses).
TABLE 2.
Association of Risk Factors With Response to SMBG Intervention
| Characteristic | Population (n [%]) | Change in Median A1C From Baseline to 4–8 months (%) | Interquartile Range Change in A1C (%) | P for Unadjusted Analysis | Adjusted Analysis 95% CI (%) | P for Adjusted Analysis |
| All patients | 139 (100) | –31 | –40 to –8 | |||
| Female patients | 72 (51.8) | –14.5 | –31 to 0 | 0.49 | ||
| Pediatric patients (<15 years of age) | 8 (5.8) | –5.5 | –12 to 1.5 | 0.05 | –1.2 to 32.4 | 0.07 |
| Diagnosed with type 1 diabetes by the age of 25 years | 82 (60.0) | –9.5 | –24 to 0 | 0.001 | 1.2–18.3 | 0.03 |
| Enrolled in care for <5 years | 79 (56.8) | –9.0 | –26 to 0 | 0.003 | 0.5–16 | 0.04 |
| Travel time to clinic >5 hours | 36 (25.9) | –9.5 | –26 to 2.5 | 0.15 | –5 to 11.9 | 0.43 |
| History of tuberculosis | 7 (5.0) | –24.0 | –30 to 7 | 0.64 | ||
| Caregiver assistance available | 88 (63.3) | –13.5 | –29.5 to 0 | 0.27 | ||
| Any alcohol consumption | 9 (6.4) | –36 | –43 to –11 | 0.1374 | –21.4 to 11.4 | 0.54 |
| History of smoking | 4 (2.9) | –37 | –37 to –18.5 | 0.21 | ||
| Any level of food insecurity | 35 (25.2) | –20 | –36 to –4 | 0.34 | ||
| Rural clinic (Webuye) | 47 (33.8) | –31 | –40 to –8 | 0.0004 | –17.5 to –0.3 | <0.01 |
| Semi-urban clinic (Eldoret-MTRH) | 92 (66.2) | –12 | –24 to –1 | 0.0004 | 4.2–20.8 | <0.01 |
Discussion
This study shows that, despite resource challenges that can impede the performance of frequent SMBG, patients in a semi-urban and rural setting in western Kenya achieved a 4-point reduction in median A1C after 6 months of participation in an intensive SMBG and insulin adjustment program. There were drastic initial reductions in blood glucose levels within the first 3 months of participation in the program. However, these reductions plateaued when A1Cs reached ∼9%. This muted response after 6 months is partially expected because of several factors inherent in the diabetes care strategy employed in the program. First, most patients were maintained on twice-daily SMBG before breakfast and dinner because of the limited availability of test strips. Second, several studies have illustrated that fasting preprandial glucose levels are the primary contributor to elevated A1C when the A1C is >9%. However, as the A1C decreases, the main contributor to elevated A1C becomes postprandial hyperglycemia (25). The initial protocol in our program limited our ability to address postprandial hyperglycemia, with the majority of patients on premixed 70/30 insulin injected twice a day (morning and night). Therefore, it was difficult to balance the achievement of lower A1C levels with the prevention of hypoglycemia. In addition, with the hot climate and most patients being farmers, many patients are unable to keep their insulin at room temperature during the day and are only able to inject themselves when they are at home in the morning and evening. This dynamic has made it difficult for us to prescribe uncoupled insulins (NPH and lispro). Therefore, lunchtime injection of lispro to reduce postprandial hyperglycemia, while often indicated, was not always possible. One of the other unique dynamics in this setting is the difficulty in maintaining the delicate balance between normoglycemia and hypoglycemia when ∼30% of the population with diabetes is food insecure (26). With all of these challenges, the aggressiveness with which clinicians push for tighter control of A1C (<7–8%) is often reduced.
These dynamics highlight some of the challenges that are encountered in the management of diabetes mellitus in sub-Saharan Africa and other low- and middle-income countries (LMICs) but are rarely found in the developed world where most diabetes research is conducted. The relatively low daily doses of insulin used in this program highlight one of the other major frequently mentioned differences seen when comparing sub-Saharan African populations to other populations (3).
The subgroup analysis of potential risk factors highlights several important trends that merit additional investigation. One especially concerning finding is the relatively lower degree of improvement in glycemic control seen in patients with type 1 diabetes coupled with a trend toward suboptimal care of pediatric patients. This finding is thought to be the result of several setting-specific challenges related to the typical school environment. Few schools have reliable infrastructure or nursing staff to store and administer insulin during the day. This limits the ease with which pediatric patients can receive more frequent injections than the standard twice-daily dosing regimen. Most schools also typically provide a carbohydrate-rich diet with limited options for lower glycemic–index foods.
To address these challenges, we have recruited pediatricians with expertise in managing diabetes to begin testing different strategies, including carbohydrate counting and insulin self-administration for children not demonstrating adequate improvements in blood glucose control. Subsequent analyses will assess the change in glycemic control seen through the introduction of these strategies for pediatric patients and all patients with type 1 diabetes.
Patients who were enrolled in care for <5 years also demonstrated statistically significantly less A1C improvement (P <0.05). For patients who had been receiving care for an extended period of time before the introduction of this novel care strategy, it is thought that they had struggled to control their glucose for many years and were beginning to face diabetes-related complications. Because of their first-hand knowledge of the consequences of prolonged hyperglycemia, it is hypothesized that these patients were more motivated to take advantage of this intensive glucose monitoring and phone-based follow-up program.
The analysis comparing patients from rural and more urban clinics highlights the feasibility of this intervention in a variety of settings, as rural patients were found to have statistically significantly greater improvements (P <0.01) in blood glucose control than the population receiving care in the more urban clinic.
Limitations
The retrospective, observational design of this study is a major limitation in that the findings are less conclusive than what might be observed in a double-blind, randomized, control trial. Because of the study design and the selection of patients with elevated glucose levels, it is also possible that some of the observed changes in glucose control might be a result of regression to the mean (27). However, there is growing support for the use of observational studies in LMICs to provide an initial assessment of the feasibility of various interventions. This study has helped to demonstrate the potential reductions in blood glucose that can be attained through an organized approach combining SMBG, phone-based follow-up for insulin adjustment, and an information system to record and track results (28).
Another limitation of this study is that participants who did not have a 6-month A1C were excluded from analysis in a per-protocol manner rather than the more desirable intention-to-treat design. This limitation could impair the ability to gauge the tolerability of this intervention because patients who did not meet the criteria were not included in the analysis. However, evaluation of enrollment revealed that only two patients were excluded during the first 6-month follow-up period.
This study used POC testing for all reported laboratory measurements. However, the limited data and experience with the use of such devices in sub-Saharan African populations raise concern over the potential for inaccuracy, as noted in previous studies in African populations (29,30). The higher rates of anemia and hemoglobin variants among sub-Saharan African populations could further confound results from POC devices (31). In an attempt to mitigate the risk of inaccurate measurements from these devices, we used one of the best performing POC A1C testing devices available on the market (32).
The 4-point reduction in median A1C between baseline and 6 months found in this study helps to demonstrate that this care strategy is feasible and effective in resource-constrained settings when adequate infrastructure is established to assist enrollees. Since the evaluation of this initial population, we have focused our efforts on expanding the service to additional high-risk patients. To improve sustainability, a cost-sharing structure has been initiated, through which patients pay a monthly user fee for continued inclusion after the initial 6 months, and other patients who do not meet the high-risk criteria can pay a monthly fee for inclusion in the program. By the end of 2014, the program had grown to include >750 patients through the combined corporate support and patient user fees.
With the unacceptably high mortality rate from diabetes in sub-Saharan Africa, replicable and scalable interventions such as the one described here must be urgently adopted throughout the region to prevent early diabetes-related complications and deaths.
Acknowledgments
This research was supported by the President’s Emergency Plan for AIDS Relief through the U.S. Agency for International Development under the terms of Cooperative Agreement No. AID-623-A-12-0001. The contents of this article are the sole responsibility of AMPATH and do not necessarily reflect the views of USAID or the U.S. government. This publication was also made possible by the Indiana Clinical and Translational Sciences Institute, funded in part by grant UL1 TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. The authors thank Abbott for providing glucose testing supplies and the Abbott Fund for providing financial support for this effort. They also thank Eli Lilly and Company for donating insulin and providing seed funding to support the authors’ overarching work in diabetes.
Duality of Interest
S.D.P. has received payment for serving as a speaker and consultant for Abbott and the Abbott Fund. No other potential conflicts of interest relevant to this article were reported.
References
- 1.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011;378:31–40 [DOI] [PubMed] [Google Scholar]
- 2.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world: a growing challenge. N Engl J Med 2007;356:213–215 [DOI] [PubMed] [Google Scholar]
- 3.Mbanya JCN, Motala AA, Sobngwi E, Assah FK, Enoru ST. Diabetes in sub-Saharan Africa. Lancet 2010;375:2254–2266 [DOI] [PubMed] [Google Scholar]
- 4.International Diabetes Federation Diabetes Atlas. 6th ed. 2013. Available from http://www.idf.org/diabetesatlas. Accessed 10 April 2014
- 5.Shiffman J. Has donor prioritization of HIV/AIDS displaced aid for other health issues? Health Policy Plan 2008;23:95–100 [DOI] [PubMed] [Google Scholar]
- 6.Pastakia SD, Karwa R, Kahn CB, Nyabundi JS. The evolution of diabetes care in the rural, resource-constrained setting of western Kenya. Ann Pharmacother 2011;6:721–726 [DOI] [PubMed] [Google Scholar]
- 7.van Vugt M, Hamers R, Schellekens O, Reiss P, Hamers R, Robias RDW. Diabetes and HIV/AIDS in sub-Saharan Africa: the need for sustainable healthcare systems. Diabetes Voice 2007;3:23–26 [Google Scholar]
- 8.Joint United Nations Program on HIV/AIDS Chronic Care of HIV and Non-Communicable Diseases. Available from www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/20110526_JC2145_Chronic_care_of_HIV.pdf. Accessed 22 January 2012
- 9.IU-Kenya Partnership AMPATH [article online], 2014. Available from http://www.ampathkenya.org/our-model. Accessed 24 January 2014
- 10.Einterz RM, Kimaiyo S, Mengech HN, et al. Responding to the HIV pandemic: the power of an academic medical partnership. Acad Med 2007;8:812–818 [DOI] [PubMed] [Google Scholar]
- 11.Mathenge W, Foster A, Kuper H. Urbanization, ethnicity and cardiovascular risk in a population in transition in Nakuru, Kenya: a population-based survey. BMC Public Health 2010;10:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pastakia SD, Schellhase EM, Jakait B. Collaborative partnership for clinical pharmacy services in Kenya. Am J Health Syst Pharm 2009;15:1386–1390 [DOI] [PubMed] [Google Scholar]
- 13.Kamano JH, Pastakia SD, Wambui CK, Park P. Integrating diabetes care into an already established HIV care program. Poster presented at the International Diabetes Federation World Diabetes Congress in Melbourne, Australia, 2013 [Google Scholar]
- 14.International Diabetes Federation Global guideline for type 2 diabetes. Available from http://www.idf.org/global-guideline-type-2-diabetes-2012. Accessed 10 January 2015
- 15.Saudek CD, Derr RL, Kalyani RR. Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA 2006;14:1688–1697 [DOI] [PubMed] [Google Scholar]
- 16.Rotchford AP, Rotchford KM. Diabetes in rural South Africa: an assessment of care and complications. S Afr Med J 2002;7:536–541 [PubMed] [Google Scholar]
- 17.Gill GV, Gebrekidan A, English PJ, Tesfave S. Improving glycaemic control in African diabetic patients on insulin: a resource-free approach. Trop Doct 2009;1:3–5 [DOI] [PubMed] [Google Scholar]
- 18.Gill GV, Huddle KR, Krige LP. Improving diabetic control in adverse social conditions: a home blood glucose monitoring study in Soweto, South Africa. Diabetes Res 1986;3:145–148 [PubMed] [Google Scholar]
- 19.Abbott Optium Xceed (blood glucose monitoring device) product information. Abbott Park, IL, Abbott Laboratories, April 2012 [Google Scholar]
- 20.Eli Lilly and Co Humalog (insulin lispro injection) and Humulin (insulin NPH and 70/30 injection) product information. Indianapolis, Ind., Eli Lilly and Company, April 2012 [Google Scholar]
- 21.Siemens CA. Vantage (HbA1c analyzer) product information. Tarrytown, N.Y, Siemens, January 2008 [Google Scholar]
- 22.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse. Alcohol Clin Exp Res 2007;7:1208–1217 [DOI] [PubMed] [Google Scholar]
- 23.Food and Nutritional Technical Assistance Project Household Food Insecurity Access Scale (HFIAS) for measurement of food access: indicator guide. Available from http://www.fao.org/fileadmin/user_upload/eufao-fsi4dm/doc-training/hfias.pdf. Accessed 10 April 2010
- 24.American Diabetes Association Standards of medical care in diabetes—2013. Diabetes Care 2013;36(Suppl. 1):S11–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woerle HJ, Neumann C, Zschau S, et al. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes: importance of postprandial glycemia to achieve target HbA1c levels. Diab Res Clin Pract 2007;77:280–285 [DOI] [PubMed] [Google Scholar]
- 26.Cheng SY, Kamano JH, Kirui NK, Buckwalter V, Ouma K, Pastakia SD. Prevalence of food insecurity in patients with diabetes in western Kenya. Diabet Med 2013;30:e215–e222 [DOI] [PubMed] [Google Scholar]
- 27.Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Intl J Epidemiol 2005;34:215–220 [DOI] [PubMed] [Google Scholar]
- 28.Baik S, Chacra AR, Yuxiu L, White J, Guler S, Latif ZA. Conducting cost-effectiveness analyses of type 2 diabetes in low- and middle-income countries: can locally generated observational study data overcome methodological limitations? Diab Res Clin Pract 2010;88 (Suppl. 1):S17–S22 [DOI] [PubMed] [Google Scholar]
- 29.Tonyushkina K, Nichols JH. Glucose meters: a review of technical challenges to obtaining accurate results. J Diabetes Sci Technol 2009;4:971–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bimenya GS, Nzarubara GR, Kiconco J, Sabuni S, Byarugaba W. The accuracy of self-monitoring blood glucose meter systems in Kampala Uganda. Afr Health Sci 2003;1:23–32 [PMC free article] [PubMed] [Google Scholar]
- 31.Kirk JK, Agostino RB, Bell RA, et al. Disparities in HbA1c levels between African-American and non-Hispanic white adults with diabetes. Diabetes Care 2006;9:2130–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerners-Westra E, Slingerland RJ. Six of eight hemoglobin A1c point of care instruments do not meet the general accepted analytical performance criteria. Clin Chem 2010;1:44–52 [DOI] [PubMed] [Google Scholar]