Abstract
Background
Phospholipase-associated neurodegeneration (PLAN) caused by PLA2G6 mutations is a recessively inherited disorder with three known phenotypes: the typical infantile onset neuroaxonal dystrophy (INAD); an atypical later onset form (atypical NAD); and the more recently recognized young-onset dystonia–parkinsonism (PLAN-DP).
Case Report
We report the clinical, radiological, and genetic findings of a young Pakistani male with PLAN-DP. We review 11 previously published case reports cited in PubMed, and summarize the demographic, clinical, genetic, and radiological data of the 23 patients described in those articles.
Discussion
PLAN-DP presents with diverse motor, autonomic, and neuropsychiatric features and should be considered in the differential diagnosis of patients with young-onset neurodegenerative disorders.
Keywords: PLA2G6, PLAN-DP, young-onset neurodegenerative disease
Introduction
The first description of infantile neuroaxonal dystrophy (INAD) was published in 1952 by the German neurologist Franz Seitelberger, who described two siblings presenting with psychomotor delay in infancy followed by progressive neuroregression, leading to severe disability and death in the first decade of life.1 Widespread distal axonal swelling with spheroid bodies—the histological feature known as neuroaxonal dystrophy (NAD)—was the pathological hallmark at autopsy of Seitelberger’s patients and of other cases described later.1–3 Twenty-seven years after Seitelberger’s report, Aicardi and Castelein2 reported eight new patients, reviewed 77 previously published cases, and proposed the first criteria for INAD. In 1999, Nardocci et al.4 described 13 patients and added a new phenotype, atypical NAD, with a more protracted course and cerebellar signs as distinctive clinical findings.
In 2006 mutations in PLA2G6 were identified in patients with INAD, and also in a group of patients with neurodegeneration with brain iron accumulation (NBIA) who did not have mutations in PANK2 (i.e. did not have pantothenate kinase-associated neurodegeneration [PKAN]).5,6 Subsequently, all phenotypes have been termed phospholipase-associated neurodegeneration (PLAN).7 In 2009, Paisán-Ruiz et al.8 broadened the phenotypic spectrum of PLAN by identifying PLA2G6 mutations in patients with early-onset levodopa-responsive dystonia–parkinsonism associated with cognitive decline, oculomotor abnormalities, psychiatric features, and pyramidal signs. Subsequently, a range of phenotypes has been reported, from pure Parkinsonism to severe generalized dystonia.9–18 In this article, we report a new patient with PLA2G6-associated dystonia–parkinsonism (PLAN-DP) and review the literature.
Case report
A 20-year-old Pakistani male was born to first-degree-relative parents. He was asymptomatic until age 14 years, when he developed blepharospasm and jaw-opening dystonia, followed by foot dragging and foot dystonia and handwriting deterioration. Over the next 4 years his symptoms progressed to generalized dystonia. At the age of 19, he was bradykinetic with hypomimia, jaw-opening dystonia, blepharospasm, facial and upper extremity myoclonic jerks, and dysarthria (Video 1). Ocular motility was normal. Strength was normal in all extremities. Muscle tone and deep tendon reflexes were increased throughout with right ankle clonus, but plantar reflexes were downgoing. Gait was short-stepped and spastic. He could not walk unassisted because of foot dystonia and severely impaired postural reflexes. Sensory and cerebellar examination was normal. The Wechsler Adult Intelligent Test revised score was 88 (verbal, 101; performance, 74). At age 20, he experienced a generalized seizure and an electroencephalogram demonstrated right frontotemporal slowing. No further seizures occurred on treatment with sodium valproate. Several drugs, including levodopa (maximum dose 800 mg/day), amantadine (maximum dose 400 mg/day), dopamine agonists, anticholinergics, baclofen, tizanidine, and diazepam (maximum doses of the medications are not known), were tried without beneficial effects.
Video 1. PLAN-DP case report. The video shows generalized bradykinesia, masked facies, blepharospasm, jaw-opening dystonia and right ankle clonus. The gait is limited by leg spasticity and dystonia, and balance is severely impaired.
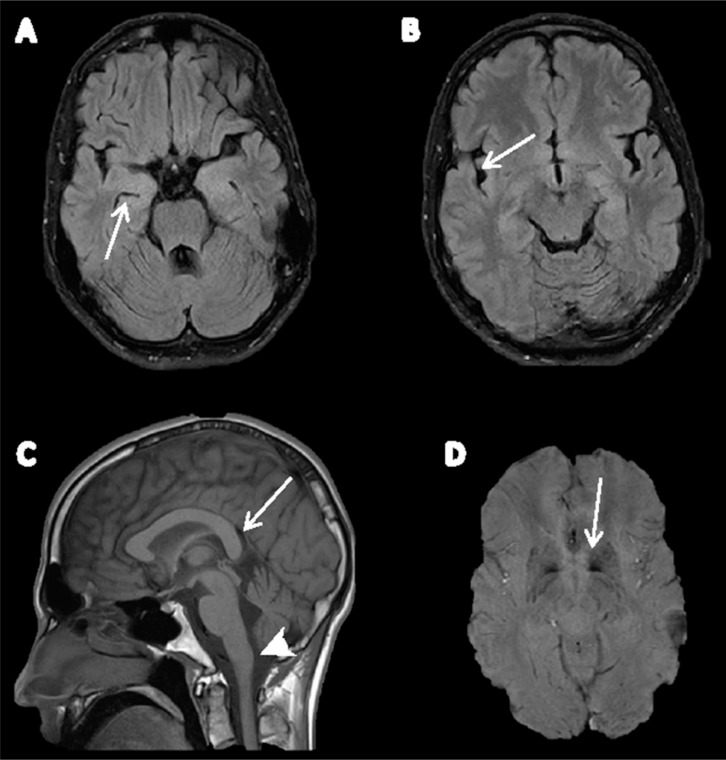
All laboratory tests including serum biochemistry, liver and thyroid function tests, serum copper and ceruloplasmin levels, and cerebrospinal fluid analysis were normal. Kayser–Fleischer rings were not detected. Brain magnetic resonance imaging (MRI) (Figure 1) showed mild generalized cortical and cerebellar atrophy, a vertically oriented splenium, and claval hypertrophy. T2* sequences demonstrated iron deposition in the basal ganglia, most prominently in the globus pallidus. Direct sequencing of the PLA2G6 gene revealed a homozygous c.2222G>A mutation resulting in a p.R741Q transition. The p.R741Q mutation was previously described as pathogenic (rs121908686)8 and is present in the ExAC database with very low frequency (0.0002277). It is only reported in the South Asian population (four out of 7,854) (http://exac.broadinstitute.org/variant/22-38508565-C-T), and all cases reported by us share a common haplotype of 769.36 kbp flanked by single nucleotide polymorphisms rs6519064 (36,171,965 bp) and rs2235265 (36,941,833 bp), likely suggesting a common ancestor between all mutation carriers.19
Figure 1. Brain Magnetic Resonance Imaging of Patient with PLA2G6-associated Dystonia–Parkinsonism. (A,B) Axial fluid-attenuated inversion recovery images show frontotemporal atrophy with widened temporal horns of lateral ventricles (arrow in A) and lateral fissure (arrow in B). (C) Mid-sagittal T1 image shows vertically oriented splenium (arrow) and apparent claval hypertrophy (arrowhead). Mild cerebellar vermis atrophy is also visible. (D) SWI demonstrates bilateral hypointensity in the globus pallidus due to iron deposition (arrow).
We searched the PubMed database using the following terms: PLA2G6 parkinsonism, PLA2G6 dystonia, PLAN, and PLA2G6 dystonia–parkinsonism. We found 11 articles describing patients with young- or adult-onset PLAN-DP, reporting 23 patients from 16 pedigrees.8–18
The youngest age of disease onset was 4 years old and the oldest was 37, and all had motor and/or neuropsychiatric features prior to age 40. Parkinsonism (rigidity and bradykinesia) was universally present. Rest tremor was found in 16 out of 24 patients.8–13,15,18 Eighteen patients had dystonia with variable severity, distribution, and time of appearance during the disease course.8–10,12–17 A summary of the demographic and sensory/motor symptoms is shown in (Table 1). The patients, ethnicity and mutations are shown in (Table 2).
Table 1. Summary of Demographic and Clinical Findings.
| Reference | Patient | Gender/Age at Onset | Presenting Symptom | Parkinsonism | Tremor at Rest | LD/LID | Ataxia | Dystonia | Pyramidal Signs | Poor balance | Myoclonus | Oculomotor Abnormality | Autonomic |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paisán-Ruiz et al.8 | F1/P1 | F/26 | Cognitive decline | + | + | +/+ | – | + | + | + | +(Facial) | SNGP/LOA | – |
| Paisán-Ruiz et al.8 | F1/P2 | F/10 | Foot drag | + | + | +/+ | – | + | NK | + | NK | NK | NK |
| Paisán-Ruiz et al.8 | F2/P1 | F/18 | Foot drag | + | + | DA/- | – | + | + | + | – | Jerky saccadic | Frequency and nocturia |
| Sina et al.9 | P1 | M/25 | Foot drag | + | + | +/+ | – | + | + | + | – | Fragmented saccades | NK |
| Sina et al.9 | P2 | M/22 | Foot drag | + | + | +/+ | – | + | + | + | – | Fragmented saccades | NK |
| Sina et al.9 | P3 | F/21 | Foot drag | + | + | +/+ | + | + | + | + | – | Fragmented saccades | – |
| Bower et al.10 | P1 | F/18 | Depression | + | – | NM | – | + | + | + | NM | – | NM |
| Bower et al.10 | P2 | F/4 | Dyslexia, stuttering, clumsiness | + | + | NM | + | + | – | + | NM | Saccadic pursuit | NM |
| Yoshino et al.11 | PA | F/20 | Tremor at rest, unsteady gait | + | + | +/+ | – | – | – | + | – | – | Urinary disturbance/Constipation/OH |
| Yoshino et al.11 | PB1 | M/25 | Bradykinesia, gait problem | + | – | +/+ | – | – | – | + | – | – | Urinary disturbance |
| Yoshino et al.11 | PB2 | M/30 | Bradykinesia, gait problem | + | – | +/+ | – | – | – | + | – | – | Urinary disturbance/Constipation/OH |
| Shi et al.12 | P | M/37 | Foot drag | + | + | +/+ | – | – | – | + | – | – | – |
| Virmani et al.13 | P1 | F/25 | Depression and psychosis | + | + | +/– | – | + | + | + | + (Face and limbs) | Jerky pursuit, OGC with levodopa | – |
| Virmani et al.13 | P2 | F/22 | Depression | + | + | +/– | – | + | + | + | – | – | – |
| Agarwal et al.14 | P1 | M/14 | Depression | + | – | + | – | + | + | – | +(Hand) | SNGP/LOA | – |
| Lu et al.15 | P1 | F/30 | Right hand awkwardness | + | – | +/+ | – | – | – | NM | – | – | – |
| Lu et al.15 | P2 | F/8 | Unsteady and slow | + | + | +/+ | + | + | – | NM | – | – | + |
| Lu et al.15 | P3 | F/19 | Unsteady and slow | + | + | +/+ | + | + | – | NM | – | Nystagmus | + |
| Kim et al.16 | P1 | F/22 | Unsteady gait and fall | + | – | +/– | + | + | + | + | – | – | – |
| Kim et al.16 | P2 | M/6 | Unsteady gait and fall | + | – | NK | + | + | – | + | – | – | – |
| Malaguti et al.17 | P | F/27 | Urge incontinence, stiff leg | + | – | +/− | – | + | + | + | – | Slow saccades | Urge incontinence |
| Xie et al.18 | PA | M/36 | Foot drag | + | + | +/NM | – | – | – | + | – | – | – |
| Xie et al.18 | PB | M/36 | Tremor at rest | + | + | +/NM | – | – | – | + | – | – | – |
| Our patient | P | M/16 | Foot drag and blepharospasm | + | + | –/– | – | + | + | + | + (Face, hands) | – | – |
DA, Dopamine agonist; F, Female; F1 and F2, Family 1 and Family 2; LD, Levodopa Response; LID, Levodopa-induced Dyskinesia; LOA, Lid Opening Apraxia; M, Male; NK, Not Known (data not available for the original article’s authors); NM, Not Mentioned (data not mentioned in the original article); OGC, Oculogyric Crisis; OH, Orthostatic Hypotension; P, Patient; PA and PB, Patient A and B; SNGP, Supranuclear Gaze Palsy; +, Positive; –, Negative.
Table 2. Mutations and Ethnicity of Patients with PLA2G6 Associated Dystonia–Parkinsonism.
| Report | Patients | Ethnicity/Consanguinity | Mutation | Result |
|---|---|---|---|---|
| Paisán-Ruiz et al.8 | Family 1 | Indian/+1 | c.2222G>A | p.R741Q |
| Paisán-Ruiz et al.8 | Family 2 | Pakistani/+ | c.2239C>T | p.R747W |
| Sina et al.9 | One family | Iranian/+ | c.1894C>T | p.R632W |
| Bower et al.10 | One family | European/– | c.4C>A/Del Ex 3 | p.Q2K/pL71_S142del |
| Yushino et al.11 | Patient A | Japanese/– | c.216C>A/c.1904G>A | p.F72L/p.R635Q |
| Yushino et al.11 | Patients B1,B2 | Japanese/– | c.1354C>T/c.1904G>A | p.Q452X/p.R635Q |
| Shi et al.12 | One patient | Chinese/+ | c.991G>T | p.D331Y |
| Virmani et al.13 | One family | Indian/+ | c.2222G>A | p.R741Q |
| Agarwal et al.14 | One family | Scandinavian/– | c.238G>A | p.A80T |
| Lu et al.15 | Two families | Han Chinese/– | c.991G>T/c.1077G>A | p.D331Y/p.M358IfsX |
| Lu et al.15 | One family | Han Chinese/+ | c.991G>T | p.D331Y |
| Kim et al.16 | One family | Korean/– | c.1039G>A/c.1670C>T | p.G347R/p.S557L |
| Malaguti et al.17 | One family | Italian/– | c.1547C>T | p.A516W |
| Xie et al.18 | Two families | Chinese/+ | c.991G>T | p.D331Y |
| This work | One Family | Pakistani/+ | c.2222G>A | p.R741Q |
+, Consanguineous Parents; –, Non-consanguineous Parents.
Thirteen patients were the result of consanguineous marriages.
Neuropsychiatric disorders such as depression, psychosis, cognitive decline, dementia, personality changes, behavioral disorders, and anxiety and obsessive compulsive disorders have all been described and can be presenting symptoms (Table 3).
Table 3. Summary of Neuropsychiatric Disorders.
| Reference | Patient number (if appropriate): Symptoms |
|---|---|
| Paisán-Ruiz et al.8 | P1: Cognitive decline, depression |
| P2: Cognitive decline, frontal execution dysfunction, personality change with aggression | |
| Sina et al.9 | Cognitive decline |
| Bower et al.10 | P1: Depression |
| P2: Behavioral difficulties, delusions, and paranoia | |
| Yoshino et al.11 | A: Frontotemporal dementia, depression, personality/behavioral changes, disordered social conduct, and apathy |
| B1, B2: Dementia | |
| Virmani et al.13 | P1: Depression with psychosis, pseudobulbar affect |
| P2: Depression and cognitive decline | |
| Agarwal et al.14 | Anxiety, obsessive compulsive disorder, depression, frontal executive dysfunction |
| Lu et al.15 | Dementia |
| Kim et al.16 | Low IQ |
| Malaguti et al.17 | Dysphoric and anosognosic behavior, executive dysfunction such as impulsive behavior, reduced strategic planning, inability to use environmental feedback to shift cognitive sets, reduced mental flexibility, and mild memory impairment |
Brain MRI was performed in all patients (Table 4). Cerebral atrophy was found in 13 patients (54%)8,9,11,13,15,17 and cerebellar atrophy in seven (29%).10,15,16 Other patients were either normal or imaging was not reported in detail. Iron was found in only eight patients (33%):10,11,14,16,17 four on gradient echo (GRE) or susceptibility-weighted imaging (SWI), two on T2, and two on both T2 and GRE. Iron was deposited in the globus pallidus10 and substantia nigra,17 or both.11,16
Table 4. Summary of clinical and radiological features of PLAN phenotypes[22].
| INAD | Atypical NAD | PLAN-DP | |
|---|---|---|---|
| Age of onset | 6 months to 3 years | Early childhood; can be as late as end of second decade | 4–36 years |
| Brain MRI | Cerebellar atrophy, cerebellar gliosis, posterior corpus callosum abnormalities (thinning, vertical orientation, elongation), apparent claval hypertrophy, iron deposition in basal ganglia (increases with age) | Iron deposition with or without cerebellar atrophy | Normal imaging, cerebral and/or cerebellar atrophy, iron deposition in basal ganglia (33%), corpus callosum changes similar to INAD (some cases) |
| Disease presentation | Gait disturbance and loss of ambulation, truncal hypotonia with hyper-reflexia and hypertonicity, neuroregression with loss of acquired motor skills | Gait impairment or ataxia; social communication difficulties, such as speech difficulties and autistic trait | Gait impairment, dystonia, Parkinsonism, tremor at rest, speech difficulties, and neuropsychiatric disorders |
| Disease progression | Spastic tetraparesis, with symmetrical pyramidal tract signs and areflexia | Dystonia and dysarthria, neuropsychiatric features, such as hyperactivity, impulsivity, emotional lability, and poor attention | Severe dystonia and/or Parkinsonism, spasticity, myoclonus, autonomic dysfunction, seizure, neuropsychiatric features, and cognitive decline |
| Ocular abnormalities | Strabismus, nystagmus, optic nerve atrophy | Strabismus, nystagmus, optic nerve atrophy | Supranuclear gaze palsy, slow saccades, fragmented saccades, nystagmus, lid opening apraxia |
INAD, Infantile Neuroaxonal Dystrophy; MRI, Magnetic Resonance Imaging; PLAN-DP, PLA2G6-associated Neurodegeneration Dystonia–Parkinsonism.
Functional imaging studies were carried out in some subjects. Single positron emission computed tomography in one patient showed frontal hypoperfusion compatible with the patient’s clinical features of frontotemporal dementia.11 In another patient with dementia and ataxia, fludeoxyglucose positron emission tomography (PET) demonstrated frontal and cerebellar hypometabolism, corresponding to cerebral and cerebellar atrophy seen on brain MRI.15
Reduced dopamine transporter (DAT) labeling was seen in all 10 patients studied using this methodology.8,12,14–18 In order to examine pre- and post-synaptic dopaminergic function, one patient received four different tracers: PET with [18]F-6-flouro-l-dopa, [11]C-dihydrotetrabenazine, and [11]C-d-thereo-methylphenidate (DAT marker) disclosed reduced presynaptic dopaminergic uptake, while [11]C-raclopride showed increased post-synaptic dopaminergic receptor labeling. Labeling with all tracers was identical to that seen in idiopathic Parkinson’s disease (IPD).14
The only mutation examined for its effects upon the catalytic function of PLA2G6 was the p.D331Y mutation. The p.D331Y mutant cells showed a 70% reduction in PLA2G6 catalytic function when compared with their wild-type counterparts.12
Discussion
We report a new case of adult-onset PLAN with dystonia, Parkinsonism, and epilepsy. On review of the literature there is notable heterogeneity in clinical presentation and phenotype. Parkinsonism (bradykinesia and rigidity) appeared uniformly during the disease course; however, the initial presentation varied greatly, and neuropsychological symptoms such as depression, psychosis, and cognitive decline preceded the motor problems on several occasions (Table 1, Table 4).10,13,14 In a couple of cases, onset was in early childhood with non-specific signs of motor slowness or speech problems.8,10,13,15
Autonomic dysfunction, specifically urinary or bowel dysfunction, has been observed in a number of cases, although not the current case, and may be an important diagnostic clue as this feature is not typical of most other neurodegenerative disorders affecting this age group. The pathophysiology of this symptom is unknown but is likely related to spinal cord neurodegeneration.
There is no evidence of a genotype–phenotype correlation, apart from possibly in patients with pure Parkinsonism and the p.D331Y mutation.12,15,18 The similarity between these four patients from different families, specifically the presence of Parkinsonism without dystonia, prompted the authors to propose a correlation between this genotype and phenotype;18 however, more cases are required to support their hypothesis.
In our patient and in the previously reported cases, most MRI images showed rather non-specific changes. Iron accumulation was found in only eight patients (33%), which is less than what is reported in INAD (40–50%).7,20 In four of the PLAN-DP cases, iron was detected on GRE/SWI sequences, emphasizing the importance of these techniques in the appropriate situation. The process of iron deposition in PLAN-DP is not yet clear. In INAD, iron accumulates over time,7 but there has been neither a prospective study in PLAN-DP imaging nor sequential imaging using a unified protocol, thus this issue requires further study. In addition to the finding of iron deposition, other helpful imaging findings are a smooth and vertically oriented posterior corpus callosum, and apparent claval hypertrophy. Although these structural alterations have been previously described in patients with INAD and atypical NAD,7,20 they were present in the current patient (Figure 1) and in those reported by Lu et al.15 and Malaguti et al.17
PET imaging demonstrated a dysfunctional presynaptic dopaminergic system and increased post-synaptic uptake in patterns similar to those in IPD, thus functional imaging techniques are not helpful in differentiating PLAN-DP from IPD.
In addition to the similarities on functional imaging and reports of an early response to levodopa,8,9,11–18 the pathology of PLAN-DP resembles that of IPD. In a post-mortem study, Paisán-Ruiz et al.21 examined brains of two PLAN phenotypes (INAD n=4; PLAN-DP n=3) and found α-synuclein pathology with Lewy bodies and Lewy neurites in all cases, and iron deposition in two PLAN-DP brain sample and one INAD brain sample. Lewy body distribution varied, from being restricted to the medulla (Braak stage 1)22 to extensive spread throughout the brain including the neocortex (Braak stage 6).22 This identical α-synuclein pathology suggests a possible final common pathway in the pathogenesis of PLAN and IPD, whether or not Parkinsonism or dystonia is present. In this study, extensive tau pathology with neurofibrillary tangles and neuropil threads was found in four cases, suggesting a link between the PLA2G6 mutation and tau hyperphosphorylation and deposition.
The protein product of PLA2G6, phospholipase-A2 group-VI (iPLA2-IVA), is an enzyme that is involved in the metabolism of glycerophospholipids,23 phospholipid remodeling, arachidonic acid release, synthesis of prostaglandins and leukotrienes, and apoptosis.24 It plays an important role in inner mitochondrial membrane homeostasis,25 in particular in maintaining normal functioning of cardiolipin, the signature phospholipid of mitochondria,26,27 which tethers electron transfer chain molecules to inner mitochondrial membrane.28 iPLA2-VIA also plays a role in capacitative Ca entry, a mechanism that is important in intracellular Ca2+ homeostasis.29 Dysfunction of mitochondrial and Ca2+ homeostasis may be key elements in the pathophysiology of PLAN, possibly similar to those which have been postulated in IPD.30–32
A dramatic reduction in catalytic activity of the p.D331Y mutated enzyme was reported.12 However, other authors found that INAD-producing mutations severely impaired iPLA2-IVA enzyme function, while mutations causing PLAN-DP either did not change enzyme activity nor increased it.33 To explain this discrepancy, we believe that other genetic factors rather than just catalytic activity of iPLA2-IVA play a substantial role in determining phenotype. A report of two siblings with identical genotype and different phenotypes supports this idea.16
In conclusion, PLAN-DP is characterized by remarkable heterogeneity in disease presentation and course. Our understanding of this disorder and its phenotypic spectrum continues to evolve and expand with the ongoing publication of case reports and series. At present, it appears that there is no clear clinical scenario typical of PLAN-DP. Basal ganglia iron deposition is helpful when present, and should be sought with the appropriate MRI sequences, but if absent does not eliminate the diagnosis. This disorder should be considered on the list of differential diagnoses for a wide range of complex, young-onset neurodegenerative disorders.
Acknowledgments
We thank the patient and his relatives for their cooperation with this work.
Footnotes
Funding: This work is supported in part by the National Institute of Neurological Disorders and Stroke of the National Institute of Health (R01NS079388; CPR).
Financial Disclosures: None.
Conflict of Interest: The authors report no conflict of interest.
References
- 1.Seitelberger F. Neuroaxonal dystrophy: Its relation to aging and neurological diseases. Handbook of clinical neurology, Extrapiramidal Disorders. In: Vinke PJ, Bruyn GW, Klawans HL, editors. 49. vol 5. Amsterdam: Elsevier Science; 1986. pp. 391–415. [Google Scholar]
- 2.Aicardi J, Castelein P. Infantile neuroaxonal dystrophy. Brain. 1979;102:727–748. doi: 10.1093/brain/102.4.727. doi: http://dx.doi.org/10.1093/brain/102.4.727. [DOI] [PubMed] [Google Scholar]
- 3.Jellinger K. Neuroaxonal dystrophy: Its natural history and related disorders. Prog Neuropathol. 1973;2:129–180. doi: http://dx.doi.org/10.1007/978-3-642-47449-1_2. [Google Scholar]
- 4.Nardocci N, Zorzi G, Farina L, et al. Infantile neuroaxonal dystrophy: Clinical spectrum and diagnostic criteria. Neurology. 1979;52:1472–1478. doi: 10.1212/wnl.52.7.1472. doi: http://dx.doi.org/10.1212/WNL.52.7.1472. [DOI] [PubMed] [Google Scholar]
- 5.Morgan NV, Westaway SK, Morton JE, et al. PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nat Genet. 2006;38:752–754. doi: 10.1038/ng1826. doi: http://dx.doi.org/10.1038/ng1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khateeb S, Flusser H, Ofir R, et al. PLA2G6 mutation underlies infantile neuroaxonal dystrophy. Am J Hum Genet. 2006;79:942–948. doi: 10.1086/508572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurian MA, Morgan NV, MacPherson L, et al. Phenotypic spectrum of neurodegeneration associated with mutations in the PLA2G6 gene (PLAN) Neurology. 2008;70:1623–1629. doi: 10.1212/01.wnl.0000310986.48286.8e. doi: http://dx.doi.org/10.1212/01.wnl.0000310986.48286.8e. [DOI] [PubMed] [Google Scholar]
- 8.Paisán-Ruiz C, Bhatia KP, Li A, et al. Characterization of PLA2G6 as a locus for dystonia-parkinsonism. Ann Neurol. 2009;65:19–23. doi: 10.1002/ana.21415. doi: http://dx.doi.org/10.1002/ana.21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sina F, Shojaee S, Elahi E, Paisan-Ruiz C. R632W mutation in PLA2G6 segregates with dystonia-parkinsonism in a consanguineous Iranian family. Eur J Neurol. 2009;16:101–104. doi: 10.1111/j.1468-1331.2008.02356.x. doi: http://dx.doi.org/10.1111/j.1468-1331.2008.02356.x. [DOI] [PubMed] [Google Scholar]
- 10.Bower MA, Bushara K, Dempsey MA, Das S, Tuite PJ. Novel mutations in siblings with later-onset PLA2G6-associated neurodegeneration (PLAN) Mov Disord. 2011;26:1768–1769. doi: 10.1002/mds.23617. doi: http://dx.doi.org/10.1002/mds.23617. [DOI] [PubMed] [Google Scholar]
- 11.Yoshino H, Tomiyama H, Tachibana N, et al. Phenotypic spectrum of patients with PLA2G6 mutation and PARK14-linked parkinsonism. Neurology. 2010;75:1356–1361. doi: 10.1212/WNL.0b013e3181f73649. doi: http://dx.doi.org/10.1212/WNL.0b013e3181f73649. [DOI] [PubMed] [Google Scholar]
- 12.Shi B-h, Tng B-s, Wang L, et al. PLA2G6 mutation in autosomal recessive early-onset parkinsonism in a Chinese cohort. Neurology. 2011;77:175–181. doi: 10.1212/WNL.0b013e318221acd3. doi: http://dx.doi.org/10.1212/WNL.0b013e318221acd3. [DOI] [PubMed] [Google Scholar]
- 13.Virmani T, Thenganatt MA, Goldman JS, Kubisch C, Greene PE, Alcalay RN. Oculogyric crisis induced by levodopa in PLA2G6 parkinsonism-dystonia. Parkinsonism Relat Disord. 2014;20:245–247. doi: 10.1016/j.parkreldis.2013.10.016. doi: http://dx.doi.org/10.1016/j.parkreldis.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal P, Hogarth P, Hayflick S, et al. Imaging striatal function in Phosphpolipase A2 Group IV-related parkinsonism. Move Disord. 2012;27:1698–1699. doi: 10.1002/mds.25160. doi: http://dx.doi.org/10.1002/mds.25160. [DOI] [PubMed] [Google Scholar]
- 15.Lu CS, Lai SC, Wu RM, et al. PLA2G6 mutations in PARK14-linked young-onset parkinsonism and sporadic Parkinson’s disease. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:183–191. doi: 10.1002/ajmg.b.32012. doi: http://dx.doi.org/10.1002/ajmg.b.32012. [DOI] [PubMed] [Google Scholar]
- 16.Kim YJ, Lyoo CH, Hong S, Kim NY, Lee MS. Parkinsonism Relat Disord. 2015. Neuroimaging studies and whole exom sequencing of PLA2G6-associated neurodegeneration in a family with intrafamilial phenotype heterogeneity. doi: http://dx.doi.org/10.1016/j.parkreldis.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Malaguti MC, Melzi V, Di Giacopo R, et al. A novel homozygous PLA2G6 mutation causes dystonia-parkinsonism. Parkinsonism Relat Disord. 2015;21:337–339. doi: 10.1016/j.parkreldis.2015.01.001. doi: http://dx.doi.org/10.1016/j.parkreldis.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Xie F, Cen Z, Ouyang Z, Wu S, Xiao J, Luo W. Parkinsonism Relat Disord. 2015. Homozygous p.D331Y mutation in PLA2G6 in two patients with pure autosomal recessive early onset parkinsonism: Further evidence of a fourth phenotype of PLA2G6-associated neurodegeneration. [DOI] [PubMed] [Google Scholar]
- 19.Paisan-Ruiz C, Guevara R, Federoff M, et al. Early-onset L-dopa responsive parkinsonism with pyramidal signs due to ATP13A2, PLA2G6, FBXO7 and spatacsin mutations. Move Disord. 2010;25:1791–1800. doi: 10.1002/mds.23221. doi: http://dx.doi.org/10.1002/mds.23221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Illingworth MA, Meyer E, Chong WK, et al. PLA2G6-associated neurodegeneration (PLAN): Further expansion of the clinical, radiological and mutation spectrum associated with infantile and atypical childhood-onset disease. Mol Genet Metab. 2014;112:183–189. doi: 10.1016/j.ymgme.2014.03.008. doi: http://dx.doi.org/10.1016/j.ymgme.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paisán-Ruiz C, Li A, Schneider SA, et al. Widespread Lewy body and tau accumulation in childhood and adult onset dystonia-parkinsonism cases with PLA2G6 mutations. Neurobiol Aging. 2012;33:814–823. doi: 10.1016/j.neurobiolaging.2010.05.009. doi: http://dx.doi.org/10.1016/j.neurobiolaging.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 23.Kurian MA, Hayflick SJ. Panthothenate kinase-associated neurodegeneration (PKAN) and PLA2G6-associated neurodegeneration (PLAN): Review of two major neurodegeneration with brain iron accumulation (NBIA) phenotypes. Int Rev Neurobiol. 2013;110:49–71. doi: 10.1016/B978-0-12-410502-7.00003-X. doi: http://dx.doi.org/10.1016/B978-0-12-410502-7.00003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balsinde J, Balboa M A. Cellular regulation and proposed biological functions of group VIA calcium-independent phospholipase A2 in activated cells. Cell Signal. 2005;17:1052–1062. doi: 10.1016/j.cellsig.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Beck G, Sugiura Y, Shinzawa K, et al. Neuroaxonal dystrophy in calcium-independent phospholipase A2β deficiency results from insufficient remodeling and degeneration of mitochondrial and presynaptic membranes. J Neurisci. 2011;31:11411–11420. doi: 10.1523/JNEUROSCI.0345-11.2011. doi: http://dx.doi.org/10.1523/JNEUROSCI.0345-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Claypool SM, Koehler CM. The complexity of cardiolipin in health and disease. Trends Biochem Sci. 2012;37:32–41. doi: 10.1016/j.tibs.2011.09.003. doi: http://dx.doi.org/10.1016/j.tibs.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zachman DK, Chicco AJ, McCune SA, Murphy RC, Moore RL, Sparagna GC. The role of calcium-independent phospholipase A2 in cardiolipin remodeling in the spontaneously hypertensive heart failure rat heart. J Lipid Res. 2010;51:525–534. doi: 10.1194/jlr.M000646. doi: http://dx.doi.org/10.1194/jlr.M000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rouault TA. Iron metabolism in the CNS: Implications for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:551–564. doi: 10.1038/nrn3453. doi: http://dx.doi.org/10.1038/nrn3453. [DOI] [PubMed] [Google Scholar]
- 29.Strokin M, Seburn KL, Cox GA, Martens KA, Reiser G. Severe disturbance in the Ca2+ signaling in astrocytes from mouse models of human infantile neuraxonal dystrophy with mutated Pla2g6. Hum Mol Gen. 2012;21:2807–2814. doi: 10.1093/hmg/dds108. doi: http://dx.doi.org/10.1093/hmg/dds108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schapira AHV, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- 31.Luth ES, Stavrovskaya IG, Bartels T, Kristal BS, Selkoe DJ. Soluble, prefibrillar α-synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction. J Biol Chem. 2014;289:21490–21507. doi: 10.1074/jbc.M113.545749. doi: http://dx.doi.org/10.1074/jbc.M113.545749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melachroinou K, Xilouri M, Emmanouilidou E, et al. Deregulation of calcium homeostasis mediated secreted α-synuclein-induced neurotoxicity. Neurobiol Aging. 2013;34:2853–2865. doi: 10.1016/j.neurobiolaging.2013.06.006. doi: http://dx.doi.org/10.1016/j.neurobiolaging.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Engel LA, Jing Z, O’Brien DE, Sun M, Kotzbauer PT. Catalytic function of PLA2G6 is impaired by mutations associated with infantile neuraxonal dystrophy but not dystonia-parkinsonism. PLoS ONE. 2010;5(9):e12897. doi: 10.1371/journal.pone.0012897. doi: http://dx.doi.org/10.1371/journal.pone.0012897. [DOI] [PMC free article] [PubMed] [Google Scholar]